Abstract
Small errors may affect the process of learning in a fundamentally different way than large errors. For example, adapting reaching movements in response to a small perturbation produces generalization patterns that are different from large perturbations. Are distinct neural mechanisms engaged in response to large versus small errors? Here, we examined the motor learning process in patients with severe degeneration of the cerebellum. Consistent with earlier reports, we found that the patients were profoundly impaired in adapting their motor commands during reaching movements in response to large, sudden perturbations. However, when the same magnitude perturbation was imposed gradually over many trials, the patients showed marked improvements, uncovering a latent ability to learn from errors. On sudden removal of the perturbation, the patients exhibited aftereffects that persisted much longer than did those in healthy controls. That is, despite cerebellar damage, the brain maintained the ability to learn from small errors and the motor memory that resulted from this learning was strongly resistant to change. Of note was the fact that on completion of learning, the motor output of the cerebellar patients remained distinct from healthy controls in terms of its temporal characteristics. Therefore cerebellar degeneration impaired the ability to learn from large-magnitude errors, but had a lesser impact on learning from small errors. The neural basis of motor learning in response to small and large errors appears to be distinct.
INTRODUCTION
Prediction error—the difference between the predicted and the actual outcome of motor commands—is a driving force of motor learning (Noto and Robinson 2001; Tseng et al. 2007; Wallman and Fuchs 1998). Yet, the specific aspects of error, including its size, can fundamentally change how the learning process occurs. For example, in a motor adaptation task one can train a subject with a perturbation that is suddenly introduced in full, versus one that is introduced gradually over many trials. The sudden introduction exposes the learner to many trials with large errors. In contrast, gradual introduction produces learning that is driven by only small errors, often in the absence of a subject's awareness. There appear to be three main differences in how the brain adapts the motor output in response to large versus small errors.
1) In young people, the two types of training result in comparable changes in motor commands and produce motor memories that can be recalled on revisiting the task (Klassen et al. 2005). Nonetheless, the memories appear to have distinct properties. In prism adaptation and reach adaptation tasks, gradual introduction (i.e., small errors) produces longer-lasting aftereffects (Hatada et al. 2006; Kagerer et al. 1997) and better retention (Huang and Shadmehr 2009; Klassen et al. 2005).
2) In older people and other animals, the ability to adapt to gradual visuomotor perturbations is often better than the ability to adapt to abrupt, large perturbations (Buch et al. 2003; Linkenhoker and Knudsen 2002).
3) The patterns of generalization following gradual perturbations are distinct from sudden perturbations (Malfait and Ostry 2004; Michel et al. 2007). For example, when force fields perturb reaching movements (Shadmehr and Mussa-Ivaldi 1994), sudden perturbations produce motor memories that generalize to the untrained arm (Criscimagna-Hemminger et al. 2003), whereas gradual perturbations have no such generalization properties (Malfait and Ostry 2004). On the other hand, gradual perturbations can lead to more robust generalization when the trained arm is used in a different context (e.g., reaching in free air after reaching with a robot), whereas this generalization is smaller if the training is in response to a sudden perturbation (Kluzik et al. 2008).
Because generalization may be a reflection of the activation fields of neurons that participate in the encoding of an internal model (Poggio and Bizzi 2004; Shadmehr 2004), the distinct generalization patterns suggest that gradual and sudden perturbations do not engage the same neural structures or mechanisms of plasticity. The cerebellum is perhaps the single most important structure for motor learning because damage to this structure generally produces severe impairments in the capabilities of humans to adapt in response to a perturbation (Baizer et al. 1999; Martin et al. 1996; Maschke et al. 2004; Morton and Bastian 2006; Rabe et al. 2009; Smith and Shadmehr 2005). There is evidence that cerebellum-dependent mechanisms that support formation and retention of motor memories are distinct for small versus large errors. One study in monkeys demonstrated that inactivation of the cerebellar dentate nucleus impaired adaptation to gradual perturbations, yet spared adaptation to sudden perturbations (Robertson and Miall 1999). In the cerebellar cortex, plasticity related to motor learning is thought to be primarily due to long-term depression (LTD) of parallel fiber–Purkinje cell synapses. In a recent motor learning study in knockout mice, the ability to maintain LTD over time was disrupted (Boyden et al. 2006). The animals could learn a motor skill in response to either large or small errors (because the formation of LTD was not disrupted), but could maintain the skill only if it was acquired via small errors. That is, disruption of LTD maintenance in the cerebellar cortex affected retention of memories acquired via large errors, but not small errors.
Here, we asked whether cerebellum-dependent adaptation in humans depends on the size of the error. Previous work has shown that damage to the human cerebellum produced profound deficits in reach adaptation to force fields (Maschke et al. 2004; Rabe et al. 2009; Smith and Shadmehr 2005). However, to our knowledge all previous adaptation experiments in cerebellar patients have used an abrupt perturbation (i.e., large errors). We considered a within-subject design to quantify the cerebellum's contributions to learning from large versus small errors.
METHODS
Thirteen individuals with cerebellar ataxia and 13 neurologically healthy age-matched controls participated in this study (Table 1). Seven of our patients were diagnosed with spinocerebellar ataxia type 6 (SCA6), one patient had both SCA6 and SCA8, one had SCA8, and one had SCA14. These are autosomal dominant diseases in which clinical symptoms of ataxia tend to manifest in mid adulthood. SCA6 is usually a pure cerebellar syndrome. SCA8 also tends to be a pure cerebellar disease, although in the minority of cases can include sensory neuropathy and spasticity. Likewise, SCA14 is often only a cerebellar disease, with a minority of cases showing myoclonus and some cognitive changes. All of our patients showed purely cerebellar signs in the arms on clinical examination, as described in the following text. Other patients had either sporadic ataxia or autosomal dominant cerebellar ataxia type III (i.e., pure cerebellar syndrome with unknown genetics).
Table 1.
Characteristics of patients with cerebellar degeneration
| Identifier | Gender | Age, yr | Handedness | Diagnosis | ICARS |
|---|---|---|---|---|---|
| 1 | M | 54 | Right | SCA6 and SCA8 | 63 |
| 2 | F | 72 | Right | SCA6 | 58 |
| 3 | F | 67 | Right | ADCA III | 55 |
| 4 | M | 75 | Left | SCA6 | 54 |
| 5 | F | 54 | Right | Sporadic | 52 |
| 6 | F | 40 | Right | SCA6 | 50 |
| 7 | M | 61 | Right | SCA14 | 47 |
| 8 | M | 37 | Left | SCA8 | 46 |
| 9 | F | 53 | Right | SCA6 | 35 |
| 10 | F | 56 | Right | SCA6 | 33 |
| 11 | F | 67 | Right | Sporadic | 27 |
| 12 | F | 57 | Right | Sporadic | 24 |
| 13 | F | 67 | Right | SCA6 | 5 |
ICARS, International Cooperative Ataxia Rating Scale; SCA, spinocerebellar ataxia; ADCA, autosomal dominant ataxia.
The severity of ataxia was rated using the International Cooperative Ataxia Rating Scale (Trouillas et al. 1997). For the purpose of data analysis, cerebellar degeneration patients were divided into two groups: mild (ataxia score <40, n = 5) and severe (ataxia score ≥40, n = 8). This division was somewhat arbitrary, but based on the natural separation of the ataxia scores in our sample. Clinical examination showed no evidence of hypertonia, sensory loss (proprioception and fine touch via monofilament), or extrapyramidal features in the arms of these individuals. Experimental procedures were approved by the Johns Hopkins University School of Medicine Institutional Review Board and all subjects signed a consent form.
Task
Subjects performed arm movements while holding the handle of a robotic device. They were asked to reach toward a target but did not have to stop at the target. Rather, a virtual “pillow” was placed behind the target and they were asked to punch it. In this way, the subject was a boxer who was given a target by a trainer. The trainer held a soft glove in hand (simulated by the virtual pillow). The task for the subject was to accurately punch the soft glove of the trainer.
A crucial feature of our task was that we did not require subjects to stop their reach at the target. This was done for a few reasons. First, our task design reduced the effect of cerebellar intention tremor, which can be marked at the end of a reaching movement at a time when the subjects are attempting to stop their hand near a target. Individuals attempt to compensate by making slower movements (Bastian et al. 1996), making it difficult to perturb their reach with forces that are speed dependent. Second, movements without the virtual pillow take cerebellar patients longer than normal to complete. This added effort could contribute to mental fatigue, making it difficult to study patients in protocols that involve many hundreds of trials. Finally, an earlier experiment demonstrated that in our punching task, cerebellar patients produced movements that were nearly as fast as those of healthy controls (Tseng et al. 2007).
Subjects held the handle of a two-joint robotic manipulandum with their dominant hand and made ballistic “punching” movements from a center starting position through a 5 × 5 mm target. The hand was covered by a horizontal screen, onto which a small cursor (5 × 5 mm) representing hand position was projected at all times. The target was presented at 10 cm from the center at either 121.5° (toward the right shoulder, 0° is at 12 o'clock) or 301.5° (random with equal probability). The target position was reflected in the sagittal axis for left-handed subjects (58.5° and 238.5°). Once through the target, the hand hit a robot-generated virtual pillow. The robot then brought the hand back to the center start position. If the movement duration was 150–400 ms, subjects were rewarded with an “explosion.” The size of the explosion and the number of points assigned were dependent on the endpoint accuracy of the movement, which was displayed as a yellow dot at the point where the hand crossed the 10 cm radius. Color feedback indicated whether the movement speed was too slow or too fast. We recorded not only force at the handle, but also the position and velocity of the hand at a rate of 100 Hz.
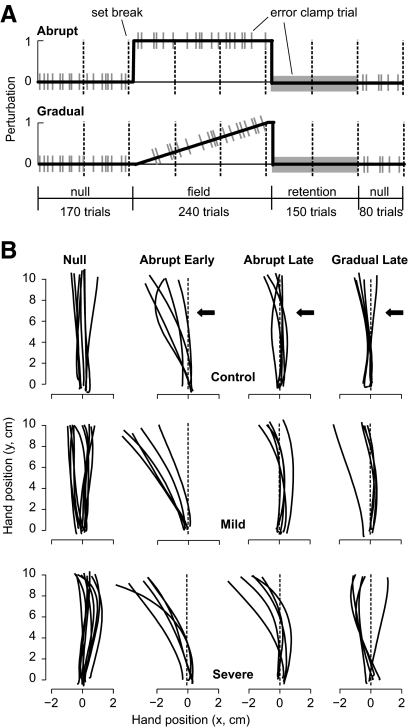
The experiment (Fig. 1A) began with training in a null field (no forces, 170 trials), followed by an adaptation phase (240 trials) in which subjects trained in a curl field in which forces were perpendicular to hand velocity f = Aẋ. The force field was either a counterclockwise curl A = {0, −11; 11, 0} N·s/m or a clockwise curl field B = {0, 11; −11, 0} N·s/m. Following the adaptation trials, we measured the rate of decay of the memory via a long sequence of error-clamp trials (retention block, 150 trials). The session ended with a washout phase in which a null field was reintroduced (80 trials).
Fig. 1.
Study protocol and performance of typical subjects. A: subjects participated in 2 experiments: abrupt and gradual introduction of a force field. They held the handle of a lightweight robotic arm and made shooting movements to a target, crossing it, and hitting a virtual pillow beyond the target. In the first 170 trials the robot produced a null field (no forces). In the subsequent 240 trials, a curl force field was introduced (field A or field B), perturbing the hand perpendicular to its direction of motion. The gray bars represent “error-clamp” trials during which the robot produced a stiff channel, guiding the hand to the target. These error-clamp trials allowed us to measure the subject's motor output perpendicular to the direction of motion. Vertical dashed lines indicate brief set breaks. B: representative trajectories from the end of the null and the beginning and end of the adaptation periods for an individual subject from the control, mild (#9), and severe (#6) groups. The movement starts at (0,0) and the target is at (0,10).
We placed error-clamp trials randomly in the baseline, adaptation, and washout blocks (1/5th probability). During the error-clamp trials, the motion of the hand was constrained to a straight line to the target by a stiff one-dimensional spring (spring coefficient = 2500 N/m; damping coefficient = 25 N·s/m) that counteracted forces perpendicular to the target direction. Error-clamp trials, however, were no different from regular trials in the type of feedback that subjects received: they were rewarded with an “explosion” for completing the movement within the specified time window. Because the field perturbed the hand perpendicular to the direction of motion, the forces that the hand produced against the “channel” wall in error-clamp trials served as a proxy for the change in the motor output.
Experimental sessions
The subjects were tested on two sessions, separated by an average of 17 days (range: 1.05 h to 105 days, as patient visit schedules permitted). Ten of the 13 patients were tested within 24 h. The two sessions for the healthy controls were separated by a maximum of 96 h, median of 24 h (range: 1.1 h to 96 h). On one of these sessions they were tested on an abrupt version of the perturbation (Fig. 1A) and on the other session they were tested on a gradual version. In the abrupt protocol, the field was presented on trial one and remained at full strength for 240 trials. In the gradual protocol, the field was linearly increased during the first 232 adaptation trials. The last 8 trials of the adaptation phase (6 field, 2 error-clamp) were performed at full field strength. Three severe patients performed an additional 20 trials at the end of adaptation at full field strength. The order of the two sessions was counterbalanced.
The abrupt condition causes large errors (lateral deviations of trajectory) when the perturbation is first introduced. When adaptation is successful, these errors then gradually decrease in size over repeated trials. The gradual condition causes small, imperceptible errors when the perturbation is first introduced. During successful adaptation, errors remain small throughout the course of learning. The major difference between abrupt and gradual condition is thus in the size of error experienced by the subject.
Data analysis
In field trials, our performance measure was the endpoint error (distance between center of target and hand position as it crossed the 10 cm boundary of the trial). In error-clamp trials, our performance measure constituted the forces that subjects produced against the channel wall. The average force profile in the null block of error-clamp trials for each target served as a baseline. We subtracted this baseline from the force that we recorded in the adaptation and retention block of error-clamp trials and represented the result as a percentage of perturbation—i.e., the ratio between the actual force produced and the ideal force—where the ideal force is the velocity-dependent force that should be produced to counteract the field.
The post-adaptation retention period was composed entirely of a sequence of error-clamp trials for which we examined the rate of decay of the force output. The decay was estimated by calculating the percentage loss in the force output during the 150 error-clamp trials. The difference in the average force during the first 6 and last 6 error-clamp trials was calculated for each subject.
RESULTS
We compared performance of individuals with cerebellar damage in two protocols (Fig. 1A): one in which the perturbation was introduced abruptly (resulting in larger errors) versus one in which the perturbation was introduced gradually (resulting in generally small errors).
Severe cerebellar patients learned better in the gradual protocol
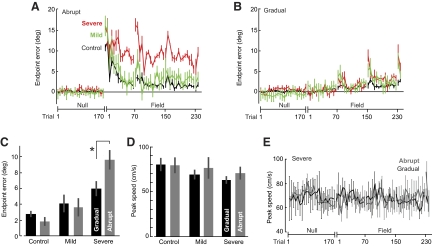
Representative trajectories from the end of null periods and the beginning and end of the adaptation periods are plotted in Fig. 1B for individual subjects from the control, mild, and severe groups. By the end of the adaptation period (trials 233–240), during which the magnitude of the force field was equal in the abrupt and gradual protocols, the subject in the control group performed equally well in the abrupt and gradual protocols (endpoint errors, two-tailed t-test, P > 0.30). Similarly, the mildly affected cerebellar patient performed equally well in the two protocols (two-tailed t-test, P > 0.30). However, the severely affected cerebellar patient had smaller endpoint errors at the end of the gradual protocol than the end of the abrupt protocol (two-tailed t-test, P < 0.05).
Indeed, the severely affected patients benefited from the gradual training protocol. A repeated-measures ANOVA on the adaptation trials (192 field trials, bin size = 16) revealed a main effect of group [F(2,48) = 8.857, P = 0.001], a main effect of condition [F(1,48) = 89.625, P < 0.001], and a main effect of trial [F(1,11) = 16.855, P < 0.001]. There was also a group × trial interaction [F(2,22) = 1.689, P = 0.026] and a condition × trial interaction [F(1,11) = 10.189, P < 0.001].
In the abrupt protocol, patients from the severe group were clearly impaired (Fig. 2A). A repeated-measures ANOVA on the abrupt adaptation trials confirmed a main effect of group [F(2,23) = 33.627, P < 0.001], a main effect of trial [F(1,11) = 18.584, P < 0.001], but no group × trial interaction [F(2,22) = 1.166, P > 0.28]. Post hoc analysis (Tukey's test) indicated a significant difference between the severe and control groups (P < 0.001) and the severe and mild groups (P < 0.001), whereas the mild and control groups performed comparably (P > 0.30). That is, adaptation was significantly impaired in the severe group in the abrupt condition. In contrast, when the same force field was introduced gradually (Fig. 2B), a repeated-measures ANOVA revealed no main effect of group [F(2,23) = 2.330, P = 0.120], but a main effect of trial [F(1,11) = 19.075, P < 0.001] and a group × trial interaction [F(2,22) = 2.907, P < 0.001]. Post hoc analysis (Tukey's test) showed no significant differences between the severe and control groups (P > 0.12), the severe and mild groups (P > 0.30), or the mild and control groups (P > 0.30). Therefore between-group comparisons suggested that, whereas the individuals in the severe group were impaired in the abrupt protocol, they were not different from the mild and control groups in the gradual protocol.
Fig. 2.
Performance during the adaptation block. A and B: angular error at the end of the movement (means ± SE) for each group during abrupt and gradual training. Set breaks are indicated by the numbered tick marks on the x-axis. Bin size is 2 trials for the trials immediately following set break and 6 trials for the remainder of the block. C: average angular error at the end of the movement (means ± SE) during the last 6 field trials when the force field was at full strength in both the abrupt and gradual conditions. Control and mild patients perform comparably in the 2 conditions. Severe patients showed an improvement in performance in the gradual condition. D: average peak speed (means ± SE) during the last 6 trials when the field was at full strength in both the abrupt and the gradual conditions. E: average peak speed (means ± SE) for the severe patients during abrupt and gradual training. Bin size is 2 trials for the trials immediately following set break and 6 trials for the remainder of the block.
To further test whether the cerebellar patients adapted better to the sequence of small errors, we compared their performance as measured by endpoint error during the trials in which the force field was at full strength in the abrupt and gradual protocols (adaptation trials 233–240). A two-way ANOVA revealed a main effect of group [F(2,23) = 22.484, P < 0.001] and a group × condition interaction [F(2,23) = 6.172, P = 0.007], but no main effect of condition [F(2,23) = 1.254, P > 0.25]. A post hoc Tukey's test revealed that the severe patients performed worse than not only the mild patients (P < 0.01) but also the control groups (P < 0.001). The mild group performed comparably to the control group (P > 0.25). More specifically, in the abrupt condition the severe patients were impaired compared with the control subjects (P < 0.001) and mild patients (P = 0.001), whereas the control subjects and mild patients performed comparably (P > 0.69). A within-subject planned comparison confirmed that the patients in the severe group had significantly larger errors at the end of adaptation in the abrupt protocol compared with the gradual protocol (two-tailed t-test, P < 0.05, Fig. 2C). However, the subjects in the control group performed equally well in these two conditions (two-tailed t-test, P > 0.15, Fig. 2C). Similarly, the performance of the patients in the mild group did not differ in the two training protocols (two-tailed t-test, P > 0.30, Fig. 2C).
Because the perturbations were velocity dependent, it was important to determine whether movement speeds were comparable during the two protocols. Figure 2D displays the average peak speed in trials at the end of the adaptation block and Fig. 2E displays the peak movement speeds for the null and adaptation blocks. For the data shown in Fig. 2D, a two-way ANOVA found no effect of condition, no effect of group, and no interaction (all P > 0.20). For the data shown in Fig. 2E, repeated-measures ANOVA during adaptation trials (bin size = 16) in the abrupt and gradual conditions for severe patients revealed no main effect of condition [F(1,14) = 0.792, P > 0.38], no main effect of trial [F(1,14) = 1.108, P > 0.35], and no interaction [F(1,14) = 1.333, P > 0.21]. Despite these similar movement speeds, performance of the severely affected patients was significantly better in the gradual protocol (Fig. 2C).
In the original protocol, there were only 8 full strength trials in the gradual condition. To test the robustness of our results, three of the patients in the severe group (4, 5, and 6) were tested in a modified protocol in which we added 20 extra trials (16 field and 4 channel) to the adaptation block. In this way, for these three severely affected patients the fields in the abrupt and gradual protocols were of equal strength for 28 trials. A within-subject planned comparison once again indicated that the individuals in the severe group benefited from the gradual protocol: endpoint errors were significantly smaller in the gradual protocol (abrupt: 0.154 ± 0.02 rad; gradual: 0.059 ± 0.03, means ± SE, two-tailed t-test, P < 0.05). In summary, movement trajectories indicated that with severe cerebellar degeneration, there was a greater impairment in adapting to large errors versus small errors.
Gradual protocol enhanced predictive adaptive control in the severe patients
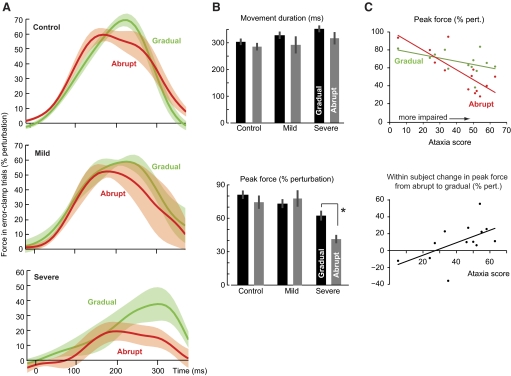
In principle, the improved performance in response to a force perturbation during reaching can be attributed to two mechanisms: one may get better at reacting to a perturbation as the movement unfolds (e.g., increased stiffness) or one may get better at predicting that perturbation and producing motor commands that compensate for it. One way to dissociate these two possibilities is via the forces that the subjects produced in error-clamp trials. During an error-clamp trial there are no perturbations. Rather, a virtual force “channel” constrains the movement to a straight line to the target, eliminating the need to correct for an error. Thus we can view the forces that the subjects produce against the channel walls as a proxy for the forces that they expect from the external perturbation.
The force profiles for the last two error-clamp trials of training are plotted in Fig. 3A. The time at which the movement crossed the target is shown in the top subplot of Fig. 3B. A two-way ANOVA on the average peak force during these error-clamp trials showed a main effect of group [F(2,23) = 11.141, P < 0.001] and group × condition interaction [F(2,23) = 3.566, P = 0.045; Fig. 3B, bottom subplot]. Indeed, the maximum force produced by the severe patients was smaller than controls in the gradual [one-way ANOVA: F(2,23) = 5.586, P < 0.05; post hoc Tukey's test: severe vs. control, P < 0.01; severe vs. mild, P > 0.25; mild vs. control, P > 0.30] and smaller than controls and mild patients in the abrupt protocols [one-way ANOVA: F(2,23) = 10.286, P = 0.001; post hoc Tukey's test: severe vs. control, P = 0.001; severe vs. mild, P = 0.004; mild vs. control, P = 0.935]. Importantly, for the severe patients a within-subject comparison of the peak forces showed a significant improvement in performance in the gradual versus the abrupt protocols (P = 0.007), but no difference for mild patients (P > 0.30) or controls (P > 0.15). Therefore the severe cerebellar patients were better able to predict the force perturbation after training in the gradual protocol versus the abrupt protocol.
Fig. 3.
Motor output in error-clamp trials at end of adaptation. A: force output, represented as percentage of perturbation (which normalizes for movement speed), during the last 2 error-clamp trials of the adaptation phase for the control, mild, and severe groups. B: average movement duration and peak force (% perturbation) during the last 2 error-clamp trials of training. C: peak force (% perturbation) during the last 2 error-clamp trials of the adaptation phase plotted as a function of ataxia score for mild and severe patients (top: abrupt and gradual; bottom: within-subject difference between abrupt and gradual). In the abrupt condition, severity predicts performance in the error-clamp trials.
In Fig. 3C, we have plotted the average peak force during the last two error-clamp trials of the adaptation phase for the mild and severe patients in the abrupt and gradual conditions. In the abrupt condition, patients with greater severity produced less force (R2 = 0.64, P = 0.001). However, in the gradual condition severity of ataxia was not a clear predictor of performance (R2 = 0.24, P = 0.09). Figure 3C also displays the within-subject change in motor output in the abrupt and gradual conditions. The data demonstrate that patients with greater severity tended to benefit the most from the gradual presentation of the perturbation (R2 = 0.30, P = 0.05).
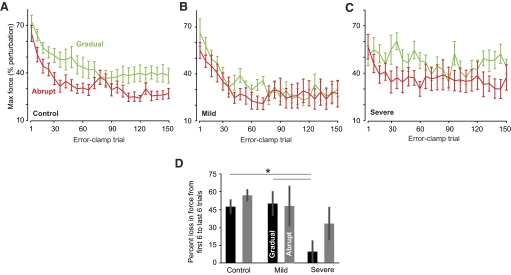
In the severely affected patients, the gradual protocol produced motor memories that resisted change
The adaptation block was followed by a long series of error-clamp trials in which we assayed the rate of decay of the adapted response. The maximum force during the post-adaptation retention block is shown for the various groups in Fig. 4. We calculated the percentage loss as the difference between the average force during the first six and last six error-clamp trials for each subject (Fig. 4D). A two-way ANOVA showed a main effect of group [F(2,23) = 7.444, P = 0.003] and no group × condition interaction [F(2,23) = 0.658, P > 0.50] or main effect of condition [F(2,23) = 1.450, P > 0.24]. This main effect of group appeared to be driven by the decreased rate of decay in the severely affected patients in the gradual condition [one-way ANOVA: F(2,23) = 7.228, P = 0.004]. A post hoc Tukey's test confirmed that the gradual training in the severely affected patients produced a motor output that decayed more slowly than those observed in the control (P = 0.005) and mildly affected (P < 0.02), whereas the mild and control groups decayed at comparable rates (P > 0.97). This result was confirmed when we quantified the decay by fitting an exponential to the individual data. In the gradual condition, the severe patients had slower decay rates in their motor output than the mild and control groups.
Fig. 4.
Motor output in postadaptation error-clamp block. A–C: maximum force output during the error-clamp trials in the post-adaptation retention block (means ± SE). Bin size is 6 trials. D: fragility of the memory. The change in force output from the beginning to the end of the post-adaptation block, expressed as percentage reduction from the first 6 trials to the last 6 trials. The changes are shown as means ± SE. In the gradual condition, the severe patients had less percentage loss of motor output than that of control and mild patients.
After completion of the retention block, all subjects were provided with a brief break and then they returned to the reaching task (Fig. 1A). However, the robot motors now no longer produced a force perturbation (null field). This allowed us to ask two questions: first, would the severe cerebellar patients show aftereffects of the prior training, and second, would these aftereffects wash out more slowly than those in healthy controls?
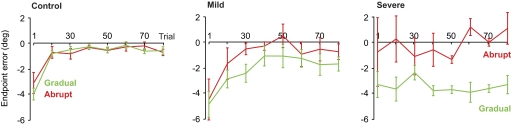
To test whether the cerebellar patients had aftereffects of prior training, we examined the endpoint error in each movement (Fig. 5; a negative value indicates an aftereffect). Aftereffects were seen in the control group, as demonstrated by the negative average endpoint error (significantly different from zero, two-tailed t-test: abrupt, P < 0.004; gradual, P < 0.001). Although the average endpoint error was negative for the mild and severe groups, the large variability in the abrupt condition resulted in aftereffects that were significantly different from zero only in the gradual condition (two-tailed t-test: mild: abrupt, P > 0.17, gradual, P < 0.01; severe: abrupt, P > 0.65, gradual, P < 0.015). Furthermore, we observed that the aftereffects of the severe group following the gradual condition showed little or no evidence of washout, despite the 80 null trials. Thus the motor memory formed by the severely affected patients not only decayed more slowly than normal in the error-clamp block of trials, it also produced aftereffects that washed out more slowly in the null block of trials.
Fig. 5.
Aftereffects during the final null block of trials. A–C: angular error at the end of the movement (means ± SE) during the null washout trials. Bin size is 8 trials. The control and mild groups demonstrated aftereffects in both abrupt and gradual conditions. The severe group showed significant aftereffects only in the gradual condition. (The total number of trials does not add up to 80 because there were also error-clamp trials in this data set, which are not shown.)
A missing component of adaptation in the cerebellar patients
Elsewhere we reported that during adaptation to a velocity-dependent force field, the optimal trajectory to the target is not a straight line, but a trajectory that overcompensates early into the movement (when the forces are weak), allowing the robot to bring the hand back toward the target when velocities are higher and the field is strong (Izawa et al. 2008). Such a trajectory minimizes effort (sum of forces exerted). Here, we found that whereas this ability to optimize the adaptive response was present in the control subjects, it was clearly missing in the severely affected patients in both the abrupt and the gradual conditions.
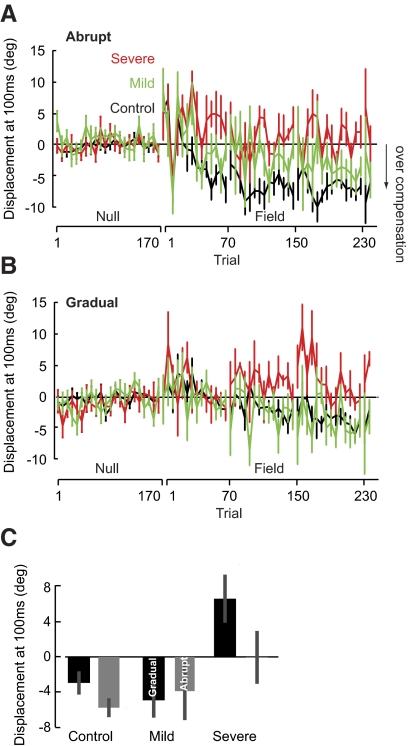
The overcompensation pattern is demonstrated by a perpendicular displacement that becomes negative early into the movement (Izawa et al. 2008). To quantify this pattern, we measured the perpendicular displacement at 100 ms (Fig. 6). In the abrupt protocol, patients from the severe group did not show a pattern of overcompensation. A repeated-measures ANOVA on the 240 abrupt adaptation trials (192 field trials, bin size = 16) showed a main effect of group [F(2,23) = 9.774, P = 0.001]. A post hoc Tukey's test indicated a significant difference between the severe and control groups (P = 0.001), whereas the severe and mild groups (P = 0.157) and the mild and control groups performed comparably (P > 0.20). Likewise, when the same force field was introduced gradually, there was a main effect of group [repeated-measures ANOVA: F(2,23) = 6.761, P = 0.005]. A post hoc Tukey's test showed that the severe group showed less overcompensation than the mild (P < 0.05) and control (P < 0.01) groups, but there were no significant differences between the mild and control groups (P > 0.30). Thus although the performance of the severe patients was better in the gradual protocol, they were unable to learn the optimal adaptive response in both the abrupt and gradual conditions.
Fig. 6.
Overcompensation for the force perturbation. A and B: angular error at 100 ms (means ± SE) for each subgroup during abrupt and gradual training. Bin size is 2 trials immediately following set break and 6 trials for the remainder of the block. C: average angular error at 100 ms (means ± SE) during the last 6 trials when the field was at full strength in both abrupt and gradual conditions. Controls showed overcompensation in both conditions. The severe patients did not show significant overcompensation in either condition.
To test whether the cerebellar patients learned optimally when the sequence of errors was small, we examined whether the average perpendicular displacement was negative (i.e., subjects overcompensated) during trials in which the magnitude of the force field was at full strength in both protocols (Fig. 6C). Overcompensation was seen in the control group, as demonstrated by the negative average perpendicular displacement (significantly different from zero, two-tailed t-test: abrupt, P < 0.001, gradual, P < 0.05). Although the average overcompensation was negative for the mild group, the large variability resulted in overcompensation that was not significantly negative (two-tailed t-test: gradual, P = 0.066; abrupt, P > 0.30). The severe patients not only failed to overcompensate in the abrupt condition (P > 0.5), but they also had a positive perpendicular displacement at 100 ms in the gradual condition (P < 0.05). We also compared the performance of the groups with each other at the end of adaptation. A two-way ANOVA revealed a main effect of group [F(2,23) = 9.887, P = 0.001], but no main effect of condition [F(2,23) = 2.235, P = 0.149] or group × condition interaction [F(2,23) = 1.187, P > 0.30]. Post hoc analysis (Tukey's test) showed that the severe patients did not overcompensate comparably to the mild (P = 0.008) or control (P = 0.001) groups, but the mild patients did overcompensate comparably to the control group (P > 0.5). The ability to find the optimal trajectory (i.e., overcompensate) was clearly absent in the severe group in both the abrupt and gradual conditions.
DISCUSSION
Damage to the cerebellum generally produces profound impairments in the ability of the brain to learn from movement errors. Previous experiments have been unequivocal: cerebellar patients are impaired in their ability to alter their motor output to compensate for a predictable perturbation (Lang and Bastian 1999; Martin et al. 1996; Nowak et al. 2007; Smith and Shadmehr 2005). Our results change this perspective by demonstrating that motor learning impairment is not a general phenotype of cerebellar damage. Rather, cerebellar degeneration has a significant effect on the ability to learn from large errors but has a lesser effect on the ability to learn from small errors. Therefore the neural bases of learning from large and small errors are likely distinct.
The patients that we studied suffered from cerebellar atrophy. We divided our population into a mild and a severe group based on their ataxia score and then tested them in a reaching task in which a force field pushed the hand perpendicular to the direction of motion. Previous work had demonstrated that the greater the severity of the cerebellar disease, the greater the learning impairment in response to an abrupt perturbation (Maschke et al. 2004). Indeed, we found that there was a trend of increasing endpoint error corresponding to degree of impairment (i.e., the healthy controls exhibited less error than did the mild patients and the mild patients exhibited less error than did the severe patients; Fig. 2C).
Our subjects were tested in two conditions: abrupt introduction of the perturbation in a single trial and gradual introduction over many trials. When the two perturbations were of equal strength, the severely affected patients had smaller endpoint errors in the gradual versus the abrupt protocol. This improved performance was due to learning of an internal model that better predicted the perturbing forces, as evidenced by the greater forces that the patients produced in the gradual protocol.
After the adaptation period, the stability of the acquired memory was tested in a long sequence of error-clamp trials, i.e., trials in which movement errors were eliminated. This assayed the sensitivity of the acquired memory to passage of time and/or trial. Following gradual training, the acquired motor memory decayed more slowly in the severe group than in the control and mild groups. When the force field was unexpectedly removed, in the severe group the resulting aftereffects persisted for nearly 80 trials, whereas the aftereffects washed out within 10 trials in healthy controls. That is, the severely affected patients not only learned better from small errors, this learning produced a motor memory that was more resistant to change compared with healthy controls.
Our observation that the adaptation in the severe patients produced a motor memory that had a slower rate of decay than that of controls is consistent with observations in another adaptation experiment. Earlier, we examined the ability of cerebellar degeneration patients to adapt the gain of their saccadic eye movements (Xu-Wilson et al. 2009). We found that although the patients (eight of whom were also in the current study) were impaired in their ability to adapt their saccades, the learning that did take place produced motor memories that exhibited little forgetting as a function of time. If we assume that there are fast and slow adaptive processes that support motor memory in healthy people (Smith et al. 2006), the ability to learn better from the gradual protocol and the resulting slower decay of motor output suggest that cerebellar degeneration has a particularly significant impact on the fast process.
One way to explain these results is to hypothesize that learning from large and small errors normally requires the integrity of the cerebellum, but there are other brain structures that contribute to motor learning and these structures are primarily engaged in response to small errors. For example, Boyden et al. (2006) reduced the capacity of Purkinje cells to maintain LTD and found that this affected retention of a vestibuloocular reflex motor skill, but only if the adaptation was due to a perturbation that introduced large errors (high-frequency rotation). It is possible that our patients were less impaired in the gradual condition because they could use generally spared neural structures outside the cerebellum to learn from small errors.
The trouble with this line of thinking is that it implies that the cerebellum is specialized for learning from large errors, something that is inconsistent with at least one neurophysiological experiment. Soetedjo et al. (2008) recently quantified encoding of movement errors in a saccade adaptation protocol. They noted that although there were Purkinje cells in the oculomotor vermis that were sensitive to only small errors (i.e., complex spikes occurred with high probability for small errors but low probability for large errors), the Purkinje cells that were sensitive to large errors were often equally sensitive to small errors. That is, in the healthy subject, small errors had a greater probability of producing complex spikes in the Purkinje cells of the vermis.
It is important to point out that as cerebellar degeneration takes place (particularly in SCA6 patients), the disease tends to have a differential effect on the hemispheres versus the vermis (Schulz et al. 2010). The generally spared ability to learn from small errors may indicate that different regions of the cerebellum are engaged in response to large and small errors.
The rationale for this idea is that large perturbations produce conscious awareness of the error, which coincides with activity in prefrontal cortical regions (Shadmehr and Holcomb 1997), whereas small perturbations often preclude this awareness. Prefrontal regions project to and receive inputs from parts of the cerebellum (Crus II) that are distinct from those regions (lobules IV–VI) that connect to the motor cortex (Kelly and Strick 2003). In principle, it is possible that large errors that produce conscious awareness not only engage nonmotor structures in the cortex, but also produce learning in regions of the cerebellum that are distinct from regions that may be connected to the motor cortical structures. Therefore the improved performance with small errors may not be a reflection of specialization of cerebellum for large errors, but rather an indication of dissociation of large and small errors within the cerebellum along with a differential rate of damage associated with neurodegeneration.
Although our data do not allow us to dissociate between these possibilities, it uncovers an unexpected and important fact: despite severe cerebellar damage, there is a latent ability in the brain to learn from small errors and the motor memory that this learning produces has a greater resistance to change. When damage to the brain affects one form of learning but not another, it generally suggests that the neural bases of the two forms of learning are distinct. Our results raise the possibility that the multiple computational processes that are thought to support motor adaptation—i.e., the so-called fast and slow processes (Smith et al. 2006)—are neurally distinct.
From a practical standpoint, gradual introduction of a perturbation may be an effective method for training of patient or elderly populations. For example, in a visuomotor rotation paradigm, healthy elderly people have smaller errors at the end of a gradual training protocol compared with an abrupt protocol (Buch et al. 2003). The longer-lasting aftereffects of gradual training (Hatada et al. 2006; Kagerer et al. 1997) coupled with its generalization to movements outside the training apparatus (Kluzik et al. 2008) suggest that this method of training might be more advantageous for rehabilitation.
Despite this ability to learn to predict the pattern of forces in a gradually imposed perturbation, the motor memory formed by cerebellar patients was missing a fundamental component: their motor output did not exhibit an overcompensation of the perturbation early in the movement. This specific feature of reaching movements in force fields has been linked to a process of optimization (Izawa et al. 2008): i.e., a process in which the brain finds the motor commands that do not merely compensate for the perturbation, but do so with minimum effort. This optimization process appears to be lost with cerebellar damage in both the mild and severe patients.
What does the current result suggest about the function of the cerebellum in motor control? Elsewhere we have proposed that the general problem of motor control is twofold (Shadmehr and Krakauer 2008): 1) to learn to predict the sensory consequences of motor commands and 2) to find motor commands that bring the maximum amount of reward at a minimum effort. From a theoretical standpoint, the first problem is one of learning a forward model, whereas the second problem is learning an optimal control strategy. Importantly, the two steps may not be independent. That is, one cannot form an optimal control strategy unless one already has formed an accurate way to predict the consequences of motor commands. An influential idea is that the cerebellum is a site for learning of forward models (Miall and Wolpert 1996; Pasalar et al. 2006). However, our results here suggest that cerebellar damage impairs one of the adaptive processes that are involved in learning (the process that depends on large errors), but there are other mechanisms, perhaps in different regions of the cerebellum or outside the cerebellum, that may contribute to learning from small errors. Furthermore, even when learning is driven by small errors, cerebellar damage prevents formation of a predictive model that has an optimal timing property. This raises the possibility that the contributions of the cerebellum go beyond formation of sensory predictions (i.e., forward models), but also play a role in the programming of motor commands that optimally (i.e., with minimum effort) produce a desired movement outcome.
GRANTS
This research was supported by National Institutes of Health Grants NS-37422 and HD-40289.
ACKNOWLEDGMENTS
We thank S. Ying, J. Savitt, and D. Zee of Johns Hopkins Neurology and S. Reich of University of Maryland Neurology for referring the cerebellar patients and the patients and families for participating in this study.
REFERENCES
- Baizer JS, Kralj-Hans I, Glickstein M. Cerebellar lesions and prism adaptation in macaque monkeys. J Neurophysiol 81: 1960–1965, 1999 [DOI] [PubMed] [Google Scholar]
- Boyden ES, Katoh A, Pyle JL, Chatila TA, Tsien RW, Raymond JL. Selective engagement of plasticity mechanisms for motor memory storage. Neuron 51: 823–834, 2006 [DOI] [PubMed] [Google Scholar]
- Buch ER, Young S, Contreras-Vidal JL. Visuomotor adaptation in normal aging. Learn Mem 10: 55–63, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criscimagna-Hemminger SE, Donchin O, Gazzaniga MS, Shadmehr R. Learned dynamics of reaching movements generalize from dominant to nondominant arm. J Neurophysiol 89: 168–176, 2003 [DOI] [PubMed] [Google Scholar]
- Hatada Y, Miall RC, Rossetti Y. Two waves of a long-lasting aftereffect of prism adaptation measured over 7 days. Exp Brain Res 169: 417–426, 2006 [DOI] [PubMed] [Google Scholar]
- Huang VS, Shadmehr R. Persistence of motor memories reflects statistics of the learning event. J Neurophysiol 102: 931–940, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa K, Watanabe M, Yoshizawa K, Fujita T, Iwamoto H, Yoshizawa T, Harada K, Nakamagoe K, Komatsuzaki Y, Satoh A, Doi M, Ogata T, Kanazawa I, Shoji S, Mizusawa H. Clinical, neuropathological, and molecular study in two families with spinocerebellar ataxia type 6 (SCA6). J Neurol Neurosurg Psychiatry 67: 86–89, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izawa J, Rane T, Donchin O, Shadmehr R. Motor adaptation as a process of reoptimization. J Neurosci 28: 2883–2891, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagerer FA, Contreras-Vidal JL, Stelmach GE. Adaptation to gradual as compared with sudden visuo-motor distortions. Exp Brain Res 115: 557–561, 1997 [DOI] [PubMed] [Google Scholar]
- Kelly RM, Strick PL. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J Neurosci 23: 8432–8444, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klassen J, Tong C, Flanagan JR. Learning and recall of incremental kinematic and dynamic sensorimotor transformations. Exp Brain Res 164: 250–259, 2005 [DOI] [PubMed] [Google Scholar]
- Kluzik J, Diedrichsen J, Shadmehr R, Bastian AJ. Reach adaptation: what determines whether we learn an internal model of the tool or adapt the model of our arm? J Neurophysiol 100: 1455–1464, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang CE, Bastian AJ. Cerebellar subjects show impaired adaptation of anticipatory EMG during catching. J Neurophysiol 82: 2108–2119, 1999 [DOI] [PubMed] [Google Scholar]
- Linkenhoker BA, Knudsen EI. Incremental training increases the plasticity of the auditory space map in adult barn owls. Nature 419: 293–296, 2002 [DOI] [PubMed] [Google Scholar]
- Malfait N, Ostry DJ. Is interlimb transfer of force-field adaptation a cognitive response to the sudden introduction of load? J Neurosci 24: 8084–8089, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TA, Keating JG, Goodkin HP, Bastian AJ, Thach WT. Throwing while looking through prisms. I. Focal olivocerebellar lesions impair adaptation. Brain 119: 1183–1198, 1996 [DOI] [PubMed] [Google Scholar]
- Maschke M, Gomez CM, Ebner TJ, Konczak J. Hereditary cerebellar ataxia progressively impairs force adaptation during goal-directed arm movements. J Neurophysiol 91: 230–238, 2004 [DOI] [PubMed] [Google Scholar]
- Miall RC, Wolpert DM. Forward models for physiological motor control. Neural Networks 9: 1265–1279, 1996 [DOI] [PubMed] [Google Scholar]
- Michel C, Pisella L, Prablanc C, Rode G, Rossetti Y. Enhancing visuomotor adaptation by reducing error signals: single-step (aware) versus multiple-step (unaware) exposure to wedge prisms. J Cogn Neurosci 19: 341–350, 2007 [DOI] [PubMed] [Google Scholar]
- Morton SM, Bastian AJ. Cerebellar contributions to locomotor adaptations during splitbelt treadmill walking. J Neurosci 26: 9107–9116, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noto CT, Robinson FR. Visual error is the stimulus for saccade gain adaptation. Brain Res Cogn Brain Res 12: 301–305, 2001 [DOI] [PubMed] [Google Scholar]
- Nowak DA, Timmann D, Hermsdorfer J. Dexterity in cerebellar agenesis. Neuropsychologia 45: 696–703, 2007 [DOI] [PubMed] [Google Scholar]
- Pasalar S, Roitman AV, Durfee WK, Ebner TJ. Force field effects on cerebellar Purkinje cell discharge with implications for internal models. Nat Neurosci 9: 1404–1411, 2006 [DOI] [PubMed] [Google Scholar]
- Poggio T, Bizzi E. Generalization in vision and motor control. Nature 431: 768–774, 2004 [DOI] [PubMed] [Google Scholar]
- Rabe K, Livne O, Gizewski ER, Aurich V, Beck A, Timmann D, Donchin O. Adaptation to visuomotor rotation and force field perturbation is correlated to different brain areas in patients with cerebellar degeneration. J Neurophysiol 101: 1961–1971, 2009 [DOI] [PubMed] [Google Scholar]
- Robertson EM, Miall RC. Visuomotor adaptation during inactivation of the dentate nucleus. Neuroreport 10: 1029–1034, 1999 [DOI] [PubMed] [Google Scholar]
- Schulz JB, Borkert J, Wolf S, Schmitz-Hübsch T, Rakowicz M, Mariotti C, Schoels L, Timmann D, van de Warrenburg B, Dürr A, Pandolfo M, Kang J-S, Mandly AG, Nägele T, Grisoli M, Boguslawska R, Bauer P, Klockgether T, Hauser T-K. Visualization, quantification and correlation of brain atrophy with clinical symptoms in spinocerebellar ataxia types 1, 3 and 6. NeuroImage 49: 158–168, 2010 [DOI] [PubMed] [Google Scholar]
- Shadmehr R. Generalization as a behavioral window to the neural mechanisms of learning internal models. Hum Mov Sci 23: 543–568, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R, Holcomb HH. Neural correlates of motor memory consolidation. Science 277: 821–825, 1997 [DOI] [PubMed] [Google Scholar]
- Shadmehr R, Krakauer JW. A computational neuroanatomy for motor control. Exp Brain Res 185: 359–381, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R, Mussa-Ivaldi FA. Adaptive representation of dynamics during learning of a motor task. J Neurosci 14: 3208–3224, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Ghazizadeh A, Shadmehr R. Interacting adaptive processes with different timescales underlie short-term motor learning. PLoS Biol 4: e179, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Shadmehr R. Intact ability to learn internal models of arm dynamics in Huntington's disease but not cerebellar degeneration. J Neurophysiol 93: 2809–2821, 2005 [DOI] [PubMed] [Google Scholar]
- Trouillas P, Takayanagi T, Hallett M, Currier RD, Subramony SH, Wessel K, Bryer A, Diener HC, Massaquoi S, Gomez CM, Coutinho P, Ben Hamida M, Campanella G, Filla A, Schut L, Timann D, Honnorat J, Nighoghossian N, Manyam B. International Cooperative Ataxia Rating Scale for pharmacological assessment of the cerebellar syndrome. The Ataxia Neuropharmacology Committee of the World Federation of Neurology. J Neurol Sci 145: 205–211, 1997 [DOI] [PubMed] [Google Scholar]
- Tseng YW, Diedrichsen J, Krakauer JW, Shadmehr R, Bastian AJ. Sensory prediction errors drive cerebellum-dependent adaptation of reaching. J Neurophysiol 98: 54–62, 2007 [DOI] [PubMed] [Google Scholar]
- Wallman J, Fuchs AF. Saccadic gain modification: visual error drives motor adaptation. J Neurophysiol 80: 2405–2416, 1998 [DOI] [PubMed] [Google Scholar]
- Xu-Wilson M, Chen-Harris H, Zee DS, Shadmehr R. Cerebellar contributions to adaptive control of saccades in humans. J Neurosci 29: 12930–12939, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]