Abstract
The cortical nucleus LMAN (lateral magnocellular nucleus of the anterior nidopallium) provides the output of a basal ganglia pathway that is necessary for acquisition of learned vocal behavior during development in songbirds. LMAN is composed of two subregions, a core and a surrounding shell, that give rise to independent pathways that traverse the forebrain in parallel. The LMANshell pathway forms a recurrent loop that includes a cortical region, the dorsal region of the caudolateral nidopallium (dNCL), hitherto unknown to be involved with learned vocal behavior. Here we show that vocal production strongly induces the IEG product ZENK in dNCL of zebra finches. Hearing tutor song while singing is more effective at inducing expression in dNCL of juvenile birds during the auditory–motor integration stage of vocal learning than is hearing conspecific song. In contrast, hearing conspecific song is relatively more effective at inducing expression in adult birds, regardless of whether they are producing song. Furthermore, ZENK+ neurons in dNCL include projection neurons that are part of the LMANshell recurrent loop and a high proportion of dNCL projection neurons express ZENK in singing juvenile birds that hear tutor song. Thus juvenile birds that are actively refining their vocal pattern to imitate a tutor song show high levels of ZENK induction in dNCL neurons when they are singing while hearing the song of their tutor and low levels when they hear a novel conspecific. This pattern indicates that dNCL is a novel brain region involved with vocal learning and that its function is developmentally regulated.
INTRODUCTION
Songbirds and humans learn the sounds used for vocal communication by forming a memory of vocal sounds from adult “tutors” based on auditory experience, during a sensitive period of development, and then using feedback of self-produced vocalizations to adjust motor commands until vocal output matches the neural memory of those acoustic patterns. A basal ganglia circuit that is necessary for vocal learning includes a projection from the striatal/pallidal region Area X to a dorsal thalamic region (DLM), which projects in turn to the cortical nucleus LMAN (lateral magnocellular nucleus of the anterior nidopallium) (Fig. 1) (Bottjer 2004; Doupe et al. 2005; Perkel 2004).
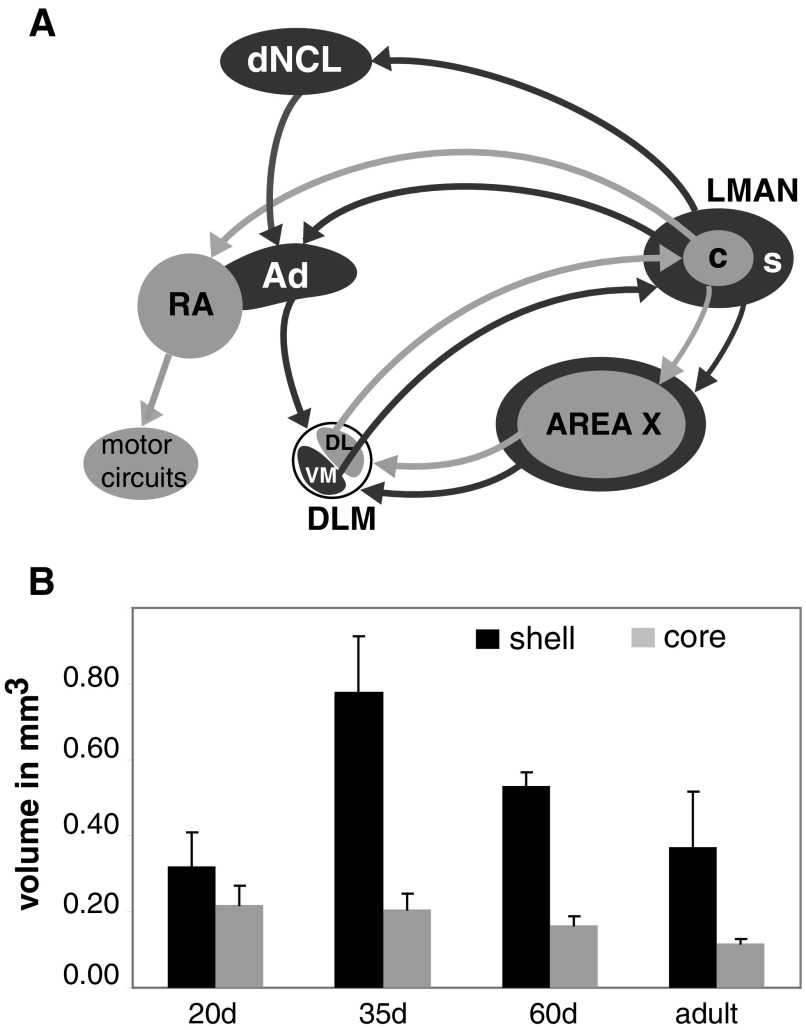
Fig. 1.
Major axonal connections made by core and shell regions of LMAN. A: the cortical nucleus LMAN provides the output of a basal ganglia pathway from Area X (and surrounding medial striatum) to DLM (in dorsal thalamus) to LMAN. Area X contains both striatal and pallidal neurons. The core region of LMAN (light gray) projects to RA within the area of avian brain that is analogous to motor cortex and RA projects in turn to descending motor circuits that activate vocal and respiratory muscles. In contrast, the shell region of LMAN (dark gray) projects to Ad, an area of motor cortex adjacent to RA. Ad does not make direct connections to hindbrain vocal-motor circuits, but makes a variety of axonal projections, including a projection to DLM that forms a recurrent loop from LMANshell → dNCL → Ad → DLMVM → LMANshell. The shell region of LMAN projects to both Ad and to a region of polymodal association cortex, dNCL, which projects in turn to Ad, such that Ad receives both direct and indirect projections from LMANshell. Separate groups of neurons in DLM (dorsolateral [DL] vs. ventromedial [VM]) project to either core or shell, respectively, and core and shell regions of LMAN project to RA vs. Ad, such that these connections form parallel, apparently independent projections that traverse the forebrain. Core and shell regions of LMAN also project to Area X and surrounding medial striatum, respectively. Not shown for the sake of clarity is a projection from Ad to DMP (part of a dorsal thalamic zone that includes both DLM and DMP); DMP projects to MMAN, which projects in turn to HVC (see Supplemental Fig. S1). B: changes in the volume encompassed by core (light gray) and shell (dark gray) regions of LMAN. The volume of LMANcore decreases slightly (but significantly) despite maintaining a fixed number of projection neurons. The volume of LMANshell exhibits pronounced growth during early stages of vocal learning (20–35 days of age), followed by substantial regression between 35 days and adulthood (90 days). Data are replotted from Johnson et al. (1995). LMAN, lateral magnocellular nucleus of the anterior nidopallium; MMAN, medial magnocellular nucleus of the anterior nidopallium; c, core region; s, shell region; Area X, Area X of the medial striatum; HVC, high vocal center; RA, robust nucleus of the arcopallium; Ad, dorsal arcopallium; DLM, medial dorsolateral nucleus of the thalamus (DL contains neurons in the dorsolateral portion of DLM that project to LMANcore, whereas VM contains neurons in the ventromedial portion of DLM that project to LMANshell); DMP, dorsomedial nucleus of the posterior thalamus; dNCL, dorsal region of the caudolateral nidopallium (see Reiner et al. 2004).
LMAN is composed of two separate subregions: a core region of mostly magnocellular neurons and a surrounding shell region containing both magnocellular and parvocellular neurons (Fig. 1A) (Johnson and Bottjer 1992; Pinaud et al. 2007). Core and shell regions of LMAN give rise to independent parallel pathways that traverse Area X and surrounding striatum, the thalamic nucleus DLM, and the robust nucleus of the arcopallium (RA) and Ad (dorsal arcopallium; adjacent brain nuclei within the region analogous to motor cortex) (Iyengar et al. 1999; Johnson et al. 1995; Luo et al. 2001; Person et al. 2008). However, the parallel pathways formed by core and shell regions of LMAN are largely discontinued in the output pathways of motor cortex: RA makes strong projections to hindbrain vocal motor and respiratory circuits (Wild 1993, 1997, 2004); Ad makes a variety of axonal connections, including a prominent projection to a dorsal thalamic zone that sends projections to LMAN as well as to the cortical motor-control nucleus high vocal center (HVC) (via the medial magnocellular nucleus of the anterior nidopallium [MMAN]; see Supplemental Fig. S1) (Bottjer et al. 2000; Foster et al. 1997).1 Thus the LMANshell pathway forms a recurrent loop that makes feedback connections to LMAN as well as feedforward connections to HVC. Damage to the LMANshell pathway in juvenile birds does not disrupt basic vocal motor production but prevents the acquisition of stereotyped vocal sequences as well as imitation of sounds from a tutor (Bottjer and Altenau 2009).
The volume of LMANshell more than doubles during early stages of vocal learning and then regresses as birds approach adulthood and the song pattern becomes stereotyped (Fig. 1B; Johnson and Bottjer 1992; Johnson et al. 1995). In contrast, the volume of LMANcore never grows, but does regress somewhat as vocal learning proceeds. The dramatic increase in overall volume of LMANshell is accompanied by pronounced regression of individual thalamic axon arbors (from DLM) within this region during early stages of vocal learning (Iyengar and Bottjer 2002a), indicating substantial refinement of connectivity in this part of the shell pathway during the sensitive period. This pattern suggests that LMANshell makes some unique contribution to mechanisms of vocal learning during development (e.g., when its volume is maximal), which could account in part for the enhanced capacity for vocal plasticity observed during the sensitive period.
LMANshell also projects strongly to a specific region within dNCL, a cortical polymodal association region similar to a region of chick brain that has been implicated in imprinting (Bottjer et al. 2000; Braun et al. 1999; Metzger et al. 1998). In zebra finches, dNCL projects to Ad, such that LMANshell projects directly to Ad and indirectly via dNCL (Fig. 1A). However, this region has never been implicated as having an involvement in neural control of vocal learning. Here we show that dNCL neurons show robust expression of the immediate early gene (IEG) ZENK following song production and that ZENK expression is high in juvenile birds that are practicing their own emerging song when they hear playback of tutor song, but is relatively low when they hear playback of novel conspecific song. In contrast, playback of conspecific songs is highly effective in inducing ZENK expression in dNCL neurons of adult birds, regardless of whether they are producing song. Additionally, a higher proportion of Ad-projecting neurons in dNCL express ZENK following playback of tutor song compared with conspecific song in juvenile singing birds. These results suggest an important role for dNCL, as part of the LMANshell circuit, in auditory–motor integration or motor learning.
METHODS
Subjects, song recording, and playback
Male zebra finches were bred in our group aviaries; they were raised by their natural parents and heard the father's tutor song up until ≥35 days, which is sufficient for making a detailed copy (Bohner 1990; Jones et al. 1996; Roper and Zann 2006). Tutors were identified by observers who watched the five nest boxes in each group aviary and identified the adult male that entered each nest box. Only one adult male (the father) enters a specific nest box (we identify each male by using unique combinations of colored leg bands). Juvenile birds were segregated from their group aviary and housed in individual cages starting at 36–54 days of age. Vocal behavior was recorded (44 kHz, Sound Analysis Pro ver. 1.04; Tchernichovski et al. 2000) from singly caged birds in individual chambers with no females present (i.e., all songs were undirected). Songs of juvenile birds were recorded at 40–57 days of age; adult birds were recorded at 90–100 days of age. All birds could hear songs and calls of other birds while they were in the recording chambers, but could not see them. Birds that were exposed to playback of specific song types (see following text) were housed in individual chambers such that they heard playback but did not hear other birds singing. Birds that heard playback were isolated for 24–48 h prior to playback. All procedures were performed in accordance with protocols approved by the USC Institutional Animal Care and Use Committee and conformed to national regulatory policies.
Experimental groups
Experiment 1.
All birds tested in experiment 1 were juveniles (n = 23; mean age: 54.2 days, range: 51–60 days). Birds were randomly assigned to one of four different treatment conditions: CON Playback, Singing, Deaf-Singing, or Control. Birds in the CON Playback group were exposed to playback of conspecific songs (CON, n = 4) for 40 min at lights on. An observer sat in the room and monitored birds to make sure that these birds did not produce song during the playback session. Birds in the Singing group (n = 7) were allowed to sing for 60 min at lights on; each bird had to meet a criterion of singing for a total time of ≥1 min to be included in this group. Birds in the Deaf-Singing group (n = 5) were surgically deafened 4–7 days prior to being allowed to sing for 60 min at lights on. Five birds served as a silent control; they did not receive any playback and were removed from the recording chamber immediately after lights on and transferred to the main lab to make sure that they did not sing. Two additional birds were exposed to playback of pure tones (TON Playback) as an alternate control condition (see following text).
Our procedure for deafening has been described previously (Iyengar and Bottjer 2002b). Briefly, birds were anesthetized with 1.5% isoflurane and an incision was made in the skin overlying the tympanic membrane. The tympanic membrane and the columella overlying the oval window were removed and a pair of fine forceps were used to remove the cochlea. The incision was closed with Collodion (Sigma).
Sequencing software (Cakewalk Pro Audio 8) was used to program playlists for song playback. The playlist for CON birds included 13 conspecific song files (average duration 6 s each) from five different birds and the interval between each song file was 45 s, for a total duration of about 11 min. The entire playlist was repeated about 3.6 times for a total playback duration of 40 min at a volume of 70 ± 5 dB. The five adult birds used for the CON playlist were taken from a nonbreeding aviary, so that the songs would be unfamiliar (novel) for juveniles; adult birds in this study are also very unlikely to have heard any of the CON songs since the songs had been recorded substantially before the onset of these experiments.
The playlist for TON birds was based on a set of 15 tones, with a ramp time of 20 ms at both the beginning and end of each tone burst (generated in Matlab): five frequencies (1–5 kHz in steps of 1 kHz) at each of three different durations (100, 250, 500 ms). Different random orders of these 15 tones were generated and used to create a playlist in which 7–8 tones were played sequentially followed by a 1-s delay followed by the remaining 7–8 tones; the interval between each set of 15 tones was 45 s. The TON playlist was created this way in an attempt to mimic the CON playlist as closely as possible and to equate the total amount of auditory exposure between the two conditions. These two birds showed very low levels of ZENK induction in dNCL (cf. Mello et al. 1992): levels of ZENK induction for both of these birds were much lower than that in any bird in the CON Playback group, but overlapped with the lower range of ZENK induction for birds in the control group. Because ZENK expression in TON Playback birds did not differ from that in control birds, these two groups were combined into a single Control group (n = 7).
One hour after the start of playback or of singing, birds in the Singing and Playback groups were injected with Equithesin and perfused transcardially for 3–5 min with 0.7% saline followed by 4% paraformaldehyde + 0.1% glutaraldehyde for 15 min. Birds in the Control group were transferred to the main lab immediately after lights on and perfused within 20 min.
Experiment 2.
The goal of experiment 2 was to examine ZENK expression in dNCL as a function of type of playback in both juvenile and adult birds. In addition, we wanted to selectively examine dNCL neurons that project to Ad to investigate whether ZENK is expressed within neurons that contribute specifically to the LMANshell pathway. The design was a 2 × 3 factorial in which one factor was age (juvenile vs. adult) and the other factor was type of song playback (conspecific song, each bird's own tutor song, or no playback). Juvenile birds (n = 21) were about 55 days of age (mean age: 58.3 days; range: 50–65 days) and adult birds (n = 34) were about 95 days (mean age: 98.1 days; range: 90–100 days). Birds were randomly assigned to one of six main groups: Tutor Playback Juvenile (TUT-JUV, n = 9), Tutor Playback Adult (TUT-ADL, n = 10), Conspecific Playback Juvenile (CON-JUV, n = 7), Conspecific Playback Adult (CON-ADL, n = 17), Control Juvenile (Cont-JUV, n = 5), and Control Adult (Cont-ADL, n = 7). Experimental birds were exposed to 40-min playback of either conspecific songs (CON) or their tutor song (TUT) at lights on, using the same parameters as those for experiment 1. Birds in the control group were removed from their recording chamber at lights on and perfused within 20–30 min; as for the silent controls in experiment 1, they neither sang nor heard playback.
Because this experiment was initiated prior to experiment 1, we did not realize in advance that production of song behavior was an important factor and thus birds in this study were not monitored to ensure that they did not sing. Therefore after the experiment was completed, we assessed whether birds sang or did not sing during the period of playback by examining ZENK expression in RA since previous studies have shown a linear relationship between production of undirected song and IEG induction in RA (Jarvis and Nottebohm 1997; Jarvis et al. 1998; Jin and Clayton 1997; Kimpo and Doupe 1997; Whitney and Johnson 2005; Whitney et al. 2000). All of our birds produced undirected song and thus would be expected to exhibit ZENK expression throughout RA. In accord with this expectation, we observed robust induction of ZENK throughout RA of both juvenile and adult singing birds and very low levels of ZENK expression in control birds. Sections containing RA from all birds were examined on a confocal microscope (without knowledge of experimental treatment) and an observer counted the number of ZENK+ neurons in RA using a ×20 objective. The exact number of ZENK+ neurons per section was recorded up until the count reached 50; if >50 neurons were counted in an individual section of RA, then the number was recorded as 50 (a total of three birds had more than 50 ZENK+ neurons in RA). The total number of ZENK+ neurons counted for each bird was divided by the number of RA sections examined (at least three sections/bird). The number of ZENK+ neurons in control birds (juvenile and adult combined) was 5.26 ± 0.50 (means ± SE; range: 2.50–7.83). Based on this average, we set a criterion that any bird with <8.0 ZENK+ neurons in RA was classified as nonsinging, whereas birds with high numbers of ZENK+ neurons in RA were classified as singing. Across all experimental groups, birds classified as nonsinging had 2.63 ± 0.47 ZENK+ neurons in RA, whereas birds classified as singing had 27.33 ± 2.40 ZENK+ RA neurons.
The majority of birds in each playback group sang and we therefore focused primarily on analysis of data from singing birds (see results). The n values for the final group were as follows: 1) among juveniles TUT Singing = 7; TUT NonSinging = 2; CON Singing = 4; CON NonSinging = 3; Controls = 5; 2) among adults TUT Singing = 7; TUT NonSinging = 3; CON Singing = 12; CON NonSinging = 5; Controls = 7.
All birds in experiment 2 received a stereotaxic surgery in advance of experimental treatment to retrogradely label Ad-projecting neurons in dNCL: biotinylated dextran amine (BDA) 3,000 MW (10% BDA-3K in sterile-filtered phosphate-buffered saline [PBS]; Invitrogen, D7135) was injected into Ad using procedures outlined previously (Bottjer et al. 2000). Briefly, animals were anesthetized using 1.5% isoflurane and placed in a stereotaxic apparatus. Injections into both left and right Ad were made through a glass micropipette (outer tip diameter: 25–30 μM) using a Nanoject (Drummond Scientific); three injections of about 10 μL each, targeted to medial, mid, and lateral Ad (respectively) were made on each side. Animals were transferred to individual recording chambers following recovery from surgery (typically within 1–3 h). Two to 3 days following surgery, CON and TUT birds were exposed to playback, whereas control birds were removed from the recording chamber immediately after lights on (as in experiment 1). Playback animals were perfused with 4% paraformaldehyde containing 0.2% sodium periodate plus 1.8% d,l-lysine about1 h after the start of playback and control animals were transferred to the main lab and perfused as quickly as possible (within 20 min).
Tissue processing
ZENK protein was detected using a rabbit polyclonal antibody raised against a peptide consisting of the last 19 amino acid residues (carboxy-terminus) predicted by rodent and human sequences (anti-egr-1, sc-189; Santa Cruz Biotechnology). This antibody provides specific labeling of zebra finch ZENK as determined by Western blot, consistent with the highly conserved predicted amino acid sequence in songbirds (Mello and Ribeiro 1998).
Brains were postfixed in 4% paraformaldehyde for 24 h and then transferred to 25% sucrose at 4°C before being frozen-sectioned in the coronal plane at a thickness of 50 μm into two alternate series: a free-floating series for ZENK immunohistochemical staining and a thaw-mounted series for Nissl staining. For peroxidase labeling of ZENK protein in experiment 1, free-floating tissue sections were rinsed three times with PBS (0.02 M PBS) followed by quenching of endogenous peroxidase activity in 1% H2O2 in PBS. Sections were again rinsed three times with PBS, blocked for 1 h in 5% normal goat serum in PBS with 0.3% Triton-X, and then incubated overnight in primary antibody (anti-egr-1 rabbit IgG 1:4,000). Approximately four control sections (distributed along the rostrocaudal axis) were removed and set aside in PBS during the incubation period. Control sections were added back to the staining trays the next morning and all sections were transferred to biotinylated goat anti-rabbit IgG (1:200 in 0.3% Triton-X in PBS) for 1 h and then incubated in avidin-biotin peroxidase reagent (ABC Elite Kit, Vector Laboratories) for 1 h. Antibody labeling was visualized with 0.05% 3,3′-diamino-benzidine solution; the concentration of H2O2 was increased incrementally from 0.003 to 0.015% to enhance the sensitivity of labeling (Bottjer et al. 2000). Sections were rinsed in PBS, mounted onto gelatin-coated slides, air-dried overnight, defatted, and coverslipped with Permount.
For double-labeling of ZENK expression and Ad-projecting neurons in dNCL in experiment 2, free-floating sections were rinsed three times with PBS and then immersed in Streptavidin Alexa 488 (1:3,000; Invitrogen, # S32354) to visualize neurons retrogradely labeled by BDA. Sections were again rinsed three times with PBS, blocked for 1 h in 5% normal goat serum in PBS with 0.3% Triton-X, and then incubated overnight in primary antibody (anti-egr-1 rabbit IgG 1:3,000). Antibody labeling was visualized with goat anti-rabbit IgG conjugated to Alexa 594 (Invitrogen, #A11012). Sections were rinsed in PBS, mounted onto gelatin-coated slides, air-dried for 1–3 h, and coverslipped with Vectastain Hardset (Vector Labs).
Quantification
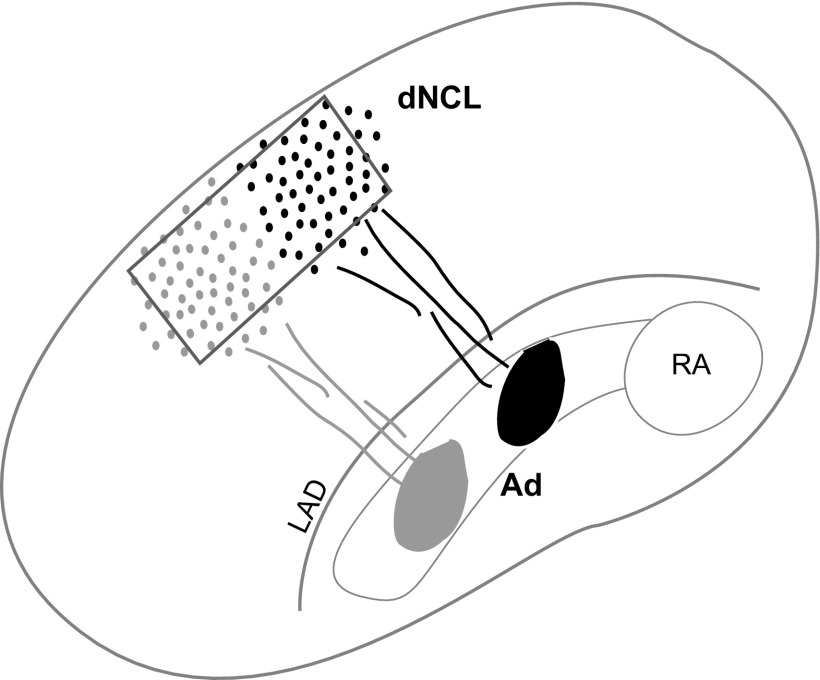
We quantified ZENK expression without knowledge of the experimental treatment received by any bird. dNCL is not visible as a distinct brain nucleus, but occupies a highly characteristic location at the dorsal margin within the lateral telencephalon extending rostrally from the level of RA and Ad toward HVC (Bottjer et al. 2000). We therefore sampled from within the borders of dNCL in sections that contained RA and Ad, two adjacent nuclei that receive a major axonal projection from LMAN (Iyengar et al. 1999; Johnson et al. 1995; Pinaud et al. 2007). Because Ad also receives a strong projection from dNCL, we extrapolated the location of dNCL based on knowing the path taken by axons from dNCL to Ad (Fig. 2; Bottjer et al. 2000). For each bird in experiment 1, we located the middle of Ad along the mediolateral axis and placed a standard sampling window 45° dorsolateral to Ad, close to the dorsal margin of the telencephalon. To ensure that we counted neurons only within dNCL, we placed the sampling window (a rectangular contour 0.42 mm tall × 0.80 mm wide) well within the boundaries of dNCL such that the area in which we counted ZENK+ neurons did not include any regions outside dNCL. Four or five contours were counted for each animal (one sampling contour per section) from within left and/or right dNCL.
Fig. 2.
Schematic illustration of dNCL. Cross section through the caudal telencephalon (after Bottjer et al. 2000) showing RA and Ad within the arcopallium and dNCL in the dorsolateral nidopallium The projection from dNCL to Ad is topographic, as shown here by injection sites in midmedial (black) and midlateral (gray) Ad that produced corresponding clusters of retrogradely labeled cells in dNCL (note that the extreme lateral and medial regions of dNCL do not include labeled cells in this figure). The borders of dNCL are not apparent in Nissl-stained sections nor in our immunostained tissue. In experiment 1, we therefore extrapolated the location of dNCL based on the location of Ad and our knowledge of the path of axons from dNCL to Ad. We counted ZENK+ cells within a sampling window (gray box), the size and placement of which were chosen to be conservative in the sense that it would exclude extreme dorsal, ventral, medial, and lateral regions of dNCL. In this way we avoided counting immunoreactive cells outside the borders of dNCL. In experiment 2, we counted cells within dNCL by locating our sampling window over regions that included the highest density of retrogradely labeled neurons, depending on the quality and location of the injection site. Neurons outside dNCL are never labeled by injections into Ad (Bottjer et al. 2000).
We used the Virtual Slice module of Neurolucida (ver. 8.01, MicroBrightField, Williston, VT) to quantify the number of ZENK+ neurons within each contour. Briefly, an image montage of the entire telencephalic lobe containing RA, Ad, and dNCL was captured in Neurolucida using a digital camera (MicroFire CX9000, Optronics), interfaced with a Leica DMRE microscope with a motorized stage (×10 objective) and saved as an 8-bit image (TIFF file). The sampling contour was then placed within the boundaries of dNCL, as described earlier for each section analyzed. The only parameter that varied between animals and sections was the threshold used to highlight ZENK+ cells; the grayscale value above which a given cluster of pixels was considered to be a signal was set individually by matching the highlighted pixels to correspond as closely as possible to labeled cells. In some cases a higher background yielded inaccurate counts using this strategy. Additionally, immunoreactive cells varied somewhat in their staining intensity and we followed the procedure of previous studies in counting all labeled cells (Mello and Ribeiro 1998; Whitney et al. 2000); qualitative inspection clearly indicated that comparable variations in staining intensity of individual cells occurred across all groups. In practice it was difficult to set a threshold that included lightly labeled cells without picking up some background. To ensure accuracy of counts in all birds, we manually added markers over labeled cells that were not detected by thresholding, to include them with the thresholded number of cells. The accuracy of cell counts obtained using the automated procedure was thus verified by manual inspection of labeled cells on every section that was counted. The absolute number of ZENK+ neurons measured for each animal was divided by the total volume of tissue measured to yield a density measure. This measure was compared between groups to obtain the relative differences in ZENK induction in the different conditions.
For experiment 2, double-labeled sections were examined using a Zeiss confocal microscope (LSM510) with a ×20 objective. Sections were scanned with an argon/helium-neon laser using excitation wavelengths of 488 for Alexa 488 and 543 for Alexa 594. Images within dNCL were captured at ×20 and saved as TIFF files; enough images were captured per bird to ensure a minimum of 50 retrogradely labeled neurons. For these sections, we chose areas within dNCL that included the highest density of retrogradely labeled cells, which was determined by the injection site (Fig. 2) The number of retrogradely labeled neurons and double-labeled neurons within a sampling window (a square contour 0.45 × 0.45 mm) placed within dNCL were counted manually. The TIFF images were then examined in Neurolucida and ZENK+ neurons were counted using the same procedures as those in experiment 1. The total numbers of retrogradely labeled neurons, ZENK+ neurons, and double-labeled neurons were each divided by the area of the tissue examined. We did not convert these areas to volumes since our optical slice was about 3 microns. Because our unit of count (whole cells or nuclei) was large in relation to section thickness (3 μm), absolute density values for volumes in this experiment would be inflated. However, because we were interested only in relative differences between groups it does not matter whether area or volume is used. Last, because the magnitude of injection sites (and thus the density of retrogradely labeled neurons) varied between birds, the incidence of double-labeled cells was expressed as the ratio of double-labeled cells to retrogradely labeled cells. In this way, projection neurons were treated as a separate (unbiased) population, thereby ensuring that the incidence of ZENK+ neurons that project to Ad would not be influenced by quality of the injection site.
Statistics
We used ANOVAs (α <0.05, two-tailed) to analyze differences between groups whenever the data satisfied the requirements for homogeneity of variance. This condition was not satisfied in experiment 1, so we performed a rank test (Kruskal–Wallis) on those data. The percentage data in experiment 2 were transformed as 2 × arcsin to meet the normality assumptions of an ANOVA.
RESULTS
Experiment 1
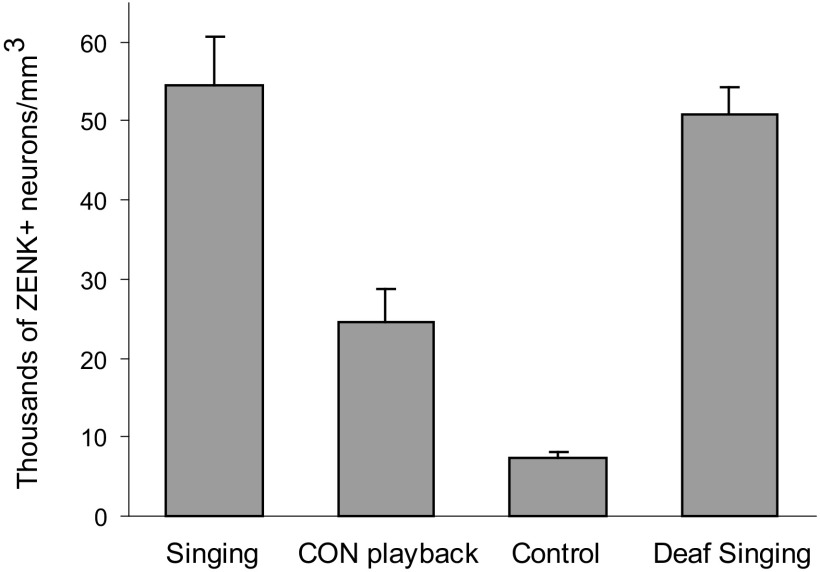
The induction of ZENK within dNCL neurons of juvenile birds varied across groups, with birds that sang for 60 min at lights on (Singing) showing high levels of expression. Figure 3 shows that the mean density of ZENK+ neurons was high in dNCL of Singing birds compared with Control birds that neither sang nor heard song playback. Nonsinging birds that were exposed to playback of conspecific song (CON Playback) showed intermediate levels of ZENK expression in dNCL that were substantially lower than those in Singing birds but higher than those in Control birds. To test whether singing behavior alone was effective in inducing ZENK expression in dNCL neurons, an additional group of birds was surgically deafened via cochlea removal and allowed to sing for 60 min at lights on. These Deaf Singing birds also showed high levels of ZENK expression, comparable to those of Singing birds with normal hearing.
Fig. 3.
ZENK induction in dNCL in response to singing and hearing. Number of ZENK+ cells per mm3 within dNCL (means ± SE) in juvenile birds (∼55 days of age) in the 4 groups of experiment 1.
One bird in the Control group was an outlier: levels of ZENK induction in that bird were more than fourfold higher than the average of the remaining six birds in that group; thus this bird was eliminated from all statistical analyses. A Kruskal–Wallis test revealed a main effect of groups (H = 16.71, P = 0.001). Individual group comparisons showed that Singing birds had significantly higher densities of ZENK+ neurons in dNCL than did those in the CON Playback group (H = 6.04, P = 0.014) and CON Playback birds in turn had higher levels of ZENK expression than did Control birds (H = 6.55, P = 0.011). Deaf Singing birds were not different from Singing birds (H = 0.06, P = 0.81).
The expression of IEGs in RA (and other sensorimotor song control nuclei, such as HVC, Area X, and LMAN) increases linearly with the amount of song produced (Jarvis and Nottebohm 1997; Jarvis et al. 1998; Jin and Clayton 1997; Kimpo and Doupe 1997). We examined the number of song bouts produced to see whether there was a positive relationship between singing and ZENK expression in dNCL. Singing birds produced 78.4 ± 30.4 bouts (means ± SE) compared with 63.2 ± 9.9 for Deaf Singing birds (U = 14, P = 0.32). Because there were no differences between these two groups, they were collapsed to test for a correlation between the density of ZENK+ neurons and number of song bouts produced: this analysis revealed no tendency for birds that sang more to show higher levels of ZENK expression in dNCL (R2 = 0.05, P > 0.20).
This robust induction of ZENK by hearing and producing song implicates dNCL as a novel region important for songbird vocal learning. It seems counterintuitive that the density of ZENK+ neurons was not higher in Singing birds than that in Deaf Singing birds, given that playback of conspecific song (CON Playback) induces the expression of ZENK at an intermediate level. That is, the similar levels of ZENK induction in both deaf and hearing singing birds suggest that ZENK induction in these neurons is due solely to singing and not hearing. In addition, induction in nonsinging CON Playback birds must be due to hearing. However, if different dNCL cells can be stimulated to express ZENK by either singing or hearing song, then singing–hearing birds would be expected to have higher levels of ZENK+ neurons than those of singing–deaf birds. We consider various alternative explanations in the discussion.
Experiment 2
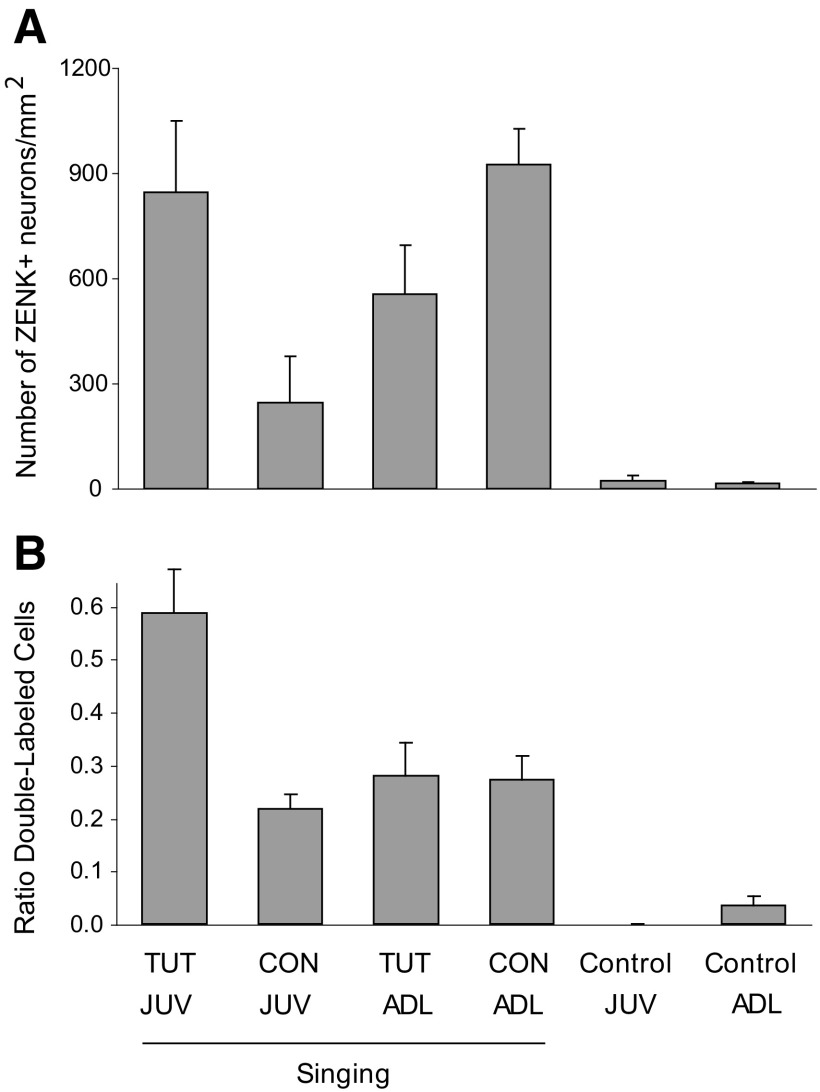
The main goal of experiment 2 was to examine ZENK expression in dNCL as a function of type of playback in both juvenile and adult birds. Juvenile (JUV) versus adult (ADL) birds heard playback of either conspecific or tutor song (CON vs. TUT). The majority of birds sang during the period of playback (birds were not monitored during playback to prevent them from singing, as in experiment 1, and thus song production was assessed after the fact by examining the level of ZENK induction in RA; see methods). The level of ZENK induction in dNCL varied as a function of both type of song playback and age in singing birds; ZENK expression was substantially higher in all birds that heard playback while singing compared with that of control birds, which neither sang nor heard playback. Figure 4A shows that juvenile birds that heard TUT playback and sang showed a substantially higher level of ZENK induction compared with those that heard CON Playback. In contrast, adult birds that heard TUT playback and sang showed a lower level of ZENK induction compared with those that heard CON Playback. Thus hearing conspecific song while singing resulted in high levels of ZENK in adult birds but not juveniles, whereas hearing tutor song while singing resulted in high levels of ZENK in juvenile birds but moderate levels in adults.
Fig. 4.
ZENK induction in dNCL as a function of age and playback in singing birds. A: number of ZENK+ cells per mm2 within dNCL (means ± SE) in singing juvenile (JUV) vs. adult (ADL) birds during playback of tutor (TUT) vs. conspecific (CON) song. B: the percentage of ZENK+ cells among retrogradely labeled neurons within dNCL. Retrogradely labeled cells (Ad-projecting neurons) were examined separately such that the incidence of double-labeled cells was expressed as the ratio of double-labeled cells to retrogradely labeled cells (see methods).
We tested whether the density of ZENK+ neurons in dNCL varied as a function of group by conducting an ANOVA with Age and Playback as between-subject factors. This analysis revealed a significant Age × Playback interaction [F(1,26) = 9.59, P = 0.005]. Individual pairwise comparisons for Playback showed that TUT playback was more effective than CON in inducing ZENK expression in dNCL neurons of juvenile singing birds [F(1,26) = 5.79, P = 0.02], whereas CON playback was only marginally more effective than TUT in singing adults [F(1,26) = 3.81, P = 0.06]. Individual pairwise comparisons for Age showed that the density of ZENK+ cells in dNCL was substantially lower for CON playback in juvenile compared with adult birds [F(1,26) = 8.76, P = 0.006], whereas ZENK expression did not differ for TUT playback in juveniles versus adults [F(1,26) = 1.85, P = 0.19].
Because experiment 1 indicated that the act of singing was especially effective in inducing ZENK expression within dNCL in juvenile birds, it was important to evaluate whether differential song production among groups contributed to playback-dependent differences in singing birds in experiment 2. We quantified the level of ZENK induction within RA as a measure of amount of singing behavior (see methods). Inspection of these data showed some tendency for level of ZENK expression in RA to vary between groups (Table 1). The interaction of Age (JUV vs. ADL) × Playback (TUT vs. CON) tended toward significance for this measure [F(1,26) = 3.52, P = 0.07]. Individual pairwise comparisons for Playback showed that ZENK expression in RA was not higher in juveniles that heard TUT song compared with CON (F = 1.92, P = 0.18); likewise, ZENK expression in RA was not higher in adult birds that heard CON compared with TUT (F = 1.63, P = 0.21). Individual pairwise comparisons for Age showed that ZENK expression in RA was not higher in juveniles that heard TUT song relative to adults (F <1); ZENK expression in RA tended to be higher for CON playback in adult birds relative to juveniles (F = 3.55, P = 0.07). Although these effects are not statistically significant, the pattern of results suggests the possibility that juvenile birds that heard playback of TUT may have tended to sing somewhat more than those that heard playback of CON, whereas adult birds may have tended to sing slightly more when they heard playback of CON as opposed to TUT. Additionally, juvenile birds that heard CON song tended to produce less song than adults that heard CON playback, whereas juvenile and adult birds that heard TUT song did not differ in terms of ZENK expression in RA.
Table 1.
Number of ZENK+ cells per RA section in singing birds
| JUV | ADL | |
|---|---|---|
| TUT | 28.85 ± 5.60 | 23.85 ± 5.53 |
| CON | 17.65 ± 4.01 | 31.69 ± 3.40 |
Values are means ± SE.
In summary, hearing a specific song while producing the bird's own song caused differential levels of ZENK expression in dNCL of adult versus juvenile birds. Playback of TUT song was more effective in inducing ZENK in dNCL of singing juvenile birds than was CON, whereas among singing adult birds playback of CON song was only marginally more effective than TUT. In addition, hearing CON playback in singing adult birds resulted in much higher levels of ZENK expression in dNCL than in singing juvenile birds, whereas hearing TUT playback was not significantly higher in juveniles compared with adults. The pattern of ZENK expression in RA described earlier suggests that differences in song production between groups might have contributed slightly to these differences in dNCL.
A second goal of experiment 2 was to selectively examine ZENK expression within Ad-projecting neurons in dNCL (dNCLAd) to investigate whether ZENK expression within neurons that contribute specifically to the LMANshell pathway varies as a function of age and playback condition. To do this, we retrogradely labeled dNCL neurons that project to Ad and then examined this population of projection neurons selectively to determine whether they also expressed ZENK differentially as a function of treatment. Figure 5 shows that many Ad-projecting neurons within dNCL expressed ZENK in singing birds (examples marked by arrows are double-labeled cells in an adult singing bird that heard conspecific song). Figure 4B shows the proportion of dNCLAd neurons that expressed ZENK for each group (number of double-labeled neurons divided by the number of retrogradely labeled neurons; see methods). Over 50% of Ad-projecting dNCL neurons in juvenile birds that sang while hearing TUT song expressed ZENK, compared with 25% for adult birds that sang while hearing TUT song. Ad-projecting neurons in dNCL of both juvenile and adult birds that sang while hearing playback of conspecific song also expressed ZENK at much lower levels (20–25%) compared with singing juvenile birds that heard tutor song. Thus a much higher incidence of Ad-projecting neurons in dNCL express ZENK in juvenile singing birds when they hear tutor song compared with any other group.
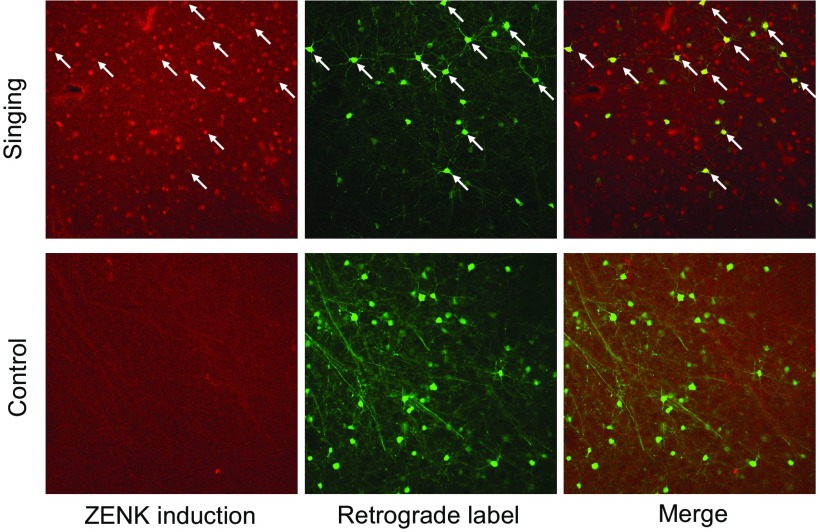
Fig. 5.
ZENK induction in dNCL neurons that project to Ad. Representative photomicrographs of ZENK+ cells, retrogradely labeled cells (showing projection neurons in dNCL that send axons to Ad), and the merge showing double-labeled cells within dNCL from an adult singing bird in the CON playback group (Dg633, top panels) and an adult control bird that neither sang nor heard playback (Lg716, bottom panels). Arrows show examples of double-labeled neurons.
An ANOVA with Age and Playback as between-subject factors revealed a significant Age × Playback interaction [F(1,26) = 5.92, P = 0.02], due to a much higher percentage of ZENK expression within projection neurons of singing juvenile birds that heard tutor song compared with the other three groups. Pairwise comparisons for Playback showed that TUT playback was more effective than CON in inducing ZENK expression in Ad-projecting dNCL neurons of juvenile singing birds [F(1,26) = 9.57, P = 0.005], whereas CON playback was not different from TUT in singing adults (F <1). Pairwise comparisons for Age showed that playback of TUT resulted in a higher incidence of double-labeled neurons in juvenile than that in adult birds [F(1,26) = 10.09, P = 0.004], whereas playback of CON did not vary as a function of age (F <1). This pattern of results shows that a higher proportion of dNCLAd projection neurons in juvenile singing birds express ZENK after hearing tutor song than after hearing conspecific song, whereas adult birds express the same percentage of ZENK+ Ad-projecting neurons regardless of whether they hear playback of TUT or CON. In addition, a higher proportion of Ad-projecting neurons in singing juvenile birds express ZENK after hearing tutor song than do those of singing adult birds, whereas there was no difference in ZENK expression within dNCLAd neurons between singing juveniles and adults that heard CON playback.
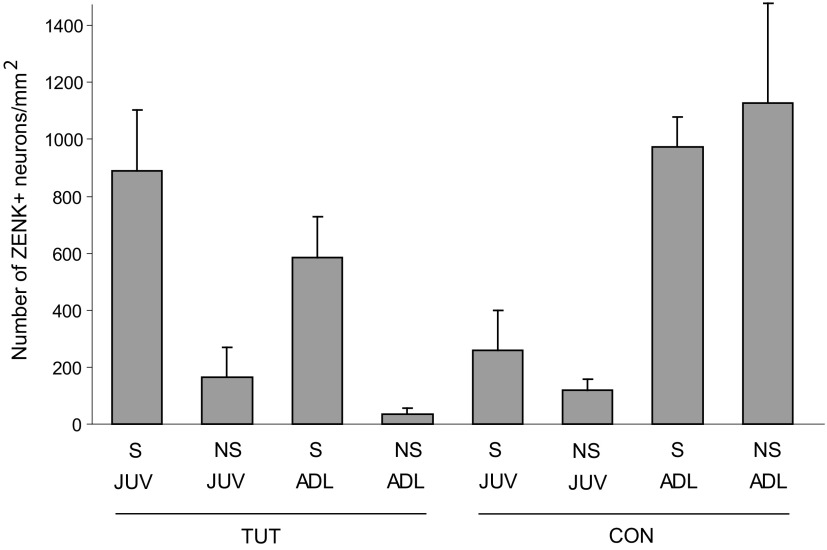
Some birds in each group did not sing, as assessed by the level of ZENK induction in RA (see methods), which enabled us to conduct preliminary comparisons of ZENK induction in singing versus nonsinging birds that heard different types of playback. A detailed investigation of the role of singing/nonsinging versus different types of playback in inducing ZENK expression in dNCL is beyond the scope of this study due to the small number of birds that did not sing in some groups. However, we were nevertheless able to make some interesting comparisons from the pattern of data obtained (Fig. 6). For both juveniles and adults, birds that heard TUT playback and sang showed substantially higher levels of ZENK induction than did birds that heard TUT playback but did not sing (and singing juvenile birds that heard tutor song had higher levels of ZENK expression than the corresponding adult group, as described earlier). Likewise, juvenile birds that heard CON playback and sang also showed a higher density of ZENK+ neurons than did juveniles that heard CON playback but did not produce song. This latter comparison replicates the finding from experiment 1 showing that singing juvenile birds had higher densities of ZENK+ neurons in dNCL than did nonsinging juveniles that heard CON playback (the ratio of ZENK expression for singing and hearing birds to that for birds that heard CON playback without singing was ∼2.2 in both experiments). In contrast, adult birds that heard CON playback showed comparable, high levels of ZENK expression in dNCL, regardless of whether they were singing.
Fig. 6.
ZENK induction in dNCL as a function of age and playback in singing and nonsinging birds. Number of ZENK+ cells per mm2 within dNCL (means ± SE) in juvenile and adult birds that heard different types of playback (tutor song vs. conspecific song: TUT vs. CON). The majority of birds in each group sang, but some did not (see text), allowing a comparison of the effects of playback in singing vs. nonsinging birds. Data from Fig. 4A are replotted here for comparison.
Interestingly, juvenile birds that heard playback without singing showed comparable levels of ZENK regardless of whether they heard TUT or CON (with the caveat that only two juvenile nonsinging birds heard TUT playback), whereas juveniles in the Singing-TUT condition exhibited much higher levels of ZENK expression than did those in Singing-CON. Among adults, TUT playback in nonsinging birds was not an effective stimulus for inducing ZENK, whereas CON playback in nonsinging birds was highly effective (and not different in singing vs. nonsinging birds). Thus juvenile birds that sing while hearing the song of their tutor show high levels of ZENK induction in dNCL neurons during active auditory–motor integration, but low levels when they sing and hear conspecific song. In contrast, playback of conspecific song is more effective at inducing ZENK expression in dNCL of adult birds, regardless of whether they are producing song.
DISCUSSION
We observed robust, developmentally regulated ZENK induction in a novel region of cortex that receives direct input from the shell subregion of LMAN. Both hearing and producing song activated the expression of ZENK in dNCL neurons, thus identifying a novel brain region involved in learned vocal behavior. Furthermore, many ZENK+ dNCL neurons in juvenile singing birds that hear tutor song project to Ad (within motor cortex), indicating that ZENK+ neurons in dNCL are part of the LMANshell circuit. The results further implicate this circuit as having a functional involvement with vocal learning whose role varies during development. LMANshell is part of a basal ganglia pathway essential for acquisition of learned vocal sounds (Bottjer and Altenau 2009) and, along with dNCL, is part of a recurrent loop that makes feedback connections to LMAN as well as feedforward connections to HVC (Fig. 1 and Supplemental Fig. S1). The pattern of results obtained here is complex, indicating that age, song exposure, and singing all play interrelated roles in inducing ZENK expression in dNCL neurons. The changes that we observed suggest that multiple components of how dNCL functions are changing across development.
Previous studies of ZENK induction have shown a dissociation in the pattern of ZENK induction due to hearing versus producing song: the act of singing induces expression of IEGs in sensorimotor nuclei of songbirds (such as HVC and RA), whereas hearing playback of song induces IEG expression in regions of higher-level auditory cortex (Jarvis and Nottebohm 1997; Jarvis et al. 1998; Kimpo and Doupe 1997; Mello and Clayton 1994; Mello and Ribeiro 1998; Mello et al. 1992; Stripling et al. 1997, 2001). Thus dNCL is the first region shown to respond to some conjoint influence of producing and hearing vocal sounds (although Whitney and Johnson found a higher level of ZENK translation in RA of singing adult birds only when they could hear other birds singing; Whitney and Johnson 2005). In addition, previous studies using song playback as a stimulus have found no evidence that different types of song (e.g., tutor song vs. bird's own song vs. conspecific song) elicit differential levels of ZENK expression or responsivity in higher-level regions of auditory cortex of male birds (but see Gentner et al. 2004; Terpstra et al. 2006). We found that juvenile singing birds had higher levels of induction when they heard tutor song versus conspecific song. Further studies will be needed to determine whether this effect is caused by facilitation of ZENK induction in singing juvenile birds that hear TUT song or by suppression of ZENK expression in singing juveniles that hear CON. In contrast, playback of conspecific song was a highly effective stimulus for inducing ZENK expression in dNCL of adult birds—irrespective of whether they were singing—suggesting a developmental regulation that is independent of song production.
It is difficult to draw conclusions about the precise functional role of any brain region based on patterns of IEG expression (Kimpo and Doupe 1997). Singing-induced expression in dNCL could indicate some direct premotor role. However, to date the only known efferent projection of dNCL in zebra finches is to Ad (Fig. 1). Thus dNCL (and the entire LMANshell recurrent loop) lack any direct motor connections, which argues against a direct role in vocal motor production (Bottjer and Altenau 2009; Bottjer et al. 2000). An alternative possibility is that dNCL receives corollary discharge and/or auditory information as birds produce song. The high level of ZENK induction seen in juvenile birds that heard tutor song while producing their own song suggests a possible role for dNCL in sensorimotor integration. However, as birds become adult their dNCL neurons no longer respond strongly to hearing tutor song by translating ZENK protein but do respond to conspecific song. This result suggests the possibility that dNCL neurons are excited by tutor song in juveniles but may come to be inhibited by tutor song in adults. Alternatively, hearing CON song may be inhibitory in juvenile birds, which could suppress expression of ZENK. Studies of the electrophysiological characteristics of dNCL neurons will elucidate how dNCL may be involved in comparing vocal motor production, auditory feedback, and the memory of the tutor song. Further investigation of the functional role of dNCL will also require measuring the exact temporal relationship between singing activity and playback for each bird.
Of course, ZENK expression may relate to developmental changes in vocal learning in some way that is not reflected by measures of excitation or inhibition. This latter possibility includes the idea that involvement of neuromodulatory systems may change developmentally as vocal learning progresses. Although the effect did not achieve statistical significance, expression of ZENK in RA suggested that juvenile birds that heard playback of tutor song may have produced more song than those that heard conspecific song. Hearing tutor song may influence the behavioral state of juvenile birds and increase the expression of neuromodulatory factors. In accord with this idea, a recent study showed that exposing juvenile birds to a familiar tutor increased the density of Fos+ cells in the ventral tegmental area (VTA), substantia nigra (SNc), and the periaqueductal gray (PAG) more than did exposure to a nontutor conspecific male (Nordeen et al. 2009). In addition, concentrations of acetylcholine (ACh), dopamine, and norepinephrine are known to be high in song-control nuclei of juvenile zebra finches; in particular, concentrations of ACh seem to peak around the period of sensorimotor integration (Sakaguchi and Saito 1991, 1996). Interestingly, Ad makes a projection to VTA and SN, thereby providing song-related information to midbrain neuromodulatory centers (Bottjer et al. 2000; Gale et al. 2008).
Our initial studies of LMANshell circuitry showed that dNCL receives its major and perhaps only input from LMANshell (Bottjer et al. 2000; Iyengar et al. 1999; Johnson et al. 1995). However, the robust induction of ZENK caused by hearing tutor song in singing juvenile birds and by hearing conspecific song in adults suggests the possibility that high-level regions of auditory cortex may project, either directly or indirectly, to dNCL. For example, dNCL might receive information regarding the tutor song from areas of higher-level auditory cortex that have been implicated as encoding the tutor song memory (such as the caudomedial nidopallium and the caudomedial mesopallium; Bolhuis and Gahr 2006; Gentner et al. 2004; London and Clayton 2008; Phan et al. 2006; Terpstra et al. 2004) and perform some comparison of that information to current versions of song. Another possibility is that LMANshell neurons in juvenile birds may respond to playback of tutor song and convey that information to dNCL. Interestingly, inspection of our tissue revealed little or no ZENK induction in LMANshell. However, it is common for synaptically connected neurons to show different patterns of IEG induction even when it is known that they both exhibit high levels of neuronal activation; for example, Fos is highly expressed in RA of singing birds but is not expressed in their synaptic targets, the vocal motor neurons (Kimpo and Doupe 1997). Alternatively, auditory inputs might indirectly contribute to activation of ZENK expression in dNCL. For example, hearing tutor song might induce changes in behavioral state of juvenile birds and cause them to sing more, as suggested earlier.
If dNCL does have some role as part of a comparator circuit during auditory–motor integration, the fact that ZENK induction was the same in hearing and deaf juvenile birds (experiment 1) argues against the idea that individual dNCL neurons are responding to a direct match (or mismatch) between their own song and tutor song. Singing birds with their hearing intact did not show higher levels of ZENK induction than deaf singing birds, despite the fact that hearing playback in nonsinging birds was alone sufficient to induce ZENK activation (Fig. 3). The pattern of results seems to rule out the idea that a single population of dNCL neurons is stimulated to express ZENK by either singing or hearing song, since that hypothesis predicts that hearing-only birds (nonsinging CON playback birds) should show the same levels of ZENK expression as singing–hearing birds. One idea is that dNCL contains two populations of ZENK-inducible neurons: one population that expresses ZENK in response either to hearing song or producing song and a separate population that expresses ZENK only when the bird produces song (regardless of hearing). Alternatively, the act of singing might gate the auditory response to hearing (McCasland and Konishi 1981; Prather et al. 2008; Schmidt and Konishi 1998). In this scenario, song production induces expression of ZENK in one population of dNCL neurons, but inhibits the ZENK response in another population of dNCL neurons (that otherwise responds to playback in nonsinging birds). Another possibility is that hearing song causes some level of (subthreshold) activation of motor circuitry and/or that ZENK is reporting some type of systemwide neuromodulation induced by hearing songs while producing the bird's own song. In accord with the idea that dNCL includes different functional populations of ZENK-inducible neurons, we observed robust ZENK induction both in Ad-projecting neurons and in other neurons (the latter likely including many nonprojecting neurons; Bottjer et al. 2000), indicating that at least two different populations of neurons in dNCL show ZENK induction in response to singing and/or hearing song. Thus the role of dNCL may rely, at least in part, on synaptic interactions between functionally segregated populations of neurons within dNCL.
Juvenile birds of the age used in this study (∼55 days) have completed the process of learning syllables from a tutor and are heavily engaged in auditory–motor integration (Bohner 1990; Roper and Zann 2006). In addition, they are at the peak of plastic song production and birds that engage in more vocal practice at this age produce songs with more stereotyped vocal sequences as adults (Johnson et al. 2002). Studies in humans have shown strong sensorimotor integration in the neural representation of speech, as predicted by the motor theory of speech perception (Liberman et al. 1967; Studdert-Kennedy et al. 1970). For example, listening to speech activates motor cortical areas (as well as effectors) involved in speech production and speech production activates cortical perceptual areas including auditory association cortex (Dhanjal et al. 2008; Fadiga et al. 2002; Watkins and Paus 2004; Wilson et al. 2004). In addition, microstimulation of motor cortical regions controlling different articulators (tongue vs. lips) facilitated perception of phonemes associated with those articulators and inhibited perception of contrasting phonemes, showing a functional interdependence of perception and production (D'Ausilio et al. 2009). Similarly, recent studies in songbirds have indicated links between perception and production in both traditional “motor” and “sensory” cortical regions (Keller and Hahnloser 2009; Prather et al. 2008). The present data suggest that higher levels of ZENK induction in dNCL during early stages of sensorimotor integration may make an important mechanistic contribution to the emergence of stereotyped adult song and contribute to the development of experience-dependent links between perception and production. Impairments in such basic types of integration may contribute both to a variety of disorders in speech perception and production and to disorders such as developmental dyslexia that include abnormalities of phonological processing (Meltzoff et al. 2009; Stein and Walsh 1997).
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke.
Supplementary Material
ACKNOWLEDGMENTS
We thank S. Butler for assistance with confocal analysis.
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Bohner J. Early acquisition of song in the zebra finch, Taeniopygia guttata. Anim Behav 39: 369–374, 1990. [Google Scholar]
- Bolhuis JJ, Gahr M. Neural mechanisms of birdsong memory. Nat Rev Neurosci 7: 347–357, 2006. [DOI] [PubMed] [Google Scholar]
- Bottjer SW. Developmental regulation of basal ganglia circuitry during the sensitive period for vocal learning in songbirds. Ann NY Acad Sci 1016: 395–415, 2004. [DOI] [PubMed] [Google Scholar]
- Bottjer SW, Altenau B. Parallel pathways for vocal learning in basal ganglia of songbirds. Nat Neurosci 13: 153–155, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottjer SW, Brady JD, Cribbs B. Connections of a motor cortical region in zebra finches: relation to pathways for vocal learning. J Comp Neurol 420: 244–260, 2000. [PubMed] [Google Scholar]
- Braun K, Bock J, Metzger M, Jiang S, Schnabel R. The dorsocaudal neostriatum of the domestic chick: a structure serving higher associative functions. Behav Brain Res 98: 211–218, 1999. [DOI] [PubMed] [Google Scholar]
- D'Ausilio A, Pulvermuller F, Salmas P, Bufalari I, Begliomini C, Fadiga L. The motor somatotopy of speech perception. Curr Biol 19: 381–385, 2009. [DOI] [PubMed] [Google Scholar]
- Dhanjal NS, Handunnetthi L, Patel MC, Wise RJ. Perceptual systems controlling speech production. J Neurosci 28: 9969–9975, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doupe AJ, Perkel DJ, Reiner A, Stern EA. Birdbrains could teach basal ganglia research a new song. Trends Neurosci 28: 353–363, 2005. [DOI] [PubMed] [Google Scholar]
- Fadiga L, Craighero L, Buccino G, Rizzolatti G. Speech listening specifically modulates the excitability of tongue muscles: a TMS study. Eur J Neurosci 15: 399–402, 2002. [DOI] [PubMed] [Google Scholar]
- Foster EF, Mehta RP, Bottjer SW. Axonal connections of the medial magnocellular nucleus of the anterior neostriatum in zebra finches. J Comp Neurol 382: 364–381, 1997. [DOI] [PubMed] [Google Scholar]
- Gale SD, Person AL, Perkel DJ. A novel basal ganglia pathway forms a loop linking a vocal learning circuit with its dopaminergic input. J Comp Neurol 508: 824–839, 2008. [DOI] [PubMed] [Google Scholar]
- Gentner TQ, Hulse SH, Ball GF. Functional differences in forebrain auditory regions during learned vocal recognition in songbirds. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 190: 1001–1010, 2004. [DOI] [PubMed] [Google Scholar]
- Hessler NA, Doupe AJ. Social context modulates singing-related neural activity in the songbird forebrain. Nat Neurosci 2: 209–211, 1999. [DOI] [PubMed] [Google Scholar]
- Iyengar S, Bottjer SW. Development of individual axon arbors in a thalamocortical circuit necessary for song learning in zebra finches. J Neurosci 22: 901–911, 2002a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyengar S, Bottjer SW. The role of auditory experience in the formation of neural circuits underlying vocal learning in zebra finches. J Neurosci 22: 946–958, 2002b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyengar S, Viswanathan SS, Bottjer SW. Development of topography within song control circuitry of zebra finches during the sensitive period for song learning. J Neurosci 19: 6037–6057, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis ED, Nottebohm F. Motor-driven gene expression. Proc Natl Acad Sci USA 94: 4097–4102, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis ED, Scharff C, Grossman MR, Ramos JA, Nottebohm F. For whom the bird sings: context-dependent gene expression. Neuron 21: 775–788, 1998. [DOI] [PubMed] [Google Scholar]
- Jin H, Clayton DF. Localized changes in immediate-early gene regulation during sensory and motor learning in zebra finches. Neuron 19: 1049–1059, 1997. [DOI] [PubMed] [Google Scholar]
- Johnson F, Bottjer SW. Growth and regression of thalamic efferents in the song-control system of male zebra finches. J Comp Neurol 326: 442–450, 1992. [DOI] [PubMed] [Google Scholar]
- Johnson F, Sablan MM, Bottjer SW. Topographic organization of a forebrain pathway involved with vocal learning in zebra finches. J Comp Neurol 358: 260–278, 1995. [DOI] [PubMed] [Google Scholar]
- Johnson F, Soderstrom K, Whitney O. Quantifying song bout production during zebra finch sensory-motor learning suggests a sensitive period for vocal practice. Behav Brain Res 131: 57–65, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AE, TenCate C, Slater PJB. Early experience and plasticity of song in adult male zebra finches (Taeniopygia guttata). J Comp Psychol 110: 354–369, 1996. [Google Scholar]
- Kao MH, Brainard MS. Lesions of an avian basal ganglia circuit prevent context-dependent changes to song variability. J Neurophysiol 96: 1441–1455, 2006. [DOI] [PubMed] [Google Scholar]
- Kao MH, Doupe AJ, Brainard MS. Contributions of an avian basal ganglia-forebrain circuit to real-time modulation of song. Nature 433: 638–643, 2005. [DOI] [PubMed] [Google Scholar]
- Keller GB, Hahnloser RH. Neural processing of auditory feedback during vocal practice in a songbird. Nature 457: 187–190, 2009. [DOI] [PubMed] [Google Scholar]
- Kimpo RR, Doupe AJ. FOS is induced by singing in distinct neuronal populations in a motor network. Neuron 18: 315–325, 1997. [DOI] [PubMed] [Google Scholar]
- Liberman AM, Cooper FS, Shankweiler DP, Studdert-Kennedy M. Perception of the speech code. Psychol Rev 74: 431–461, 1967. [DOI] [PubMed] [Google Scholar]
- London SE, Clayton DF. Functional identification of sensory mechanisms required for developmental song learning. Nat Neurosci 11: 579–586, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M, Ding L, Perkel DJ. An avian basal ganglia pathway essential for vocal learning forms a closed topographic loop. J Neurosci 21: 6836–6845, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCasland JS, Konishi M. Interaction between auditory and motor activities in an avian song control nucleus. Proc Natl Acad Sci USA 78: 7815–7819, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello CV, Clayton DF. Song-induced ZENK gene expression in auditory pathways of songbird brain and its relation to the song control system. J Neurosci 14: 6652–6666, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello CV, Ribeiro S. ZENK protein regulation by song in the brain of songbirds. J Comp Neurol 393: 426–438, 1998. [DOI] [PubMed] [Google Scholar]
- Mello CV, Vicario DS, Clayton DF. Song presentation induces gene expression in the songbird forebrain. Proc Natl Acad Sci USA 89: 6818–6822, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzoff AN, Kuhl PK, Movellan J, Sejnowski TJ. Foundations for a new science of learning. Science 325: 284–288, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger M, Jiang S, Braun K. Organization of the dorsocaudal neostriatal complex: a retrograde and anterograde tracing study in the domestic chick with special emphasis on pathways relevant to imprinting. J Comp Neurol 395: 380–404, 1998. [PubMed] [Google Scholar]
- Nordeen EJ, Holtzman DA, Nordeen KW. Increased Fos expression among midbrain dopaminergic cell groups during birdsong tutoring. Eur J Neurosci 30: 662–670, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkel DJ. Origin of the anterior forebrain pathway. Ann NY Acad Sci 1016: 736–748, 2004. [DOI] [PubMed] [Google Scholar]
- Person AL, Gale SD, Farries MA, Perkel DJ. Organization of the songbird basal ganglia, including area X. J Comp Neurol 508: 840–866, 2008. [DOI] [PubMed] [Google Scholar]
- Phan ML, Pytte CL, Vicario DS. Early auditory experience generates long-lasting memories that may subserve vocal learning in songbirds. Proc Natl Acad Sci USA 103: 1088–1093, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinaud R, Saldanha CJ, Wynne RD, Lovell PV, Mello CV. The excitatory thalamo-“cortical” projection within the song control system of zebra finches is formed by calbindin-expressing neurons. J Comp Neurol 504: 601–618, 2007. [DOI] [PubMed] [Google Scholar]
- Prather JF, Peters S, Nowicki S, Mooney R. Precise auditory–vocal mirroring in neurons for learned vocal communication. Nature 451: 305–310, 2008. [DOI] [PubMed] [Google Scholar]
- Reiner A, Perkel DJ, Mello CV, Jarvis ED. Songbirds and the revised avian brain nomenclature. Ann NY Acad Sci 1016: 77–108, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper A, Zann R. The onset of song learning and song tutor selection in fledgling zebra finches. Ethology 112: 458–470, 2006. [Google Scholar]
- Sakaguchi H, Saito N. Developmental change of cholinergic activity in the forebrain of the zebra finch during song learning. Brain Res Dev Brain Res 62: 223–228, 1991. [DOI] [PubMed] [Google Scholar]
- Sakaguchi H, Saito N. Developmental changes in axon terminals visualized by immunofluorescence for the growth-associated protein, GAP-43, in the robust nucleus of the archistriatum of the zebra finch. Brain Res Dev Brain Res 95: 245–251, 1996. [DOI] [PubMed] [Google Scholar]
- Schmidt MF, Konishi M. Gating of auditory responses in the vocal control system of awake songbirds. Nat Neurosci 1: 513–518, 1998. [DOI] [PubMed] [Google Scholar]
- Stein J, Walsh V. To see but not to read; the magnocellular theory of dyslexia. Trends Neurosci 20: 147–152, 1997. [DOI] [PubMed] [Google Scholar]
- Stripling R, Kruse AA, Clayton DF. Development of song responses in the zebra finch caudomedial neostriatum: role of genomic and electrophysiological activities. J Neurobiol 48: 163–180, 2001. [DOI] [PubMed] [Google Scholar]
- Stripling R, Volman SF, Clayton DF. Response modulation in the zebra finch neostriatum: relationship to nuclear gene regulation. J Neurosci 17: 3883–3893, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studdert-Kennedy M, Liberman AM, Harris KS, Cooper FS. Theoretical notes. Motor theory of speech perception: a reply to Lane's critical review. Psychol Rev 77: 234–249, 1970. [DOI] [PubMed] [Google Scholar]
- Tchernichovski O, Nottebohm F, Ho CE, Pesaran B, Mitra PP. A procedure for an automated measurement of song similarity. Anim Behav 59: 1167–1176, 2000. [DOI] [PubMed] [Google Scholar]
- Terpstra NJ, Bolhuis JJ, den Boer-Visser AM. An analysis of the neural representation of birdsong memory. J Neurosci 24: 4971–4977, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terpstra NJ, Bolhuis JJ, Riebel K, van der Burg JM, den Boer-Visser AM. Localized brain activation specific to auditory memory in a female songbird. J Comp Neurol 494: 784–791, 2006. [DOI] [PubMed] [Google Scholar]
- Vates GE, Vicario DS, Nottebohm F. Reafferent thalamo-“cortical” loops in the song system of oscine songbirds. J Comp Neurol 380: 275–290, 1997. [PubMed] [Google Scholar]
- Watkins K, Paus T. Modulation of motor excitability during speech perception: the role of Broca's area. J Cogn Neurosci 16: 978–987, 2004. [DOI] [PubMed] [Google Scholar]
- Whitney O, Johnson F. Motor-induced transcription but sensory-regulated translation of ZENK in socially interactive songbirds. J Neurobiol 65: 251–259, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney O, Soderstrom K, Johnson F. Post-transcriptional regulation of zenk expression associated with zebra finch vocal development. Brain Res Mol Brain Res 80: 279–290, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild JM. Descending projections of the songbird nucleus robustus archistriatalis. J Comp Neurol 338: 225–241, 1993. [DOI] [PubMed] [Google Scholar]
- Wild JM. Neural pathways for the control of birdsong production. J Neurobiol 33: 653–670, 1997. [DOI] [PubMed] [Google Scholar]
- Wild JM. Functional neuroanatomy of the sensorimotor control of singing. Ann NY Acad Sci 1016: 438–462, 2004. [DOI] [PubMed] [Google Scholar]
- Wilson SM, Saygin AP, Sereno MI, Iacoboni M. Listening to speech activates motor areas involved in speech production. Nat Neurosci 7: 701–702, 2004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.