Abstract
This study examines how signals generated in the oculomotor cerebellum could be involved in the control of gaze shifts, which rapidly redirect the eyes from one object to another. Neurons in the caudal fastigial nucleus (cFN), the output of the oculomotor cerebellum, discharged when monkeys made horizontal head-unrestrained gaze shifts, composed of an eye saccade and a head movement. Eighty-seven percent of our neurons discharged a burst of spikes for both ipsiversive and contraversive gaze shifts. In both directions, burst end was much better timed with gaze end than was burst start with gaze start, was well correlated with eye end, and was poorly correlated with head end or the time of peak head velocity. Moreover, bursts accompanied all head-unrestrained gaze shifts whether the head moved or not. Therefore we conclude that the cFN is not part of the pathway that controls head movement. For contraversive gaze shifts, the early part of the burst was correlated with gaze acceleration. Thereafter, the burst of the neuronal population continued throughout the prolonged deceleration of large gaze shifts. For a majority of neurons, gaze duration was correlated with burst duration; for some, gaze amplitude was less well correlated with the number of spikes. Therefore we suggest that the population burst provides an acceleration boost for high acceleration (smaller) contraversive gaze shifts and helps maintain the drive required to extend the deceleration of large contraversive gaze shifts. In contrast, the ipsiversive population burst, which is less well correlated with gaze metrics but whose peak rate occurs before gaze end, seems responsible primarily for terminating the gaze shift.
INTRODUCTION
Gaze shifts, which redirect the line of sight, rapidly aim the foveae at different targets of interest in the visual world. Within approximately ±20° from the primary direction of gaze, they usually are accomplished by rapid, accurate, and rather stereotyped saccadic eye movements (Becker 1989). In 1975, Robinson proposed an imaginative brain stem model that nicely captured saccade characteristics. His local feedback circuit, which compared a desired eye position signal with a local estimate of the actual ongoing eye position, produced movements that had trajectories resembling those of actual saccades and that also landed accurately on a target. Furthermore, the model elegantly simulated certain pathological conditions such as the slowed, but accurate, saccades of patients with spino-cerebellar atrophy (Zee et al. 1976) and the interrupted but accurate saccades produced by intrasaccadic microstimulation of the inhibitory omnipause neurons in the brain stem saccade generator (OPNs) (Keller et al. 1996).
At about the time this model appeared, however, evidence was accumulating that the midline cerebellum, vermis lobules VIc and VII, and the caudal fastigial nucleus (cFN) to which they project (Yamada and Noda 1987) also are crucial for saccade generation. Damage to the oculomotor vermis caused saccades to become very dysmetric (Optican and Robinson 1980; Ritchie 1976; Takagi et al. 1998; Vilis and Hore 1981) and more variable than normal (Barash et al. 1999). Inactivation of the cFN by muscimol, a GABAA agonist, caused contraversive saccades to become hypometric and ipsiversive saccades to become hypermetric (Goffart et al. 2003, 2004; Iwamoto and Yoshida 2002; Ohtsuka et al. 1994; Robinson et al. 1993); injections of bicuculline, a GABA antagonist, caused the opposite deficits (Sato and Noda 1992). Because the majority of cFN neurons discharged a burst that preceded contraversive and lagged ipsiversive saccades (Fuchs et al. 1993; Helmchen et al. 1994; Ohtsuka and Noda 1991), these data, gathered with the head restrained from moving, suggested that the oculomotor cerebellum provides signals to help accelerate contraversive saccades and decelerate ipsiversive ones. Such signals have direct access to Robinson's brain stem saccade generator (Noda et al. 1988, 1990; Scudder et al. 2000).
For natural gaze shifts to targets located at eccentricities greater than ±30°, however, the eye saccade requires the help of a head movement. Moreover, the eye and head movement components must be carefully coordinated to provide normal, consistent head-unrestrained gaze shifts (Freedman and Sparks 1997b; Phillips et al. 1995). An on-going debate is whether it is the overall gaze shift that is controlled during orienting behaviors (Choi and Guitton 2006; Sylvestre and Cullen 2006) or its individual eye and head components (Freedman 2001). The major input to the brain stem burst generator for saccades originates in the superior colliculus (SC), which is the node for descending saccade commands from a variety of cortical structures. The burst of activity in SC neurons signals the overall displacement of a head-unrestrained gaze shift (Freedman and Sparks 1997a). Eventually, the gaze signal must be decomposed into head and eye movement signals, at which stage they could be processed independently (Sparks 1999).
Recent studies showed that inactivation of the monkey cFN compromises head-unrestrained gaze shifts by creating independent deficits of their eye and head movement components (Quinet and Goffart 2005, 2007). These authors attributed the effect on head movement to unwanted spread of the muscimol to the rostral fastigial nucleus and concluded that cFN neurons are involved “in the generation of appropriate saccadic oculomotor commands” (Quinet and Goffart 2005). Moreover, their stimulation in the cFN evoked only eye saccades at all but one site, but little if any head movement, even though they used very long stimulus trains (Quinet and Goffart 2009). However, we felt that the data drawn from their inactivation studies (Quinet and Goffart 2005, 2007) might have been more robust if they had tested larger gaze shifts that always require a substantial head movement component. Therefore we recorded from cFN neurons during much larger head-unrestrained gaze shifts to examine whether there is evidence of head-related activity or eye or gaze movement activity alone.
Our diagnosis is facilitated because head-unrestrained gaze shifts can be much larger and therefore have much longer durations than even the very largest saccades made with the head restrained (Phillips et al. 1995; Tomlinson and Bahra 1986). Consequently, the timing of the burst relative to saccade onset and end can be more clearly delineated, providing a more sensitive condition to test the hypothesis of cerebellar function proposed on the basis of head-restrained saccades. For such head-unrestrained gaze shifts, we unexpectedly show that the cFN burst is best timed with the end of all horizontal gaze shifts, whether they are ipsi- or contraversive.
Taken together, our data suggest that the cFN not only helps facilitate the acceleration of gaze shifts that use no head movement, as was previously proposed, but also helps maintain the deceleration phase of gaze shifts that do require a head movement. Moreover, the termination of the gaze shift when the eye reaches the target would be caused by both the removal of the drive maintaining deceleration and a late burst of inhibition from the opposite cFN.
Preliminary reports of these experiments have appeared earlier (Brettler and Fuchs 2001; Brettler et al. 2003).
METHODS
Surgical procedures
Four rhesus macaques (Macaca mulatta) were implanted with a single scleral search coil (Fuchs and Robinson 1966; Robinson 1963) to monitor gaze movements and a head coil to measure head position. To allow access to both the left and right fastigial nuclei, we implanted a stainless steel recording chamber vertically over a craniotomy centered on the midline and located 8 mm posterior to the intra-aural axis. The chamber, stabilization lugs to allow the head to be held, and connectors for the eye and head coil were secured to the head with stainless steel screws and dental acrylic. Details of these surgical procedures are available in previous publications from our laboratory (Fuchs et al. 2005). Behavioral sessions, which began 1–2 wk after recovery from surgery, were carried out in a sound-attenuated booth.
Methods
We trained each monkey to redirect its gaze rapidly between sequentially illuminated LED targets, which subtended 0.25°, on a hemispheric array positioned 24 in (61 cm) in front of the eyes. The LEDs were mounted at 1° intervals on eight curved arcs that lay along the horizontal and vertical meridians and along the meridians halfway between, i.e., at every 45° angle from 0 (right) to 315°. The maximum possible target excursion from straight ahead was ±80° horizontally, ±32° vertically, and ±45° along the oblique axes. If the animal's gaze remained within ±2° of the target continually for an average of 1.2 s, it received a small dollop of applesauce. The accuracy requirement was disabled for 700 ms after the target step to allow for the reaction time of the primary saccade and the latency to the corrective saccade.
Head-unrestrained data were obtained with the head completely free to rotate. To minimize body movements and shoulder rotations, we installed either hip or shoulder restraints on the primate chairs in which the animals sat. Even for the largest horizontal eye rotations, we could measure eye position accurately (to the nearest 0.5° in a well-trained subject) with our rotating magnetic field coil system (CNC Engineering).
We recorded single-unit activity extracellularly with a homemade tungsten microelectrode (∼100–200 kΩ at 1 kHz), which was advanced by a hydraulic drive into the cerebellum through the recording chamber. To provide a search stimulus as the electrode was lowered, we required the animal to make horizontal gaze shifts to a variety of large target steps, usually between 50 and 80°, with the head unrestrained. We identified the cFN by its characteristic bursting activity for saccades in all directions (Fuchs et al. 1993; Helmchen et al. 1994; Ohtsuka and Noda 1991). To facilitate the search for the cFN, we also occasionally turned on the booth lights to elicit spontaneous omni-directional saccades, which occurred in rapid succession, thereby making it easier to hear saccade-related bursts of activity over the audio monitor. Once a putative cFN neuron had been isolated, we attempted to collect horizontal gaze shifts to target steps that ranged in size pseudo-randomly from 10 to 100° in 10° increments. Saccades could start from a variety of initial target positions, which, in part, were determined by the final eye position on the previous trial. Such target steps elicited both a large primary and a smaller secondary corrective saccade. In our analyses, we considered responses associated with both primary and corrective saccades but not spontaneous saccades.
Data collection and analysis
Horizontal and vertical gaze position (eye position in space), head position, and target location were digitized at 1 kHz, and the time of spike occurrence was specified with 10 μs accuracy using a 16-bit analog/digital board (NB-MIO-16x, National Instruments, Austin, TX) either on-line and/or saved to tape (Model 4000A, Vetter Digital, Rebersburg, PA) for off-line processing. Eye position in the head was reconstructed off-line by subtracting head position from gaze position.
Digitized data were displayed and analyzed with a customized program. The gaze, head, and eye position traces were differentiated to create velocities. The occurrence of a gaze shift was detected when gaze velocity exceeded ±50°/s. A velocity criterion of 5°/s was used to mark the onset and offset of the gaze shift and of its accompanying eye and head movements. In addition, the program marked the peak velocities of each movement component. Each of the gaze shifts was checked manually to verify the appropriateness of the marking of each movement component. In ∼15% of trials where remarking was required, it was usually because there was either a particularly slow head movement or an upward then downward deflection of the eye during a large horizontal gaze shift.
To mark the burst, the customized program located saccade onset and searched both earlier and later in time to find the peak firing rate. From the peak firing rate, it searched for the two times at which the firing rate fell below 40 spikes/s. The earlier time was taken as burst onset and the later as burst end. On occasion, a cFN neuron exhibited an early increase in activity, a later second increase and bridging activity in excess of the resting rate (also observed by Scudder and McGee 2003). In these cases, all three components together were taken as the burst. The magnitude of peak firing rate was defined as the average frequency of the five consecutive spikes that occurred within the shortest total duration. We used this measure instead of a two-spike peak frequency measure to deemphasize the occasional short interspike intervals, which would cause uncharacteristically high peak rates. As previously reported in head-restrained studies (Fuchs et al. 1993; Ohtsuka and Noda 1991), most cFN neurons exhibited low discharge rates between gaze-shift related bursts. To construct spike density functions (SDFs), we convolved Gaussians (width 20 ms) with a train of unity pulses positioned at the occurrence of a spike.
We exported the analyzed data into another customized computer program and Microsoft Excel to perform linear regression analyses on metrics of the burst (e.g., start time, end time, peak frequency, number of spikes, duration) and on associated metrics of the saccade (e.g., start time, end time, peak velocity, amplitude, duration). We combined all similar-sized saccades irrespective of their initial positions because cFN burst characteristics largely reflect the metrics of the saccade and not the initial or final eye position (Fuchs et al. 1993; Kleine et al. 2003; Ohtsuka et al. 1994).
All experiments were performed in accordance with the recommendations of the National Research Council (1997, 2003) and the Society for Neuroscience and exceeded the minimal requirements recommended by the Institute of Laboratory Animal Resources and the Association for Assessment and Accreditation of Laboratory Animal Care International. All procedures were evaluated and approved by the local Animal Care and Use Committee of the University of Washington.
RESULTS
We recorded the activity of 38 cFN neurons that exhibited phasic changes in activity associated with head-unrestrained (referred to from here on as head-free) gaze shifts to targets jumping along the horizontal meridian. The vast majority (33) exhibited a burst of spikes for all gaze shifts to the left and right as has been reported for cFN unit activity during saccades with the head restrained from moving (hereafter referred to as head-fixed). During the fixation intervals between saccades, all these bidirectional burst neurons displayed a tonic discharge, which did not consistently vary with gaze, eye, or head position. As we reported previously (Fuchs et al. 1993), the bursts of some cFN neurons (22/33 in this study) occurred in association with pauses in tonic firing. In this study, the pause usually preceded the burst (21/22), and for many neurons, the pause was either more prominent (longer in duration) or appeared only for large gaze shifts. Although the bidirectional burst was a constant feature of all our cFN neurons, the pre- and postburst activities were quite idiosyncratic from unit to unit. Therefore it was impossible to segregate the units into subtypes by considering the discharge patterns during the time course of an entire saccade trial.
Of the remaining 5 of the 38 neurons, 3 (all recorded in 1 monkey) discharged a very weak burst but only for larger ipsiversive gaze shifts, which usually were made in association with a head movement. All three lay ∼1.5–2 mm dorsal to the bidirectional burst neurons but did not display the complex spikes that are characteristic of Purkinje cells. Of the other two neurons, one exhibited unreliable pauses for contraversive gaze shifts and the other unreliable pauses for both contra- and ipsiversive gaze shifts.
We will examine the discharge characteristics of only the 33 bidirectional burst neurons in relation to the horizontal component of gaze shifts evoked by horizontal target steps. With these large gaze shifts, we will show that the timing of the burst not only is sensitive to the direction of the gaze shift but also its duration.
General discharge features
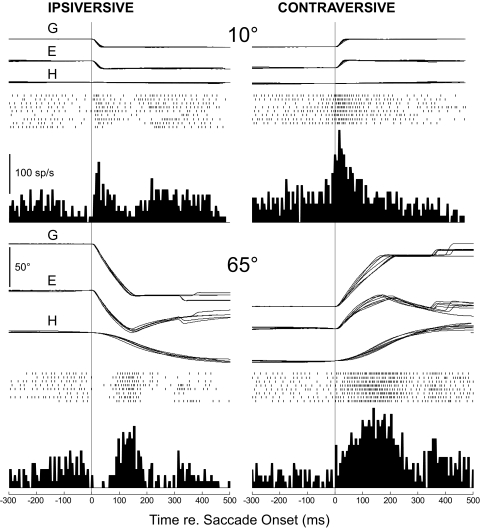
Figure 1 shows the discharge patterns of a cFN neuron during head-free horizontal gaze shifts of 10 (above) and 65° (below). For the 10° gaze shifts, which exhibited no head movement component, contraversive saccades were associated with a leading burst and ipsiversive saccades with a burst that began at or after the onset of the gaze shift. Therefore for this neuron, the burst timing for small gaze shifts with the head-free resembled that reported for the majority of cFN neurons with the head fixed (Fuchs et al. 1993; Helmchen et al. 1994; Ohtsuka and Noda 1991). Also, the bursts associated with nearly identical 10° gaze shifts showed considerable variability, like that reported for bursts accompanying head-fixed saccades (Fuchs et al. 1993; Inaba et al. 2003).
Fig. 1.
Discharge pattern of an exemplar caudal fastigial nucleus (cFN) neuron (S12-1) during small (10°) and large (65°) horizontal head-free gaze shifts. For both amplitudes, we show 8 or 9 very similar ipsiversive and contraversive gaze shifts in the left and right columns, respectively. In all 4 examples, traces from top to bottom are the horizontal components of gaze, eye, and head movement, the associated discharge patterns shown as rasters and an average histogram (bin width = 8 ms). The amplitude calibration bar applies to all movements. All traces aligned on the start of the gaze shift (thin vertical lines).
For 65° gaze shifts (Fig. 1, lower gaze shifts), there always was a prominent head movement component, and our exemplar neuron again discharged a vigorous burst of spikes for saccades in both horizontal directions. Because the eye movement component is larger in amplitude and hence also longer in duration, large gaze shifts clarified some features of burst timing and revealed others. For large ipsiversive gaze shifts, it now became quite clear that the burst is indeed better timed with the ends of the eye movement or gaze shift, which were difficult to distinguish in our paradigm, than with their onsets. Unlike the situation for the small contraversive gaze shifts, where the burst began before or near saccade onset, the peak of the burst for the large contraversive gaze shift now was shifted toward the ends of the gaze and/or eye movement.
In what follows, we first will examine the relations of the timing of different aspects of the burst relative to gaze start, e.g., burst end, time to peak burst rate, with the times of occurrence of salient features of the gaze shift, e.g., gaze end time, time to peak gaze velocity, for movements of different sizes in the two horizontal directions. Then we will evaluate possible relations of the metrics of the burst such as burst duration and number of spikes with the metrics of the movements such as movement duration and size.
Correlations of burst timing with movement timing
TIMING WITH GAZE MOVEMENT END.
In studies performed on head-fixed monkeys, cFN bursts were shown to be associated with the start of contraversive saccades and the end of ipsiversive saccades (Fuchs et al. 1993; Ohtsuka and Noda 1991). In head-free monkeys, in contrast, the timing of the end of the burst is well correlated with the end of both the ipsiversive and contraversive gaze shift. This is shown in Figs. 2 and 3, which show data from two other cFN neurons for which we were able to gather many head-free gaze shifts at a variety of amplitudes and hence durations. Here we present the rasters accompanying gaze shifts of different sizes and arrange them from bottom to top in order of increasing gaze shift duration.
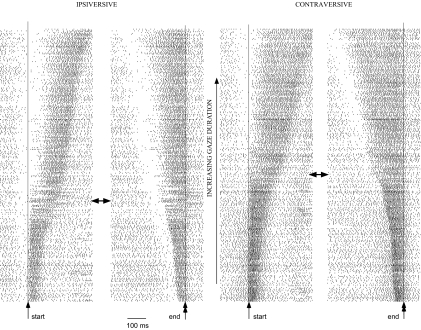
Fig. 2.
Burst pattern of an exemplar cFN neuron (W53-1) for head-free horizontal gaze shifts ranging from 10 to 100°. Ipsiversive gaze shifts appear in the left 2 columns and contraversive gaze shifts in the right 2 columns. For each column, the rasters associated with either 326 ipsi- or 327 contraversive gaze shifts are aligned (see thin vertical lines) either on gaze start (1st and 3rd columns) or gaze end (2nd and 4th columns). The rasters are organized from bottom to top in order of increasing gaze duration. All gaze shifts above the horizontal double-headed arrows have total head movement components of ≥5°.
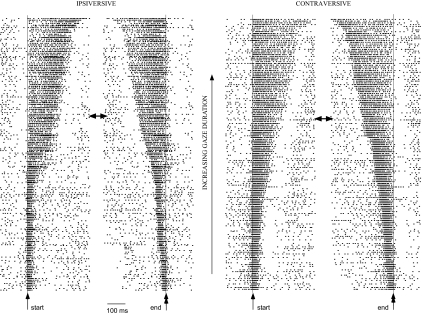
Fig. 3.
Burst pattern of another cFN neuron (W59-6) for head-free horizontal gaze shifts ranging from ∼2 to 80°. There are 177 and 150 ipsi- and contraversive gaze shifts, respectively. Presentation and traces as in Fig. 2.
The cFN neuron shown in Fig. 2 shows an increase in burst latency relative to gaze onset (thin vertical line) as both ipsiversive and contraversive gaze duration increase (left columns for each direction). The end of the burst also occurs later and later as burst duration increases. When the rasters instead are aligned with the end of the gaze shift (right columns), the end of the burst now consistently occurs near the end of both the ipsiversive and contraversive gaze shifts. The correlation of gaze end time and burst end time is quite strong for both ipsiversive and contraversive saccades, as can be seen in Fig. 4, A and B, respectively. The linear regressions of ipsiversive and contraversive gaze end time with burst end time have correlation coefficients of 0.95.
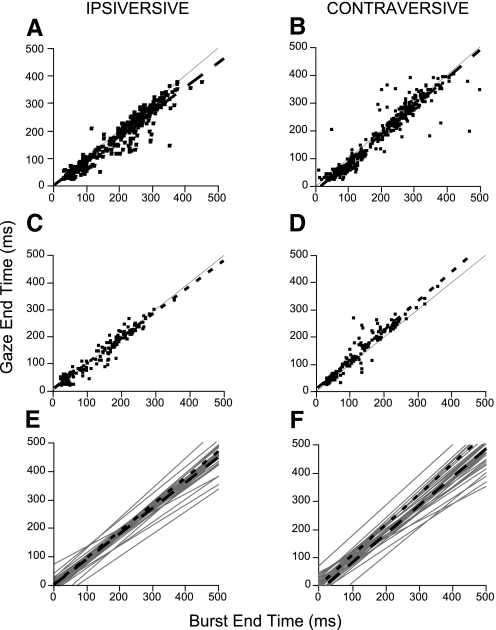
Fig. 4.
Correlation of the timing of the end of the burst with the end of the gaze shift re gaze onset. A–D: linear regressions of gaze end time with burst end time for ipsi- and contraversive gaze shifts, left and right columns, respectively, for the exemplar neurons in Figs. 2 (A and B) and 3 (C and D). The equations for the linear regressions in A–D: gaze end time (GET) = 0.9 × burst end time (BET) − 5.43 (r = 0.95), GET = 1.01 × BET − 18.75 (r = 0.95), GET = 0.94 × BET + 2.84 (r = 0.97), and GET = 1.08 × BET + 2.42 (r = 0.96), respectively. Thin lines in A–D have a unity slope. E and F: gaze end time vs. burst end time linear fits for all 33 burst neurons; fits for the units in Figs. 2 and 3 are shown as long and short dashed lines, respectively.
A similar tight correlation between the end of the burst and the end of the gaze shift exists for the unit shown in Fig. 3. For ipsiversive movements, the most intense part of the burst occurs later relative to gaze onset as gaze duration increases. For contraversive gaze shifts, burst onset is not delayed as gaze duration increases but rather the burst increases in duration. Again, the correlation of gaze end time with burst end time is quite robust for both ipsiversive and contraversive saccades as can be seen in Fig. 4, C and D, respectively. The linear regressions of burst end time with ipsiversive and contraversive gaze end time have correlation coefficients of 0.97 and 0.96, respectively.
It should be emphasized that the neurons shown in Figs. 1–3 were selected to show the timing properties of the saccade-related burst, which is the consistent discharge feature that characterizes every cFN neuron considered in this study. In contrast, the nature of the activity occurring before and after the burst differs from unit to unit and therefore should not be taken as representative of our cFN population as a whole.
Across our entire population of 33 neurons, the correlation coefficients of the relations between the end of ipsiversive and contraversive gaze shifts and the end of the burst averaged 0.92 ± 0.08 and 0.92 ± 0.07 (SD) in the ipsi- and contraversive directions, respectively. The correlation coefficients exceeded 0.82 for 62 of the 66 possible relations (33 neurons × 2 directions) in the two directions.
Figure 4, E and F, shows the linear regressions of gaze end time versus burst end time relative to gaze onset for ipsiversive and contraversive gaze shifts for all 33 neurons. The slopes averaged 0.90 ± 0.09 and 0.95 ± 0.12 for ipsi- and contraversive gaze shifts, respectively, with average intercepts near zero (−1.66 ± 25.37 and 3.19 ± 28.97, respectively). Therefore on average, the burst ended approximately at gaze end for small ipsiversive and contraversive saccades but ended later and later after gaze end as gaze duration increased. Others have reported that the burst of some cFN neurons extends beyond the end of contraversive saccades made with the head fixed (Scudder and McGee 2003).
BURST TIMING WITH GAZE MOVEMENT END AND ONSET.
On the basis of previously reported data with the head fixed (Fuchs et al. 1993; Ohtsuka and Noda 1991), the finding of a strong correlation between the end of ipsiversive gaze shifts and the end of the burst was expected. However, the correlation between the end of the burst and the end of contraversive gaze shifts was not. For contraversive head-fixed saccades ≤20–25°, burst onset for most cFN neurons appeared to be best timed with saccade onset and not saccade end (Fuchs et al. 1993; Ohtsuka and Noda 1991; also see Scudder and McGee 2003). However, with the head free, not only was the end of the contraversive gaze shift highly correlated with the end of the burst, its beginning often was not well correlated with the onset of the burst. To show this, we compared the variance of the onset of the burst relative to the start of the contraversive gaze shift (gaze start latency) with the variance of the end of the burst relative to the end of the contraversive gaze shift (gaze end latency). We used an F-test that determines whether the SD of two populations is unequal (Rosner 1995). For 20 of 33 (61%) cFN neurons, the variance of gaze end latency (median: 539.32; range: 62.18–2,839.55) was statistically less than that for gaze start latency (median: 3,076.39; range: 554.5–8,610.38; P < 0.001). For the remaining 13 neurons, 11 either had bursts that began before the gaze shift and lasted until it ended (7 neurons) or had both an early weak burst at gaze start and a later stronger one associated with saccade end (4 neurons). Double bursts, which can occur not only for large contraversive gaze shifts but large ipsiversive gaze shifts as well, also have been reported for large saccades with the head fixed (Scudder and McGee 2003).
The variance of end latency for ipsiversive gaze shifts (median: 886.02; range: 368.30–3,620.64) also was statistically less than that for start latency (median: 3,762.49; range: 1,256.92–8,510.93; F-test for unequal variances, P < 0.001) for 25 of 33 cFN neurons. For seven of the remaining eight, the variance of end latency and start latency was not statistically different (P > 0.001).
Even though the end of the burst is well timed to occur near the end of both contra- and ipsiversive gaze shifts, the bursts of some units clearly ended after the movement (Fig. 4, E and F) and therefore seem ill suited to influence its termination. In these cFN neurons, perhaps the low rates at the end of the burst are insufficient to drive downstream target neurons, and only high rates are successful. Therefore in the next section we consider the timing of the peak burst rate relative to gaze end. Also, perhaps it is the differential influence of the contra- and ipsiversive cFN activity that influences the burst produced by the saccade generator, a possibility that we consider later in the discussion of Figs. 6 and 7.
Fig. 6.
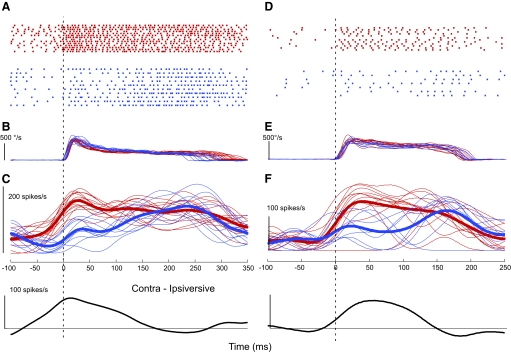
Comparison of discharge patterns of 2 exemplar neurons during large ipsi- and contraversive gaze shifts matched for peak gaze velocity. For both neurons (W39-2: A–C and S9-1: D–F), traces from top to bottom are unit rasters from individual trials associated with contraversive (red) and ipsiversive (blue) gaze shifts, time courses of the associated absolute gaze velocities of the individual trials, and spike density functions (20 ms Gaussian) of unit activity. In C and F, thick red and blue lines show the averages for all contra- and ipsiversive trials, respectively. The lowest traces (Contra-Ipsiversive) are the difference between the averages of the contra- and ipsiversive gaze shifts for each neuron. All traces are aligned on the start of the gaze shift (t = 0, dashed lines). Gaze amplitude ranged from 70 to 85 and 60 to 73° for W39-2 and S9-1, respectively.
Fig. 7.
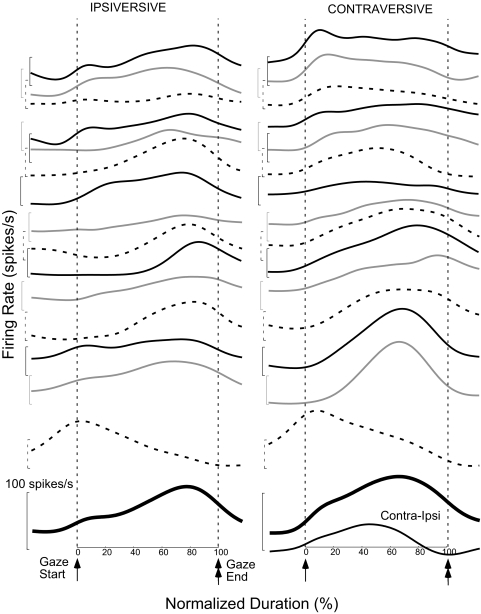
Comparison of average spike density functions for 15 neurons with sufficient data. Spike density functions for ipsi- and contraversive gaze shifts, left and right columns, respectively, have been adjusted so that the associated gaze shifts have equal average 100 ms durations. Single and double tipped arrows indicate the beginning and end, respectively, of the duration-normalized gaze shift. All traces are aligned on the start of the gaze shift (t = 0). Paired side-by-side traces with lines of the same quality (black, gray, and dashed) in the 2 columns refer to the same unit. The thick curves at the bottom of each column are the ipsi- and contraversive population averages across all 15 neurons; at the bottom of the right column, the thin curve labeled Contra-Ipsi shows their difference. The 1st and 3rd units from the top are those described in detail in the left and right columns, respectively, of Fig. 6. All brackets represent 100 spikes/s; the bottom of the bracket indicates the unit's 0 firing rate. The units shown in Figs. 1–3 and 6 (S9-1 and W39-2) are the 9th, 12th, 2nd, 3rd, and 1st pairs of traces, respectively.
TIMING OF GAZE MOVEMENT END WITH PEAK BURST RATE AND BURST ONSET.
In contrast to the end of the burst, the peak burst rate usually occurred well before the end of the gaze shift. Gaze end time was well correlated with the time of occurrence of peak burst rate for most units. For ipsiversive gaze shifts, the correlation coefficient (r) averaged 0.87 ± 0.18 even though three units had r < 0.44. For contraversive gaze shifts, r averaged 0.80 ± 0.22, but six units had r < 0.62. For five of these six, the burst continued throughout the contraversive gaze shift as it did for the unit in Fig. 3; the remaining unit had a low r for both contraversive and ipsiversive gaze shifts. The average slopes of the relation between gaze end and the time to peak burst rate were 0.99 ± 0.14 and 1.05 ± 0.30 for ipsi- and contraversive gaze shifts, respectively. The average intercepts (the time by which peak burst time preceded gaze end) of 42.14 ± 33.97 and 64.15 ± 36.28 ms, respectively, were significantly different (P = 0.01, 2-tailed t-test). Therefore peak burst rate occurred well before both ipsi- and contraversive gaze end for small gaze shifts and continued to maintain a substantial lead as gaze amplitude increased. On average, peak burst rate occurred 22 ms earlier for contraversive than ipsiversive gaze shifts.
To determine whether the ipsi- or contraversive burst could have the earliest influence on the end of the gaze shift, we calculated the times by which the bursts started relative to gaze end. Bursts began ∼20 ms earlier for contraversive than ipsiversive gaze shifts. The average intercepts of the relations between gaze end and burst start (times by which burst start preceded gaze end) were 104.07 ± 38.35 and 83.8 ± 32.73 ms for contra- and ipsiversive gaze shifts, respectively. The difference in burst leads relative to (hereafter abbreviated as re) gaze end was significant (P = 0.02, 2-tailed t-test).
Therefore, relative to the end of the gaze shift, the burst associated with contraversive movements began before and reached its peak discharge ∼20 ms earlier than did the burst associated with ipsiversive saccades.
TIMING WITH EYE MOVEMENT CONTRIBUTION.
For most head-free gaze shifts in the monkey, the end of the eye saccade, which usually is terminated by an eye counter-rotation associated with the continuing head movement, occurs near the end of the gaze movement or slightly before it (Phillips et al. 1995; Tomlinson and Bahra 1986). Therefore it is not surprising that the end of the cFN burst is also well correlated with the end of the eye component. To determine whether gaze or eye end was better correlated with burst end, we compared the variances of the time from the end of the gaze movement to the end of the burst (gaze end re burst end interval) with the time from the end of the eye movement to the end of the burst (eye end re burst end interval) for all gaze shifts for each cFN neuron. For ipsiversive gaze shifts, 25 of 33 neurons showed no statistical difference (F-test, P < 0.001) between the gaze and eye end re burst end interval variances; for the remaining 8, the gaze end re burst end interval was less variable than the eye end re burst end interval. For contraversive gaze shifts, 27 of 33 neurons showed no statistical difference (F-test, P < 0.001) between the gaze end re burst end interval and the eye end re burst end interval variances; for the remaining 6, the gaze end re burst end interval was less variable than the eye end re burst end interval. Therefore for more than three quarters of our cFN neurons, the end of the burst was as reliably correlated with the end of the eye movement as with the end of the gaze movement. For the remainder, the end of the burst was more tightly correlated with the end of the gaze movement.
TIMING WITH HEAD MOVEMENT CONTRIBUTION.
Because the timing of the end of the burst is well correlated with the end of both the gaze shift and its eye component, it seems likely that the timing of the burst would show little correlation with the timing of the head movement. Indeed, Fig. 1 shows that a burst can occur even when a head movement is not present at all. To study a possible influence of burst timing on the head component, we compared the variance of the gaze end re burst end interval with the variance of the time from the end of the head movement to the end of the burst (head end re burst end interval). For both contra- and ipsiversive gaze shifts, the variance of the gaze end re burst end interval always was significantly less than that of the head end re burst end interval (F-test, P < 0.001). For contraversive gaze shifts, the median variances of the gaze end re burst end interval and the head end re burst end interval were 1,130.75 (range: 218.89–8,935.62) and 17,160.76 (8,622.28–44,857.04), respectively. For ipsiversive gaze shifts, the medians were 926.66 (range: 319.74–8,446.28) and 18,263.13 (5,824.05–42,100.38) respectively.
It also is possible that the cFN burst is involved only with accelerating the head movement to its peak velocity. If the acceleration of the head was appropriately controlled, its inertia could carry it to its final destination. In this scenario, the burst should be timed to stop near the end of the acceleration phase, i.e., at peak head velocity, and the variability of the time from the end of the burst to the time of peak head velocity (peak head velocity time re burst end interval) would be less than that of the gaze end re burst end interval. However, the variance of the contraversive peak head velocity time re burst end interval was greater than that of the gaze end re burst end interval for 27 of 33 neurons (F-test, P < 0.001). Likewise, the variance of the ipsiversive peak head velocity time re burst end interval was greater than that of the gaze end re burst end interval for 28 of 33 neurons (F-test, P < 0.001). The five neurons for which burst end was equally well timed with ipsiversive gaze end and peak head velocity were different from the six for which burst end was equally well timed with contraversive gaze end and peak head velocity. In summary, the timing of the end of the burst always was better correlated with the end of the gaze shift than with the time to peak head velocity and, only occasionally (11/66 of all possible contra- and ipsiversive relations), was equally well timed with both.
We examined other possible relations between the burst and the head movement with two final analyses. First, we performed multiple linear regressions of the number of spikes with gaze and head movement amplitude and of burst duration with gaze duration and the duration of the head movement contribution at peak head velocity. We tested 14 neurons that were recorded during ≥100 gaze shifts to the right and left and had amplitudes distributed over the range between ∼10 and >65°. For the relations of number of spikes with gaze and head amplitude, 13 of the 14 cFN neurons showed either no statistically significant correlations with contraversive head amplitude (n = 5) or substantially better (the magnitude of the gaze coefficient was ≥2 and most often 3 orders of magnitude greater than that of the head coefficient) correlations with contraversive gaze amplitude (n = 8). For ipsiversive gaze shifts, 12 of 14 showed either no statistically significant correlations with head amplitude (n = 4) or much better correlations with gaze amplitude (n = 8). For the relations of burst duration with gaze duration and the head duration at peak head velocity, all 14 showed either no significant correlations with head duration at peak head velocity or substantially better correlations with gaze duration for both ipsi- and contraversive movements. Second, we examined whether peak head velocity was correlated with the change in burst rate (peak minus resting rate). For gaze shifts with total head movement components of ≥5°, the correlation coefficient of the relation ranged from −0.36 to 0.30 (0.02, on average) and −0.22 to 0.51 (0.1 average) for ipsiversive and contraversive gaze shifts, respectively (the only 2 negative correlation coefficients occurred in opposite directions for the same unit).
In summary, several observations indicate that the burst of cFN neurons is unrelated to metrics of the head movement component of a gaze shift. First, the end of the burst is equally well timed with the end of the gaze shift and its eye component but is timed poorly, if at all, with either the end of the head movement or the time that the head reaches its peak velocity. Second, multiple regressions of burst properties with gaze and head metrics in units with a broad distribution of gaze amplitudes show substantially better correlations with gaze than head movement. Third, peak head velocity is poorly correlated with the peak change in burst rate. The final, and we feel most compelling, observation is that vigorous bursts also occur when there is no head movement at all (Fig. 1) even though the head is free to move. Consequently, there is not an obligatory link between the occurrence of a burst and a head movement. Therefore in the remainder of the results, we no longer will consider the head movement but will evaluate the correlations of the properties of the burst with regard to the metrics of the gaze movement.
Correlations of gaze metrics with burst metrics
Neurons in the cFN project to neurons in the brain stem saccade generator (Noda et al. 1988, 1990; Scudder et al. 2000), which, in turn, provides direct inputs to abducens motoneurons (see Scudder et al. 2002 for summary). The firing patterns of both the excitatory and inhibitory burst neurons (EBNs and IBNs, respectively) and the omnidirectional pause neurons (OPNs) are tightly related to saccade duration (Moschovakis et al. 1996; Scudder et al. 2002). Also, the number of spikes in the burst of both EBNs and IBNs is tightly correlated with saccade size. Some authors (Ohtsuka and Noda 1991) have reported that the bursts of many cFN neurons exhibit similar tight correlations with saccade metrics, such as amplitude and duration, when the head is fixed, whereas we (Fuchs et al. 1993) and others (Kleine et al. 2003) concluded that such strong relations occur infrequently. However, with the head free, as in this study, the resulting greater range of both gaze duration and amplitude might make such correlations easier to detect.
GAZE DURATION WITH BURST DURATION.
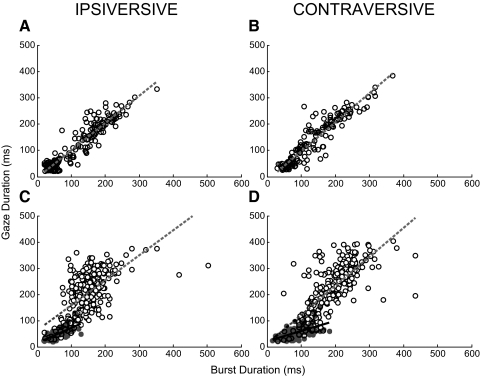
All but one of our cFN neurons exhibited monotonic increases of gaze duration with burst duration in at least one of the horizontal directions. For many, e.g., the unit shown in Fig. 3, the entire relation appeared to be well captured by a single linear regression (Fig. 5, A and B). However, for others, e.g., the unit shown in Fig. 2, the relation was better captured by two lines: one with a shallow slope for small burst durations followed by a steeper one for larger burst durations (Fig. 5, C and D, black and dashed lines, respectively). For the unit shown in Fig. 5, C and D, and other neurons with similar characteristics, the small and large burst duration data could reasonably well be divided according to whether or not there was an associated head movement. In Fig. 5, the gray data points identify the data for which the total head movement was ≤5° and the open circles the data for which it was >5°. The slope of the line fit to the small duration data (gray circles) was less than that fit to the large duration data (open circles) for 29 of 32 relations in the ipsiversive and 31 of 32 relations in the contraversive directions, respectively. The means of the slopes of the small versus large duration data fits were 0.24 ± 0.25 versus 0.64 ± 0.29 in the ipsiversive direction and 0.22 ± 0.22 versus 0.80 ± 0.42 in the contraversive directions, respectively. For the small duration data, the slopes were not significantly different from zero (P > 0.05) for 14/32 ipsiversive and 13/32 contraversive relations. In contrast, for the large duration data, the slopes for 26/32 ipsiversive and 31/32 contraversive relations were significantly different from zero. Also, the large and small duration slopes were significantly different (P < 0.05; 2-sample t-test). Taken together, these data suggest that when gaze shifts have an attendant head movement, many cFN neurons display a clear monotonic relation between gaze duration and burst duration, whereas when there is little head movement, fewer cells display a significant relation, and it is less robust (lower average slope).
Fig. 5.
Relation of gaze duration with burst duration. A–D: gaze duration as a function of burst duration for ipsi- and contraversive gaze shifts, left and right columns, respectively, for the exemplar neurons in Figs. 3 (A and B) and 2 (C and D). Data are divided into those obtained when head movements were ≤5° (gray data points; only those within ±3 SD of the mean were considered) and those that were not (open circles). Linear regressions for the small and large head movement data are shown as solid black and dashed lines, respectively. Linear regressions—A: gaze duration (GD) = 1.04 × burst duration (BD) − 2.71 (r2 = 0.88); B: GD = 1.10 × BD − 12.69 (r2 = 0.86); C: small head movements: GD = 0.56 × BD + 15.04 (r2 = 0.53); large head movements: GD = 0.96 × BD + 63.59 (r2 = 0.40); and D: small head movements: GD = 0.38 × BD + 21.39 (r2 = 0.31); large head movements: GD = 1.1 × BD + 13.05 (r2 = 0.56).
GAZE AMPLITUDE WITH NUMBER OF SPIKES.
For our cFN neurons, gaze amplitude was not as often or as well correlated with the number of spikes in the burst. For our exemplar neurons, both contra- and ipsiversive gaze amplitude showed only modest correlations with the number of spikes. R values for relations with contraversive gaze amplitude were 0.6 and 0.76 and with ipsiversive gaze amplitude were 0.58 and 0.84, respectively, for the units shown in Figs. 2 and 3. For all 33 neurons, correlation coefficients ranged from −0.55 to 0.88 (mean = 0.61 ± 0.33) for contraversive gaze shifts and from −0.54 to 0.89 (mean = 0.42 ± 0.39) for ipsiversive gaze shifts. However, the correlation coefficients of the gaze amplitude versus number of spikes relation was >0.7 for contraversive gaze shifts in 21/33 neurons but for ipsiversive gaze shifts in only 8/33 units. For the neurons with r > 0.7, the average slope of the regression was 2.70 ± 1.04 and 2.39 ± 0.58, with average intercepts of −6.03 ± 12.00 and −2.95 ± 5.61 for contra- and ipsiversive gaze shifts, respectively. Six neurons had good relations with both contra- and ipsiversive gaze amplitude. In sum, these data indicate that the majority of our cFN neurons (64%) exhibited a modest correlation of contraversive gaze amplitude with the number of spikes in the burst, but ipsiversive gaze amplitude was modestly well correlated with the number of spikes for far fewer neurons (24%).
SUMMARY.
Thus far, our data showed several characteristics that distinguish ipsi- and contraversive bursts. With regard to timing, bursts for contraversive gaze shifts began significantly earlier (by ∼20 ms, on average) and reached their peak discharge significantly sooner (by ∼22 ms, on average) than did bursts for ipsiversive gaze shifts. With regard to relations between movement and burst metrics, all but one cFN neuron showed a significant increase of gaze duration with burst duration for larger contraversive gaze shifts that used head movements, whereas fewer cFN neurons showed the increase for larger ipsiversive gaze shifts. Moreover, the average increase, as indicated by the slope of the relation, was significantly greater in the contraversive direction. Also, many more cFN neurons exhibited better correlations between contraversive gaze amplitude and the number of spikes (64%) than between ipsiversive gaze amplitude and the number of spikes (24%). Taken together, these data suggest that the earlier onset and earlier peak rate of burst activity associated with contraversive gaze shifts might be more involved in controlling movement metrics than the later onset and peak rate of burst activity associated with ipsiversive gaze shifts.
The occurrence of a pause preceding the burst in some neurons, e.g., for the ipsiversive gaze shifts in Figs. 1 and 2, might support this suggestion. For our 33 cFN burst neurons, pauses preceded the burst in 21 (64%), but, for some units, became apparent only after the gaze shift had reached a sufficient duration or size (e.g., in association with the longer duration contraversive gaze shifts in Fig. 2). For these 21 neurons, pauses occurred only for ipsiversive gaze shifts in 10 (e.g., the unit in Fig. 3), for both ipsi- and contraversive gaze shifts in 7 (e.g., the unit in Fig. 2) and only for contraversive gaze shifts in 4. Therefore when a preceding pause occurred, it more often was in association with an ipsiversive (17/21; 81%) than a contraversive (11/21; 52%) gaze shift. The preceding pause could serve to enhance the effect of the late ipsiversive activity by causing a greater change in burst rate.
Is there a differential role for bursts associated with contra- and ipsiversive gaze shifts?
Thus far, we showed that as gaze shifts become longer in duration, the bursts associated with both contraversive and ipsiversive saccades tend to be shifted toward the end of the movement. Indeed, the ends of their bursts are equally well timed with the end of a gaze shift, suggesting that neurons in both cFNs might be involved with insuring that the gaze saccade ends on time. Now we consider whether the average discharge patterns for similar ipsi- and contraversive movements support this suggestion or lead to other conclusions.
COMPARISON OF IPSI- AND CONTRAVERSIVE BURSTS FOR SIMILAR GAZE SACCADES.
For 15 of our 33 neurons, there were sufficient data to compare the unit activity associated with ≥10 ipsi- and contraversive large gaze shifts with similar metrics. For different neurons, mean gaze amplitudes varied from 50 to 70°, with average ranges about the means of ±6°. It was not always possible to find ipsi- and contraversive gaze shifts with identical metrics for all parameters. We began by identifying gaze shifts of nearly equal size and selected those with very similar initial peak velocities. Last, we tried to match gaze durations. For some units (e.g., Fig. 6E), it was possible to match the entire time course of the velocity profiles of ipsi- and contraversive saccades. When the match was not perfect, it usually failed because gaze shifts in one direction had longer decelerations than in the other (Fig. 6B). After we had identified matching ipsi- and contraversive gaze shifts, we convolved each action potential of their associated bursts with a 20 ms Gaussian function to produce smoothed spike density functions (SDFs) for each trial. We averaged the SDFs for the ipsi- and contraversive gaze shifts separately for each unit.
Figure 6 shows the raw rasters and the associated SDFs from two representative units recorded in different monkeys. As we reported for head-fixed saccades (Fuchs et al. 1993), although the gaze shifts were very similar, the related cFN bursts exhibited considerable variability from trial to trial (see rasters). Nevertheless, certain features of the SDFs were relatively consistent from trial to trial. Generally, the SDFs increased before the onset of a contraversive gaze shift and remained elevated throughout the movement (Fig. 6, C and F, red traces). In contrast, most ipsiversive gaze shifts were associated with a modest increase in activity early in the movement and showed their greatest discharge toward the end (Fig. 6, C and F, blue traces). The difference in neuronal behavior for the two directions is clear in the averages of all the SDFs (thick lines). This difference is highlighted by subtracting the average ipsiversive from the average contraversive SDFs (bottom panel, Contra-Ipsiversive curves).
To compare the net responses of all 15 neurons for ipsi- and contraversive gaze shifts, we calculated the population response in each direction. Even for gaze shifts with similar sizes and peak velocities, the durations of the movements varied substantially from animal to animal (Fig. 6, cf. B and E) and even from day to day in the same monkey. Therefore we normalized the response durations for individual neurons to produce the population response. First we determined the average gaze duration for the ipsi- and contraversive data sets for each neuron separately and normalized all averages to 100 ms. From these duration-normalized curves for each unit, we calculated separate grand averages of the neuronal activity associated with ipsi- and contraversive gaze shifts.
Figure 7 shows the averaged SDFs for each of the 15 neurons in the ipsi- and contraversive directions (adjacent curves with the same quality line). Clearly, the SDFs vary from unit to unit, but some features are qualitatively similar across units. In the ipsiversive direction, all neurons but one (last curve in 1st column) discharge their peak activity late in the movement before gaze ends; seven show a weaker increased activity by or at movement onset. In the contraversive direction, the SDFs are not as similar, but most (12/15) show an increase in activity by or at movement onset, followed by activity that is maintained or increases somewhat later in the gaze shift. The three lowermost neurons (2 obtained in the same monkey) show patterns that are opposite to these trends in one or both directions. The lowest thick curves show the population averages for all 15 neurons in each direction. Before a contraversive gaze shift, the population average begins to show an increase in activity before movement onset and continues the increased activity until the gaze shift ends. Toward the middle of the gaze shift, the cFN on the other side (as reflected by the ipsiversive population average) exhibits a net increase in activity, which is substantially reduced by the time that the gaze movement ends. Their anatomical connections suggest that the two cFNs contribute a differential signal to the brain stem burst generator during a gaze shift (Fig. 10 of Goffart et al. 2004). The lowest curve in the contraversive column, the difference between the contra- and ipsiversive population averages (Contra-Ipsi), is our estimate of that signal.
DIFFERENCES IN THE METRICS OF SMALL AND LARGE GAZE SHIFTS.
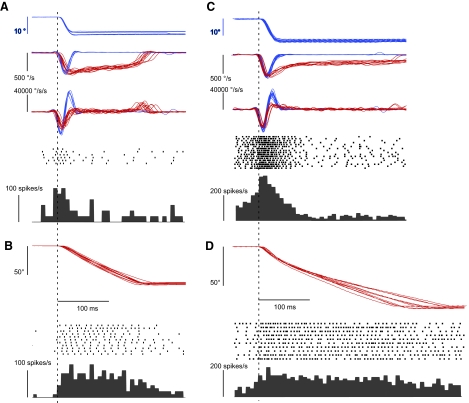
Our data show that the timing and shape of the cFN burst associated with contraversive gaze shifts seems to vary with the size of the movement (e.g., contraversive gaze shifts in Fig. 1). Is there a difference in the initial phase of large and small gaze shifts that could account for the difference? As we (Phillips et al. 1995) and others (McCluskey and Cullen 2007; Tomlinson and Bahra 1986) have shown, large simian gaze shifts have a very asymmetric velocity profile that is skewed toward a longer deceleration phase (also see our Fig. 6, B and E), whereas small gaze shifts have more bell-shaped velocity profiles with nearly equal acceleration and deceleration phases. In Fig. 8, we compare the discharge patterns associated with small and large gaze shifts that have been selected to have comparable early peak velocities. Both units (the examples in Fig. 6) discharged a vigorous burst near the onset of small (∼10°) contraversive saccades. For larger contraversive gaze shifts (60–80°) with matched peak velocities, the discharge near saccade onset was reduced for both neurons. Indeed, the discharge near saccade onset was less for large than small gaze shifts for 14 of the 15 cFN neurons with sufficient data to make the comparison.
Fig. 8.
Comparison of discharge patterns associated with small and large contraversive head-free gaze shifts for the exemplar neurons in Fig. 6. A and B: data from the unit shown in Fig. 6, D–F. C and D: data from the unit shown in Fig. 6, A–C. In A and C, traces from top to bottom are contraversive horizontal gaze position, velocity, and acceleration; the associated spike rasters and average response histograms are for the smaller gaze shifts only. All traces aligned on gaze position start time. The blue traces are position, velocity, and acceleration traces are for the smaller gaze shifts; the red traces are the position, velocity, and acceleration profiles of the larger shifts that are shown in B and D with their associated spike rasters and average response histograms. For each unit, trials for each gaze amplitude were selected to have similar peak velocities. Vertical dashed lines indicate gaze start time. Histogram bin widths are 8 ms.
This difference in peak rates was related to the acceleration of the gaze shift. Although for each unit the large and small amplitude datasets were matched for peak gaze velocities, those peaks usually were reached slightly earlier (see velocity traces in Fig. 8, A and C) for the smaller than for the larger gaze shifts. Across all 15 neurons, small gaze shifts (range: 6–14°) had an average acceleration of 30,037 ± 7,503°/s2 (n = 15), whereas large gaze shifts (range: 55–80°) had an average acceleration of only 18,323 ± 6,632°/s2. The accelerations were highly statistically different (P = 5 × 10−5, 2-sample t-test). In 14 of the 15 neurons, the maximum average firing rate that occurred in the interval from 10 ms before the start of the gaze shift to the time of peak gaze velocity, i.e., the initial peak burst rate, was greater for the smaller, higher acceleration, gaze shifts by a factor that ranged from 1.2 to 26 (average = 5.71 ± 8.27, n = 14). Therefore the early part of the discharge in a burst is strongly shaped by, or more likely helps determine, the acceleration of a contraversive gaze shift.
Gaze acceleration was greater for larger initial peak burst rates not only for the extreme case of relatively small and large gaze shifts, but also throughout the entire range of gaze shifts. To show this, we plotted the relation between peak gaze acceleration and the value of the spike density function at the time of peak acceleration (initial burst rate) for all movements with comparable peak gaze velocities. For all 14 cFN neurons considered above, the relation between gaze acceleration and initial burst rate was significant (P < 0.05). As expected, the remaining neuron was the one for which the relation between gaze acceleration and initial burst rate also had failed when only the two gaze shifts with very different sizes were considered.
DISCUSSION
Our data show that cFN neurons discharge a burst of spikes for large gaze shifts that require a head movement. However, neither the end of the head movement nor the time that it reaches its peak velocity is correlated with the end of either the ipsi- or contraversive burst. Multiple linear regression analyses show substantially better correlations of the features of the burst with gaze than head metrics for virtually every comparison. Nor is peak burst rate correlated with peak head velocity. Moreover, cFN neurons discharge vigorous bursts when a head-free gaze shift is made with no head contribution at all (Fig. 1, 10° gaze shifts). Therefore the cFN burst and head movements do not have an obligatory linkage. Instead, for all size gaze movements, the timing of the end of the burst is highly correlated with the end of the gaze shift (Fig. 4) and, for most neurons, also with the end of its eye movement component, which were difficult to distinguish in our monkeys. Therefore the most parsimonious conclusion is that, as with the head fixed, the cFN is involved with controlling the overall gaze shift or possibly its eye movement component. The cerebellar control of head movements is not routed through cFN burst neurons.
Our finding that the discharge of cFN neurons is not related to the metrics of the head movement supports the conclusion reached after inactivation (Quinet and Goffart 2007) and stimulation (Quinet and Goffart 2009) of the cFN. In this study, we deliberately examined visually elicited gaze shifts of ≥60° and ≤80°. Therefore our conditions not only required larger head movement components, but also a greater range of head movements than was obtained with their smaller gaze shifts. Even under these more optimal conditions, the properties of the burst always were much better correlated with gaze than head metrics.
Whether cFN activity is better related to the overall gaze shift or its eye movement component was difficult to determine from our unit data. For some monkeys, the eye component ends considerably before the gaze shift, making it possible to determine whether the firing rate is better timed with one or the other (Fuchs et al. 2005). Unfortunately, that fortuitous situation did not occur consistently for any of the monkeys in this study. Therefore, although all units showed high correlations of the end of the gaze shift with the end of the burst, correlations between the end of the eye movement were as good for 82 and 76% of the contra- and ipsiversive relations, respectively. Moreover, during large gaze shifts, the eye saccade is probably truncated by the counter-rotations of the vestibulo-ocular reflex, so our “weaker” correlations with the end of the eye saccade may have been caused, in part, by an inaccurate estimation of eye end time. Consequently, our data do not allow us to support or reject the suggestion that cFN neurons influence only oculomotor commands in primates (Quinet and Goffart 2005, 2007, 2009). A resolution of this issue will require unit recording during behavioral conditions that are specifically designed to divorce the usually tight coupling between the timing of the gaze movement and its eye component (Freedman and Sparks 1997b; Gandhi and Sparks 2001).
Influence of head movement on cFN discharge
Although the timing of the cFN burst does not seem to control head movement, the timing of the burst first began to be delayed relative to gaze onset when a head component occurred consistently. This can be appreciated in Figs. 2 and 3, where the trials above the horizontal arrows all had total head movements of ≥5°. For such gaze shifts, the eye movement develops an extended deceleration phase (e.g., Figs. 6 and 8). Possibly, for gaze shifts with significant head contributions, the oculomotor system uses a different control strategy, which involves a stretching of the eye component to allow the slower head component to make a contribution. In this scenario, the cFN would not be involved with generating or controlling the head movement per se, but a signal related to head movement would serve to extend the duration of a basically saccade-related signal. However, to our knowledge, there currently is no evidence that cFN neurons receive any signals related to head movement.
Muscimol injections into the cFN cause the amplitudes and timing of the eye and head components of gaze shifts to be affected in independent ways (Quinet and Goffart 2007). Taken at face value, this observation suggests that the cFN does not process a gaze signal because, in that case, the eye and head would be affected similarly. However, Quinet and Goffart (2007) concluded that there are “several lines of evidence indicating that the control of eye and head movements involves separate population of neurons in the fastigial nucleus.” They speculate that the changes in the head movement components are caused by diffusion of the muscimol to the rostral cFN, which contains neurons that discharge with head movements (Büttner et al. 1991; Siebold et al. 1997). This speculation would be consistent with our study, which shows no units in the cFN that discharge solely with head movements.
Extended deceleration phases also occur for large head-fixed saccades (Becker 1989), although they are much more prominent when the head is free to participate. Therefore it is unlikely that the prolongation of deceleration with the head free is caused solely by a head movement influence on cFN discharge. The cFN activity that continues through gaze deceleration also could partially originate in the SC and reach the oculomotor cerebellum via the nucleus reticularis tegmenti pontis (NRTP). For large gaze shifts, the duration of the SC burst continues to just before the end of large gaze shifts (Freedman and Sparks 1997a; e.g., their Fig. 11), probably because of feedback from the brain stem burst generator (Choi and Guitton 2006; Soetedjo et al. 2002).
In conclusion, although head movement seems to have an influence on the discharge of cFN neurons, the mechanism for such an effect is unknown.
Expanded role for the cFN in gaze shifts
In contrast to the good correlations of gaze end with burst end, gaze onset was poorly timed with burst onset for both ipsi- and contraversive gaze shifts over the entire range of gaze amplitudes in most of our units. The tighter timing of the burst with the end than the beginning of large contraversive gaze shifts seems to be at odds with earlier studies with the head fixed. In those studies, we (Fuchs et al. 1993) and others (Helmchen et al. 1994; Ohtsuka and Noda 1991) noted that the burst of cFN firing for most neurons (also see Scudder and McGee 2003) usually began either before or early in a contraversive saccade and more toward the middle of an ipsiversive saccade. Moreover, pharmacological inactivation of one cFN caused contraversive saccades to fall short of their targets, whereas ipsiversive saccades overshot them (Goffart et al. 2004; Robinson et al. 1993). Taken together, these data suggested a model in which one cFN helps to accelerate contraversive saccades, and the late burst of neurons in the other cFN is responsible for decelerating those saccades so they land accurately on target (Fuchs et al. 1993; Robinson et al. 1993). The data we present here suggest that this model does not adequately account for the unit behavior during head-free gaze shifts. We now consider our single unit data and population averages for contra- and ipsiversive gaze shifts separately and use the difference in population responses to suggest how the model may be expanded.
BURST FOR CONTRAVERSIVE GAZE SHIFTS.
Our results contribute three new insights about the role of the burst for contraversive gaze shifts: the initial part of the burst depends on gaze acceleration, the burst is continued throughout the velocity plateau of the gaze shift, and the burst ends near the end of the gaze shift. First, for 14 of 15 cFN neurons with sufficient data, we show directly that the early component of the contraversive burst is related to gaze acceleration. For gaze shifts matched for peak velocity, smaller gaze shifts reached higher average accelerations than did large gaze shifts. For these small gaze shifts with higher accelerations, the early maximum burst rate was substantially greater than that reached during larger gaze shifts, which have lower accelerations (Fig. 8). Moreover, the relation between peak gaze acceleration and the corresponding value of the SDF holds over the entire range of gaze shifts with the same peak velocities but varying amplitudes. Second, we report, as have others (McCluskey and Cullen 2007; Phillips et al. 1995), that large gaze shifts reach their targets by means of an extended gradual deceleration phase and that, during such velocity plateaus, there is an associated prolongation of burst activity in the cFN contralateral to the movement direction (Fig. 6, C and F, thick black traces; Fig. 7, average SDFs in lowest traces). Although there is variability from unit to unit and even trial to trial, the population average of the SDFs of our 15 neurons showed a firing rate profile that roughly recapitulated the velocity profiles, i.e., a modest initial peak followed by a plateau until gaze end. These observations suggest that the contraversive burst participates not only in the acceleration phase of a gaze shift but also helps to prolong the gaze shift throughout its deceleration phase.
The relations between contraversive gaze and burst metrics for most of our cFN neurons support our suggestion of this expanded role for the contraversive burst. Although others have described cFN neurons with similar correlations for head-fixed saccades (Ohtsuka and Noda 1991), we previously found few such neurons (Fuchs et al. 1993). However, in our previous study, we examined saccades ranging in amplitude from 5 to only 20°, whereas in this study, the range was much larger, often from 5 to 80°, making it easier to reveal subtle correlations. Our findings here that the relation of gaze duration with burst duration is not robust for gaze shifts that use little if any head movement is consistent with data reported by us (Fuchs et al. 1993) and others (Kleine et al. 2003) in the head-fixed monkey. However, it should be mentioned that the range of duration data when there is little head movement (Fig. 5, A and C, filled circles) was smaller than that when the head movement was large, and many of these small duration data were obtained from corrective saccades. In contrast, for larger gaze shifts requiring head movement contributions, burst duration does increase monotonically for almost all of our cFN neurons. For large gaze shifts therefore the population of cFN neurons might help control saccade duration and hence size by helping to maintain the late velocity plateau during the prolonged gaze deceleration.
BURST FOR IPSIVERSIVE GAZE SHIFTS.
Whereas neurons in the left cFN could help launch and continue a contraversive rightward gaze shift, those in the opposite right cFN, where the same saccade would be ipsiversive, would retain their previously hypothesized role as the saccade terminator. For larger gaze shifts, the burst for ipsiversive saccades continues to be well timed with the end of the gaze shift as it was for smaller gaze shifts, either those made with the head free (Figs. 1 to 3) or the head fixed (Fuchs et al. 1993; Ohtsuka and Noda 1991; Ohtsuka et al. 1994; Scudder and McGee 2003). Although the burst of some cFN neurons also is well correlated with ipsiversive gaze duration and occasionally with amplitude, they are much fewer in number than those with good relations with contraversive gaze shifts. Finally, the population of neurons in the opposite right cFN is not very active at gaze onset, but the population activity increases during the course of an ipsiversive movement to reach a peak before the end of the gaze saccade (Fig. 7, left column). The tight timing of the end of the burst with the end of the ipsiversive gaze shift (r > 0.82) occurred for 94% of individual cFN neurons. Therefore we suggest that the major role of the ipsiversive burst is to help terminate the gaze shift at the appropriate time. Of course, the tight timing of the end of the burst with the end of the contraversive gaze shift indicates that timely removal of that facilitation could also be important in saccade termination.
RATIONALE FOR USING POPULATION AVERAGES.
As with our past studies with the head fixed, we found a trial-to-trial variability in the discharge of a single unit and a variation in the discharge patterns from one unit to another (Fuchs et al. 1993). The trial-by-trial variability seems to make cFN neurons unsuitable for the precision control of saccade accuracy. Although more cFN units in this study showed good correlations between contraversive burst and gaze saccade metrics than did those in our previous head-fixed study, those correlations are, nevertheless, not as tight as those for the burst generator neurons to which they project. Moreover, they result, in part, because of the larger range of movement parameters when the head is free. However, lesions of the cFN not only produce saccade dysmetria but also greatly increase the variability of saccades to the same target steps (Robinson et al. 1993). Perhaps the trial-by-trial variability of cFN bursts reflects the cerebellum's attempt to compensate for variable signals that it receives from the SC.
Differences in the discharge patterns across our population of cFN neurons were present even after we calculated SDFs. The lowest three units in Fig. 7 are particularly egregious examples, displaying burst patterns that differ markedly from those of the other 12 in at least the ipsi- or contraversive direction. The cFN projects to both the SC (May et al. 1990) and the brain stem burst generator, so it is possible that units with qualitatively different discharge patterns constitute separate efferent populations. However, such a separation is rather arbitrary. Therefore we reasoned that the most objective way of determining the downstream influence of our cFN activity was to assume that all units contributed equally. Consequently, we determined both the contraversive and ipsiversive population averages across the 15 neurons described in Fig. 7. Such population averages also have been shown to improve the precision of the timing of the cFN burst (Scudder and McGee 2003).
We used the population averages (Fig. 7) to support our proposal that the bursts associated with contra- and ipsiversive gaze shifts play different roles. Moreover, because the two cFNs send crossed projections to the brain stem burst generator, their net influence can be taken as the difference in the population averages (Contra-Ipsi curve in Fig. 7). Clearly, the net cFN signal that reaches the burst generator must produce a coherent effect to account for the havoc its absence wreaks on head-fixed saccades. This difference signal drops to zero before gaze end and so may help explain the conundrum that the burst in some cFN units continues beyond the end of the gaze shift, whose termination, we hypothesized, the cFN controls.
Suggested relative roles of the cFN and SC in gaze shifts
Previous papers have discussed the established connections from the SC both directly to neurons of the burst generator and indirectly via the nucleus reticularis tegmenti pontis (NRTP) and the midline cerebellum (for summary, see Scudder et al. 2002). We suggest that the principal role of SC input to the burst generator is to initiate the gaze shift and to provide a continuing drive that alone is insufficient to extend the deceleration phase of larger gaze shifts. Based on our data, the expanded role of the cFN is to aid primarily in the acceleration of small gaze shifts, to boost the SC drive during the deceleration of large gaze shifts to maintain their velocities, and to remove that boost near the end of the gaze shift to help stop it in time. For small gaze shifts, we suggest that the excitatory drive from the SC is insufficient to produce their high accelerations, and therefore the cFN must supplement that drive. cFN neurons consistently show their largest burst during the acceleration phase of small saccades (e.g., Fig. 8, middle rasters and histograms). For large gaze shifts, the cFN discharge during acceleration is relatively smaller (Fig. 8, lowest rasters and histograms), so the supplement it provides is much weaker. Therefore even though larger gaze shifts can reach similar peak velocities, they require more time to do it, i.e., they accelerate more slowly. As the larger gaze shifts continue past peak velocity, it seems that the SC contribution late in the gaze shift is inadequate to maintain the lengthened deceleration phase, and the cFN must be mobilized to help sustain the deceleration.
To date, there are few studies that support or refute our “model,” which should be considered with at least two caveats. First, it is possible that the activity of SC neurons alone could account for the difference in accelerations of large and small gaze shifts. It is likely that burst neurons in the rostral SC, which is responsible for small gaze shifts, discharge at higher peak rates than do those in the caudal SC, where large gaze shifts are represented. However, projections from the rostral SC to the brain stem also are sparser than those from the caudal SC (Moschovakis et al. 1998), so it is unclear whether the actual SC drive to the burst generator actually differs during gaze shifts of different size. Second, the cFN sends a modest projection back to the SC so they also could influence each other via a feedback loop. However, it is unclear whether the cFN innervates only the rostral zone (May et al. 1990) or the more caudal regions as well (Batton et al. 1977). Indeed, during behaviorally induced adaptations of saccade amplitude, changes in discharge patterns occur not only in many cFN neurons (Inaba et al. 2003; Scudder and McGee 2003) but also in most SC burst neurons as well (Takeichi et al. 2007). Currently, there is insufficient information to warrant speculation about the role of the cFN feedback to the SC in saccade generation.
EFFECTS OF BILATERAL cFN INACTIVATION.
If the only two drives to the BG originate in the SC and cFN, bilateral inactivation of the cFN should show the contribution of the SC in isolation. Bilateral injections have been performed only in head-fixed monkeys, and the two cFNs probably were not disabled equally (Robinson et al. 1993). Nonetheless, saccades in both horizontal directions became hypermetric (saccades ipsiversive to the first side injected were more affected; Robinson et al. 1993). Moreover, the accelerations and decelerations of hypermetric saccades to 10° target steps in both directions were significantly lower than normetric 10° targeting saccades (Robinson et al. 1993). Taken together, these data support the suggestion that the cFN is required for the normal acceleration of small saccades. Also, for small saccades, it is the absent braking activity in the opposite cFN that seems primarily responsible for hypermetric small gaze shifts. Therefore we suggest that, for small head-fixed gaze shifts, the saccade command from the SC, if acting without influence of the cFN, produces a saccade that would overshoot the target. With the head free, bilateral inactivation would eliminate both the contralateral activity, which we suggest is involved with maintaining gaze deceleration and also the braking activity provided by the opposite cFN. In this circumstance, we might expect the absence of contralateral activity to lead to shortened (smaller) gaze shifts but the absence of braking activity to lengthened (larger) gaze shifts. These opposite expectations have not yet been tested, because there are no studies that examine the effect of bilateral cFN inactivation on large head-free gaze shifts. After unilateral inactivation, however, contraversive head-free gaze shifts to 40° target steps become hypometric and ipsiversive gaze shifts become hypermetric (Quinet and Goffart 2005, 2007), just as for smaller head-fixed saccades (Goffart et al. 2003; Iwamoto and Yoshida 2002; Ohtsuka et al. 1994; Robinson et al. 1993).
GRANTS
This study was supported by National Institutes of Health (NIH) Grants EY-00745 to A. F. Fuchs and RR-00166 from the National Center for Research Resources (NCRR), a component of the NIH.
ACKNOWLEDGMENTS
We thank M. Ibarreta for the daily experimental contributions, B. Cent for the computer programs, and the animal resources division of the WaNPRC for extraordinary support. We also thank R. Soetedjo for producing Fig. 1. We received valuable comments from S. Bierer, E. Buzunof, C.R.S. Kaneko, Y. Kojima, A. Mueller, J. Phillips, F. Robinson, R. Soetedjo, and A. Weiss on the first version of this manuscript. Finally, we appreciate the efforts of the three reviewers whose critiques made this paper much better than it was.
REFERENCES
- Barash S, Melikyan A, Sivakov A, Zhang M, Glickstein M, Thier P. Saccadic dysmetria and adaptation after lesions of the cerebellar cortex. J Neurosci 19: 10931–10939, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batton R, Jayaraman A, Ruggiero D, Carpenter M. Fastigial efferent projections in the monkey: an autoradiographic study. J Comp Neurol 174: 281–306, 1977 [DOI] [PubMed] [Google Scholar]
- Becker W. Metrics. In: The Neurobiology of Saccadic Eye Movements, edited by Wurtz B, Goldberg M. Amsterdam: Elsevier, 1989, p. 13–67 [PubMed] [Google Scholar]
- Brettler S, Fuchs A. Activity of caudal fastigial nucleus neurons during head-unrestrained gaze shifts in rhesus monkeys. Soc Neurosci Abstr 27: 405.11, 2001 [Google Scholar]
- Brettler S, Fuchs A, Ling L. Discharge patterns of cerebellar output neurons in the caudal fastigial nucleus during head-free gaze shifts in primates. Ann NY Acad Sci 1004: 61–68, 2003 [PubMed] [Google Scholar]
- Büttner U, Fuchs A, Markert-Schwab G, Buckmaster P. Fastigial nucleus activity in the alert monkey during slow eye movements and head movements. J Neurophysiol 65: 1360–1371, 1991 [DOI] [PubMed] [Google Scholar]
- Choi WY, Guitton D. Responses of collicular fixation neurons to gaze shift perturbations in head-unrestrained monkey reveal gaze feedback control. Neuron 50: 491–505, 2006 [DOI] [PubMed] [Google Scholar]
- Freedman E. Interactions between eye and head control signals can account for movement kinematics. Biol Cybern 84: 453–462, 2001 [DOI] [PubMed] [Google Scholar]
- Freedman E, Sparks D. Activity of cells in the deeper layers of the superior colliculus of the rhesus monkey: evidence for a gaze displacement command. J Neurophysiol 78: 1669–1690, 1997a [DOI] [PubMed] [Google Scholar]
- Freedman E, Sparks D. Eye-head coordination during head-unrestrained gaze shifts in rhesus monkeys. J Neurophysiol 77: 2328–2348, 1997b [DOI] [PubMed] [Google Scholar]
- Fuchs A, Robinson D. A method for measuring horizontal and vertical eye movements chronically in the monkey. J Appl Physiol 21: 1068–1070, 1966 [DOI] [PubMed] [Google Scholar]
- Fuchs A, Ling L, Phillips J. Behavior of the position vestibular pause (PVP) interneurons of the vestibuloocular reflex during head-free gaze shifts in the monkey. J Neurophysiol 94: 4481–4490, 2005 [DOI] [PubMed] [Google Scholar]
- Fuchs A, Robinson F, Straube A. Role of the caudal fastigial nucleus in saccade generation. I. Neuronal discharge patterns. J Neurophysiol 70: 1723–1740, 1993 [DOI] [PubMed] [Google Scholar]
- Gandhi R, Sparks D. Experimental control of eye and head positions prior to head-unrestrained gaze shifts in monkey. Vis Res 41: 3243–3254, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffart L, Chen L, Sparks D. Saccade dysmetria during functional perturbation of the caudal fastigial nucleus in the monkey. Ann NY Acad Sci 1004: 220–228, 2003 [DOI] [PubMed] [Google Scholar]
- Goffart L, Chen L, Sparks D. Deficits in saccades and fixation during muscimol inactivation of the caudal fastigial nucleus in the Rhesus monkey. J Neurophysiol 92: 3351–3367, 2004 [DOI] [PubMed] [Google Scholar]
- Helmchen C, Straube A, Büttner U. Saccade-related activity in the fastigial oculomotor region of the macaque monkey during spontaneous eye movements in light and darkness. Exp Brain Res 98: 474–482, 1994 [DOI] [PubMed] [Google Scholar]
- Inaba N, Iwamoto Y, Yoshida K. Changes in cerebellar fastigial burst activity related to saccadic gain adaptation in the monkey. Neurosci Res 46: 359–368, 2003 [DOI] [PubMed] [Google Scholar]
- Iwamoto Y, Yoshida K. Saccadic dysmetria following inactivation of the primate fastigial oculomotor region. Neurosci Lett 325: 211–215, 2002 [DOI] [PubMed] [Google Scholar]
- Keller E, Gandhi N, Shieh J. Endpoint accuracy in saccades interrupted by stimulation in the omnipause region in monkey. Vis Neurosci 13: 1059–1067, 1996 [DOI] [PubMed] [Google Scholar]
- Kleine J, Guan Y, Büttner U. Saccade-related neurons in the primate fastigial nucleus: what do they encode? J Neurophysiol 90: 3137–3154, 2003 [DOI] [PubMed] [Google Scholar]
- May P, Hartwich-Young R, Nelson J, Sparks D, Porter J. Cerebellotectal pathways in the macaque: implications for collicular generation of saccades. Neuroscience 36: 305–325, 1990 [DOI] [PubMed] [Google Scholar]
- McCluskey M, Cullen K. Eye, head and body coordination during large gaze shifts in Rhesus monkeys: movement kinematics and the influence of posture. J Neurophysiol 97: 2976–2991, 2007 [DOI] [PubMed] [Google Scholar]
- Moschovakis A, Kitama T, Dalezios Y, Petit J, Brandi A, Grantyn A. An anatomical substrate for the spatiotemporal transformation. J Neurosci 18: 10219–10229, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moschovakis A, Scudder C, Highstein S. The microscopic anatomy and physiology of the mammalian saccadic system. Prog Neurobiol 50: 133–254, 1996 [DOI] [PubMed] [Google Scholar]
- Noda H, Murakami S, Yamada J, Tamaki Y, Aso T. Saccadic eye movements evoked by microstimulation of the fastigial nucleus of macaque monkeys. J Neurophysiol 60: 1036–1052, 1988 [DOI] [PubMed] [Google Scholar]
- Noda H, Sugita S, Ikeda Y. Afferent and efferent connections of the oculomotor region of the fastigial nucleus in the Macaque monkey. J Comp Neurol 302: 330–348, 1990 [DOI] [PubMed] [Google Scholar]
- Ohtsuka K, Noda H. Saccadic burst neurons in the oculomotor region of the fastigial nucleus of macaque monkeys. J Neurophysiol 65: 1422–1434, 1991 [DOI] [PubMed] [Google Scholar]
- Ohtsuka K, Sato H, Noda H. Saccadic burst neurons in the fastigial nucleus are not involved in compensating for orbital nonlinearities. J Neurophysiol 71: 1976–1980, 1994 [DOI] [PubMed] [Google Scholar]
- Optican L, Robinson D. Cerebellar-dependent adaptive control of primate saccadic system. J Neurophysiol 44: 1058–1079, 1980 [DOI] [PubMed] [Google Scholar]
- Phillips J, Ling L, Fuchs A, Siebold C, Plorde J. Rapid horizontal gaze movement in the monkey. J Neurophysiol 73: 1632–1651, 1995 [DOI] [PubMed] [Google Scholar]
- Quinet J, Goffart L. Saccade dysmetria in head-unrestrained gaze shifts after muscimol inactivation of the caudal fastigial nucleus in the monkey. J Neurophysiol 93: 2343–2349, 2005 [DOI] [PubMed] [Google Scholar]
- Quinet J, Goffart L. Head-unrestrained gaze shifts after muscimol injection in the caudal fastigial nucleus of the monkey. J Neurophysiol 98: 3269–3283, 2007 [DOI] [PubMed] [Google Scholar]
- Quinet J, Goffart L. Electrical microstimulation of the fastigial oculomotor region in the head-unrestrained monkey. J Neurophysiol 102: 320–336, 2009 [DOI] [PubMed] [Google Scholar]
- Ritchie L. Effects of cerebellar lesions on saccadic eye movements. J Neurophysiol 39: 1246–1256, 1976 [DOI] [PubMed] [Google Scholar]
- Robinson D. A method of measuring eye movement using a scleral search coil in a magnetic field. IEEE Trans Biomed Electron BME 10: 137–145, 1963 [DOI] [PubMed] [Google Scholar]
- Robinson D. Oculomotor control signals. In: Basic Mechanisms of Ocular Motility and Their Clinical Implications, edited by Lennerstrand G, Bach-y-Rita P. Oxford, UK: Pergamon, 1975, p. 337–374 [Google Scholar]
- Robinson F, Straube A, Fuchs A. Role of the caudal fastigial nucleus in saccade generation. II. Effects of muscimol inactivation. J Neurophysiol 70: 1741–1758, 1993 [DOI] [PubMed] [Google Scholar]
- Rosner B. Hypothesis testing: two sample inference. In: Fundamentals of Biostatistics Belmont, CA: Wadsworth, 1995, p. 264–270 [Google Scholar]
- Sato H, Noda H. Saccadic dysmetria induced by transient functional decortication of the cerebellar vermis. Exp Brain Res 88: 455–458, 1992 [DOI] [PubMed] [Google Scholar]
- Scudder C, Kaneko C, Fuchs A. The brainstem burst generator for saccadic eye movements: a modern synthesis. Exp Brain Res 142: 439–462, 2002 [DOI] [PubMed] [Google Scholar]
- Scudder C, McGee D. Adaptive modification of saccade size produces correlated changes in the discharges of fastigial nucleus neurons. J Neurophysiol 90: 1011–1026, 2003 [DOI] [PubMed] [Google Scholar]
- Scudder C, McGee D, Balaban C. Connections of monkey saccade-related fastigial nucleus neurons revealed by anatomical and physiological methods. Soc Neurosci Abstr 26: 971, 2000 [Google Scholar]
- Siebold C, Glonti L, Glasauer S, Büttner U. Rostral fastigial nucleus activity in the alert monkey during three-dimensional passive head movements. J Neurophysiol 77: 1432–1446, 1997 [DOI] [PubMed] [Google Scholar]
- Soetedjo R, Kaneko C, Fuchs A. Evidence that the superior colliculus participates in the feedback control of saccadic eye movements. J Neurophysiol 87: 679–695, 2002 [DOI] [PubMed] [Google Scholar]
- Sparks D. Conceptual issues related to the role of the superior colliculus in the control of gaze. Curr Opin Neurobiol 9: 698–707, 1999 [DOI] [PubMed] [Google Scholar]
- Sylvestre P, Cullen K. Premotor correlates of integrated feedback control for eye-head gaze shifts. J Neurosci 26: 4922–4929, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi J, Zee D, Tamargo R. Effects of lesions of the oculomotor vermis on eye movements in primate: saccades. J Neurophysiol 80: 1911–1931, 1998 [DOI] [PubMed] [Google Scholar]
- Takeichi N, Kaneko C, Fuchs A. Changes in activity of burst neurons in the superior colliculus during saccade adaptation in the monkey. J Neurophysiol 97: 4096–4107, 2007 [DOI] [PubMed] [Google Scholar]
- Tomlinson R, Bahra P. Combined eye-head gaze shifts in the primate. I. Metrics. J Neurophysiol 56: 1542–1557, 1986 [DOI] [PubMed] [Google Scholar]
- Vilis T, Hore J. Characteristics of saccadic dysmetria in monkeys during reversible lesions of medial cerebellar nuclei. J Neurophysiol 46: 828–839, 1981 [DOI] [PubMed] [Google Scholar]
- Yamada J, Noda H. Afferent and efferent connections of the oculomotor cerebellar vermis in the macaque monkey. J Comp Neurol 265: 224–241, 1987 [DOI] [PubMed] [Google Scholar]
- Zee D, Optican L, Cook J, Robinson D, King Engel W. Slow saccades in spinocerebellar degeneration. Arch Neurol 33: 243–251, 1976 [DOI] [PubMed] [Google Scholar]