Abstract
We recorded the activity of pontine omnipause neurons (OPNs) in two macaques during saccadic eye movements and blinks. As previously reported, we found that OPNs fire tonically during fixation and pause about 15 ms before a saccadic eye movement. In contrast, for blinks elicited by air puffs, the OPNs paused <2 ms before the onset of the blink. Thus the burst in the agonist orbicularis oculi motoneurons (OOMNs) and the pause in the antagonist levator palpabrae superioris motoneurons (LPSMNs) necessarily precede the OPN pause. For spontaneous blinks there was no correlation between blink and pause onsets. In addition, the OPN pause continued for 40–60 ms after the time of the maximum downward closing of the eyelids, which occurs around the end of the OOMN burst of firing. LPSMN activity is not responsible for terminating the OPN pause because OPN resumption was very rapid, whereas the resumption of LPSMN firing during the reopening phase is gradual. OPN pause onset does not directly control blink onset, nor does pause offset control or encode the transition between the end of the OOMN firing and the resumption of the LPSMNs. The onset of the blink-related eye transients preceded both blink and OPN pause onsets. Therefore they initiated while the saccadic short-lead burst neurons were still fully inhibited by the OPNs and cannot be saccadic in origin. The abrupt dynamic change of the vertical eye transients from an oscillatory behavior to a single time constant exponential drift predicted the resumption of the OPNs.
INTRODUCTION
Pontine omnipause neurons (OPNs) are located in the nucleus raphe interpositus (Büttner-Ennever et al. 1988). They fire tonically during fixation and slow eye movements and pause just before and during a saccade or quick phase eye movement (Busettini and Mays 2003; Everling et al. 1998; Evinger et al. 1982). Electrical microstimulation of the OPNs delays the execution of a saccade and interrupts a saccade that has already started (Becker et al. 1981; Keller 1977; Keller et al. 1996; King and Fuchs 1977). This evidence strongly suggested that OPNs might act as saccadic triggers. However, experimental damage of the OPNs in the macaque monkey caused saccades to be abnormally slow, but otherwise, they were normal (Kaneko 1996; Soetedjo et al. 2002). These results are consistent with the actual saccadic trigger being provided by other pathways and the primary function of the OPNs to gate the release of the upstream saccadic motor command to the saccadic short-lead burst neurons at an optimal time with regard to saccadic dynamics.
Blinks heavily interact with saccades. They are known to facilitate the execution of saccades and vice versa (Zee et al. 1983). Large saccades are often associated with blinks (Evinger et al. 1994) and stimulation of the OPNs is reported to inhibit the execution of air puff–elicited trigeminal blinks (Mays and Morrisse 1995). Several studies confirmed slowing of saccades during blinks, which is consistent with a blink-related pause of the OPNs interfering with the timing of the saccadic-related pause (Gandhi and Bonadonna 2005; Goossens and Van Opstal 2000a; Rambold et al. 2002, 2004; Rottach et al. 1998). In fact, some preliminary reports indicate that OPNs also stop during blinks (Cohen and Henn 1972; Hepp et al. 1989; Mays and Morrisse 1994).
Overall, the current evidence strongly supports a crossed interaction between oculomotor and blink systems through the OPNs and it is thus logical to hypothesize that OPNs have similar function and similar pause timings for both saccades and blinks. A major motivation for the present blink–OPN study was that recent data opened the possibility that OPNs actually have different roles for blinks and saccades and perhaps different timings as well. Using antidromic stimulation from the OPN area, Gandhi and Keller (1997) showed that the more rostral fixation and buildup neurons of the deep layer of the superior colliculus have the greatest density of connections to the OPNs. This density decreases moving caudally. It was thus expected that stimulation of the rostral area would be the most effective in inhibiting reflex blink excitability because that area has the strongest influence on the OPNs; however, this was not the case. Gnadt et al. (1997) confirmed that stimulation of the deep layers of the superior colliculus inhibits blinks, with an average latency of 26 ms, but the rostral area was the least effective in blink inhibition. Studies in rats aimed at exploring the source of the reflex blink hyperexcitability in Parkinson's disease indicated that the rat brain regulates the excitability of blinks through a modulation of the nucleus raphe magnus by the basal ganglia and the inhibitory nigrostriatal pathway (Basso and Evinger 1996; Basso et al. 1996). It is therefore possible that the reported suppressive effect of OPNs on blinks, which would be consistent with an OPN gating role similar to that for saccades, may instead be an indirect action on these pathways.
In this report, by using air puff–elicited trigeminal blinks in alert behaving macaque monkeys, we compare OPN behavior during blinks while the animal is fixating a stationary target and during conjugate saccades. We also specifically quantify the timing relationship between the OPN pause and the blink-related eye movement transients (Bergamin et al. 2002; Bour et al. 2000; Collewijn et al. 1985; Evinger et al. 1984; Goossens and Van Opstal 2000a; Rambold et al. 2002, 2004) to test the hypothesis that they originate from the saccadic system. When present, we analyze spontaneous blinks as well, which might have a different triggering mechanism, because they are elicited outside the trigeminal reflex arc. Combined blink–saccades or blink–vergence responses are not included in this study because our analysis is limited to blinks during fixation. Preliminary results of this study were previously published in abstract form (Busettini et al. 2006).
METHODS
Electrical activity of pontine omnipause neurons, eye movements, and eyelid movements were recorded in two juvenile rhesus monkeys (Macaca mulatta) weighing 6–8 kg. All procedures and experimental protocols were approved by the University of Alabama–Birmingham Institutional Animal Care and Use Committee and complied with USDA, AAALAC, and U.S. Public Health Service Policy on the humane care and use of laboratory animals.
Surgical procedures
After being trained to enter a primate chair and being acclimated to the laboratory environment, a series of aseptic surgical procedures were performed. In the first surgery, custom-made and thermally shaped polyether ether ketone (PEEK) strips were attached to the skull with ceramic bone screws. After full recovery, the strips were used as an attachment for an external head post crafted from PEEK and dental acrylic and designed to immobilize the animal's head. The animals were then fully trained to do simple saccadic tasks using a binocular video eye tracker (ISCAN, Woburn, MA). Since a video eye tracker cannot be used to measure eye movements during blinks, a surgery was performed to implant a coil of fine wire (Biomed Wire AS633) underneath the conjunctiva of the right eye for the purpose of eye tracking via the magnetic search coil technique (Fuchs and Robinson 1966). One of the animals (A1) also had a coil in the left eye. Two custom-made PEEK recording chambers were then implanted on each animal's head with acrylic cement and ceramic bone screws over 15 mm holes trephined into their skulls. The chambers were stereotaxically positioned bilaterally over the brain stem at 20° to the sagittal plane, 14.5 mm lateral from the midline and 2 mm anterior to ear-bar zero.
Behavioral task
With their heads immobilized, animals were trained to make saccades between visual targets (white Maltese crosses 1.2° in size on a black background) for a reward. The optical system was a mirror haploscope system (Walton and Mays 2003). The angular position in depth and blur of the visual targets were maintained at optical infinity to require the animal to generate far conjugate saccades. Each trial, a standard no-gap saccadic task, consisted of the animal fixating an initial target at primary position (0°) and then a subsequent target at a horizontal or vertical secondary position of 5, 10, or 15°. The duration of the fixation periods and the position of the second target were randomized to avoid anticipation by the animal. During the fixation periods on the first or the second target, randomly occurring gentle puffs of air were delivered to the animal to elicit trigeminal reflex blinks. The percentage of fixation periods with an air puff was, on average, ≤30%. The intensity and duration of the air puffs were set just at the threshold required for a reliable blink reflex to cause minimal aggravation to the animals. The air, generated by an air compressor (Doerr Electric), was released from a small tube pointed at the right eye and had a wide area of impact, covering roughly a third of the right side of the animal's face. The puff delivery was controlled by a solenoid valve driven by a transistor-to-transistor logic (TTL) triggered circuit. The compressor and solenoid were located behind two closed doors from the animal to avoid conditioned blinks elicited by the solenoid clicks. A miniature wide-band Brüel & Kjær microphone was placed where the monkey's right eye was during the experiments and the time between the TTL command and the first change in the microphone signal determined the air puff delivery delay (39 ms). The microphone indicated the duration of the air puffs to be around 80 ms. The location in our records of the TTL pulse driving the solenoid differentiated the puff-elicited blinks from the spontaneous blinks. Blinks elicited by the air puff will be termed “trigeminal.” All blinks that did not occur in close association with an air puff will be labeled “spontaneous.” Based on preliminary tests showing no relevant timing changes to eye or eyelid movements when switching the tube between the right and left sides of the face, or with the location of the search coil used to measure the movement of the upper eyelid, the tube was kept pointing at the right eye and the eyelid coil was also placed on the right eyelid. This is consistent with what was observed by Stava et al. (1994) and VanderWerf et al. (2003).
Data acquisition and single-unit recordings
A real-time Linux computer presented the visual stimuli, administered juice rewards, and acquired eye, eyelid, and unit signals. Horizontal and vertical eye positions, along with vertical right eyelid position, were acquired at 1 kHz, with the analog filter of the phase detectors set at 300 Hz, 18 dB/octave. Another 1 kHz channel was used to acquire the TTL pulse triggering the air puff. This was manually controlled by the investigator using a push button. The analog signal from the electrode was sampled at 20 kHz, and recorded along with the occurrence of neuronal spikes, detected via a window discriminator (BAK Electronics). The TTL pulses from the window discriminator were acquired as digital events with a time resolution of 0.1 ms. The 1 kHz traces were linearly resampled at 10 kHz before the actual analysis to preserve the full time resolution of the spike events. The electrode signal was hardware-filtered above 9 kHz to reduce interference from the magnetic field coils of the eye tracking system and below 10 or 100 Hz to reduce low frequency noise and low frequency fluctuations in the cell polarization. Low-impedance tungsten microelectrodes (0.2–0.5 MΩ; MicroProbe) insulated with parylene and additional Teflon tubing were mounted in 26-gauge steel tubing and advanced by a Kopf microdrive through a 21-gauge hypodermic needle, which acted as a guide tube and pierced the dura during the insertion to avoid bending of the microelectrode's tip. Cells were considered to be omnipause neurons when they maintained a constant firing rate during fixation and paused during saccades in all directions. All cells stopped for blinks as well and no cell was found to stop only for blinks. To further verify that these cells were in the omnipause area, some cells were stimulated (maximum current 40 μA at 250 Hz for 200 ms) to confirm the block of the occurrence of saccades or the transient stopping of ongoing saccades. Marking lesions were made in both animals at the end of the recordings and they were found in the omnipause area during histological reconstruction.
Data analysis
For each trial, right eye movement analysis (conjugate saccades with no blinks and blink-related eye transients while the animal was steadily fixating the first or the second target), right eyelid analysis, and OPN analysis were done in separate interactive steps with plots showing only the needed traces at each step to avoid potential visual cross-biases in the timing measures. In animal A1, we also acquired and analyzed the blink-related transient movements of the left eye. The programs used for the analysis were developed in-house using RedHat Linux and ANSI C language.
SACCADIC EYE MOVEMENTS.
Horizontal and vertical eye position traces were linearized with third-order polynomials in both horizontal and vertical coefficients to compensate for possible horizontal/vertical phase detector cross talks and were fit with a cubic spline with weight 1 × 108 (0.1 with time expressed in milliseconds). Velocity signals were then calculated using a two-point backward differentiation of the splined traces. The same spline and differentiation procedures were also applied to the eyelid traces. Rightward and upward eye movements are considered positive, whereas leftward and downward movements are considered negative. Saccades were manually identified and the two-segment fitting process outlined in Busettini and Mays (2005) was applied to the Pythagorean velocity, defined as to identify saccadic onset and offset. Once onset and offset were identified, the peak of the Pythagorean velocity, direction, size, and duration of the saccade were automatically determined.
BLINK-RELATED EYE TRANSIENTS.
An example of a blink trial is reported in Fig. 1. Figure 1A shows the horizontal component and Fig. 1B shows the vertical component of the blink-related transient change in the left eye position for the blink illustrated in Fig. 1C. The position traces are in green and the velocity traces are in black. Once a blink was manually identified, the onsets of the transient eye movements associated with the blink were analyzed in a similar manner, but the vertical and horizontal components were studied separately. Blinks with eye transients that resulted in an overall Pythagorean change in eye position >3° were considered contaminated by a saccade and were not further analyzed. A preliminary velocity analysis of the transient profiles showed that a 3° positional threshold was sufficiently low to eliminate most of the saccade-contaminated blinks. Saccades were identifiable as high-velocity artifacts superimposed on the more gradual and highly stereotyped transients and blinks that did not match the change in position criteria, but presented such artifacts, were manually discarded during the analysis. Because these transients often had multiple velocity peaks, special care was taken to ensure that the onset fitting algorithm was searching the onset before the first peak when calculating the horizontal and vertical transient onsets.
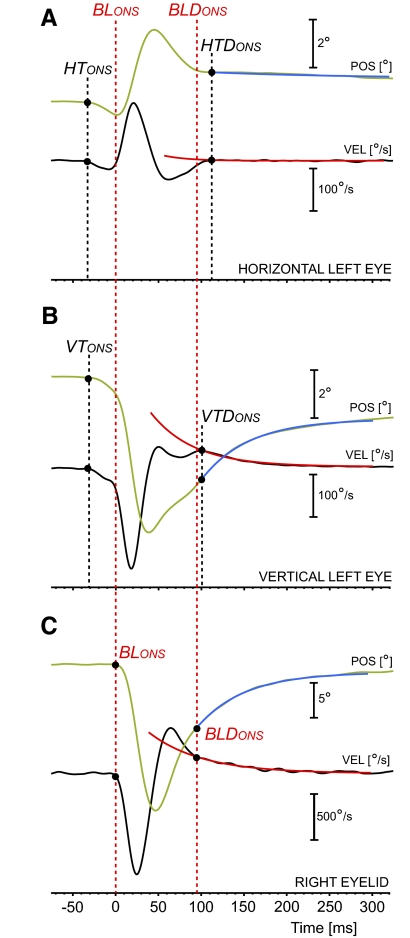
Fig. 1.
Example of blink and associated eye transients. A: horizontal left eye transient. B: vertical left eye transient. C: right eyelid. Position traces are in green and velocity traces in black. It was a general feature, as in this example, to have the eye transients starting before the blink. How the single exponential fit of the monotonic drifts (in blue for the position traces, in red for the velocity traces) was computed is reported in the text. BLONS, HTONS, and VTONS indicate the onset of the blink, of the horizontal eye transient, and of the vertical eye transient, respectively. BLDONS, HTDONS, and VTDONS indicate the onsets of their exponential drifts.
Both the horizontal and vertical components of the eye transients, after a more dynamic phase, ended with prolonged slow monotonic drifts similar to the glissades that are observed after a saccade when there is a pulse-step mismatch (Optican and Robinson 1980). We decided to test the hypothesis that the switch from the more dynamic phase of the eye transients to the monotonic drift is linked to the resumption of the firing of the OPNs and represents the end of the “saccadic” phase of the transients. Our assumption is that the blink-related eye transients drive the eyes off the tonic equilibrium established by the oculomotor neural integrator. The resumption of the OPN firing, by inhibiting the neural activity in the saccadic short-lead burst neurons, blocks any further active attempt by the saccadic system to compensate for the perturbation. A passive glissade follows until the eye reaches a balance between the tonic forces generated by the extraocular muscles. We will determine the onset of the exponential drift, which we also identified as the offset of the eye transient proper, and the time constant of the drift, which we fitted with a single exponential. The onset of this exponential drift, in both the horizontal and vertical domains, was identifiable in the eye velocity trace as an abrupt dynamic change and was objectively determined as follows: While viewing the eye position and velocity traces (horizontal traces first, vertical traces after, to avoid crossed biases in the timing measures), the operator selected a point in the velocity trace that was close to the potential onset, but clearly inside the slow drift. Then, the algorithm automatically fitted with a single exponential the segment of the velocity trace starting from this point and extending 200 ms further in time. A minimum square error procedure was used to quantify the fitting parameters. Using these parameters, the program then extended the exponential fit backward by 60 ms from the selected point. Drift onset was determined as the point where the separation between the velocity trace and the fit had an abrupt increase, again using the two-segment fitting, applied now on the deviation trace. Finally, using the drift onset estimate from the velocity trace as the starting point, the exponential fitting was applied to the position trace to determine the amplitude and time constant of the exponential fits. In the blink example in Fig. 1, the single exponential fits of the eye monotonic drifts, horizontal component in Fig. 1A, vertical component in Fig. 1B, are illustrated in blue (position) and red (velocity). The few drifts that were too small for a reliable onset measure were excluded from the data sets. The amplitude and dynamic profiles of the blink-related eye transients are known to vary with eye position (Bergamin et al. 2002; Bour et al. 2000; Collewijn et al. 1985). With our focus centered on transient onset, onset, and time constant of the monotonic drift after the more transient period, and their timing relationship with the OPN firing, we did not analyze either the dynamic profile of the transient proper or its dependence with eye position. A postanalysis control showed eye position had no significant effects on the timing of such events or the time constant. Eye position affected only the amplitude of the exponential drift of the horizontal component of the eye transients.
EYELID MOVEMENTS.
At the beginning of the recording session, a 3 mm diameter search coil with three wire loops was taped near the bottom of the right upper eyelid of the animal to record vertical eyelid position. Downward motion is defined as negative, with upward motion defined as positive. Following a procedure similar to that used by Gnadt et al. (1997), a protractor was used to rotate the eyelid coil through known values to precalibrate the eyelid coil signal. Although Wouters et al. (1995) reported that the small daily changes in the location of the coil with respect to the eyelid edge can significantly affect the coil gain, the eyelid time events and measures of interest in this study are not affected by the eyelid gain calibration.
Onsets of blinks were determined from the velocity trace of the eyelid using the same fitting process as that for saccadic onset. Several spontaneous blinks were preceded by a small preparatory drift. The selection of the fitting interval was done to ignore this drift and thus to identify as blink onset the onset of the main closing phase of the blink. An exploratory analysis to see whether these preparatory drifts, albeit small, correlated with an early onset of the OPN pause, or a more pronounced slowing of the tonic firing of the cell just before the pause, failed to identify any consistent relationship. A slow upward monotonic drift of the eyelid (Fig. 1C) similar to the monotonic upward drift of the vertical eye transient (Fig. 1B) occurred during the last part of the reopening phase of the blink. The onset of this drift, which we also identified as the end of the main dynamic phase of the eyelid reopening, its amplitude, and its time constant were analyzed in the same manner as that for the eye drifts. These values were compared with the values of the eye exponential drifts for possible correlations. There is a tight functional linkage between vertical eye position and upper eyelid position (Becker and Fuchs 1988) and a drift in the vertical eye position is likely matched by a similar upper eyelid drift. However, the presence of these monotonic drifts in the horizontal eye position as well suggests that they might have a different, perhaps passive, nature. The alternative hypothesis is that they are, directly or indirectly, related to the postblink resumption of activity of the levator palpabrae superioris motoneurons (LPSMNs). Fuchs and colleagues (1992) published a detailed analysis of the LPSMNs during blinks and we will compare their timing and dynamics of the LPSMN resuming with our timing and dynamics of the monotonic drifts of the eye transients.
OPN ACTIVITY AND TIMING MEASURES.
All unit recordings were first closely inspected to ensure that all action potentials were labeled correctly by our window discriminator and spike marks were inserted or deleted as needed using the electrode 20 kHz analog trace as reference. Trials with spike activity that could not be confidently isolated were not included in our data and, for each cell, only saccadic, trigeminal, and spontaneous data subsets containing at least eight valid trials were used in the OPN population statistical analysis. For each point in time, firing frequency was defined as the reciprocal of the duration of the interspike interval (ISI) containing that point.
To quantify the effects of the saccade and of the blink outside the OPN pause proper, we computed the relative change in firing rate with respect to fixation (estimated baseline firing) just before and just after the pause. Eye position has a small and irregular, but sometimes significant, effect on the tonic firing rate of OPNs (Busettini and Mays 2003). Additionally, in several cells we observed, for the same eye position, a slow but often very significant tonic firing rate variation over time, i.e., with trial number. This might have been due to changes in alertness of the animal during the data acquisition, fluctuations in the cell bias, or to changes in the position of the electrode tip with respect to the cell. To have a reliable estimate of the baseline firing rate, we used a linear regression in Systat, with independent coefficients for horizontal and vertical eye position and trial number. When, from the residuals, a second- or third-order polynomial fit for the effect of trial number was needed, a nonlinear model was used instead. The data for this regression came from tonic firing measures at various fixation points selected manually for each trial to be far enough from saccades and/or blinks for the cell's firing not to be affected by them. At each of these points, the average firing rate over 100 ms was measured along with its associated average eye position and trial number. The baseline parameters extracted from the merging of these static measures were then used to estimate the baseline rate of the cell at each point of each trial. For both saccadic and blink trials, the firing frequency (1/duration) of the last three ISIs before the pause and of the first six ISIs after the pause were normalized with respect to the estimated baseline firing rate using the eye position values at the start of each of the ISIs and the trial number. By using normalized firing data, we were able to compare the relative changes in firing rate just before the onset and just after the offset of the OPN pause for blinks and saccades. The time interval between the last spike prior to the pause and saccadic onset determined the saccadic pause lead. Saccadic trigger lead was defined as pause lead minus half of the duration of the last ISI before the pause. The average of this value is a better statistical estimate of the average time of the actual arrival of the inhibitory signal to the cell (Busettini and Mays 2003). In the simplified hypothesis of the trigger signal driving the cell to inhibition being very fast and the cell firing steadily until the arrival of the trigger, we have a uniform random distribution of the relative timing of the trigger arrival with respect to the last spike. This distribution has, as limits, the last spike itself (the trigger arrived immediately after the last spike) and one ISI after the last spike (the trigger arrived at the same time of the next spike, inhibiting it), with center value, for steady firing, half of the last ISI. Any additional slowing of the cell firing after the last spike translates in the actual trigger arrival to occur further in time with respect to our estimate. Similar pause onset timing measures were computed for the eye transients and for blinks. A positive value means that pause (or estimated trigger) onset preceded movement onset.
Pause offset measures used in our study are the position of the first resumption spike with respect to saccadic offset, the onset of the monotonic eye drifts, and the onset of the monotonic eyelid drift. A positive value means that the first resumption spike occurred before the associated movement event. For blinks, we also tested the correlation of the first resumption spike with other blink events, which will be described in results.
Statistical tests were standard single value, paired, or unpaired t-test on the means or linear regressions, with statistical significance threshold P = 0.01 (Systat). Unless specified otherwise, all statistical tests illustrated herein reached the significance threshold.
RESULTS
OPN pause and movement onsets
A total of 102 OPNs were recorded from the nucleus raphe interpositus of two rhesus monkeys. Not all cells had the minimum number of trials (8) for all types of events (saccades, puff-elicited trigeminal blinks, and spontaneous blinks) and we will report, in each case, the number of cells included in that specific measure or comparison.
All OPNs paused abruptly and completely for saccades and, except for some trials in a few cells that will be discussed later on, also for blinks. Surprisingly, it was immediately clear that the time of pause onset differed significantly between saccades and blinks. When we separated trigeminal from spontaneous blinks, the timing also differed between these two types of blinks. This is illustrated in Fig. 2. Figure 2A shows, from a cell from animal A1, horizontal right eye position, horizontal right eye velocity, firing rate, and single spikes for all the 9 to 11° rightward saccades in this data set (n = 14 trials). The vertical dotted lines indicate saccadic onset (SONS) or blink onset (BLONS), depending on the type of trial. In agreement with Busettini and Mays (2003) and Everling et al. (1998), this OPN cell always stopped firing before the saccade. Considering the natural scatter of one ISI in the position of the last spike before the pause, the timing was tightly correlated with saccadic onset. Figure 2B illustrates 14 of the 33 trigeminal blinks in this data set. The panel reports eyelid position, eyelid velocity, firing rate, and single spikes for these 14 trials. Similar tight correlation between movement onset and pause onset was observed for trigeminal blinks. However, the last spike occurred near or after the onset of the blink. For trigeminal blinks, the sum of the neural and mechanical delays between the onset of the firing of the orbicularis oculi motoneurons (OOMNs), which drive the blink, and the actual eyelid onset is, in cats, around 6.7 ms (Trigo et al. 1999). No data are available for the monkey, but we would expect them to be similar and likely longer. This implies that, for the trigeminal blinks in Fig. 2B, the onset of the firing of the OOMNs preceded the OPN pause of the cell by several milliseconds. Figure 2C illustrates 17 of the 28 spontaneous blinks in this data set with the same layout as that of Fig. 2B. For spontaneous blinks, there was a much larger scatter in the timing, with the pause of the OPN cell sometimes starting much earlier than that for saccades as well as after the onset of the blink. For the earliest pauses, we carefully verified the absence of saccades preceding the blink. Figure 2D reports the pause lead distributions for the full data sets. The similarity of the shape of the distributions of the pause leads for saccades and for trigeminal blinks is truly remarkable, as well as the evident shift in time. The much wider distribution of pause leads for spontaneous blinks superimposed on both saccadic and trigeminal distributions. The average values for the three distributions were: for saccades, 11.7 ms (±3.5 ms SD; range: 1.7 to 20.3 ms); for trigeminal blinks, −3.3 ms (±3.3 ms SD; range: −10.6 to 4.1 ms); and for spontaneous blinks, 5.9 ms (±7.5 ms SD; range: −4.9 to 27.5 ms). A positive value means that the onset of the pause preceded the onset of the saccade or of the blink. Spontaneous blinks were also found to be, on average, slightly smaller and slower than trigeminal blinks, as can be seen by comparing the eyelid traces in Fig. 2, B and C. For the 33 trigeminal blinks, the average blink size was −40.8° (±4.0° SD; range: −46.5 to −31.7°) and the peak velocity was −1,596°/s (±148°/s SD; range: −1,851 to −1,244°/s). For the 28 spontaneous blinks, the average blink size was −34.6° (±4.6° SD; range: −42.0 to −25.2°) and the peak velocity was −1,275°/s (±179°/s SD: range: −1,714 to −996°/s).
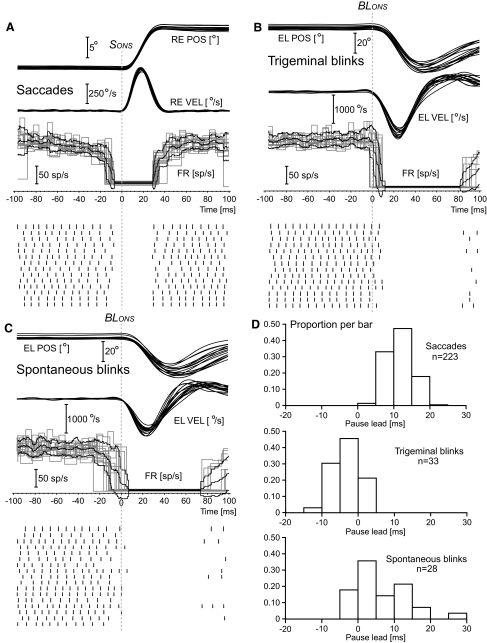
Fig. 2.
Pontine omnipause neuron (OPN) pause onset: single trials. The first 3 panels illustrate movement position and velocity traces, OPN firing frequency, and OPN spike events as a function of time for subsets of single trials from cell c51026.A1. All traces are synchronized with the onset of the movement, identified with the vertical dotted lines labeled SONS (saccades) and BLONS (blinks). A: 9 to 11° rightward saccades. Top traces: right eye horizontal position (RE POS) and right eye horizontal velocity (RE VEL). Bottom traces: firing rate (FR) and associated spike events. B: trigeminal blinks. C: spontaneous blinks. The top traces in B and C are right eye eyelid position (EL POS) and right eye eyelid velocity (EL VEL). The pause leads, i.e., the time intervals between the last spike before the pause, identifying pause onset, and the movement onset clearly differ for saccades and blinks. D illustrates the distribution of the pause lead values for all the trials from this cell in 5 ms bins. The shift in time between saccadic pause leads and the pause leads for trigeminal blinks is quite evident. The pause lead distribution for spontaneous blinks was much wider and encompassed both saccadic and trigeminal distributions.
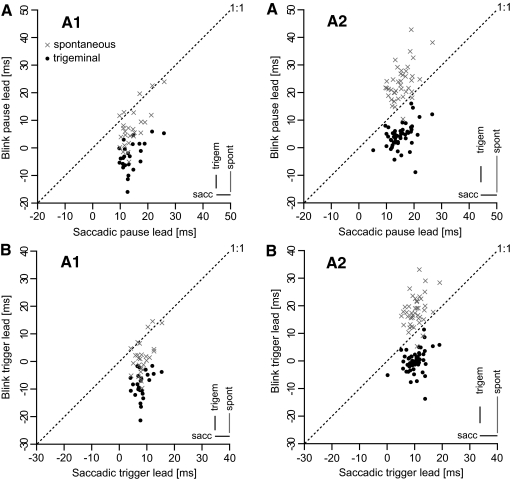
The behavior of the cell shown in Fig. 2 extended to all the OPN cells of both animals. The two top panels in Fig. 3 compare cell-average saccadic pause lead with cell-average blink pause lead for trigeminal blinks (black dots) and for spontaneous blinks (gray  symbols) for animal A1 (Fig. 3AA1) and animal A2 (Fig. 3AA2). The key observation is that, for all cells, the pause leads for the trigeminal blinks (black dots) were below the 1:1 line, indicating that the pause onset occurred later for blinks than for saccades, often well after the onset of the blink (negative values). On the contrary, the average pause leads for the spontaneous blinks were much more variable. Some cells in animal A2 stopped firing, on average, 30–40 ms before the onset of the spontaneous blink, and some cells in animal A1 had negative pause leads. The averages, for the three trial types, of the SDs of each cell subset are reported on the bottom right corner of the two plots as visual indicators of the between-trials data scatter. The combined population pause lead average for saccades was 14.5 ms (±3.9 ms SD, n = 102 cells), for trigeminal blinks was 1.7 ms (±5.8 ms SD, n = 79 cells), and for spontaneous blinks was 16.9 ms (±10.5 ms SD, n = 69 cells). The results for each animal are illustrated in Table 1. For the 75 cells with sufficient saccadic and trigeminal blink measures, with the exclusion of one cell in animal A2, all cells (98.7%) had significantly shorter pause leads for trigeminal blinks than for saccades. The 16.9 ms average pause lead for spontaneous blinks was not statistically different from the 14.5 ms average pause lead for saccades, but the scatter in lead values for spontaneous blinks was much more pronounced both at the single trial level—compare (sacc) and (spont) bars in Fig. 3—and as cell averages. As already observed in Fig. 2, the scatter of the pause lead values was quite similar for saccades and trigeminal blinks. The onset of the OPN pauses for spontaneous blinks differed somewhat in the two animals. In animal A1, 72% of the cells had their pause leads still significantly shorter for spontaneous blinks when compared with saccades. For animal A2, 76% of the cells had statistically longer pause leads for spontaneous blinks than for saccades.
symbols) for animal A1 (Fig. 3AA1) and animal A2 (Fig. 3AA2). The key observation is that, for all cells, the pause leads for the trigeminal blinks (black dots) were below the 1:1 line, indicating that the pause onset occurred later for blinks than for saccades, often well after the onset of the blink (negative values). On the contrary, the average pause leads for the spontaneous blinks were much more variable. Some cells in animal A2 stopped firing, on average, 30–40 ms before the onset of the spontaneous blink, and some cells in animal A1 had negative pause leads. The averages, for the three trial types, of the SDs of each cell subset are reported on the bottom right corner of the two plots as visual indicators of the between-trials data scatter. The combined population pause lead average for saccades was 14.5 ms (±3.9 ms SD, n = 102 cells), for trigeminal blinks was 1.7 ms (±5.8 ms SD, n = 79 cells), and for spontaneous blinks was 16.9 ms (±10.5 ms SD, n = 69 cells). The results for each animal are illustrated in Table 1. For the 75 cells with sufficient saccadic and trigeminal blink measures, with the exclusion of one cell in animal A2, all cells (98.7%) had significantly shorter pause leads for trigeminal blinks than for saccades. The 16.9 ms average pause lead for spontaneous blinks was not statistically different from the 14.5 ms average pause lead for saccades, but the scatter in lead values for spontaneous blinks was much more pronounced both at the single trial level—compare (sacc) and (spont) bars in Fig. 3—and as cell averages. As already observed in Fig. 2, the scatter of the pause lead values was quite similar for saccades and trigeminal blinks. The onset of the OPN pauses for spontaneous blinks differed somewhat in the two animals. In animal A1, 72% of the cells had their pause leads still significantly shorter for spontaneous blinks when compared with saccades. For animal A2, 76% of the cells had statistically longer pause leads for spontaneous blinks than for saccades.
Fig. 3.
OPN pause and trigger onset leads: cell averages. AA1 and AA2 compare average saccadic pause leads (x-axis) and average blink pause leads (y-axis) for the OPN cells acquired from animal A1 and animal A2, respectively. Trigeminal blink averages are identified by the black dots and spontaneous blink averages are identified by the gray × symbols. Symbols below the 1:1 dotted lines indicate pause onset for blinks occurring, on average, later than pause onset for saccades. BA1 and BA2 compare, with the same layout of the top panels, saccadic trigger leads and blink trigger leads, as defined in methods. The bars on the bottom right corners are the averages of the SDs of each cell subset for the 3 types of pause (trigger) lead measures. The shift in time for all cells of the trigeminal pause (trigger) lead values when compared with the saccadic pause (trigger) lead values is quite remarkable, clearly indicating that OPN pause onset has different functions for saccades and trigeminal blinks. The 2 animals present evident differences in their spontaneous blink lead values.
Table 1.
OPN pause and trigger leads
| OPN Pause Lead |
OPN Trigger Lead |
|||
|---|---|---|---|---|
| Trial Type | Mean ± SD, ms | Range, ms | Mean ± SD, ms | Range, ms |
| A. Animal A1 | ||||
| Sacc (31) | 13.8 ± 3.9 | 9.5 to 25.7 | 8.4 ± 2.6 | 4.4 to 15.7 |
| Trigem (25) | −3.4 ± 5.4 | −16.3 to 5.6 | −8.3 ± 4.9 | −21.3 to −1.5 |
| Spont (27) | 6.8 ± 7.2 | −5.3 to 23.7 | 1.4 ± 6.1 | −9.5 to 14.7 |
| B. Animal A2 | ||||
| Sacc (71) | 14.9 ± 3.9 | 5.3 to 26.7 | 10.0 ± 3.3 | 0.3 to 19.1 |
| Trigem (54) | 4.1 ± 4.3 | −9.0 to 15.9 | −0.2 ± 4.1 | −13.9 to 11.3 |
| Spont (42) | 23.4 ± 6.3 | 10.4 to 42.8 | 18.1 ± 5.5 | 5.3 to 33.1 |
Values are in milliseconds. Combined averages, with associated SD and range, of the averages for each cell of the pontine omnipause neuron (OPN) pause and trigger leads for saccades (Sacc), trigeminal blinks (Trigem), and spontaneous (Spont) blinks. The numbers in parentheses in the Trial Type column are the numbers of cells used for the average computation. Positive values indicate that the OPN pause/trigger preceded the onset of the movement.
The biological significance of the shorter pause leads for trigeminal blinks with respect to saccades becomes even more evident when we use the average trigger lead estimates (Busettini and Mays 2003; see methods). With the same layout of the top panels, the bottom panels of Fig. 3 plot cell-average saccadic trigger lead versus the two types of blink trigger leads. The overall population average of the saccadic trigger leads was 9.5 ms (±3.1 ms SD, n = 102 cells), for trigeminal blinks was −2.7 ms (±5.8 ms SD, n = 79 cells), and for spontaneous blinks was 11.6 ms (±10.0 ms SD, n = 69 cells). The results for each animal are illustrated in Table 1. When the more veridical average timing estimate of when the cells receive the inhibitory signal is used, the saccadic trigger timing is still strongly positive, consistent with the fact that the OPN group has to be inhibited for a saccade to start. On the contrary, the average trigger lead for trigeminal blinks now has a negative average; i.e., the signal driving the OPNs to inhibition follows blink onset.
The possibility that pause onset for blinks could instead be related to the onset of the eye transients occurring in association with blinks was easily rejected by the observation that the eye transients, in both animals and for both trigeminal and spontaneous blinks, preceded the onset of the blink by several milliseconds, as can also be seen in the example in Fig. 1. This translates into an even more extended prepause period of baseline OPN activity following the onsets of the eye transients, particularly for trigeminal blinks. A similar earlier onset of the eye transients with respect to the onset of spontaneous blinks in humans was reported by Bergamin et al. (2002).
Although the eye transients are a behavioral property, and thus are independent of the cell under study at that moment, during their analysis we kept the data sets separate, to estimate the reliability of the blink onset measures between sessions and blink types. We had two main concerns. The first was that the daily variability in the placing of the search coil on the eyelid might have introduced large changes in the detection of blink onset between sessions. The second concern, which applies specifically to trigeminal blinks, was that the air puffs, by possibly deflecting the eyelid coil wires, could have caused an anticipated detection of blink onset. With the eye coils permanently implanted under the conjunctiva and their connecting wires under the skin, the eye transient onsets were most likely invariant between sessions and mechanically insensitive to the air puffs. This made the eye transient onsets well suited as control values. As shown in Table 2, all eye transient lead times were positive, indicating that they, on average, preceded blink onset. With the exclusion of the value marked with a +, all time differences were statistically different from zero. The small SDs of the time differences indicate a precise timing correlation between blinks and eye transients that remained largely invariant between sessions. The positioning of the eyelid coil on the eyelid therefore had little or no effect on the detection of blink onset. Trigeminal blink onsets occurred, on average, only 4.4 ms (±5.5 ms SD of the difference, n = 6) earlier with respect to spontaneous blink onsets for the same eye transient onset value, which was not significantly different from zero. This earlier onset for trigeminal blinks probably reflects the more synchronized signal driving the trigeminal blinks compared with spontaneous blinks. As observed earlier, spontaneous blinks are usually slightly slower than reflex blinks and this may also have small effects on the onset timing. Therefore we can exclude that the earlier eyelid onsets with respect to pause onset for trigeminal blinks (see also Figs. 2 and 3) were due to mechanical or electrical artifacts associated with the air puffs.
Table 2.
Eye transient leads with respect to blink onset
| Animal A1 |
Animal A2 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Trial Type | Number of Cells | Right Hor, ms Mean ± SD | Right Vert, ms Mean ± SD | Left Hor, ms Mean ± SD | Left Vert, ms Mean ± SD | Number of Cells | Right Hor, ms Mean ± SD | Right Vert, ms Mean ± SD |
| Trigem | 25 | 1.3+ ± 3.1 | 5.4 ± 3.4 | 17.1 ± 6.6 | 11.1 ± 5.0 | 54 | 7.5 ± 2.5 | 4.4 ± 2.2 |
| Spont | 27 | 6.3 ± 4.9 | 12.5 ± 4.3 | 12.1 ± 4.4 | 15.3 ± 6.7 | 42 | 10.7 ± 3.1 | 16.0 ± 4.9 |
Values are in milliseconds. Combined differences, with associated SD of the difference, between the averages for each cell of the eye transient onsets and blink onsets. Positive values indicate that the onset of the eye transient preceded blink onset. All values, with the exclusion of the one marked with +, are significantly different from zero.
The main conclusion of the OPN pause onset analysis is that, contrary to saccades, trigeminal blinks and associated eye transients started while the OPN population was still firing near baseline values. For spontaneous blinks, OPN pause leads covered a much larger range of values, both at the single trial level and between cells, indicating a functional dissociation between blink onset and OPN pause onset.
Prepause OPN behavior
Possibly important clues to the nature and dynamics of the neural signal driving the OPNs to inhibition during blinks can be obtained by observing the change in OPN firing rate just before the pause. We concentrated our attention on the normalized firing frequency of the last three ISIs, as described in methods. The average results are illustrated in Table 3. As already observed in Busettini and Mays (2003) and Everling et al. (1998), the OPN population fires close to baseline up to the very last spike prior to the saccade-related pause. Although both animals presented a significant (indicated by the asterisk) presaccadic slowing of the firing at the last ISI, it was only minor (85.4% and 90.2% of the baseline). The second and third ISIs were close to 100% or slightly higher. The slowing for trigeminal blinks was very similar, with some minor slowing for animal A1 and no slowing for animal A2. A slightly more pronounced and gradual slowing was present for spontaneous blinks, but again with the overall population not going <80.4% of the baseline estimate. This likely excludes the possibility that the earlier and more scattered pause onsets for the spontaneous blinks are due to some kind of preblink slow-building anticipatory activity gradually inhibiting the OPN population. The signal driving the OPNs to inhibition for both trigeminal and spontaneous blinks shares the same fast dynamics observed for saccades. Curiously, the onset of this fast signal appears to be only loosely related to the actual blink onset for spontaneous blinks.
Table 3.
Prepause OPN behavior
| Trial Type | ISI-1 Mean ± SD | ISI-2 Mean ± SD | ISI-3 Mean ± SD |
|---|---|---|---|
| A. Animal A1 | |||
| Sacc (31) | 85.4* ± 5.8 | 94.7* ± 5.6 | 97.3 ± 5.4 |
| Trigem (25) | 88.2* ± 7.1 | 102.6 ± 10.3 | 101.3 ± 6.7 |
| Spont (27) | 86.2* ± 9.6 | 93.3* ± 8.9 | 97.6 ± 7.1 |
| B. Animal A2 | |||
| Sacc (71) | 90.2* ± 9.3 | 100.9 ± 8.5 | 106.7+ ± 9.5 |
| Trigem (54) | 101.1 ± 9.7 | 107.9+ ± 9.3 | 105.3+ ± 8.3 |
| Spont (42) | 80.4* ± 8.0 | 87.6* ± 8.2 | 94.2* ± 9.6 |
Combined averages, with associated SD, of the averages for each cell of the firing rate during the last three interspike intervals before pause onset, expressed as a percentage of the firing rate with respect to the estimated baseline. The numbers in parentheses in the Trial Type column are the numbers of cells used for the average computation. The asterisks indicate that the average was significantly <100% and the crosses indicate that the average was significantly >100%.
OPN pause and movement offsets
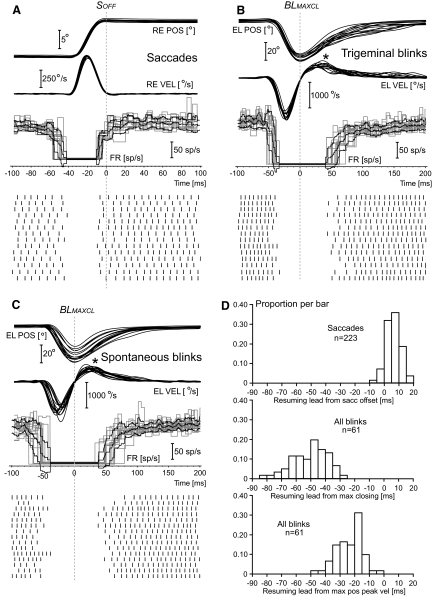
It is generally accepted that the resumption of the firing of the OPNs for saccades is associated with the end of the activity in the short-lead saccadic burst neurons (Scudder 1988) and, accordingly, it occurs around the end of the saccade, although both Busettini and Mays (2003) and Everling et al. (1998) reported a surprisingly high variability in the resumption timing between cells. We confirmed that this variability was not correlated with the baseline firing rate of the cell. Cells with higher baseline firing (i.e., with stronger excitatory bias) did not necessarily resume earlier than cells with lower baseline firing. Using the same saccadic trials of Fig. 2, but now synchronized with the end of the saccade (SOFF), Fig. 4A shows that this cell resumed its firing just before saccadic end. The average resumption lead for the rightward 9 to 11° saccades of the data set in Fig. 4A was 3.7 ms (±2.2 ms SD; range: 0.8 to 7.9 ms, n = 14 trials). Positive values mean that the cell resumed its firing before the movement event, in this case saccadic offset. When this average was extended to all 223 saccades of the data set (Fig. 4D, top histogram), the resumption lead was 6.2 ms (±4.7 ms SD; range: −5.5 to 18.5 ms).
Fig. 4.
OPN resumption leads: single trials. The first 3 panels illustrate movement position and velocity traces, OPN firing frequency, and OPN spike events as a function of time for subsets of single trials from cell c51026.A1, the same cell of Fig. 2. The layout of these 3 panels is identical to that of Fig. 2, with the following difference: the saccadic traces (A) are now synchronized with the offset of the saccade, identified with the vertical dotted line labeled SOFF, and the blink traces are synchronized with respect to the time of maximum downward closing of the eyelid, identified with the vertical dotted line labeled BLMAXCL. The cell resumed around the end of the saccade (A), which is also near when the saccadic short-lead burst neurons end their activity. For both trigeminal (B) and spontaneous (C) blinks, the cell resumed several 10 s of milliseconds after the maximum closing of the eyelid, which human electromyographic (EMG) data indicate it occurs near the end of the activity in the orbicularis oculi motoneurons (OOMNs). This striking difference is also evident in the histograms in D. Positive values mean that the cell resumed before the specific movement event. For the blink histograms, trigeminal and spontaneous data are pooled together. The third histogram illustrates that the OPN resumption occurred even after the time of the maximum positive velocity peak of the reopening phase, indicated by the asterisks in B and C. The human EMG data indicate that this event is reached upto 50 ms after the end of the activity in the orbicularis oculi muscle, as the result of passive elastic forces and the resumption of activity in the levator palpabrae superioris muscle.
Using the saccadic system as reference (Scudder 1988), our initial hypothesis was that the OPNs are maintained inhibited during the blink by a copy of the premotor command driving the OOMNs, encoded in blink equivalents to the saccadic short-lead burst neurons. At the end of this activity, monosynaptically transmitted downstream to the OOMNs, the OPNs would resume their baseline firing. There are no data available in the literature on the firing profiles of these putative blink premotor burst neurons or of the OOMNs for nonhuman primates, but we have human electromyographic data from the orbicularis oculi muscles (Bour et al. 2000; VanderWerf et al. 2003) that give some indirect information. These data suggest that the activity in the orbicularis oculi muscle ends in humans around the time the eyelid reaches the maximum eyelid closure (BLMAXCL in Fig. 4, B and C) and roughly 50 ms before the maximum (positive) peak velocity of the reopening phase. We randomly selected trigeminal blink trials (Fig. 4B) and spontaneous blink trials (Fig. 4C) from the same cell in Fig. 2 and synchronized the traces with respect to the time of maximum eyelid closure. The cell remained inhibited well after the end of the main reopening phase of the blink in all trials. For the 33 trigeminal blinks in this data set, the resumption lead with respect to the time of maximum eyelid closing was, on average, −51.9 ms (±11.9 ms SD; range: −84.2 to −36.7 ms). For the 28 spontaneous blinks it was, on average, −48.2 ms (±12.4 ms SD; range: −79.6 to −28.4 ms). There was no statistical difference between the two averages and we confirmed that this was a general feature among cells. Thus we merged the trigeminal and spontaneous blinks data for all the resumption-related measures. The middle histogram in Fig. 4D shows that the resumption of activity followed the eyelid maximum closing for all 61 blinks, with an average resumption lead of −50.2 ms (±12.2 ms SD; range: −84.2 to −28.4 ms). When we measured the cell resumption with respect to the maximum positive velocity peak of the blink reopening phase (asterisks in Fig. 4), the average resumption lead remained deeply negative, with mean −23.7 ms (±8.9 ms SD; range: −49.0 to −4.9 ms). Based on what we can infer from the human EMG data, the cell illustrated in Figs. 2 and 4 remained inhibited several 10s of milliseconds after all activity in the orbicularis oculi motoneurons has ceased. The OPNs cannot be responsible for the initiation of the OOMN interblink suppression. As shown in Table 4, this behavior was shared by all the OPNs we acquired from the two animals. As expected, all cells resumed their firing around the end of the saccade (SOFF). From the resumption leads with respect to the time of maximum eyelid closure (BLMAXCL) and with respect to the time of the maximum positive velocity peak of the blink reopening phase (BLMPPK), it is quite clear that the OPN population, as a whole, continued to remain inhibited for quite some time after any activity in the OOMNs and associated premotor network has likely ceased.
Table 4.
OPN resumption leads
| Animal A1 |
Animal A2 |
|||||
|---|---|---|---|---|---|---|
| Resumption Lead | Number of Cells | Mean ± SD, ms | Range, ms | Number of Cells | Mean ± SD, ms | Range, ms |
| SOFF | 33 | −0.3 ± 7.6 | −21.8 to 10.8 | 71 | 2.1 ± 7.6 | −19.1 to 13.6 |
| BLMAXCL | 27 | −64.0 ± 17.2 | −105.8 to −28.7 | 59 | −41.3 ± 14.6 | −74.2 to 1.3 |
| BLMPPK | 27 | −32.8 ± 14.9 | −72.1 to −7.5 | 59 | −9.2 ± 14.2 | −39.6 to 29.1 |
Values are in milliseconds. Combined averages, with associated SD and range, of the average OPN resumption leads for each cell with respect to saccade offset (SOFF) for saccadic trials and with respect to the time of maximum eyelid closing (BLMAXCL) and the time of the maximum positive peak velocity of the eyelid reopening phase (BLMPPK) for blink trials. Positive values indicate that the cell resumed before the movement event. The measures for trigeminal and spontaneous blinks are merged together.
If the OPN resumption for blinks is driven by the end of an indirect saccadic mechanism unrelated to the end of the OOMN activity, we expected a similar relationship between saccades and blinks in the velocity of the resumption of the firing to baseline. Table 5 reports the average OPN population behavior during the first six ISIs after pause end, expressed as percentage of the firing rate with respect to the estimated baseline. As was observed for pause onset, only the very first ISI showed a consistently lower firing rate than the estimated baseline. Animal A2, after the first ISI, presented a rebound effect above baseline, often significant. Animal A1 presented a more gradual buildup for blinks, but already the second ISI was on average 87.7% of baseline. Thus as for saccades, the resumption for blinks was quite rapid. In animal A2, there was a strong linear correlation between the saccadic leads and the blink resumption leads with respect to the time of maximum eyelid closure (slope: 1.30, ±0.19 SE of the slope, t = 7.0, n = 59 cells). This correlation did not reach significance in animal A1 (slope: 0.93, ±0.39 SE of the slope, t = 2.4 equivalent to P = 0.02, n = 27 cells). Cells that resumed earlier with respect to saccadic offset often resumed earlier also with respect to the time of maximum eyelid closure during blinks. We interpreted this result as the indirect effect of a timing relationship, weaker in animal A1, between the time of maximum eyelid closure and the end of this putative blink-related saccadic process. This process, being saccadic, shares the same variability in resumption leads between cells found for visually driven saccades, which we can observe as an indirect correlation between saccadic leads and blink resumption leads with respect to the time of maximum eyelid closure.
Table 5.
Postpause OPN behavior
| Trial Type | ISI + 1 Mean ± SD | ISI + 2 Mean ± SD | ISI + 3 Mean ± SD | ISI + 4 Mean ± SD | ISI + 5 Mean ± SD | ISI + 6 Mean ± SD |
|---|---|---|---|---|---|---|
| A. Animal A1 | ||||||
| Sacc (31) | 87.3* ± 23.7 | 107.3 ± 15.9 | 107.0+ ± 12.4 | 108.5+ ± 9.9 | 108.4+ ± 8.3 | 109.3+ ± 8.8 |
| Blink (27) | 61.4* ± 12.1 | 87.7* ± 17.6 | 90.3* ± 10.9 | 94.8 ± 12.2 | 95.7 ± 11.4 | 98.1 ± 11.5 |
| B. Animal A2 | ||||||
| Sacc (71) | 79.2* ± 15.0 | 101.4 ± 12.0 | 101.8 ± 8.4 | 102.6+ ± 7.3 | 103.8+ ± 7.3 | 104.2+ ± 6.8 |
| Blink (59) | 71.1* ± 15.6 | 104.2 ± 16.7 | 108.1+ ± 12.5 | 111.1+ ± 12.2 | 112.3+ ± 12.2 | 113.9+ ± 14.6 |
Combined averages, with associated SD, of the averages for each cell of the firing rate during the first six interspike intervals after pause offset, expressed as a percentage of the firing rate with respect to the estimated baseline. The numbers in parentheses in the Trial Type column are the numbers of cells used for the average computation. The asterisks indicate that the average was significantly <100% and the crosses indicate that the average was significantly >100%. The measures for trigeminal and spontaneous blinks are merged together.
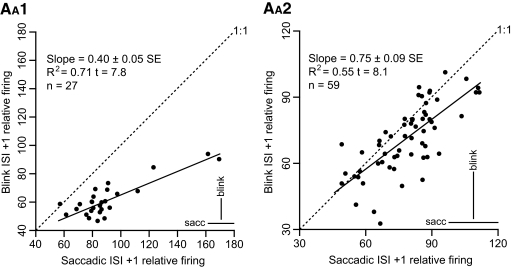
Additional evidence for a blink-related saccadic process controlling the OPN resumption can be inferred from the relationship illustrated in Fig. 5, which focuses on the first resumption ISI. The two panels demonstrate a significant correlation between the normalized firing frequency with respect to baseline of the first resumption ISI (ISI +1) for saccades (x-axis) and blinks (y-axis), for animal A1 and animal A2, respectively. The slope for animal A1 was 0.40 and for animal A2 it was 0.75. Cells that had a slow resumption after the pause for saccades were also slow to resume after a blink and vice versa. The slopes, particularly for animal A1, were lower than the 1:1 ratio, indicating that cells with faster saccadic resumption rates also resumed faster for blinks, but not as fast. This slower resumption rate for blinks was limited to the first ISI. As illustrated in Table 5, the second ISI was close or even above baseline for both saccades and blinks. The SDs of the normalized frequencies were also similar between saccades and blinks, as shown by the bottom right bars in the panels in Fig. 5.
Fig. 5.
Relative interspike interval (ISI) +1 firing: cell averages. The 2 panels illustrate the average relative firing with respect to baseline of the first ISI after the first resumption spike for saccades on the x-axis and for blinks on the y-axis for the 27 cells from animal A1 (AA1) and for the 59 cells from animal A2 (AA2) for which we have both average values (n ≥ 8). The 2 panels have different scaling factors for clarity. The bars on the bottom right corners report the average of the SDs of the resumption lead subsets, as indicators of the between-trials scatter of the data. The results of the linear regression are reported on the top left corners of the plots. Cells with faster initial resumption for saccades also showed faster initial resumption for blinks.
Our OPN pause offset analysis presents two major findings. The first is that the OPN resumption occurred well after any activity in the OOMNs and, by consequence, in the premotor network controlling the OOMNs, has ended. The second is that the similarities of the OPN resumption modalities between saccades and blinks were quite remarkable. We see this as evidence that the event that controls the end of the OPN pause during blinks is saccadic in nature.
Eyelid and eye transient drifts
Are the onsets of the monotonic drifts observed for the eyelid and for the eye transients (Fig. 1), and which follow their more dynamic phases, reliable indicators of the resumption of activity of the OPNs? As described in methods, we fitted these late drifts with single time constant exponential functions.
EYELID REOPENING DRIFTS.
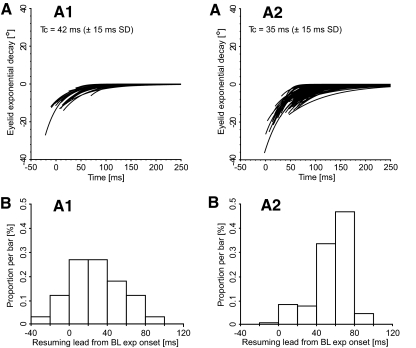
Figure 6 illustrates the exponential fits of the monotonic drifts of the eyelid position traces for the blinks of a cell data set from animal A1 (Fig. 6AA1) and from a cell data set from animal A2 (Fig. 6AA2). The default value (t → ∞) of the exponential fits is artificially set to zero for clarity. Both trigeminal and spontaneous blinks are included and only the traces with a clear breaking point in the eyelid velocity dynamics (56 and 63% of all traces, respectively) are reported. The traces are synchronized with respect to the first resumption spike after the pause in that trial. Figure 6, BA1 and BA2 are the associated distribution histograms of the resumption lead values. Positive values mean that the onset of the exponential drift followed the first resumption spike. For animal A1 the average lead value was 29.1 ms (±26.8 ms SD; range: −21.3 to 82.3 ms, n = 34 trials), whereas for animal A2 the average lead value was 55.6 ms (±18.6 ms SD; range: −2.2 to 89.2 ms, n = 160 trials). In the first data set (Fig. 6, AA1 and BA1), the average value would support a causal association, albeit somewhat delayed, between the start of the eyelid exponential drift and the resumption of the cell firing, but the scatter in the values is too pronounced to support such a conclusion. The second data set had most of the lead values in the 40 to 80 ms range, which would indicate that the effect of the cell resumption is seen on the eyelid trace far too late to be causally related. Extending the lead measures to all cells, the histograms in Fig. 7, AA1 and AA2 show that, for many cells in both animals, the onsets of the eyelid exponential drifts occurred too late to be causally associated with the OPN resumption. As before, both trigeminal and spontaneous blinks were included and only trials with a clear onset of the eyelid monotonic drift on the velocity trace were used to compute each cell average. For animal A1, the population average value was 37.2 ms (±19.6 ms SD; range: −4.5 to 64.4 ms, n = 29 cells, cell average acceptance ratio: 58% of trials). A paired t-test showed that the average OPN resumption lead with respect to the onset of the eyelid exponential drifts in animal A1 was 37.9 ms (±19.8 ms SD of the difference, n = 29 cells) longer than the average saccadic resumption lead. There was no correlation between the saccadic and the eyelid lead values. Animal A2 showed very similar results, with the average value of the OPN resumption lead from the onset of the eyelid exponential drifts equal to 44.5 ms (±44.4 ms SD; range: 0.4 to 94.8 ms, n = 59 cells, cell average acceptance ratio: 78% of trials). The paired t-test comparison with the average saccadic resumption leads resulted in a value of 42.5 ms (±19.4 ms SD of the difference, n = 59 cells). These data add further evidence that there is no direct functional relationship between the eyelid temporal profile and the OPN behavior and that none of the eyelid events can be used as a reliable estimate of when the OPN population returns to baseline after the pause.
Fig. 6.
Eyelid exponential drifts: single trials. AA1 illustrates, for the blinks (n = 34) of a cell data set from animal A1, the exponential fits of the eyelid position drifts, as defined in methods. AA2 illustrates the fits for the blinks (n = 160) of a cell data set from animal A2. Both trigeminal and spontaneous blinks are included and only the trials with a clear breaking point in the eyelid velocity dynamics (56 and 63% of all eyelid traces, respectively) are reported. The traces are synchronized with respect to the first resumption spike after the pause. The default value (t → ∞) of the exponentials is artificially set to zero for clarity. The bottom panels are the distribution histograms of the associated resumption lead values. Positive values indicate that the onset of the exponential drifts followed the first resumption spike of the cell. Tc is the time constant average (±SD) of the exponential drifts.
Fig. 7.
OPN resumption leads from the onsets of the eyelid exponential drifts: cell averages. Histograms of the distributions of the OPN resumption leads with respect to the onset of the eyelid exponential drifts for the 29 cells from animal A1 (AA1) and the 59 cells from animal A2 (AA2) for which we have data. Positive values mean that the onset of the eyelid exponential drift followed, on average, the first resumption spike of the cell after the pause.
EYE TRANSIENT DRIFTS.
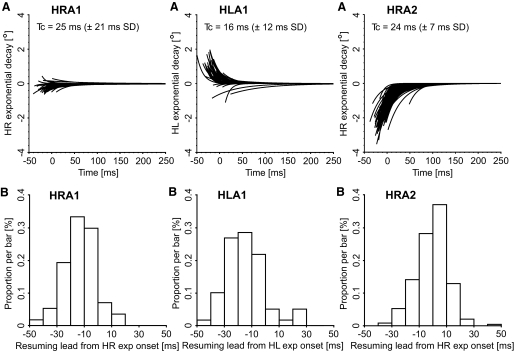
We have shown that the onset of the eye transients for trigeminal blinks, and for many spontaneous blinks, occurs before the onset of the blink and of the OPN pause. This indicates their nonsaccadic nature, in the sense that they cannot be delivered to the motoneurons of the extraocular muscles by the saccadic short-lead burst neurons. We proposed that this early eye transient component is perhaps linked to the globe retraction during blinks (Collewijn et al. 1985; Evinger and Manning 1993; Evinger et al. 1984) and our data would indicate that the retraction commands bypass the saccadic system. This observation does not rule out a subsequent activation of the saccadic system, perhaps in response to the perturbation in eye alignment caused by the globe retraction. With trigeminal and spontaneous blinks being highly stereotyped movements, it seems reasonable to expect some kind of adaptive, short-latency eye realigning mechanism, with the saccadic system likely the main player. In fact, our OPN resumption analysis supports a blink-related saccadic activation. At the end of the OPN pause, any residual mismatch between the tonic forces acting on the globe will produce a passive exponential-like drift toward equilibrium, similar to what is observed during saccadic pulse-step mismatches (Optican and Robinson 1980). Figure 8 illustrates, for the same cells used in Fig. 6 and with the same layout, the exponential fits of the horizontal eye transient drifts, whereas Fig. 9 shows the exponential fits of the vertical eye transient drifts. Both trigeminal and spontaneous blink trials are used in these plots. For animal A2, we have only the right eye data. All traces are aligned with the time of the first resumption spike of the cell in that trial. The default value (t → ∞) of the exponentials is artificially set to zero for clarity.
Fig. 8.
Horizontal exponential drifts of the eye transients: single trials. Same cells and layout as those in Fig. 6. AHRA1 illustrates the horizontal exponential fits of the eye position drifts for the right eye in animal A1 (n = 58), whereas AHLA1 illustrates the horizontal exponential fits of its left eye in the same trials (n = 58). AHRA2 illustrates the horizontal exponential fits for the right eye in animal A2 (n = 243). Both trigeminal and spontaneous blinks are included and only the blinks with clear breaking points in both the horizontal and vertical eye velocity dynamics—and in both eyes in animal A1—(95% and 97% of all blinks, respectively) are reported. The traces are synchronized with respect to the first resumption spike after the pause. The default value (t → ∞) of the exponentials is artificially set to zero for clarity. The bottom panels are the distribution histograms of the associated resumption lead values. Positive values indicate that the onset of the exponential drifts followed the first resumption spike of the cell. Tc is the time constant average (±SD) of the exponential drifts.
Fig. 9.
Vertical exponential drifts of the eye transients: single trials. AVRA1 illustrates the vertical exponential fits of the eye position drifts for the right eye in animal A1 (n = 58), whereas AVLA1 illustrates the vertical exponential fits of its left eye (n = 58). AVRA2 illustrates the vertical exponential fits for the right eye in animal A2 (n = 243). Same layout and same trials as those of Fig. 8. The vertical histograms were narrower and shifted later in time than the horizontal histograms in Fig. 8.
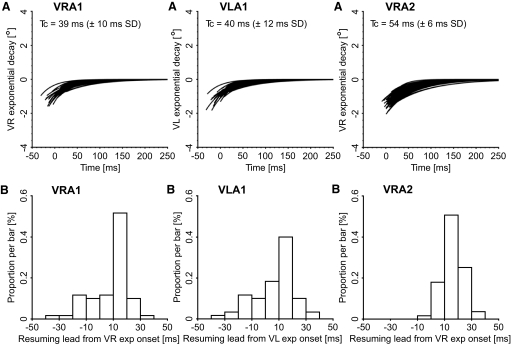
By comparing the horizontal exponential drifts (Fig. 8) with the vertical exponential drifts (Fig. 9), three significant differences are readily apparent. The first is that the horizontal drifts were more variable. Several publications report that the eyes transiently move inward and downward during blinks (Bergamin et al. 2002; Bour et al. 2000; Collewijn et al. 1985), although Goossens and Van Opstal (2000a,b) report outward and upward eye transients. In our ±15° oculomotor range, most horizontal transients in the left eye of animal A1 were inward and in the right eye of animal A2 all of them. Accordingly, the exponential drifts were mostly outward (i.e., abducting). The right eye in animal A1 had smaller and more irregular horizontal exponential drifts. All three horizontal data sets showed a weak, but significant, dependence of the drift amplitude with respect to the horizontal eye position at drift onset. The difference between HRA1 and HLA1 was mostly a positional shift of the intercept value of this correlation. The vertical transients in our oculomotor range were always downward and very similar in the three measured eyes. The exponential drifts were therefore upward, with no dependence on the horizontal or vertical eye position at drift onset in our range of eye positions. For all data sets, the vertical time constants were approximately twice the horizontal ones (Tc values in the top panels) and the difference was, from a paired t-test, strongly significant in all three eyes. The most puzzling result was that, for all three eyes, the horizontal drift onsets occurred much earlier than the vertical ones and there was no time correlation between the horizontal and the vertical onsets in the same trial (compare the resumption lead distributions in the bottom panels of Figs. 8 and 9). For the 58 trials (95% of total trials) from the cell from animal A1, the HR lead was −13.5 ms (±12.0 ms SD; range: −40.6 to 17.4 ms) and the VR lead was 7.6 ms (±13.6 ms SD; range: −30.9 to 30.1 ms). Very similar results were obtained for HL, with average −14.1 ms (±14.6 ms SD; range: −49.7 to 27.4 ms), and VL, with average 6.4 ms (±14.9 ms SD; range: −31.8 to 36.7 ms). For the 243 trials (97% of total trials) from the cell from animal A2, HR had an average of −1.3 ms (±11.5 ms SD; range: −37.8 to 48.8 ms), and VR had an average of 15.5 ms (±7.9 ms SD; range: −8.8 to 38.1 ms). As indicated by the percentage of accepted trials from these two cells, these eye transient timing measures were very robust, with the onset of the exponential drifts clearly identifiable. In fact, for the 88 cells, we had data for 95.1% of the trials. The time shift between the horizontal and the vertical exponential onsets is a behavioral property and, accordingly, showed little variation between sessions. For animal A1, the overall population average of the right-eye time shift between the horizontal and the vertical exponential onsets was 21.7 ms (±12.2 ms SD of the difference; n = 29 cells) and of the left-eye shift was 35.8 ms (±15.5 ms SD). For animal A2, the population average of the right-eye time shift was 16.7 ms (±3.6 ms SD; n = 59 cells). The third and most important result was that the histograms of the resumption leads for the vertical exponential drifts presented a clear peak in the 10 to 20 ms resumption lead bin, consistent with a synchronization of this onset with the resumption of the cell. The onsets of the horizontal drifts were in negative territory (animal A1) or near zero (animal A2), indicating they preceded the OPN resumption and the distribution, overall, was more scattered. The comparison with the eyelid data, with average drift onsets occurring later in time with respect to the eye drifts, matches the observation of Collewijn et al. (1985) that the eye transients have a shorter duration than that of the associated eyelid movement.
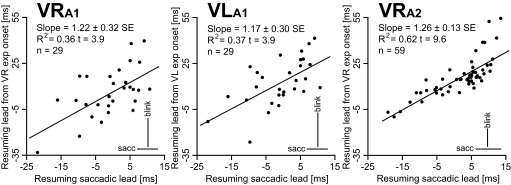
Our hypothesis is that the onset of the vertical exponential drift indicates the end of the saccadic phase of the eye transient and the resumption of activity in the OPNs. A key feature that would support a causal relationship between the resumption of the OPNs and the onset of the vertical exponential drifts is the scatter in the lead values of the vertical drifts to parallel the scatter in the saccadic resumption lead times. This is illustrated in Fig. 10, with the average (Pythagorean) saccadic resumption lead of each cell plotted on the x-axis and the average resumption lead of the onset of the vertical exponential drift on the y-axis. The correlation between the saccadic and the vertical exponential drift leads was highly significant in all three data sets. As shown in Fig. 10, the slopes were also remarkably similar. The overall averages, SDs, and ranges of the cell averages are reported in the top portion of Table 6. A positive value means that the OPN resumed its firing before the onset of the respective event. A paired t-test showed that the vertical exponential drift onsets occurred later with respect to saccadic offset for matching OPN resuming times. These values, together with the SD of the difference, are reported in the bottom portion of Table 6. The similarity of these values with the average OPN pause onset leads for saccades (Table 1), which can be seen as the time delay between a change in the OPN firing (pause onset) and its effect on the eye traces (saccade onset), further supports a causal linkage between OPN pause offset and vertical exponential drift onsets. These results are quite extraordinary, considering how variable the saccadic resumption lead is at the trial-by-trial level and between cells and the potential pitfalls in determining the onsets of such small exponential drifts.
Fig. 10.
Comparison between saccadic and eye vertical drift resumption leads. For each cell, the average saccadic resumption lead is plotted on the x-axis and the average resumption lead of the vertical exponential drift on the y-axis. A positive value means that the OPN resumed its firing before saccadic offset and before the onset of the exponential drift of the vertical eye transient, respectively. VRA1 illustrates the right eye data for the 29 cells from animal A1 for which we have data and VLA1 the left eye data. VRA2 reports the right eye data for the 59 cells from animal A2 for which we have data. We have no data for the left eye. The bars on the bottom right corners report the average of the SDs of the resumption lead subsets, as indicators of the between-trial scatter of the data. The results of the linear regression are reported on the top left corners of the plots. Cells that resumed their firing later with respect to saccadic offset also resumed their firing later with respect to the onset of the vertical eye drifts and vice versa.
Table 6.
OPN resumption lead times with respect to the onsets of the vertical exponential drifts
| Animal A1 (n = 27 Cells) |
Animal A2 (n = 59 Cells) |
|||
|---|---|---|---|---|
| Resumption Lead | Mean ± SD, ms | Range, ms | Mean ± SD, ms | Range, ms |
| SOFF | −0.3 ± 7.6 | −21.8 to 10.8 | 2.0 ± 7.7 | −19.1 to 13.6 |
| REVDONS | 5.8 ± 16.1 | −33.3 to 39.5 | 16.1 ± 12.6 | −10.0 to 55.1 |
| LEVDONS | 12.4 ± 15.3 | −26.5 to 41.3 | — | — |
| Time difference between vertical drift onset and saccadic offset for matching OPN resumption times | ||||
| REVDONS | 6.6+ ± 13.0 | 14.1 ± 8.2 | ||
| LEVDONS | 13.1 ± 12.3 | — | ||
Values are in milliseconds. The top section of the table shows combined averages, with associated SD and range, of the average OPN resumption leads for each cell with respect to saccade offset (SOFF) for saccadic trials and with respect to the onset of the vertical exponential drifts of the right eye (REVDONS) and of the left eye (LEVDONS) for blink trials. The bottom section shows the combined differences, with associated SD of the difference, between the averages for each cell of the vertical drift onset and of the saccadic offset for matching OPN resumption times. Positive values indicate that the cell resumed before the movement event. The measures for the trigeminal and spontaneous blinks are merged together. The cross indicates that the difference was not significantly different from zero.
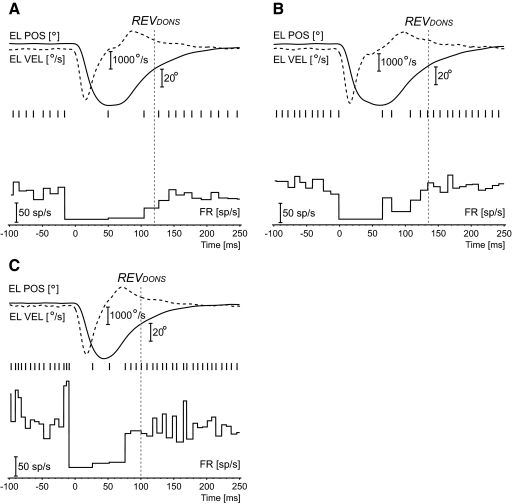
Qualitative evidence for two inhibitory phases of the OPNs during blinks
Some blink trials presented firing patterns that were slightly different from the ones described earlier. Only three cells in animal A1 and only four cells in animal A2 presented these patterns for >10% (max 26%) of their trials. The other cells had none or one or two occurrences at most. Three examples are illustrated in Fig. 11. All are from animal A2, but similar patterns were observed in animal A1. Time zero identifies blink onset. By far, the most common pattern was the one described in Fig. 11A. These trials had pause onset and pause offset that were not different from normal trials, but with a single spike around the time of maximum eyelid closing or slightly after and always well after the maximum (negative) peak velocity of the closing period. Figure 11B illustrates a less common pattern, from another cell, of two intermediate spikes. Two cells, one in animal A1 and one in animal A2, presented randomly occurring trials with a firing pattern like the one illustrated in Fig. 11C. These two cells again presented a normal pause onset and a brisk resumption of their firing that matched the resumption in the trials with complete OPN inhibition, but there was sometimes a low-level activity starting 10–15 ms after the maximum (negative) peak velocity of the closing period. This activity could just be a pair of spikes, as in Fig. 11C, or a series of spikes. The firing patterns in Fig. 11, A and B would suggest that the OPN inhibition during blinks is comprised of two phases, with the OPN able to sometimes fire one or, less likely, two spikes during the transition between the two. The pattern in Fig. 11C would be consistent with the second phase being too weak to fully maintain the inhibition of the cell. The vertical dotted lines indicate the onset of the right eye vertical exponential drift (REVDONS), well predicting the full resumption of the cell.
Fig. 11.
Examples of intermediate spikes. The panels illustrate 3 examples of intermediate spikes, which randomly occurred in some blink trials in some cells. In the first 2 examples, from 2 different cells, an intermediate spike, most often alone (A), but sometimes followed by another one (B), was roughly centered at the time of maximum closing of the eyelid or soon after. The isolated single spike, like the example in A, was the most common pattern. Only 2 cells, one in animal A1 and one in animal A2, had the first intermediate spike following the maximum (negative) peak velocity of the closing phase by 10–15 ms, as in C. This first spike was followed by a low-frequency activity until the more typical resumption, which was easily seen as an abrupt increase in the firing rate. The vertical dotted lines indicate the onset of the right eye vertical exponential drift (REVDONS).
DISCUSSION
Our results quantitatively confirm that OPNs do stop for both trigeminal and spontaneous blinks. The quite unexpected finding is that the timing of the OPN pause shows that these cells play no direct role in blink onset or in the interblink transition from the end of the burst of activity in the OOMNs to the resumption of the LPSMN firing.
Blink triggering
Intracellular recordings in the alert cat (Yoshida et al. 1999) have shown that the pause of the OPNs during saccades has two components. The first component is driven by a very fast trigger signal of fixed duration, which is responsible for the pause onset. The second component is driven by an inhibitory signal that closely follows the firing profile of saccadic burst neurons. This finding is in agreement with the latch theory (Moschovakis 1994; Moschovakis et al. 1996; Robinson 1975; Scudder 1988). Once the excitatory short-lead burst neurons are freed from OPN inhibition by the action of the trigger, which quickly drives the OPNs to silence, the reciprocal action of the inhibitory short-lead burst neurons on the OPNs maintains the inhibition of the OPNs until the end of the movement. Accordingly, we found, as in previous reports, that there was little scatter in the pause onset lead times for saccades, with the onset of the pause being causally linked to the start of the saccade, and a very small prepause slowing of the cell firing up to the last ISI before pause onset (Table 3).
Our evidence that blinks can start with the OPNs still firing at or near baseline levels excludes the possibility that OPN activity, per se, directly controls the occurrence or the progress of blinks. A time-locked triggering signal preceding the onset of blinks was clearly absent in our OPN data. The onset of the OPN pause occurred, for trigeminal blinks, too late for the OPNs to gate the blink circuitry in a manner similar to their inhibitory role in the saccadic system (Figs. 2 and 3). For spontaneous blinks, there was no direct correlation between OPN pause onset and blink onset. This difference between saccades and blinks seems surprising in light of the findings of Mays and Morrisse (1995) that electrical stimulation of the OPNs can suppress the generation of trigeminal blinks with currents only slightly higher than the currents needed to stop the generation of saccades. It is possible that their electrical stimulation was activating other nearby raphe nuclei or their afferents/efferents. The nucleus raphe magnus lies just ventral of the nucleus raphe interpositus (Horn et al. 1994) and has a known role in rat blink excitability (Basso and Evinger 1996). A suppressive effect caused by the antidromic stimulation of cells projecting to the OPNs is also possible.
During air-puff trigeminal blinks in monkeys, the inhibition of the motoneurons of the LPS muscle (Fuchs et al. 1992) precedes blink onset by 29.2 ms on average (range: 10 to 59 ms). Our data show that the OPNs paused <2 ms before the onset of trigeminal blinks. Thus as for the onset of the OOMN burst, the onset of the OPN pause also follows the inhibition of the LPSMNs.
OPN pause onset
If OOMN onset precedes OPN pause onset, it is reasonable to ask whether an efference copy of the OOMN activity during the lid closure inhibits the OPNs. It is not known when the activity in the OOMNs starts with respect to blink onset in primates, but the pause leads in Fig. 2D and in Fig. 3, AA1 and AA2 have values compatible with all OPN cells being fully inhibited soon after the onset of the burst in the OOMNs. The resumption leads also indicate that no cell resumed firing earlier than the end of the activity in the OOMNs, with the vast majority resuming much later. This also applies to the two cells with the trials with the earliest resumption times (Fig. 11C), where the first resumption spike always occurred after the maximum negative peak velocity of the closing phase. Even in these extreme cases, the OOMNs were likely near the end of their firing. The tight timing correlation between blink onset and pause onset for trigeminal blinks, similar to that observed for saccades—compare the averages of the SDs for saccades and trigeminal blinks in Fig. 3, AA1 and AA2—also supports the idea that the onset of the OPN pause for trigeminal blinks is driven by the onset of the blink. There is no anatomical or electrophysiological evidence for a pathway, direct or indirect, from the OOMNs to the OPNs in the monkey (Langer and Kaneko 1990). Perhaps this is achieved through inhibitory relay cells similar to the inhibitory burst neurons for saccades, or some other blink-related cells causally linked to blink onset, such as the Vi/Vc group (Henriquez and Evinger 2007). Although it seems reasonable to think that these hypothetical blink-related inhibitory relay cells might be the same cells that also inhibit the LPSMNs during the closing of the eyelid, Fuchs et al. (1992) reported that, for air-puff trigeminal blinks in the monkey, LPSMNs stop firing 29.2 ms, on average, before blink onset. There is therefore a clear timing discrepancy between the onset of the pause of the LPSMNs and the onset of the pause of the OPNs for trigeminal blinks. The observation that the onset of the LPSMN inhibition precedes blink onset and outlasts the time of maximum eyelid closing also applies to spontaneous blinks (Bjork and Kugelberg 1953). Wang et al. (2009) recently reported that the central mesencephalic reticular formation, a major target of the superior colliculus, has a direct inhibitory action on the OPNs. Unfortunately, the behavior of this formation during blinks is not known.
This putative reversal of triggering roles between OPNs and blinks clearly does not apply for spontaneous blinks, with pause onsets that can precede blink onsets by several 10s of milliseconds. In this case, some other mechanism, not linked to blink onset, drives the OPNs to inhibition. Fuchs et al. (1992) reported a gradual decline in LPSMN activity that precedes the full pause by 40 ms on average, even for trigeminal blinks. For spontaneous blinks, we often observed some small eye and/or eyelid drifts, usually downward, prior to the main onset. These premotor effects were too gradual to correlate with the very fast prepause OPN slowing (Table 3). An attempt to predict OPN onset from the amplitude of these preblink eye and eyelid drifts also failed to show any meaningful correlation.
Interestingly, the correlation between the pause onset of the fixation neurons in the rostral superior colliculus and saccade onset (Everling et al. 1998) is also quite poor and shifted in time, with the OPNs acting as downstream synchronizing elements for saccades by means of an external trigger signal. Is the lack of a trigger signal for spontaneous blinks simply unmasking poor timings in the upstream blink pathways acting on the OPNs? A key clinical observation is the necessity of blinking to elicit saccades in Huntington's patients (Topper et al. 1998). This suggests that at least part of the OPN pause is driven by the blink circuitry, irrespective of the loss of a proper saccadic triggering mechanism. An exclusively saccadic mechanism controlling the OPN behavior during blinks is therefore unlikely.
Resumption of firing of the OPNs
For saccades, the OPN resumption occurs around saccadic offset. Human EMG data (VanderWerf et al. 2003) indicate that the OO activity ends around the time of maximum eyelid closing. The OPNs resumed, on average, 64.0 ms (animal A1) and 41.3 ms (animal A2) after this point (Fig. 4 and Table 4), which is much too late for the two to be functionally related. An additional mechanism maintains the inhibition of the OPNs for quite some time after the OOMN command has ended. An obvious candidate for controlling the OPN resumption of activity during the reopening phase of the blink is the circuitry controlling the LPSMNs. Two alternative mechanisms associated with blinks, but not directly related to the motor commands to the eyelids, are the retraction of the globe during blinks and the saccadic compensatory responses to the perturbation in visual fixation caused by the eye transients.
LPSMN RESUMPTION.
Fuchs et al. (1992) reported that the onset of the LPSMN resumption of firing ranged from 26 ms before the first detectable upswing of eyelid reopening to 58 ms after, with an average value of 23 ms after. With our very brief air puffs, the first detectable upswing in the eyelid trace practically matched the time of the maximum eyelid closing (Fig. 4). The OPN resumption with respect to this point ranged from 1.3 ms before to 105.8 ms after, with an average value 48.4 ms after. The two resumption timings are not so dissimilar as to exclude a shared resumption mechanism, but we found that the OPN resumption rate was much faster than the LPSMN resumption rate and comparable to the one observed for saccades.
Could the OPNs control the resumption of the LPSMNs instead? Both trigeminal and spontaneous blinks are extremely repeatable trial by trial and we expect the resumption of the firing of the LPSMNs, although quite scattered in time (Fuchs et al. 1992), to be, on average, relatively time invariant with respect to the time of maximum eyelid closing. The large between-trials and between-cells scatter of the OPN resumption leads from the maximum eyelid closing (Fig. 4 and Table 4) suggests that we are in the presence of uncorrelated events.
GLOBE CO-CONTRACTION.
During blinks, the globe is transiently pulled into the eye socket by a co-contraction of some of the extraocular muscles. Indirect studies have concluded that most of the co-contraction in primates is driven by the superior and inferior vertical rectus muscles and that the eyelid movement and the co-contraction appear to be driven by a common neural mechanism (Bergamin et al. 2002; Bour et al. 2000; Evinger and Manning 1993).
Our data offer insights about the nature of the eye transients and their relationship with the co-contraction. The pause of the OPNs associated with the blink has been considered the most likely origin for the transient eye movements observed during blinks (Mays and Morrisse 1995). Any activity present in the upstream saccadic circuitry is, during the OPN pause, freely transmitted downstream. As already observed in humans (Bergamin et al. 2002), we found that monkey eye transients preceded blink onsets, with both trigeminal and spontaneous blinks showing similar lead times. The timing correlation between blink onsets and eye transient onsets was very strong, indicating they likely originated from the same motor command. Although we did not quantify the initial segment of the transients, several reports (Bergamin et al. 2002; Bour et al. 2000) indicated that they are very stereotyped and depend on the eye position at blink onset. This makes the eye transients as the result of a blink-unrelated upstream saccadic activity unmasked by the OPN pause quite unlikely. Furthermore, our onset analysis showed that, for trigeminal blinks, the eye transients had an even larger negative lead with respect to OPN pause onset than eyelid movements. Thus at least the initial component of the eye transients occurred while the saccadic burst neurons were still fully inhibited by the OPNs. We propose that the initial component of the eye transients is an indirect result of the globe retraction, with the co-contraction pulse delivered to the motoneurons of the vertical rectus muscles by nonsaccadic circuits. The saccadic system is exquisitely organized in an agonist–antagonist arrangement. Sending the co-contraction command directly to the motoneurons overrides this mechanism and also avoids the integration of this pulse by the neural integrator. The duration of this command is not known, but if it is comparable with the duration of the command driving the OOMNs, the end of the co-contraction has no role in the OPN resumption.
SACCADIC REACTION TO THE EYE TRANSIENTS.
The co-contraction drives the eyes off the positional equilibrium of forces determined by the oculomotor neural integrator. Inhibition of the OPNs would be the required first step for a corrective saccade. These saccades are different from the interblink voluntary saccades that result in a net change in eye position. For trigeminal blinks elicited by unpredictable air puffs, this saccade-related OPN inhibition will likely follow blink onset and perhaps be masked by a true blink-related inhibition of the OPNs that is time-locked to blink onset. For spontaneous blinks, which are initiated by the subject, there is time for the saccadic system to start to prepare for the disturbance—both trigeminal and spontaneous blinks are highly stereotyped movements—irrespective of when the blink will actually occur. The relatively asynchronous saccadic preblink activity for spontaneous blinks could generate the observed scatter in OPN onsets. A comparative analysis of the onset and amplitude of the vertical exponential drifts between trigeminal and spontaneous blinks showed no evidence for an earlier and more efficient saccadic correction of the eye transients for spontaneous blinks, with similar amplitude of the drifts after the resumption of the OPNs. Supporting the hypothesis of blink-related corrective saccades is the fact that some saccadic short-lead burst neurons fire weakly during blinks (Mays and Morrisse 1995) and that the resumption of activity in the OPNs is associated with the onset of the exponential drifts in the vertical eye traces (Fig. 9). This indicates that the vertical saccadic system is active during the blink up to the resumption of firing of the OPNs. If there was any saccadic activity in the horizontal burst neurons, it seemed to terminate before the OPN resumption.
A higher-level control of the end of the OPN pause for both trigeminal and spontaneous blinks with no direct linkage to any actual saccadic or blink motor command is also a possibility. OPNs receive direct connections from the frontal eye fields (Stanton et al. 1988) and from the supplementary eye fields (Shook et al. 1988). Imaging data (Bodis-Wollner et al. 1999; Yoon et al. 2005) show voluntary and spontaneous blink-related activation in the same areas.
Onsets of the eye exponential drifts as markers of OPN resumption of firing
One of the major goals of this study was to search for any element on the eyelid or eye transient traces that can be used as a reliable indicator of the resumption of the OPN activity. We concentrated our attention on the onset of the exponential drifts that follow the more dynamic phases of the eyelid reopening and of the eye transients. The breaking point that marks the onset of the exponential drift for the eyelids (Fig. 6) occurred too late to explain the OPN pause offset and contained too much scatter over the OPN population to be usable as a good predictor of OPN behavior (Fig. 7). We expected the eye transient behavior to be affected by the pause of the OPNs, since the pause determines when upstream saccadic commands, whether functionally associated with the blink or random, are transmitted downstream. Horizontal and vertical exponential drifts differed in many aspects. Horizontal drifts began prior to the resumption of OPN firing (Fig. 8), whereas vertical drifts began after the resumption of OPN firing (Fig. 9). Vertical time constants were also almost twice as long in both animals and the horizontal drifts showed much more variance in terms of timing and time constants. This may be related to the evidence that only the superior and inferior rectus muscles are associated with the globe retraction in primates, with the inward movements being mostly a passive effect of the co-contraction of the vertical rectus muscles. The fact that the amplitude of the horizontal exponential drifts correlates with the horizontal eye position, and thus with the alignment of the vertical rectus muscles with respect to the line of sight, further supports this view. The key observation was that the onset of the vertical exponential drifts tightly correlated with the resumption of the OPN activity, indicating the interruption of an ongoing saccadic activity. We described earlier that the onset of the eye transients precedes the onset of the OPN pause by several milliseconds, which requires the commands generating them to be delivered to the extraocular motoneurons outside the saccadic system. The resumption results show that a vertical saccadic component develops later in time and it is this component that is curtailed by the OPN resumption.
There is a known synergy between vertical eye movements and eyelids (Becker and Fuchs 1988) and we attempted to use this functional linkage to better understand the nature of the vertical eye drifts. The time constants of the final section of the eyelid reopening, which we fitted with a single exponential (Figs. 1 and 6), were very similar to the time constants of the vertical eye drifts (Fig. 9)—between 39 and 54 ms—and we estimated from the LPSMNs plots in Fuchs et al. (1992) that the time constant of the LPSMN resumption was around 47 ms. Does this mean that the late vertical eye drifts are neutrally encoded and drive the LPSMNs and, consequently, the later eyelid reopening, or vice versa? With the OPNs back to baseline firing, these eye signals would necessarily be delivered to the extraocular motoneurons outside the saccadic pathway. At the same time, the passive postsaccadic drifts associated with a saccadic pulse-step mismatch (Optican and Robinson 1980) also have a time constant around 40 ms, a value that the authors found quite puzzling, considering that the main time constant of the orbital mechanics is about 200 ms. In another report (Robinson 1968), at the end of a brief stimulation of the oculomotor nerve the eye returned to the initial position, with a vertical time constant of 33 ms and a horizontal time constant of 11 ms. The time constant values support either an active or a passive nature of these drifts and only direct recordings in the extraocular motoneurons during blinks will elucidate the nature of these drifts.
Our results open more issues than they solve regarding what drives OPN activity during blinks and leave many questions about saccade-blink interaction unanswered. However, we have verified that all OPNs do stop for blinks and that the onsets of the vertical exponential drifts during the reopening phase of the blink are a reliable indicator of the end of the OPN pause. The quite unexpected dissociation between the behavior of the OPNs and the OOMN firing, with an extended additional period of OPN inhibition after the end of the OOMN activity, indicates that there is a quite complex interaction between the blink and the saccadic circuitry. This clearly needs to be explored to understand the precise sequence of events during blinks and during combined blink/eye movements and how they are modified during pathologies involving abnormal blink or saccadic triggering.
GRANTS
This research was supported by National Eye Institute Grants R01 EY-017283 to C. Busettini and P30 EY-03039 (Core Grant) to the University of Alabama at Birmingham (UAB), Vision Science Research Center, and The EyeSight Foundation of Alabama Grant FY2006-2007-42 to C. Busettini. K. Schultz was a graduate student of the UAB Vision Science Graduate Program.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
We thank S. Hayley for computer programming, L. Millican and A. Yildirim for technical assistance, L. Phillips and M. Robbins for administrative assistance, and Drs. Paul May and Paul Gamlin for comments and suggestions.
REFERENCES
- Basso MA, Evinger C. An explanation for reflex blink hyperexcitability in Parkinson's disease. II. Nucleus raphe magnus. J Neurosci 16: 7318–7330, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso MA, Powers AS, Evinger C. An explanation for reflex blink hyperexcitability in Parkinson's disease. I. Superior colliculus. J Neurosci 16: 7308–7317, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker W, Fuchs AF. Lid–eye coordination during vertical gaze changes in man and monkey. J Neurophysiol 60: 1227–1252, 1988 [DOI] [PubMed] [Google Scholar]
- Becker W, King WM, Fuchs AF, Jürgens R, Johanson G, Kornhuber HH. Accuracy of goal-directed saccades and mechanisms of error correction. In: Progress in Oculomotor Research, edited by Fuchs AF, Becker W. Amsterdam: Elsevier/North-Holland, 1981, p. 29–37 [Google Scholar]
- Bergamin O, Bizzarri S, Straumann D. Ocular torsion during voluntary blinks in humans. Invest Ophthalmol Vis Sci 43: 3438–3443, 2002 [PubMed] [Google Scholar]
- Bjork A, Kugelberg E. The electrical activity of the muscles of the eyes and eyelids in various positions and during movement. Electroencephalogr Clin Neurophysiol 5: 595–602, 1953 [DOI] [PubMed] [Google Scholar]
- Bodis-Wollner I, Bucher SF, Seelos KC. Cortical activation patterns during voluntary blinks and voluntary saccades. Neurology 53: 1800–1805, 1999 [DOI] [PubMed] [Google Scholar]
- Bour LJ, Aramideh M, Ongerboer de Visser BW. Neurophysiological aspects of eye and eyelid movements during blinking in humans. J Neurophysiol 83: 166–176, 2000 [DOI] [PubMed] [Google Scholar]
- Busettini C, Mays LE. Pontine omnipause activity during conjugate and disconjugate eye movements in macaques. J Neurophysiol 90: 3838–3853, 2003 [DOI] [PubMed] [Google Scholar]
- Busettini C, Mays LE. Saccade–vergence interactions in macaques. I. Test of the omnipause multiply model. J Neurophysiol 94: 2295–2311, 2005 [DOI] [PubMed] [Google Scholar]
- Busettini C, Williams CR, Schultz KP. Behavior of saccadic omnipause neurons during blinks in the macaque. Soc Neurosci Abstr 345.26, 2006 [Google Scholar]
- Büttner-Ennever JA, Cohen B, Pause M, Fries W. Raphe nucleus of the pons containing omnipause neurons of the oculomotor system in the monkey, and its homologue in man. J Comp Neurol 267: 307–321, 1988 [DOI] [PubMed] [Google Scholar]
- Cohen B, Henn V. Unit activity in the pontine reticular formation associated with eye movements. Brain Res 46: 403–410, 1972 [DOI] [PubMed] [Google Scholar]
- Collewijn H, van der Steen J, Steinman RM. Human eye movements associated with blinks and prolonged eyelid closure. J Neurophysiol 54: 11–27, 1985 [DOI] [PubMed] [Google Scholar]
- Everling S, Paré M, Dorris MC, Munoz DP. Comparison of the discharge characteristics of brain stem omnipause neurons and superior colliculus fixation neurons in monkey: implications for control of fixation and saccade behavior. J Neurophysiol 79: 511–528, 1998 [DOI] [PubMed] [Google Scholar]
- Evinger C, Kaneko CRS, Fuchs AF. Activity of omnipause neurons in alert cats during saccadic eye movements and visual stimuli. J Neurophysiol 47: 827–844, 1982 [DOI] [PubMed] [Google Scholar]
- Evinger C, Manning KA. Pattern of extraocular muscle activation during reflex blinking. Exp Brain Res 92: 502–506, 1993 [DOI] [PubMed] [Google Scholar]
- Evinger C, Manning KA, Pellegrini JJ, Basso MA, Powers AS, Sibony PA. Not looking while leaping: the linkage of blinking and saccadic gaze shifts. Exp Brain Res 100: 337–344, 1994 [DOI] [PubMed] [Google Scholar]
- Evinger C, Shaw MD, Peck CK, Manning KA, Baker R. Blinking and associated eye movements in humans, guinea pigs, and rabbits. J Neurophysiol 52: 323–339, 1984 [DOI] [PubMed] [Google Scholar]
- Fuchs AF, Becker W, Ling L, Langer TP, Kaneko CRS. Discharge patterns of levator palpebrae superioris motoneurons during vertical lid and eye movements in the monkey. J Neurophysiol 68: 233–243, 1992 [DOI] [PubMed] [Google Scholar]
- Fuchs AF, Robinson DA. A method for measuring horizontal and vertical eye movement chronically in the monkey. J Appl Physiol 21: 1068–1070, 1966 [DOI] [PubMed] [Google Scholar]
- Gandhi NJ, Bonadonna DK. Temporal interactions of air-puff-evoked blinks and saccadic eye movements: insight into motor preparation. J Neurophysiol 93: 1718–1729, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi NJ, Keller EL. Spatial distribution and discharge characteristics of superior colliculus neurons antidromically activated from the omnipause region in monkey. J Neurophysiol 78: 2221–2225, 1997 [DOI] [PubMed] [Google Scholar]
- Gnadt JW, Lu S-M, Breznen B, Basso MA, Henriquez VM, Evinger C. Influence of the superior colliculus on the primate blink reflex. Exp Brain Res 116: 389–398, 1997 [DOI] [PubMed] [Google Scholar]
- Goossens HHLM, Van Opstal AJ. Blink-perturbed saccades in monkey. I. Behavioral analysis. J Neurophysiol 83: 3411–3429, 2000a [DOI] [PubMed] [Google Scholar]
- Goossens HHLM, Van Opstal AJ. Blink-perturbed saccades in monkey. II. Superior colliculus activity. J Neurophysiol 83: 3430–3452, 2000b [DOI] [PubMed] [Google Scholar]
- Henriquez VM, Evinger C. The three-neuron corneal reflex circuit and modulation of second-order corneal responsive neurons. Exp Brain Res 179: 691–702, 2007 [DOI] [PubMed] [Google Scholar]
- Hepp K, Henn V, Vilis T, Cohen B. The neurobiology of saccadic eye movements. Brainstem regions related to saccade generation. Rev Oculomot Res 3: 105–212, 1989 [PubMed] [Google Scholar]
- Horn AKE, Büttner-Ennever JA, Wahle P, Reichenberger I. Neurotransmitter profile of saccadic omnipause neurons in nucleus raphe interpositus. J Neurosci 14: 2032–2046, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko CRS. Effect of ibotenic acid lesions of the omnipause neurons on saccadic eye movements in rhesus macaques. J Neurophysiol 75: 2229–2242, 1996 [DOI] [PubMed] [Google Scholar]
- Keller EL. Control of saccadic eye movements by midline brain stem neurons. In: Control of Gaze by Brain Stem Neurons, edited by Baker R, Berthoz A. Amsterdam: Elsevier/North Holland, 1977, p. 327–336 [Google Scholar]
- Keller EL, Gandhi NJ, Shieh JM. Endpoint accuracy in saccades interrupted by stimulation in the omnipause region in monkey. Vis Neurosci 13: 1059–1067, 1996 [DOI] [PubMed] [Google Scholar]
- King WM, Fuchs AF. Neuronal activity in the mesencephalon related to vertical eye movements. In: Control of Gaze by Brain Stem Neurons, edited by Baker R, Berthoz A. Amsterdam: Elsevier/North Holland, 1977, p. 319–326 [Google Scholar]
- Langer TP, Kaneko CRS. Brainstem afferents to the oculomotor omnipause neurons in monkey. J Comp Neurol 295: 413–427, 1990 [DOI] [PubMed] [Google Scholar]
- Mays LE, Morrisse DW. Activity of pontine omnipause neurons during eye blinks. Soc Neurosci Abstr 20: 1404, 1994 [Google Scholar]
- Mays LE, Morrisse DW. Electrical stimulation of the pontine omnipause area inhibits eye blink. J Am Optom Assoc 66: 419–422, 1995 [PubMed] [Google Scholar]
- Moschovakis AK. Neural network simulations of the primate oculomotor system. I. The vertical saccadic burst generator. Biol Cybern 70: 291–302, 1994 [DOI] [PubMed] [Google Scholar]
- Moschovakis AK, Scudder CA, Highstein SM. The microscopic anatomy and physiology of the mammalian saccadic system. Prog Neurobiol 50: 133–254, 1996 [DOI] [PubMed] [Google Scholar]
- Optican LM, Robinson DA. Cerebellar-dependent adaptive control of primate saccadic system. J Neurophysiol 44: 1058–1076, 1980 [DOI] [PubMed] [Google Scholar]
- Rambold H, El Baz I, Helmchen C. Differential effects of blinks on horizontal saccade and smooth pursuit initiation in humans. Exp Brain Res 156: 314–324, 2004 [DOI] [PubMed] [Google Scholar]
- Rambold H, Sprenger A, Helmchen C. Effects of voluntary blinks on saccades, vergence eye movements, and saccade–vergence interactions in humans. J Neurophysiol 88: 1220–1233, 2002 [DOI] [PubMed] [Google Scholar]
- Robinson DA. A note on the oculomotor pathway. Exp Neurol 22: 130–132, 1968 [DOI] [PubMed] [Google Scholar]
- Robinson DA. Oculomotor control signals. In: Basic Mechanisms of Ocular Motility and Their Clinical Implications, edited by Lennerstrand G, Bach-Y-Rita P. Oxford, UK: Pergamon Press, 1975, p. 337–374 [Google Scholar]
- Rottach KG, Das VE, Wohlgemuth W, Zivotofsky AZ, Leigh RJ. Properties of horizontal saccades accompanied by blinks. J Neurophysiol 79: 2895–2902, 1998 [DOI] [PubMed] [Google Scholar]
- Scudder CA. A new local feedback model of the saccadic burst generator. J Neurophysiol 59: 1455–1475, 1988 [DOI] [PubMed] [Google Scholar]
- Shook BL, Schlag-Rey M, Schlag J. Direct projections from the supplementary eye field to the nucleus raphe interpositus. Exp Brain Res 73: 215–218, 1988 [DOI] [PubMed] [Google Scholar]
- Soetedjo R, Kaneko CRS, Fuchs AF. Evidence that the superior colliculus participates in the feedback control of saccadic eye movements. J Neurophysiol 87: 679–695, 2002 [DOI] [PubMed] [Google Scholar]
- Stanton GB, Goldberg ME, Bruce CJ. Frontal eye field efferents in the macaque monkey: II. Topography of terminal fields in midbrain and pons. J Comp Neurol 271: 493–506, 1988 [DOI] [PubMed] [Google Scholar]
- Stava MW, Huffman MD, Baker RS, Epstein AD, Porter JD. Conjugacy of spontaneous blinks in man: eyelid kinematics exhibit bilateral symmetry. Invest Ophthalmol Vis Sci 35: 3966–3971, 1994 [PubMed] [Google Scholar]
- Topper R, Schwarz M, Lange HW, Hefter H, Noth J. Neurophysiological abnormalities in the Westphal variant of Huntington's disease. Mov Disord 13: 920–928, 1998 [DOI] [PubMed] [Google Scholar]
- Trigo JA, Gruart A, Delgado-García JM. Discharge profiles of abducens, accessory abducens, and orbicularis oculi motoneurons during reflex and conditioned blinks in alert cats. J Neurophysiol 81: 1666–1684, 1999 [DOI] [PubMed] [Google Scholar]
- VanderWerf F, Brassinga P, Reits D, Aramideh M, Ongerboer de Visser BW. Eyelid movements: behavioral studies of blinking in humans under different stimulus conditions. J Neurophysiol 89: 2784–2796, 2003 [DOI] [PubMed] [Google Scholar]
- Walton MMG, Mays LE. Discharge of saccade-related superior colliculus neurons during saccades accompanied by vergence. J Neurophysiol 90: 1124–1139, 2003 [DOI] [PubMed] [Google Scholar]
- Wang N, Warren S, May PJ. Ultrastructural evidence the central mesencephalic reticular formation may depress omnipause neurons activity for tectally evoked saccades. Soc Neurosci Abstr 851.9/V18, 2009 [Google Scholar]
- Wouters RJ, van den Bosch WA, Stijnen T, Bubberman AC, Collewijn H, Lemij HG. Conjugacy of eyelid movements in vertical eye saccades. Invest Ophthalmol Vis Sci 36: 2686–2694, 1995 [PubMed] [Google Scholar]
- Yoon HW, Chung JY, Song MS, Park H. Neural correlates of eye blinking; improved by simultaneous fMRI and EOG measurements. Neurosci Lett 381: 26–30, 2005 [DOI] [PubMed] [Google Scholar]
- Yoshida K, Iwamoto Y, Chimoto S, Shimazu H. Saccade-related inhibitory input to pontine omnipause neurons: an intracellular study in alert cats. J Neurophysiol 82: 1198–1208, 1999 [DOI] [PubMed] [Google Scholar]
- Zee DS, Chu FC, Leigh RJ, Savino PJ, Schatz NJ, Reingold DB, Cogan DG. Blink-saccade synkinesis. Neurology 33: 1233–1236, 1983 [DOI] [PubMed] [Google Scholar]