Abstract
Most visual scenes are complex and crowded, with several different objects competing for attention and action. Thus a complete understanding of the production of goal-directed actions must incorporate the higher-level process of target selection. To examine the neural substrates of target selection for visually guided reaching, we recorded the activity of isolated neurons in the dorsal premotor area (PMd) of monkeys performing a reaction-time visual search task. In this task, monkeys reached to an odd-colored target presented with three distractors. We found that PMd neurons typically discriminate the target before movement onset, ∼150–200 ms after the appearance of the search array. In one subset of neurons, discrimination occurred at a consistent time after search array onset regardless of when the reaching movement occurred, suggesting that these neurons are involved in target selection. In a second group of neurons, discrimination time depended on reach reaction time, consistent with involvement in movement production but not in target selection. To look for physiological corroboration of these two functionally defined groups, we analyzed the extracellular spike waveforms of recorded neurons. This analysis showed a population of neurons with narrow action potentials that carried signals related to target selection. A second population with broader action potentials was more heterogeneous, with some neurons showing activity related to target selection and others showing only movement production activity. These results suggest that PMd contains signals related to target selection and movement execution and that different signals are carried by distinct neural subpopulations.
INTRODUCTION
In the real world, most visual scenes are complex and crowded with several different objects competing for attention and action. To interact efficiently with objects in the environment, motor systems must be capable of selecting a single target for action from the multitude of possibilities. Although the neural basis of target selection has been studied extensively for the oculomotor system (Basso and Wurtz 1998; Bichot and Schall 1999, 2002; Ipata et al. 2006; Kim and Basso 2008; McPeek and Keller 2002, 2004; Schall and Hanes 1993; Schiller and Tehovnik 2005; Thomas and Pare 2007; Thompson et al. 1996), fewer studies have investigated this issue for the skeletal motor systems involved in reaching movements. In this study, we examined neural activity in the dorsal premotor area (PMd) in a task in which monkeys reached to an odd-colored target presented with distractors. Such visual search tasks have been used previously in behavioral studies of reach target selection in both humans and monkeys (Song and Nakayama 2006, 2007a,b, 2008; Song et al. 2008).
The neural substrates of reach target selection are thought to involve higher-order movement-related areas in frontal and parietal cortex, including the dorsal premotor area (PMd) and parietal reach region (PRR) (Cisek 2006; Cisek and Kalaska 2002b, 2005; Hoshi and Tanji 2000; Pesaran et al. 2008; Scherberger and Andersen 2007). In studies of reaches to single, isolated targets, PMd activity has been shown to be related to the direction, amplitude, and speed of upcoming limb movements (Boussaoud and Wise 1993a; Churchland et al. 2006; Crammond and Kalaska 1996; Kurata and Hoffman 1994). In more complex reaching tasks, neural correlates of the stimulus-response mapping and of virtual action plans have been observed in PMd (Caminiti et al. 1998; Crammond and Kalaska 2000; Nakayama et al. 2008; Shen and Alexander 1997; Wise et al. 1996).
Cisek and Kalaska (2005) examined PMd activity in a selective reaching task in which two stimuli representing potential goals were initially presented. After a delay, a central cue identified one of the stimuli as the reach goal. After another delay, a “go” signal triggered the monkey's reach to the target. They found that, at the population level, PMd activity initially represented both potential goals but that, after the cue identified the reach target, PMd activity began to signal only the selected goal. They also found that some cells registered the selected target location before the go signal, whereas other cells began to increase their activity only after the go signal was presented. This pattern of results suggests that different groups of PMd neurons are involved in target selection and movement production.
In this study, we used a reaction time color-oddity search task to investigate the role of PMd in reach target selection. Reaction-time search tasks have been used extensively to study saccade target selection in both humans and monkeys, providing substantial information about the behavioral and neural mechanisms (Basso and Wurtz 1998; Bichot and Schall 1999, 2002; Ipata et al. 2006; Kim and Basso 2008; McPeek 2006; McPeek and Keller 2002, 2004; Schall and Hanes 1993; Thomas and Pare 2007; Thompson et al. 1996). Thus using this task to study reach target selection has the advantage of allowing direct comparisons between the mechanisms underlying target selection for reaching and for saccades. A previous study has shown that the reach target in a color-oddity task can be decoded from PMd activity (Santhanam et al. 2006). However, in this study, a delay period was imposed between the onset of the search array and the execution of the reach, precluding an analysis of the relationship between PMd activity and reach reaction time. In contrast, our task encourages immediate target selection with no enforced delay period between stimulus and movement onset. Thus it may provide a more natural situation in which monkeys are free to respond whenever they are ready, potentially reducing the influence of anticipatory activity on cell responses. Finally, it allows us to examine the connection between PMd activity and variations in reach reaction time, providing an independent method for distinguishing cells with activity related to target selection from cells with activity that is simply related to movement production. Specifically, we adopted the logic developed by Thompson et al. (1996; see also, DiCarlo and Maunsell 2005): we reasoned that, if a neuron is involved in target selection, the time at which the neuron discriminates the target from the distractors should be largely independent of trial-to-trial variations in reaction time. On the other hand, if a neuron is only involved in movement production, neural target/distractor discrimination time should be correlated with reaction time (Ipata et al. 2006; Lee and Keller 2006; McPeek and Keller 2002; Thomas and Pare 2007; Thompson et al. 1996).
In addition to studying the time course of neural discrimination of the target, we also analyzed the extracellular voltage waveforms of the recorded neurons. Extracellular spike waveforms are related to the shapes of intracellular potentials (Gold et al. 2006; Henze et al. 2000), which, in turn, can differ for different types of neurons (Connors and Gutnick 1990; Gonzalez-Burgos et al. 2005; McCormick et al. 1985). For example, some classes of local inhibitory interneurons produce narrow spike waveforms, whereas excitatory pyramidal cells produce wider spikes. Analyses of the shapes of extracellular spike waveforms have been used to distinguish different types of neurons in a variety of cortical regions (Bartho et al. 2004; Chen et al. 2008; Cohen et al. 2009; Constantinidis and Goldman-Rakic 2002; Mitchell et al. 2007; Mountcastle et al. 1969). We used this analysis to look for converging evidence of a physiological distinction between the PMd cells that carry a target selection signal and those that only carry signals involved in movement production.
METHODS
All experimental protocols were approved by the Institutional Animal Care and Use Committee at the Smith-Kettlewell Eye Research Institute and complied with the guidelines of the Public Health Service policy on Humane Care and Use of Laboratory Animals. A head-holder system and stainless steel recording chamber were implanted under isofluorane anesthesia and aseptic surgical conditions in two rhesus monkeys (Macaca mulatta). The chambers were placed in the left hemisphere, centered between the arcuate and central sulci, which were visualized through the intact dura during surgery. To locate PMd physiologically, we first located the frontal eye field (FEF), which was identified as the area of cortex from which saccades could be elicited using low-current microstimulation (<50 μA with a 333 Hz pulse frequency; 80 ms train duration). Microstimulation always consisted of constant-current biphasic (cathodal leading) pulses with 0.2 ms pulse width per pulse.
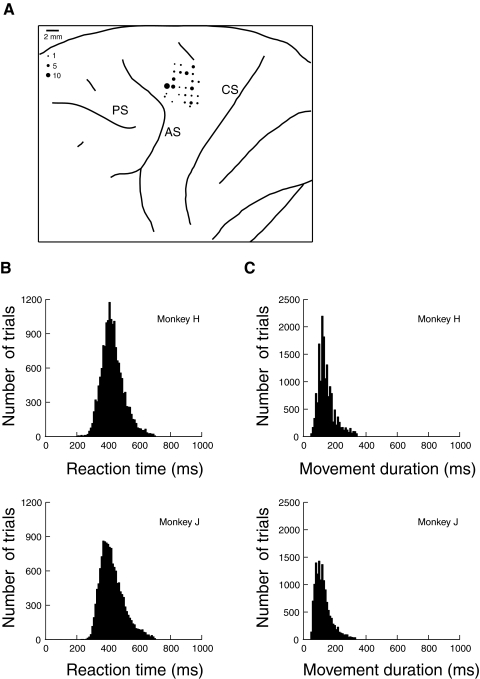
Using the known medio-lateral topographic progression in FEF from large amplitude saccades to small amplitude saccades (Bruce et al. 1985; Sommer and Wurtz 2000), the anterior bank of the arcuate sulcus was mapped out by microstimulating to elicit saccades and recording saccade-related activity in a series of electrode tracks. We located our PMd tracks in the cortical surface posterior to the superior limb of the arcuate sulcus at anterior-posterior locations near the estimated location of the genu. We recorded primarily from shoulder, elbow, and wrist representations in PMd, which we verified by evoking movements with microstimulation at high current thresholds (>80 μA; 333 Hz train frequency; 100 ms train duration). Eye movements were not evoked at any of the recorded sites. Recording sites were histologically verified in monkey H (Fig. 1A). Monkey J is currently participating in further studies.
Fig. 1.
A: location of electrode penetrations in dorsal premotor area (PMd) of monkey H. The size of the dots represents the number of neurons recorded. CS, central sulcus; AS, arcuate sulcus; PS, principal sulcus. B: histogram of reach reaction times in the search task. C: histogram of reach durations in the search task.
In each recording session, a tungsten microelectrode (FHC), with impedance ranging from 1 to 2 MΩ at 1 kHz, was lowered into PMd using a motorized microdrive (NAN Instruments). A Plexon MAP system amplified and band-pass filtered the microelectrode signal and was used to identify action potentials. Spike waveforms were digitized at 40 kHz and saved to disk. Spike occurrences were recorded with a resolution of 1 kHz and were stored in register with behavioral measurements. Spike-sorting was verified off-line using the Plexon Off-line Analysis software. Only well-isolated single units were included in the analyses.
Behavioral procedures
Testing was performed in a dimly illuminated room. Data collection and storage were controlled by a Power Mac G4, which also generated the visual displays using software constructed with PsychToolbox (Brainard 1997; Pelli 1997). Visual stimuli were presented on a 17 in color CRT touch sensitive monitor (ELO Touch Systems), positioned 25.5 cm in front of the monkeys. Eye position was sampled at 1 kHz using an EyeLink 1000 infrared video tracker (SR Research). The monkeys’ left arms were loosely restrained and heads fixed during each testing session.
VISUAL SEARCH.
At the beginning of each trial in the search task, a yellow square subtending 1–2° with a luminance of 1.5 cd/m2 was presented on a black homogenous background of 0.2 cd/m2. The monkeys touched this square with the index (D2; monkey J) or middle (D3; monkey H) digit of the right hand to initiate the trial and were required to maintain contact with the square for 500 ms. After this 500 ms interval, the yellow square was extinguished and an odd-colored target along with three distractors was presented. The stimuli consisted of red and green discs subtending 2.5° of visual angle (1.1 cm in diameter on the screen) with a luminance of 1.2 cd/m2. The color of the target was randomly selected in each trial to be either red or green, and the distractors were of the opposite color. The stimuli were presented at an eccentricity of 12° (5.4 cm from the center), separated by angles of 90°. The position of the target was randomly chosen from the four oblique directions (45, 135, 225, and 315°). The search array remained onscreen until a response was made. The monkeys were rewarded if the first point at which the hand touched the screen (after lifting off from the center point) was within 5° of the correct target location and remained at the target location for ≥500 ms. If no response was made within 2 s after the search array onset, the trial was aborted.
We tested the monkeys in two variants of this basic task. In the free-gaze variant, eye movements were not constrained and did not have any effect on the monkeys’ reward for reaching to the target. In the fixation variant, a white square subtending 0.5° with a luminance of 1.2 cd/m2 was presented above the central yellow square at the beginning of the trial and remained continuously visible throughout the trial. The monkeys were required to maintain fixation within 1.5–2° of this white square for the duration of each trial. If the eyes moved outside this window, or if radial eye velocity exceeded 40°/s, the trial was immediately aborted and no reward was given.
SINGLE TARGET DELAY TASK.
Trials began as in the visual search task, except that after the initial 500 ms interval, a single target was presented without distractors and the central hand fixation point remained illuminated. After a 500 ms delay period, the hand fixation point disappeared, cueing the monkey to reach to the target. Monkeys were rewarded for maintaining eye fixation at the center and touching the screen within 5° of the target location within 2 s after the hand fixation point was removed. The monkeys were also required to maintain contact with the reach target for 500 ms.
Behavioral data analysis
The onset of the reaching movement was measured as the time at which the hand was lifted from its initial position in the center of the touchscreen, and reach reaction time was defined as the interval between the onset of search array and the reaching movement. Reach movement duration was measured as the time from reach onset to contact with the touchscreen. The reach endpoint was measured as the first location contacted on the touchscreen after movement onset. A trial was classified as an error when the reach endpoint was >5° away from the target, and error trials were omitted from analysis. We also excluded trials in which the reach reaction time differed from the mean reaction time by >3 SD, a criterion that typically resulted in the exclusion of no more than one to two trials per cell.
Neural data analysis
Some selection of neurons occurred during experiments, and recordings of nonresponsive neurons were aborted. Further selection was performed to concentrate our analysis on neurons showing task-related activity. Because we were interested in activity related to target selection, our analysis focused on the interval from 50 ms after stimulus onset until 50 ms before movement execution (the premovement period). For a cell to be considered task-related, there had to be at least one target direction for which the cell's mean premovement firing rate was ≥10 spikes/s greater than its baseline rate, and this elevated activity had to be statistically significantly different from the baseline (Wilcoxon signed-rank test, P < 0.05). Each cell's preferred direction (PD) was determined as the direction eliciting the greatest response during the premovement period.
We also analyzed the duration of extracellular spike waveforms to examine whether different subtypes of PMd neurons make different contributions to the task. For each neuron, 700 μs analog recordings of each action potential were digitized at 40 kHz. The spikes for each neuron were aligned relative to their troughs and averaged. The average waveform for each neuron was then spline-interpolated to give a temporal precision of 1.25 μs. Waveform duration was defined as the interval from the trough to the peak of the average waveform (Mitchell et al. 2007).
ROC analysis of target/distractor discrimination
We used signal detection theory (Green and Swets 1966) to quantitatively analyze target/distractor discrimination at the single-neuron level. This technique has been used extensively to examine neural discrimination in a variety of visual, eye movement, and hand movement tasks (Bradley et al. 1987; Britten et al. 1992; Kim and Shadlen 1999; Lee and Keller 2006; McPeek and Keller 2002; Scherberger and Andersen 2007; Thompson et al. 1996). To quantify discrimination, we compared the activity of each neuron when the target was in the cell's PD versus in the opposite direction and computed the area under the ROC curve to provide an unbiased estimate of the separation between the firing rate distributions in the two conditions. ROC area ranges from 0.5, indicating complete overlap in firing rates to a maximum of 1.0, which indicates nonoverlapping distributions.
To determine the earliest time at which each neuron discriminated the target (discrimination time), we adapted a method developed by Thompson et al. (1996). Trials were aligned on the presentation of the search array. Spike density was computed by convolving spike trains with a Gaussian kernel (σ = 20 ms). ROC curves were constructed at each millisecond using the spike densities of trials in which the target was in the PD and trials in which the target was opposite the PD. To extract discrimination time, we determined when ROC area first indicated that the spike density distributions were significantly nonoverlapping (P < 0.05) and remained so for ≥50 ms. This statistical discrimination threshold was established at each time point using a permutation test (2,000 iterations) (Britten et al. 1996; Efron 1993; Lee and Keller 2006; Scherberger and Andersen 2007).
To determine whether discrimination time was better correlated with the onset of the search array or with reach reaction time (RT), we partitioned the trials for each neuron according to reach reaction time into short and long RT groups (median split). We separately estimated discrimination time for each of the two groups. If discrimination time is related to reach reaction time, it should increase proportionately for the long RT group compared with the short RT group. On the other hand, if discrimination time is related to the onset of the search array, it should remain the same for the short and long RT groups. For statistical reliability, only neurons having at least six correct trials for each target location and reaction time condition were included in the analyses, and the average number of trials per cell for each location and reaction time condition was 21.7 correct trials.
The above analysis served to determine whether neuronal discrimination time was correlated with stimulus onset, reach reaction time, or neither. However, we performed an additional analysis to check the consistency of the results, as well as to consider the possibility that a neuron might have two distinct phases of discrimination activity, with the first phase correlated with stimulus onset and the second phase correlated with reach onset. To do this, we performed the same analysis as described above, except that we realigned activity for each of the two reaction time groups on the onset of the reaching movement and searched backward in time from reach onset until the neuron did not significantly discriminate the target. In this analysis, if the neuron exhibits a phase of activity which discriminates the target at a time related to the onset of the reaching movement, it should occur a consistent time before movement onset for both RT groups when the data are aligned on reach onset. On the other hand, if discrimination time is independent of RT, discrimination should occur earlier before movement onset in long RT trials versus short RT trials.
RESULTS
The monkeys performed the task well during the recording sessions, with monkey H reaching to the correct target in 87% of trials and monkey J in 89%. Histograms of reach reaction time and the duration of the reaching movements are shown in Fig. 1, B and C. RTs were fairly long and variable [mean = 430 ± 72 (SD) ms in monkey H and mean = 428 ± 77 ms in monkey J], whereas movement durations were shorter and more consistent (mean = 146 ± 45 ms in monkey H and mean = 127 ± 43 ms in monkey J).
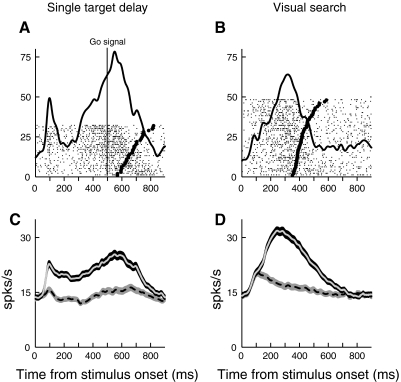
We recorded 231 isolated PMd neurons (121 from monkey H and 110 from monkey J), and 183 of these neurons (79%) met our criteria for task-related activity (see methods). All subsequent data analyses were restricted to this subset of neurons, which included 91 neurons recorded in the fixation search task, 73 neurons recorded in the free-gaze task, and 19 neurons recorded in both tasks. We first compared activity in the search task with activity in the single target delay task, to examine how PMd responses are affected by the search task. This comparison involved 145 cells that were recorded in both tasks. The most notable difference in activity was that the initial short-latency visual burst related to the onset of a target in the cell's PD was significantly lower in search than in the single-target task. Figure 2 shows the activity of representative a cell in both tasks. Activity is aligned on the presentation of the search array, and reach onset is indicated in each trial by a thick tick mark. In the neuron on the left, activity shows a transient increase after search array onset when the target was in the PD. In the single-target task (Fig. 2A), the cell shows a prominent burst of activity in the first 100 ms after the onset of a target in its PD. However, in search (Fig. 2B), this initial visual activity is substantially reduced, despite the fact that the position and visual properties of the target in the PD were identical in the two tasks.
Fig. 2.
A: activity of a cell in the single-target delay task. The target is presented in the cell's preferred direction (PD) at time 0, and the solid vertical line indicates the “go” signal. In this and all subsequent figures of this type, each raster represents neural activity in an individual trial, with tick marks indicating action potentials. The mean spike density function is overlaid (σ = 20 ms). Reach onset is indicated in each trial by a thick tick mark. B: activity of the same cell in the visual search task. A target in the cell's PD is presented at time 0, along with distractors at the other 3 locations. C and D: plots of PMd population activity in the single target delay (C) and search (D) tasks, aligned on the onset of the visual stimuli. The ribbons indicate mean activity ±SE. The solid lines represent activity when the target was in the PD, whereas the dotted lines represent activity when the target was in the opposite direction.
Similar results are seen in the mean population activity across our sample of neurons in the single-target (Fig. 2C) and search tasks (Fig. 2D). This reduction in the initial burst of activity occurs despite the fact that monkeys are free to respond immediately in the search task, whereas they must wait to respond in the single-target delay task. A similar reduction in the initial visual response for search tasks has been found for the oculomotor system (Basso and Wurtz 1998; McPeek and Keller 2002; Schall et al. 1995).
Target/distractor discrimination in PMd neurons
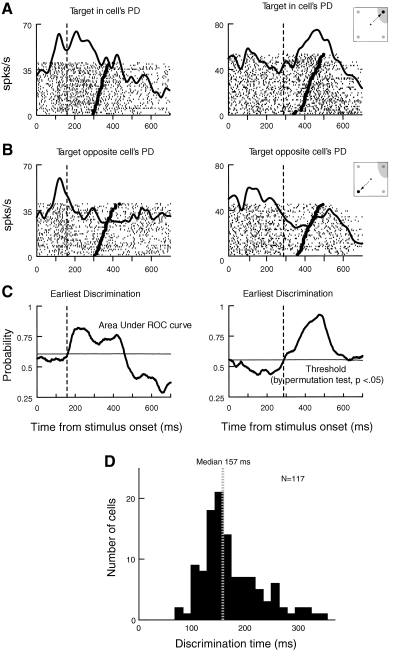
Next, we examined neural discrimination of the reach target in the search task. Figure 3 shows activity in two PMd neurons when the search target was in the cells’ preferred direction (PD; Fig. 3A) and when the target was in the opposite direction (Fig. 3B). Activity is aligned on the presentation of the search array, and reach onset is indicated in each trial by a thick tick mark. In the neuron on the left, activity shows a transient increase after search array onset when the target was in the PD. In the neuron on the right, the activity increase begins later, and continues until reach onset. When the target was in the direction opposite the PD (Fig. 3B), both cells show lower levels of activity.
Fig. 3.
Target/distractor discrimination. In A–C, each column represents results from an individual PMd neuron. The vertical dashed lines show target/distractor discrimination time [the time at which receiver operating characteristic (ROC) area 1st reliably crosses the statistical threshold of P < 0.05]. A: activity of PMd cells aligned on stimulus onset when the target was in the PD. B: activity of the same cells when the target was in the opposite direction. C: ROC analysis of target/distractor discrimination. The thick lines indicate the area under the ROC curve as a function of time from stimulus onset. The thin horizontal lines indicate the P < 0.05 statistical threshold generated at each time point using a permutation test. D: distribution of discrimination times observed in PMd.
To determine whether and when individual PMd neurons begin to reliably discriminate the target from distractors, we conducted receiver operator characteristic (ROC) analysis (see methods). For the two example cells shown above, Fig. 3C compares each cell's level of activity for trials in the PD versus the opposite direction by showing the area under the ROC curve, computed at each millisecond after array onset. At first, ROC area is near 0.5, indicating that PMd activity does not initially discriminate whether a target or distractor is in the cell's PD. As time goes on, ROC area gradually grows and eventually exceeds the threshold (horizontal line), indicating that the neurons reliably (P < 0.05) discriminate the target from the distractor. The vertical dashed line shows target/distractor discrimination time, defined as the time at which ROC area first reliably crosses the threshold (see methods).
We found that 117 (71%) of task-related PMd neurons reliably discriminated the target from the distractor. As shown in Fig. 3D, neural discrimination of the target typically occurred 150–200 ms after the onset of the search array (median, 157 ms). Note that this result was not influenced by target color (red vs. green), because color was randomized across trials, and equal numbers of correct trials with red and green targets were collected for each target direction for each cell. Furthermore, when we sorted trials according to target color, we found that PMd activity during the premovement period was not significantly different for red and green targets (P = 0.58). Finally, we observed that reach reaction times also did not differ significantly for red and green targets (Wilcoxon signed-rank test, P = 0.23).
Influence of oculomotor behavior on target/distractor discrimination in PMd
Previous studies have shown that some PMd neurons are active during oculomotor tasks or are modulated by gaze position (Batista et al. 2007; Boussaoud et al. 1998; Cisek and Kalaska 2002a; Fujii et al. 2000). For example, Cisek and Kalaska (2002a) reported a gaze-modulation effect accounting for <20% of the discharge variance and a small shift of PD by ∼1.5°. Furthermore, Pesaran et al. (2006) showed that PMd neurons encode the relative position of the target, hand, and eye, using the differences in locations between all three variables.
Because we recorded the activity of PMd neurons separately in free-gaze search, in which eye movements are unconstrained, and fixation search, in which monkeys must maintain fixation throughout the trial, we wondered whether eye movements affect target/distractor discrimination in PMd. During free-gaze search, monkeys typically executed one or two saccades, with the eyes virtually always landing on the target and remaining fixated until the reach was complete (Song and McPeek 2009).
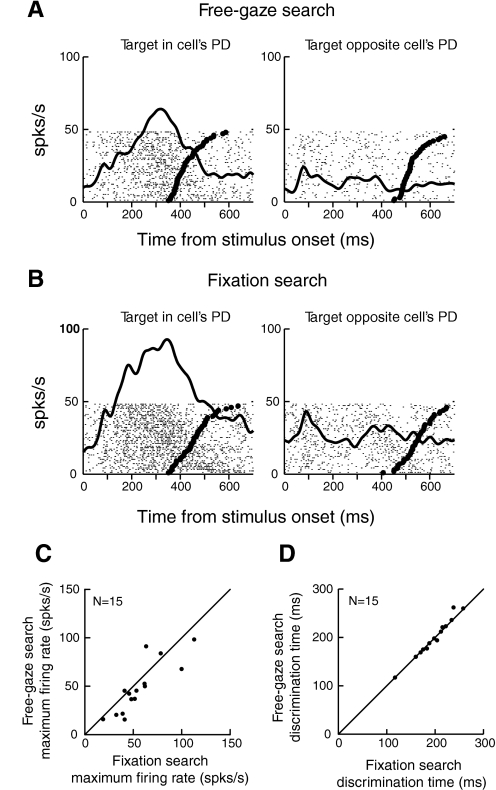
To assess the differences in PMd activity for free-gaze and fixation search, we recorded from 25 PMd neurons during both tasks. ROC analysis was applied to 19 of these neurons, which showed significant task-related activity, and we found that 15 neurons reliably discriminated the target from the distractor in both tasks. Figure 4, A and B, shows the responses of a representative PMd neuron in free-gaze (A) and fixation search (B). Activity when the target was in the cell's PD and in the opposite direction is shown in the left and right panels, respectively. The thick raster ticks indicate the reach reaction time in each trial. Compared with Fig. 4A, in which eye movements were unconstrained, Fig. 4B shows that the cell's overall activity is higher when fixation was required.
Fig. 4.
Comparison of PMd activity in free-gaze and fixation search. A: neural activity aligned on stimulus onset in free-gaze search. B: activity of the same cell aligned on stimulus onset in fixation search. In A and B, the left column represents neural activity when the target was in the cell's PD, whereas the right column represents activity when the target was in the opposite direction. C: comparison of maximum firing rates in fixation and free-gaze search. D: comparison of discrimination time in fixation and free-gaze search.
To confirm and quantify this observation, for each cell, we compared the maximum neural activity in free-gaze and fixation search during the premovement period when the target was in cell's PD. As shown in Fig. 4C, neural activity was modestly, but significantly, lower in free-gaze search than in fixation search: mean 48.2 ± 7.1 (SE) spikes/s in free-gaze search versus 56.6 ± 6.6 spikes/s in fixation search (13% decrease; Wilcoxon signed-rank test, P < 0.025). Nonetheless, as shown in Fig. 4D, there seems to be no difference in target/distractor discrimination time between the two tasks (Wilcoxon signed-rank test, P = 0.63). Because the eye movement condition (free-gaze vs. fixation) did not affect neural discrimination time, we combined the sample of cells across the two tasks.
Relationship between target/distractor discrimination time and reach reaction time
Because individual PMd neurons can reliably discriminate the target from distractors, we asked whether these neurons are simply involved in movement production or whether they might also be involved in target selection. To study this question, we examined the relationship between PMd neuronal discrimination time and reach reaction time (Ipata et al. 2006; Lee and Keller 2006; McPeek and Keller 2002; Sato et al. 2001; Thomas and Pare 2007; Thompson et al. 1996). Specifically, we divided the trials for each cell according to reach reaction time into short and long reaction time groups (median split). Then we performed ROC analysis on each group to determine the time of target/distractor discrimination separately for trials with short and long reaction times (see methods).
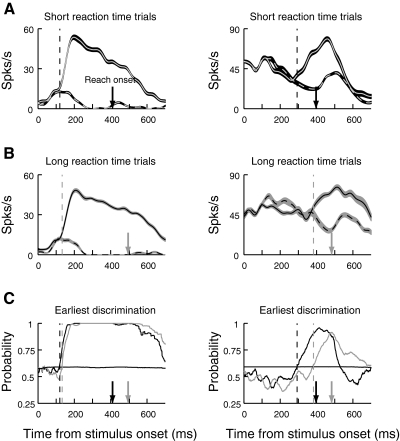
The two columns in Fig. 5 show results for two representative cells. Activity is aligned on stimulus onset and is plotted separately for the short (A) and long (B) reaction time groups. Solid lines indicate activity when the target was in the cells’ PD, and dashed lines show activity when the target was in the opposite direction. Figure 5C compares the growth in area under the ROC curve for the short (black line) and long (gray line) reaction time groups for the two cells. The downward arrows indicate mean reaction time, and the vertical dashed lines show discrimination time for each group. Of interest is the relationship between discrimination time and reach reaction time. In some cells, such as the cell on the left in Fig. 5, discrimination time is virtually the same for the short and long reaction time groups. This suggests that the cell carries a signal involved in target selection. However, in other cells, such as the cell on the right in Fig. 5, discrimination time is well correlated with reaction time, occurring later for the long reaction time group. This pattern of results suggests that the cell's role is limited to movement production.
Fig. 5.
Target/distractor discrimination for the short and long reaction time groups. Neural activity is aligned on the onset of the stimulus array, and each column in the figure shows data from a single neuron. Black corresponds to the short reaction time group and gray corresponds to the long reaction time group. The arrows on the abscissas represent the mean reach reaction time for each group. The vertical dashed lines show discrimination time for the corresponding groups. A: neural activity for the short reaction time group. B: activity of the same cells for the long reaction time group. In A and B, mean ± SE firing rates are plotted. The solid line represents activity when the target was in the cell's PD, and the dotted line represents activity when the target was in the opposite direction. C: ROC analysis of target/distractor discrimination. The thick lines show area under the ROC curve as a function of time from stimulus onset for both short (black) and long (gray) reaction time groups. In the cell on the left, ROC area 1st reliably crosses the threshold at about the same time regardless of reaction time, indicating that discrimination in this neuron is not correlated with reaction time. However, in the cell on the right, discrimination occurs proportionally later for the longer reaction time group.
Overall, 99 PMd neurons reliably discriminated the target for both short and long reach reaction time groups. Although the number of trials involved in the analysis varied somewhat from cell to cell, we found no significant correlation between the number of trials included in each condition and target discrimination time (r = −0.005, P = 0.91).
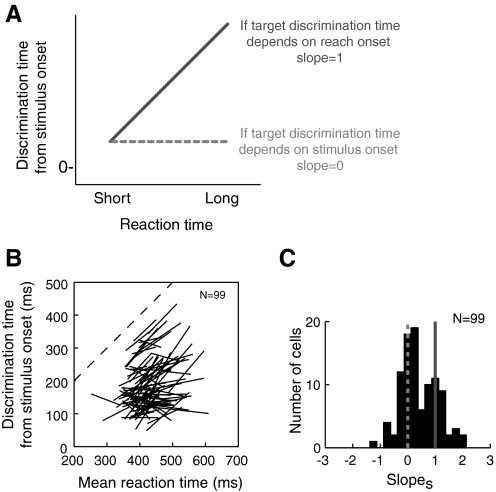
For each cell, we plotted discrimination time as a function of mean reaction time for the two reaction time groups. Each cell's data points were connected by a line, and we extracted the slopes of these lines, which we denote slopes. As shown in the schematic in Fig. 6A, we reasoned that if neural discrimination is correlated with the onset of the reach, it should occur proportionally earlier in short RT trials than in long RT trials, resulting in a slopes of 1. On the other hand, for PMd neurons showing activity related to target selection, target discrimination should occur at a consistent time regardless of reaction time, resulting in a slopes of 0.
Fig. 6.
Discrimination time vs. reaction time when activity is aligned on stimulus onset. A: predicted relationships of discrimination time and reaction time for short and long RT groups. Dotted line indicates the prediction for neurons involved in target selection, whereas solid line indicates the prediction for neurons involved only in movement production. B: discrimination time in individual PMd neurons as a function of reaction time for short and long reaction time groups. C: histogram of slopes for each cell (the slope of the lines in B).
Figure 6B shows the discrimination time/reaction time data for each cell. Some PMd neurons show slopes close to 1, indicating that target discrimination time is correlated with reach onset. However, others have slopes near 0, indicating that target discrimination time in these neurons occurs a consistent time after stimulus onset. In Fig. 6C, a histogram summarizing the slopes obtained for our sample of neurons shows that the slopes appear to form two populations: one clustered near a slope of 0, the prediction for a cell that carries a target selection signal; and the other clustered near a slope of 1, the prediction for a cell that is only involved in producing the movement. To verify that these slope differences were not caused by differences in behavior, we examined the mean reaction times and movement durations of trials collected for cells in the two populations. We found that there were no significant differences (Mann-Whitney U tests; reaction time: P = 0.66; movement time: P = 0.91), consistent with the idea that these different slopes reflect differences in neural function.
To quantitatively test for bimodality in the histogram of slopes, we computed the Akaike information criterion (AIC) to compare unimodal and bimodal Gaussian fits (Akaike 1974). The AIC for a unimodal Gaussian fit (μ = 0.43 and σ = 0.41) was 194.2, whereas for a bimodal fit (μ = 0.2, σ = 0.13 and μ = 1.0, σ = 0.035) was 192.6. This suggests that the bimodal fit provides a better model. To obtain converging evidence for bimodality including a statistical significance level, we conducted the kernel-density test for multimodality (Silverman 1981). The critical window width for one mode was 0.24 and for two modes was 0.12. To obtain a significance level, we conducted a bootstrapping analysis to test the null hypothesis that the underlying density has at most k modes against the alternative that it has more than k modes (Efron 1993; Silverman 1981). With 2,000 replications, the P value for a single mode hypothesis was P = 0.17, whereas that for the two mode hypothesis was P < 0.0001, thus supporting a bimodal distribution.
We performed an additional analysis to check the consistency of these results and to consider the possibility that a neuron might discriminate the target in two distinct phases of discrimination activity, with one phase correlated with stimulus onset and another phase correlated with reach onset. To do this, we performed the same analysis as described above, except that we realigned activity for each of the two reaction time groups on the onset of the reaching movement and searched backward in time from reach onset until the neuron no longer significantly discriminated the target (see methods). When trials are aligned in this fashion, if a neuron exhibits a phase of discrimination activity that is related to movement production, discrimination should occur a consistent time before movement onset for both RT groups. On the other hand, if discrimination time is independent of RT, neural discrimination of the target should occur earlier before movement onset in long RT trials versus short RT trials.
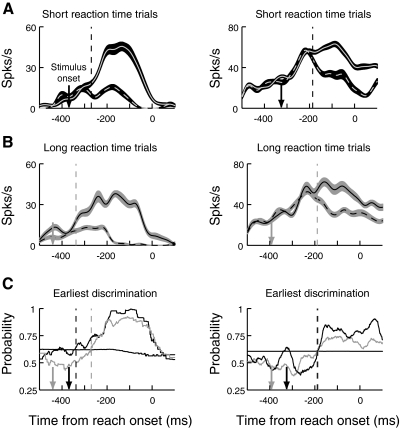
When activity was aligned on the onset of the reaching movements, 91 of our task-related PMd neurons reliably discriminated the target from distractors for both short and long reaction time groups. Figure 7 shows the activity of the two example neurons aligned on movement onset for short (A) and long reach reaction groups (B), as well as the results of the ROC analyses (C). Here, the downward-pointing arrows indicate the mean onset times of the stimulus array. For the cell on the left, target discrimination occurs earlier relative to reach execution for the long reaction time group than for the short. This would be expected if discrimination occurred a consistent time after the onset of the stimulus array. In contrast, in the cell on the right, discrimination of the target occurs at a similar time before movement onset in the two reaction time groups, indicating that discrimination time in this cell is correlated with movement production.
Fig. 7.
Target/distractor discrimination in the short and long reaction time groups when neural activity is aligned on reach onset. Figure conventions are the same as in Fig. 5 except that neural activity is aligned on reach onset.
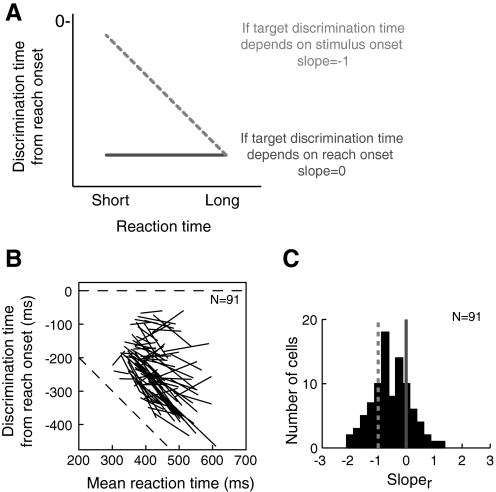
Figure 8A shows the predicted relationships between reaction time and target discrimination time when activity is aligned on reach onset (sloper). If discrimination occurs a consistent time after stimulus onset, we would expect a sloper of −1, whereas if discrimination is correlated with movement onset, we would expect a sloper of 0. Figure 8B shows the results for our sample of neurons: some cells have slopes near 0, indicating that discrimination time is correlated with reach onset, whereas others have slopes near −1, indicating that discrimination time is independent of reaction time. The distribution of slopes across cells is shown in the histogram in Fig. 8C. As before, we evaluated the evidence for bimodality in this histogram using the AIC and kernel density test. According to the AIC, a bimodal Gaussian fit (μ = −0.9, σ = 0.26 and μ = 0, σ = 0.14) provides a better model than a unimdal Gussian fit (μ = −0.53, σ = 0.44) for the distribution of slopes in Fig. 8C (165.7 for unimodal Gaussian fit vs. 161.5 for bimodal Gaussian fit). In accordance with this, the kernel-density test for multimodality indicates that two modes (critical window width = 0.13, P < 0.0001) explain this distribution of slopes significantly better than a single mode (critical window width = 0.19, P = 0.56).
Fig. 8.
Discrimination time vs. reaction time when activity is aligned on reach onset. Figure conventions are the same as in Fig. 6 except that neural activity is aligned on reach onset.
Target selection and movement production in PMd
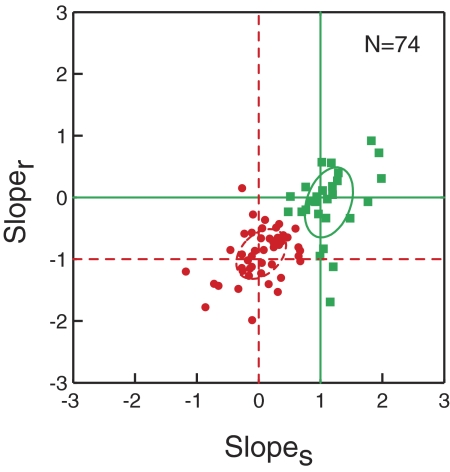
To simultaneously visualize the results of both the stimulus- and movement-aligned analyses, we plotted the joint distribution of slopes in Fig. 9 for the 74 task-related cells that reliably discriminated the target for both reaction time groups and both alignments. Here, each data point represents one cell, with the abscissa indicating the slope obtained when activity was aligned on stimulus onset (slopes), and the ordinate indicating the slope when activity was aligned on reach onset (sloper). If the slopes obtained using both alignments indicate that discrimination of the target occurs a consistent time after stimulus onset regardless of reaction time, the cell will be plotted at the junction corresponding to a slopes of 0 and a sloper of −1. On the other hand, if the slopes obtained from both alignments indicate that target discrimination time is correlated with reach onset, the cell will be plotted at the junction corresponding to a slopes of 1 and a sloper of 0.
Fig. 9.
Joint plot of discrimination time/reaction time slopes obtained when the data are aligned on stimulus onset and reach onset. The abscissa indicates the slope obtained from the stimulus-onset alignment (slopes), and the ordinate indicates the slope obtained from the reach-onset alignment (sloper). For a given cell, if the slopes obtained from both alignments are consistent with a discrimination time that is correlated with reaction time, the cell will be plotted near the junction of slopes = 1 and sloper = 0 (solid green circle). On the other hand, if the slopes obtained from both alignments indicate that discrimination time occurs a consistent time after stimulus onset regardless of reaction time, the cell will be plotted near the junction of slopes = 0 and sloper = −1 (dotted red circle). Circles indicate SD around the mean for each category.
This joint analysis also provided the opportunity to identify neurons with separate phases of discrimination activity related to target selection and movement production; such cells would be plotted near the junction corresponding to a slopes of 0 and a sloper of 0. As is evident in Fig. 9, however, such cells were rarely found. Instead, there seems to be the two clusters corresponding to the predictions for target selection and movement production. To classify each neuron, we applied the k-means algorithm (with k = 2), which partitions data points such that the sum of squares from the data points to cluster centers is minimized. In Fig. 9, each cell is color-coded according to its assigned cluster, with red points representing neurons in which discrimination time is independent of reaction time and green points representing neurons in which discrimination time is correlated with reaction time. The circles indicate SD around the mean for each category. We found that the centroid of the 50 neurons in the first group (red) was at a slopes of 0.1 ± 0.06 (SE) and a sloper of −0.9 ± 0.05, which is close to the predicted slopes for neurons carrying a target selection signal (slopes of 0 and sloper of −1). In contrast, the centroid of the 24 neurons in the other group is at a slopes of 1.1 ± 0.08 and a sloper of 0.067 ± 0.08 in accord with the predictions for cells that are only involved in movement production (slopes of 1 and sloper of 0).
To alleviate concerns about whether the choice of each cell's PD influenced these classifications, we repeated the same analyses using the two off-axis directions, rather than the PD and opposite directions. In this analysis, the off-axis direction that elicited the greatest response from the neuron was used in place of the PD, and the remaining direction was used in place of the opposite direction. We found that 69% of the neurons reliably discriminated the target from the distractor for both target and movement alignments. Of these neurons, 88% were classified with the same category membership as in the initial classification (rφ = 0.68, P < 10−11).
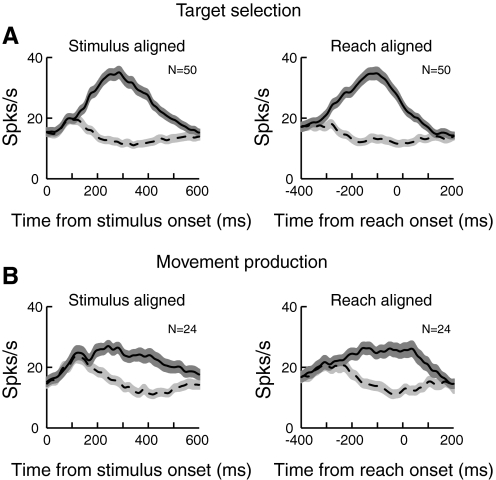
To summarize the characteristics of these two classes of neurons shown in Fig. 9, we computed population mean spike density functions for each class when the target was in the PD and when it was in the opposite direction (Fig. 10). When the activity is aligned on the onset of search array (Fig. 10, left), cells having activity related to target selection (Fig. 10A) discriminate the target from the distractor earlier than cells having only movement production activity (Fig. 10B) at the population level (145 vs. 204 ms), consistent with results obtained at the single cell level [152 ± 7.9 vs. 198 ± 13 (SE) ms; Mann-Whitney U test, P < 0.0015]. In addition, when the activity is aligned on reach onset (Fig. 10, right), the maximum discrimination between the target and the distractor, as measured by the maximum of the area under the ROC curve, occurs near reach onset in the movement production cells (−63 ms; Fig. 10B) compared with cells having target selection activity (−159 ms; Fig. 10A), which is again consistent with results obtained at the single cell level (−53 ± 1.57 vs. –148 ± 13.7 ms; Mann-Whitney U test, P < 0.03).
Fig. 10.
Population activity of target selection and movement production neurons. A: mean ± SE firing rates for cells classified as target selection neurons when the activity is aligned on stimulus onset (left) and reach onset (right). The solid line represents the mean activity when the target was in the PD, whereas the dotted line represents activity when the target was in the opposite direction. B: mean population activity for cells classified as movement production neurons. Conventions are the same as in A.
Narrow- and broad-spiking neurons in target selection and movement production
In the previous sections, we identified PMd neurons with target selection activity and those with only movement production activity in the search task. Here, we sought converging evidence of this distinction by looking for anatomical or physiological differences between these functionally defined groups. A post hoc examination of the locations of the electrode tracks within PMd and the depths of the recorded cells did not show any notable differences in location or depth of neurons classified as involved in target selection and movement production. However, it should be noted that we did not explore as large a rostro-caudal extent of PMd as Cisek and Kalaska (2005), who reported finding more selection-related cells in rostral PMd and more movement-related cells in caudal PMd.
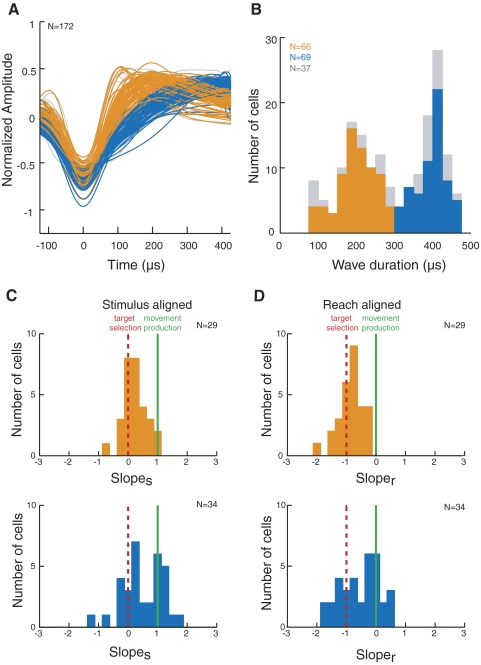
Next, we analyzed the widths of the extracellular spike waveforms of our recorded neurons, a feature that can be used to distinguish different classes of neurons (Connors and Gutnick 1990; Gold et al. 2006; Gonzalez-Burgos et al. 2005; Henze et al. 2000; McCormick et al. 1985). Specifically, distinct narrow- and broad-spiking neuronal populations have been observed in several cortical areas, including prefrontal, somatosensory, primary visual, and inferior temporal cortex (Bartho et al. 2004; Chen et al. 2008; Cohen et al. 2009; Constantinidis and Goldman-Rakic 2002; Mitchell et al. 2007; Mountcastle et al. 1969). Extracellular waveforms were analyzed from 190 of our neurons, 135 of which showed task-related activity. Figure 11A shows the mean waveform of each neuron, temporally aligned relative to its trough (Mitchell et al. 2007). The magnitude of the waveforms has been normalized to aid in comparing spike widths as well as the height of each waveform's peak and the depth of its trough. Eighteen waveforms that did not have a biphasic shape consisting of a trough followed by a clear peak were excluded from further analysis.
Fig. 11.
Analysis of extracellular spike widths. A: mean waveforms of all analyzed neurons, aligned relative to their troughs. The heights of the waveforms have been normalized. Waveforms that did not have a biphasic shape with a trough followed by a clear peak were excluded from analysis (n = 18). B: distribution of waveform durations, measured from trough to peak. In A and B, task-related neurons are indicated in orange and blue, for narrow- and broad-spiking units, respectively, whereas non–task-related neurons are indicated in gray. C: histograms of slopes for narrow- (top) and broad-spiking neurons (bottom). D: histograms of sloper for narrow- (top) and broad-spiking neurons (bottom).
We first examined the distribution of spike widths to determine whether it was bimodal and could therefore be divided into narrow- and broad-spiking subpopulations (Fig. 11B). We found that the distribution was significantly bimodal (Hartigan's dip test, P < 0.003) (Hartigan and Hartigan 1985; Mechler and Ringach 2002; Mitchell et al. 2007), and narrow- and broad-spiking neurons were separated on the basis of the two modes of the distribution, with narrow-spiking neurons defined as those ranging in duration from 100 to 300 μs and broad-spiking neurons defined as those ranging in duration from 301 to 500 μs. These spike widths are longer than those found by Mitchell et al. (2007) in extrastriate area V4 but are comparable to the range of widths found in the FEF by Cohen et al. (2009). In Fig. 11, A and B, the neurons exhibiting task-related activity are indicated in orange and blue, corresponding to narrow- and broad-spiking neurons, respectively, whereas non–task-related neurons are indicated in gray. We found that narrow-spiking neurons exhibited a higher level of spontaneous activity (mean, 10.9 Hz) during the period before the stimuli were presented than broad-spiking neurons (6.7 Hz; Mann-Whitney U test, P < 0.005). Furthermore, when we calculated the trough-peak ratio of each cell's mean normalized waveform by dividing the height of the waveform peak by the depth of its trough, we found that broad-spiking neurons exhibited significantly shallower waveform peaks relative to their troughs (mean trough-peak ratio = 0.54) compared with narrow-spiking neurons (mean = 0.66; Mann-Whitney U test, P < 0.05). These physiological properties of narrow- and broad-spiking neurons are consistent with what has been reported in previous studies (Connors and Gutnick 1990; Henze et al. 2000; McCormick et al. 1985; Mitchell et al. 2007).
To determine whether narrow- and broad-spiking neurons play different roles in our task, we examined the relationship between the neural discrimination time/reaction time slopes and spike width. Of the 74 neurons shown in Fig. 9 in which slopes were obtained using both the stimulus and reach onset alignments, spike waveforms were recorded in 63 neurons. Of these, 29 cells were classified as narrow-spiking and 34 neurons were classified as broad-spiking. Figure 11C shows histograms of slopes for the narrow- (top) and broad-spiking neurons (bottom), whereas Fig. 11D shows histograms of sloper for the narrow- (top) and broad-spiking neurons (bottom). The dotted red lines indicate the predicted slopes for cells involved in target selection (a slopes of 0 in Fig. 11C and a sloper of −1 in Fig. 11D), whereas the solid green lines indicate the predicted slopes for cells involved in movement production (a slopes of 1 in Fig. 11C and a sloper of 0 in Fig. 11D). The slopes of the majority of narrow-spiking neurons are clustered near the red lines associated with target selection, whereas slopes of broad-spiking neurons fall near both the target selection and movement production predictions. The differences in the distributions of narrow- and broad-spiking neurons in both slopes and sloper were confirmed by Kruskal-Wallis tests (both P < 0.05). This suggests that most narrow-spiking, and some broad-spiking, neurons carry target selection signals, whereas other broad-spiking neurons carry movement production signals but lack target-selection signals.
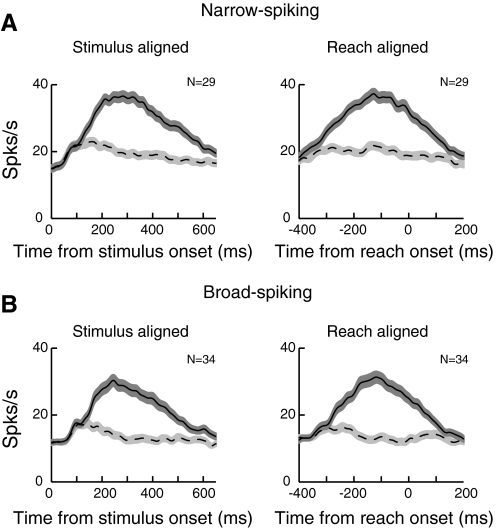
To show the activity of narrow- and broad-spiking neurons in this task, we calculated the population activity separately for narrow- and broad-spiking neurons from cells showing task-related activity (Fig. 12). We found that overall, narrow-spiking neurons (Fig. 12A) show higher firing rates compared with broad-spiking neurons (Fig. 12A): mean activity between 50 and 300 ms after the stimulus onset was 29.6 ± 2.4 spikes/s (SE) in narrow-spiking neurons and 23.2 ± 2.5 spikes/s in broad-spiking neurons (Mann-Whitney U test, P < 0.015). We also examined target discrimination time for the classified neurons shown in Fig. 9. Because we previously showed that discrimination time for target selection cells is earlier than for movement production neurons (Fig. 11), we predicted that the narrow-spiking cells, consisting mostly of target selection neurons, would discriminate the target earlier than broad-spiking neurons, which are a mixture of both target selection and movement production cells. Consistent with this, we found that narrow-spiking neurons discriminated the target significantly earlier than broad-spiking neurons (median, 147 ± 9.6 vs. 170 ± 11.08 ms, Mann-Whitney U test, P < 0.02).
Fig. 12.
Mean population activity of narrow-spiking neurons (A) and broad-spiking neurons (B). Conventions are the same as in Fig. 10.
DISCUSSION
We studied the role of PMd in a reaction-time task in which a reach target was selected from distractors. We found that PMd neurons reliably discriminated the target from distractors ∼150–200 ms after the onset of the stimuli. Our use of a reaction-time task allowed us to relate neural discrimination of the target with the reaction time of the reaching movement. We found that some neurons first discriminated the target at a time that was independent of reaction time, suggesting that they are involved in target selection. Discrimination in other neurons was fairly tightly linked with reaction time, suggesting that they are only involved in movement production and not in target selection. Of course, neurons involved in target selection could also potentially contribute to movement production. Our analysis allowed for the possibility that neurons might show two distinct phases of target discrimination, with one phase related to target selection and the other phase related to movement production. However, we seldom identified such neurons in our sample of recorded cells. Nonetheless, it is possible that neurons identified as target-selection neurons could produce activity that contributes to movement production that is not picked up by our analysis. Overall, target selection neurons showed neural discrimination times that were significantly faster than those of movement production neurons. This raises the possibility that the target selection neurons might transfer reach goal information to the movement production cells.
An analysis of the waveforms of the recorded cells showed a bimodal distribution of spike widths, with populations of narrow- and broad-spiking neurons. The narrow-spiking neurons virtually all showed activity consistent with target selection, whereas the population of broad-spiking neurons was more heterogeneous, consisting of some neurons with activity related to target selection and others related to movement production. These results indicate that PMd contains both target selection and movement execution signals and suggest that these signals are carried by distinct neural subpopulations.
Role of PMd in selective reaching
Our results are generally in accordance with previous findings that PMd activity is involved not only in reach execution, but also in more abstract higher-level cognitive processes such as encoding potential movement goals and motor intention, and representing and transforming a virtual action plan into an executable motor plan (Boussaoud and Wise 1993b; Cisek and Kalaska 2002b, 2005; Nakayama et al. 2008). Cisek and Kalaska (2005) investigated target selection in a delayed reaching task and distinguished several classes of cells, some of which were involved in specifying the goal and others in producing the movement. Although our task differs from theirs in that we used a reaction-time task rather than a delay task and a color pop-out target among multiple distractors rather than a central cue with a single distractor, our results are in general agreement with theirs. This is an important observation, because it indicates that the mechanisms of reach target selection in PMd are not strongly task dependent. Specifically, our target-selection cells could correspond to their selected-response (SR) and potential-response (PR) cells, which show directionally tuned activity during the delay period in their task. Similarly, cells in which neural discrimination of the target is correlated with reaction time in our task likely correspond to their movement cells, which exhibited direction tuning only after the go signal in their task.
Nakayama et al. (2008) also examined PMd responses in a target choice task, showing that PMd neurons respond to the appearance of a symbolic instruction that informs whether the left or right of two stimuli will be the reach target in upcoming trials, even when the specific locations of the stimuli change across trials. Thus in this task, the selection of right or left at an abstract level is dissociated from the physical motor plan. Nakayama et al. showed that the activity of PMd neurons is initially modulated by the symbolic instruction, reflecting a virtual action plan, even before the physical location of the target is shown. Later activity indicates that this virtual action plan is transformed into an executable motor plan.
Other neural areas involved in reach target selection
In addition to PMd, the parietal reach region (PRR), which has reciprocal connections with PMd (Wise et al. 1997), is likely also involved in reach target selection. For example, Scherberger and Andersen (2007) recently showed that when two potential targets are sequentially presented in opposite directions, activity in PRR is linked to the monkey's target choice. Furthermore, Pesaran et al. (2008) simultaneously recorded activity in PMd and PRR in free choice and instructed search and found evidence that information is transferred between the two areas during decision making. They suggested that this signal could underlie the coordination of target selection in PMd and PRR toward a common goal. Finally, Baldauf et al. (2008) showed that PRR encodes subsequent movement goals as well as an immediate goal, suggesting its role in more complex sequential target-selection processes.
Comparison to saccade target selection in visual search
Visual search tasks similar to the one used here have been used to study the neural substrates of saccade target selection in the superior colliculus (SC), FEF, and lateral intraparietal area (LIP) (Basso and Wurtz 1998; Bichot and Schall 1999, 2002; Ipata et al. 2006; Kim and Basso 2008; McPeek and Keller 2002, 2004; Schall and Hanes 1993; Schiller and Tehovnik 2005; Thomas and Pare 2007; Thompson et al. 1996). Indeed, our results are similar in many respects to what has been found for saccade target selection in the FEF and SC. For example, Schall and colleagues found that many FEF visuomovement neurons discriminate the saccade target from distractors at a time that is independent of saccade latency, suggesting that these cells are involved in target selection (Thompson et al. 1996). A smaller group of visuomovement neurons shows neural discrimination of the target that is linked to saccade onset, indicating that these neurons are involved in initiating saccades (Schall 2004). Using similar logic, McPeek and Keller (2002) found distinct subpopulations of SC neurons involved in target selection and movement initiation. In LIP, neuronal discrimination of the target was found to vary proportionally with reaction time, indicating a role for LIP in saccade initiation (Ipata et al. 2006; Thomas and Pare 2007).
In addition to these classical oculomotor areas, a recent study showed that the ventral premotor cortex (PMv) is also involved in target selection for saccades. Pardo-Vazquez et al. (2008) showed transient activity in PMv reflecting decision making when monkeys were required to compare the orientation of sequentially presented stimuli.
Gaze-dependent modulation in PMd
Fujii et al. (2000) reported that some rostral PMd neurons are active during performance of oculomotor tasks as well as limb-movement tasks. A more recent study (Pesaran et al. 2006) dissociated the effects of target location and initial hand and eye positions on PMd reach-related neurons. They found that PMd neurons encode the relative positions of the target, hand, and eye. We did not systematically vary gaze and hand position, but in our task, we observed that allowing eye movements versus enforcing fixation resulted in a modest, but significant, modulation of PMd activity. However, the time course of target/distractor discrimination was the same in the two conditions.
We conjecture that the greater activity in fixation search might be related to the fact that, with fixation, the peripheral targets are more eccentric, activating PMd neurons more because some PMd neurons have been reported to increase firing rate with eccentricity (Messier and Kalaska 2000). This is also in accordance with previous studies showing that the activity of reach-related PMd cells is affected by the gaze position (Batista et al. 2007; Boussaoud et al. 1998; Cisek and Kalaska 2002a; Pesaran et al. 2006).
Narrow- and broad-spiking neurons in PMd
Several studies have analyzed the widths of extracellularly recorded spike waveforms to discriminate different subpopulations of cortical neurons (Bartho et al. 2004; Chen et al. 2008; Cohen et al. 2009; Constantinidis and Goldman-Rakic 2002; Mitchell et al. 2007; Mountcastle et al. 1969). For example, in extrastriate area V4, Mitchell et al. (2007) found distinct subpopulations of neurons with narrow and broad spike widths and showed that the narrow-spiking neurons had higher firing rates and were more strongly affected by attention than the broad-spiking neurons.
In PMd, we also found evidence for distinct subpopulations of narrow- and broad-spiking neurons, with associated differences in spontaneous firing rates and in the ratios of the amplitudes of the peaks and troughs of the spikes. When we examined the roles of the neurons in our task, we found that nearly all narrow-spiking neurons showed activity consistent with target selection, whereas some broad-spiking neurons showed activity consistent with movement production, and others showed activity consistent with target selection.
A number of studies combining intra- and extracellular recordings have shown that some classes of local cortical inhibitory neurons produce narrow action potentials, whereas long-range, excitatory pyramidal cells typically have broad spikes (Connors and Gutnick 1990; Gold et al. 2006; Gonzalez-Burgos et al. 2005; Henze et al. 2000; McCormick et al. 1985). Thus it is tempting to posit that the narrow-spiking neurons in our study correspond to local inhibitory neurons and the broad-spiking neurons to pyramidal cells. Along these lines, we speculate that both narrow-spiking inhibitory and broad-spiking excitatory neurons may be involved in target selection because it requires both facilitating the target and inhibiting distractors. On the other hand, movement execution presumably involves broad-spiking PMd output pyramidal cells. However, it is important to note that we cannot verify whether the narrow- and broad-spiking neurons indeed correspond to local inhibitory and pyramidal cells, respectively. Furthermore, the narrow- and broad-spiking populations that we observe are likely composed of a number of different subtypes of neurons that cannot be distinguished on the basis of their extracellular spike waveforms.
In FEF, Cohen et al. (2009) compared the widths of extracellular spike waveforms across three functionally defined cell types involved in saccades. They found that movement neurons had the broadest spikes, visual neurons had narrower spikes, and visuomovement neurons had the narrowest spikes. Although they did not measure the activity of their cells in a target selection task, previous studies have identified FEF visuomovement neurons as involved in saccade target selection and have shown that these neurons typically discriminate the target at a time that is independent of reaction time (Thompson et al. 1996). This fits well with our conclusion that narrow-spiking PMd neurons in our task are involved in reach target selection. In contrast, FEF movement neurons, corresponding to the broad-spiking neurons in Cohen et al., show activity that is correlated with saccadic reaction time (Hanes and Schall 1996). Again, this fits with our observation that broad-spiking PMd neurons formed the vast majority of neurons in which neural discrimination of the target was correlated with reaction time.
GRANTS
This study was supported by National Eye Institute Grant R01 EY-014885 to R. M. McPeek, Core Grant P30 EY-006883, and an R. C. Atkinson Fellowship Award to J.-H. Song.
ACKNOWLEDGMENTS
We thank J. F. Mitchell and J. H. Reynolds for kindly providing the source code for the waveform analysis and N. Takahashi for animal care.
REFERENCES
- Akaike H. A new look at the statistical model identification. Automatic control. IEEE Transactions on 19: 716–723, 1974 [Google Scholar]
- Baldauf D, Cui H, Andersen RA. The posterior parietal cortex encodes in parallel both goals for double-reach sequences. J Neurosci 28: 10081–10089, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartho P, Hirase H, Monconduit L, Zugaro M, Harris KD, Buzsaki G. Characterization of neocortical principal cells and interneurons by network interactions and extracellular features. J Neurophysiol 92: 600–608, 2004 [DOI] [PubMed] [Google Scholar]
- Basso MA, Wurtz RH. Modulation of neuronal activity in superior colliculus by changes in target probability. J Neurosci 18: 7519–7534, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista AP, Santhanam G, Yu BM, Ryu SI, Afshar A, Shenoy KV. Reference frames for reach planning in macaque dorsal premotor cortex. J Neurophysiol 98: 966–983, 2007 [DOI] [PubMed] [Google Scholar]
- Bichot NP, Schall JD. Saccade target selection in macaque during feature and conjunction visual search. Vis Neurosci 16: 81–89, 1999 [DOI] [PubMed] [Google Scholar]
- Bichot NP, Schall JD. Priming in macaque frontal cortex during popout visual search: feature-based facilitation and location-based inhibition of return. J Neurosci 22: 4675–4685, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boussaoud D, Jouffrais C, Bremmer F. Eye position effects on the neuronal activity of dorsal premotor cortex in the macaque monkey. J Neurophysiol 80: 1132–1150, 1998 [DOI] [PubMed] [Google Scholar]
- Boussaoud D, Wise SP. Primate frontal cortex: effects of stimulus and movement. Exp Brain Res 95: 28–40, 1993a [DOI] [PubMed] [Google Scholar]
- Boussaoud D, Wise SP. Primate frontal cortex: neuronal activity following attentional versus intentional cues. Exp Brain Res 95: 15–27, 1993b [DOI] [PubMed] [Google Scholar]
- Bradley A, Skottun BC, Ohzawa I, Sclar G, Freeman RD. Visual orientation and spatial frequency discrimination: a comparison of single neurons and behavior. J Neurophysiol 57: 755–772, 1987 [DOI] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spat Vis 10: 433–436, 1997 [PubMed] [Google Scholar]
- Britten KH, Newsome WT, Shadlen MN, Celebrini S, Movshon JA. A relationship between behavioral choice and the visual responses of neurons in macaque MT. Vis Neurosci 13: 87–100, 1996 [DOI] [PubMed] [Google Scholar]
- Britten KH, Shadlen MN, Newsome WT, Movshon JA. The analysis of visual motion: a comparison of neuronal and psychophysical performance. J Neurosci 12: 4745–4765, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce CJ, Goldberg ME, Bushnell MC, Stanton GB. Primate frontal eye fields. II. Physiological and anatomical correlates of electrically evoked eye movements. J Neurophysiol 54: 714–734, 1985 [DOI] [PubMed] [Google Scholar]
- Caminiti R, Ferraina S, Mayer AB. Visuomotor transformations: early cortical mechanisms of reaching. Curr Opin Neurobiol 8: 753–761, 1998 [DOI] [PubMed] [Google Scholar]
- Chen Y, Martinez-Conde S, Macknik SL, Bereshpolova Y, Swadlow HA, Alonso JM. Task difficulty modulates the activity of specific neuronal populations in primary visual cortex. Nat Neurosci 11: 974–982, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchland MM, Santhanam G, Shenoy KV. Preparatory activity in premotor and motor cortex reflects the speed of the upcoming reach. J Neurophysiol 96: 3130–3146, 2006 [DOI] [PubMed] [Google Scholar]
- Cisek P. Integrated neural processes for defining potential actions and deciding between them: a computational model. J Neurosci 26: 9761–9770, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisek P, Kalaska JF. Modest gaze-related discharge modulation in monkey dorsal premotor cortex during a reaching task performed with free fixation. J Neurophysiol 88: 1064–1072, 2002a [DOI] [PubMed] [Google Scholar]
- Cisek P, Kalaska JF. Simultaneous encoding of multiple potential reach directions in dorsal premotor cortex. J Neurophysiol 87: 1149–1154, 2002b [DOI] [PubMed] [Google Scholar]
- Cisek P, Kalaska JF. Neural correlates of reaching decisions in dorsal premotor cortex: specification of multiple direction choices and final selection of action. Neuron 45: 801–814, 2005 [DOI] [PubMed] [Google Scholar]
- Cohen JY, Pouget P, Heitz RP, Woodman GF, Schall JD. Biophysical support for functionally distinct cell types in the frontal eye field. J Neurophysiol 101: 912–916, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors BW, Gutnick MJ. Intrinsic firing patterns of diverse neocortical neurons. Trends Neurosci 13: 99–104, 1990 [DOI] [PubMed] [Google Scholar]
- Constantinidis C, Goldman-Rakic PS. Correlated discharges among putative pyramidal neurons and interneurons in the primate prefrontal cortex. J Neurophysiol 88: 3487–3497, 2002 [DOI] [PubMed] [Google Scholar]
- Crammond DJ, Kalaska JF. Differential relation of discharge in primary motor cortex and premotor cortex to movements versus actively maintained postures during a reaching task. Exp Brain Res 108: 45–61, 1996 [DOI] [PubMed] [Google Scholar]
- Crammond DJ, Kalaska JF. Prior information in motor and premotor cortex: activity during the delay period and effect on pre-movement activity. J Neurophysiol 84: 986–1005, 2000 [DOI] [PubMed] [Google Scholar]
- DiCarlo JJ, Maunsell JH. Using neuronal latency to determine sensory-motor processing pathways in reaction time tasks. J Neurophysiol 93: 2974–2986, 2005 [DOI] [PubMed] [Google Scholar]
- Efron BTR. An Introduction to the Bootstrap New York: Chapman and Hall, 1993 [Google Scholar]
- Fujii N, Mushiake H, Tanji J. Rostrocaudal distinction of the dorsal premotor area based on oculomotor involvement. J Neurophysiol 83: 1764–1769, 2000 [DOI] [PubMed] [Google Scholar]
- Gold C, Henze DA, Koch C, Buzsaki G. On the origin of the extracellular action potential waveform: a modeling study. J Neurophysiol 95: 3113–3128, 2006 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Krimer LS, Povysheva NV, Barrionuevo G, Lewis DA. Functional properties of fast spiking interneurons and their synaptic connections with pyramidal cells in primate dorsolateral prefrontal cortex. J Neurophysiol 93: 942–953, 2005 [DOI] [PubMed] [Google Scholar]
- Green DM, Swets JA. Signal Detection Theory and Psychophysics Oxford, UK: Wiley, 1966 [Google Scholar]
- Hanes DP, Schall JD. Neural control of voluntary movement initiation. Science 274: 427–430, 1996 [DOI] [PubMed] [Google Scholar]
- Hartigan JA, Hartigan PM. The dip test of unimodality. Ann Stat 13: 70–84, 1985 [Google Scholar]
- Henze DA, Borhegyi Z, Csicsvari J, Mamiya A, Harris KD, Buzsaki G. Intracellular features predicted by extracellular recordings in the hippocampus in vivo. J Neurophysiol 84: 390–400, 2000 [DOI] [PubMed] [Google Scholar]
- Hoshi E, Tanji J. Integration of target and body-part information in the premotor cortex when planning action. Nature 408: 466–470, 2000 [DOI] [PubMed] [Google Scholar]
- Ipata AE, Gee AL, Goldberg ME, Bisley JW. Activity in the lateral intraparietal area predicts the goal and latency of saccades in a free-viewing visual search task. J Neurosci 26: 3656–3661, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, Basso MA. Saccade target selection in the superior colliculus: a signal detection theory approach. J Neurosci 28: 2991–3007, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JN, Shadlen MN. Neural correlates of a decision in the dorsolateral prefrontal cortex of the macaque. Nat Neurosci 2: 176–185, 1999 [DOI] [PubMed] [Google Scholar]
- Kurata K, Hoffman DS. Differential effects of muscimol microinjection into dorsal and ventral aspects of the premotor cortex of monkeys. J Neurophysiol 71: 1151–1164, 1994 [DOI] [PubMed] [Google Scholar]
- Lee KM, Keller EL. Symbolic cue-driven activity in superior colliculus neurons in a peripheral visual choice task. J Neurophysiol 95: 3585–3595, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Connors BW, Lighthall JW, Prince DA. Comparative electrophysiology of pyramidal and sparsely spiny stellate neurons of the neocortex. J Neurophysiol 54: 782–806, 1985 [DOI] [PubMed] [Google Scholar]
- McPeek RM. Incomplete suppression of distractor-related activity in the frontal eye field results in curved saccades. J Neurophysiol 96: 2699–2711, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPeek RM, Keller EL. Saccade target selection in the superior colliculus during a visual search task. J Neurophysiol 88: 2019–2034, 2002 [DOI] [PubMed] [Google Scholar]
- McPeek RM, Keller EL. Deficits in saccade target selection after inactivation of superior colliculus. Nat Neurosci 7: 757–763, 2004 [DOI] [PubMed] [Google Scholar]
- Mechler F, Ringach DL. On the classification of simple and complex cells. Vision Res 42: 1017–1033, 2002 [DOI] [PubMed] [Google Scholar]
- Messier J, Kalaska JF. Covariation of primate dorsal premotor cell activity with direction and amplitude during a memorized-delay reaching task. J Neurophysiol 84: 152–165, 2000 [DOI] [PubMed] [Google Scholar]
- Mitchell JF, Sundberg KA, Reynolds JH. Differential attention-dependent response modulation across cell classes in macaque visual area V4. Neuron 55: 131–141, 2007 [DOI] [PubMed] [Google Scholar]
- Mountcastle VB, Talbot WH, Sakata H, Hyvarinen J. Cortical neuronal mechanisms in flutter-vibration studied in unanesthetized monkeys. Neuronal periodicity and frequency discrimination. J Neurophysiol 32: 452–484, 1969 [DOI] [PubMed] [Google Scholar]
- Nakayama Y, Yamagata T, Tanji J, Hoshi E. Transformation of a virtual action plan into a motor plan in the premotor cortex. J Neurosci 28: 10287–10297, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo-Vazquez JL, Leboran V, Acuna C. Neural correlates of decisions and their outcomes in the ventral premotor cortex. J Neurosci 28: 12396–12408, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis 10: 437–442, 1997 [PubMed] [Google Scholar]
- Pesaran B, Nelson MJ, Andersen RA. Dorsal premotor neurons encode the relative position of the hand, eye, and goal during reach planning. Neuron 51: 125–134, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesaran B, Nelson MJ, Andersen RA. Free choice activates a decision circuit between frontal and parietal cortex. Nature 453: 406–409, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhanam G, Ryu SI, Yu BM, Afshar A, Shenoy KV. A high-performance brain-computer interface. Nature 442: 195–198, 2006 [DOI] [PubMed] [Google Scholar]
- Sato T, Murthy A, Thompson KG, Schall JD. Search efficiency but not response interference affects visual selection in frontal eye field. Neuron 30: 583–591, 2001 [DOI] [PubMed] [Google Scholar]
- Schall JD. On the role of frontal eye field in guiding attention and saccades. Vision Res 44: 1453–1467, 2004 [DOI] [PubMed] [Google Scholar]
- Schall JD, Hanes DP. Neural basis of saccade target selection in frontal eye field during visual search. Nature 366: 467–469, 1993 [DOI] [PubMed] [Google Scholar]
- Schall JD, Hanes DP, Thompson KG, King DJ. Saccade target selection in frontal eye field of macaque. I. Visual and premovement activation. J Neurosci 15: 6905–6918, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherberger H, Andersen RA. Target selection signals for arm reaching in the posterior parietal cortex. J Neurosci 27: 2001–2012, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller PH, Tehovnik EJ. Neural mechanisms underlying target selection with saccadic eye movements. Prog Brain Res 149: 157–171, 2005 [DOI] [PubMed] [Google Scholar]
- Shen L, Alexander GE. Preferential representation of instructed target location versus limb trajectory in dorsal premotor area. J Neurophysiol 77: 1195–1212, 1997 [DOI] [PubMed] [Google Scholar]
- Silverman BW. Using kernel density estimates to investigate multimodality. J R Stat Soc Series B (Methodological) 43: 97–99, 1981 [Google Scholar]
- Sommer MA, Wurtz RH. Composition and topographic organization of signals sent from the frontal eye field to the superior colliculus. J Neurophysiol 83: 1979–2001, 2000 [DOI] [PubMed] [Google Scholar]
- Song J-H, McPeek RM. Eye-hand coordination during target selection in a pop-out visual search. J Neurophysiol 102: 2681–2692, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J-H, Nakayama K. Role of focal attention on latencies and trajectories of visually guided manual pointing. J Vis 6: 982–995, 2006 [DOI] [PubMed] [Google Scholar]
- Song J-H, Nakayama K. Automatic adjustment of visuomotor readiness. J Vis 7: 1–9, 2007a [DOI] [PubMed] [Google Scholar]
- Song J-H, Nakayama K. Fixation offset facilitates saccades and manual reaching for single but not multiple target displays. Exp Brain Res 177: 223–232, 2007b [DOI] [PubMed] [Google Scholar]
- Song J-H, Nakayama K. Target selection in visual search as revealed by movement trajectories. Vision Res 48: 853–861, 2008 [DOI] [PubMed] [Google Scholar]
- Song J-H, Takahashi N, McPeek RM. Target selection for visually guided reaching in macaque. J Neurophysiol 99: 14–24, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas NW, Pare M. Temporal processing of saccade targets in parietal cortex area LIP during visual search. J Neurophysiol 97: 942–947, 2007 [DOI] [PubMed] [Google Scholar]
- Thompson KG, Hanes DP, Bichot NP, Schall JD. Perceptual and motor processing stages identified in the activity of macaque frontal eye field neurons during visual search. J Neurophysiol 76: 4040–4055, 1996 [DOI] [PubMed] [Google Scholar]
- Wise SP, Boussaoud D, Johnson PB, Caminiti R. Premotor and parietal cortex: corticocortical connectivity and combinatorial computations. Annu Rev Neurosci 20: 25–42, 1997 [DOI] [PubMed] [Google Scholar]
- Wise SP, di Pellegrino G, Boussaoud D. The premotor cortex and nonstandard sensorimotor mapping. Can J Physiol Pharmacol 74: 469–482, 1996 [DOI] [PubMed] [Google Scholar]