Abstract
Visual working memory (VWM) helps to temporarily represent information from the visual environment and is severely limited in capacity. Recent work has linked various forms of neural activity to the ongoing representations in VWM. One piece of evidence comes from human event-related potential studies, which find a sustained contralateral negativity during the retention period of VWM tasks. This contralateral delay activity (CDA) has previously been shown to increase in amplitude as the number of memory items increases, up to the individual's working memory capacity limit. However, significant alternative hypotheses remain regarding the true nature of this activity. Here we test whether the CDA is modulated by the perceptual requirements of the memory items as well as whether it is determined by the number of locations that are being attended within the display. Our results provide evidence against these two alternative accounts and instead strongly support the interpretation that this activity reflects the current number of objects that are being represented in VWM.
INTRODUCTION
Our ability to represent information in an active state is facilitated by the visual working memory (VWM) system. This system is capacity-limited such that only a small amount of information can be represented simultaneously. Neural measures of VWM have provided critical evidence regarding fundamental attributes of this system. One form of evidence comes from single-unit recording studies with monkeys. Neurons across a wide range of cortical areas show a sustained increase in firing rate above baseline during the retention period of VWM tasks (Funahashi et al. 1989a; Fuster and Alexander 1971; Kubota and Niki 1971), an effect often referred to as delay activity. The delay activity in many cells have been shown to be highly sensitive to properties of the remembered material such as its spatial position (Chafee and Goldman-Rakic 1998; Funahashi et al. 1989; Umeno and Goldberg 2001), and identity (Rainer and Miller 2002; Warden and Miller 2007) and is correlated with behavioral outcome (Funahashi et al. 1989). Similar activity has been reported in human neuroimaging studies showing sustained activations during the retention period of VWM tasks in regions such as the prefrontal cortex (PFC), intraparietal sulcus (IPS), lateral occipital cortex (LOC), and primary visual cortex (V1) (Courtney et al. 1998; Curtis and D'Esposito 2006; Ferber et al. 2005; Harrison and Tong 2009; Postle et al. 2000; Serences et al. 2009; Srimal and Curtis 2008). Of these regions, the IPS has recently gained much attention because it is strongly modulated by the number of items being remembered in VWM but reaches an asymptote once memory capacity is exhausted (Todd and Marois 2004).
Similar evidence can be observed using human event-related potential (ERP) recordings in which a large negative wave is observed over posterior electrode sites that are contralateral to the position of the remembered items that persists during the retention period. This contralateral delay activity (CDA) is strongly modulated by the number of items in memory but reaches an asymptote once capacity is reached and is highly predictive of the individual's specific memory capacity (Drew and Vogel 2008; Robitaille et al. 2009; Vogel and Machizawa 2004; Vogel et al. 2005). This suggests that the CDA provides a measure of the number of objects that are in VWM. However, there are two important alternative accounts of this activity that preclude such a conclusion. First, CDA amplitude modulations may reflect the increasing perceptual demands of the display. That is, as the number of items in the display increases, the perceptual difficulty of the display also increases, and it may be these increased encoding demands that may be the factor that drives increasing CDA amplitude rather than reflecting increased memory representations. Second, the CDA may reflect a spatial indexing process that represents the number of locations that are currently being attended. All previous CDA studies have used displays that have confounded the number of objects with the number of positions. In the present study, we seek to evaluate these two alternative accounts of this activity in an attempt to determine what aspect of WM performance the CDA reflects.
METHODS
Overview
In the first experiment, we will test whether the CDA is modulated by the amount of perceptual effort required for the display rather than the number of items in memory. To do this, we will compare the CDA amplitude for arrays of items that are presented either in high contrast or very low contrast while also manipulating the number of items in the display. We expect that the low contrast displays will be much more effortful to perceive than the high contrast displays, thus if the CDA is primarily sensitive to this increasing perceptual effort then we would expect that low contrast items should produce an increase in amplitude as compared with high contrast displays with the same number of items.
In the second experiment, we will test whether the CDA is sensitive to the number of objects in VWM or if it is instead sensitive only to the number of locations that are currently being attended. Here we will attempt to decouple the number of memory items from the number of attended positions by presenting the memory items as a sequence of two arrays separated by 500 ms. We have previously shown that separating the memory items into two two-item arrays results in a “step-like” function for CDA amplitude: initially amplitude is at the two-item level, then quickly ramps up to the level of four items that were simultaneously presented (Vogel et al. 2005). Experiment 2 will be similar, with the critical exception being that we will also present a condition in which the items in the second array will be presented in the same locations as those in the first array. If the CDA amplitude is determined solely by the number of locations, we would expect that remembering 4w objects presented at two locations would be equivalent to remembering two objects at two locations. Because subjects made a same/different response for each array in the sequential conditions, on a quarter of these trials a change was presented in each array. This resulted in four equally probable trial types for the sequential conditions: array 1 same/ array 2 same; array 1 same/ array 2 different; array 1 different/ array 2 same; array 1 different/ array 2 different. Thus detection of a change on array 1 provided the subject no information regarding whether or not array 2 would have a change.
Subjects
All subjects were between 18 and 30 yr old, had normal or corrected-to-normal vision, and had no history of neurological disorders or color blindness. Subjects were recruited from the University of Oregon community and were paid $10 per hour for their participation. A unique set of subjects participated in each experiment with 17 in experiment 1 and 18 in experiment 2. Subjects with eye-blink or -movement artifacts in excess of 25% of trials were excluded from further analysis. Two subjects in experiment 1 and three subjects in experiment 2 exceeded this threshold.
Stimuli and procedure for experiment 1
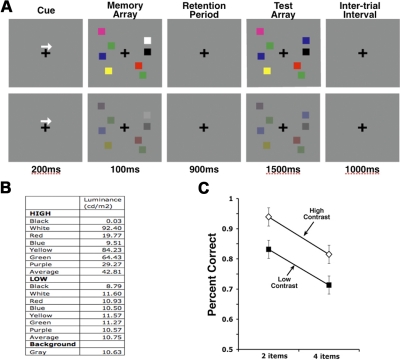
In all experiments, the stimuli were presented with Presentation software (Neurobehavioral Systems) on a CRT screen in a semi-dark room. Items were presented within 4 × 7.3° rectangular regions bilaterally, centered 3° to the left and right of the middle of the screen. A black fixation cross was presented in the center of the screen throughout the trial against the gray background. An arrow was presented above the fixation point. Colored squares (0.65 × 0.65°) were randomly chosen without replacement from a set of seven colors (black, white, red, blue, yellow, green, and purple). Luminance for each color is shown in Fig. 1B. On average, high contrast objects had four times as much contrast as low contrast objects (high = 42.81 cd/m2, low = 10.75 cd/m2).
Fig. 1.
Experiment 1. A: trial schematics for high contrast (top) and low contrast (bottom) conditions, set size 4. Trials were intermixed. B: luminance of colored squares and background. C: accuracy for high contrast (◇) and low contrast (■) conditions. Average working memory capacities (K) across subjects were 2.5 and 1.71 for high and low contrast conditions, respectively, and difference was significant paired t-test, t(14) = 6.763, P < 0.001.
The schematic of a trial is illustrated in Fig. 1A. Subjects were instructed to fixate the black cross from 80 cm of viewing distance. Each trial consisted of an arrow cue (200 ms), memory array (100 ms), retention period (900 ms), a test array (1,500 ms), and the intertrial interval (ITI: 1,000 ms). Subjects attended to the cued visual field and remembered the colors of the memory array items. At the onset of the test array, subjects responded whether the memory and test arrays were identical by a button press (same vs. different). Subjects were instructed to make a button press as accurately as possible. Item positions were randomized between the trials with a constraint that no square was present within 2° of one another. We used a 2 (set size: 2 vs. 4) × 2 (contrast: high vs. low) design, and all conditions were intermixed within blocks. All subjects completed a total of eight blocks of 100 trials each, resulting in 200 trials per condition.
Stimuli and procedure for experiment 2
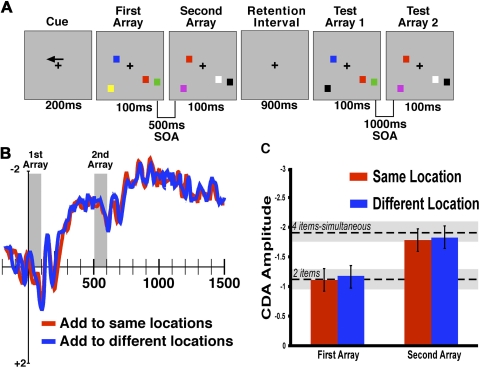
Experiment 2 used highly similar stimuli and procedures as those described in the high contrast condition of experiment 1. There were four primary conditions: two items simultaneous; four items simultaneous; sequential same locations; sequential different locations (Fig. 4A). In the sequential conditions, two items were presented in the memory array for 100 ms, and after a blank interval of 400 ms, a second memory array was presented. The items in this second array could be presented either in the same positions as those in the first array or at different locations within the same hemifield. 900 ms following the second array, a test array for the first memory set was presented for 100 ms that was then followed by a test array for the second memory set. Subjects in these conditions were instructed to make a same/different response for each test array presented.
Fig. 4.
experiment 2. A: trial schematics for “same location” trial. B: ERP data from trials in which 4 items were remembered sequentially, either at the same location (red) or different location (blue) as the 1st memory array. Time-locked to the onset of memory array 1. C: CDA amplitudes 300–500 ms following the 1st and 2nd memory array. Mean CDA amplitudes from simultaneous 2- and 4-item conditions are shown in dashed lines. Error bars = 95% confidence intervals. Significant main effect of time window was found (1st delay vs. 2nd delay, P < 0.001), but no effect of locations was found. Regardless of the location, CDA amplitude after the second delay was statistically not different from simultaneous presentation of 4 items.
Electrophysiology (EEG) recording and analyses
Twenty tin recording electrodes were mounted on an elastic cap to record EEG during the task. Electrode placements followed the International 10/20 system; F3/4, C3/4, P3/4, O1/2, T3/4, T5/6, Fz, Cz, and Pz. In addition, OL/R (half way in between O1 and T5, and O2, and T6, respectively), PO3/4 (halfway between P3 and O1, and P4 and O2, respectively), and POZ (half way between PO3 and PO4). These sites and a right mastoid site were referenced against the left mastoid reference, and data were re-referenced to the average of the left- and right mastoids.
A horizontal electrooculogram (EOG) was recorded from electrodes placed next to each eye, and vertical EOG was recorded from an electrode placed below the left eye. EEG and EOG were amplified by SA Instrumentation amplifier with a band-pass of 0.01–80 Hz, and data were collected at a sampling rate of 250 Hz. EOG was scanned for artifacts related to blinks and eye movements using an algorithm that detected large (>100 μV) peak-to-peak deflections or eye movements of >0.5°. All trials containing these artifacts were excluded from further analysis. Participants with trial rejection rates that exceeded 25% were excluded from the analyses. Two subjects in experiment 1 were excluded on this basis.
ERPs were time-locked at the onset of the memory array (in experiment 4, onset of the 1st memory array) and recorded throughout the retention period. CDA mean amplitude was analyzed using mean amplitude of difference wave (contralateral - ipsilateral) using time window of between 300 and 900 ms from the onset of the memory array. In addition to CDA, we examined amplitudes of N2pc between 200 and 280 ms. The N2pc is a transient contralateral wave observed over the posterior sites during the target selection period (Drew and Vogel 2008; Eimer 1996; Luck and Hillyard 1994).
RESULTS
Experiment 1
BEHAVIORAL PERFORMANCE.
As Fig. 1C shows, change detection accuracy was better for two-item arrays than for four-item arrays [F(1,14) = 126,71, P < 0.001] and also better for the high contrast than the low contrast arrays [F(1,14) = 107.35, P < 0.001]. In general, the contrast effect amounted to a roughly 10% decline in accuracy. Furthermore, two-item low contrast performance was not significantly different from four-item high contrast performance [planned contrast, t(56) = −0.539, P > 0.05]. Finally, there was no significant interaction between set size and contrast [F(1,14) = 0.434, P > 0.05].
Electrophysiological results
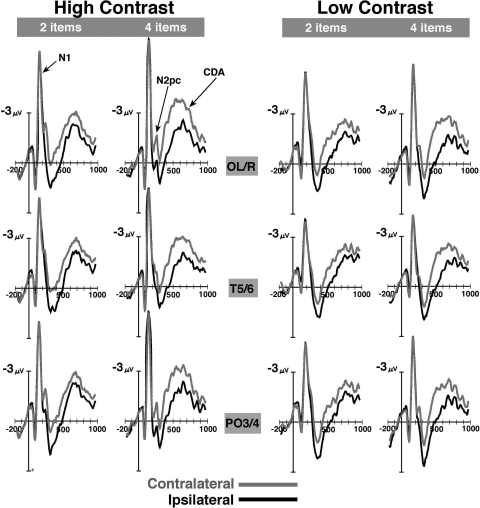
Figure 2 illustrates the ipsi- and contralateral waveforms for each condition. Beginning around 250 ms, strong negative contralateral waves arose over posterior electrode sites and continued throughout the retention period. Figure 3A shows mean difference waves (contralateral minus ipsilateral) averaged over three posterior sites with particularly strong contralateral negativity; OL/R, T5/6, and PO3/4. Two ERP components are evident: the N2pc, which is a component related to the selection of targets (Drew and Vogel 2008; Eimer 1996; Jolicouer et al. 2008; Luck and Hillyard 1994); and the CDA, which is related to the number of items in VWM.
Fig. 2.
Event-related potential (ERP) data from experiment 1, time-locked to the onset of the memory array. Posterior lateral recording sites (OL/R, T5/6, PO3/4) are shown. Purple and black lines are data from contralateral and ipsilateral sites, respectively. Negative is plotted up.
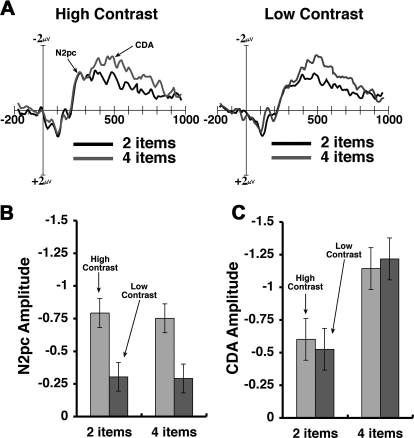
Fig. 3.
Difference wave averaged across 3 channels shown in Fig. 2. A: averaged difference wave across time for high (left) and low (right) contrast conditions. Set size 2 and 4 are shown in black and blue, respectively. In both contrast conditions, N2pc followed by contralateral delay activity (CDA) are visible. B: N2pc amplitude in the time window between 200 and 280 ms after the memory array onset. Error bars = 95% confidence intervals. Notice there is a significant difference between contrasts (P < 0.001) but not between set sizes. C: CDA amplitude in the time window between 300 and 900 ms after the memory array onset. There is a significant difference between set sizes (P < 0.02) but not between contrasts.
N2pc amplitude was significantly larger in the high contrast condition than in the low contrast condition [F(1,14) = 33.71, P < 0.001], but there was no significant main effect of set size [F(1,14) = 0.07, P > 0.05]. The larger N2pc for high contrast arrays suggests that the memory items were initially selected from the display more consistently when the contrast was sufficiently high. The lack of a set size effect on the N2pc is consistent with prior results (McCollough et al. 2007; but see Drew and Vogel 2008). Of course, a reduced N2pc for low contrast objects does not necessarily indicate that the attentional selection process was eliminated for these items. Similar results would be expected if that process was simply more variable in its latency from trial to trial (Luck 2005).
Consistent with prior work (Drew and Vogel 2008; McCollough et al. 2007; Vogel and Machizawa 2004; Vogel et al. 2005), the CDA emerged at ∼300 ms and persisted throughout the retention period. CDA amplitude was significantly larger for four-item arrays than for two-item arrays [F(1,14) = 8.18, P < 0.02]. However, there was no significant main effect of contrast, [F(1,14) <1.0] nor was there a significant interaction between set size and contrast [F(1,14) <1.0]. These results demonstrate that significantly increasing the perceptual demands of the memory items did not modulate CDA amplitude. However, increasing the number of items irrespective of stimulus contrast did indeed result in significant increases in CDA amplitude. This dissociation between perceptual difficulty and CDA amplitude is clearest when comparing between the low contrast two-item arrays with the high contrast four-item arrays. These two conditions yielded equivalent performance levels, likely engendering similar levels of perceptual difficulty, yet the CDA amplitude was considerably greater for four-item arrays. In addition, the large behavioral performance decrement (∼10%), along with a reduced N2pc, suggests that the contrast manipulation used here was sufficiently large to significantly affect both behavior as well as attentional selection.
These results also provide a decoupling of behavioral performance levels and CDA amplitude. That is, poorer performance for the low contrast arrays does not appear to be the consequence of a reduction of the number of representations that are held in memory. Instead these results are more consistent with the proposal that low contrast objects yield poorer memory performance because the resolution of the representations may not have been sufficient to accurately discriminate the remembered color from the color of the changed item. Moreover, the low contrast of the items in the test array also likely contributed to reduced memory performance during the comparison process at the end of the trial. This is consistent with recent evidence that memory items that are highly similar to one another often are susceptible to comparison errors in change detection tasks (Awh et al. 2007; Scolari et al. 2008). These results indicate that contrast manipulations such as this one yield the consequences of insufficient precision of the representation rather than a reduction of the number of items held in WM.
Experiment 2: does the CDA reflect number of items or number of locations in memory?
The results of experiment 1 demonstrate that while CDA amplitude was sensitive to the number of objects in the memory array, it was not modulated by the contrast of the objects despite a fairly large performance decrement for the low contrast objects. However, one alternative account of these results is that the CDA may not actually be sensitive to the number of objects in VWM but instead may be simply sensitive to the number of locations that are currently being attended or remembered (Xu and Chun 2006). That is, in all previous experiments examining this activity, the number of objects has always been confounded with the number of locations. If this was the case, the lack of a contrast effect on the CDA would be ambiguous because low contrast objects may still provide sufficient spatial information for representing location despite consuming a larger proportion of maintenance resources.
To address this alternative account of the CDA, in experiment 2, we presented subjects with a sequence of two memory arrays separated by 500 ms (Fig. 4A). Each memory array contained two high contrast colored squares for a total memory load of four items. In one sequential memory condition, the items in the second array were presented in the same locations as those in the first array. Thus four objects in two locations needed to be maintained in memory. In the other sequential condition, the items in the second array were presented in different locations in the hemifield from those used in the first array. Thus four objects in four locations needed to be maintained in memory. We contrasted these sequential conditions with two conditions in which either two or four items were presented simultaneously in a single memory array.
In a previous study using a procedure that is highly similar to the sequential-different locations condition, we found that the CDA initially has an amplitude that is similar to two items but rose up to the level of 4 simultaneous items shortly following the onset of the second array (Vogel et al. 2005). If the CDA was sensitive only to the number of locations in memory, we would expect this same increase to the level of four items only in the different locations condition because four locations must be remembered. Alternatively, if the CDA is sensitive to the total object load in VWM, we would expect to see this amplitude increase equivalently in both the “same location” and “different location” conditions because four objects must be remembered in each.
Behavioral performance
Change detection accuracy was better for two-item arrays (92%) than for four-item simultaneous arrays (82%; P < 0.001) as well as the sequential-same locations (79%; P < 0.001) and the sequential-different locations (77%; P < 0.001). However, performance for the four-items simultaneous condition was not significantly different from either of the sequential conditions (both F's > 1) nor were the two sequential conditions significantly different from one another (F < 1).
Electrophysiological results
As shown in Fig. 4, B and C, CDA amplitude in the sequential conditions was initially equivalent to a two-item level but then increased to the level of the four-item simultaneous condition shortly following the onset of the second memory array. We tested this pattern by comparing mean amplitude for an early time window (300–500 ms following memory array 1) in the sequential conditions to the same time window in the simultaneous two-item and found that they were not significantly different (F < 1). Moreover, we compared mean amplitude for a late time window (300–500 ms following memory array 2) in the sequential conditions to the same time window in the simultaneous four-item condition and again found that they were not significantly different (P > 0.35).
We compared the rise between the same location and different location conditions by measuring amplitude during two time windows, early (300–500 ms following memory array 1) and late (300–500 ms following memory array 2). We found a highly significant main effect of time window (memory array 2 is greater than array 1; P < 0.001) but no significant main effect of condition (F < 1) and no interaction between these factors (F < 1). That is, even though the “same location” required the subjects to remember four objects across only two positions, it yielded identical amplitudes to the different location condition, which required four objects across four locations to be remembered. Thus these results indicate that the CDA amplitude is modulated by the number of objects that are being held in memory, irrespective of the number of distinct locations that are being remembered or attended within the display.
DISCUSSION
The present study examined the aspects of WM performance that results in CDA amplitude modulations associated with increasing numbers of items to be remembered. We tested two viable alternative hypotheses of this activity. The first was that the CDA reflects perceptual demands for resources that increase as the number of items increases. However, in experiment 1, we found that increasing the perceptual demands of the items (by greatly lowering contrast), did not modulate CDA amplitude. While an increase in set size is obviously not identical to a reduction in contrast, both manipulations engender an substantial increase in perceptual difficulty and resulted in equivalent decreases in behavioral performance. Nevertheless, CDA amplitude was only modulated by the number of items in the display, which is consistent with a memory load-based interpretation. The second hypothesis we tested was whether the CDA charted the number of locations being attended rather than reflecting the total number of objects being remembered. Experiment 2 provided evidence against this interpretation by showing that CDA amplitude could be decoupled from the number of locations that are relevant for the task. Together, these results lead us to conclude that modulations of CDA amplitude across memory set size reflect the current number of object representations that are being held in VWM. However, that is not to say that this activity is entirely insensitive to other attributes of the WM representation, such as the identity of the memoranda. Indeed we and others have already reported preliminary evidence that this activity may indeed be sensitive to the information content of the items in memory (Luria et al. 2009; Woodman and Vogel 2008).
At present, it is not entirely clear whether the CDA reflects the ongoing output of a WM maintenance process or if it instead reflects a limited-capacity “pointer system” that helps to keep task-relevant representations individuated from one another by linking some coarse identity information with a spatial position. Some evidence for the latter view comes from recent work examining the multiple object tracking (MOT) task in which a subject must attentively track several targets as they move among identical distractors (Cavanagh and Alvarez 2005; Drew and Vogel 2008; Pylyshyn and Storm 1988). Drew and Vogel (2008) found a sustained CDA that was modulated by the number of targets that were being tracked on a given trial, and this activity showed similar capacity limitations that predicted an individual's tracking ability. That is, despite negligible memory maintenance requirements, similar activity can still be obtained if attention is continuously being allocated to each target. In this regard, a limited capacity pointer system may be at play in both WM and MOT tasks as a means of keeping a small number of object representations individuated. For WM tasks, this pointer system may simply require sustaining the representations in an active state. For MOT tasks, these pointers may interface with updating mechanisms that reflect the changing position of the targets as they move through space.
GRANTS
This work was supported by grants from the National Science Foundation and the National Institute of Mental Health to E. K. Vogel.
REFERENCES
- Awh E, Barton B, Vogel EK. Visual working memory represents a fixed number of items regardless of complexity. Psychol Sci 18: 622–628, 2007 [DOI] [PubMed] [Google Scholar]
- Cavanagh P, Alvarez GA. Tracking multiple targets with multifocal attention. Trends Cogn Sci 9: 349–354, 2005 [DOI] [PubMed] [Google Scholar]
- Chafee MV, Goldman-Rakic PS. Matching patterns of activity in primate prefrontal area 8a and parietal area 7ip neurons during a spatial working memory task. J Neurophysiol 79: 2919–2940, 1998 [DOI] [PubMed] [Google Scholar]
- Courtney SM, Petit L, Maisog JM, Ungerleider LG, Haxby JV. An area specialized for spatial working memory in human frontal cortex. Science 279: 1347–1351, 1998 [DOI] [PubMed] [Google Scholar]
- Curtis CE, D'Esposito M. Selection and maintenance of saccade goals in the human frontal eye fields. J Neurophysiol 95: 3923–3927, 2006 [DOI] [PubMed] [Google Scholar]
- Drew T, Vogel EK. Neural measures of individual differences in selecting and tracking multiple moving objects. J Neurosci 28: 4183–4191, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eimer M. The N2pc component as an indicator of attentional selectivity. Electroencephalogr Clin Neurophysiol 99: 225–234, 1996 [DOI] [PubMed] [Google Scholar]
- Engle RW, Tuholski SW, Laughlin JE, Conway AR. Working memory, short-term memory, and general fluid intelligence: a latent-variable approach. J Exp Psychol Gen 128: 309–331, 1999 [DOI] [PubMed] [Google Scholar]
- Ferber S, Humphrey GK, Vilis T. Segregation and persistence of form in the lateral occipital complex. Neuropsychologia 43: 41–51, 2005 [DOI] [PubMed] [Google Scholar]
- Fukuda K, Vogel EK. Human variation in overriding attentional capture. J Neurosci 29: 8726–8733, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the monkey's dorsolateral prefrontal cortex. J Neurophysiol 61: 331–349, 1989 [DOI] [PubMed] [Google Scholar]
- Fuster JM, Alexander GE. Neuron activity related to short-term memory. Science 173: 652–654, 1971 [DOI] [PubMed] [Google Scholar]
- Harrison SA, Tong F. Decoding reveals the contents of visual working memory in early visual areas. Nature 458: 632–635, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolicoeur P, Brisson B, Robitaille N. Dissociation of the N2pc and sustained posterior contralateral negativity in a choice response task. Brain Res 1215: 160–172, 2008 [DOI] [PubMed] [Google Scholar]
- Kubota K, Niki H. Prefrontal cortical unit activity and delayed alternation performance in monkeys. J Neurophysiol 34: 337–347, 1971 [DOI] [PubMed] [Google Scholar]
- Kyllonen PC, Christal RE. Reasoning ability is (little more than) working-memory capacity?! Intelligence 14: 389, 1990 [Google Scholar]
- Luck SJ. An Introduction to the Event-Related Potential Technique. Cambridge, MA: MIT Press, 2005 [Google Scholar]
- Luck SJ, Hillyard SA. Spatial filtering during visual search: evidence from human electrophysiology. J Exp Psychol Hum Percept Perform 20: 1000–1014, 1994 [DOI] [PubMed] [Google Scholar]
- Luria R, Sessa P, Gotler A, Jolicoeur P, Dell'acqua R. Visual short-term memory capacity for simple and complex objects. J Cogn Neurosci 22: 496–512, 2009 [DOI] [PubMed] [Google Scholar]
- McCollough AW, Machizawa MG, Vogel EK. Electrophysiological measures of maintaining representations in visual working memory. Cortex 43: 77–94, 2007 [DOI] [PubMed] [Google Scholar]
- Postle BR, Berger JS, Taich AM, D'Esposito M. Activity in human frontal cortex associated with spatial working memory and saccadic behavior. J Cogn Neurosci 12, Suppl 2: 2–14, 2000 [DOI] [PubMed] [Google Scholar]
- Pylyshyn ZW, Storm RW. Tracking multiple independent targets: Evidence for a parallel tracking mechanism. Spat Vis 3: 179–197, 1988 [DOI] [PubMed] [Google Scholar]
- Rainer G, Miller EK. Timecourse of object-related neural activity in the primate prefrontal cortex during a short-term memory task. Eur J Neurosci 15: 1244–1254, 2002 [DOI] [PubMed] [Google Scholar]
- Robitaille N, Grimault S, Jolicoeur P. Bilateral parietal and contralateral responses during the maintenance of unilaterally-encoded objects in visual short-term memory: evidence from magnetoencephalography. Psychophysiology 46: 1090–1099, 2009 [DOI] [PubMed] [Google Scholar]
- Scolari M, Vogel EK, Awh E. Perceptual expertise enhances the resolution but not the number of representations in working memory. Psychonom Bull Rev 15: 215–222, 2008 [DOI] [PubMed] [Google Scholar]
- Serences JT, Ester EF, Vogel EK, Awh E. Stimulus-specific delay activity in human primary visual cortex. Psychol Sci 20: 207–214, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srimal R, Curtis CE. Persistent neural activity during the maintenance of spatial position in working memory. NeuroImage 1: 455–468, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd JJ, Marois R. Capacity limit of visual short-term memory in human posterior parietal cortex. Nature 428: 751–754, 2004 [DOI] [PubMed] [Google Scholar]
- Umeno MM, Goldberg ME. Spatial processing in the monkey frontal eye field. II. Memory responses. J Neurophysiol 86: 2344–2352, 2001 [DOI] [PubMed] [Google Scholar]
- Unsworth N, Brewer GA, Spillers GJ. There's more to the working memory capacity–fluid intelligence relationship than just secondary memory. Psychon Bull Rev 16: 931–937, 2009 [DOI] [PubMed] [Google Scholar]
- Vogel EK, Machizawa MG. Neural activity predicts individual differences in visual working memory capacity. Nature 428: 748–751, 2004 [DOI] [PubMed] [Google Scholar]
- Vogel EK, McCollough AW, Machizawa MG. Neural measures reveal individual differences in controlling access to working memory. Nature 438: 500–503, 2005 [DOI] [PubMed] [Google Scholar]
- Warden MR, Miller EK. The representation of multiple objects in prefrontal neuronal delay activity. Cereb Cortex 17, Suppl 1: i41–i50, 2007 [DOI] [PubMed] [Google Scholar]
- Woodman GF, Vogel EK. Selective storage and maintenance of an object's features in visual working memory. Psychon Bull Rev 15: 223–229, 2008 [DOI] [PubMed] [Google Scholar]
- Xu Y, Chun MM. Dissociable neural mechanisms supporting visual short-term memory for objects. Nature 440: 91–95, 2006 [DOI] [PubMed] [Google Scholar]