Abstract
Control of the human walking pattern normally requires little thought, with conscious control used only in the face of a challenging environment or a perturbation. We have previously shown that people can adapt spatial and temporal aspects of walking to a sustained perturbation generated by a split-belt treadmill. Here we tested whether conscious correction of walking, versus distraction from it, modifies adaptation. Conscious correction of stepping may expedite the adaptive process and help to form a new walking pattern. However, because walking is normally an automatic process, it is possible that conscious effort could interfere with adaptation, whereas distraction might improve it by removing competing voluntary control. Three groups of subjects were studied: a control group was given no specific instructions, a conscious correction group was instructed how to step and given intermittent visual feedback of stepping during adaptation, and a distraction group performed a dual-task during adaptation. After adaptation, retention of aftereffects was assessed in all groups during normal treadmill walking without conscious effort, feedback, or distraction. We found that conscious correction speeds adaptation, whereas distraction slows it. Subjects trained with distraction retained aftereffects longest, suggesting that the training used during adaptation predicts the time course of deadaptation. An unexpected finding was that these manipulations affected the adaptation rate of spatial but not temporal elements of walking. Thus conscious processes can preferentially access the spatial walking pattern. It may be that spatial and temporal controls of locomotion are accessible through distinct neural circuits, with the former being most sensitive to conscious effort or distraction.
INTRODUCTION
Human locomotion normally requires little conscious effort, even when switching patterns (i.e., running to walking). We don't think about the exact motion of our legs but instead focus on other important things: the car approaching the crosswalk or the ball that we are running to catch. This automatic control of walking is critical to our daily activities. It is only in the face of injury or novel situations (i.e., stepping on ice for the first time) that we actually “think” about walking. In other words, explicitly thinking about walking is normally the exception rather than the rule. However, during rehabilitation of walking, thinking about the pattern is heavily emphasized. It is unknown whether encouraging conscious control of movement will assist in learning a largely automatic task like walking.
Prior work has shown that attending to movements and providing explicit feedback can be beneficial when it comes to learning a novel motor skill (Todorov et al. 1997; Wulf and Prinz 2001). Direct feedback about the desired behavior enables subjects to reduce errors and enhance performance for skill learning (Salmoni et al. 1984; Schmidt 1991). After continued practice, however, the action becomes automatic, and thinking about the movement can actually interfere with performance (Wulf and Prinz 2001). This suggests that conscious correction can be beneficial or detrimental depending on whether the skill is novel or well-practiced.
When well-practiced skills become automatic, conscious resources can be used for other activities. This automaticity of tasks has been studied with dual-task paradigms in learning novel skills, motor adaptations, sensory discrimination tasks, and walking (Collette et al. 2005; Lang and Bastian 2002; Taylor and Thoroughman 2007; Yogev-Seligmann et al. 2008). Dual-tasking requires subjects to complete unrelated tasks simultaneously, which involves the coordination and integration of different processing mechanisms (Collette et al. 2005). Studies hypothesize that the dorsolateral prefrontal cortex is responsible for coordinating and allocating resources in these dual-tasks, thus enabling “multi-tasking” (Collette et al. 2005; Schubert and Szameitat 2003). Although healthy adults can complete dual tasks, there is still a detrimental effect on performance—they make more errors and take longer to learn the skill of interest (Collette et al. 2005; Lang and Bastian 2002). Taylor and Thoroughman hypothesized that a secondary task can interfere with feed-forward mechanisms of error correction (Taylor and Thoroughman 2007), which could result in more errors and a slower learning rate. To our knowledge, the effects of dual-tasking on the adaptation of an automatic task, like walking, have not been studied.
There are also likely to be distinctions between types of motor learning (i.e., motor adaptation vs. skill learning) which may be important for understanding effects of conscious effort and distraction. Motor adaptation is an error driven learning process that adjusts the sensorimotor mappings of an already well-learned movement (Anderson et al. 2002; Bastian 2008; Krakauer 2009); skill learning requires creation of de novo spatiotemporal muscle activations (Anderson et al. 2002). Thus it is possible that effects of conscious correction on de novo skill learning may differ from effects on motor adaptation. Some studies have shown a positive effect from feedback on skill acquisition, such as playing ping-pong (Todorov et al. 1997), playing tennis (Maddox et al. 1999), skiing (Wulf and Weigelt 1997), and playing golf (Perkins-Ceccato et al. 2003). Although feedback has been shown to benefit skill learning, the gait changes induced by the split-belt treadmill are better classified as motor adaptation; subjects are adjusting an already learned walking pattern to novel demands. Prior work on visuomotor and gait adaptation has suggested that an explicit conscious strategy cannot substitute for the more implicit processes normally used for these forms of motor learning (Mazzoni and Krakauer 2006; Reynolds and Bronstein 2003). Thus locomotor adaptation may prove different from skill acquisition in how it is affected by explicit feedback about how to change the movement.
To study this, we used a split-belt treadmill, which has two belts driving each leg at a different speed. Previous work has shown that people initially “limp” (i.e., show temporal and spatial asymmetry of leg motions) when exposed to split-belt conditions; within 10–15 min of split-belt walking, symmetry is restored (Reisman et al. 2005). This modified pattern is retained when treadmill conditions are returned to normal (i.e., when the belts move at the same speed), which results in subjects limping in the opposite direction. In previous studies, subjects have adapted to the split-belt treadmill without instruction on how to change their gait.
We hypothesized that individuals would adapt at different rates when using conscious effort versus being distracted, with the former adapting faster and the latter slower. Based on the work of Huang and Shadmehr (2009), we thought that these manipulations might also alter the rate of deadaptation (i.e., retention) of the new locomotor pattern. We specifically thought that use of more subconscious processes for adaptation in the distraction condition might lead to longer retention.
METHODS
Subjects
Thirty-three healthy volunteers (19 males and 14 females; mean age, 23.6 yr) participated in this study. All subjects gave informed written consent before participating. The protocols were approved by the Johns Hopkins Institutional Review Board.
Data collection
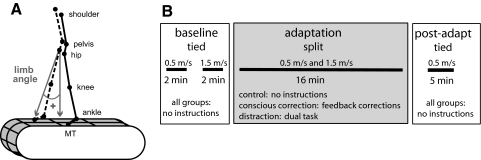
Kinematic data were collected at 100 Hz using Optotrak (Northern Digital). Infared-emitting markers were placed bilaterally over the toe (5th metatarsal head), ankle (lateral malleolus), knee (lateral femoral epicondyle), hip (greater trochanter), pelvis (iliac crest), and shoulder (acromion process; Fig. 1A). Foot contacts were determined using four contact switches per foot: two on the forefoot and two on the heel. Analog data (foot switches and treadmill speed) was synchronized with the kinematic data and collected at 1,000 Hz.
Fig. 1.
A: diagram of marker location and limb angle convention. B: experimental paradigm showing the periods of split-belt walking and conditions.
Experimental protocol
Split-belt walking adaptation was studied using a custom-built treadmill (Woodway) that had two separate belts driven by independent motors. Speed commands for each belt were sent to the treadmill through a custom MATLAB (MathWorks) computer interface. Subjects were positioned in the middle of the treadmill with one leg on each belt and wore a safety harness that was suspended from the ceiling. The safety harness did not support their body weight. At the beginning of each trial, the belts were stationary, and subjects were not informed of the speeds of the belts. Subjects were instructed to initially hold onto a ground-referenced rail when the belts were started and to lift their hands off the rail and cross their arms as soon as they felt comfortable (within 5 s). They were also instructed to refrain from looking down at the belts. In the “tied-belt” condition, the belts moved at the same speed (0.5 or 1.5 m/s). In the “split-belt” condition, the belt under the dominant leg moved at 0.5 m/s and the other at 1.5 m/s.
The experimental paradigm is shown in Fig. 1B. All subjects began the experiment with two baseline periods during which the belts were tied at slow and fast speeds. Following this, the subjects were adapted to split-belts for 16 min. Subjects were randomly assigned to one of three groups, and each group was given different instructions during adaptation. Subjects in the control group were given no instructions (n = 11). The conscious correction group was given on-line visual feedback of their steps and instructed to “keep their step lengths equal on both sides” during the entire adaptation block (n = 11). To allow subjects to develop their own error monitoring and correction mechanisms, the experimenter demonstrated what was defined as a step length until the subject had an understanding of the parameter, but allowed the subject to monitor his/her own errors (i.e., the experimenter did not comment on the step lengths) once the experiment began.
Step length was defined as the anterior-posterior distance between the ankle markers at heel contact—slow step length was measured at heel contact of the slow leg; fast step length was measured at heel contact of the fast leg (Fig. 2). The on-line visual feedback was provided via a video camera that recorded a sagittal view of the subjects' lower calves and feet—the video image was displayed in real-time on a television in front of them. Subjects received the visual feedback of their walking pattern for 25% of the adaptation block, alternating 30 s on and 90 s off. This was done based on prior work suggesting that intermittent feedback is most helpful in learning and retaining new movements (REF) (Cirstea and Levin 2007; Schmidt 1991). The distraction group (n = 11) watched a television program unrelated to walking and were told to count the number of times a particular word was said using a handheld counter. Additionally, subjects in the distraction group were asked to focus their attention on the television so that they could answer questions about the program's visual scenes after the adaptation block finished. Therefore the subjects were distracted by audio and visual stimuli. After the adaptation period, the belts were tied at 0.5 m/s for 5 min (deadaptation).
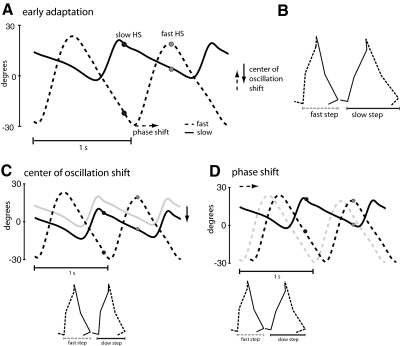
Fig. 2.
A: limb angle trajectories plotted as a function of time in early split-belt adaptation—2 cycles are shown. Positive limb angles are when the limb is in front of the trunk (flexion). Two time points are marked—slow heel strike (HS) in black and fast HS in gray. The spread between the limb angles is directly proportional to the step lengths shown in B. Step lengths can be changed through alterations in phasing (lag time at peak cross-correlation) or through a shift in the center of oscillation (midpoint angle of the limb between heel strike and toe off for each leg). B: stick figure diagram of the legs taking 2 consecutive steps. C: step lengths can be equalized through a shift in the center of oscillation (purely spatial change). Gray trajectory represents the slow limb in early adaptation. A spatial shift in the slow limb's center of oscillation can result in equal step lengths. D: a phase lag (purely temporal change) in the fast limb from the gray trajectory (early adaptation) to the black trajectory will also equalize the step lengths.
Importantly, during deadaptation, all subjects were under control conditions, where the distracter and visual feedback were removed, and subjects were asked to “just walk,” without looking down at the belts. We did not use a drape to block the subject's view of the belts, because we wanted all subjects to be as unconstrained as possible on the treadmill (i.e., nothing attached to them and not holding on). For this reason, we asked subjects to look straight ahead when on the treadmill and monitored their performance to make sure they did not focus on the belt speeds during the experiment.
Data analysis
The primary measurement used in this study was step length symmetry, because subjects could be instructed to change their step lengths consciously and because prior studies have shown that this measure adapts robustly (Reisman et al. 2005, 2007). There are two ways that subjects can alter their step lengths in this task: by changing phasing between the legs and by shifting the center of oscillation for each leg. These are further described below. For all parameters, the data were analyzed starting from the time subjects removed their hands from the safety bar, where maximum step asymmetry occurred during early adaptation and early deadaptation.
Step symmetry (SS) was defined as the normalized difference between the step lengths (SL) of the fast and slow leg (e.g., Fig. 2), or
A positive step symmetry value meant that the fast step was larger than the slow step and vice versa for negative values. A value of 0 indicates symmetry. Normalization was done so subjects of different height who take different sized steps could be compared.
Figure 2 shows the two ways that a subject can alter step symmetry. First, subjects can change step lengths by shifting the angle about which each limb oscillates. The “center of oscillation” was determined to ascertain whether the leg was oscillating about a flexed, extended, or neutral (i.e., vertical) axis. This was calculated on a stride-by-stride basis as the midpoint of the limb angle between heel strike and toe off for each leg. Limb angle was defined as the angle between a vertical line and the vector from the hip to the foot on an x-y plane (Fig. 1B); it was positive when the foot is in front of the hip (flexion) and negative behind (extension). When the limb was oscillating symmetrically around a vertical axis at the top of the pelvis, the center of oscillation value was defined as zero. The center of oscillation of the fast leg was subtracted from that of the slow leg to give the center of oscillation difference between the two legs. When the center of oscillation difference is zero, stepping in the spatial realm is symmetric.
Second, subjects can change phasing (i.e., shift timing) between the legs' motions. Phasing was determined using the time series of limb angles for each leg (Choi and Bastian 2007). It was calculated as the lag time at peak cross-correlation (Signal Processing Toolbox, MATLAB) of the limb angle trajectories over one stride cycle (Choi and Bastian 2007). Possible phasing values ranged from 0 to 1 stride cycles, with symmetric walking having a value of 0.5 (i.e., out-of-phase walking). The slow leg was the reference leg in this analysis.
The rates of adaptation and deadaptation for each parameter were quantified on a subject-by-subject basis. Individual curves were smoothed with a moving average and binned by three strides, and a calculation on the number of strides it took for each subject to reach a plateau in his/her behavior was done. The plateau range was defined as the mean ± SD of the last 30 strides. In a few cases, an individual subject's variability decreased at the end of adaptation. In this situation, we calculated the plateau range, once the behavior reached plateau but before the variability decreased. The beginning of the plateau was determined as the first point at which five consecutive strides fell within the plateau range for each period. The advantage of this rate calculation is that it is agnostic—it does not assume that the subjects follow a specific pattern as, for example, would a single or double exponential fit.
Statistical analysis
One-way ANOVAs were used to compare the first 5 strides (early) and the last 30 strides (late) in adaptation and deadaptation across the three groups. Post hoc pairwise comparisons were done using a Fisher LSD test. The rates of adaptation and deadaptation were analyzed using the number of strides to plateau, as described above. One-way ANOVAs were also used to compare the adaptation and deadaptation rates across groups. Statistica (StatSoft, Tulsa, OK) was used for all statistical analysis.
RESULTS
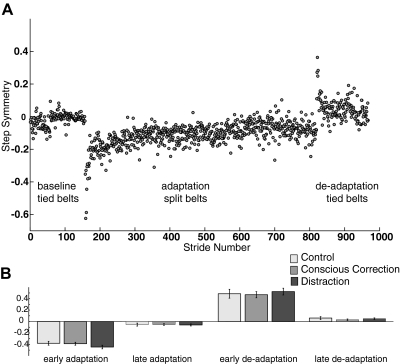
All subjects were able to complete the walking task without difficulty, regardless of group assignment. Figure 3A shows a single subject example of step symmetry plotted stride-by-stride. This subject came from the control group and shows important elements of this behavior. Baseline (tied belt) walking was symmetric when both belts moved together at slow or fast speeds. During early adaptation, the split-belts caused a marked step asymmetry that changed with increasing stride number. By the end of adaptation, the subject's performance returned to near baseline values. In the deadaptation period with belts tied, this subject showed a robust aftereffect that was washed out by the end of this period.
Fig. 3.
A: example of single subject (control) step symmetry data for the entire experiment. In baseline and deadaptation periods, the belts were tied (same speed). In the adaptation block, belts were split (3:1 speed ratio). The visual feedback and dual-task conditions were only used during the adaptation block. B: subjects did not differ in step symmetry during periods of early adaptation (P = 0.27), late adaptation (P = 0.96), early deadaptation (P = 0.83), and late deadaptation (P = 0.52).
To fairly compare data from the three groups, we first checked to make sure that their initial perturbations were comparable. Figure 3B shows no significant difference in the size of the initial asymmetry during early adaptation [F(2,30) = 1.37, P = 0.27], indicating all groups were similarly perturbed when the belts were first split.
We checked to see how much each group changed over the course of the split-belt adaptation period. All groups reached plateau at the same level, as determined from the average of the last 30 strides in late adaptation [Fig. 3B; F(2,30) = 0.04, P = 0.96]. In other words, no group learned more than the others by the end of adaptation. To quantify the storage of a new walking pattern, we analyzed the step symmetry in the early deadaptation period, when the belts are tied again and all groups are walking without distraction or conscious corrections. Figure 3B shows that the aftereffect (average of the 1st 5 strides in deadaptation) was the same across groups, indicating that training method did not affect the amount stored during adaptation [F(2,30) = 0.19, P = 0.83]. Figure 3B also shows that all groups washed out the learned pattern to similar extents by the end of the deadaptation period and resumed a near symmetric walking pattern [F(2,30) = 0.67, P = 0.52]. Thus the magnitude of change in behavior during adaptation and deadaptation was similar across groups.
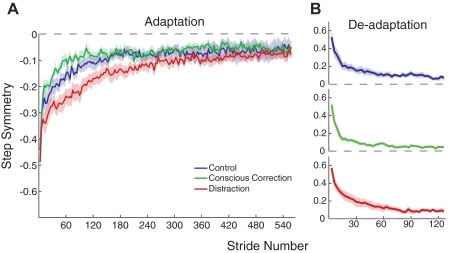
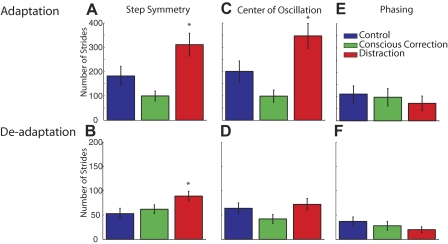
What did differ across the groups was the rate of adaptation and deadaptation (Fig. 4). Rate was quantified as the number of strides it took to plateau for each subject, as described in the methods. Figure 4 shows group behavior for step symmetry in adaptation and deadaptation. During adaptation, the conscious correction group reached plateau slightly faster than the control group, which was faster than the distraction group [F(2,30) = 7.95, P < 0.01; Fig. 4A]. Post hoc analysis (Fig. 5A) showed that the distraction group rate was different from both the control group (P = 0.02) and conscious correction group (P < 0.01), while the difference between control and conscious correction was not significant (P = 0.14). Thus, all groups adapted to the split-belt perturbation, but the rate at which subjects adapted differed depending on the training method that was used, with distraction being slowest.
Fig. 4.
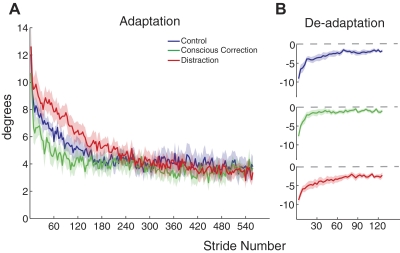
Adaptation and deadaptation curves for step symmetry. A: average adaptation curves for the 3 groups, with SE indicated by the shaded area. Baseline values are subtracted out from curves (i.e., symmetry is indicated by a value of 0). B: average deadaptation curves for the 3 groups. Recall that all groups deadapted under the same condition (no feedback or distraction). Curves are shown individually to more clearly show the plateau level.
Fig. 5.
The adaptation and deadaptation rate expressed as the number of strides until a plateau is reached for each measure. A: for step symmetry, distraction subjects adapted slower than control and conscious correction subjects. Whereas conscious correction subjects tended to adapt faster than control subjects, this difference did not reach significance. B: for deadaptation, distraction subjects also took longer to reach a plateau than the control and conscious correction subjects. The rate of deadaptation was similar for conscious correction and control subjects. C: similar trends are shown for rate of adaptation in center of oscillation difference, compared with step symmetry. Distraction subjects took the longest to adapt vs. control subjects and conscious correction. D: deadaptation in center of oscillation difference is similar to adaptation. The conscious correction group adapted fastest, then control, and distraction was the slowest. E: all groups adapted phasing at similar rates. F: deadaptation rate for phasing was also similar across groups.
It is not entirely surprising that we found differences in the adaptation rate across the groups, because they encountered different demands during training. We were intrigued, however, to find differences in the rates of deadaptation. Recall that in the deadaptation period, all groups walked in the same control condition (i.e., no visual feedback of foot motion; no distraction). Figure 4B shows group mean data in deadaptation, with ANOVA results showing a difference in group deadaptation rates [F(2,30) = 3.52, P = 0.04]. Generally speaking, subjects deadapted much faster than they adapted, regardless of group. However, post hoc analysis shown in Fig. 5B showed that distraction was different from control (P = 0.02) and trended toward different from conscious correction (P = 0.07), whereas there was no difference between control and conscious correction (P = 0.51).
There are two strategies that subjects could use to adapt their step symmetry: they could shift the center of oscillation (i.e., spatial) of each limb or alter the phasing (i.e., temporal) of motion between the limbs. Figure 6 shows the adaptation and deadaptation pattern for the center of oscillation difference. For adaptation, the conscious correction group adapted fastest, the control group was intermediate, and the distraction group was slowest [F(2,30) = 9.16, P < 0.01]. The rates are similar to those calculated for step symmetry during adaptation (Fig. 5C). Post hoc analysis on the rate of adaptation for center of oscillation difference showed that distraction was significantly different from control (P = 0.02) and conscious correction (P < 0.01), while the difference between the control and conscious correction group was not significant (P = 0.09). The same pattern held in deadaptation: the conscious correction group was fastest, followed by the control group and the distraction group (Fig. 5D). ANOVA results in group rates of deadaptation for center of oscillation difference only showed a trend toward significance [F(2,30) = 2.24, P = 0.12]. In sum, our manipulations had a clear effect on spatial elements of limb motions.
Fig. 6.
Adaptation and deadaptation curves for the center of oscillation difference. A: average adaptation curves for the 3 groups plotted as in Fig. 4. Trends seen in the center of oscillation are comparable to those seen in step symmetry. B: average deadaptation curves, shown individually.
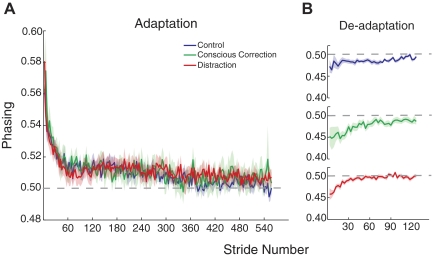
In contrast, there was very little effect on the time course of adapting temporal elements of limb motion. Figure 7 shows the average plots of phasing adaptation and deadaptation for each group. All three groups adapted (Fig. 5E) and deadapted (Fig. 5F) phasing at similar rates. ANOVA effects of phasing rate for adaptation [F(2,30) = 0.35, P = 0.71] and deadaptation [F(2,30) = 1.33, P = 0.28] showed no difference in group. Moreover, it is interesting to note that the phasing adaptation rate differs markedly from that observed for step symmetry—it is at least twice as fast as that for spatial parameter rates.
Fig. 7.
Adaptation and deadaptation curves for phasing. A: average curves for the 3 groups plotted as in Fig. 4. No differences were found in the adaptation rate. B: average deadaptation curves shown individually. Again, no differences were found in phasing deadaptation rate.
DISCUSSION
Here we showed that conscious corrections speed up split-belt walking adaptation, whereas distraction slows it down. Perhaps more surprisingly, deadaptation followed a similar pattern despite removal of the visual feedback or distracter during that period of the experiment. One interpretation of this is that how one learns affects the rate of unlearning (Huang and Shadmehr 2009), possibly because subjects engage neural circuits with different time courses under these conditions. We also found that our manipulations affected the adaptation rate of spatial elements of walking (i.e., stepping distance) but had minimal effects on the adaptation rate of temporal elements of walking (i.e., phasing). This unexpected result suggests that the control of spatial and temporal patterns of walking may be accessible through different neural circuits.
Conscious versus automatic control
It has been shown that the prior experience of the subject and the type of feedback provided to them are both critical factors affecting how conscious corrections will impact motor learning. Feedback that directs subjects' attention to internal parameters, such as the force of their feet (Wulf et al. 1998), the specific location of their feet (Wulf et al. 1998), or the swing of their arms (Wulf et al. 1999) can interfere with learning. These studies instead suggest that inexperienced subjects should receive feedback with an external focus of attention, which will direct subjects to the effects of their movements [e.g., the pendulum-like motion of a golf club (Wulf et al. 1999) or the accuracy in hitting a particular target (Perkins-Ceccato et al. 2003)]. With focus on a movement's effects on the environment, one can use the natural control processes inherent to the motor system, as opposed to when focus is on particular joints and muscles, which can constrain a limb's degrees of freedom (McNevin et al. 2003). Here we tried to keep the subjects as unconstrained as possible—we did not ask subjects to step in a particular location, but instead asked them to equalize their steps. This could be accomplished by changing the center of oscillation or phasing. This feedback was appropriate to allow subjects in our conscious correction group to improve their spatial symmetry adaptation rate, without constraining the possible ways to adjust their limbs.
Conscious or executive control can to play a role in the very initial stages of reaching adaptation (Taylor and Thoroughman 2008). In this study, we also saw that conscious processes engaged in the conscious correction group affected the initial epoch of adaptation most (i.e., 1st 60 strides). Additionally, we saw a trend that the conscious correction group deadapted more quickly, suggesting that this conscious modulation of walking can be rapidly disengaged. This is most obvious in our measure of purely spatial control of the limbs (i.e., the center of oscillation). In contrast, reducing conscious correction by requiring dual-task performance leads to slower adaptation.
It is not surprising that adaptation still occurs in the distraction group—previous studies have shown that it can be driven using more implicit and automatic processes (Bronstein et al. 2009; Frensch 1998; Mazzoni and Krakauer 2006). Indeed, studies of balance and locomotion, such as in the “broken escalator phenomenon,” have shown that adaptation and aftereffects occur even when subjects have declarative knowledge about the upcoming platform movement (Bronstein et al. 2009). In other words, declarative systems can be at odds with procedural motor learning systems, and yet adaptation still occurs. This is also shown in tasks that use gradual perturbations that can drive adaptation even without conscious awareness and correction of errors (Mazzoni and Krakauer 2006). This is thought to occur because the motor system responds to the error in the planned versus executed trajectory, not just errors in task performance (Mazzoni and Krakauer 2006; Tseng et al. 2007). We suggest that these automatic adaptive processes may be more responsible for the slower adaptation and also seem to improve retention as seen by the longer deadaptation rate.
Distinct control of spatial versus temporal patterns of walking
The differences in the adaptation rates of spatial and temporal elements of walking were unexpected—based on our prior work, we had assumed that they were controlled by overlapping neural systems, and would therefore adapt together (Choi and Bastian 2007). Instead, this dissociation shows that conscious correction processes may have preferential access to spatial and not temporal control of the limbs during walking. Evidence from our control group in this study supports this notion: they showed differences in the adaptation rate of temporal versus spatial parameters, with the former adapting nearly 2 times faster (cf. blue bars, Fig. 5, C and E).
Patient studies also support the notion of distinct control mechanisms. Children with hemispherectomy (i.e., have 1 cerebral hemisphere removed) show temporal asymmetry under normal conditions and have deficits adapting temporal features of walking on the split-belt treadmill (Choi et al. 2008). However, they adapt spatial features of walking normally. Patients with spinal cord injury have not been tested in our split-belt adaptation paradigm, but these individuals show fairly normal temporal symmetry in walking under normal conditions (Ivanenko et al. 2009). Taken together, these studies suggest that the basic symmetric timing for locomotion can be set at the spinal cord level but that asymmetric supraspinal influences can influence spinal half-centers and result in temporal gait asymmetry. Why the children with hemispherectomy have temporal but not spatial adaptation deficits is not fully understood—although it is worth noting that these children show crossed cerebellar degeneration and substantial CNS reorganization, which could contribute to their pattern of deficits. Nonetheless, these results show another instance where there is a dissociation of temporal and spatial adaptive mechanisms.
Why did our manipulations influence adaptation of the spatial pattern only? One possibility is that it was driven by the feedback that we gave (i.e., make your steps even). Step symmetry can be changed via limb phasing (i.e., timing), but perhaps it is easiest to envision the change in the spatial domain (i.e., center of oscillation). That being said, feedback was only given to the conscious correction group; the distraction group was told nothing about the task, yet spatial parameters were affected, whereas timing was not. Thus we think that the form of the feedback in the conscious correction group cannot be driving the observed dissociation. A second reason why the phasing adaptation rate was not altered could be that it is inherently less modifiable. Antiphase timing of leg motions may be most essential for maintaining upright balance in walking and perhaps naturally adapts at a faster rate than the spatial parameter. We do not know if the rate could be changed using more specific feedback, such as an auditory tempo, and plan to investigate this in the future. A third possibility is that the neural computation for spatial adaptation is simpler (e.g., an offset in limb movement) than that in the temporal domain, and therefore more easily adjusted voluntarily. If so, then the nervous system may be able to adjust it faster or slower depending on the amount of conscious effort allowed. We think that the latter two possibilities are likely and may both be operational in this study.
Potential neural substrates
Locomotion normally involves interactions between cerebral centers, the cerebellum, the brain stem, spinal circuits, and afferent sensory feedback. The basic alternating muscle pattern appropriate for walking can be seen in decerebrate and deafferented animals because of the presence of spinal central pattern generators (CPGs) (Goulding 2009; Grillner 2006). Humans may also have CPGs, although it is more difficult to prove. Infants show a basic alternating stepping pattern on a treadmill before the myelination of their corticospinal pathways (Yang et al. 1998), which suggests that supraspinal control is not needed. Overall, it is thought that supraspinal centers and afferent feedback may play more of a role in adjusting the spinal circuits to perturbations and different environments.
Our observation of differences in spatial versus timing adaptation begs the question of whether different neural structures contribute to these processes. We suggest that it is plausible—for example, at the spinal cord level, a two-level CPG has been proposed where separate neural networks are responsible for rhythm control and pattern generation (Lafreniere-Roula and McCrea 2005; Rybak et al. 2006). This could be a substrate for differential control of timing and spatial features of locomotion. Additionally, tonic stimulation of the mecenscephalic locomotor region can adjust the timing of gait (Shik et al. 1969), while descending drive from vestibular systems in decerebrate cats can change the balance of flexor and extensor muscle activity (Gottschall and Nichols 2007), possibly reflecting a change in the spatial pattern. Lesion data from our laboratory suggest that supraspinal centers are needed for this adaptation in humans. In particular, cerebellar damage impairs adaptation of both timing and spatial features of walking (Morton and Bastian 2006).
It is possible is that distinct cerebellar regions preferentially influence different levels of spinal control of locomotion. Cerebellar control of the timing of locomotion could require the spinocerebellum. Indeed, stimulation of Deiter's nucleus (termination region of fastigiovestibular fibers) has been shown to influence the timing of the locomotor pattern (Russell and Zajac 1979). Descending projections through the vestibulospinal and reticulospinal pathways could influence the rhythm generator in the two-level CPG (Arshavsky et al. 1978a-c). Conversely, control of spatial pattern of locomotion could be adjusted through the lateral hemispheres of the cerebellum, which has projections to cerebral structures via the thalamus. These cortical structures (particularly motor cortex) can influence the pattern generator of the CPG through corticospinal pathways.
This organization could explain why only the spatial features of locomotion (i.e., center of oscillation) were influenced by our manipulations. If the spatial pathway was accessible through cerebral centers, it may be more affected by conscious effort toward the task versus cognitive demands away from it. In contrast, if the temporal features were controlled by midbrain/brain stem structures, they would be more difficult to access with our conscious control or distraction mechanisms.
Conclusion
Understanding the adaptation and learning processes for locomotion is essential for rehabilitation. Although it is known that locomotor patterns can be learned at a spinal level in many animals (e.g., cat, Rossignol et al. 1999), there is evidence that compensation for injuries, such as peripheral nerve damage, may require descending control from the brain (Bouyer and Rossignol 2003). While our prior work showed that split-belt adaptation in humans requires supraspinal structures (i.e., cerebellum, Morton and Bastian 2006), we have shown that this form of adaptation can still be used to improve gait asymmetries from focal cerebral lesions caused by stroke (Reisman et al. 2007). We therefore think that split-belt training may be useful for rehabilitation of walking asymmetry from hemiparesis.
This study highlights two important issues. First, therapists commonly instruct patients to think about their walking after an injury, increasing descending cortical control. This may enable people to change their walking pattern quickly, but it may be that learning in this manner may result in more transient changes (especially in spatial characteristics). Second, training with distraction (i.e., dual-tasking) may be more difficult, but produces longer lasting effects, at least on a single session basis. It may also lead to more functional walking, because other work has shown that a dual cognitive task can interfere with walking performance after stroke (Haggard et al. 2000). We do not know how these manipulations will play out after stroke or during long-term training. Our current thought is that the best strategy for training an automatic behavior like walking is in a way that reduces the conscious contributions to the process.
It is interesting that temporal and spatial parameters of walking adapt at different rates and show different sensitivities to conscious corrections and distraction. This suggests that there may be differential accessibility to neural mechanisms responsible for learning in these domains. Future work will be aimed at understanding the neural underpinnings associated with these differences and how best to leverage them for therapeutic purposes.
GRANTS
This work was supported by National Institute of Child Health and Human Development Grant R01 HD-048741.
ACKNOWLEDGMENTS
We thank A. Gurbani, R. Pallegadda, and P. Trautman for assistance with data collection.
REFERENCES
- Anderson BJ, Eckburg PB, Relucio KI. Alterations in the thickness of motor cortical subregions after motor-skill learning and exercise. Learn Mem 9: 1–9, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arshavsky YI, Gelfand IM, Orlovsky GN, Pavlova GA. Messages conveyed by descending tracts during scratching in the cat. I. Activity of vestibulospinal neurons. Brain Res 159: 99–110, 1978a [DOI] [PubMed] [Google Scholar]
- Arshavsky YI, Gelfand IM, Orlovsky GN, Pavlova GA. Messages conveyed by spinocerebellar pathways during scratching in the cat. I. Activity of neurons of the lateral reticular nucleus. Brain Res 151: 479–491, 1978b [DOI] [PubMed] [Google Scholar]
- Arshavsky YI, Gelfand IM, Orlovsky GN, Pavlova GA. Messages conveyed by spinocerebellar pathways during scratching in the cat. II. Activity of neurons of the ventral spinocerebellar tract. Brain Res 151: 493–506, 1978c [DOI] [PubMed] [Google Scholar]
- Bastian AJ. Understanding sensorimotor adaptation and learning for rehabilitation. Curr Opin Neurol 21: 628–633, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouyer LJ, Rossignol S. Contribution of cutaneous inputs from the hindpaw to the control of locomotion. II. Spinal cats. J Neurophysiol 90: 3640–3653, 2003 [DOI] [PubMed] [Google Scholar]
- Bronstein AM, Bunday KL, Reynolds R. What the “broken escalator” phenomenon teaches us about balance. Ann NY Acad Sci 1164: 82–88, 2009 [DOI] [PubMed] [Google Scholar]
- Choi JT, Bastian AJ. Adaptation reveals independent control networks for human walking. Nat Neurosci 10: 1055–1062, 2007 [DOI] [PubMed] [Google Scholar]
- Choi JT, Vining EP, Reisman DS, Bastian AJ. Walking flexibility after hemispherectomy: split-belt treadmill adaptation and feedback control. Brain 103: 1–9, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirstea MC, Levin MF. Improvement of arm movement patterns and endpoint control depends on type of feedback during practice in stroke survivors. Neurorehabil Neural Repair 21: 398–411, 2007 [DOI] [PubMed] [Google Scholar]
- Collette F, Olivier L, Van der LM, Laureys S, Delfiore G, Luxen A, Salmon E. Involvement of both prefrontal and inferior parietal cortex in dual-task performance. Brain Res Cogn Brain Res 24: 237–251, 2005 [DOI] [PubMed] [Google Scholar]
- Frensch PA. One concept, multiple meanings: on how to define the concept of implicit learning. In: Handbook of Implicit Learning, edited by Standler M, Frensch PA. Thousand Oaks, CA: Sage, 1998, p. 47–104 [Google Scholar]
- Gottschall JS, Nichols TR. Head pitch affects muscle activity in the decerebrate cat hindlimb during walking. Exp Brain Res 182: 131–135, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulding M. Circuits controlling vertebrate locomotion: moving in a new direction. Nat Rev Neurosci 10: 507–518, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S. Biological pattern generation: the cellular and computational logic of networks in motion. Neuron 52: 751–766, 2006 [DOI] [PubMed] [Google Scholar]
- Haggard P, Cockburn J, Cock J, Fordham C, Wade D. Interference between gait and cognitive tasks in a rehabilitating neurological population. J Neurol Neurosurg Psychiatry 69: 479–486, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang VS, Shadmehr R. Persistence of motor memories reflects statistics of the learning event. J Neurophysiol 102: 931–940, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanenko YP, Poppele RE, Lacquaniti F. Distributed neural networks for controlling human locomotion: lessons from normal and SCI subjects. Brain Res Bull 78: 13–21, 2009 [DOI] [PubMed] [Google Scholar]
- Krakauer JW. Motor learning and consolidation: the case of visuomotor rotation. Adv Exp Med Biol 629: 405–421, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafreniere-Roula M, McCrea DA. Deletions of rhythmic motoneuron activity during fictive locomotion and scratch provide clues to the organization of the mammalian central pattern generator. J Neurophysiol 94: 1120–1132, 2005 [DOI] [PubMed] [Google Scholar]
- Lang CE, Bastian AJ. Cerebellar damage impairs automaticity of a recently practiced movement. J Neurophysiol 87: 1336–1347, 2002 [DOI] [PubMed] [Google Scholar]
- Maddox MD, Wulf G, Wright DL. The effect of an internal vs. external focus of attention on the learning of a tennis stroke. J Exerc Psychol 21: S78, 1999 [Google Scholar]
- Mazzoni P, Krakauer JW. An implicit plan overrides an explicit strategy during visuomotor adaptation. J Neurosci 26: 3642–3645, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNevin NH, Shea CH, Wulf G. Increasing the distance of an external focus of attention enhances learning. Psychol Res 67: 22–29, 2003 [DOI] [PubMed] [Google Scholar]
- Morton SM, Bastian AJ. Cerebellar contributions to locomotor adaptations during splitbelt treadmill walking. J Neurosci 26: 9107–9116, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins-Ceccato N, Passmore SR, Lee TD. Effects of focus of attention depend on golfers' skill. J Sports Sci 21: 593–600, 2003 [DOI] [PubMed] [Google Scholar]
- Reisman DS, Block HJ, Bastian AJ. Interlimb coordination during locomotion: what can be adapted and stored? J Neurophysiol 94: 2403–2415, 2005 [DOI] [PubMed] [Google Scholar]
- Reisman DS, Wityk R, Silver K, Bastian AJ. Locomotor adaptation on a split-belt treadmill can improve walking symmetry post-stroke. Brain 130: 1861–1872, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds RF, Bronstein AM. The broken escalator phenomenon. Aftereffect of walking onto a moving platform. Exp Brain Res 151: 301–308, 2003 [DOI] [PubMed] [Google Scholar]
- Rossignol S, Drew T, Brustein E, Jiang W. Locomotor performance and adaptation after partial or complete spinal cord lesions in the cat. Prog Brain Res 123: 349–365, 1999 [DOI] [PubMed] [Google Scholar]
- Russell DF, Zajac FE. Effects of stimulating Deiters' nucleus and medial longitudinal fasciculus on the timing of the fictive locomotor rhythm induced in cats by DOPA. Brain Res 177: 588–592, 1979 [DOI] [PubMed] [Google Scholar]
- Rybak IA, Shevtsova NA, Lafreniere-Roula M, McCrea DA. Modelling spinal circuitry involved in locomotor pattern generation: insights from deletions during fictive locomotion. J Physiol 577: 617–639, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmoni AW, Schmidt RA, Walter CB. Knowledge of results and motor learning: a review and critical reappraisal. Psychol Bull 95: 355–386, 1984 [PubMed] [Google Scholar]
- Schmidt RA. Frequent augmented feedback can degrade learning: evidence and interpretations. In: Tutorials in Motor Neuroscience, edited by Requin J, Stelmach GE. Dordrecht, The Netherlands: Kluwer, 1991, p. 59–75 [Google Scholar]
- Schubert T, Szameitat AJ. Functional neuroanatomy of interference in overlapping dual tasks: an fMRI study. Brain Res Cogn Brain Res 17: 733–746, 2003 [DOI] [PubMed] [Google Scholar]
- Shik ML, Severin FV, Orlovsky GN. Control of walking and running by means of electrical stimulation of the mesencephalon. Electroencephalogr Clin Neurophysiol 26: 549, 1969 [PubMed] [Google Scholar]
- Taylor JA, Thoroughman KA. Divided attention impairs human motor adaptation but not feedback control. J Neurophysiol 98: 317–326, 2007 [DOI] [PubMed] [Google Scholar]
- Taylor JA, Thoroughman KA. Motor adaptation scaled by the difficulty of a secondary cognitive task. PLoS One 3: e2485, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorov E, Shadmehr R, Bizzi E. Augmented feedback presented in a virtual environment accelerates learning of a difficult motor task. J Motor Behav 29: 147–158, 1997 [DOI] [PubMed] [Google Scholar]
- Tseng YW, Diedrichsen J, Krakauer JW, Shadmehr R, Bastian AJ. Sensory prediction errors drive cerebellum-dependent adaptation of reaching. J Neurophysiol 98: 54–62, 2007 [DOI] [PubMed] [Google Scholar]
- Wulf G, Hosz M, Prinz W. Instructions for motor learning: differential effects of internal versus external focus of attention. J Motor Behav 30: 169–179, 1998 [DOI] [PubMed] [Google Scholar]
- Wulf G, Lauterbach B, Toole T. Learning advantages of an external focus of attention in golf. Res Q Exerc Sport 70: 120–126, 1999 [DOI] [PubMed] [Google Scholar]
- Wulf G, Prinz W. Directing attention to movement effects enhances learning: a review. Psychol Bull Rev 8: 648–660, 2001 [DOI] [PubMed] [Google Scholar]
- Wulf G, Weigelt C. Instructions about physical principles in learning a complex motor skill: to tell or not to tell. Res Q Exerc Sport 68: 362–367, 1997 [DOI] [PubMed] [Google Scholar]
- Yang JF, Stephens MJ, Vishram R. Infant stepping: a method to study the sensory control of human walking. J Physiol 507: 927–937, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogev-Seligmann G, Hausdorff JM, Giladi N. The role of executive function and attention in gait. Mov Disord 23: 329–342, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]