Abstract
Aims
This article reviews the importance of the renin-angiotensin-aldosterone system (RAAS) in the cardiometabolic continuum; presents the pros and cons of dual RAAS blockade with angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs); and examines the theoretical and practical benefits supporting the use of direct renin inhibitors (DRIs) in combination with ACEIs or ARBs.
Main methods
The author reviewed the literature for key publications related to the biochemical physiology of the RAAS and the pharmacodynamic effects of ACEIs, ARBs, and DRIs, with a particular focus on dual RAAS blockade with these drug classes.
Key findings
Although ACEI/ARB combination therapy produces modest improvement in BP, it has not resulted in the major improvements predicted given the importance of the RAAS across the cardiorenal disease continuum. This may reflect the fact that RAAS blockade with ACEIs and/or ARBs leads to exacerbated renin release through loss of negative-feedback inhibition, as well as ACE/aldosterone escape through RAAS and non–RAAS-mediated mechanisms. Plasma renin activity (PRA) is an independent predictor of morbidity and mortality, even for patients receiving ACEIs and ARBs. When used alone or in combination with ACEIs and ARBs, the DRI aliskiren effectively reduces PRA. Reductions in BP are greater with these combinations, relative to the individual components alone.
Significance
It is possible that aliskiren plus either an ACEI or ARB may provide greater RAAS blockade than monotherapy with ACEIs or ARBs, and lead to additive improvement in BP and clinically important outcomes.
Keywords: hypertension, renin-angiotensin-aldosterone system (RAAS) inhibition, angiotensin II, angiotensin-(1–7), combination therapy, aliskiren, prorenin/renin receptor
Introduction
The renin-angiotensin-aldosterone system (RAAS) plays a key role in the pathophysiology and development of hypertension, atherosclerosis, congestive heart failure (CHF), type 2 diabetes mellitus (DM), and renal disease (Weir and Dzau 1999). Specifically, angiotensin II (Ang II) is a major effector of vasoconstriction, cell growth, sodium and water retention, and sympathetic activation; it appears to promote endothelial dysfunction, inflammation, oxidative stress, insulin resistance, and reduced β-cell responsiveness. The close relationship between the RAAS and hypertension has led to compelling indications to block the formation or activity of Ang II through use of angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) (Table 1) (Chobanian et al. 2003; Mancia et al. 2007).
Table 1.
Indications and contraindications for the use of ACEIs and ARBs (Chobanian et al. 2003; Mancia et al. 2007)
| ACEIs | ARBS | |||
|---|---|---|---|---|
| Guideline | 2007 ESH/ESC | JNC 7 | 2007 ESH/ESC | JNC 7 |
| Indications | CHF | CHF | CHF | CHF |
| Diabetes/DN | Diabetes | Diabetes/DN | Diabetes | |
| CKD | CKD | CKD | CKD | |
| Post-MI | Post-MI | Post-MI | ||
| Proteinuria/MA | Prevention of recurrent stroke | Proteinuria/MA | ||
| LVH | High risk of coronary disease | LVH | ||
| Recurrent AF | Recurrent AF | |||
| Metabolic syndrome | Metabolic syndrome | |||
| Prevention of recurrent stroke | Prevention of recurrent stroke | |||
| Carotid atherosclerosis | ACEI-induced cough | |||
| LVD | ||||
| Nondiabetic nephropathy | ||||
| Contraindications | Pregnancy | Pregnancy | ||
| Hyperkalemia | Hyperkalemia | |||
| Bilateral renal artery stenosis | Bilateral renal artery stenosis | |||
| Angioneurotic edema | ||||
Abbreviations: ACEI = angiotensin-converting enzyme inhibitor; AF = atrial fibrillation; ARB = angiotensin receptor blocker; CHF = congestive heart failure; CKD = chronic kidney disease; DN = diabetic nephropathy; ESC = European Society of Cardiology; ESH = European Society of Hypertension; JNC 7 = Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; LVD = left ventricular dysfunction; LVH = left ventricular hypertrophy; MA = microalbuminuria; MI = myocardial infarction.
Although ACEIs and ARBs are among the most effective and safe antihypertensives, when used as monotherapy they control blood pressure (BP) effectively (<140/90 mm Hg in uncomplicated patients, <130/80 mm Hg in diabetics/complicated patients) in only 40% to 60% of patients with mild-to-moderate hypertension (Ibrahim 2006). Furthermore, Weber and Giles (Weber and Giles 2006) noted that ACEIs and ARBs have not produced the major reductions in clinical outcomes that were predicted based on the centrality of the RAAS in the pathophysiology of cardiorenal disease. They speculate that additional novel methods of RAAS blockade may yield better control of hypertension and improved organ protection. One such approach explored combination therapy with ACEIs and ARBs. Although some studies have shown this combination to provide modest benefits beyond monotherapy with either class of agent, other studies, and particularly ONTARGET (Yusuf et al. 2008; Mann et al. 2008), have cast doubt on the long-term safety and effectiveness of this strategy.
Direct renin inhibitors (DRIs), when used in combination with ACEIs or ARBs, may provide more complete RAAS blockade, greater BP control, and better target-organ protection. This article reviews the importance of the RAAS in the cardiometabolic continuum, presents the pros and cons of dual RAAS blockade with ACEIs and ARBs, and examines the theoretical and practical benefits supporting the use of DRIs in combination with ACEIs or ARBs.
The Biochemical Physiology of the RAAS
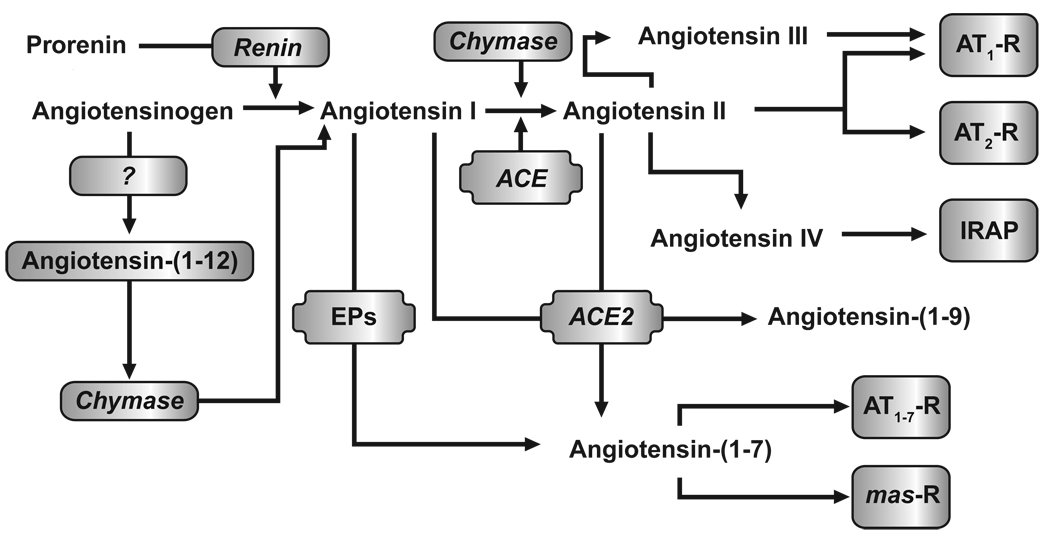
Figure 1 illustrates the current biochemical pathways involved in the production of biologically active angiotensins. In the RAAS, enzymatically inactive prorenin, primarily synthesized in the kidney and accounting for 70% to 90% of the renin in the circulation, is proteolytically converted to enzymatically active renin in response to renal baroreceptor signaling, sodium concentration changes, sympathetic nerve stimulation, and negative feedback by Ang II on juxtaglomerular cells (Atlas 2007). Angiotensinogen, an α2-globulin produced mainly in the liver, is cleaved by renin to generate angiotensin 1 (Ang I; Ang[1–10]). Plasma renin activity (PRA) clinically provides a measure of the endogenous rate of Ang I production. This assay appears to be an important predictor of adverse cardiovascular outcomes and death across the cardiovascular continuum. For example, untreated hypertensive patients with high PRA (vs low) had a 2.4-fold higher risk for cardiovascular disease (Alderman et al. 1997), and patients with severe coronary artery disease and high PRA (vs low) had a significantly greater risk for CHF hospitalization and all-cause death (Bair et al. 2009).
Figure 1.
Biochemical pathways denoting the enzymes and intermediate peptides involved in the formation of active angiotensin peptides. ACE = angiotensin-converting enzyme; AT1-R = angiotensin type 1 receptor; AT2-R = angiotensin type 2 receptor; IRAP = insulin-regulated aminopeptidase; mas-R = Mas receptor.
[Note: for black & white reproduction/printing only]
Ang I is then metabolized by membrane-bound angiotensin-converting enzyme (ACE), found in vascular endothelial, neuroepithelial, and renal proximal tubule cells to produce biologically active Ang II (Ang[1–8]). Although the ACE pathway is important for Ang II generation, Ang II can be formed by non-ACE pathways in various tissues via chymase, cathepsin G, and kallikrein-like enzymes. In a study of healthy volunteers, although virtually all Ang II produced in the kidneys was renin dependent, >40% of circulating Ang II was derived from non-ACE pathways (Hollenberg et al. 1998). Ihara and colleagues (Ihara et al. 1999) reported that >80% of Ang II in normal and atherosclerotic aortas was derived from chymase-dependent pathways. This suggests that abnormal activation of local tissue RAAS through non-ACE pathways may have more of a role than originally thought and be clinically significant.
The pressor action of Ang II is chiefly the result of Ang II binding to the Ang II type 1 receptor (AT1-R) in the heart, vasculature, kidneys, adrenal glands, brain, and adipocytes. Formation of the Ang II:AT1-R complex results in the negative-feedback inhibition of renin release and the production and release of aldosterone from the adrenal cortex. The Ang II:AT1-R complex also is responsible for receptor-mediated increases in contractile force, hypertrophy, and fibrosis in the heart; increases in vascular tone; constriction of renal arterioles; and increased reabsorption of sodium in the proximal segments of kidney nephrons. In contrast, the interaction of Ang II with the Ang II type 2 receptor (AT2-R), which normally is present only in low levels in adults, has been hypothesized to provide some degree of cardiorenal protection through receptor-mediated vasodilatation, nitric-oxide (NO) release, kinin-mediated antiproliferative and pro-apoptotic effects in the heart and vasculature, and beneficial effects on sodium resorption by the proximal tubules in the kidney (Lifton et al. 2001). However, data from a meta-analysis have suggested that treatment with ARBs may significantly increase the risk for myocardial infarction (MI) by 8%, and that this “ARB-MI paradox” may be due in part to ARB-mediated upregulation and stimulation of the AT2-R, resulting in growth promotion, fibrosis, hypertrophy, and atherogenic and proinflammatory effects (Strauss and Hall 2006). In contrast to AT1-R blockade with ARBs, ACE inhibitors block the conversion of Ang I to Ang II. Because of this, ACE inhibitors are not associated with the increased levels of Ang II required for AT2-R activation. However, due to the non-discriminating nature of ACE in terms of substrates, its inhibition increases the level of several ACE substrates, including bradykinin, substance P, and enkephalins. Of note, increases in bradykinin and substance P are thought to be responsible for ACE inhibitor-induced cough and angioedema (Dicpinigaitis 2006).
Work from our laboratory first identified angiotensin-(1–7) (Ang[1–7]) as an active product of the RAAS and demonstrated that this peptide functions to oppose the endogenous action of tissue-borne Ang II (Ferrario et al. 1998; Ferrario et al. 1997; Ferrario et al. 2005b; Schiavone et al. 1988). In initial studies, the generation of Ang[1–7] was identified as a product of the metabolism of Ang I via the action of tissue endopeptidases, particularly vascular-endothelium–derived neprilysin (Santos et al. 1992; Welches et al. 1993; Yamamoto et al. 1992). Subsequently, Ang[1–7] is degraded to inactive fragments by ACE. Ang[1–7] provides cardiorenal protection (eg, vasodilation, antiproliferation, anti-fibrosis, and natriuresis) through binding to the Mas proto-oncogene receptor (Santos et al. 2003). Inhibition of the MAP kinase-phosphatase pathway via the Mas receptor appears to be the primary mechanism responsible for the inhibitory effects of Ang[1–7] on cardiac and vascular remodeling (Gallagher et al. 2008; Tallant et al. 2005).
The complexity of the biochemical cascade accounting for the biological actions of the RAAS in the control of cardiovascular function is underscored by additional sequential cleavage of Ang II into the equally potent aldosterone secretagogue angiotensin III (Ang III; Ang[2–8]) and to two other active angiotensins: angiotensin IV (Ang IV; Ang[3–8]) and Ang[1–7]. Although the production of Ang III and Ang IV is highest in the brain and kidney, their direct role in the maintenance of high BP in hypertension has not been demonstrated. In animal models, the Ang IV:Ang II type 4 receptor (AT4-R) complex improved endothelial function and provided protection against acute cerebral ischemia (Faure et al. 2008; Vinh et al. 2008). On the other hand, recent research has buttressed the importance of the opposing role of Ang-(1–7) in physiology and pathology through the characterization of an ACE homologue (ACE2) that acts as a mono carboxypeptidase cleaving Ang II into Ang(1–7) (Vickers et al. 2002). In contrast to ACE, ACE2 is not inhibited by ACEIs such as captopril or lisinopril, nor does it share the same catalytic properties. ACE2 exhibits the highest efficiency (kcat/km) among Ang(1–7)-forming enzymes and a 500-fold greater kcat/km for Ang II compared with Ang I. Similar to ACE, ACE2 exists in both soluble and membrane-associated forms with high expression in the kidney, heart, brain and testes. In this regard, ACE2 contains a single catalytic site that corresponds to the C-terminal domain of somatic ACE (Turner et al. 2002; Vickers et al. 2002). A critical step in the further understanding of ACE2 role in cardiovascular function was achieved by our demonstration that ACE2 maps to a defined quantitative trait locus (QTL) on the X chromosome in three different rat models of hypertension and that, in three salt-sensitive hypertensive rat strains, ACE2 messenger RNA and protein expression are markedly reduced (Crackower et al. 2002).
Although angiotensinogen has remained the undisputed substrate at which renin selectively cleaves the Leu10-Leu11 bond of the glycoprotein to generate Ang I, recent studies revealed the existence of a peptide that derived fromangiotensinogen contains the first 12 amino acids within the N-terminus of the substrate. Nagata et al. (Nagata et al. 2006) showed the endogenous presence of the peptide, now termed angiotensin-(1–12) (Ang[1–12]), in a Japanese-derived strain of Wistar rats and its ability to serve as a substrate for the in vitro and in vivo generation of Ang II. The ability of Ang[1–12] to act as an endogenous substrate for the production of Ang II was documented by showing that the vasoconstrictor effects of Ang[1–12] in the isolated aorta and the systemic circulation were prevented by previous blockade with either captopril or the AT1 receptor antagonist candesartan (Nagata et al. 2006). Moreover, studies from our laboratory showed increased content of Ang[1–12] in cardiac myocytes from spontaneously hypertensive rats (Jessup et al. 2008) and that generation of Ang II from Ang[1–12] is regulated by a non-renin enzyme (Ferrario et al. 2009; Trask et al. 2008). In a more recent study, Prosser et al. (Prosser et al. 2009) showed that cardiac chymase converted Ang[1–12] into Ang II. These findings may explain the observation that aliskiren does not produce complete suppression of plasma concentrations of Ang II and that the combination of aliskiren with losartan or valsartan induces greater blood-pressure responses and further target-organ protection (Chrysant et al. 2008; Dechend et al. 2007; Kamoi 2008; Legrand et al. 2008; Nussberger et al. 2002; Oparil et al. 2007a; Oparil et al. 2007b; Pool et al. 2007; Yarows et al. 2008). The enhanced antihypertensive response induced by the combination of aliskiren with an Ang II receptor antagonist suggests that Ang[1–12] may act as an alternate pathway for the generation of Ang I and Ang II during blockade of renin. Evidence of this possibility has been obtained from bilateral nephrectomized rats in which loss of renal renin was associated with increases in cardiac levels of Ang[1–12] and no changes in tissue levels of Ang II (Ferrario et al. 2009).
Although not confirmed in humans, additional studies in animals indicate that activation of the RAAS may occur via pathways that are Ang II–independent and therefore not subject to inhibition by ACEIs and ARBs. For example, two studies showed that there is a receptor in the heart, brain, kidney, and liver that binds both prorenin and renin, resulting in a 4-fold increase in the activity of renin as well as non-proteolytic activation of prorenin, presumably through unmasking of the catalytic site (Nguyen et al. 2002; Ichihara et al. 2004). In vitro, binding of renin to this receptor increased mitogen-activated protein (MAP) kinases, which were not suppressed by addition of an ACEI or ARB (Nguyen et al. 2002). MAP-kinase increases are implicated in the development of interstitial fibrosis and cardiac hypertrophy. These findings, and the extensive preclinical work on Ang[1–7], ACE2, and now Ang[1–12], present exciting possibilities for developing new therapeutic agents that block the RAAS (Trask and Ferrario 2007).
RAAS Blockade by ACEIs, ARBs, and DRIs
The direct inhibition of renin is a logical target for pharmacologic suppression of the RAAS, because renin-mediated cleavage of angiotensinogen to form Ang I is a rate-limiting first step in the RAAS pathway. However, early attempts to develop DRIs met with little success, and research subsequently focused on developing ACEIs and ARBs, with approval of ACEIs throughout the 1980s and ARBs beginning in the mid 1990s. Table 2 summarizes the effects of DRIs, ACEIs, and ARBs on the RAAS.
Table 2.
Biochemical differences in the various methods used to inhibit the the renin-angiotensin-aldosterone system (RAAS). Note the additive effects (⬆+⬆ [increases] or ⇩+⇩ [decreases]) of dual RAAS blockade. Adapted from Azizi et al (Azizi et al. 2006)
| Component | Single-site RAAS blockade | Dual RAAS blockade | ||||
|---|---|---|---|---|---|---|
| ACEI | ARB | DRI | ACEI + ARB | DRI + ARB | DRI + ACEI* | |
| Enzymes | ||||||
| Plasma renin activity | ⬆ | ⬆ | ⇩ | ⬆+⬆ | ⇩ | ⇩ |
| Plasma renin concentration | ⬆ | ⬆ | ⬆ | ⬆+⬆ | ⬆+⬆ | ⬆+⬆ |
| Renal immunoreactivity | ⬆ | ⬆ | ⬆ | ⬆+⬆ | ⬆+⬆ | ⬆+⬆ |
| Plasma prorenin | ⬆ | ⬆ | ⬆ | ⬆+⬆ | ⬆+⬆ | ⬆+⬆ |
| Plasma ACE | ⇩ | ↔ | ↔ | ⇩ | ↔ | ⇩ |
| Tissue ACE | ⇩ | ↔ | ↔ | ⇩ | ↔ | ⇩ |
| Substrate concentrations | ||||||
| Angiotensinogen | ⇩ | ⇩ | ↔ | ⇩+⇩ | ↔ | ↔ |
| Angiotensin I | ⬆ | ⬆ | ⇩ | ⬆+⬆ | ⇩ or ↔ | ⇩ or ↔ |
| Bradykinin | ⬆ | ↔ | ↔ | ⬆ | ↔ | ⬆ |
| AcSDKP | ⬆ | ↔ | ↔ | ⬆ | ↔ | ⬆ |
| Receptors | ||||||
| Angiotensin type 1 receptor | ↔ | ☒ | ↔ | ↔ and ☒ | ↔ and ☒ | ↔ |
| Angiotensin type 2 receptor | ↔ | ⬆ | ↔ | ⬆ | ↔ | ↔ |
| Bradykinin B2 | ⬆ | ⬆ | ↔ | ⬆+⬆ | ↔ | ⬆ |
| End products | ||||||
| Angiotensin II | ⇩ | ⬆ | ⇩ | ⇩ or ↔ | ⇩ or ↔ | ⇩+⇩ |
| Non–ACE dependent angiotensin II | (A) | ☒ | ☒ | ☒ | ☒ | |
| Angiotensin III | ⇩ | ⬆ | ⇩ | ⇩ or ↔ | ⇩ or ↔ | ⇩+⇩ |
| Angiotensin IV | ⇩ | ⬆ | ⇩ | ⇩ or ↔ | ⇩ or ↔ | ⇩+⇩ |
| Angiotensin[1–7] | ⬆ | ⬆ | ⬆ | ⬆+⬆ | ⬆ or ↔ | ⬆ |
| Aldosterone | ⇩ | ⇩ | ⇩ | (B) | ⇩+⇩ | ⇩+⇩ |
| Miscellaneous | ||||||
| Tissue RAAS | ⇩ | ☒ | ⇩ | ⇩ and ☒ | ⇩ and ☒ | ⇩+⇩ |
Based on theoretical considerations.
Abbreviations: (A) = present; (B) = no major additive effect; ⇩ = decreased or inhibited; ⬆ = increased or stimulated; + = additive effect; ↔ = normal, no change, or not inhibited or stimulated; ☒ = blocked; ACE = angiotensin converting enzyme; ACEI = angiotensin-converting enzyme inhibitor; AcSDKP = acetyl-Ser-Asp-Lys-Pro tetrapeptide; ARB = angiotensin receptor blocker; DRI = direct renin inhibitor; RAAS = renin-angiotensin-aldosterone system.
As mentioned above, ACEIs, but not ARBs, lead to significant accumulation of bradykinin and substance P as ACE mediates the metabolism of these peptides. This potentially contributes to increased vasodilation and decreased thrombosis, atherogenesis, and tissue proliferation, but also is likely responsible for ACEI-related, dose-limiting side effects, such as angioedema and dry cough. Although acute administration of ACEIs effectively reduces Ang II levels, chronic administration can lead to “ACE escape” (Wong et al. 2004). In addition, the ability of glucocorticoids and estrogenic hormones to increase hepatic angiotensinogen production (Lalouel et al 2001) may lead to large increases in Ang I production and subsequent ACE escape.
Reactive increases in plasma renin concentration (PRC) and PRA during ACEI treatment may result in increased Ang II production via non-ACE pathways. Moreover, higher levels of Ang I may overcome the ability of ACEIs to effectively suppress ACE activity. ACE escape also may relate to the relatively low binding affinity of ACEIs for ACE and the relatively low levels of the dosing of ACEIs used in clinical practice to avoid drug-related adverse events. With ARBs, the reactive elevations in PRC and PRA lead to increases in Ang II levels (Schindler et al. 2007). This may result in greater competition and displacement of ARBs from AT1-R sites and reduced antihypertensive efficacy (Burnier and Brunner 2000). Unlike ACEIs, ARBs block the effect of non–ACE-dependent Ang II on the AT1-R and increase the interaction of Ang II with the AT2-R, the benefits and risks of which are not completely understood.
As mentioned previously, in addition to other effects, AT1-R activation results in the release of aldosterone from the adrenal cortex, which contributes to cardiorenal damage (Brown 2005; Remuzzi et al. 2008), and aldosterone antagonists provide benefits related to morbidity and mortality (Pitt et al. 1999; Pitt et al. 2003). Both ACEIs and ARBs inhibit aldosterone production. However, “aldosterone escape” is observed with both drug classes (Athyros et al. 2007), possibly owing to increased serum potassium levels (ACEIs or ARBs), ACE escape (ACEIs), or increased Ang II competing with the AT1-R or binding to the AT2-R (ARBs).
The discovery of novel, nonpeptide, renin antagonists in the early 1980s led to resurgent interest in the development of DRIs, culminating with the approval of aliskiren for the treatment of hypertension in 2007 (Jensen et al. 2008). DRIs provide more complete blockade of the RAAS (Fisher and Hollenberg 2005). Furthermore, although plasma prorenin and PRC increase due to interruption of the Ang II:AT1-R–mediated negative-feedback inhibition of renin release, PRA remains reduced. It appears that DRIs are associated with a low incidence of hyperkalemia, similar to that observed in placebo recipients (Weir et al. 2007), and therefore may reduce the potential for aldosterone escape. Similar to ARBs (and unlike ACEIs), DRIs have no appreciable effect on bradykinin metabolism, and therefore may have better tolerability than ACEIs.
ACEIs and ARBs: Antihypertensive Effects and Clinical Benefits Beyond BP Control
Monotherapy with ACEIs and ARBs effectively controls BP in approximately 40% to 60% of patients with mild-to-moderate hypertension (Ibrahim 2006). Both drug classes reduce the risk of adverse cardiovascular outcomes and are considered suitable for the initiation and maintenance of antihypertensive treatment, either as monotherapy or in combination with other antihypertensives (Chobanian et al. 2003; Mancia et al. 2007).
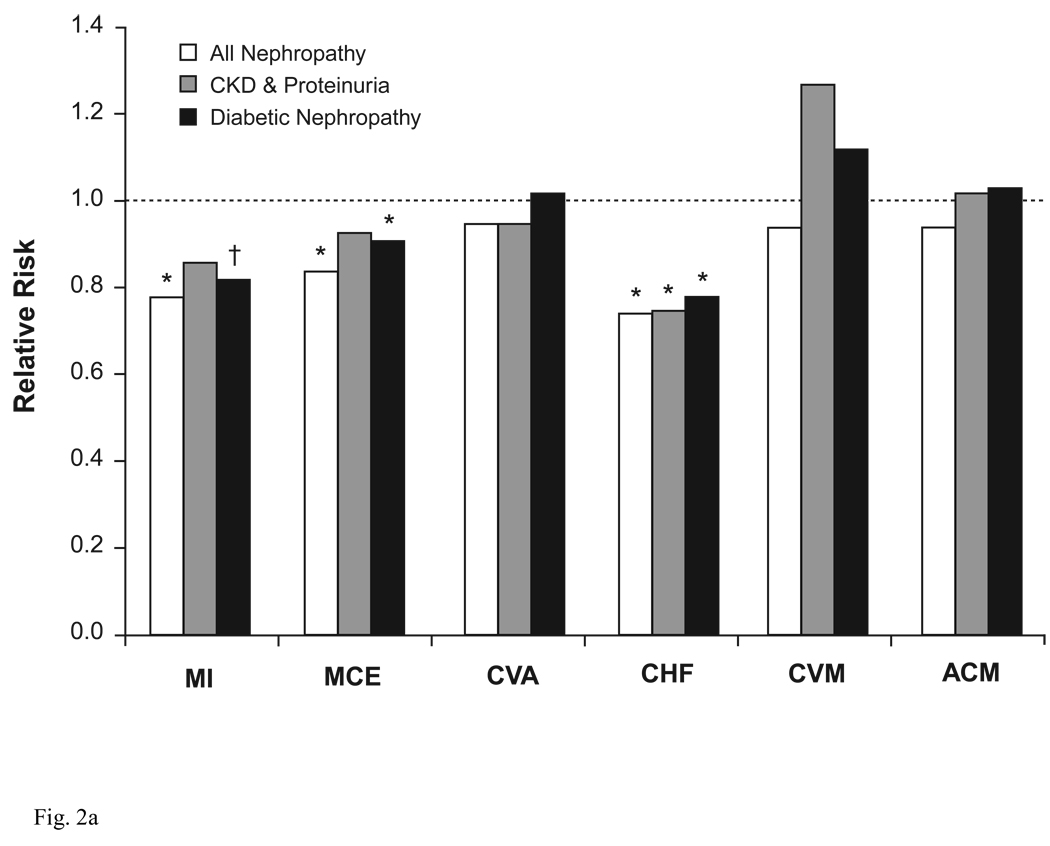
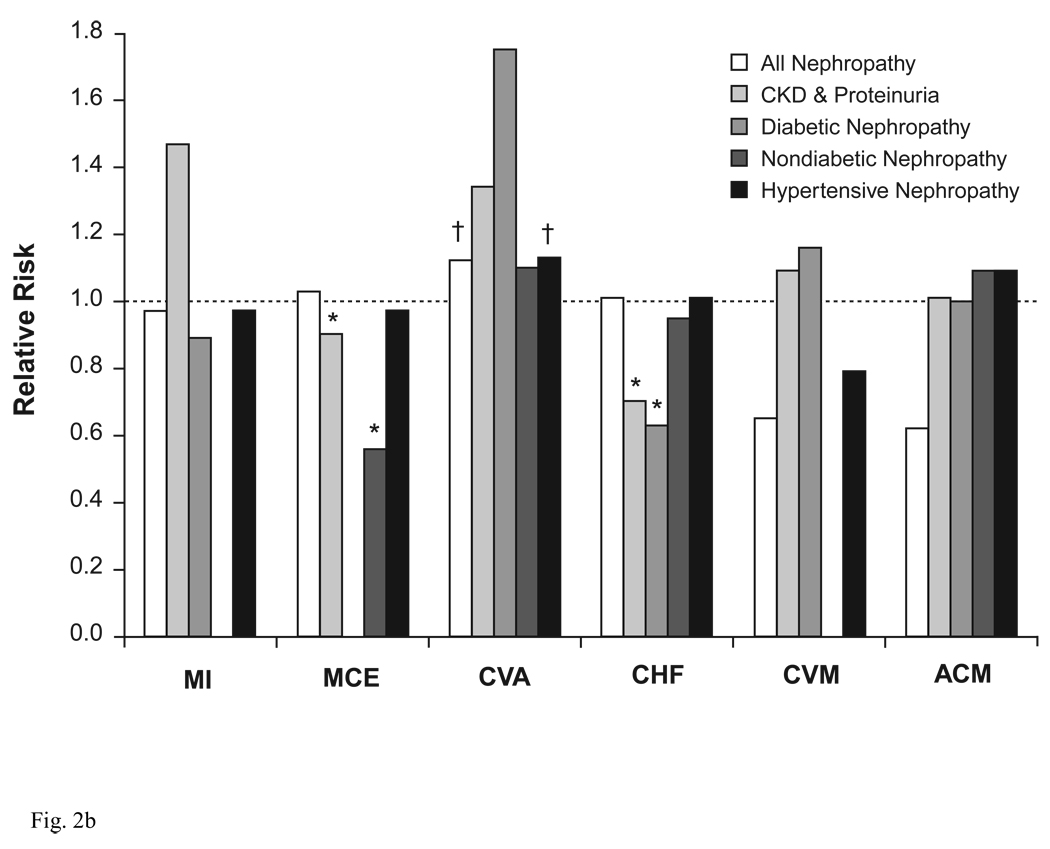
A number of compelling indications exist for using ACEIs and ARBs, which extend beyond simple control of BP (Table 1) (Chobanian et al. 2003; Mancia et al. 2007). ACEIs and ARBs provide protective benefits in target organs, independent of their BP-lowering effects. Many of these benefits arise because the RAAS is abnormally activated in various forms of renal disease, as part of an adaptive response to loss of renal mass and subsequent changes in renal hemodynamics (Schiffrin et al. 2007). Patients who have renal disease, with or without concurrent DM, frequently experience hypertension, dyslipidemia, inflammation, atherosclerosis, vascular calcification, and/or acceleration in the development and progression of cardiovascular disease (Schiffrin et al. 2007). Because of this cardiorenal connection, ACEIs and ARBs are particularly useful for reducing proteinuria, preserving renal function, delaying renal disease, reducing left ventricular hypertrophy (LVH), and attenuating the fibrotic component of LVH (Mancia et al. 2007). As a result, guidelines from the National Kidney Foundation (National Kidney Foundation 2007) strongly recommend that patients with DM, chronic kidney disease (CKD), and hypertension be treated with ACEIs or ARBs, usually in combination with a diuretic, as a means for controlling BP, slowing the progression of CKD, and improving clinical outcomes. A meta-analysis of cardiovascular outcomes data from 25 trials involving nearly 46 000 patients with CKD and proteinuria showed that treatment with ACEIs or ARBs significantly reduced the risk for several cardiovascular outcomes (Figure 2) (Balamuthusamy et al. 2008). These agents also may reduce the incidence of new-onset DM (Andraws and Brown 2007).
Figure 2.
Relative risk for cardiovascular outcomes in patients with chronic kidney disease. (A) ACEIs or ARBs Versus Placebo; (B) ACEIs or ARBs Versus Non-RAAS Antihypertensives. *P<0.05, †P=0.05. ACEI = angiotensin-converting enzyme inhibitor; ACM = all-cause mortality; ARB = angiotensin receptor blocker; CHF = congestive heart failure; CKD = chronic kidney disease; CVA = cerebrovascular accident; CVM = cardiovascular mortality; MCE = major cardiovascular event (eg, MI, CVA, coronary revascularization, unstable angina); MI = myocardial infarction; RAAS = renin-angiotensin-aldosterone system. Adapted from Balamuthusamy et al. (Balamuthusamy et al. 2008)
[Note: for black & white reproduction/printing only]
Dual RAAS Blockade with ACEIs and ARBs
As reviewed above, the existence of multiple pathways for the generation of the biologically active angiotensin peptides posits the question as to how effective current approaches are to suppress the activity of Ang II. At each of the points within the cascade of the RAAS, alternate enzymatic pathways can bypass the blockade of the primary enzyme while it is also possible that intracellularly the formation of angiotensin peptides does not follow what has been characterized in the circulation or the extracellular compartment. Stimulation or repression of a physiological pathway brings about compensatory responses so that the all-or-nothing effect of a particular drug is rendered ineffective. As a response, the idea of combining drugs acting at different sites within the biochemical cascade of the RAAS gained acceptance and, among possible combinations the use of ACEI and ARB became a favorite.
Although the theoretical benefits of combined ACEI- and ARB-based therapy may enhance BP control and improve clinical outcomes in some subpopulations of patients, Weber and Giles (Weber and Giles 2006) observed that, for most patients, these RAAS-based therapies do not reduce the risk for major cardiovascular events (MCEs) to the extent expected based on the central role of the RAAS in the pathophysiology of hypertension and cardiorenal disease. They speculated that this may indicate that the RAAS is not as widely dysregulated in hypertension as previously thought, or that the current strategies and/or agents available for RAAS suppression are not as effective as they could or should be. With respect to the second argument, dual RAAS blockade at different sites in the RAAS pathway theoretically would have provided additive protective effects by further reducing systemic and local levels of some or all angiotensin peptides, which could further inhibit formation of the Ang II:AT1-R effector complex and potentially avoid ACE and aldosterone escape mechanisms (van de Wal et al. 2005; Wolf and Ritz 2005; Unger and Stoppelhaar 2007; Cohn and Goldman 2008).
Although several studies have shown that ACEI/ARB combinations result in modest improvement in BP and proteinuria compared with treatment with only one of these drug classes (Ferrari et al. 2002; McMurray et al. 2003; Nakao et al. 2003; Krum et al. 2004; Kunz et al. 2008), others do not support this combination because of concerns about efficacy (modest improvement in BP with limited or no additional benefit on outcomes) and safety (Arici and Erdem 2009). In a crossover study of 64 hypertensive patients not achieving BP control with full-dose valsartan, the addition of amlodipine reduced ambulatory 24-hour BP to a greater extent than the addition of benazepril (−15.2/−9.9 mm Hg vs −8.6/−6.3 mm Hg, respectively; P<.05) (Stergiou et al. 2005). Similarly, ACEI/ARB combination therapy was less effective for lowering BP than ARB/thiazide diuretic combination therapy in a study of 327 hypertensive patients uncontrolled by ARB monotherapy (Waeber et al. 2001) and in a study of 88 African Americans with hypertension (Weir et al. 2001). In the VALIANT study of 14 703 elderly patients with left ventricular systolic dysfunction, CHF, or both after MI, combination ACEI/ARB therapy was no more effective than monotherapy with either agent for reducing the risk for death and MCEs (White et al. 2005). Two meta-analyses of patients with CHF or left ventricular dystrophy (LVD; including CHARM-Added, Val-HeFT, and VALIANT) also showed that ACEI/ARB combination therapy significantly increases the risk for adverse events (eg, hypertension, worsening renal function, and hyperkalemia), necessitating treatment discontinuation (Phillips et al. 2007; Lakhdar et al. 2008). The largest study of an ACEI/ARB combination conducted to date is ONTARGET, in which >25 000 patients at high risk for MCEs (eg, vascular disease, high-risk DM without CHF) were randomized to receive the ACEI ramipril or the ARB telmisartan in monotherapy or ACEI/ARB combination therapy at maximum recommended doses. After the median follow-up period of 4.7 years, the rates for the primary composite outcome (cardiovascular-related death, MI, stroke, and hospitalization for CHF) were similar for the 3 treatment arms (16.3% to16.7%), but the risk for worsening renal function was higher in the combination arm, as was the rate of discontinuation due to hypotension, syncope, decreased kidney function, and hyperkalemia (Yusuf et al. 2008). In a subsequent analysis of renal outcomes, the risk for the primary composite outcome (dialysis, doubling of serum creatinine, and death) was 9% higher with combination therapy than monotherapy (P=.038), despite significant benefits of combination therapy on indices of proteinuria (Mann et al. 2008). However, these results have been questioned, given that ONTARGET was primarily a cardiovascular-outcomes study; renal outcomes were not rigorously or consistently defined, and few patients had microalbuminuria (13%) or macroalbuminuria (4%) (Sarafidis and Bakris 2008; Berns 2009). Nevertheless, ONTARGET has raised questions regarding the place in therapy for the ACEI/ARB combination (Messerli 2009), a finding that is in keeping with previous experimental studies from my laboratory. In these studies we found that the combination of lisinopril and losartan abrogated the upregulation of cardiac ACE2 mRNA that was observed when animals were administered the single treatments (Ferrario et al. 2005a).
There are several possible clinical and experimental reasons why dual RAAS blockade with ACEIs and ARBs has not resulted in the expected benefits. These include study-design issues as well as theoretical mechanistic considerations. As noted by Arici and Erdem (Arici and Erdem 2009), many clinical studies have been small and of short duration, and most used submaximal doses of ACEIs and ARBs both alone and in combination. Most combination studies were not designed to maximize BP control and, in fact, achieved only modest improvement in BP (~3 to 4 mm Hg) over monotherapy with an ACEI or ARB (Doulton et al. 2005). Additionally, many early studies used once-daily dosing with short-acting ACEIs. Therefore, it is possible that low ACEI concentrations at trough in combination studies using short-acting ACEIs could have increased the likelihood of both acute (method-related) and chronic (mechanistic-mediated) ACE escape. Administration of diuretics also has resulted in increases in PRA (Lijnen et al. 1981), and the use of diuretics as concurrent medications usually is permitted in ACEI and ARB studies.
Increases in both PRA and ACE escape have been associated with adverse clinical outcomes in patients on ACEI or ARB therapy. For example, in a study of 70 patients with CHF, elevated PRA despite 6 months of treatment with an ACEI was an independent predictor of elevated Ang II levels (P=.0004), and elevated plasma Ang II levels were an independent predictor of death or worsening CHF (P=.002) (Roig et al. 2000). In another study, 699 patients with CHF underwent a complete clinical and biochemical workup at baseline and were monitored for a median of 23 months; 81% of them were receiving an ACEI or ARB (Vergaro et al. 2008). Elevated baseline PRA was an independent predictor of death or the need for cardioversion in patients with implantable cardioverter devices (P<.001), and PRA was higher in patients on RAAS inhibitors relative to those not receiving RAAS inhibitors (P=.017). In Val-HeFT, analysis of 4300 patients with CHF who had neurohormonal measurements showed that increased baseline PRA was an independent predictor of all-cause mortality (P=.011) and of combined mortality and morbidity (P=.0025) (Latini et al. 2004). This was observed despite the fact that the majority of patients were receiving treatment with an ACEI and approximately half were also receiving treatment with an ARB. A recent post hoc analysis of Val-HeFT data showed that PRA was 3.7 times higher in patients receiving ACEIs (vs those not receiving them), and that higher PRA at baseline was associated with greater mortality among patients receiving ACEIs (P=.0005) (Masson et al. 2009). Taken together, these findings strongly indicate that PRA is related to adverse clinical outcomes even for patients receiving treatment with ACEIs and/or ARBs, and further raises the possibility that DRIs may be useful alone or in combination with ACEIs or ARBs for reducing the risk for these outcomes.
Knowledge of the complexity of the biochemical pathways mediating the formation of angiotensin peptides suggests that combined ACEI/ARB therapy may induce greater synthesis of intracellular Ang II due to a direct effect of the increased plasma or tissue renin on the prorenin/renin receptor (Pro-RR). I advance this hypothesis from the lessons learned from previous experiments conducted in our laboratory (Ferrario et al. 2005b) and the emerging evidence that the binding of prorenin and renin to the Pro-RR (Nguyen and Contrepas 2008) can lead to stimulation of intracellular formation of Ang II or even activation of growth-promoting signaling pathways via a non-Ang II dependent pathway. By promoting the reversible activation of prorenin and enhancing the enzyme activity of mature renin the Pro-RR activates mitogen-activated protein kinase and hypertrophic, hyperplastic, profibrotic, and cyclooxygenase-2-signaling (Nguyen and Contrepas 2008).
DRIs: Suppression of PRA and Potential Role in Dual RAAS Blockade
As reviewed in the following sections, PRC increases in response to monotherapy with aliskiren, ACEIs, ARBs, amlodipine, aldosterone antagonists, or thiazide diuretics (or combination therapy with aliskiren plus any of these agents), whereas PRA decreases to below baseline when aliskiren is used alone or in combination with other antihypertensives (studies with thiazide diuretics are included because these agents increase PRA levels (Lijnen et al. 1981). Results are summarized below and in Table 2, and only the studies that assessed RAAS biomarkers are described below.
Studies in healthy volunteers
The effects of aliskiren in combination with an ARB on components of the RAAS were explored in a double-blind crossover study in 12 mildly sodium-depleted, healthy, normotensive volunteers who received aliskiren 300 mg, valsartan 160 mg, or aliskiren/valsartan 150/80 mg (Azizi et al. 2004). Twenty-four hours after dosing, PRC increased 14.1-fold and 5.7-fold in response to aliskiren and valsartan monotherapy, respectively, and PRA decreased by 39% with aliskiren but increased 3-fold with valsartan. Levels of Ang I and Ang II decreased by 26% and 36%, respectively, with aliskiren and increased 10-fold and 6-fold with valsartan. The combination of aliskiren and valsartan resulted in a 12-fold increase in PRC and attenuation of the valsartan-induced increases in PRA (1.4–2.1 ng/mL/h), Ang I (11–44 pg/mL), and Ang II (9–19 pg/mL). The rate of urinary aldosterone excretion was greater for subjects receiving valsartan 160 mg than those on either aliskiren 300 mg or aliskiren/valsartan 150/80 mg (18, 13, and 12 µg/24 h, respectively; both P<0.05).
In a pharmacodynamic study in 12 normotensive subjects on a high-sodium diet, levels of PRA, Ang I, and Ang II increased (vs baseline) 48 hours after administration of valsartan 320 mg, but decreased 48 hours after administration of aliskiren 300 mg (Azizi et al. 2007). Aliskiren 300 mg stimulated PRC to a greater extent than valsartan 320 mg, decreased urinary aldosterone excretion for a longer period, and resulted in similar improvements in BP. Compared with valsartan 320 mg, aliskiren/valsartan 150/160 mg resulted in higher PRC (4,080 vs 2201 pg/h/mL area under the curve over 48 h [AUC0–48]; P<0.05), lower PRA (11 vs 49 ng/mL/h; P<0.05), lower Ang I (235 vs 643 pg/h/mL; P<0.05), lower Ang II (109 vs 255 pg/h/mL; P<0.05), and a lower rate of urinary aldosterone excretion (7.15 vs 9.91 µg/48 h; P<0.05).
Studies in patients with hypertension, cardiovascular disease, or diabetes
Three clinical studies evaluated aliskiren alone or in combination with an ARB (valsartan), thiazide diuretic (hydrochlorothiazide [HCTZ]), or both on RAAS biomarkers in patients with mild-to-moderate hypertension (Oparil et al. 2007a; Villamil et al. 2007; Geiger et al. 2009). Another study evaluated aliskiren in combination with a calcium channel blocker (amlodipine) in patients with mild-to-moderate hypertension (Drummond et al. 2007), and a further trial evaluated aliskiren alone and in combination with an ACEI (ramipril) on RAAS biomarkers in patients with mild-to-moderate hypertension and DM (Table 3) (Uresin et al. 2007). In all of these studies, monotherapy with valsartan, amlodipine, or ramipril generally resulted in marked increases in PRC and PRA, whereas treatment with HCTZ either increased these parameters or, in patients who had already received 4 weeks of treatment, had little effect on these parameters. Aliskiren monotherapy (150 or 300 mg) yielded even greater increases in PRC, but in contrast, PRA decreased by ~70% relative to baseline. A reduction in PRA was observed when aliskiren was administered in combination with valsartan, amlodipine, ramipril, or HCTZ, whereas triple therapy with aliskiren/valsartan/HCTZ blunted the increase in PRA associated with valsartan/HCTZ. In all 5 studies, reductions in mean sitting (ms) systolic blood pressure (SBP) and diastolic blood pressure (DBP) were significantly greater for recipients of aliskiren combination therapy than for patients on aliskiren alone or the active comparator alone (Table 3). In the study by Oparil and colleagues of 1797 hypertensive patients (Oparil et al. 2007a), levels of plasma aldosterone increased slightly in placebo subjects (+7%). In contrast, these levels decreased significantly in patients on valsartan 320 mg (−25%; P=0.0007 vs placebo) and aliskiren/valsartan 300/320 mg (− 31%; P<0.0001), and remained relatively unchanged in those on aliskiren 300 mg (−6%; P>0.05). In the study by Uresin and coworkers (Uresin et al. 2007) (837 patients with hypertension and DM), levels of plasma aldosterone were lower than baseline after 8 weeks of aliskiren/ramipril 300/10 mg (−18%; P=0.034), but not after aliskiren 300 mg or ramipril 10 mg alone (−8% and −2%, respectively).
Table 3.
Effects of treatment with aliskiren and amlodipine on PRC, PRA, and BP in randomized, double-blind studies in patients with hypertension, cardiovascular disease, or diabetes
| Study | Drug | N | Geometric mean % change from baseline [n] |
Mean sitting SBP/DBP (mm Hg) | ||||
|---|---|---|---|---|---|---|---|---|
| PRC | PRA | Baseline | Change from baseline |
|||||
| Nussberger 2007 (adults with mild-to-moderate hypertension) (Nussberger et al. 2007) | ||||||||
| Week 8 | Placebo | 111 | −9 | −11 | 152/NA | −5/NA | ||
| ALI 150 mg | 112 | +157 | −69* | 151/NA | −9/NA | |||
| ALI 300 mg | 115 | +246* | −71* | 152/NA | −15/NA | |||
| ALI 600 mg | 113 | +497* | −75* | 153/NA | −16/NA | |||
| Irbesartan 150 mg | 118 | +105 | +109 | 153/NA | −13/NA | |||
| Duprez 2009 (elderly patients with systolic hypertension) (Duprez et al. 2009) | ||||||||
| Week 12 | ALI 150–300 mg | 451 | +246‡ | [59] | −79‡ | [59] | NA | −14.0*/−5.1† |
| RAM 5–10 mg | 439 | +100 | [62] | +147 | [62] | NA | −11.6/−3.6 | |
| Week 36 (±HCTZ±AMLO) |
ALI 150–300 mg | 451 | +469‡ | [65] | −70‡ | [65] | NA | NA |
| RAM 5–10 mg | 439 | +173 | [66] | +244 | [66] | NA | NA | |
| Andersen 2008, Andersen in press (adults with mild-to-moderate hypertension) (Andersen et al. 2008,Andersen et al. 2009) | ||||||||
| Week 12 | ALI 150–300 mg | 420 | ND | ND | 151.3/98.8 | −14.0†/−11.3† | ||
| RAM 5–10 mg | 422 | ND | ND | 151.5/98.9 | −11.3/−9.7 | |||
| Week 26 (±HCTZ) |
ALI 150–300 mg | 420 | +224 | [39] | −63 | [103] | 151.3/98.8 | −17.9†/−13.2* |
| RAM 5–10 mg | 422 | +145 | [33] | +143 | [100] | 151.5/98.9 | −15.2/−12.0 | |
| Oparil 2007 (adults with mild-to-moderate hypertension) (Oparil et al. 2007a) | ||||||||
| Week 8 | Placebo | 459 | +19 | [51] | +18 | [51] | 154.2/100.5 | −4.6/−4.1 |
| ALI 150 mg | 437 | +468 | [51] | −73 | [51] | 154.0/100.3 | −13.0/−9.0 | |
| VAL 160 mg | 455 | +138 | [59] | +160 | [59] | 154.2/100.4 | −12.8/−9.7 | |
| ALI 150 mg + VAL 160 mg |
446 | +912† | [60] | −44‡ | [60] | 152.7/100.1 | −17.2‡/−12.2‡ | |
| Villamil 2007 (adults with mild-to-moderate hypertension) (Villamil et al. 2007) | ||||||||
| Week 8 | Placebo | 195 | +30 | +1 | 152.7/99.3 | −7.5/−6.9 | ||
| ALI 75 mg | 184 | +164 | −54 | 153.2/99.4 | −9.4/−8.7 | |||
| ALI 150 mg | 185 | +192 | −65 | 153.4/98.8 | −12.2/−8.9 | |||
| ALI 300 mg | 183 | +348 | −58 | 154.4/99.3 | −15.7/−10.3 | |||
| HCTZ 6.25 mg | 194 | +10 | +4 | 153.4/99.3 | −11.0/−9.1 | |||
| HCTZ 12.5 mg | 188 | +26 | +45 | 153.4/99.1 | −13.9/−10.1 | |||
| HCTZ 25 mg | 176 | +108 | +72 | 154.5/99.1 | −14.3/−9.4 | |||
| ALI 75 mg + HCTZ 6.25 mg |
188 | NA | −55 | 154.5/98.9 | −14.3*/−10.8* | |||
| ALI 150 mg + HCTZ 12.5 mg |
186 | NA | −50 | 154.1/99.1 | −17.6*/−11.9* | |||
| ALI 300 mg + HCTZ 25 mg |
173 | +1211 | −62 | 154.6/99.3 | −21.2*/−14.3* | |||
| Geiger 2009 (adults with mild-to-moderate hypertension unresponsive to HCTZ monotherapy) (Geiger et al. 2009) | ||||||||
| Week 8 | HCTZ 25 mg | 152 | −29 | [43] | −13 | [43] | 154.1/99.9 | −6/−6 |
| ALI 150–300 mg + HCTZ 25 mg |
166 | +490 | [47] | −41 | [47] | 153.3/99.3 | −15‡/−11‡ | |
| VAL 160–320 mg + HCTZ 25 mg |
155 | +561 | [42] | +509 | [42] | 156.7/99.9 | −18‡/−14‡ | |
| ALI 150–300 mg + VAL 160–320 mg + HCTZ 25 mg |
168 | +1760 | [52] | +39 | [52] | 152.7/99.2 | −22‡§/−16‡§ | |
| Drummond 2007 (mild-to-moderate hypertension in amlodipine non-responding adults) (Drummond et al. 2007) | ||||||||
| Week 6 | ALI 150 mg + AMLO 5 mg |
187 | NA | −74.4 | [55] | 150.5/95.7 | −11.0/−8.5 | |
| AMLO 5 mg | 180 | NA | −9.9 | [48] | 150.5/96.2 | −5.0/−4.8 | ||
| AMLO 10 mg | 178 | NA | +58.0 | [48] | 150.8/96.5 | −9.6/−8.0 | ||
| Uresin 2007 (adults with mild-to-moderate hypertension + diabetes mellitus) (Uresin et al. 2007) | ||||||||
| Week 8 | ALI 300 mg | 282 | +139 | [84] | −66 | [84] | 157.4/98.4 | −14.7/−11.3 |
| RAM 10 mg | 278 | +72 | [72] | +106 | [72] | 155.9/98.2 | −12.0/−10.7 | |
| ALI 300 mg + RAM 10 mg |
277 | +331* | [77] | −48* | [77] | 156.5/98.4 | −16.6¶/−12.8* | |
| McMurray 2008 (adults with NYHA class II to class IV CHF receiving a BB + an ACEI or ARB) (McMurray et al. 2008) | ||||||||
| Month 3 | Placebo | 146 | −10 | −9 | 128/76 | −1.3/−0.2 | ||
| ALI 150 mg | 156 | +142 | −77 | 130/78 | −4.1/−2.9 | |||
Studies are sorted by order of appearance in text.
P<.05 vs active monotherapy comparator(s);
P<.01 vs active monotherapy comparator(s);
P<.001 vs active monotherapy comparator(s);
P<.01 vs either dual therapy;
P<.001 vs ramipril monotherapy.
Abbreviations: ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin receptor blocker; ALI = aliskiren; AMLO = amlodipine; BB = beta-blocker; BP = blood pressure; CHF = congestive heart failure; DBP = diastolic blood pressure; HCTZ = hydrochlorothiazide; NA = not available; ND = not done; NYHA = New York Heart Association; PRA = plasma renin activity; PRC = plasma renin concentration; RAM = ramipril; SBP = systolic blood pressure; VAL = valsartan.
In all studies, the administration of aliskiren alone or in combination with other antihypertensive agents was well tolerated. The ASPIRE HIGHER clinical program will further define the role of aliskiren in the management of cardiovascular and renal diseases (Sever et al. 2009). Of particular interest are large-scale studies that will examine the benefits of aliskiren on morbidity and mortality, including its combination with ACEIs or ARBs. These include the ATMOSPHERE and ASTRONAUT studies in patients with CHF, the ALTITUDE trial in patients with DM, and the APOLLO study in elderly patients with or without previous cardiovascular events.
Benefits Beyond BP Control
The potential benefits of dual RAAS therapy with aliskiren appear to extend beyond simple control of BP. Aliskiren is a potent, long-acting, renal vasodilator with a pronounced natriuretic effect in normotensive healthy volunteers on a low-sodium diet; its renal vasodilator effects are approximately twice as large as those of ACEIs and 40% greater than those of ARBs (Fisher et al. 2008). This suggests that aliskiren may provide greater and more effective blockade of the RAAS in the kidney. Evidence for renoprotective effects stem from the 6-month, double-blind, placebo-controlled AVOID study, which compared aliskiren 300 mg versus placebo when combined with losartan 100 mg and optimal antihypertensive therapy in 599 patients with type 2 DM, diabetic nephropathy, and relatively well-controlled msSBP/msDBP (~135/78 mm Hg at baseline) (Parving et al. 2008). Combination therapy reduced albuminuria (mean urinary albumin:creatinine ratio [UACR]) by 20% relative to losartan alone (P<0.001) despite the negligible differences in BP between the 2 arms (2/1 mm Hg; P>0.05). Additionally, the UACR was reduced by ≥50% in 24.7% of patients receiving combination therapy compared with 12.5% on losartan monotherapy (Pπ.001). These findings are similar to those of a 3-month, open-label study of aliskiren monotherapy in 15 patients with type 2 DM and albuminuria (Persson et al. 2008), in which aliskiren 300 mg resulted in a 44% reduction from baseline in the UACR (P<0.001). Persson and colleagues recently reported the results of a randomized, double-blind, crossover study in 26 hypertensive patients with type 2 DM and albuminuria (Persson et al. 2009). Two months of treatment with aliskiren 300 mg or irbesartan 300 mg similarly reduced albuminuria by 48% and 58%, respectively, compared with placebo (P<0.001). The combination of aliskiren/irbesartan reduced albuminuria by 71% compared with placebo (P<.001), an antiproteinuric effect that was significantly greater than that of either monotherapy (P<0.05).
Dual RAAS therapy with aliskiren may help slow or reverse cardiac end-organ damage. In the ALLAY study of 465 patients with hypertension and LVH, treatment with aliskiren 300 mg or losartan 100 mg for 9 months reduced left ventricular mass −4.9 and − 4.8 g/m2, respectively (both P<0.0001 vs baseline; P<0.0001 for noninferiority) (Solomon et al. 2009). Combination aliskiren/losartan 300/100 mg resulted in modestly greater improvements in left ventricular mass (−5.8 g/m2; P<0.0001 vs baseline; P=.52 vs losartan). In the ALOFT study mentioned previously, aliskiren 150 mg (plus ACEI or ARB) produced a greater reduction in plasma brain natriuretic peptide (BNP) compared with placebo (−61.0 vs −12.2 pg/mL; P=0.011) (McMurray et al. 2008). This may have clinical consequences for patients with CHF, because the Val-HeFT study suggests that there is a 1.2% increase in the risk of death and the risk for CHF-related hospitalization associated with each 10-pg/mL increment in BNP (Latini et al. 2004). The beneficial effect of aliskiren on BNP levels appeared independent of the changes from baseline in BP and heart rate, which were similar for the 2 study groups.
Summary
From a theoretical standpoint, direct inhibition of renin has long been recognized as a promising target for inhibiting the RAAS, because renin is the first rate-limiting enzymatic step in the RAAS pathway. From a practical standpoint, ACEIs and ARBs have not provided the major improvements in clinical outcomes that might be predicted based on the central role of the RAAS in the cardiorenal disease continuum. Moreover, the value of combination ACEI/ARB therapy has been called into question, particularly in light of the results from ONTARGET. Based on what is known to date, it may be suggested that DRIs in combination with ACEIs or ARBs may yield better clinical outcomes through more effective RAAS blockade at distinct and complementary sites. The ultimate role of aliskiren in combination therapies with ACEIs, ARBs, and other antihypertensives will be better defined through future studies, which are being conducted as part of the ASPIRE HIGHER clinical program.
Acknowledgements
Editorial support was provided by John Leinen, PhD, at Oxford PharmaGenesis Inc., Newtown, PA, and was funded by Novartis Pharmaceuticals Corporation, East Hanover, NJ. In addition to support provided by NHLBI grant PO1 HL051952, the author gratefully acknowledges grant support in part provided by Unifi, Inc., Greensboro, NC, and Farley-Hudson Foundation, Jacksonville, NC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alderman MH, Ooi WL, Cohen H, Madhavan S, Sealey JE, Laragh JH. Plasma renin activity: a risk factor for myocardial infarction in hypertensive patients. American Journal of Hypertension. 1997;10(1):1–8. doi: 10.1016/s0895-7061(96)00301-9. [DOI] [PubMed] [Google Scholar]
- Andersen K, Weinberger MH, Constance CC, Ali MA, Jin JF, Prescott MF, Keefe DL. Comparative effects of aliskiren-based and ramipril-based therapy on the renin system during long-term (6 months) treatment and withdrawal in patients with hypertension. Journal of the Renin Angiotensin Aldosterone System. 2009 doi: 10.1177/1470320309342407. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Andersen K, Weinberger MH, Egan B, Constance CM, Ali MA, Jin J, Keefe DL. Comparative efficacy and safety of aliskiren, an oral direct renin inhibitor, and ramipril in hypertension: a 6-month, randomized, double-blind trial. Journal of Hypertension. 2008;26(3):589–599. doi: 10.1097/HJH.0b013e3282f3ad9a. [DOI] [PubMed] [Google Scholar]
- Andraws R, Brown DL. Effect of inhibition of the renin-angiotensin system on development of type 2 diabetes mellitus (meta-analysis of randomized trials) American Journal of Cardiology. 2007;99(7):1006–1012. doi: 10.1016/j.amjcard.2006.10.068. [DOI] [PubMed] [Google Scholar]
- Arici M, Erdem Y. Dual blockade of the renin-angiotensin system for cardiorenal protection: an update. American Journal of Kidney Disease. 2009;53(2):332–345. doi: 10.1053/j.ajkd.2008.11.021. [DOI] [PubMed] [Google Scholar]
- Athyros VG, Mikhailidis DP, Kakafika AI, Tziomalos K, Karagiannis A. Angiotensin II reactivation and aldosterone escape phenomena in renin-angiotensin-aldosterone system blockade: is oral renin inhibition the solution? Expert Opinion on Pharmacotherapy. 2007;8(5):529–535. doi: 10.1517/14656566.8.5.529. [DOI] [PubMed] [Google Scholar]
- Atlas SA. The renin-angiotensin aldosterone system: pathophysiological role and pharmacologic inhibition. Journal of Managed Care Pharmacy. 2007;13(8 Suppl S-b):S9–S20. doi: 10.18553/jmcp.2007.13.s8-b.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azizi M, Ménard J, Bissery A, Guyene TT, Bura-Rivière A. Hormonal and hemodynamic effects of aliskiren and valsartan and their combination in sodium-replete normotensive individuals. Clinical Journal of the American Society of Nephrology. 2007;2(5):947–955. doi: 10.2215/CJN.00360107. [DOI] [PubMed] [Google Scholar]
- Azizi M, Ménard J, Bissery A, Guyenne TT, Bura-Rivière A, Vaidyanathan S, Camisasca RP. Pharmacologic demonstration of the synergistic effects of a combination of the renin inhibitor aliskiren and the AT1 receptor antagonist valsartan on the angiotensin II-renin feedback interruption. Journal of the American Society of Nephrology. 2004;15(12):3126–3133. doi: 10.1097/01.ASN.0000146686.35541.29. [DOI] [PubMed] [Google Scholar]
- Azizi M, Webb R, Nussberger J, Hollenberg NK. Renin inhibition with aliskiren: where are we now, and where are we going? Journal of Hypertension. 2006;24(2):243–256. doi: 10.1097/01.hjh.0000202812.72341.99. [DOI] [PubMed] [Google Scholar]
- Bair TL, May HT, Prescott MF, Anderson JL, Horne BD, Penman J, Muhlestein JB. Association between baseline levels of plasma renin activity and risk of cardiovascular events. Journal of the American College of Cardiology. 2009;53(10 Suppl A):A383. [Abstract 1028-12] [Google Scholar]
- Balamuthusamy S, Srinivasan L, Verma M, Adigopula S, Jalandara N, Hathiwala S, Smith E. Renin angiotensin system blockade and cardiovascular outcomes in patients with chronic kidney disease and proteinuria: a meta-analysis. American Heart Journal. 2008;155(5):791–805. doi: 10.1016/j.ahj.2008.01.031. [DOI] [PubMed] [Google Scholar]
- Berns JS. Is angiotensin-converting enzyme inhibitor and angiotensin receptor blocker combination therapy better than monotherapy and safe in patients with CKD? American Journal of Kidney Disease. 2009;53(2):192–196. doi: 10.1053/j.ajkd.2008.11.016. [DOI] [PubMed] [Google Scholar]
- Brown NJ. Aldosterone and end-organ damage. Current Opinion in Nephrology and Hypertension. 2005;14(3):235–241. doi: 10.1097/01.mnh.0000165889.60254.98. [DOI] [PubMed] [Google Scholar]
- Burnier M, Brunner HR. Angiotensin II receptor antagonists. Lancet. 2000;355(9204):637–645. doi: 10.1016/s0140-6736(99)10365-9. [DOI] [PubMed] [Google Scholar]
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42(6):1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- Chrysant SG, Murray AV, Hoppe UC, Dattani D, Patel S, Hsu H, Zhang J. Long-term safety, tolerability and efficacy of aliskiren in combination with valsartan in patients with hypertension: a 6-month interim analysis. Current Medical Research and Opinion. 2008;24(4):1039–1047. doi: 10.1185/030079908x280581. [DOI] [PubMed] [Google Scholar]
- Cohn JN, Goldman JM. Establishing a new option for target-organ protection: rationale for ARB plus ACE inhibitor combination therapy. American Journal of Hypertension. 2008;21(3):248–256. doi: 10.1038/ajh.2007.56. [DOI] [PubMed] [Google Scholar]
- Crackower MA, Sarao R, Oudit GY, Yagil C, Kozieradzki I, Scanga SE, Oliveira-dos-Santos AJ, da Costa J, Zhang L, Pei Y, Scholey J, Ferrario CM, Manoukian AS, Chappell MC, Backx PH, Yagil Y, Penninger JM. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417(6891):822–828. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- Dechend R, Shagdarsuren E, Gratze P, Fiebeler A, Pilz B, Meiners S, Derer W, Feldman DL, Webb R, Muller DN. Low-dose renin inhibitor and low-dose AT1-receptor blocker therapy ameliorate target-organ damage in rats harbouring human renin and angiotensinogen genes. Journal of the Renin Angiotensin Aldosterone System. 2007;8(2):81–84. doi: 10.3317/jraas.2007.008. [DOI] [PubMed] [Google Scholar]
- Dicpinigaitis PV. Angiotensin-converting enzyme inhibitor-induced cough: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1 Suppl):169S–173S. doi: 10.1378/chest.129.1_suppl.169S. [DOI] [PubMed] [Google Scholar]
- Doulton TW, He FJ, MacGregor GA. Systematic review of combined angiotensin-converting enzyme inhibition and angiotensin receptor blockade in hypertension. Hypertension. 2005;45(5):880–886. doi: 10.1161/01.HYP.0000161880.59963.da. [DOI] [PubMed] [Google Scholar]
- Drummond W, Munger MA, Rafique EM, Maboudian M, Khan M, Keefe DL. Antihypertensive efficacy of the oral direct renin inhibitor aliskiren as add-on therapy in patients not responding to amlodipine monotherapy. Journal of Clinical Hypertension (Greenwich) 2007;9(10):742–750. doi: 10.1111/j.1524-6175.2007.06614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duprez DA, Botha J, Charney AN, Prescott MF. Comparison of the effects of aliskiren-versus ramipril-based treatment regimens of plasma renin concentration and plasma renin activity in elderly patients with systolic hypertension. Presented at the 24th Annual Scientific Meeting and Exposition of the American Society of Hypertension; May 6–9; San Francisco, CA, USA. 2009. [Google Scholar]
- Faure S, Bureau A, Oudart N, Javellaud J, Fournier A, Achard JM. Protective effect of candesartan in experimental ischemic stroke in the rat mediated by AT2 and AT4 receptors. Journal of Hypertension. 2008;26(10):2008–2015. doi: 10.1097/HJH.0b013e32830dd5ee. [DOI] [PubMed] [Google Scholar]
- Ferrari P, Marti HP, Pfister M, Frey FJ. Additive antiproteinuric effect of combined ACE inhibition and angiotensin II receptor blockade. Journal of Hypertension. 2002;20(1):125–130. doi: 10.1097/00004872-200201000-00018. [DOI] [PubMed] [Google Scholar]
- Ferrario CM, Chappell MC, Dean RH, Iyer SN. Novel angiotensin peptides regulate blood pressure, endothelial function, and natriuresis. Journal of the American Society of Nephrology. 1998;9(9):1716–1722. doi: 10.1681/ASN.V991716. [DOI] [PubMed] [Google Scholar]
- Ferrario CM, Chappell MC, Tallant EA, Brosnihan KB, Diz DI. Counterregulatory actions of angiotensin-(1–7) Hypertension. 1997;30(3 Pt 2):535–541. doi: 10.1161/01.hyp.30.3.535. [DOI] [PubMed] [Google Scholar]
- Ferrario CM, Jessup J, Chappell MC, Averill DB, Brosnihan KB, Tallant EA, Diz DI, Gallagher PE. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005a;111(20):2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- Ferrario CM, Trask AJ, Jessup JA. Advances in biochemical and functional roles of angiotensin-converting enzyme 2 and angiotensin-(1–7) in regulation of cardiovascular function. American Journal of Physiology-Heart and Circulatory Physiology. 2005b;289(6):H2281–H2290. doi: 10.1152/ajpheart.00618.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CM, Varagic J, Habibi J, Nagata S, Kato J, Chappell MC, Trask AJ, Kitamura K, Whaley-Connell A, Sowers JR. Differential regulation of angiotensin-(1–12) in plasma and cardiac tissue in response to bilateral nephrectomy. American Journal of Physiology-Heart and Circulatory Physiology. 2009;296(4):H1184–H1192. doi: 10.1152/ajpheart.01114.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher ND, Hollenberg NK. Renin inhibition: what are the therapeutic opportunities? Journal of the American Society of Nephrology. 2005;16(3):592–599. doi: 10.1681/ASN.2004100874. [DOI] [PubMed] [Google Scholar]
- Fisher ND, Jan Danser AH, Nussberger J, Dole WP, Hollenberg NK. Renal and hormonal responses to direct renin inhibition with aliskiren in healthy humans. Circulation. 2008;117(25):3199–3205. doi: 10.1161/CIRCULATIONAHA.108.767202. [DOI] [PubMed] [Google Scholar]
- Gallagher PE, Ferrario CM, Tallant EA. MAP kinase/phosphatase pathway mediates the regulation of ACE2 by angiotensin peptides. American Journal of Physiology Cell Physiology. 2008;295(5):C1169–C1174. doi: 10.1152/ajpcell.00145.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger H, Barranco E, Gorostidi M, Taylor A, Zhang X, Xiang Z, Zhang J. Combination therapy with various combinations of aliskiren, valsartan, and hydrochlorothiazide in hypertensive patients not adequately responsive to hydrochlorothiazide alone. Journal of Clinical Hypertension (Greenwich) 2009;11(6):324–332. doi: 10.1111/j.1751-7176.2009.00114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenberg NK, Fisher ND, Price DA. Pathways for angiotensin II generation in intact human tissue: evidence from comparative pharmacological interruption of the renin system. Hypertension. 1998;32(3):387–392. doi: 10.1161/01.hyp.32.3.387. [DOI] [PubMed] [Google Scholar]
- Ibrahim MM. RAS inhibition in hypertension. Journal of Human Hypertension. 2006;20(2):101–108. doi: 10.1038/sj.jhh.1001960. [DOI] [PubMed] [Google Scholar]
- Ichihara A, Hayashi M, Kaneshiro Y, Suzuki F, Nakagawa T, Tada Y, Koura Y, Nishiyama A, Okada H, Uddin MN, Nabi AH, Ishida Y, Inagami T, Saruta T. Inhibition of diabetic nephropathy by a decoy peptide corresponding to the "handle" region for nonproteolytic activation of prorenin. Journal of Clinical Investigation. 2004;114(8):1128–1135. doi: 10.1172/JCI21398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihara M, Urata H, Kinoshita A, Suzumiya J, Sasaguri M, Kikuchi M, Ideishi M, Arakawa K. Increased chymase-dependent angiotensin II formation in human atherosclerotic aorta. Hypertension. 1999;33(6):1399–1405. doi: 10.1161/01.hyp.33.6.1399. [DOI] [PubMed] [Google Scholar]
- Jensen C, Herold P, Brunner HR. Aliskiren: the first renin inhibitor for clinical treatment. National Reviews Drug Discovery. 2008;7(5):399–410. doi: 10.1038/nrd2550. [DOI] [PubMed] [Google Scholar]
- Jessup JA, Trask AJ, Chappell MC, Nagata S, Kato J, Kitamura K, Ferrario CM. Localization of the novel angiotensin peptide, angiotensin-(1–12), in heart and kidney of hypertensive and normotensive rats. American Journal of Physiology-Heart and Circulatory Physiology. 2008;294(6):H2614–H2618. doi: 10.1152/ajpheart.91521.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamoi K. Aliskiren combined with losartan in diabetes and nephropathy. New England Journal of Medicine. 2008;359(10):1069–1070. [PubMed] [Google Scholar]
- Krum H, Carson P, Farsang C, Maggioni AP, Glazer RD, Aknay N, Chiang YT, Cohn JN. Effect of valsartan added to background ACE inhibitor therapy in patients with heart failure: results from Val-HeFT. European Journal of Heart Failure. 2004;6(7):937–945. doi: 10.1016/j.ejheart.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Kunz R, Friedrich C, Wolbers M, Mann JF. Meta-analysis: effect of monotherapy and combination therapy with inhibitors of the renin angiotensin system on proteinuria in renal disease. Annals of Internal Medicine. 2008;148(1):30–48. doi: 10.7326/0003-4819-148-1-200801010-00190. [DOI] [PubMed] [Google Scholar]
- Lakhdar R, Al-Mallah MH, Lanfear DE. Safety and tolerability of angiotensin-converting enzyme inhibitor versus the combination of angiotensin-converting enzyme inhibitor and angiotensin receptor blocker in patients with left ventricular dysfunction: a systematic review and meta-analysis of randomized controlled trials. Journal of Cardiac Failure. 2008;14(3):181–188. doi: 10.1016/j.cardfail.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Lalouel J-M, Rohrwasser A, Terreros D, Morgan T, Ward K. Angiotensinogen in essential hypertension: from genetics to nephrology. J Am Soc Nephrol. 2001;12:606–615. doi: 10.1681/ASN.V123606. [DOI] [PubMed] [Google Scholar]
- Latini R, Masson S, Anand I, Salio M, Hester A, Judd D, Barlera S, Maggioni AP, Tognoni G, Cohn JN. The comparative prognostic value of plasma neurohormones at baseline in patients with heart failure enrolled in Val-HeFT. European Heart Journal. 2004;25(4):292–299. doi: 10.1016/j.ehj.2003.10.030. [DOI] [PubMed] [Google Scholar]
- Legrand D, Krzesinski JM, Scheen AJ. [What is the purpose of dual or triple inhibition of the renin-angiotensin-aldosterone system?] Revue Médicale Suisse. 2008;4(168):1792–1797. [PubMed] [Google Scholar]
- Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell. 2001;104(4):545–556. doi: 10.1016/s0092-8674(01)00241-0. [DOI] [PubMed] [Google Scholar]
- Lijnen P, Fagard R, Staessen J, Amery A. Effect of chronic diuretic treatment on the plasma renin-angiotensin-aldosterone system in essential hypertension. British Journal of Clinical Pharmacology. 1981;12(3):387–392. doi: 10.1111/j.1365-2125.1981.tb01231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, Narkiewicz K, Ruilope L, Rynkiewicz A, Schmieder RE, Boudier HA, Zanchetti A. 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Journal of Hypertension. 2007;25(6):1105–1187. doi: 10.1097/HJH.0b013e3281fc975a. [DOI] [PubMed] [Google Scholar]
- Mann JF, Schmieder RE, McQueen M, Dyal L, Schumacher H, Pogue J, Wang X, Maggioni A, Budaj A, Chaithiraphan S, Dickstein K, Keltai M, Metsarinne K, Oto A, Parkhomenko A, Piegas LS, Svendsen TL, Teo KK, Yusuf S. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet. 2008;372(9638):547–553. doi: 10.1016/S0140-6736(08)61236-2. [DOI] [PubMed] [Google Scholar]
- Masson S, Solomon S, Angelici L, Anand IS, Prescott M, Maggioni AP, Tognoni G, Cohn JN. Plasma renin activity retains a strong prognostic value in patients with chronic HF, independent of ACE inhibitor or beta-blocker therapy. Data from the Valsartan Heart Failure (Val-HeFT) trial; Presented at the 31th Annual Congress of the European Society of Cardiology; August 29–September 2; Barcelona, Spain. 2009. [Google Scholar]
- McMurray JJ, Ostergren J, Swedberg K, Granger CB, Held P, Michelson EL, Olofsson B, Yusuf S, Pfeffer MA. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet. 2003;362(9386):767–771. doi: 10.1016/S0140-6736(03)14283-3. [DOI] [PubMed] [Google Scholar]
- McMurray JJ, Pitt B, Latini R, Maggioni AP, Solomon SD, Keefe DL, Ford J, Verma A, Lewsey J. Effects of the oral direct renin inhibitor aliskiren in patients with symptomatic heart failure. Circulation Heart Failure. 2008;1:17–24. doi: 10.1161/CIRCHEARTFAILURE.107.740704. [DOI] [PubMed] [Google Scholar]
- Messerli FH. The sudden demise of dual renin-angiotensin system blockade or the soft science of the surrogate end point. Journal of the American College of Cardiology. 2009;53(6):468–470. doi: 10.1016/j.jacc.2008.10.036. [DOI] [PubMed] [Google Scholar]
- Nagata S, Kato J, Sasaki K, Minamino N, Eto T, Kitamura K. Isolation and identification of proangiotensin-12, a possible component of the renin-angiotensin system. Biochemical and Biophysical Research Communications. 2006;350(4):1026–1031. doi: 10.1016/j.bbrc.2006.09.146. [DOI] [PubMed] [Google Scholar]
- Nakao N, Yoshimura A, Morita H, Takada M, Kayano T, Ideura T. Combination treatment of angiotensin-II receptor blocker and angiotensin-converting-enzyme inhibitor in non-diabetic renal disease (COOPERATE): a randomised controlled trial. Lancet. 2003;361(9352):117–124. doi: 10.1016/S0140-6736(03)12229-5. [DOI] [PubMed] [Google Scholar]
- National Kidney Foundation. KDOQI clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. American Journal of Kidney Disease. 2007;49(2 Suppl 2):S12–S154. doi: 10.1053/j.ajkd.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Nguyen G, Contrepas A. Physiology and pharmacology of the (pro)renin receptor. Current Opinion in Pharmacology. 2008;8(2):127–132. doi: 10.1016/j.coph.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Nguyen G, Delarue F, Burckle C, Bouzhir L, Giller T, Sraer JD. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. Journal of Clinical Investigation. 2002;109(11):1417–1427. doi: 10.1172/JCI14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussberger J, Gradman AH, Schmieder RE, Lins RL, Chiang Y, Prescott MF. Plasma renin and the antihypertensive effect of the orally active renin inhibitor aliskiren in clinical hypertension. International Journal of Clinical Practice. 2007;61(9):1461–1468. doi: 10.1111/j.1742-1241.2007.01473.x. [DOI] [PubMed] [Google Scholar]
- Nussberger J, Wuerzner G, Jensen C, Brunner HR. Angiotensin II suppression in humans by the orally active renin inhibitor Aliskiren (SPP100): comparison with enalapril. Hypertension. 2002;39(1):E1–E8. doi: 10.1161/hy0102.102293. [DOI] [PubMed] [Google Scholar]
- Oparil S, Yarows SA, Patel S, Fang H, Zhang J, Satlin A. Efficacy and safety of combined use of aliskiren and valsartan in patients with hypertension: a randomised, double-blind trial. Lancet. 2007a;370(9583):221–229. doi: 10.1016/S0140-6736(07)61124-6. [DOI] [PubMed] [Google Scholar]
- Oparil S, Yarows SA, Patel S, Zhang J, Satlin A. Dual inhibition of the renin system by aliskiren and valsartan. Lancet. 2007b;370(9593):1126–1127. doi: 10.1016/S0140-6736(07)61508-6. [DOI] [PubMed] [Google Scholar]
- Parving HH, Persson F, Lewis JB, Lewis EJ, Hollenberg NK. Aliskiren combined with losartan in type 2 diabetes and nephropathy. New England Journal of Medicine. 2008;358(23):2433–2446. doi: 10.1056/NEJMoa0708379. [DOI] [PubMed] [Google Scholar]
- Persson F, Rossing P, Reinhard H, Juhl T, Stehouwer CD, Schalkwijk C, Danser AJ, Boomsma F, Frandsen E, Parving HH. Renal effects of aliskiren compared to and in combination with irbesartan in patients with type 2 diabetes, hypertension and albuminuria. Diabetes Care. 2009 doi: 10.2337/dc09-0168. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson F, Rossing P, Schjoedt KJ, Juhl T, Tarnow L, Stehouwer CD, Schalkwijk C, Boomsma F, Frandsen E, Parving HH. Time course of the antiproteinuric and antihypertensive effects of direct renin inhibition in type 2 diabetes. Kidney International. 2008;73(12):1419–1425. doi: 10.1038/ki.2008.68. [DOI] [PubMed] [Google Scholar]
- Phillips CO, Kashani A, Ko DK, Francis G, Krumholz HM. Adverse effects of combination angiotensin II receptor blockers plus angiotensin-converting enzyme inhibitors for left ventricular dysfunction: a quantitative review of data from randomized clinical trials. Archives of Internal Medicine. 2007;167(18):1930–1936. doi: 10.1001/archinte.167.18.1930. [DOI] [PubMed] [Google Scholar]
- Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. New England Journal of Medicine. 2003;348(14):1309–1321. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. New England Journal of Medicine. 1999;341(10):709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- Pool JL, Schmieder RE, Azizi M, Aldigier JC, Januszewicz A, Zidek W, Chiang Y, Satlin A. Aliskiren, an orally effective renin inhibitor, provides antihypertensive efficacy alone and in combination with valsartan. American Journal of Hypertension. 2007;20(1):11–20. doi: 10.1016/j.amjhyper.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Prosser HC, Forster ME, Richards AM, Pemberton CJ. Cardiac chymase converts rat proAngiotensin-12 (PA12) to angiotensin II: effects of PA12 upon cardiac haemodynamics. Cardiovascular Research. 2009;82(1):40–50. doi: 10.1093/cvr/cvp003. [DOI] [PubMed] [Google Scholar]
- Remuzzi G, Cattaneo D, Perico N. The aggravating mechanisms of aldosterone on kidney fibrosis. Journal of the American Society of Nephrology. 2008;19(8):1459–1462. doi: 10.1681/ASN.2007101079. [DOI] [PubMed] [Google Scholar]
- Roig E, Perez-Villa F, Morales M, Jimenez W, Orus J, Heras M, Sanz G. Clinical implications of increased plasma angiotensin II despite ACE inhibitor therapy in patients with congestive heart failure. European Heart Journal. 2000;21(1):53–57. doi: 10.1053/euhj.1999.1740. [DOI] [PubMed] [Google Scholar]
- Santos RA, Brosnihan KB, Jacobsen DW, DiCorleto PE, Ferrario CM. Production of angiotensin-(1–7) by human vascular endothelium. Hypertension. 1992;19(2 Suppl):II56–II61. doi: 10.1161/01.hyp.19.2_suppl.ii56. [DOI] [PubMed] [Google Scholar]
- Santos RA, Simoes e Silva AC, Maric C, Silva DM, Machado RP, de B, I, Heringer-Walther S, Pinheiro SV, Lopes MT, Bader M, Mendes EP, Lemos VS, Campagnole-Santos MJ, Schultheiss HP, Speth R, Walther T. Angiotensin-(1–7) is an endogenous ligand for the G protein-coupled receptor Mas. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(14):8258–8263. doi: 10.1073/pnas.1432869100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarafidis PA, Bakris GL. Renin-angiotensin blockade and kidney disease. Lancet. 2008;372(9638):511–512. doi: 10.1016/S0140-6736(08)61212-X. [DOI] [PubMed] [Google Scholar]
- Schiavone MT, Santos RA, Brosnihan KB, Khosla MC, Ferrario CM. Release of vasopressin from the rat hypothalamo-neurohypophysial system by angiotensin-(1–7) heptapeptide. Proceedings of the National Academy of Sciences of the United States of America. 1988;85(11):4095–4098. doi: 10.1073/pnas.85.11.4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffrin EL, Lipman ML, Mann JF. Chronic kidney disease: effects on the cardiovascular system. Circulation. 2007;116(1):85–97. doi: 10.1161/CIRCULATIONAHA.106.678342. [DOI] [PubMed] [Google Scholar]
- Schindler C, Bramlage P, Kirch W, Ferrario CM. Role of the vasodilator peptide angiotensin-(1–7) in cardiovascular drug therapy. Vascular Health and Risk Management. 2007;3(1):125–137. [PMC free article] [PubMed] [Google Scholar]
- Sever PS, Gradman AH, Azizi M. Managing cardiovascular and renal risk: the potential of direct renin inhibition. Journal of the Renin Angiotensin Aldosterone System. 2009;10(2):65–76. doi: 10.1177/1470320309104662. [DOI] [PubMed] [Google Scholar]
- Solomon SD, Appelbaum E, Manning WJ, Verma A, Berglund T, Lukashevich V, Cherif PC, Smith BA, Dahlöf B. Effect of the direct renin inhibitor aliskiren, the angiotensin receptor blocker losartan, or both on left ventricular mass in patients with hypertension and left ventricular hypertrophy. Circulation. 2009;119(4):530–537. doi: 10.1161/CIRCULATIONAHA.108.826214. [DOI] [PubMed] [Google Scholar]
- Stergiou GS, Makris T, Papavasiliou M, Efstathiou S, Manolis A. Comparison of antihypertensive effects of an angiotensin-converting enzyme inhibitor, a calcium antagonist and a diuretic in patients with hypertension not controlled by angiotensin receptor blocker monotherapy. Journal of Hypertension. 2005;23(4):883–889. doi: 10.1097/01.hjh.0000163159.22116.ab. [DOI] [PubMed] [Google Scholar]
- Strauss MH, Hall AS. Angiotensin receptor blockers may increase risk of myocardial infarction: unraveling the ARB-MI paradox. Circulation. 2006;114(8):838–854. doi: 10.1161/CIRCULATIONAHA.105.594986. [DOI] [PubMed] [Google Scholar]
- Tallant EA, Ferrario CM, Gallagher PE. Angiotensin-(1–7) inhibits growth of cardiac myocytes through activation of the mas receptor. American Journal of Physiology-Heart and Circulatory Physiology. 2005;289(4):H1560–H1566. doi: 10.1152/ajpheart.00941.2004. [DOI] [PubMed] [Google Scholar]
- Trask AJ, Ferrario CM. Angiotensin-(1–7): pharmacology and new perspectives in cardiovascular treatments. Cardiovascular Drug Reviews. 2007;25(2):162–174. doi: 10.1111/j.1527-3466.2007.00012.x. [DOI] [PubMed] [Google Scholar]
- Trask AJ, Jessup JA, Chappell MC, Ferrario CM. Angiotensin-(1–12) is an alternate substrate for angiotensin peptide production in the heart. American Journal of Physiology-Heart and Circulatory Physiology. 2008;294(5):H2242–H2247. doi: 10.1152/ajpheart.00175.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner AJ, Tipnis SR, Guy JL, Rice G, Hooper NM. ACEH/ACE2 is a novel mammalian metallocarboxypeptidase and a homologue of angiotensin-converting enzyme insensitive to ACE inhibitors. Canadian Journal of Physiology and Pharmacology. 2002;80(4):346–353. doi: 10.1139/y02-021. [DOI] [PubMed] [Google Scholar]
- Unger T, Stoppelhaar M. Rationale for double renin-angiotensin-aldosterone system blockade. American Journal of Cardiology. 2007;100(3A):25J–31J. doi: 10.1016/j.amjcard.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Uresin Y, Taylor AA, Kilo C, Tschöpe D, Santonastaso M, Ibram G, Fang H, Satlin A. Efficacy and safety of the direct renin inhibitor aliskiren and ramipril alone or in combination in patients with diabetes and hypertension. Journal of the Renin Angiotensin Aldosterone System. 2007;8(4):190–198. doi: 10.3317/jraas.2007.028. [DOI] [PubMed] [Google Scholar]
- van de Wal RM, Van Veldhuisen DJ, Van Gilst WH, Voors AA. Addition of an angiotensin receptor blocker to full-dose ACE-inhibition: controversial or common sense? European Heart Journal. 2005;26(22):2361–2367. doi: 10.1093/eurheartj/ehi454. [DOI] [PubMed] [Google Scholar]
- Vergaro G, Fontana M, Poletti R, Giannoni A, Iervasi AL, Masi L, Mammini C, Gabutti A, Passino C, Emdin M. Plasma renin activity is an independent prognostic factor in chronic heart failure. European Heart Journal. 2008;29 Suppl 1:393. Abstract 2493. [Google Scholar]
- Vickers C, Hales P, Kaushik V, Dick L, Gavin J, Tang J, Godbout K, Parsons T, Baronas E, Hsieh F, Acton S, Patane M, Nichols A, Tummino P. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. Journal of Biological Chemistry. 2002;277(17):14838–14843. doi: 10.1074/jbc.M200581200. [DOI] [PubMed] [Google Scholar]
- Villamil A, Chrysant SG, Calhoun D, Schober B, Hsu H, Matrisciano-Dimichino L, Zhang J. Renin inhibition with aliskiren provides additive antihypertensive efficacy when used in combination with hydrochlorothiazide. Journal of Hypertension. 2007;25(1):217–226. doi: 10.1097/HJH.0b013e3280103a6b. [DOI] [PubMed] [Google Scholar]
- Vinh A, Widdop RE, Drummond GR, Gaspari TA. Chronic angiotensin IV treatment reverses endothelial dysfunction in ApoE-deficient mice. Cardiovascular Research. 2008;77(1):178–187. doi: 10.1093/cvr/cvm021. [DOI] [PubMed] [Google Scholar]
- Waeber B, Aschwanden R, Sadecky L, Ferber P. Combination of hydrochlorothiazide or benazepril with valsartan in hypertensive patients unresponsive to valsartan alone. Journal of Hypertension. 2001;19(11):2097–2104. doi: 10.1097/00004872-200111000-00022. [DOI] [PubMed] [Google Scholar]
- Weber MA, Giles TD. Inhibiting the renin-angiotensin system to prevent cardiovascular diseases: do we need a more comprehensive strategy? Reviews in Cardiovascular Medicine. 2006;7(2):45–54. [PubMed] [Google Scholar]
- Weir MR, Bush C, Anderson DR, Zhang J, Keefe D, Satlin A. Antihypertensive efficacy, safety, and tolerability of the oral direct renin inhibitor aliskiren in patients with hypertension: a pooled analysis. Journal of the American Society of Hypertension. 2007;1(4):264–277. doi: 10.1016/j.jash.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Weir MR, Dzau VJ. The renin-angiotensin-aldosterone system: a specific target for hypertension management. American Journal of Hypertension. 1999;12(12 Pt 3):205S–213S. doi: 10.1016/s0895-7061(99)00103-x. [DOI] [PubMed] [Google Scholar]
- Weir MR, Smith DH, Neutel JM, Bedigian MP. Valsartan alone or with a diuretic or ACE inhibitor as treatment for African American hypertensives: relation to salt intake. American Journal of Hypertension. 2001;14(7 Pt 1):665–671. doi: 10.1016/s0895-7061(01)01296-1. [DOI] [PubMed] [Google Scholar]
- Welches WR, Brosnihan KB, Ferrario CM. A comparison of the properties and enzymatic activities of three angiotensin processing enzymes: angiotensin converting enzyme, prolyl endopeptidase and neutral endopeptidase 24.11. Life Sciences. 1993;52(18):1461–1480. doi: 10.1016/0024-3205(93)90108-f. [DOI] [PubMed] [Google Scholar]
- White HD, Aylward PE, Huang Z, Dalby AJ, Weaver WD, Barvik S, Marin-Neto JA, Murin J, Nordlander RO, Van Gilst WH, Zannad F, McMurray JJ, Califf RM, Pfeffer MA. Mortality and morbidity remain high despite captopril and/or valsartan therapy in elderly patients with left ventricular systolic dysfunction, heart failure, or both after acute myocardial infarction: results from the Valsartan in Acute Myocardial Infarction Trial (VALIANT) Circulation. 2005;112(22):3391–3399. doi: 10.1161/CIRCULATIONAHA.105.551143. [DOI] [PubMed] [Google Scholar]
- Wolf G, Ritz E. Combination therapy with ACE inhibitors and angiotensin II receptor blockers to halt progression of chronic renal disease: pathophysiology and indications. Kidney International. 2005;67(3):799–812. doi: 10.1111/j.1523-1755.2005.00145.x. [DOI] [PubMed] [Google Scholar]
- Wong J, Patel RA, Kowey PR. The clinical use of angiotensin-converting enzyme inhibitors. Progress in Cardiovascular Diseases. 2004;47(2):116–130. doi: 10.1016/j.pcad.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Chappell MC, Brosnihan KB, Ferrario CM. In vivo metabolism of angiotensin I by neutral endopeptidase (EC 3.4.24.11) in spontaneously hypertensive rats. Hypertension. 1992;19(6 Pt 2):692–696. doi: 10.1161/01.hyp.19.6.692. [DOI] [PubMed] [Google Scholar]
- Yarows SA, Oparil S, Patel S, Fang H, Zhang J. Aliskiren and valsartan in stage 2 hypertension: subgroup analysis of a randomized, double-blind study. Advances in Therapy. 2008;25(12):1288–1302. doi: 10.1007/s12325-008-0123-x. [DOI] [PubMed] [Google Scholar]
- Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, Schumacher H, Dagenais G, Sleight P, Anderson C. Telmisartan, ramipril, or both in patients at high risk for vascular events. New England Journal of Medicine. 2008;358(15):1547–1559. doi: 10.1056/NEJMoa0801317. [DOI] [PubMed] [Google Scholar]