Abstract
Receptive fields of neurons in somatosensory area 3b of monkeys are typically described as restricted to part of a single digit or palm pad. However, such neurons are likely involved in integrating stimulus information from across the hand. To evaluate this possibility, we recorded from area 3b neurons in anesthetized owl monkeys with 100-electrode arrays, stimulating two hand locations with electromechanical probes simultaneously or asynchronously. Response magnitudes and latencies of single- and multiunits varied with stimulus conditions, and multiunit responses were similar to single-unit responses. The mean peak firing rate for single neurons stimulated within the preferred location was estimated to be ∼26 spike/s. Simultaneous stimulation with a second probe outside the preferred location slightly decreased peak firing rates to ∼22 spike/s. When the nonpreferred stimulus preceded the preferred stimulus by 10–500 ms, peak firing rates were suppressed with greatest suppression when the nonpreferred stimulus preceded by 30 ms (∼7 spike/s). The mean latency for single neurons stimulated within the preferred location was ∼23 ms, and latency was little affected by simultaneous paired stimulation. However, when the nonpreferred stimulus preceded the preferred stimulus by 10 ms, latencies shortened to ∼16 ms. Response suppression occurred even when stimuli were separated by long distances (nonadjacent digits) or long times (500 ms onset asynchrony). Facilitation, though rare, occurred most often when the stimulus onsets were within 0–30 ms of each other. These findings quantify spatiotemporal interactions and support the hypothesis that area 3b is involved in widespread stimulus integration.
INTRODUCTION
As tactile object recognition depends on the integration of information from different parts of the hand, it is logical to presume that neurons somewhere in the somatosensory system integrate information from locations across the hand. Primary somatosensory cortex (S1) is the first cortical level at which this integration could take place. In primates, anterior parietal cortex can be subdivided into somatosensory areas 3a, 3b, 1, and 2 with area 3b, the homologue of S1 in other mammals (Kaas 1983), as the first site for processing tactile information. We selected New World owl monkeys for these studies because the cortical area of interest, area 3b, is not buried in a central sulcus in this primate and the somatosensory cortex has been well studied (e.g., Cusick et al. 1989; Garraghty et al. 1989; Merzenich et al. 1978; Nicolelis et al. 2003). An evaluation of the extent of this integration in area 3b is a critical step in determining its role in object perception and other somatosensory abilities.
Although area 3b receptive fields in monkeys have been consistently described as restricted in size and usually confined to part of a single digit phalanx or palm pad (e.g., DiCarlo et al. 1998; Iwamura et al. 1983; Pons et al. 1987; Sur 1980; Sur et al. 1985), there is evidence that stimuli spatially distant to the receptive fields modulate neuron responses. For example, in primary visual cortex of monkeys, stimuli “beyond the classical receptive field” are now well known to modulate responses of neurons to stimuli within the receptive field (e.g., Allman et al. 1985; Brown et al. 2003; Walker et al. 2000; Wang et al. 1995). As for the classical receptive field in vision research, the tactile minimal receptive field (mRF) (e.g., Merzenich et al. 1983; Xerri et al. 1999), the excitatory field to near-threshold stimuli, of the monkey hand can be surrounded by regions that, when stimulated, modulate responses to stimuli within the mRF (e.g., DiCarlo et al. 1998; Mountcastle 2005; Sripati et al. 2006). In S1 of rats, neuronal responses are modulated by stimulating whiskers beyond the traditional receptive field of a primary whisker and immediately adjacent whiskers (e.g., Armstrong-James and Fox 1987; Ghazanfar and Nicolelis 1997, 1999; Moore and Nelson 1998; Zhu and Connors 1999) and even by stimulating the forepaw (Berwick et al. 2004). Studies in the forelimb representation of the somatosensory system in several species have examined effects of stimuli within the receptive field and at sites near, but outside, the excitatory receptive field of neurons in cortex (e.g., Burton et al. 1998; DiCarlo et al. 1998, 2000, 2002; Gardner and Costanzo 1980a,b; Greek et al. 2003; Laskin and Spencer 1979; Mountcastle and Powell 1959; Sripati et al. 2006), thalamus (e.g., Canedo and Aguilar 2000; Greek et al. 2003; Jänig et al. 1979), and brain stem (e.g., Canedo and Aguilar 2000; Jänig et al. 1977). Although the proximity of the second stimulus to the receptive field varies, the overall finding is that inhibition caused by the second stimulus is maximal at the receptive field center and decreases with distance from center. Similarly, human psychophysics (e.g., Braun et al. 2005; Schweizer et al. 2000) and evoked potential and magnetic field responses (e.g., Hoechstetter et al. 2001; Pilz et al. 2004; Tanosaki et al. 2002a,b) indicate that a second stimulus will interfere with the response to the first stimulus in a topographic manner, such that near stimuli have a greater effect than distant stimuli.
Here we extend these studies by determining how paired stimuli within and across digits on the monkey hand interact to evoke and modify neuronal responses in area 3b. As temporal factors influence spatial interactions, we presented two stimuli at the same time or at different offset times. Temporal stimulus relationships may signal object relatedness and also play a role in forming cortical topography and receptive fields (Wiemer et al. 2000; Xing and Gerstein 1996). For example, Merzenich and colleagues (1988, 1995) found that increasing co-activation of the digits resulted in multidigit receptive fields and concluded that receptive fields do not simply reflect anatomical connections but are derived from a subset of inputs based on temporal correlations of activity. To examine the effects of temporal stimulus relationships on neuronal response properties, we used a modification of condition-test paradigms in which the conditioning stimulus is presented outside the minimal receptive field and the test stimulus is within or outside of the suprathreshold receptive field for a given neuron. We selected onset delays between the conditioning stimulus and the test stimulus based on studies in raccoons (e.g., Chowdhury and Rasmusson 2003; Greek et al. 2003) and macaque monkeys (Burton et al. 1998; Gardner and Costanzo 1980b) that involved similar condition-test paradigms. Condition-test paradigms have also been studied extensively in the rat whisker barrel system with wide-ranging intervals between the conditioning and test stimuli (e.g., Boloori and Stanley 2006; Shimegi et al. 1999; Simons 1985).
In our design, a conditioning stimulus is referred to as the “nonpreferred stimulus” because this stimulus is the one that evokes fewer spikes from the reference neuron than the “test” or “preferred stimulus.” We presented tactile stimuli that indented the skin for 500 ms, followed by 2.0 s off of the skin, in blocks of 100–120 trials so that each stimulus had the potential to cause sustained activation. Then we selected paired skin sites and delivered pulses simultaneously (0 ms delay) and at selected stimulus onset asynchronies of 10, 30, 50, 100, and 500 ms to study the effects of varying the temporal proximity of stimuli. Thus the paired 500 ms stimuli variably overlapped in time. (For the 500 ms stimulus onset asynchrony condition, as the conditioning stimulus is lifting off of the skin, the test stimulus is contacting the skin. Depending on the spatial proximity of the stimuli, this conditioning stimulus may not affect the response to the test stimulus because the stimuli occur far apart in time.) When the presence of a nonpreferred stimulus affects the responses of a neuron to a preferred stimulus, a stimulus “interaction” occurs. The term “widespread interaction” can then be applied when the effects on the reference neuron occur when stimuli are presented widely separated in space or time. Thus differences in response properties due to paired stimulation with varying spatial and temporal parameters allow us to quantify spatiotemporal stimulus interactions. The current study extends previous studies by investigating spatial stimulus interactions in area 3b neurons of monkeys to compare the effects of stimuli presented to locations within individual digits and stimuli presented to locations on different digits in conjunction with investigating temporal stimulus interactions by presenting the stimuli at varying condition-test intervals. While there are some similar experiments that set the precedents for this study, this is the first report to our knowledge that quantifies widespread spatiotemporal stimulus interaction effects across a large number of systematically sampled neurons in the primate somatosensory cortex hand representation.
METHODS
The present study examines the effects on firing rate and latency of neurons in area 3b of owl monkeys (Aotus nancymaae) when two 500 ms skin indentations were presented simultaneously or at stimulus onset asynchronies to adjacent or nonadjacent hand locations. One stimulus was within the “response field” and the other was within or outside of the response field of the reference neuron (see response field).
Preparation for recording
Two adult male owl monkeys (cases 1 and 2; each 1 kg) and one adult female owl monkey (case 3; 1.2 kg) were prepared for electrophysiological recordings in primary somatosensory cortex following procedures detailed previously (Reed et al. 2008). Monkeys 1 and 2 were part of a previous study related to spike timing correlations (Reed et al. 2008). All procedures followed the guidelines established by the National Institutes of Health and the Animal Care and Use Committee at Vanderbilt University. Each monkey was given a ketamine injection (10–30 mg/kg, intramuscular) for sedation during surgical preparations. Anesthesia was induced with 2–4% halothane gas and maintained with propofol (10 mg·kg−1·h−1 iv) during surgery. Rectal temperature was monitored and maintained between 37 and 39°C with a servo-controlled heating pad. The monkey was secured in a stereotaxic device for surgery and throughout the experiment. After anesthetizing the animal, paralysis was induced with 1–3 ml vecuronium bromide and maintained by intravenous vecuronium bromide (0.1–0.3 mg·kg−1·h−1) mixed with 5% dextrose and lactated Ringer solution. Once paralyzed, animals were artificially ventilated with a mixture of N2O-O2-CO2 (75:23.5:1.5) at a rate sufficient to maintain peak end tidal CO2 at ∼4%. Paralysis was induced to keep the hand stable during long stimulus blocks. Electrocardiograms (ECGs) and electroencephalograms (EEGs) were monitored to gauge depth of anesthesia. The skull and dura overlying primary somatosensory cortex were removed. The pneumatic inserter for the electrode array was set to a depth of 600 μm, so that electrode tips were expected to be within cortical layer 3. After the insertion of the electrode array, the opening was covered with 1% agar mixed with Ringer solution to provide electrode stability and prevent desiccation. Following surgical procedures, supplemental anesthesia during recordings was provided by 0.3 mg·kg−1·h−1 propofol. Similar methods have been described elsewhere (Samonds et al. 2003; Xu et al. 2003). In one monkey (case 3), 1.2 mg/kg sufentanil was added to the lactated Ringer solution for slow infusion during the surgical procedures to stabilize anesthetic depth. Following delivery of this amount of sufentanil, the monkey was maintained under propofol anesthesia and vecuronium bromide paralysis without supplemental sufentanil during extracellular recordings. All monkeys were maintained approximately within sleep stage 2 during the recording experiments, as estimated by inspection of the EEG and ECG.
Stimulation procedures
We focused on comparing neuron responses to paired stimulation on adjacent digits versus nonadjacent digits; however, we also collected responses to paired stimulation on adjacent and nonadjacent phalanges within a single digit. Stimuli were provided by two independent force- and position-feedback controlled motor systems (300B, Aurora Scientific, Aurora, ON, CA). The lever arms of the motors were tipped with round Teflon probes 1 mm in diameter; thus the stimulus probes represented a small contact surface. Stimuli consisted of square wave pulses that indented the skin 0.5 mm for 0.5 s, followed by 2.0 s off of the skin, repeated for 255–300 s (100–120 trials). We used the minimal ramp time allowed by the stimulation equipment, which had a length step response time of 1.3 ms. The long duration of the indentation allowed us to record phasic and sustained responses (Sur et al. 1984). Paired skin locations were selected for stimulation and the indentations were delivered simultaneously (0 ms delay) and at selected stimulus onset asynchronies of 10, 30, 50, 100, and 500 ms, with the conditioning stimulus preceding the test stimulus, to study the effects of varying the temporal proximity of stimuli. Single-site control stimuli were delivered to each of the skin locations prior to paired stimulation. These stimulus parameters were selected to analyze spatiotemporal effects on response properties of neurons in primary somatosensory cortex. For practical purposes, reference units were identified, and probes were positioned so that one probe was inside and one probe was outside the minimal receptive field (mRF) of the reference neuron. The mRF was defined as the region of skin where a near-threshold light touch with a probe reliably evoked spikes from the recorded neurons. Procedures for mRF mapping have been published elsewhere (e.g., Merzenich et al. 1978; Nelson et al. 1980), and our general procedures have been published (Reed et al. 2008). Receptive field mapping also helped locate electrodes as within and outside of area 3b in a manner that complemented our subsequent histological identification of area 3b. The fingernails were glued (cyanoacrylic) to Teflon screws fixed in plasticine to keep the hand in place during stimulus blocks. Adhesive was removed from the fingernails using acetone.
Data acquisition
Recordings were made using the 10 × 10 “Utah” array and the Bionics data acquisition system (Blackrock Microsystems, Salt Lake City, UT). The signals on each channel were amplified by 5,000 and band-pass filtered between 250 Hz and 7.5 kHz. The threshold for each electrode was automatically set for 3.25 times the mean activity and the waveforms were sampled at 30 kHz for 1.5 ms windows (Samonds et al. 2003).
Histology
Following data collection, animals were perfused with saline followed by fixative, and the brains were prepared for histological analysis as described previously (Jain et al. 2001). The cortex was flattened, frozen, and cut parallel to the surface at 40 μm. Sections were processed for myelin to aid in determining the electrode sites relative to the area 3b hand representation (see Reed et al. 2008 for an example of the tissue quality that may be obtained using the 100-electrode array and a description of how electrode depths and locations are estimated). We estimated electrode depth by tracking the appearance and disappearance of electrode tracks across serial sections.
Data analysis
SPIKE SORTING.
The details of the spike sorting procedures have been described previously (Reed et al. 2008). Recorded signals were sorted off-line with an automatic spike classification program based on the t-distribution expectation maximization algorithm (Shoham et al. 2003), which is part of the data acquisition system. The recordings for a given stimulus block were sorted together to standardize sorting across recordings. We used a second spike sorter program, Plexon off-line sorter (Plexon, Dallas, TX) to verify the quality of unit isolation such that single units had refractory periods ≥1.2 ms; P values ≤0.05 for multivariate ANOVA related to cluster separation; and distinct waveform amplitudes and shapes when compared with other activity on the same electrode (Nicolelis et al. 2003). Single- and multiunits were categorized separately but grouped together for factor analysis. If multiple single units were collected from one electrode, all were included in the analysis; but at most only one multiunit cluster per electrode was included in the analysis. Typically, our multiunit recordings contained a cluster of spikes of several amplitudes that could not be isolated into individual units, but the waveforms had typical neuron-like negative and positive deflections.
PEAK FIRING RATE.
As described in Reed et al. (2008), spike trains were smoothed with a spike density function to determine peak firing rate using Matlab (The Mathworks, Natick, MA). A spike density function was produced by convolving the spike train from each trial with a function resembling a postsynaptic potential specified by τg, the time constant for the growth phase, and τd, the time constant for the decay phase as: R(t) = [1 – exp(−t/τg)] × exp(−t/τd).
Based on physiological data from excitatory synapses, τg was set to 1 ms (e.g., Mason et al. 1991; Moore and Nelson 1998). We set τd to 5 ms rather than the commonly used 20 ms value (Mason et al. 1991; Moore and Nelson 1998; Veredas et al. 2005) because the transient nature of the on and off responses in primary somatosensory neurons was excessively smoothed at larger values, particularly for cases in which stimuli were presented at stimulus onset asynchronies of 10 and 30 ms. For stimuli presented with onset asynchronies, we focused on responses that occurred within a response time window of 50 ms following the onset of the second stimulus; thus the first stimulus acted as “conditioning stimulus” as used in many other studies (e.g., Chowdhury and Rasumusson 2003; Gardner and Costanzo 1980b; Greek et al. 2003).
For excitatory responses, the peak firing rate was determined as the maximum of the spike density function within the response time window, and the average baseline firing rate (calculated over a 500 ms window prior to stimulation onset) was subtracted from this value. This peak firing rate value was required to be greater than a threshold value, which was the average baseline firing rate plus 2 SD of this baseline with a minimum value of 5 spike/s. To determine if this value was significant, the nonparametric Mann-Whitney U test and the parametric Student's t-test with two tails were performed and results compared. The U test and t-test resulted in the same categorizations of significance (α = 0.05). If no excitatory response was detected in the response window, responses were examined for possible suppressive effects. Our criteria for suppressive responses were average firing rates from the spike density function less than the average baseline firing rate <1.65 times the SD of the baseline, and this low firing had to be sustained for ≥10 ms.
We used the average peak firing rate over 100 trials as our measure of interest because neuron responses in area 3b (for rapidly and slowly adapting responses) tend to show an initial transient onset response, followed by a variable amount of firing suppression (e.g., Sur 1980). Therefore peak firing rate acts as a general measure of responsiveness that would not depend on the presence of sustained responsiveness to the stimuli and would indicate if the onset responses were influenced by the presence of the conditioning stimulus.
RESPONSE LATENCY.
Response latencies were calculated using Matlab and determined from spike density function histograms as the initial time when the rate meets the half-height over the threshold value of the peak firing rate within the response time window (see preceding text). The peak latency was calculated using the greater of the half-height or the threshold value. Because the data were not continuous, polynomial fitting of the spike density function was used to determine the time of the half-height. The value that was found at the least distance from the time of the peak and preceding the peak such that the rate values followed an increasing trend (upward slope toward the peak) was called the response latency. This method of determining latency is similar to measures that determine the width or duration of peaks in histograms (e.g., Davidson et al. 2007; Tutunculer et al. 2006). Latencies were only calculated when response criteria were met for excitatory or suppressive responses (see peak firing rate). Results were checked individually to ensure proper assignments.
FIRING RATE MODULATION INDEX.
A modulation index was developed for Matlab based on methods used to describe multisensory integration (Alvarado et al. 2007; Stanford et al. 2005). The observed average response in the 50 ms window of interest during a given two-site stimulation condition was compared with the distribution of expected average responses based on simple summation of the unit's response to the two stimuli presented individually. The expected distribution is based on the sum of two control values computed for every combination of trials, for 100 trials (100 × 100). From the distribution, 100 trials were randomly selected (without replacement) and averaged to give the predicted average response to two-site stimulation. This was repeated 10,000 times to build a reference distribution of predicted responses, and the observed response was compared with this distribution, following the methods of Alvarado et al. (2007). The Kolmogorov-Smirnov test of normality was used for the observed responses (100 trials each) and the predicted responses (100 trials selected from 10,000 bootstrapped samples). When normality assumptions were met, paired t-test for matched samples were used; otherwise, nonparametric Wilcoxon signed-rank tests were used (α = 0.05). The modulation categories were superadditive, subadditive, additive, suppressive, or no difference from the response to the preferred single stimulus, similar to the methods of Stanford et al. (2005). Superadditive responses were greater than the predicted distribution by two times the SD of the distribution, and subadditive responses were less than the predicted distribution by two times the SD of the distribution. Responses were classified as suppressive when the magnitude of the response to two-site stimulation was less than that of the response to the preferred single stimulus. These categories were determined for individual neuron units across conditions and tallied.
RESPONSE FIELD.
While the placement of our stimulus probes was guided by the previously determined mRF, we found it useful to define excitatory response fields within which neurons fired at high rates to above-threshold skin indentations with a stimulus probe. Response fields were typically larger than mRFs. Because we recorded from multiple neurons simultaneously using a 100-electrode array, the stimulus locations that evoked excitatory responses did not always coincide with the mRFs of each neuron. To describe the region of skin where suprathreshold tactile stimulation was highly effective in evoking spikes as separate from weakly effective skin locations, we defined an excitatory response field as the region of skin where stimuli evoked a response such that peak firing rate was at least three times higher than the SD of the firing rate of the entire population of neurons recorded from the 100 electrode array, after each neuron's firing rate was corrected by subtracting the baseline spontaneous rate.
To do this, the peak firing rate of each neuron was first corrected by subtracting the average spontaneous firing rate of that neuron. Then the corrected peak firing rates of all of the neurons recorded from the array were averaged, resulting in a population average firing rate. When a single location on the hand was stimulated, that location was classified as inside the unit's excitatory response field (IN) if the corrected peak firing rate for neurons at that site was at least three times higher than the SD of the population average firing rate. For a given unit, responses for all of the hand locations that were stimulated for a given experiment were compared and the maximum firing rate was found, with that location becoming designated as in the center of that unit's response field (CN). When the unit firing rate did not meet the criteria to be categorized as inside, the skin location was considered outside of the response field (OUT). This response field is a firing-rate-based estimate similar to the concept of the receptive field (as measured by suprathreshold stimulation) and was used to examine and classify spatial integration. Although two skin locations were routinely stimulated together, the response field classification was based on the responses to stimulation at the individual locations. The concept of the response field was used to see how dual-site stimulation results related to how effective the individual stimulation locations were in evoking responses from the recorded neurons.
DATA SELECTION AND CLASSIFICATION.
The data were classified based on several factors that were used in a statistical model to estimate the contributions of those factors to the observed latencies and firing rates. These factors were the temporal proximity of the stimuli, the spatial proximity of the stimuli, and the neuron's response field relationship to the stimuli.
Temporal asynchrony condition.
For each stimulus block, two locations on the hand were selected and stimulated individually for comparison with paired stimulus presentations. The firing rates in response to stimulation on the two hand locations individually were compared. The “nonpreferred” location was the skin location to which the neuron responded with the lower peak firing rate. The “preferred control” was the skin location to which the neuron responded with the higher peak firing rate. The firing rates of individual neurons during stimulation at the preferred location were compared with paired stimulation at the selected preferred and nonpreferred locations. Results from stimulation at the preferred control location alone represented the first level of the temporal condition factor for analysis. Paired stimuli were presented simultaneously (0 ms delay) and at selected stimulus onset asynchronies of 10, 30, 50, 100, and 500 ms, with the nonpreferred stimulus preceding the preferred control stimulus, for a total of seven levels. See Fig. 1A. The data were selected such that the preferred stimulus for a given neuron was presented second and the nonpreferred stimulus was presented first.
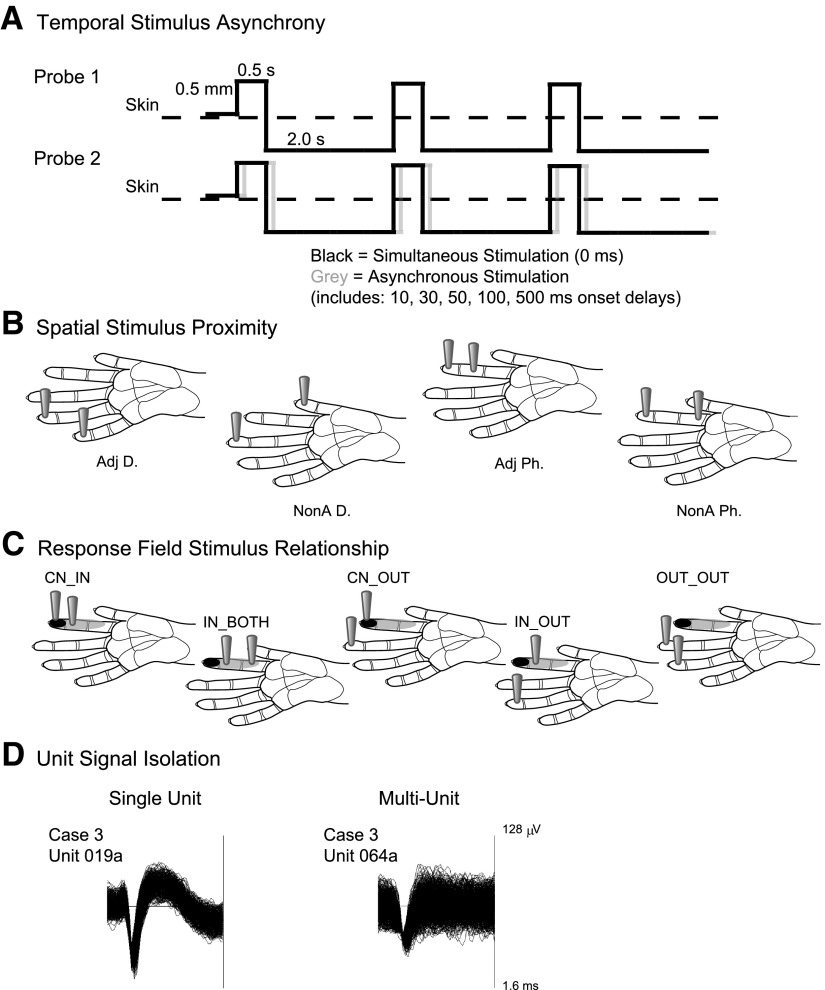
Fig. 1.
Schematic representations of data categories. A: the pattern of stimulation is depicted by solid lines indicating the duration the stimulus probe indents the skin (0.5 s), the depth of indentation (0.5 mm), and the duration the stimulus probe is off of the skin per stimulus cycle (2.0 s). Paired stimulation, indicated by the schematics of probes 1 and 2, may be simultaneous or asynchronous. To depict asynchronous stimulation generally, the gray solid line representing probe 2 is shifted relative to the black solid line. The 2 probes are presented to different skin sites on the hand; however, the schematic depicts the overlap in contact time of the stimuli presented via probes 1 and 2. B: the spatial stimulus relationships were divided into 4 categories. Locations of the 2 stimulus probes on schematics of the owl monkey hand illustrate the proximity category. Scale bars on hand diagrams are 1 mm. Adj D, adjacent digits (or palm pads); NonA D, nonadjacent digits (or palm pads); Adj P, adjacent phalanges; NonA Ph, nonadjacent phalanges. C: the response field category is determined by the unit's response field relative to the stimulation location. Black shading on schematics of the owl monkey hand indicates the center of the response field for a hypothetical unit. Gray shading on the hand indicates locations inside the response field but outside the center “hotspot” for the hypothetical reference unit. The locations of the 2 gray probes indicate the location of the stimulation relative to the response field for each response field category. Scale bars on hand diagrams are 1 mm. D: data were also classified by the quality of the signal isolation into single or multiunits. Examples of each unit type are shown from monkey case 3. The trace window for each unit type shown spanned 128 μV and 1.6 ms.
Spatial proximity condition.
The paired hand locations stimulated for each monkey case can be found in Table 1. These stimulus conditions allowed us to have four levels of the spatial proximity condition, which we abbreviated as follows: Adj D, adjacent digits; NonA D, nonadjacent digits; Adj Ph, within a digit, adjacent phalanges; and NonA Ph, within a digit, nonadjacent phalanges. See Fig. 1B.
Table 1.
Summary of stimulus locations and conditions
| Condition | Paired Location | Monkey |
|---|---|---|
| Adjacent hand sites | dD2 + dD3 | 1–3 |
| dD3 + dD4 | 2, 3 | |
| dD4 + dD5 | 3 | |
| dPTh + P1 | 1 | |
| Nonadjacent hand sites | dD2 + dD4 | 2 |
| dD3 + dD5 | 3 | |
| dD2 + dD5 | 3 | |
| mD1 + dD3 | 2 | |
| dPTh + P2 | 1 | |
| Adjacent phalanges | dD2 + mD2 | 2 |
| mD2 + pD2 | 2 | |
| dD4 + mD4 | 3 | |
| Nonadjacent phalanges | dD2 + pD2 | 2 |
| dD4 + pD4 | 3 | |
| dD4 + P3 | 3 |
D, digit; P, Palm pad; d, distal; m, middle; p, proximal; Th, thenar.
RESPONSE FIELD CONDITION.
The relationship of the paired stimuli to the response field of the reference unit was included as a factor that could influence the firing rate and latency of the unit. We collected the following relationships under our stimulus conditions: one probe in the center of the unit's response field, and the other probe also inside but not in the center “hotspot” of the response field, abbreviated as CN_IN; both probes inside the unit's response field (but not in the center), abbreviated as IN_BOTH; one probe in the center and one probe outside the response field, abbreviated as CN_OUT; one probe inside the response field and one probe outside the response field, abbreviated as IN_OUT; and both probes outside the response field, abbreviated as OUT_OUT. We never had an instance in which both stimulus probes were located in the center of a unit's response field. Thus the response field factor had five levels. See Fig. 1C.
SIGNAL ISOLATION CATEGORY.
We classified the data as single unit (SU) and multiunit (MU) signal isolation based on our spike sorting criteria and included this two-level factor in the analysis to determine the influence on the response latency and peak firing rate. See Fig. 1D.
Summary statistics
Data from Matlab calculations for firing rate and latency measures were compiled and summarized in Excel to select units and conditions in which the nonpreferred location was stimulated first in the series. Data for which the preferred location was stimulated first and the nonpreferred location stimulated second were not included in this analysis of firing rate and latency. To be included in the summary analysis for firing rate and latency, units had to respond to at least one single-site stimulation condition and at least one dual-site stimulation condition. If a unit's response was suppressed in all stimulus conditions after the control stimulation so that no significant peak firing rates or latencies could be detected, then that unit was not included in the analysis. Only excitatory responses were included in this analysis. The data subset was imported into SPSS 17.0 (SPSS, Chicago, IL) for further statistical analysis. The α level for all significance tests was 0.05. We examined the raw data values for firing rate and latency, but for comparison across stimulus conditions, we also duplicated the data and normalized the values to those for the control stimulation for each unit to evaluate how the response properties changed when two sites were stimulated compared with the single preferred control site.
Across all conditions we performed the Kolmogorov-Smirnov one-sample distribution test (SPSS 17.0) on the values we obtained for response latency and firing rate, and we determined that the distributions differed from the normal and Poisson distributions; therefore we analyzed firing rate and latency using an extension of the generalized linear model, which is becoming more widely used in psychological sciences (Tuerlinckx et al. 2006; review), known as the generalized estimating equation approach (e.g., Liang and Zeger 1986; Zeger and Liang 1986). We specifically employed generalized estimating equations because this method estimates the contributions of multiple factors resulting in the observed responses and is able to take into account the presence of correlated data in the sample, unlike other methods which require that observations are independent. This analysis has fewer assumptions regarding the data distribution than parametric ANOVA (e.g., Liang and Zeger 1986), and although it accounts for the correlated data, it does not specify the within-subject effects (Tuerlinckx et al. 2006; review). Both the firing rate and latency data were better fit by a gamma distribution function than the normal distribution, and the generalized estimating equations analysis using SPSS allowed us to select the gamma distribution with a log link function (e.g., Hardin and Hilbe 2003; p. 58–76) as the model distribution.
We used this approach on our peak firing rate and response latency data sets to estimate the sources of variance related to the temporal stimulus condition, the spatial proximity of the stimuli, and the response field relationship of the neuron unit subjects. In our case, neuron properties were measured across the temporal conditions and therefore could be correlated within subjects (neuron units). A model was made for the dependent variable (response latencies or peak firing rates), and the effects of the between-subjects predictors are estimated by solving the generalized estimating equation based on the parameters provided. Thus in these models, latency or peak firing rate acted as the dependent variable, the within-subject variable was the stimulation condition, while the predictor factors included: the temporal stimulation condition, the spatial proximity of stimuli (Adj D, NonA D, Adj Ph, NonA Ph), the relationship of the stimulus to the Response Field of the neuron unit, and the classification of the unit as a single- or multi-unit. Then we analyzed single unit data separately from multiunit data in additional statistical analyses.
The model parameters for both latency and firing rate results were based on a gamma probability distribution with the log link function, a first-order autoregressive correlation matrix, and a robust model estimator. We tested other probability distributions including Poisson and Gaussian (normal); however, the gamma distribution with the log link fit the data best (according to data fitting and model goodness-of-fit tests). We tested possible working correlation matrix structures to determine which estimate of the dependency within the data resulted in the best fitting models. We used an autoregressive correlation matrix for the model because this incorporates the time dependence of the repeated measures, but we also tested other correlation matrix structures for the best fit (e.g., Hardin and Hilbe 2003; p. 58–76). We selected a robust model estimator rather than a model-based estimator because the robust model estimator is consistent even when the working correlation matrix structure is mis-specified (Hardin and Hilbe 2003; p. 30). We applied the Bonferroni correction for multiple comparisons. The Wald χ2 statistic was used to test the significance of the model effects resulting from the generalized estimating equations analysis. The results of the generalized estimating equations analysis provide estimated values to fit the observed data and calculations to indicate the likely significance of the differences; therefore the values reported in results are the predictions from the analysis. These predicted values did not differ greatly from the observed values and followed the same trends. See supplemental material1 for plots of the observed data values.
RESULTS
We recorded from 100-electrode arrays implanted in layer 3 of anterior parietal cortex, primarily area 3b, of three anesthetized owl monkeys during stimulation on selected hand locations and analyzed the contributions of spatiotemporal stimulus properties to the observed response latencies and peak firing rates. Figure 2 depicts the placement of the electrode arrays in each of the three monkey cases as determined from patterns of myelin staining in sections of flattened cortex and from receptive field mapping during the recording procedures. Several electrodes recorded single-unit activity in each monkey (45 electrodes in case 1 and 65 electrodes in both cases 2 and 3); however, not all single units responded significantly under each stimulus condition. For many electrodes, more than one neuron was isolated, and activity from multiunits was recorded from multiple electrodes. Thus the numbers of single units reported for firing rate and latency are higher than the numbers of electrodes recording single units, as more than one single unit could be recorded from the same electrode. In addition, the same units were not always maintained during the 1–3 days of recording; thus different units could be isolated at different times and the units analyzed represent the total units isolated over the experiment duration. The numbers of units in each summary analysis are those studied for each stimulus condition. The numbers of units meeting the criteria for analysis were as follows: 19 single units (SUs) and 64 multiunits (MUs) from monkey 1; 44 SUs and 71 MUs from monkey 2; and 99 SUs and 71 MUs from monkey 3. Data from SUs and MUs were collapsed for summary analysis to determine statistical differences between SU and MU responses for peak firing rate and latency, and then these categories were analyzed separately. Typical response histograms of a single neuron are shown in Fig. 3.
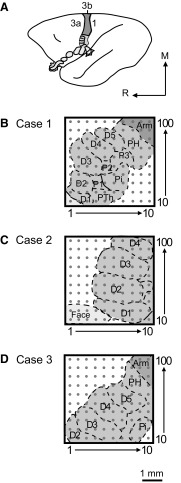
Fig. 2.
Schematic reconstructions show 100-electrode array placement in area 3b of 3 owl monkey cases. A: a schematic of the owl monkey brain is shown with the area 3b body representations highlighted as area 3a and area 1 neurons tended not to respond well to light tactile stimulation under the anesthetic conditions of these experiments. Gray shading indicates the area 3b hand representation. Light gray shading indicates the area 3b face and oral cavity representations, portions of which are hidden from the cortical surface, but are revealed by “unfolding” the schematic representation. Dark gray indicates the rest of 3b. B–D: electrode locations in each owl monkey case were approximated based on examination of myelin-stained sections of flattened cortex in which area 3b stains more darkly than surrounding areas, with a myelin-poor septum indicating the border of the hand and face representation. Occasionally, myelin-poor septa can seen between digit representations in flattened cortex preparations, but in our cases, we used the results of receptive field mapping during recording experiments to estimate the digit and palm pad representations. The placements of the 4 × 4 mm array in each case tended to cover a large part of the hand representation. The 1 mm scale bar corresponds to the size of the array schematics for all 3 owl monkey cases.
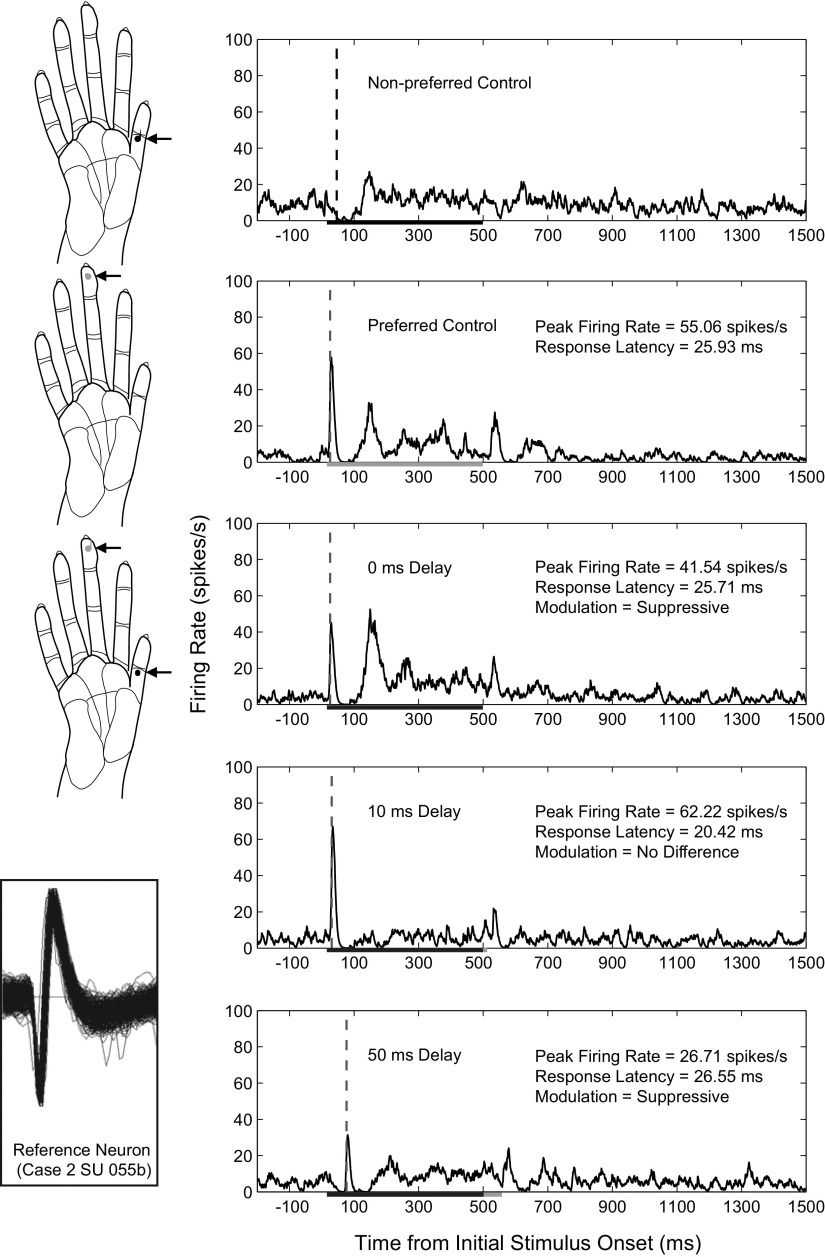
Fig. 3.
Example histograms from a single unit depict peak firing rate and latency changes across selected spatiotemporal stimulus conditions. Arrows point to dots on the diagrams of the owl monkey hand that indicate the stimulus locations on digits 1 and 3 for a series of recordings in monkey case 2. Histograms are shown for the neuron's responses when the 2 locations were stimulated individually, then for both locations simultaneously, and finally for 2 stimulus blocks in which the nonpreferred, or conditioning, stimulus was presented at 10 and 50 ms before the onset of the preferred stimulus. Stimulation was presented in blocks of 100 trials. Histograms are smoothed by a spike density function as described in methods. Vertical dashed lines indicate when the latency was determined as described in methods. The duration for each stimulus was 500 ms as indicated by the line on the x axis; therefore paired stimulation overlapped in time for all stimulus onset delays tested except for 500 ms (not shown). The measures we examined included the peak firing rate within 50 ms of the preferred stimulus onset and the associated latency of that response. The peak firing rates and response latencies following stimulation of the preferred location are given for each excitatory response. The firing rate modulation category is listed for examples of paired stimulation. While the 10 ms delay resulted in an average peak firing rate value that was ∼13% larger than the response during control stimulation alone, the analysis for firing rate modulation found that this difference was not statistically significant in this example. Inset: the trace of the single unit in each histogram is shown at the lower left corner in a trace window that spanned 128 μV and 1.6 ms.
Spatiotemporal stimulus effects on peak firing rate and response latency
Peak firing rates varied with spatiotemporal features of the stimulus conditions. We collected 1,385 SU firing rate responses and 2,213 MU firing rate responses that met the inclusion criteria under the spatiotemporal stimulus conditions. These values are slightly higher than the number of latencies collected because firing rates could have a zero value when the firing rate did not differ from the spontaneous rate; however, latencies could not be given zero values. We collected 1,098 SU response latencies and 1,771 MU response latencies that met our inclusion criteria under the spatiotemporal stimulus conditions. The mean of the observed peak firing rates for the dataset was 28.70 ± 36.04 (SD) spike/s, and the median of the peak firing rates was 14.95 spike/s. The mean of the observed response latency for the dataset over all conditions was 20.67 ± 6.31 ms, and the median was 19.80 ms.
In Supplemental Fig. S1, the group medians of the peak firing rates and latencies are plotted in three dimensions to show the distributions along the four main categories: temporal asynchrony, spatial proximity, response field relationship (see methods), and signal isolation (SU vs. MU). Peak firing rates tended to cluster based on these categories with a tendency to be lowest when stimulation probes were both outside the response field (OUT_OUT), highest when both were located in the center (CN) or inside (IN) the response field (CN_IN and IN_BOTH), and intermediate when one stimulus probe was inside (CN or IN) and one probe was outside the response field (CN_OUT and IN_OUT). Latencies tended to be longer when both stimuli were located outside the response field (OUT_OUT), but latencies were dispersed across the categories rather than falling into clusters.
We used generalized estimating equations analysis on the full data set to determine the contributions of these categories on the variability of peak firing rate responses and response latencies. Our analysis indicated that several factors influenced the neuron response properties of peak firing rate and latency.
SIGNAL ISOLATION CATEGORY.
As one might expect, the MU peak firing rates were significantly higher than those of SUs (MU = 27.46 ± 2.21 spike/s; SU = 19.17 ± 1.81 spike/s; P = 0.002). Excitatory response latencies in a MU cluster were slightly but significantly shorter than those of SUs (MU = 19.48 ± 0.26 ms; SU = 21.24 ± 0.36 ms; P < 0.0005). The differences between SU and MU peak firing rates and latencies are plotted in Supplemental Fig. S2 along with the effects of the spatiotemporal stimulus conditions on the population of SUs and MUs combined. Although our analysis indicated that SUs and MUs did not follow different trends, we separated SUs from MUs for the subsequent analysis to determine the effects on the variance of SU responses and MU responses separately (see Supplemental Tables S1–S4).
TEMPORAL ASYNCHRONY CATEGORY.
For the temporal stimulation condition, peak firing rates were suppressed by co-stimulation with a nonpreferred stimulus compared with stimulation by the preferred control stimulus alone (see SU results in Fig. 4A; combined results in Supplemental Fig. S2A). Significant decreases in peak response magnitudes compared with single-site control stimulation (SU = 26.11 ± 1.15 spike/s) occurred for all of the paired stimulation conditions at stimulus onset asynchronies as found in Table 2. On average, peak firing rate responses of SUs were suppressed by the presence of the nonpreferred stimulus at all delays tested, with maximal suppression when the nonpreferred stimulus preceded the preferred stimulus by 30 ms. Thus peak firing rates indicate that stimulus information is integrated across time ≤500 ms with strong effects when the nonpreferred stimulus is presented between 10 and 50 ms before the preferred stimulus. MUs followed the same trend, and these data are presented in the supplemental material.
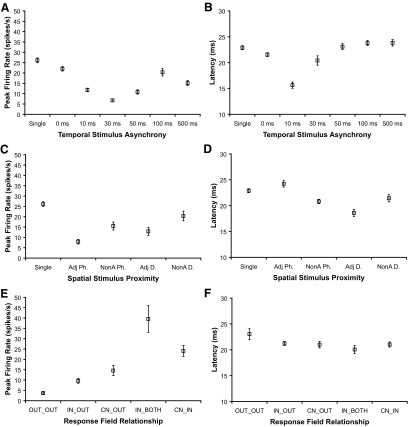
Fig. 4.
Main effects for peak firing rate and response latency measures from single units across spatial and temporal stimulus conditions. Estimated mean values from generalized estimating equations analysis of 309 single units are shown with error bars representing ±1 SE for all panels. Results for peak firing rate are shown in A, C, and E; results for latency are shown in B, D, and F. A: Means of peak firing rate values (spike/s) are plotted for each temporal stimulation category comparing the preferred control stimulation (single) to paired stimulation at 0, 10, 30, 50, 100, and 500 ms stimulus onset asynchronies. Dual-site stimulation suppressed peak firing rates relative to single-site stimulation. A stimulus onset asynchrony of 30 ms resulted in the greatest suppression of peak firing rate, and this suppression was significantly different from all other stimulus categories (P < 0.0005 for all comparisons). B: means of response latencies (ms) plotted for each temporal stimulation condition show that 0 and 10 ms stimulus onset delays resulted in faster latencies than single-site control stimulation (P = 0.003 and P < 0.0005, respectively). C: means of peak firing rates are plotted for each spatial proximity condition in which the stimuli were presented on separate adjacent digit sites (Adj D), separate nonadjacent digit sites (NonA D), within a single digit on adjacent phalanges (Adj Ph), or within a single digit on nonadjacent phalanges (NonA Ph). Dual-site stimulation suppressed peak firing rates compared with single-site stimulation for all conditions except when nonadjacent digits were stimulated (P = 0.074). On average, peak firing rates were higher in response to stimulation on NonA D than Adj Ph, (P < 0.0005). D: latencies did not vary greatly with spatial stimulus proximity, but latencies were slightly faster in response to stimulation on Adj D and NonA Ph compared with single-site stimulation (P < 0.0005 for both comparisons). E: means of peak firing rate values are plotted for each type of relationship of the response field to the stimulus location. Peak firing rates were lower when the stimuli were outside of the response field (OUT_OUT) compared with all other conditions (P < 0.0005 for all comparisons). CN_IN was not different from IN_BOTH (P = 0.272) or CN_OUT (P = 0.087), and IN_OUT was not different from CN_OUT (P = 0.713). F: Latencies were slightly but not significantly longer when both stimuli were presented outside of the neuron's response field (OUT_OUT) compared with all other response field stimulus relationships. CN, response field center; IN, in response field; OUT, outside response field.
Table 2.
Estimated means of peak firing rates for the factor temporal stimulus asynchrony and tests of significant differences compared to single-site control stimulation
| Temporal Asynchrony | Estimated Mean | Significance (to Control) |
|---|---|---|
| Control | 26.11 ± 1.15 | |
| 0 | 22.04 ± 1.08 | 0.007 |
| 10 | 11.78 ± 0.88 | <0.0005 |
| 30 | 6.76 ± 0.68 | <0.0005 |
| 50 | 10.85 ± 1.03 | <0.0005 |
| 100 | 20.34 ± 1.79 | 0.033 |
| 500 | 15.10 ± 1.17 | <0.0005 |
Estimated means for the dependent variable, peak firing rate (spike/s), under the independent factor, temporal stimulus asynchrony, Values are means ± SE. n = 309 single neurons from three monkeys.
In contrast to the results for peak firing rates, we found shortened response latencies occurred when stimuli were paired and presented simultaneously (SU = 21.55 ± 0.37 ms; P = 0.003) or when the preferred (test) stimulus was presented at a 10 ms delay (SU = 15.64 ± 0.61 ms; P < 0.0005) compared with single-site stimulation (SU = 22.89 ± 0.38 ms), as shown in Fig. 4B. Latencies in response to stimulations at other onset delays were not significantly different from the latency during single-site stimulation as shown in Table 3. MUs followed the same trend (see supplemental material). Thus latencies were reduced by paired stimulus presentations within 10 ms and did not show clear relationships to stimulus integration across time.
Table 3.
Estimated means of response latencies for the factor temporal stimulus asynchrony and tests of significant differences compared to single-site control stimulation
| Temporal Asynchrony | Estimated Mean | Significance (to Control) |
|---|---|---|
| Control | 22.89 ± 0.38 | |
| 0 | 21.55 ± 0.37 | 0.003 |
| 10 | 15.64 ± 0.61 | <0.0005 |
| 30 | 20.41 ± 0.91 | 0.235 |
| 50 | 23.09 ± 0.58 | 1.000 |
| 100 | 23.79 ± 0.47 | 1.000 |
| 500 | 23.83 ± 0.63 | 1.000 |
Estimated means for the dependent variable, response latency (ms), under the independent factor, temporal stimulus asynchrony. n = 309 single neurons from three monkeys.
RELATIONSHIP BETWEEN RESPONSE LATENCY AND FIRING RATE.
The shortened mean latency we observed when the nonpreferred stimulus preceded the preferred stimulus by 10 ms compared with the latency in response to the preferred control stimulus alone (Fig. 4B) appeared to conflict with the decreased peak firing rate in response to the 10 ms onset delay compared with the response to the preferred control stimulus alone (Fig. 4A). To investigate this result further, we performed a Wilcoxon signed-ranks test between matched samples such that the peak firing rate for each neuron was compared between the control stimulation condition and the condition in which the onset of the preferred stimulus was delayed by 10 ms relative to the onset of the nonpreferred stimulus. We performed the same test for the response latency for each neuron in our data set. From the test of firing rate values, 294 values were negative differences and 127 values were positive differences (0 ties); therefore the majority of firing rates decreased in the 10 ms delay group relative to the control group (Z = −7.788, P < 0.0005). For the Wilcoxon test of latency values, 235 values were negative differences and 69 values were positive differences (0 ties); therefore most of the latencies were shortened in the 10 ms delay group compared with the control group (Z = −10.503, P < 0.0005). Thus the results in Fig. 4, A and B, reflect the response properties of the majority of the neurons in the dataset; however, some neurons responded differently. We determined the nonparametric Spearman correlation coefficient (ρ) to relate response latency with peak firing rates paired for each neuron across all of the spatiotemporal stimulus conditions. A weak negative correlation existed between latency and peak firing rate (ρ = −0.141, P < 0.0005), such that latencies shortened as peak firing rates increased overall, as expected.
SPATIAL PROXIMITY CATEGORY.
Peak firing rates were generally suppressed by paired stimulation compared with single-site stimulation, shown in Table 4, with the greatest suppression when stimuli were presented to adjacent phalanges (Fig. 4C). While latencies were slightly delayed when stimuli were presented to adjacent phalanges (Adj Ph: SU = 24.23 ± 0.64 ms) compared with single-site stimulation (single-site: SU = 22.89 ± 0.38 ms), this difference was not statistically significant (P = 0.660). However, latencies were slightly shortened when adjacent digits were stimulated (Adj D: SU = 18.59 ± 0.69 ms) compared with single-site stimulation (single-site: SU = 22.89 ± 0.38 ms; P < 0.0005; see Table 5 and Fig. 4D). For both SUs and MUs, paired stimuli on adjacent digits reduced the latencies of the neural responses, while paired stimuli on adjacent phalanges within a digit increased the latencies of these responses (see supplemental material for MU results). Thus widespread integration of stimulus information from different parts of the hand was demonstrated by differences in peak firing rates in response to single-site stimulation compared with dual-site stimulations even when paired locations were nonadjacent digits. Response latencies were somewhat affected by the spatial proximity of paired stimuli and therefore also demonstrated some relationship to stimulus integration across the hand.
Table 4.
Estimated means of peak firing rates for the factor spatial stimulus proximity and tests of significant differences compared to single-site control stimulation
| Spatial Proximity | Estimated Mean | Significance (to Control) |
|---|---|---|
| Control | 26.11 ± 1.15 | |
| Adj D | 12.92 ± 1.92 | <0.0005 |
| NonA D | 20.37 ± 2.33 | 0.074 |
| Adj Ph | 7.94 ± 1.08 | <0.0005 |
| NonA Ph | 15.53 ± 1.90 | <0.0005 |
Estimated means for the dependent variable, peak firing rate (spike/s), under the independent factor, spatial stimulus proximity. n = 309 single neurons from three monkeys. Adj, adjacent; D, digits; NonA, nonadjacent; Ph, phalanges.
Table 5.
Estimated means of response latencies for the factor spatial stimulus proximity and tests of significant differences compared to single-site control stimulation
| Spatial Proximity | Estimated Mean | Significance (to Control) |
|---|---|---|
| Control | 22.89 ± 0.38 | |
| Adj D | 18.59 ± 0.69 | <0.0005 |
| NonA D | 21.43 ± 0.70 | 0.364 |
| Adj Ph | 24.23 ± 0.64 | 0.660 |
| NonA Ph | 20.81 ± 0.43 | <0.0005 |
Estimated means for the dependent variable, response latency (ms), under the independent factor, spatial stimulus proximity. n = 309 single neurons from three monkeys.
RESPONSE FIELD RELATIONSHIP CATEGORY.
The relationship of the location of the stimulation sites to the response field affected peak firing rates (Fig. 4E, Table 6) but did not affect latencies (Fig. 4F, Table 7). As may be expected, the peak firing rates were significantly lower when both stimuli were outside (OUT) of the response field (OUT_OUT: SU = 3.72 ± 0.49 spike/s) compared with other conditions when one or both stimuli were inside (IN or CN) the response field, as shown in Table 6. The highest firing rates were found when both stimuli were within the response field (IN_BOTH) of the reference unit, or in the center (CN) and just in the response field (IN). The responses in these two categories were not significantly different (IN_BOTH and CN_IN, P = 0.272). Similarly, there was no significant difference in firing rates when the response field was classified as IN_OUT compared with CN_OUT (P = 0.713). Overall, peak firing rates were lowest when both stimuli were outside the response field and highest when both stimuli were within the response field and presenting one stimulus outside the response field reduced peak firing rates ∼50%. When both stimuli were outside of the response field, the latencies were slightly longer (OUT_OUT: SU = 23.02 ± 1.06 ms) than when both stimuli were inside the response field (IN_BOTH: SU = 20.05 ± 0.76 ms), but this small difference was not statistically significant (P = 0.228). Thus the response field relationship influenced peak firing rates but not latencies (and these results were also found in MUs as reported in supplemental material). We expected that the categorization of the response field relationship with the stimulation parameters would be related to the ways neurons integrate widespread stimulus information in time and space.
Table 6.
Estimated means of peak firing rates for the factor response field relationship and tests of significant differences compared to stimulation when both probes are outside the response field
| Response Field | Estimated Mean | Significance OUT_OUT |
|---|---|---|
| OUT_OUT | 3.72 ± 0.49 | |
| IN_OUT | 9.64 ± 1.19 | <0.0005 |
| CN_OUT | 14.60 ± 2.48 | <0.0005 |
| IN_BOTH | 39.56 ± 6.50 | <0.0005 |
| CN_IN | 24.08 ± 2.63 | <0.0005 |
Estimated means for the dependent variable, peak firing rate (spike/s), under the independent factor, response field relationship. n = 309 single neurons from three monkeys. CN = center. OUT_OUT, both probes outside the response field; IN_OUT, one probe inside, one probe outside the response field; CN_OUT, one probe in the center, one probe outside the response field; IN_BOTH, both probes inside the response field; CN_IN, one probe in the center of the response field, the other inside but not in the center.
Table 7.
Estimated means of response latencies for the factor response field relationship and tests of significant differences compared to stimulation when both probes are outside the response field
| Response Field | Estimated Mean | Significance OUT_OUT |
|---|---|---|
| OUT_OUT | 23.02 ± 1.06 | |
| IN_OUT | 21.22 ± 0.39 | 1.000 |
| CN_OUT | 20.99 ± 0.64 | 1.000 |
| IN_BOTH | 20.05 ± 0.76 | 0.228 |
| CN_IN | 21.02 ± 0.50 | 1.000 |
Estimated means for the dependent variable, response latency (ms), under the independent factor, temporal stimulus asynchrony. n = 309 single neurons from three monkeys.
Because we recorded from multiple neuron units simultaneously using the 100-electrode array, we expected that not all of the neurons recorded under each condition would behave the same way, given the differences in response field relationships to the stimulus locations. For visualization of the activity patterns across the spatial extent of the electrode array under different stimulus conditions, Fig. 5 shows color map plots of the peak firing rate values for SUs and MUs recorded from the 100-electrode array during a subset of the stimulus parameters for each block. Individual units had different changes in peak firing rates with each change in stimulation parameters. To address these individual changes and determine the population effects across spatiotemporal stimulation parameters, we calculated a modulation index for each unit across the levels of our temporal stimulation variable. For further treatment of spatiotemporal interactions, see supplemental material and Supplemental Tables S5 and S6.
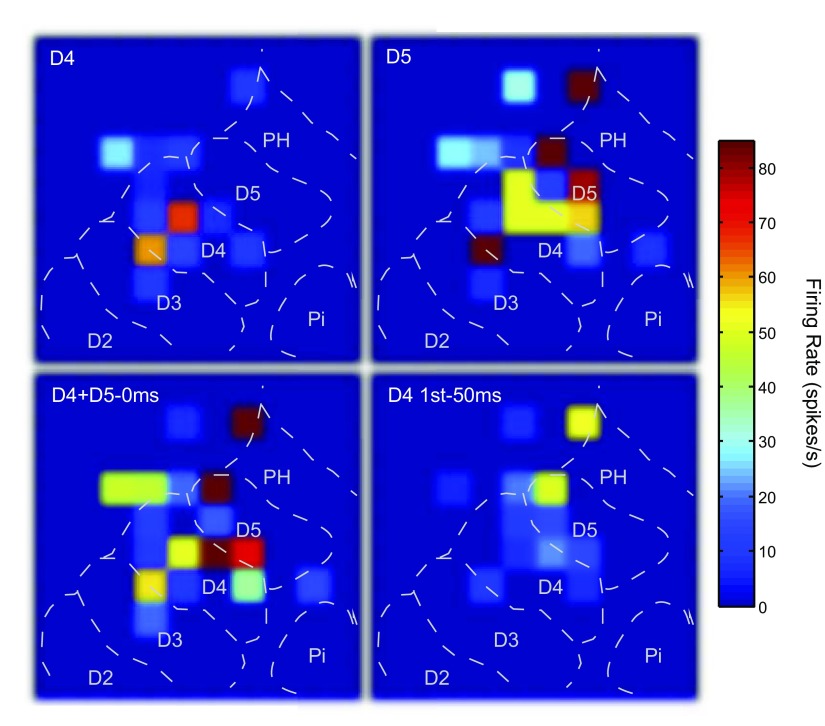
Fig. 5.
Peak firing rate values represented across the 100-electrode array under selected stimulus conditions. Color maps of the peak firing rate responses across the array were generated in Matlab with hot colors representing higher peak firing rates. Each square represents an electrode in the array and the peak firing rate value during the 50 ms response window. Electrodes from which no significant responses were obtained during the stimulations shown are indicated in dark blue (no squares). The dashed lines indicate approximate locations of the representations within area 3b in monkey 3. Top left: the responses when a single site on distal digit 4 (D4) was stimulated for 100 trials. Top right: the responses when a single site on the adjacent digit 5 (D5) was stimulated. Activity overall was greater during D5 stimulation, but both single site stimulation conditions show that the hotspots of activity occurred in the expected representations, but the activity could cross into adjacent representations and be nonuniform. Bottom left: both single site locations were stimulated simultaneously, resulting in a pattern of activity that one would not expect from simple summation across electrodes. Bottom right: the responses when D4 was stimulated 50 ms before D5 was stimulated, with the responses after D5 stimulation captured. The responding electrodes appear to show suppressed or unchanged responses.
Firing rate modulation index
To further describe the effects of spatiotemporal stimulation on peak firing rate, we used a modulation index to categorize how the observed response to the paired stimulation compared with the expected firing rate based on the sum of the responses to both of the single-site control stimuli. In this analysis, mean firing rate responses to each single-site stimulus alone were summed for comparison to mean firing rate responses to co-stimulation as described in methods.
For these data, there was no significant difference between SUs and MUs (P = 0.948), so the results are plotted together. In total across the conditions, 51.1% (n = 1,671 of 3,272) of the responses were classified as suppressive; 39.2% (n = 1,283) of the responses were classified as no difference; 5.7% (n = 187) were classified as superadditive; and 4.0% (n = 131) were classified as subadditive. We found no instances of additive modulation in which the response to paired stimulation was not statistically different from the linear sum of the two single-site responses. The counts of neurons for each modulation index category are shown in Fig. 6 divided into the stimulation categories on the x-axis and grouped by the proximity of the stimuli in each panel. We collected fewer conditions for stimulation within a digit (n = 543, Adj Ph; n = 389, NonA Ph) compared with stimulation across two digits (n = 936, Adj D; n = 1,402, NonA D); however, the majority of the responses under all conditions were no difference from the preferred control response and suppressive compared with the preferred control response. An interesting effect due to the temporal stimulation parameters appears prominent when reviewing the panels for stimulation on adjacent (Adj D) and nonadjacent (NonA D) digit pairs in Fig. 6. As the stimulus onset asynchrony increases from 0 to 500 ms, the number of responses classified as no difference decreases and the number of responses classified as suppressive increases.
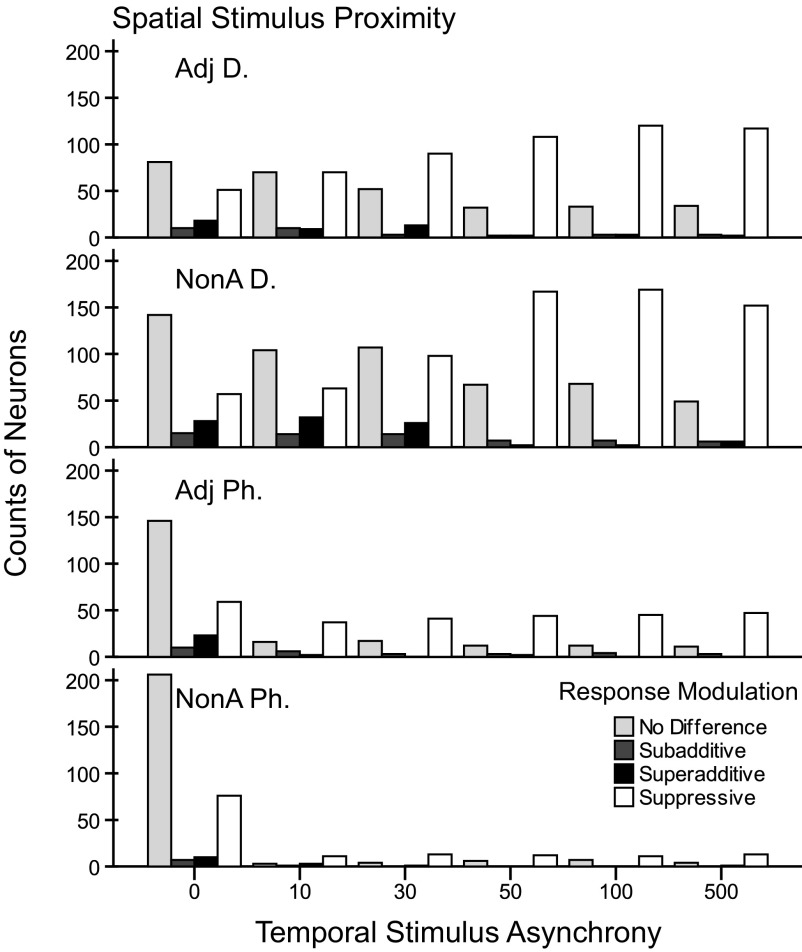
Fig. 6.
Counts of responses to dual-site stimulation categorized by spatial and temporal stimulation parameters. Individual bar shades indicate the classes of firing rate modulation in response to paired stimulation which occurred in these data: “no difference” compared with the response to the preferred control, “subadditive” compared with the summation of the responses of both controls, “superadditive” compared with the summation of the responses of the controls, and “suppressive” compared with the response to the preferred control. The types of paired stimulation conditions are grouped on the x axis referring to the stimulus onset asynchronies from 0 to 500 ms in which the preferred stimulus was always presented after the nonpreferred stimulus. The panels show the total counts of these categories for each of the four tested stimulus proximity categories. Fewer recordings were made with paired stimuli within a single digit (Adj Ph, NonA Ph). Suppression and no difference compared with the preferred control stimulation dominate the modulation classification types. Note the pattern such that many responses are classified as no difference during stimulation at short stimulus onset delays (0–30 ms) and there are fewer at longer delays (50–500 ms). Responses classified as suppressive show the reverse effect, particularly in the panels for adjacent and nonadjacent digit stimulation conditions. Facilitation (subadditive, superadditive) occurred less often, but was predominantly found when the delays were short (0–30 ms).
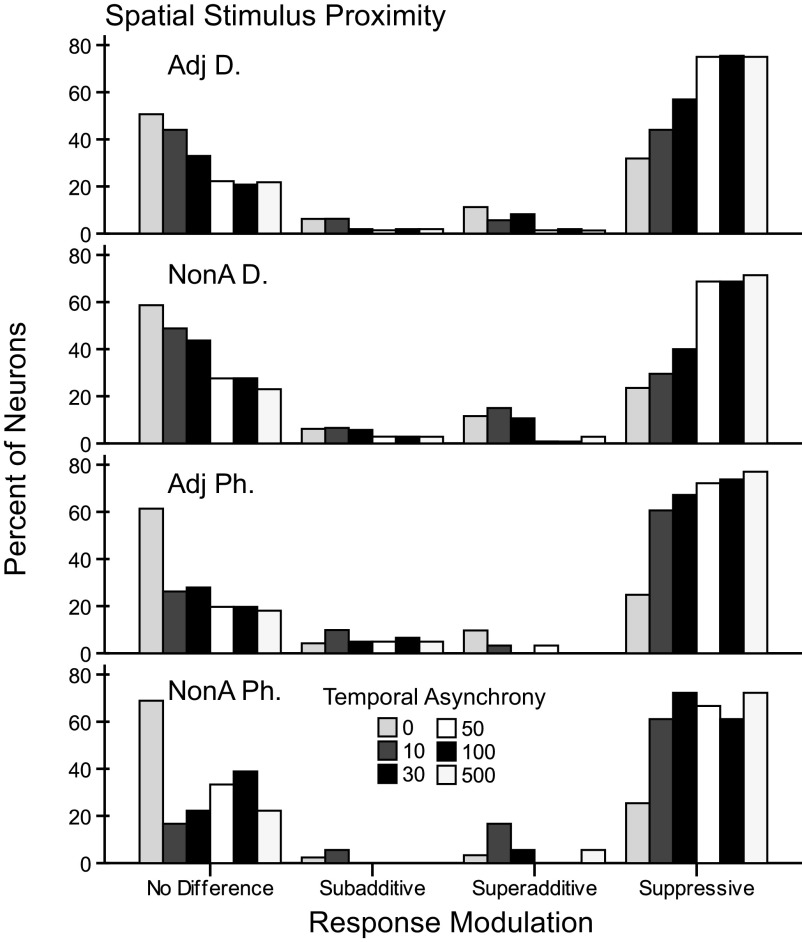
The same data presented in a second way in Fig. 7 illustrate the findings based on the percent of responses within each stimulation category. Suppression dominated the response modulation types averaged across categories (∼59% for Adj D, ∼50% for NonA D, ∼50% for Adj Ph, and ∼35% for NonA Ph). But this effect was not found uniformly across the stimulus conditions as also indicated by the counts in Fig. 6. For example, we found that when paired stimuli were presented simultaneously, most of the responses showed no difference compared with the preferred control site across each of the four stimulus proximity factors (∼51% for Adj D, ∼59% for NonA D, ∼61% for Adj Ph, and ∼69% for NonA Ph). Facilitation was rare. Summing the proportions of total facilitation (from subadditive and superadditive categories), we found that stimuli presented on nonadjacent digits resulted in the highest proportion of facilitation (∼11%); followed by stimulation within a digit on adjacent phalanges (∼10%), stimulation on separate, adjacent digits (∼8%), and stimulation within a digit on nonadjacent phalanges (∼6%). However, when facilitation occurred (subadditive and superadditive categories), the stimuli were more likely to be presented at short stimulus onset asynchronies (0, 10, 30 ms) across each of the proximity factors (∼81% for Adj D, ∼81% for NonA D, ∼79% for Adj Ph, and ∼96% for NonA Ph). Thus using this response modulation index, we found that responses were suppressed in large proportions across the temporal stimulation and spatial proximity categories. In addition, spatiotemporal characteristics of stimulus presentations influenced the proportions of neurons showing no difference, suppression, and subadditive and superadditive facilitation of firing rate when two locations were stimulated compared with the sum of the rates when each location was stimulated separately.
Fig. 7.
Percentages of response types for firing rate modulation categorized by spatial and temporal stimulation parameters. Four classes of firing rate modulation in response to paired stimulation occurred in these data: no difference, subadditive, superadditive, and suppressive, and these are displayed on the x axis. Individual bar shades indicate the paired stimulation type referring to the stimulus onset asynchronies in which the preferred stimulus was always presented after the nonpreferred stimulus. The panels show the total counts of these categories for each of the 4 tested stimulus proximity categories. Note that fewer recordings were made for the categories stimulating within a single digit (Adj Ph, NonA Ph). Percent on the y axis refers to the percentage of occurrences of each response type category, calculated within each panel for the four proximity categories. This view of the same data from Fig. 6 shows that when stimuli were presented at short delays (0–30 ms), response types tended to be no difference and facilitative (subadditive, superadditive). At these short delays, paired stimuli presented within a single digit (Adj Ph, NonA Ph) were suppressive modulators of firing rate in greater proportions than when paired stimuli were presented on adjacent or nonadjacent digits.
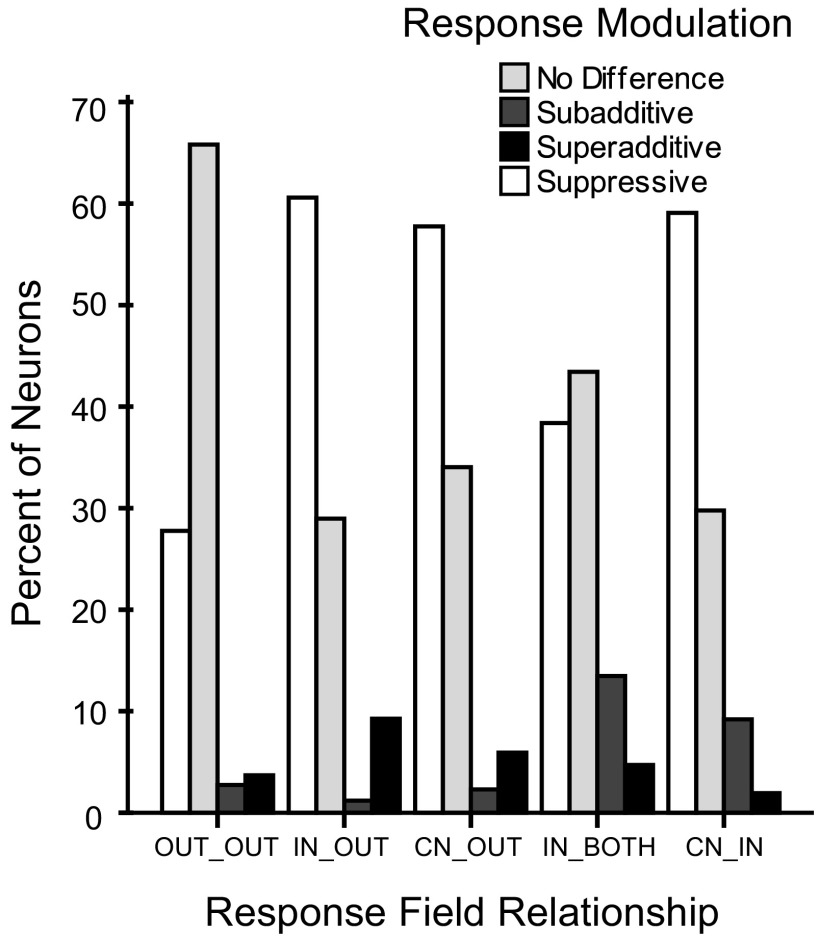
The proportion of SU and MU neurons falling into the response modulation categories of suppression, no difference, subadditive facilitation, and superadditive facilitation were similar across the relations of the stimulation locations to the neurons response fields (Fig. 8). The response modulation types were distributed across the response field categories such that no one category contained the majority of any one response modulation type. Neurons that responded with facilitation, for example, did not fall into one specific category. The response categories of suppression, no difference, subadditive facilitation, and superadditive facilitation were similarly distributed across the response field categories.
Fig. 8.
Percentages of response types for firing rate modulation categorized by response field relationships. The response field categories are shown on the x axis. On the y axis, the percentages of occurrences of the four response classes of “no difference”, “subadditive”, “superadditive”, and “suppressive” were determined within each response field relationship category. The response classes were distributed across the response field categories rather than grouped within the categories.
DISCUSSION
In this study, we examined peak firing rate and excitatory response latency during stimulation conditions that varied in spatiotemporal characteristics to quantify how neurons in primary somatosensory cortex integrate information from pairs of stimuli presented inside and outside their receptive fields at various stimulus onset asynchronies. The ranges of stimulus onset asynchronies along with the pairings of the stimuli on locations within single digits and across digits have not been reported previously, nor has the categorization of the response field been applied to extracting the contributions of stimulus properties to the response properties.
The hypothesis that both spatial and temporal stimulus properties shape responses to paired stimuli was supported and the intricacies of the effects were explored. As predicted, we found that peak firing rate and latency were affected by the spatial and temporal stimulus parameters, but some of the effects were unexpected, including how far in space and time paired stimuli can interact to influence neuron responses. Our stimuli were two 1-mm-diam probes placed on different phalanges within a digit or on separate digits, providing indentations 0.5 mm in depth. Because our stimuli were placed on different phalanges, we do not believe that the skin indentations provided by our stimuli interacted in the skin in the ways reported in previous investigations by Vega-Bermudez and Johnson (1999) in which multiple stimulus probes were placed within the distal digit of macaque monkeys. While some interactions between indentations of adjacent phalanges seem possible, we did not observe visible spread of skin indentations beyond a single phalange using our stimuli. Our results are consistent with those from previous studies, but we also provide new information regarding spatiotemporal stimulus integration. Here we treat each aspect of the analysis individually and then conclude with a discussion of the possible mechanisms that mediate the results.
Response field mapping and cortical topography
The first step in our analysis of the data from each monkey was to reconstruct the location of the electrodes in the array relative to the hand representation. This was done based on histological reconstruction from myelin stained sections of flattened cortex, minimal receptive field mapping data, and the classification of the response field based on the mechanical stimulation. Locations of multidigit receptive fields based on minimal receptive field mapping helped us to draw approximate borders between representations. Our classification of the excitatory response field was based on neuron firing rate to suprathreshold stimuli. While minimal receptive fields studied with near-threshold stimuli have been described as small and typically localized within a single digit, the suprathreshold response fields we characterized can be large, often encompassing different digits. The idea that primary somatosensory cortex, area 3b, responds only to small and discrete body locations is now supplemented by a growing awareness of how stimulation “beyond the classical receptive field” influences neuron responses (e.g., Chen et al. 2003; Clark et al. 1988; Friedman et al. 2008; Nicolelis et al. 1998; Reed et al. 2008). The inclusion of separate digits within the response field indicates that the large receptive fields are not simply artifacts caused by the spread of deformations in the skin. Because near-threshold stimuli are commonly used to map minimal receptive fields in the somatosensory system, small receptive fields usually contained within a single digit have been primarily reported within area 3b (e.g., DiCarlo et al. 1998; Iwamura et al. 1983; Pons et al. 1987; Sur 1980; Sur et al. 1985). We used mechanical stimulation that indented the skin 0.5 mm, and this stimulus would be suprathreshold for most responsive neurons; therefore two-digit receptive fields may not be surprising, even for single neurons. Ours is not the first report of receptive fields encompassing two or more digits. For example, Tremblay et al. (1996) reported 37 of 96 single neurons recorded from area 3b in macaque monkeys had multiple digit receptive fields, although there was a higher proportion of single digit receptive fields in 3b than found in areas 1 and 2. Because our study used suprathreshold stimuli to define a larger excitatory receptive field than would have been obtained with threshold stimuli, our study may have revealed fewer or different “surround” effects than would be reported using near-threshold measures of the receptive field and suprathreshold stimulation outside the minimal receptive field.
Topographic maps can be modified by sensory experience; it seems unlikely that in our anesthetized preparation with passive stimulation, the topography of area 3b would change significantly due to the spatiotemporal stimulation (e.g., Darian-Smith 2009). For the somatotopy of cortical maps in area 3b to be modified, stimuli need to be behaviorally relevant (Clark et al. 1988; Pilz et al. 2004; Xerri et al. 1999) or applied for extended periods of time (Kalisch et al. 2007; Pilz et al. 2004). While we applied stimuli for extended periods of time, the locations to which the stimuli were applied varied throughout the experiment. However, the basic techniques we applied could be used to investigate possible changes in cortical topography due to spatiotemporal features of stimulation.
Spatiotemporal contributions to peak firing rate and response latency changes
Our analyses support the hypothesis that spatiotemporal interactions modulate response magnitudes during paired stimulation (Supplemental Tables S1 and S2). Notably, these results indicate that presentation of a conditioning stimulus affects the response to a test stimulus that may be presented far apart in space (nonadjacent digits) and time (50–500 ms). These results quantify the relationships of specific spatiotemporal stimulus characteristics to neuron response properties and demonstrate that widespread spatiotemporal interactions occur within area 3b. We also related the response fields of neurons to the locations of the paired stimulation locations. As expected, the peak firing rates differed among these categories such that when both probes were outside the response field (OUT_OUT), the peak firing rate was low and when both probes were inside the response field (CN_IN, IN_BOTH), the peak firing rate was much higher (Fig. 4E). Using this categorization, we were able to find statistically significant interactions of this factor with the other prediction factors of temporal and spatial stimulus proximity (Supplemental Tables S1 and S2 and Supplemental Fig. S3).
These results indicate that peak firing rates are generally suppressed by a preceding conditioning stimulus, particularly when test stimuli within the neuron's response field occurred after a 30 or 50 ms delay. Co-stimulating adjacent phalanges resulted in strong suppression of the peak firing rate across all response field relationships when the conditioning stimulus was presented 30 or 50 ms ahead of the test stimulus. Overall, stimulating nonadjacent digits resulted in less suppression, particularly when one stimulus was inside and the other was outside the response field. Suppression of the response peak was found when the stimulation locations were close spatially (within a digit) or both within the neuron's suprathreshold receptive field. The suppression was lessened (or more facilitation was present) when stimulation locations were distant (nonadjacent digits) and the conditioning stimulus was located outside the neuron's response field. We hypothesized that the relationship of the response field to the stimulus sites might account for the different response types and reveal why most neuron responses are suppressed in condition-test paradigms, while subsets of others are facilitated or unchanged. We found, however, that each of the response field categories included units showing no change, suppression, or facilitation of response peaks (Fig. 8).
The influences of the relationship of the stimulation sites to the response field on response latencies were mostly predictable. Paired stimuli outside the response field resulted in the longest latencies, and paired stimuli inside the response field resulted in the shortest latencies (Fig. 4F). The response was primed for paired stimulation within a single digit across all of the response field categories and also for paired stimulation on adjacent digits when the conditioning stimulus was within the neuron's response field. This indicates a facilitative effect specific for when the stimulated locations are close spatially or both within the neuron's suprathreshold receptive field. In these situations in which latencies are shortened, excitatory drive must outweigh the “in-field inhibition” (e.g., DiCarlo et al. 1998; Gardner and Costanzo 1980a,b; Helmstaedter et al. 2009; Jänig et al. 1979; Mountcastle and Powell 1959; Sripati et al. 2006) and lateral inhibition effects (e.g., Chowdhury and Rasmusson 2003; Helmstaedter et al. 2009; Hicks and Dykes 1983) that depress firing and instead allow the spiking to occur a few milliseconds quicker than when the conditioning stimulus is not present.
We found the maximal suppression of firing rates when stimulus onsets were presented 30–50 ms apart (Fig. 4A and Supplemental Fig. S2A), and this coincides well with results from other studies. Condition-test intervals resulting in maximum suppression in S1 cortex were 10–30 ms for adjacent whisker stimulation in rats (Moore et al. 1999; review) and 20–40 ms for adjacent digit stimulation in raccoons (Greek et al. 2003). Within the large receptive fields on the arm in awake macaque monkeys, Gardner and Costanzo (1980b) found maximum suppression intervals of 10–20 ms using air jet stimulation (mostly to hairs). Our suppressive effects for tactile stimulation on paired locations within a digit and across separate digits, rather than on adjacent whiskers or within larger receptive fields, are most similar to the values found for adjacent digit stimulation in raccoons (Greek et al. 2003).
We expected that latencies would be generally lengthened by co-stimulating digits with varying spatiotemporal stimulus parameters as firing rates were typically reduced. Instead response latencies decreased compared with single-site stimulation on the preferred location when the two stimuli were presented simultaneously or when a conditioning, nonpreferred stimulus preceded the test stimulus by ≤30 ms. The shortest latencies occurred when the conditioning stimulus preceded the test stimulus by 10 ms. This result occurred across the spatial categories of stimulus proximity and the relationship of the response field to the stimuli (Fig. 4B and Supplemental Fig. S2B). Latencies were longer when the test stimulus was preceded by a conditioning stimulus by >30 ms, but most latencies were not significantly longer compared with the latency to stimulation on the preferred site alone (Fig. 4B and Supplemental Fig. S2B). Boloori and Stanley (2006), who used stimulation of pairs of whiskers in rats to examine the spatiotemporal response properties in S1 neurons (in layers 2/3 and 4), found that the conditioning stimulus increased the latencies of the test responses, and they did not describe neurons with shortened latencies. In addition, Boloori and Stanley (2006) found that response latency was longer than baseline for short interstimulus delays but recovered to baseline as the delay lengthened. Our finding that the shortest latencies occur when stimuli are presented within 10 ms of each other demonstrates a facilitative effect in which the response to conditioning stimulus facilitates the response to the preferred stimulus. The facilitative effect peaked when the conditioning stimulus preceded the test stimulus by 10 ms and decreased in strength when the delay increased to 30 ms. Stimuli presented farther apart in time had insignificant effects on response latencies.
Latencies were shortened when paired locations were stimulated on separate digits compared with paired locations within a single digit (Fig. 4D). Co-stimulating adjacent digits resulted in the shortest latencies, and co-stimulating adjacent phalanges within a single digit resulted in the longest latencies. The result from stimulating adjacent digits is similar to the finding in the superior colliculus of cats that multisensory stimulation shortens response latencies of neurons compared with the preferred unisensory stimulus (Rowland et al. 2007). Instead of providing two stimuli from different sensory modalities, our pairing tactile stimuli at short delays likely resulted in the summation of subthreshold inputs to allow the spiking threshold to be reached sooner.
SU and MU data comparisons
MU data potentially include responses from neurons with slightly different receptive fields recorded from the same electrode. Thus integration from MU data may differ from integration within single neurons. Interestingly, SU and MU data showed similar trends under our experimental conditions. These similarities in results between SU and MU data may be due to the columnar organization of the cortex, as the MU activity recorded from one electrode would come from neurons within a particular cortical column. An electrophysiological study in primary somatosensory cortex of cats has shown that there were no systematic differences in the receptive field positions for recordings from the same column (Favorov and Diamond 1990).
Our results from SU recordings of peak firing rates and latencies were similar to those from MU recordings as described in the supplemental material. However, the MU recordings produced higher peak firing rates than those from SUs (MU = 27.46 ± 2.21 spike/s; SU = 19.17 ± 1.81 spike/s; P = 0.002), and latencies from the MU sample were slightly shorter than those from SUs (MU = 19.48 ± 0.26 ms; SU = 21.24 ± 0.36 ms; P < 0.0005). A higher firing rate in MU recordings simply reflects the combined rates of two or more neurons; and slightly shorter latencies would reflect the earlier detection of a significant change from spontaneous activities when response rates are higher. Other MU results closely reflected the single unit results; therefore we do not expect that SU activity represented an unusual subset of neurons sampled by the 100-electrode array. Using single electrodes that can be adjusted in depth may result in sampling bias as experimenters isolate the spikes from the largest neurons; however, our method used immovable electrodes. Thus isolated SUs were likely to be those that happened to be closest to the recording electrodes, all of which were within cortical layer 3.
Relationship of peak response magnitudes and latencies
The relationship between response latency and peak firing rate (paired neuron by neuron) followed expectations overall, such that across all spatiotemporal stimulation conditions tested, latencies tended to shorten as firing rates increased (ρ = −0.141, P < 0.0005). Our findings differ somewhat from those of Boloori and Stanley (2006) who found a correlation of −0.76 (P < 0.00001) between latency and firing rate, such that longer response latencies were strongly associated with firing rate suppression in rat S1 neurons (for the 2nd whisker deflection in a stimulus pair). Because our summary analysis of the effects of the spatiotemporal stimulus conditions on response latency (Fig. 4B) and peak firing rate (Fig. 4A) did not consistently reveal the expected inverse relationship between latency and firing rate, we used Wilcoxon signed-ranks tests for paired samples to reveal conditions in which the trend was violated. When the nonpreferred stimulus preceded the preferred stimulus by 10 ms, some neurons responded with shorter latencies but lower firing rates compared with the response to the preferred stimulus alone. However, in a subset of units, we found relative increases in firing rates when paired stimuli were presented within short delays (Figs. 6 and 7), so some underlying facilitative drive must exist to result in shortened latencies and firing rate increases compared with single-site stimulation on the preferred skin location. The underlying cause for the response diversity within our dataset is uncertain. We suspect that in a subset of neurons, latencies were shortened when firing rates decreased due to the time course of facilitation and suppression in individual cortical neurons as described by Sripati et al. (2006). The facilitative component due to stimulus drive from the first conditioning stimulus could reach the neuron of interest, initiating early spikes in response to the test stimulus. The facilitative component is followed by an inhibitory component, which prevents increased spiking in response to the test stimulus. When the stimuli were presented at longer delays, the inhibitory component mostly dominated the dynamic response, resulting in the firing rate suppression we observed in most of the recorded neurons.
Spatiotemporal contributions to deviations from simple summation of peak firing rates in response to paired stimulation
Based on the analyses of firing rate modulation, small subsets of neurons were facilitated by the presence of a preceding conditioning stimulus, primarily when test stimuli were presented after the shortest delays of 0, 10, and 30 ms (Fig. 7). Although the average value of the peak firing rate was maximally suppressed for 30 ms delays across all spatial parameters, this delay also resulted in response facilitation in a subset of neurons; and particularly for conditions in which stimulation occurred on separate digits. Additionally, we found that with increasing stimulus onset asynchronies, peak response suppression increased (Fig. 6). Even at 100 and 500 ms delays, the instances of suppression were still high, particularly for stimulation on adjacent and nonadjacent digits. Studies with condition-test paradigms typically do not use intervals as long as 500 ms. At their longest interval tested of 260 ms, Boloori and Stanley (2006) found that test responses had not fully recovered to control levels. Nevertheless, we may have been more capable of detecting interactions over such large time intervals because stimulus durations were long (500 ms) and the 0.5 mm indentations provided suprathreshold stimulation for most responsive neurons. Thus our stimuli likely recruited more neurons and activated them for longer periods than would occur for brief stimulus pulses at low intensities.
While suppression tends to dominate the results of condition-test paradigms (e.g., Brumberg et al. 1996; Burton et al. 1998; Chowdhury and Rasmusson 2003; Derdikman et al. 2003; Gardner and Costanzo 1980b; Greek et al. 2003; Jänig et al. 1977, 1979; Laskin and Spencer 1979; Melzer et al. 2006; Mirabella et al. 2001; Moore et al. 1999; Simons 1985; Simons and Carvell 1989), we and others (e.g., Greek et al. 2003; Shimegi et al. 1999, 2000) report mixed effects of the conditioning stimulus on the responses of neurons to the test stimulus after the conditioning stimulus. We hypothesized that the relationship of the response field to the stimulus sites might account for the different response types and reveal why most neuron responses are suppressed in condition-test paradigms, while subsets of others are facilitated or unchanged. We found, however, that each of the response field categories included units showing no change, suppression, or facilitation of response peaks (Fig. 8).
While their studies focused on other issues, results from optical imaging experiments in squirrel monkeys (Chen et al. 2003; Friedman et al. 2008) and a supporting sample of single electrode recordings (Friedman et al. 2008) apply to the issue of widespread spatial interactions in area 3b. Optical imaging results showed that simultaneous stimulation on two adjacent digits reduced the signal amplitude at the individual cortical sites for digits. As the signal reduction was not significant when nonadjacent digits were stimulated (Chen et al. 2003; Friedman et al. 2008), suppressive effects were likely stronger when the cortical locations were closer in proximity. While no effect, facilitatory, and suppressive categories were calculated differently than our modulation index categories, more than one-third of the neuron responses in their sample (n = 27 area 3b neurons) were suppressed by simultaneous stimulation on adjacent or nonadjacent digits, 30% of the neuron responses were facilitated, and one-third of the responses were unchanged by paired stimulation (Friedman et al. 2008). The differences in the proportions of responses categorized as no effect, facilitative, or suppressive in the two studies may relate to the sample sizes and differences in categorization calculations. Together with our findings, these studies suggest a spatial gradient of inhibition-facilitation interactions exists such that simultaneous stimulation on adjacent digits results in stronger suppression than that on nonadjacent digits. Our results further suggest that suppression is strongest between locations on adjacent phalanges within a single digit.
Possible origins of spatiotemporal interactions in 3b hand representation
Our study clearly indicates that there are facilitative and suppressive components contributing to the responses of neurons across the digit representation in area 3b. Suppressive effects of conditioning stimuli on firing rate were found over all spatial and temporal stimulus conditions; but the largest proportion of suppressive effects occurred with stimulation of adjacent phalanges within a single digit, followed by nonadjacent phalanges within a single digit. The shortened latencies and increased firing rates indicate that facilitation occurred when adjacent digits were stimulated; and facilitation occurred more often when two stimuli were presented within 0–30 ms of each other. Interactions between stimuli presented to nonadjacent digits resulted in the highest proportions of neurons with facilitated firing rates in response to co-stimulation (11%); however, because the response latencies and peak firing rates showed facilitative effects when adjacent digits were stimulated (Figs. 4B and 7B), we estimated that facilitation between stimuli presented to nonadjacent digits is similar in strength to that when adjacent digits are stimulated.
Intracortical connections are the most likely sources of the spatiotemporal interactions we observed. Primate area 3b has dense horizontal connections overall (Burton and Fabri 1995; De Felipe et al. 1986; Manger et al. 1997) with particularly dense connections within individual digit regions and between digit and adjoining palm regions rather than across adjacent digits (Fang et al. 2002). However, horizontal connections also extend across digit representations (Burton and Fabri 1995; Manger et al. 1997), although these projections likely decrease in density with distance from the soma (Tanosaki et al. 2002a,b). We found that co-stimulating adjacent phalanges on the same digits had a suppressive effect. Yet sites within a single digit are close in physical proximity and have dense intracortical connections in area 3b (Fang et al. 2002). Thus our results suggest that intrinsic, horizontal connections within the cortical representation of a digit primarily have suppressive effects that result in both delayed latencies and decreased firing rates, while the less dense horizontal connections between the representations of digits have mainly a facilitative effect (Fig. 9). There is considerable evidence that intrinsic horizontal connections contribute to the inhibitory surrounds of cortical receptive fields and allow stimuli beyond the classical receptive field to modulate responses to stimuli within the receptive field (e.g., Brecht and Sakmann 2002; Fox 2002; Fox et al. 2003; Gilbert 1992; review: Moore and Nelson 1998; Smits et al. 1991).
Fig. 9.
A summary diagram of the spatiotemporal interactions resulting from functional interactions between parts of the hand representation in area 3b. Representations of 3 digits (D2, D3, D4) in area 3b are shown above the schematic of the owl monkey brain indicating the location of area 3b. These represent stimulation applied to adjacent and nonadjacent locations rather than to the specific digits. One digit is divided into distal (d), middle (m), and proximal (p) sections to represent the stimulation on phalanges within each digit. Line thickness between sites is an approximate representation of the proportion of suppression or facilitation recorded from the selected locations. Black lines refer to suppressive interactions (−) that occurred when stimuli were presented on the paired locations indicated, and these lines are solid to indicate that suppressive interactions were found at all stimulus onset asynchronies tested, from 0 to 500 ms. Gray lines refer to facilitative interactions (+) that occurred when stimuli were presented on the paired sites indicated. All of the gray lines are dashed to indicate that facilitative interactions tended to dominate during short stimulus onset delays from 0 to 30 ms. The most suppressive interactions tended to occur when adjacent phalanges were stimulated and the most facilitative interactions tended to occur when adjacent and nonadjacent digits were stimulated.
The responses of neurons in the present study to spatially distributed stimuli are not necessarily only the result of interactions through cortical connections within area 3b. Area 3b neurons have other ways of being responsive to spatially separate stimuli in addition to using intra-areal connections. 1) The ascending system is divergent in its connections, and receptive field sizes are maintained in part by lateral inhibition with relays in the brain stem (cuneate nucleus) and thalamus (ventroposterior nucleus) (e.g., Garraghty and Sur 1990; Hicks and Dykes 1983; Jones et al. 1982, 1997; Rausell et al. 1995, 1998; Xu and Wall 1999). Thalamocortical axon arbors have been traced in owl monkeys (Garraghty et al. 1989) and macaque monkeys (Garraghty and Sur 1990) to reveal that these arbors can span millimeters of cortex and have secondary branches terminating away from the main branch. Adjacent thalamic neurons can project to targets ≤1.5 mm apart in 3b of macaque monkeys (Rausell and Jones 1995), reflecting dispersion greater than 3b receptive field mapping reveals. These thalamocortical projections could account for some of the interactions within digits and possibly across adjacent digits. Most notably, theoretical modeling of neuron responses in primary visual cortex suggests that many response properties are mediated by thalamocortical connections, including some of the suppression caused by stimuli considered outside the classical receptive field (Carandini et al. 2002); these findings could be relevant to S1 as well. 2) Cortical feedback to thalamic and subthalamic levels appears to sharpen or diminish the receptive field size in visual (e.g., Murphy and Sillito 1987, 1999; Sillito and Jones 2002), auditory (e.g., Yan and Suga 1996; Zhang and Suga 1997, 2000), and somatosensory systems (e.g., Ergenzinger et al. 1998; Ghazanfar and Nicolelis 1997; Ghazanfar et al. 1999, 2001; Ghosh et al. 1994; Shin and Chapin 1990; Temereanca and Simons 2004). Thus spatial interactions of some extent occur subcortically. Feedback that adjusts subcortical receptive fields will in turn influence cortical responses. 3) Area 3b receives feedback connections from other somatosensory representations, especially those in areas 3a, 1, 2, S2, and PV (e.g., Burton and Fabri 1995; Burton et al. 1995; Cusick et al. 1989; Darian-Smith et al. 1993; Krubitzer and Kaas 1990; Qi et al. 2002; Wu and Kaas 2003) that influence response properties. 4) Finally, callosal connections reach 3b directly (albeit, few) (see Killackey et al. 1983 for owl monkeys) and via feedback from other fields with denser callosal connections (Calford 2002; review). Our stimuli were presented only within the contralateral hand; therefore these callosal connections are expected to contribute little to the response properties observed.
Functional relevance of spatiotemporal interactions in the hand representation
The spatiotemporal effects on neuron responses to somatosensory stimuli we report here affect stimulus processing and therefore perception. Although our procedure of stimulating one or two hand locations does not closely mimic many natural stimulation situations, this simplified stimulation allowed us to quantitatively evaluate how sensory information from the hand is integrated in area 3b. In the somatosensory system, spatiotemporal effects of features of stimuli on neuron response characteristics have been suggested to account for motion detection (e.g., Gardner et al. 1992; Hyvärinen and Poranen 1978; Warren et al. 1986; Whitsel et al. 1972), motion direction perception (e.g., Hyvärinen and Poranen 1978; Warren et al. 1986), feature detection regardless of stimulus velocity (DiCarlo and Johnson 2002), and orientation detection (e.g., DiCarlo et al. 2002; Hyvärinen and Poranen 1978; Warren et al. 1986).
From our results, neurons that exhibited facilitation or suppression of firing rates in response to test stimulation preceded by conditioning stimulation on a different hand location could have properties necessary to convey stimulus motion information. Neurons in area 3b that were not modulated by the presence of a preceding conditioning stimulus may not play a direct role in motion detection. While our stimuli are unlikely to elicit perceptions of continuous motion across the paired sites on the digits, these simple stimuli allowed us to investigate basic neuron response properties that likely underlie more complex stimulus discriminations. Our results revealed that neurons in area 3b were affected by stimuli presented far apart in space (nonadjacent digits) and time (50–500 ms apart), reflecting the ability of these neurons to integrate spatiotemporal stimulus information from beyond the classical receptive field.
GRANTS
This work was supported by James S. McDonnell Foundation funding to J. H. Kaas and National Institute of Health Grants NS-16446 to J. H. Kaas, F31-NS-053231 to J. L. Reed, EY-014680-03 to A. B. Bonds, and T32-GM-07347 to M. J. Burish.
Supplementary Material
ACKNOWLEDGMENTS
Matlab scripts were written primarily by Dr. Pierre Pouget and J. Haitas in collaboration with J. L. Reed and J. Cockhren. Dr. Omar Gharbawie and C. Camalier helped collect data from two of the three monkey cases.
The present address of Dr. Z. Zhou: Research and Development, Epic Systems, 1979 Milky Way, Verona, WI 53593.
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Allman J, Miezin F, McGuinness E. Stimulus specific responses from beyond the classical receptive field: neurophysiological mechanisms for local-global comparisons in visual neurons. Annu Rev Neurosci 8: 407–430, 1985. [DOI] [PubMed] [Google Scholar]
- Alvarado JC, Vaughan JW, Stanford TR, Stein BE. Multisensory versus unisensory integration: contrasting modes in the superior colliculus. J Neurophysiol 97: 3193–205, 2007. [DOI] [PubMed] [Google Scholar]
- Armstrong-James M, Fox K. Spatiotemporal convergence and divergence in the rat S1 “barrel” cortex. J Comp Neurol 236: 265–281, 1987. [DOI] [PubMed] [Google Scholar]
- Arnold PB, Li CX, Waters RS. Thalamocortical arbors extend beyond single cortical barrels: an in vivo intracellular tracing study in rat. Exp Brain Res 136: 152–168, 2001. [DOI] [PubMed] [Google Scholar]
- Berwick J, Redgrave P, Jones M, Hewson-Stoate N, Martindale J, Johnston D, Mayhew JEW. Integration of neural responses originating from different regions of the cortical somatosensory map. Brain Res 1030: 284–293, 2004. [DOI] [PubMed] [Google Scholar]
- Boloori AR, Stanley GB. The dynamics of spatiotemporal response integration in the somatosensory cortex of the vibrissa system. J Neurosci 26: 3767–3782, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun C, Hess H, Burkhardt M, Wühle A, Preissl H. The right hand knows what the left hand is feeling. Exp Brain Res 162: 366–373, 2005. [DOI] [PubMed] [Google Scholar]
- Brecht M, Sakmann B. Dynamic representation of whisker deflection by synaptic potentials in spiny stellate and pyramidal cells in the barrels and septa of layer 4 rat somatosensory cortex. J Physiol 543: 49–70, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown HA, Allison JD, Samonds JM, Bonds AB. Nonlocal origin of response suppression from stimulation outside the classic receptive field in area 17 of the cat. Vis Neurosci 20: 85–96, 2003. [DOI] [PubMed] [Google Scholar]
- Brumberg JC, Pinto DJ, Simons DJ. Spatial gradients and inhibitory summation in the rat whisker barrel system. J Neurophysiol 76: 130–140, 1996. [DOI] [PubMed] [Google Scholar]
- Burton H, Fabri M. Ipsilateral intracortical connections of physiologically defined cutaneous representations in area 3b and 1 of macaque monkeys: projections in the vicinity of the central sulcus. J Comp Neurol 355: 508–538, 1995. [DOI] [PubMed] [Google Scholar]
- Burton H, Fabri M, Alloway K. Cortical areas within the lateral sulcus connected to cutaneous representations in areas 3b and 1: a revised interpretation of the second somatosensory area in macaque monkeys. J Comp Neurol 355: 539–562, 1995. [DOI] [PubMed] [Google Scholar]
- Burton H, Sinclair RJ, Whang K. Vibrotactile stimulus order effects in somatosensory cortical areas of rhesus monkeys. Somatosens Mot Res 15: 316–324, 1998. [DOI] [PubMed] [Google Scholar]
- Calford MB. Dynamic representational plasticity in sensory cortex. Neuroscience 111: 709–738, 2002. [DOI] [PubMed] [Google Scholar]
- Canedo A, Aguilar J. Spatial and cortical influences exerted on cuneothalamic and thalamocortical neurons of the cat. Eur J Neurosci 12: 2515–2533, 2000. [DOI] [PubMed] [Google Scholar]
- Carandini M, Heeger DJ, Senn W. A synaptic explanation of suppression in visual cortex. J Neurosci 22: 10053–10065, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LM, Friedman RM, Roe AW. Optical imaging of a tactile illusion in area 3b of the primary somatosensory cortex. Science 302: 881–885. [DOI] [PubMed] [Google Scholar]
- Chowdhury SA, Rasmusson DD. Corticocortical inhibition of peripheral inputs within primary somatosensory cortex: the role of GABAA and GABAB receptors. J Neurophysiol 90: 851–856, 2003. [DOI] [PubMed] [Google Scholar]
- Clark SA, Allard T, Jenkins WM, Merzenich MM. Receptive fields in the body-surface map in adult cortex defined by temporally correlated inputs. Nature 332: 444–445, 1988. [DOI] [PubMed] [Google Scholar]
- Cusick CG, Wall JT, Felleman DJ, Kaas JH. Somatotopic organization of the lateral sulcus of owl monkeys: area 3b, S-II, and a ventral somatosensory area. J Comp Neurol 282: 169–190, 1989. [DOI] [PubMed] [Google Scholar]
- Darian-Smith C. Somatosensory plasticity. In: Encyclopedia of Neuroscience, edited by Squire LR. Elsevier, 2009, p. 99–110. [Google Scholar]
- Darian-Smith C, Darian-Smith I, Burman K, Ratcliffe N. Ipsilateral cortical projections to areas 3a, 3b, and 4 in the macaque monkey. J Comp Neurol 335: 200–213, 1993. [DOI] [PubMed] [Google Scholar]
- Davidson AG, O'Dell R, Chan V, Schieber MH. Comparing effects in spike-triggered averages of rectified EMG across different behaviors. J Neurosci Methods 163: 283–294, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felipe J, Conley M, Jones EG. Long-range focal collateralization of axons arising from corticocortical cells in monkey sensory-motor cortex. J Neurosci 6: 3749–3766, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derdikman D, Hildesheim R, Ahissar E, Arieli A, Grinvald A. Imaging spatio-temporal dynamics of surround inhibition in the barrels somatosensory cortex. J Neurosci 23: 3100–3105, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCarlo JJ, Johnson KO. Spatial and temporal structure of receptive fields in primate somatosensory area 3b: effects of stimulus scanning direction and orientation. J Neurosci 20: 495–510, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCarlo JJ, Johnson KO. Receptive field structure in cortical area 3b of the alert monkey. Behav Brain Res 135: 167–178, 2002. [DOI] [PubMed] [Google Scholar]
- DiCarlo JJ, Johnson KO, Hsiao SS. Structure of receptive fields in area 3b of primary somatosensory cortex in the alert monkey. J Neurosci 18: 2626–2645, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ergenzinger ER, Glasier MM, Hahm JO, Pons TP. Cortically induced thalamic plasticity in the primate somatosensory system. Nat Neurosci 1: 226–229, 1998. [DOI] [PubMed] [Google Scholar]
- Fang P-C, Jain N, Kaas JH. Few intrinsic connections cross the hand-face border of area 3b of new world monkeys. J Comp Neurol 454: 310–319, 2002. [DOI] [PubMed] [Google Scholar]
- Favorov OV, Diamond ME. Demonstration of discrete place-defined columns—segregates—in the cat SI. J Comp Neurol 298: 97–112, 1990. [DOI] [PubMed] [Google Scholar]
- Fox K. Anatomical pathways and molecular mechanisms for plasticity in the barrel cortex. Neuroscience 111: 799–814, 2002. [DOI] [PubMed] [Google Scholar]
- Fox K, Wright N, Wallace H, Glazewski S. The origin of cortical surround receptive fields studied in the barrel cortex. J Neurosci 23: 8380–8391, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman RM, Chen LM, Roe AW. Responses of areas 3b and 1 in anesthetized squirrel monkeys to single- and dual-site stimulation of the digits. J Neurophysiol 100: 3185–3196, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner EP, Costanzo RM. Spatial integration of multiple-point stimuli in primary somatosensory cortical receptive fields of alert monkeys. J Neurophysiol 43: 420–443, 1980a. [DOI] [PubMed] [Google Scholar]
- Gardner EP, Costanzo RM. Temporal integration of multiple-point stimuli in primary somatosensory cortical receptive fields of alert monkeys. J Neurophysiol 43: 444–468, 1980b. [DOI] [PubMed] [Google Scholar]
- Gardner EP, Palmer CI, Hämäläinen HA, Warren S. Simulation of motion on the skin. V. Effect of stimulus temporal frequency on the representation of moving bar patterns in primary somatosensory cortex of monkeys. J Neurophysiol 67: 37–63, 1992. [DOI] [PubMed] [Google Scholar]
- Garraghty PE, Pons TP, Sur M, Kaas JH. The arbors of axons terminating in middle cortical layers of somatosensory area 3b in owl monkeys. Somatosens Motor Res 6: 401–411, 1989. [DOI] [PubMed] [Google Scholar]
- Garraghty PE, Sur M. Morphology of single intracellularly stained axons terminating in area 3b of macaque monkeys. J Comp Neurol 294: 583–593, 1990. [DOI] [PubMed] [Google Scholar]
- Ghazanfar AA, Krupa DJ, Nicolelis MAL. Role of cortical feedback in the receptive field structure and nonlinear response properties of somatosensory thalamic neurons. Exp Brain Res 141: 88–100, 2001. [DOI] [PubMed] [Google Scholar]
- Ghazanfar AA, Nicolelis MAL. Nonlinear processing of tactile information in the thalamocortical loop. J Neurophysiol 78: 506–510, 1997. [DOI] [PubMed] [Google Scholar]
- Ghazanfar AA, Nicolelis MAL. Spatiotemporal properties of layer V neurons of the rat primary somatosensory cortex. Cereb Cortex 9: 348–361, 1999. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Murray GM, Turman AB, Rowe MJ. Corticothalamic influences on transimission of tactile information in the ventroposterolateral thalamus of the cat: effect of reversible inactivation of somatosensory cortical areas I and II. Exp Brain Res 100: 276–286, 1994. [DOI] [PubMed] [Google Scholar]
- Gilbert CD. Horizontal integration and cortical dynamics. Neuron 9: 1–13, 1992. [DOI] [PubMed] [Google Scholar]
- Greek KA, Chowdhury SA, Rasmusson DD. Interactions between inputs from adjacent digits in somatosensory thalamus and cortex of the raccoon. Exp Brain Res 151: 364–371, 2003. [DOI] [PubMed] [Google Scholar]
- Hardin JW, Hilbe JM. Generalized Estimating Equations. Boca Raton, FL: Chapman and Hall/CRC, 2003. [Google Scholar]
- Helmstaedter M, Sakmann B, Feldmeyer D. Neuronal correlates of local, lateral, and trans-laminar inhibition with reference to cortical columns. Cereb Cortex 19: 926–937, 2009. [DOI] [PubMed] [Google Scholar]
- Hicks TP, Dykes RW. Receptive field size for certain neurons in primary somatosensory cortex is determined by GABA-mediated intracortical inhibition. Brain Res 274: 160–164, 1983. [DOI] [PubMed] [Google Scholar]
- Hoechstetter K, Rupp A, Stančák A, Meinck H-M, Stippich C, Berg P, Scherg M. Interaction of tactile input in the human primary and secondary somatosensory cortex—a magnetoencephalographic study. Neuroimage 14: 759–767, 2001. [DOI] [PubMed] [Google Scholar]
- Hyvärinen J, Poranen A. Movement-sensitive and direction and orientation-selective cutaneous receptive fields in the hand area of the post-central gyrus in monkeys. J Physiol 283: 523–537, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamura Y, Tanaka M, Sakamoto M, Hikosaka O. Functional subdivisions representing different finger regions in area 3b or the first somatosensory cortex of the conscious monkey. Exp Brain Res 51: 315–326, 1983. [Google Scholar]
- Jain N, Qi H-X, Kaas JH. Long-term chronic multichannel recordings from sensorimotor cortex and thalamus of primates. In: Progress in Brain Research. New York: Elsevier Science B.V, 2001, chapt. 5, p. 1–10. [PubMed] [Google Scholar]
- Jänig W, Schoultz T, Spencer WA. Temporal and spatial parameters of excitation and afferent inhibition in cuneothalamic relay neurons. J Neurophysiol 40: 822–835, 1977. [DOI] [PubMed] [Google Scholar]
- Jänig W, Spencer WA, Younkin SG. Spatial and temporal features of afferent inhibition of thalamocortical relay cells. J Neurophysiol 42: 1450–1460, 1979. [DOI] [PubMed] [Google Scholar]
- Jones EG, Friedman DP, Hendry SHC. Thalamic basis of place- and modality-specific columns in monkey somatosensory cortex: a correlative anatomical and physiological study. J Neurophysiol 48: 545–568, 1982. [DOI] [PubMed] [Google Scholar]
- Jones EG, Manger PR, Woods TM. Maintenance of a somatotopic cortical map in the face of diminishing thalamocortical inputs. Proc Natl Acad Sci USA 94: 11003–11007, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas JH. What, if anything, is SI? Organization of first somatosensory area of cortex. Physiol Rev 63: 206–231, 1983. [DOI] [PubMed] [Google Scholar]
- Kalisch T, Tegenthoff M, Dinse HR. Differential effects of synchronous and asynchronous multifinger coactivation on human tactile performance. BMC Neurosci 8: 58, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killackey HP, Gould HJ, 3rd, Cusick CG, Pons TP, Kaas JH. The relation of corpus callosum connections to architectonic fields and body surface maps in sensorimotor cortex of new and old world monkeys. J Comp Neurol 219: 384–419, 1983. [DOI] [PubMed] [Google Scholar]
- Krubitzer LA, Kaas JH. The organization and connections of somatosensory cortex in marmosets. J Neurosci 10: 952–974, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskin SE, Spencer WA. Cutaneous masking. II. Geometry of excitatory and inhibitory receptive fields of single units in somatosensory cortex of the cat. J Neurophysiol 42: 1061–1082, 1979. [DOI] [PubMed] [Google Scholar]
- Li CX, Waters RS. In vivo intracellular recording and labeling of neurons in the forepaw barrel subfield (FBS) of rat somatosensory cortex: possible physiological and morphological substrates for reorganization. Neuroreport 7: 2261–2272, 1996. [DOI] [PubMed] [Google Scholar]
- Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika 73: 13–22, 1986. [Google Scholar]
- Manger PR, Woods TM, Muñoz A, Jones EG. Hand/face border as a limiting boundary in the body representation in monkey somatosensory cortex. J Neurosci 17: 6338–6351, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason A, Nicoll A, Stratford K. Synaptic transmission between individual pyramidal neurons of the rat visual cortex in vitro. J Neurosci 11: 72–84, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer P, Champney GC, Maguire MJ, Ebner FF. Rate code and temporal code for frequency of whisker stimulation in rat primary and secondary somatic sensory cortex. Exp Brain Res 172: 370–386, 2006. [DOI] [PubMed] [Google Scholar]
- Merzenich MM, Kaas JH, Sur M, Lin CS. Double representations of the body surface within cytoarchitectonic areas 3b and 1 in “SI” in the owl monkey (Aotus trivirgatus). J Comp Neurol 181: 41–74, 1978. [DOI] [PubMed] [Google Scholar]
- Mirabella G, Battiston S, Diamond ME. Integration of multiple-whisker inputs in rat somatosensory cortex. Cereb Cortex 11: 164–170, 2001. [DOI] [PubMed] [Google Scholar]
- Moore CI, Nelson SB. Spatio-temporal subthreshold receptive fields in the vibrissa representation of rat primary somatosensory cortex. J Neurophysiol 80: 2882–2892, 1998. [DOI] [PubMed] [Google Scholar]
- Moore CI, Nelson SB, Sur M. Dynamics of neuronal processing in rat somatosensory cortex. Trends Neurosci 22: 513–520, 1999. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB. The Sensory Hand: Neural Mechanisms of Somatic Sensation. Cambridge, MA: Harvard University Press, 2005, p. 320–322; 461–462. [Google Scholar]
- Mountcastle VB, Powell TPS. Neural mechanisms subserving cutaneous sensibility, with special reference to the role of afferent inhibition in sensory perception and discrimination. Bull Johns Hopkins Hosp 105: 201–232, 1959. [PubMed] [Google Scholar]
- Murphy PC, Duckett SG, Sillito AM. Feedback connections to the lateral geniculate nucleus and cortical response properties. Science 286: 1552–1554, 1999. [DOI] [PubMed] [Google Scholar]
- Murphy PC, Sillito AM. Corticofugal feedback influences the generation of length tuning in the visual pathway. Nature 329: 727–729, 1987. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, Sur M, Felleman DJ, Kaas JH. Representations of the body surface in postcentral parietal cortex of Macaca fascicularis. J Comp Neurol 192: 611–643, 1980. [DOI] [PubMed] [Google Scholar]
- Nicolelis MAL, Ghazanfar AA, Stambaugh CR, Oliveira LMO, Laubach M, Chapin JK, Nelson RJ, Kaas JH. Simultaneous encoding of tactile information by three primate cortical areas. Nat Neurosci 1: 621–630, 1998. [DOI] [PubMed] [Google Scholar]
- Nicolelis MAL, Dimitrov D, Carmena JM, Crist R, Lehew G, Kralik JD, Wise SP. Chronic, multisite, multielectrode recordings in macaque monkeys. Proc Natl Acad Sci USA 100: 11041–11046, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilz K, Veit R, Braun C, Godde B. Effects of co-activation on cortical organization and discrimination performance. Neuroreport 15: 2669–2672, 2004. [DOI] [PubMed] [Google Scholar]
- Pons TP, Wall JT, Garraghty PE, Cusick CG, Kaas JH. Consistent features of the representation of the hand in area 3b of macaque monkeys. Somatosens Res 4: 309–331, 1987. [DOI] [PubMed] [Google Scholar]
- Qi H-X, Lyon DC, Kaas JH. Cortical and thalamic connections of the parietal ventral somatosensory area in marmoset monkeys (Callithrix jacchus). J Comp Neurol 443: 168–182, 2002. [DOI] [PubMed] [Google Scholar]
- Rausell E, Bickford L, Manger PR, Woods TM, Jones EG. Extensive divergence and convergence in the thalamocortical projection to monkey somatosensory cortex. J Neurosci 18: 4216–4232, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausell E, Jones EG. Extent of intracortical arborization of thalamocortical axons as a determinant of representational plasticity in monkey somatic sensory cortex. J Neurosci 15: 4270–4288, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JL, Pouget P, Qi H-X, Zhou Z, Bernard MR, Burish MJ, Haitas J, Bonds AB, Kaas JH. Widespread spatial integration in primary somatosensory cortex. Proc Natl Acad Sci USA 105: 10233–10237, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland BA, Quessy S, Stanford TR, Stein BE. Multisensory integration shortens physiological response latencies. J Neurosci 27: 5879–5884, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samonds JM, Allison JD, Brown HA, Bonds AB. Cooperation between Area 17 neuron pairs enhances fine discrimination of orientation. J Neurosci 23: 2416–2425, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer R, Maier M, Braun C, Birbaumer N. Distribution of mislocalizations of tactile stimuli on the fingers of the human hand. Somatosens Motor Res 17: 309–316, 2000. [DOI] [PubMed] [Google Scholar]
- Shimegi S, Akasaki T, Ichikawa T, Sato H. Physiological and anatomical organization of multiwhisker response interactions in the barrel cortex of rats. J Neurosci 20: 6241–6248, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimegi S, Ichikawa T, Akasaki T, Sato H. Temporal characteristics of response integration evoked by multiple whisker stimulations in the barrel cortex of rats. J Neurosci 19: 10164–10175, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H-C, Chapin JK. Mapping the effects of SI cortex stimulation on somatosensory relay neurons in the rat thalamus: direct responses and afferent modulation. Somatosens Motor Res 7: 421–434, 1990. [DOI] [PubMed] [Google Scholar]
- Shoham S, Fellows MR, Normann RA. Robust, automatic spike sorting using mixtures of multivariate t-distributions. J Neurosci Methods 127: 111–122, 2003. [DOI] [PubMed] [Google Scholar]
- Sillito AM, Jones HE. Corticothalamic interactions in the transfer of visual information. Philos Trans R Soc Lond B Biol Sci 357: 1739–1752, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons DJ. Temporal and spatial integration in the rat SI vibrissa cortex. J Neurophysiol 54: 615–633, 1985. [DOI] [PubMed] [Google Scholar]
- Simons DJ, Carvell GE. Thalamocortical response transformation in the rat vibrissa/ barrel system. J Neurophysiol 61: 311–330, 1989. [DOI] [PubMed] [Google Scholar]
- Smits E, Gordon DC, Witte S, Rasmusson DD, Zarzecki P. Synaptic potentials evoked by convergent somatosensory and corticocortical inputs in raccoon somatosensory cortex: substrates for plasticity. J Neurophysiol 66: 688–695, 1991. [DOI] [PubMed] [Google Scholar]
- Sripati AP, Yoshioka T, Denchev P, Hsiao SS, Johnson KO. Spatiotemporal receptive fields of peripheral afferents and cortical area 3b and 1 neurons in the primate somatosensory system. J Neurosci 26: 2101–2114, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford TR, Quessy S, Stein BE. Evaluating the operations underlying multisensory integration in the cat superior colliculus. J Neurosci 25: 6499–6508, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur M. Receptive fields of neurons in areas 3b and 1 of somatosensory cortex in monkeys. Brain Res 198: 465–471, 1980. [DOI] [PubMed] [Google Scholar]
- Sur M, Garraghty PE, Bruce CJ. Somatosensory cortex in macaque monkeys: laminar differences in receptive field size in areas 3b and 1. Brain Res 342: 391–395, 1985. [DOI] [PubMed] [Google Scholar]
- Sur M, Wall JT, Kaas JH. Modular distribution of neurons with slowly adapting and rapidly adapting responses in area 3b of somatosensory cortex in monkeys. J Neurophysiol 51: 724–744, 1984. [DOI] [PubMed] [Google Scholar]
- Tanosaki M, Suzuki A, Kimura T, Takino R, Haruta Y, Hoshi Y, Hashimoto I. Contribution of primary somatosensory area 3b to somatic cognition: a neuromagnetic study. Neuroreport 13: 1519–1522, 2002a. [DOI] [PubMed] [Google Scholar]
- Tanosaki M, Suzuki A, Takino R, Kimura T, Iguchi Y, Kurobe Y, Haruta Y, Hoshi Y, Hashimoto I. Neural mechanisms for generation of tactile interference effects on somatosensory evoked magnetic fields in humans. Clin Neurophysiol 113: 672–680, 2002b. [DOI] [PubMed] [Google Scholar]
- Temereanca S, Simons DJ. Functional topography of corticothalamic feedback enhances thalamic spatial response tuning in the somatosensory whisker/barrel system. Neuron 41: 639–651, 2004. [DOI] [PubMed] [Google Scholar]
- Tremblay F, Ageranioti-Bélanger SA, Chapman CE. Cortical mechanisms underlying tactile discrimination in the monkey. I. Role of primary somatosensory cortex in passive texture discrimination. J Neurophysiol 76: 3382–3403, 1996. [DOI] [PubMed] [Google Scholar]
- Tuerlinckx F, Rijmen F, Verbeke G, De Boeck P. Statistical inference in generalized linear mixed models: a review. Br J Math Stat Psychol 59: 225–255, 2006. [DOI] [PubMed] [Google Scholar]
- Tutunculer B, Foffani G, Himes BT, Moxon KA. Structure of the excitatory receptive fields of infragranular forelimb neurons in the rate primary somatosensory cortex responding to touch. Cereb Cortex 16: 791–810, 2006. [DOI] [PubMed] [Google Scholar]
- Vega-Bermudez F, Johnson KO. Surround suppression in the responses of primate SA1 and RA mechanoreceptive afferents mapped with a probe array. J Neurophysiol 81: 2711–2719, 1999. [DOI] [PubMed] [Google Scholar]
- Veredas FJ, Vico FJ, Alonso JM. Factors determining the precision of the correlated firing generated by a monosynaptic connection in the cat visual pathway. J Physiol 567. 3: 1057–1078, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker GA, Ohzawa I, Freeman RD. Suppression outside the classical cortical receptive field. Vis Neurosci 17: 1–11, 2000. [DOI] [PubMed] [Google Scholar]
- Wang X, Merzenich MM, Sameshima K, Jenkins WM. Remodelling of hand representation in adult cortex determined by timing of tactile stimulation. Nature 378: 71–75, 1995. [DOI] [PubMed] [Google Scholar]
- Warren S, Hämäläinen HA, Gardner EP. Objective classification of motion- and direction-sensitive neurons in primary somatosensory cortex of awake monkeys. J Neurophysiol 56: 598–622, 1986. [DOI] [PubMed] [Google Scholar]
- Whitsel BL, Roppolo JR, Werner G. Cortical information processing of stimulus motion on primate skin. J Neurophysiol 35: 691–717, 1972. [DOI] [PubMed] [Google Scholar]
- Wiemer J, Spengler F, Joublin F, Stagge P, Wacquant S. Learning cortical topography from spatiotemporal stimuli. Biol Cybern 82: 173–187, 2000. [DOI] [PubMed] [Google Scholar]
- Wu CW-H, Kaas JH. Somatosensory cortex of prosimian galagos: physiological recording, cytoarchitecture, and cortiocortical connections of anterior parietal cortex and cortex of the lateral sulcus. J Comp Neurol 457: 263–292, 2003. [DOI] [PubMed] [Google Scholar]
- Xerri C, Merzenich MM, Jenkins W, Santucci S. Representational plasticity in cortical area 3b paralleling tactual-motor skill acquisition in adult monkeys. Cereb Cortex 9: 264–276, 1999. [DOI] [PubMed] [Google Scholar]
- Xing J, Gerstein GL. Networks with lateral connectivity. III. Plasticity and reorganization of somatosensory cortex. J Neurophysiol 75: 217–232, 1996. [DOI] [PubMed] [Google Scholar]
- Xu J, Wall JT. Functional organization of tactile inputs from the hand in the cuneate nucleus and its relationship to organization in the somatosensory cortex. J Comp Neurol 411: 369–389, 1999. [PubMed] [Google Scholar]
- Xu X, Collins CE, Kaskan PM, Khaytin I, Kaas JH, Casagrande VA. Optical imaging of visually evoked responses in prosimian primates reveals conserved features of the middle temporal visual area. Proc Natl Acad Sci USA 101: 2566–2571, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Suga N. Corticofugal modulation of time-domain processing of biosonar information in bats. Science 273: 1100–1103, 1996. [DOI] [PubMed] [Google Scholar]
- Zeger SL, Liang K-Y. Longitudinal data analysis for discrete and continuous outcomes. Biometrics 42: 121–130, 1986. [PubMed] [Google Scholar]
- Zhang Y, Suga N. Modulation of responses and frequency tuning of thalamic and collicular neurons by cortical activation in mustached bats. J Neurophysiol 84: 325–333, 2000. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Suga N, Yan J. Corticofugal modulation of frequency processing in bat auditory system. Nature 387: 900–903, 1997. [DOI] [PubMed] [Google Scholar]
- Zhu JJ, Connors BW. Intrinsic firing patterns and whisker-evoked synaptic responses of neurons in the rat barrel cortex. J Neurophysiol 81: 1171–1183, 1999. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.