Abstract
Blood oxygen level dependent–functional magnetic resonance imaging (BOLD–fMRI) and magnetoencephalographic (MEG) signals are both coupled to postsynaptic potentials, although their relationship is incompletely understood. Here, the wide range of BOLD–fMRI and MEG responses produced by auditory cortex was exploited to better understand the BOLD–fMRI/MEG relationship. Measurements of BOLD and MEG responses were made in the same subjects using the same stimuli for both modalities. The stimuli, 24-s sequences of click trains, had duty cycles of 2.5, 25, 72, and 100%. For the 2.5% sequence, the BOLD response was elevated throughout the sequence, whereas for 100%, it peaked after sequence onset and offset and showed a diminished elevation in between. On the finer timescale of MEG, responses at 2.5% consisted of a complex of transients, including N1m, to each click train of the sequence, whereas for 100% the only transients occurred at sequence onset and offset between which there was a sustained elevation in the MEG signal (a sustained field). A model that separately estimated the contributions of transient and sustained MEG signals to the BOLD response best fit BOLD measurements when the transient contribution was weighted 8- to 10-fold more than the sustained one. The findings suggest that BOLD responses in the auditory cortex are tightly coupled to the neural activity underlying transient, not sustained, MEG signals.
INTRODUCTION
Postsynaptic potentials (PSPs) are thought to drive much of the blood oxygen level dependent (BOLD) response, which can be mapped with millimeter spatial resolution by functional magnetic resonance imaging (fMRI) (Logothetis et al. 2001). They are also the source of the high temporal-resolution signals detected with magnetoencephalography (MEG; Creutzfeld et al. 1966; Hämäläinen et al. 1993). The common activity (PSPs) underlying both BOLD–fMRI and MEG signals and the differential sensitivity of these techniques for the spatial and temporal details of this activity, respectively, imply that a composite and highly informative view of postsynaptic brain activity may be obtained by “fusing” fMRI and MEG data, to benefit from both. Although PSPs likely underlie both BOLD and MEG responses, there are a number of factors that preclude a simple relationship between the two modalities. For example, significant uncertainty stems not only from cancellation of excitatory and inhibitory PSPs (EPSPs and IPSPs) in MEG, but also from cancellation between neighboring sites, depending on details of the individual anatomy (Ahlfors and Simpson 2004). Currently, there is no explicit model to relate BOLD–fMRI and MEG responses at the macroscopic level, leaving considerable uncertainty for approaches aiming at their integration.
The present study examined the relationship between BOLD–fMRI and MEG by comparing BOLD and MEG signal time courses in the auditory cortex (AC). In response to a prolonged sequence of sound stimuli, the BOLD response in AC can range from being elevated throughout the sequence to highly phasic, with prominent peaks after sequence onset and offset (Harms and Melcher 2002). These response patterns depend on temporal parameters of the sequence, such as the interval between stimuli comprising the sequence (interstimulus interval [ISI]), the duration of the individual stimuli, or stimulus repetition rate (Giraud et al. 2000; Harms and Melcher 2002; Harms et al. 2005). These factors also influence MEG signals from AC (Hari et al. 1982; Imada et al. 1997) that, like BOLD responses, comprise both sustained elevations and transients (Hari et al. 1980; Pantev et al. 1996), but on a much finer temporal scale. The rich variety of BOLD and MEG responses to sounds makes the auditory cortex an excellent test bed to study the relationship between the two. Although a few studies have compared MEG and fMRI measures from AC (Ahveninen et al. 2007; Gutschalk et al. 2007; Jääskeläinen et al. 2004; Lin et al. 2004; Mathiak et al. 2002; Woldorff et al. 1999), none has exploited the uniquely wide variety of waveforms produced by this brain area.
In this study, BOLD–fMRI and MEG were measured in the same subjects using the same stimuli so the two types of data could be directly compared. Stimuli were sequences of click trains, which are known to produce robust responses in both MEG and fMRI (e.g., Gutschalk et al. 2004; Harms et al. 2005). To produce a wide variety of BOLD time courses and MEG source waveforms, the click trains were presented in sequences with four different duty cycles controlled by adjusting ISI and train duration. The results indicate that transient, rather than sustained, MEG activity is predominantly reflected in the BOLD signal time courses.
METHODS
Subjects
We report data from six subjects (three women, three men; mean age: 27.7 yr; range: 20–35 yr). Subjects provided written informed consent before their participation. The study was approved by the review boards of Massachusetts Eye and Ear Infirmary, Massachusetts General Hospital, and Massachusetts Institute of Technology.
Stimulus presentation
The stimuli were presented in a block paradigm during both fMRI and MEG; 24 s of binaural sound stimulation were alternated with silent periods of equal duration. Stimulus level at the ear was 75 dB SPL. During fMRI, stimuli were presented via piezoelectric headphones (BAE Systems Electronics [formerly Marconi Electronic Systems], Farnborough, UK). The scanner's coolant pump was switched off during fMRI to eliminate the background acoustic noise it produces (Ravicz and Melcher 2001). For MEG, the stimuli were delivered via foam earpieces connected to 3-m-long plastic tubes. The sound was created by a speaker system outside of the magnetically shielded room (ADU1b; Unides, Helsinki, Finland). During both MEG and fMRI, subjects watched a self-selected silent movie without subtitles and were asked not to attend to the auditory stimuli.
Eight click trains were presented during each 24-s stimulation period, resulting in a stimulus onset asynchrony of 3 s. Train duration, held constant during any given 24-s period, was either 75, 750, or 2,150 ms. The clicks of each train were presented at a rate of 500 Hz. An additional stimulus condition comprised a continuous, 24-s train of 500-Hz clicks. Thus there were four stimulus conditions with duty cycles of 2.5, 25, 72, or 100%. The stimulus conditions, separated from one another by 24-s periods of no stimulation, were presented in pseudorandomized order.
The stimuli for this experiment were generated at a 24-kHz sampling rate. The duration of each click was one sample. Each generated 24-s sequence was low-pass filtered at 10 kHz (high-frequency roll-off: 18 dB/octave).
Data acquisition
MRI data were acquired with a 3-T head-and-neck scanner (Magnetom Allegra, Siemens, Erlangen, Germany), equipped with a standard, bird-cage head coil. First, two whole-head anatomical magnetization-prepared rapid gradient echo (MP-RAGE) sequences were acquired (sagittal in-plane resolution = 256 × 256; field of view [FOV] = 256 × 256 mm; slice thickness = 1.3 mm). Based on these images, the volume for functional imaging was chosen as 11 near-coronal slices (perpendicular to the Sylvian fissure) covering auditory cortex from the posterior end of planum temporale to the anterior aspect of the superior temporal gyrus, including Heschl's gyrus in both hemispheres. For coregistration, T2-weighted anatomical images were obtained for the same volume with a high in-plane resolution (387 × 387; FOV = 200 × 200 mm). Functional imaging was performed using an echo-planar imaging sequence (gradient echo; echo time [TE] = 30 ms; flip angle = 90°; in-plane resolution = 64 × 64; FOV = 200 × 200 mm; slice thickness = 4 mm; gap = 1.32 mm). The repetition time (TR) between functional acquisitions was 8 s and the acquisitions were compressed into approximately 1-s-long clusters to prevent effects of imager acoustic noise on brain activation (Edminster et al. 1999; Hall et al. 1999). To allow for the reconstruction of the time course of activation, the timing of image acquisition relative to the sound stimulus was staggered by 2 s across stimulation periods (Belin et al. 1999).
MEG data were acquired with a Neuromag Vectorview system (Elekta Neuromag Oy, Helsinki, Finland), which comprises 204 planar gradiometers and 102 magnetometers at 102 positions, equally spaced around the head. The system is housed in a six-layer magnetically shielded room with active shielding (Cohen et al. 2002). The data were sampled continuously at 600 Hz with a 160-Hz low-pass filter and without high-pass filtering (direct coupled [DC]). The data were averaged across all presentations of a given condition, excluding epochs with amplitudes exceeding 5,000 fT/cm (considered artifact). Prior to MEG, four head position indicator (HPI) coils were fixed to the subject's head and their location was digitized relative to landmarks on the head surface. Positions of the HPI coils were detected by the Neuromag system at the beginning of the MEG session, allowing registration of the location and orientation of the head with respect to the MEG sensor array. Eye movements were recorded from four electrodes fixed to the outer canthi and above and below the left eye.
Data analysis
Activation maps for the fMRI data were based on a multivariate, linear regression analysis using basis functions designed to capture the range of BOLD response time courses produced by auditory cortex (Harms and Melcher 2003). All epochs of stimulation were contrasted with epochs of no stimulation. The resulting activation maps were coregistered with the whole-head T1-weighted volumes, which were processed with Freesurfer (Athinoula A. Martinos Center for Biomedical Imaging, Charlestown, MA) to create an inflated projection of the cortical surface (Fischl et al. 1999). The analysis was restricted to the AC; voxels within this region of interest (ROI) that were significantly activated (P < 0.001; not corrected for multiple comparisons) were used to compute activation time courses after excluding any voxels with a signal change exceeding ±8% (these were considered artifactual). Grand-average time courses were subsequently generated by averaging the time courses of each single subject.
In the source analysis of the MEG data, we used a single-compartment boundary-element forward model of the head, with the inner skull surface reconstructed from the MP-RAGE data (Hämäläinen and Sarvas 1989). Two dipoles were fit iteratively and simultaneously using XFit software (Elekta Neuromag Oy), starting with the two dipoles positioned symmetrically about the midplane in the left and right superior temporal lobe, respectively. No constraints for symmetry were used in the iterative fitting procedure. The final dipole solutions were then used to define a spatial filter to derive source waveforms over the whole 24-s epoch (Scherg 1990). The spatial filter was defined by dipoles fit to the N1m in a grand average across the individual trains of all stimulus conditions and across presentations. The dipole positions and orientations were fit for 15-ms time windows centered on the peak of each N1m waveform. The dipoles were fit to the N1m because it provided better signal-to-noise ratio than that of other alternatives and accordingly the most stable estimates dipole location. Dipole locations for the N1m and sustained fields were not systematically different from each other. Importantly, there were only minor variations of relative source magnitudes for N1m and sustained fields, depending on whether N1m or sustained-field dipoles were used. This insensitivity to precise dipole fit is in agreement with previous MEG studies using click train stimuli, where the N1m and the sustained field generally showed similar source configurations, such that decompositions based on the N1m and the sustained field result in similar source waveforms (Gutschalk et al. 2004). Once a spatial filter was determined for a given subject and experiment, it was used for all stimulus conditions of a data set. Grand-average waveforms were generated by averaging the source waveforms obtained separately in each single subject. For side-to-side presentation with the fMRI activation, dipole positions were displayed on the cortical surface using MNE software (Athinoula A. Martinos Center for Biomedical Imaging).
Low-frequency, external artifacts in the magnetometers were corrected with a signal-subspace projection (SSP), including three orthogonal topographies corresponding approximately to the three components of homogeneous magnetic fields as detected by the magnetometers. These topographies were also included in the inverse matrix of the two-dipole model. Additionally, drifts in the baseline epoch succeeding each stimulus of a sequence were modeled by SSP, including one topography for gradiometers and another, similarly defined topography for magnetometers. This correction of slow artifacts assumes that the activity always returns to baseline within about 500 ms. The validity of this assumption was supported not only by the poststimulus return to baseline of signals averaged across the individual stimuli of a sequence, but also by similar previous data from other investigators (Lammertmann and Lütkenhöner 2001; Pantev et al. 2004; Picton et al. 1978). Finally, the topographies removed were not dipolar and not well explained by the AC source model. For the continuous, 24-s stimulus (100%), an additional SSP was applied only at the end of the whole block, in the interval 2–5 s after stimulus offset.
Models of the BOLD time courses
For a quantitative comparison of BOLD and MEG measurements, the rectified MEG source waveforms were convolved with a standard hemodynamic response function (HRF). We compared two HRFs: HRF 1 was adapted to match earlier BOLD fMRI studies of human AC activation (Glover et al. 1999; Harms and Melcher 2002; Robson et al. 1998). This HRF had a peak latency of 4 s that was then followed by a negative undershoot with trough latency of 12 s. The amplitude of the negative undershoot was 20% of the positive peak. HRF 2 was a gamma function, as in Dale and Buckner (1997), with delta = 2.25 s and tau = 1.25 s, leading to a peak latency of 4 s. This HRF comprised only positive values.
To evaluate the differential contribution of transient and sustained components, the MEG response to individual stimuli, i.e., individual click trains, was divided into epochs that included: (A) the onset response, (B) the sustained response, (C) the offset response, and (D) the period after the offset response to a click train and before the onset response to the next. The epochs were overlapping and multiplied with 50-ms raised cosine windows at their intersections. The off epoch, during which the MEG noise fluctuates around the baseline level, was set to zero and not included in the fit because we considered its contribution implausible. The offset-response epoch was additionally high-pass filtered at 3 Hz (24 dB, zero-phase shift Butterworth filter) to help separate the offset response from the sustained field. The contribution of the components was reconstructed with a least-squares fit, minimizing the difference between the simulated BOLD response and the measured one. For plausibility, the fit was constrained to positive weights.
RESULTS
BOLD activation maps and MEG source locations in auditory cortex
Maps of significant BOLD activity, shown for each subject in Fig. 1, revealed widespread activation in the AC, including Heschl's gyrus, planum temporale, the circular sulcus, and the superior temporal gyrus. MEG dipole locations are superimposed on BOLD activation maps for each hemisphere and subject in Fig. 1. The dipole locations generally fall in AC, but are variably located in either Heschl's gyrus or planum temporale. This variability may be related to the fact that the widespread activation in these cases includes gray matter with a range of orientations, different orientations across subjects, resulting in changes in the “center of mass” of activity reflected by the net dipole fit.
Fig. 1.
BOLD activation maps and MEG source location for individual subjects. Each panel shows inflated views of the left and right hemispheres of a given subject. Light and dark gray indicate portions of the cortical surface lying within gyri and on sulci, respectively. Bold activation maps (color) are based on a contrast between all stimulus conditions combined and epochs without stimulation. In the maps, significance of the difference between conditions is coded on a red (P ≤ 0.001) to yellow (P ≤ 0.00001) scale. The activation is largely confined to Heschl's gyrus (HG), planum temporale (PT), the circular sulcus, and the superior temporal gyrus (STG), the regions for which BOLD time courses were analyzed. The locations of current dipoles fit to the MEG data are indicated with white circles. BOLD, blood oxygenation level–dependent; MEG, magnetoencephalographic.
Dependence of BOLD time courses on duty cycle and stimulus type
The time course of the BOLD response for each stimulus condition is shown in Fig. 2. Since the time courses showed only small systematic differences across anatomically defined ROIs within the AC, Fig. 2 shows only time courses for the whole AC. Note that time courses for the various stimulus conditions were all calculated from the same voxels, that is, those showing activation in Fig. 1.
Fig. 2.
Time courses of the BOLD responses in auditory cortex to each stimulus sequences with different duty cycles, indicated by the percentages above each panel. The gray shading indicates the 24-s duration of each sequence. The individual click trains comprising each sequence are depicted near the bottom of the gray shading. Time courses (% signal change vs. time) were determined for the significantly activated voxels of each hemisphere, then averaged across hemispheres and subjects to yield the thick trace in each panel. Thin traces indicate the mean ± 1SE. Importantly, the same voxels were used to calculate the time courses for all of the stimuli of the experiment for a given subject.
The data in Fig. 2 illustrate progressive changes in the BOLD time course with increasing stimulus duty cycle. The time course for the condition with the shortest click trains (resulting in a sequence with the lowest duty cycle: 2.5%) is elevated throughout the 24-s duration of the sequence with only a small peak shortly after sequence onset. Conversely, the continuous 24-s click train (duty cycle = 100%) produces a highly phasic time course with prominent peaks just after the onset and offset of the sound, with a comparatively weak (<1% signal change) intervening sustained elevation in BOLD signal. The 25 and 72% duty-cycle conditions show time courses between the two extremes defined by the 2.5 and 100% duty-cycle time courses. The sustained elevation in signal is slightly stronger for the 72% duty cycle, but the difference is subtle. The shape of the time courses for a given duty cycle was generally very reproducible across subjects, as reflected by the small standard errors in Fig. 2.
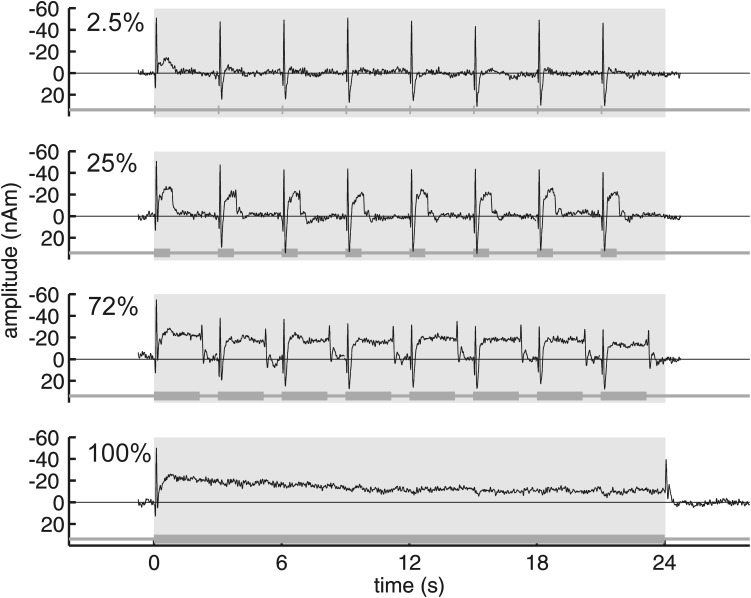
Dependence of MEG source waveforms on duty cycle and stimulus type
MEG source waveforms for the same stimuli and subjects used for fMRI are shown in Fig. 3. Each trace shows the entire response to a 24-s-long stimulus averaged over subjects and hemispheres. The MEG source waveforms, like the BOLD time courses, show clear changes with increasing stimulus duty cycle. For instance, the 2.5% duty-cycle condition shows a transient response to each of the eight click trains comprising the 24-s stimulus with the signal returning to baseline from one response to the next. In contrast, the 100% duty-cycle condition (clicks throughout the entire 24 s of the stimulus) produced a long sustained field, with the only transients being at the onset and after the offset of the clicks. In the case of the 25 and 72% duty cycles, a transient onset response, sustained field, and offset response are observed for each of the click trains of the sequence.
Fig. 3.
MEG source waveforms as a function of duty cycle as indicated by the percentage to the left of each waveform. The source waveforms are an average across hemispheres and subjects. An indication of SE has been omitted for clarity. A schematic stimulus is shown below each trace. The gray shading marks the time interval 0–24 s for a convenient comparison with the BOLD data in Fig. 2.
A detailed analysis of the MEG responses to the individual click trains of sequences is provided in Fig. 4. The most prominent peak of the transient response to click train onset was the N1m (labeled in Fig. 4B; black circles in Fig. 4A). It was followed, except in the 2.5% duty-cycle condition, by a sustained field of the same polarity (labeled SF in Fig. 4B; white circles in Fig. 4A). The amplitudes of these components were not constant, but decreased across the successive click trains of a sequence (Fig. 4A). The first N1m elicited during a sequence had approximately the same amplitude for all duty cycles. For the subsequent trains of the sequence, the N1m decreased in amplitude, most prominently for the 72% duty-cycle condition.
Fig. 4.
Details of the MEG onset (A, B) and offset (C, D) responses for each successive click train of a sequence. A and C: the mean amplitudes (and SEs) across subjects measured at the peak. Sustained field amplitudes were measured as the maximum in the interval from 400 ms after the onset until the end of the click train. For the continuous click train (100% duty cycle), the time interval was 400–3,000 ms with respect to train onsets in the other conditions. A includes the onset components N1m (black circles), P2m (gray circles), and the sustained fields (white circles). C: the components N1m-off (black squares) and P2m-off (gray squares). B and D: the response waveforms evoked by the eight successive click trains of a sequence aligned to train onset (B) or offset (D). The response evoked by the 1st click train of the sequence is plotted in black, the response evoked by the 2nd by a bold gray line, and those evoked by the 3rd to 8th by a thinner gray line. The most prominent change of the onset response occurs from the 1st to the 2nd train: whereas an N2m is observed between the N1m and the sustained field for the first stimulus, all subsequent stimuli evoke a P2m instead.
A decline in amplitude over the course of a sequence was also observed for the sustained field, with the greatest decline occurring for the continuous clicks (100% duty cycle) condition; the sustained-field source amplitude decreased to half its initial value by the end of the sequence. Some decrease of sustained-field amplitude is also observed for the 72% duty cycle, whereas nearly no decrease is seen for the 25% duty cycle.
Between the N1m and sustained field, a prominent P2m (latency ∼200 ms) is observed from the second click train on. This P2m became prominently more positive from the first to the second click train and then continued to become slightly more positive across subsequent trains of the sequence. The increased positivity of P2m could reflect diminished cancellation of P2m by the N2m wave, which could be defined in the response only to the first click train of each sequence (Fig. 4B).
In contrast to the transient onset response to each click train, the offset response varied little from one train to the next in a sequence, as shown in Fig. 4, C and D. The offset response consisted of peaks N1m, P2m, and N2m, which were most prominent for the 100 and 72% duty cycles. These peaks were fairly equal in prominence across successive trains. With the presence of N2m, the overall morphology of the off response more closely resembled the onset response to the first click train than the onset response of subsequent trains of a sequence (compare respective panels in Fig. 4, B and D). The N1m of the off response to the continuous, 24-s click train (100% duty cycle; Fig. 4, C and D, bottom right) approaches the size of the onset N1m for this condition (Fig. 4, A and B, bottom right). The peak-to-peak amplitude of the off response (N1m to P2m) for each of the 72% duty-cycle click trains is nearly as strong as that for the 100% duty cycle. It appears, however, that the relative amplitude of N1m and P2m differs, such that the N1m is larger for the 100% duty cycle, whereas the P2m is larger for the 72% duty cycle. Because the transient off response overlaps with the decrease of the sustained field, the exact amplitude relationship of these waves varies, depending on the baseline definition used to measure their amplitude.
Comparison of BOLD and MEG responses
Several trends are clear from a comparison of the BOLD time courses in Fig. 2 and the MEG source waveforms of Figs. 3 and 4.
1) A clear BOLD response was produced by the 2.5% duty-cycle sequence, whereas the MEG response to this stimulus consisted almost exclusively of transients.
2) The sustained elevation of the BOLD time courses and the sustained field in the MEG waveforms do not increase and decrease in accordance with one another. The lack of covariation between the two can be seen from the 2.5 and 100% duty-cycle conditions: whereas the BOLD response in the 2.5% duty-cycle condition is mainly a sustained elevation (Fig. 2, top left), there is little or no sustained field in the MEG waveform for this stimulus (Fig. 3, top). At the same time, the BOLD response for the 100% duty-cycle condition (Fig. 2, bottom right) shows slightly less sustained elevation (on which the on and off peaks ride) than that of the 2.5% condition, whereas the sustained field in MEG is greatest for this condition (Fig. 3, bottom).
3) Whereas a distinct BOLD off peak was observed only for the 100% duty-cycle condition, prominent MEG offset responses with similar peak-to-peak magnitude were seen not only following each individual click train in the 72% duty-cycle sequence but also at the end of the 100% duty-cycle sequence. Thus the relationship between the MEG and BOLD offset phenomena is not obvious. There are some subtle differences in the response morphology, between the 72 and 100% duty-cycle conditions, which might be of some importance, however. For example, the N1m increases from 72 to 100% duty cycles (most prominent when measured from the sustained field level), but at the same time the P2m is more prominent for the 72% condition.
4) The transient MEG response to the onset of the first click train of a sequence differs from that of subsequent trains, raising the possibility that the neural activity responsible for this difference may play a role in the strong BOLD onset transient.
A model relating MEG activity to BOLD time courses
Several alternative models were used to further investigate the relationship between the BOLD time courses and MEG source waveforms. The first (model 1) convolved a hemodynamic response function (HRF 1; Fig. 5F) with a rectified version of the MEG source waveform for each stimulus condition (Fig. 5E). The result was scaled to provide the best linear fit between measured and simulated BOLD time courses. The simulated BOLD time courses are superimposed on the measured ones in Fig. 5A. The same scale factor was applied to all four conditions. The fit is not satisfactory, particularly for the lower duty-cycle conditions. The poor fit arises from the relatively large area under the sustained field compared with transient components of the MEG source waveform, which makes the sustained field dominate the fit: preventing an overestimate of the BOLD time course in the 100 and 72% duty-cycle conditions leads to an underestimate in the 2.5 and 25% conditions. Note that the fit was not significantly improved by using the alternative HRF 2 (Fig. 5C), which was based on a gamma function (Fig. 5G). Implementing the model in individual subjects did not improve the fit either, as can be seen in Table 1 where the results of two implementations are given: 1) one based on BOLD and MEG source waveforms averaged across hemispheres and subjects (Table 1, left) and 2) another based on individual data for each subject averaged between hemispheres (Table 1, right). For the latter, the values given in the table, including residual variance (RV), were determined separately for each subject and then averaged across subjects.
Fig. 5.
Comparison of BOLD responses derived from MEG signals (red and blue) and the BOLD responses actually measured (black). The unweighted model (model 1, A, C) convolved the rectified MEG data (E) with standard, empirical hemodynamic response functions (HRF1, F; HRF2, G). This model underestimates conditions with short sustained fields in MEG, or overestimates conditions with long ones. When the transient (orange in E) and the sustained fields (blue in E) are separately convolved with the HRFs (model 2, B, D) and their relative contribution is determined by a least-squares fit, the BOLD data are explained best when the sustained field contribution is weighted less than the transient contribution by a factor of 8–10 (cf. Table 1). Sustained and transient contributions to the modeled BOLD response are plotted as blue and orange dashed lines, respectively, in B and D.
Table 1.
Weights and residual variance (RV) of MEG model for the BOLD response
| Grand-Average Analysis Relative Weighting, % |
Single-Subject Analysis Relative Weighting, % |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Model | HRF | On | Off | SF | N2m | RV, % | On | Off | SF | N2m | RV, % |
| MEG full-wave rectified | |||||||||||
| 1 | Empirical | Unweighted | 38.9 | Unweighted | 45.9 ± 11.5 | ||||||
| Gamma | Unweighted | 36.0 | Unweighted | 42.3 ± 12.3 | |||||||
| 2 | Empirical | 91.4 | (on + off) | 8.6 | 18.4 | 91.2 ± 4.8 | (on + off) | 8.8 ± 4.8 | 24.8 ± 4.8 | ||
| Gamma | 89.3 | (on + off) | 10.7 | 20.7 | 89.1 ± 5.4 | (on + off) | 10.9 ± 5.4 | 26.4 ± 8.0 | |||
| 3 | Empirical | 35.9 | 60.6 | 3.5 | 18.0 | 38.3 ± 13.5 | 57.9 ± 14.1 | 3.8 ± 2.2 | 24.1 ± 5.1 | ||
| Gamma | 49.2 | 45.0 | 5.9 | 20.7 | 52.7 ± 22.7 | 41.0 ± 24.4 | 6.3 ± 3.6 | 25.9 ± 7.7 | |||
| 4 | Empirical | 21.9 | 57.6 | 2.3 | 18.1 | 16.0 | 24.0 ± 6.9 | 52.7 ± 10.3 | 2.7 ± 1.7 | 20.6 ± 10.5 | 22.2 ± 4.0 |
| Gamma | 14.1 | 40.5 | 1.9 | 43.5 | 12.9 | 15.6 ± 6.4 | 34.2 ± 12.3 | 2.4 ± 1.6 | 47.8 ± 11.0 | 18.7 ± 6.0 | |
| MEG squared | |||||||||||
| 1 | Empirical | Unweighted | 34.0 | Unweighted | 46.7 ± 13.2 | ||||||
| Gamma | Unweighted | 29.6 | Unweighted | 39.9 ± 14.0 | |||||||
| 2 | Empirical | 87.4 | (on + off) | 12.6 | 20.0 | 87.5 ± 8.4 | (on + off) | 12.5 ± 8.4 | 29.4 ± 2.3 | ||
| Gamma | 84.3 | (on + off) | 15.8 | 19.6 | 84.7 ± 9.6 | (on + off) | 15.4 ± 9.6 | 26.2 ± 7.0 | |||
| 3 | Empirical | 14.8 | 80.6 | 2.6 | 16.9 | 16.8 ± 10.0 | 80.6 ± 12.8 | 2.6 ± 3.3 | 24.9 ± 3.1 | ||
| Gamma | 20.2 | 76.3 | 3.6 | 18.8 | 26.1 ± 24.8 | 67.8 ± 34.1 | 6.2 ± 9.7 | 24.2 ± 6.8 | |||
| 4 | Empirical | 11.3 | 80.9 | 1.5 | 6.4 | 15.8 | 13.2 ± 6.6 | 75.8 ± 11.0 | 1.8 ± 1.4 | 9.3 ± 8.2 | 22.8 ± 2.5 |
| Gamma | 8.1 | 70.0 | 1.3 | 20.6 | 12.7 | 10.1 ± 6.4 | 60.9 ± 22.3 | 2.3 ± 2.8 | 26.8 ± 16.9 | 19.1 ± 4.5 | |
Values for single-subject analysis are means ± SD. The region of interest is the whole auditory cortex.
A far better fit to the data is achieved when the onset and offset transients of the MEG data on the one hand, and the MEG sustained field on the other, are separated (orange and blue, respectively in Fig. 5E) and allowed separate weightings in the least-square fit (model 2). The results from this weighted model using HRF 1 are shown in Fig. 5B. Most obviously, the weighted model improves the explanation of the 2.5 and the 100% duty-cycle conditions. In the case of the 2.5% condition, the amplitude of the measured BOLD time course is better matched. For the 100% duty-cycle condition, the latency of the BOLD onset, previously overestimated, is now correct. The reduction in residual variance from the unweighted to the weighted model was 20.5% for the empirical HRF 1 and 15.3% for the gamma function, HRF 2 (cf. Table 1). The relative weighting assigned to transient MEG components was eight- to tenfold greater than the weighting assigned to sustained MEG activity. The dominance of transient MEG activity over sustained activity in accounting for the BOLD time courses can be seen from the separate traces for the transient (dashed orange trace) and sustained (dashed blue) contributions in Fig. 5, B and D. Note that this same result—a dominance of transient MEG signals in explaining of the BOLD response using model 2—was also observed for eight additional listeners, who participated in pilot experiments reported in the Supplemental Materials (available online).1
Although the sustained part of the BOLD response was similarly well estimated using either of the two HRFs, the empirical HRF 1 performed better in modeling the transient BOLD onset response, which was not well modeled when the gamma-function, HRF 2, without undershoot was used (compare Fig. 5, B and D). On the other hand, the empirical HRF 1 produces some undershoot after sequence offset, whereas the undershoot in the measured BOLD was quite variable across listeners; thus the overall residual variance is not much different between the empirical and the gamma-function HRF.
In the model just described, contributions of the onset and offset MEG transients were assumed to contribute with the same weighting, the result being a notable failure to fit the prominent offset response in the BOLD time course for the 100% duty-cycle condition. This motivated a variation (model 3) in which the onset and offset transients of MEG activity were assigned separate weighting factors, resulting in three free parameters, two for MEG transients and one for the sustained field. This model produced only a minimal improvement over the previous weighted model (model 2), yielding <1% reduction in residual variance (Table 1). This lack of improvement is primarily because of the prominent offset responses following each click train in the 72% duty-cycle condition; the weighting of these off responses had to be low enough to avoid overestimation of the BOLD time course prior to sequence offset, which resulted in too low a weighting to account for the BOLD off peak. Thus the weighting of onset and offset transients remained relatively balanced in model 3 despite being free to differ.
We also considered the possibility of cancellation between N2m and P2m at sequence onset (model 4). In particular, it was assumed that P2m would have been as prominent in the onset response to the first click train as in the response to subsequent trains except that a positive wave partially canceled it, resulting in an attenuated P2m and in N2m. Thus the onset response to the first click train was assumed to have two contributions, each of which was independently rectified and weighted in the model. For this separation of onset responses, the N2m was estimated as the half-wave rectified difference between the response to the first and the last four click trains in the interval after the N1m and before the sustained field onset (150–300 ms). An average of the N2m estimated for duty cycles 2.5–75% was used for the 100% condition, for which N2m could not be similarly estimated. The estimated N2m was then subtracted from the onset transients in model 3, thus revealing the putative P2m evoked by the first click train. In sum, one component of the onset transients included the waves P1m, N1m, and P2m evoked by all subsequent click trains, whereas the second component included only the N2m, putatively evoked by the first click train only. This separation of onset transients (model 4) resulted in a better fit to the transient BOLD responses (Fig. 6), but reduced the residual variance by only 2–7% compared with that of model 3 (Table 1). However, the BOLD off response was still poorly fit and the weighting of the MEG sustained field was far less than that of transient MEG components.
Fig. 6.
Measured BOLD responses (thick black trace) superimposed on BOLD responses derived by convolving MEG signals with HRF1 (A, red) or HRF2 (B, blue). The MEG signals were divided into 4 separately weighted components, 3 transient and one sustained (model 4), each of which was then convolved with the HRFs. The resulting contribution of each MEG component to the derived BOLD response is shown by the dashed traces.
Because previous studies comparing evoked responses in the somatosensory cortex with BOLD (Nangini et al. 2008, 2009) and optical imaging (Franceschini et al. 2008) reported that squaring the evoked-response data considerably improved modeling of hemodynamic measures, we tested whether using the squared instead of the rectified MEG data yielded similar improvements in fit here. The results are summarized in the bottom half of Table 1: overall there were no strong or consistent differences between squared and rectified. Finally, with the MEG signal squared instead of rectified, the sustained MEG response was still assigned a substantially lower weighting than that of the transient responses (Table 1) and the BOLD off peak could not be generated from the MEG signal.
DISCUSSION
Our results indicate that the neural activity giving rise to transient MEG responses to sound is likely a major, and perhaps primary, contributor to BOLD responses of auditory cortex; the sustained field measured with MEG appears to contribute much less to the BOLD signal produced by auditory cortex. This conclusion is based on the following observations: 1) A stimulus producing transient MEG responses with little or no sustained field (the 2.5% duty-cycle sequence) also produced a robust, sustained elevation in BOLD signal. 2) The BOLD responses to a range of stimuli that produced transient and sustained MEG activity in varying proportions were simulated faithfully when the contribution of transient activity to the BOLD signal was weighted eight- to tenfold more than sustained activity. This latter result was confirmed by the experiments described in the Supplemental Materials.
Our conclusion regarding the relationship between transient versus sustained MEG signals and BOLD response is based on two assumptions: 1) The neural activity underlying transient MEG responses, and not some other activity correlated with it, is the generator of the BOLD signal (but see Brugge et al. 2009 for an example of gamma-band activity observed in intracranial recordings that is temporally correlated with long-latency transients); and 2) the neural activity contributing to the BOLD responses also contributes measurably to MEG signals. This assumption is supported by data suggesting that both BOLD responses and long-latency MEG signals like those recorded here are driven by PSPs (Creutzfeld et al. 1966; Hämäläinen et al. 1993; Logothetis et al. 2001). In addition, there is evidence that the two responses may be driven by activity in similar cortical layers. In particular, studies in monkey AC have suggested that long-latency responses such as N1 recorded from the scalp via electroencephalography (EEG, the counterpart of N1m in MEG) are mainly related to activity in supragranular layers of cortical gray matter (Javitt et al. 1994; Steinschneider et al. 1998). In addition, studies of somatosensory cortex relating hemodynamic measures to local field potentials (LFPs), a measure of extracellular synaptic potentials, suggest that supragranular LFP activity is more closely coupled to the hemodynamic response than infragranular LFP activity (Franceschini et al. 2008). Thus both the MEG and the BOLD responses of the present study may be due to PSPs in supragranular cortical layers.
Although we compared MEG and BOLD responses to specific stimuli, there are reasons to believe that our conclusions have broader pertinence. Importantly, the forms of MEG and BOLD responses seen here for sequences of click trains are typical for many types of stimuli. For instance, a transient N1m–P2m complex is a typical MEG response to noise or tone bursts of short duration, as well as brief click trains, as is the emergence of a sustained field with increasing noise or tone duration (Gutschalk et al. 2002; Hari et al. 1980; Pantev et al. 1996; Picton et al. 1978). In BOLD, sequences of repeated noise or tone bursts, for instance, produce the same kind of responses seen here for sequences of click trains (Harms and Melcher 2002; Harms et al. 2005; Seifritz et al. 2002). It is possible but unlikely that this rich variety of responses arises from completely different neural mechanisms. Therefore the insights from the present study are likely to be generally applicable to similar responses produced by other stimuli.
Neural activity underlying the MEG and BOLD responses
The pyramidal, cortical neurons thought to generate the PSPs from which MEG signals arise (Creuzfeld et al. 1966; Hämäläinen et al. 1993) are also the targets of single-neuron recordings from auditory cortex. One such study is especially pertinent to interpreting the data presented here. The study by Lu et al. (2001) dissociated two populations of sustained firing neurons in extracellular single-cell recordings in awake monkeys, one that fires in a manner synchronized to the sound stimulus and strongly decreases its activity for stimulus repetition rates above about 20 Hz and another that increases its rate above about 20 Hz, but is not synchronized to the stimulus. In MEG, the two populations could both be reflected in the sustained field in principle, but at the high click rate of 500 Hz used in this study, no significant sustained response from synchronized neurons would be expected, leaving only the non-stimulus-synchronized neurons of AC to respond throughout most of each click train. In contrast, many neurons that are not spiking during the sustained portion of the train would be expected to produce action potentials at train onset (Wang et al. 2005). It would therefore appear that the transient and sustained MEG responses of the present study could have been driven by different populations of neurons: those producing onset responses (and possibly stimulus-synchronized activity in general) on the one hand, and those responding in a sustained, non-time-locked fashion on the other hand. Given our finding that transient MEG responses contribute far more effectively to the BOLD response than the sustained field does, it follows that the population of AC neurons that responds in a sustained, non-stimulus-locked manner may have contributed little to the BOLD responses of the present study, whereas stimulus-synchronized neurons and other neurons responsive to stimulus onsets may account for the bulk of the BOLD response. This suggestion must be tempered, however, by the fact that MEG and fMRI are not directly coupled to spiking activity but rather to the PSPs driving the spiking activity.
Importantly, spiking arises from an interplay between excitatory PSPs (EPSPs) and inhibitory PSPs (IPSPs) that may manifest differently in MEG and BOLD signals. How EPSPs and IPSPs shape the different characteristics of AC neurons is still being unraveled, but some findings are potentially important for the different observations made here in MEG and fMRI: in many auditory-cortex neurons, EPSPs and IPSPs are closely cotuned in space and time (Tan et al. 2004; Wehr and Zador 2003). EPSPs precede IPSPs by less than a millisecond and spikes are generated within this temporal gap only (Wehr and Zador 2003). Closely timed EPSPs and IPSPs such as these will produce canceling contributions to MEG signals, but likely additive contributions to the BOLD response since both EPSPs and IPSPs have a hemodynamic demand (but see following text). Thus if the activity of cotuned neurons was more prevalent during the transient MEG onset response than during the sustained field, it would help explain why the transients contribute more strongly to the BOLD signal. Other neuronal populations in the AC have been reported that show broader frequency tuning of IPSPs than EPSPs to produce lateral inhibition (Wu et al. 2008). De la Rocha et al. (2008) suggested that these two neuronal types (cotuned and lateral inhibition) are intermingled and that the lateral-inhibition type produces broadly tuned onset neural firing and subsequent, more frequency-specific sustained firing. The latter features are characteristic of the specific, sustained-firing units reported by Wang et al. (2005) of which the non-stimulus-locked, sustained-firing neuron type observed with fast click trains (Lu et al. 2001) is supposedly a subtype. A consequence for MEG could be that the more broadly tuned IPSPs would not be completely canceled by EPSPs such that the nonstimulus locked neurons would produce a more sustained MEG response than that of the cotuned type. In summary, different synaptic mechanisms producing different amounts of cancellation between EPSPs and IPSPs might underlie the strong weighting of MEG transients over sustained fields that was needed to account for the BOLD responses of the present study.
The preceding discussion makes a common assumption: that EPSPs and IPSPs contribute similarly to the BOLD response based on their energy demand (Logothetis et al. 2001; Niessing et al. 2005; Viswanathan and Freeman 2007). However, it is worth noting that this view has recently been challenged by findings of vessel constriction at sites of isolated IPSP activity (Devor et al. 2007), suggesting that EPSPs and IPSPs might oppositely and very directly control perfusion. In this case, the balance of EPSPs and IPSPs would need to be considered for the generation of the BOLD response, which would have strong implications for the integration of BOLD and MEG. The link between neurons and local perfusion is thought to be mediated by astrocytes and is best established for the excitatory transmitter glutamate (Schummers et al. 2008). There is some evidence for an involvement of GABAergic interneurons (Cauli 2004), with subtypes producing both vasodilation or vasoconstriction. Thus although a relationship between MEG and fMRI can be expected based on EPSPs, the role of IPSPs is unclear at this point. Several scenarios could therefore be constructed to explain the data of this study. For example, if the BOLD response was primarily coupled to EPSPs, whereas the sustained field reflected IPSPs (cf. previous paragraph), little direct correlation between the MEG sustained field and BOLD response would be expected. Conversely, if EPSPs and IPSPs were antagonistic for BOLD then a closer correlation with MEG than expected might be possible.
BOLD offset response: no clear correlate in MEG
When the contribution from transient MEG activity was strongly weighted in our simulations of BOLD responses, the simulated responses provided a good explanation for much of the measured BOLD responses, but not the off-peak occurring after sequence offset. The reason for this discrepancy remains unclear at present, but several explanations might apply. Perhaps the most tempting would be to attribute the off-peak entirely to the hemodynamic mechanisms coupling neural activity to the measured BOLD signal. For example, we cannot rule out the possibility of a nonlinear relation between neural activity and BOLD response accounting for the off-peak—i.e., a situation in which the increase in BOLD exceeds what one would expect from the driving increase in neural activity, a scenario supported by empirical data (Devor et al. 2003).
An alternative possibility is that the neural activity driving the BOLD off-peak is only subtly reflected in the MEG signal or is not reflected at all. This could occur if the BOLD off-peak reflected activity in neuronal populations that are not temporally synchronized or spatially aligned in a manner suitable for contributing effectively to the MEG signal. It could also be that PSP contributions to the MEG signal cancel out, as discussed earlier, and reinforce one another in the BOLD signal. An indication of such a process is the observation that the N1m-off is larger for the 100% compared with the 72% condition, whereas the P2m-off is smaller. Considering that a decrease of the P2m-off is unlikely here, one might suggest that this is rather an indication of enhanced (cortical) surface-negative activity, respective the N1m-off and N2m-off. Inclusion of such an interpretation of the MEG signal peaks in the model would significantly enhance the explanation of the BOLD offset transient.
Significance for future fMRI studies
The physiological basis of the BOLD response is of general interest for the design and interpretation of many fMRI experiments. Experiments in which fMRI is combined with MEG or EEG need to consider the differential contribution of separate evoked response components to BOLD, especially when source models for MEG/EEG are constrained by BOLD data (Dale et al. 2000). For instance, the sustained activity might be of more interest than transient activity, but reflect only a minor fraction of the overall BOLD magnitude. Wang et al. (2005) have shown that sustained firing units in AC reflect specificity for higher-level sound features. Most of these neurons also generate transient onset responses, although these transient responses are nonspecifically observed across many neurons. Our data suggest that these feature-specific, sustained-firing neurons may contribute only weakly to the BOLD response in fMRI and the consequential low sensitivity for these neurons will need to be considered for the design of studies seeking feature specificity in AC with fMRI.
Conclusion
We investigated the relationship of MEG and BOLD time series at the auditory cortex using click trains with parametrically varied click trains as stimuli. Our results demonstrate that MEG time series cannot be transformed into BOLD time series by convolution with hemodynamic response functions without additional constraints. One such constraint suggested by our study is to weight transient MEG activity more strongly than sustained (near DC) activity contributions to the BOLD signal. Further studies of the hemodynamic coupling to transient versus sustained activity at the microscopic level are required to more fully understand the neurophysiological underpinnings of our functional imaging data.
GRANTS
This research was supported by Deutsche Forschungsgemeinschaft Fellowship GU 593/2-1 to A. Gutschalk; National Institutes of Health Grants P41-RR-14075, P30-DC-005209, and R01-EB-006385; and Bundesministerium für Bildung und Forschung Grant 01EV0712 to A. Gutschalk.
Supplementary Material
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Ahlfors SP, Simpson GV. Geometrical interpretations of fMRI-guided MEG/EEG inverse estimates. NeuroImage 22: 323–332, 2004. [DOI] [PubMed] [Google Scholar]
- Ahveninen J, Jaaskelainen IP, Raij T, Bonmassar G, Devore S, Hämäläinen M, Levänen S, Lin FH, Sams M, Shinn-Cunningham BG, Witzel T, Belliveau JW. Task-modulated “what” and “where” pathways in human auditory cortex. Proc Natl Acad Sci USA 103: 14608–14613, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin P, Zatorre RJ, Hoge R, Evans AC, Pike B. Event-related fMRI of the auditory cortex. NeuroImage 10: 417–429, 1999. [DOI] [PubMed] [Google Scholar]
- Brugge JF, Nourski KV, Oya H, Reale RA, Kawasaki H, Steinschneider M, Howard MA., 3rd Coding of repetitive transients by auditory cortex on Heschl's gyrus. J Neurophysiol 102: 2358–2374, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauli B, Tong XK, Rancillac A, Serluca N, Lambolez B, Rossier J, Hamel E. Cortical GABA interneurons in neurovascular coupling: relays for subcortical vasoactive pathways. J Neurosci 24: 8940–8949, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen D, Schläpfer U, Ahlfors S, Hämäläinen M, Halgren E. New six-layer magnetically-shielded room for MEG. In: BIOMAG 2002, Proceedings of the 13th International Conference on Biomagnetism, edited by Nowak H, Haueisen J, Gieβler F, Hounker R. Berlin: VDE Verlag, 2002, p. 919–921. [Google Scholar]
- Creutzfeldt OD, Watanabe S, Lux HD. Relations between EEG phenomena and potentials of single cortical cells. I. Evoked responses after thalamic and epicortical stimulation. Electroencephalogr Clin Neurophysiol 20: 1–18, 1966. [DOI] [PubMed] [Google Scholar]
- Dale AM, Buckner RL. Selective averaging of individual trials using fMRI. Hum Brain Mapp 5: 329–340, 1997. [DOI] [PubMed] [Google Scholar]
- Dale AM, Liu AK, Fischl BR, Buckner RL, Belliveau JW, Lewine JD, Halgren E. Dynamic statistical parametric mapping: combining fMRI and MEG for high-resolution imaging of cortical activity. Neuron 26: 55–67, 2000. [DOI] [PubMed] [Google Scholar]
- De la Rocha J, Marchetti C, Schiff M, Reyes AD. Linking the response properties of cells in auditory cortex with network architecture: cotuning versus lateral inhibition. J Neurosci 28: 9151–9163, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devor A, Dunn AK, Andermann ML, Ulbert I, Boas DA, Dale AM. Coupling of total hemoglobin concentration, oxygenation, and neural activity in rat somatosensory cortex. Neuron 39: 353–359, 2003. [DOI] [PubMed] [Google Scholar]
- Devor A, Tian P, Nishimura N, Teng IC, Hillman EMC, Narayanan SN, Ulbert I, Boas DA, Kleinfeld D, Dale AM. Suppressed neuronal activity and concurrent arteriolar vasoconstriction may explain negative blood oxygenation level-dependent signal. J Neurosci 27: 4452–4459, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edminster WB, Talavage TM, Ledden PL, Weisskoff RM. Improved auditory cortex imaging using clustered volume acquisition. Hum Brain Mapp 7: 89–97, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II. Inflation, flattening and a surface-based coordinate system. NeuroImage 9: 195–207, 1999. [DOI] [PubMed] [Google Scholar]
- Franceschini MA, Nissila I, Wu W, Diamond SG, Bonmassar G, Boas DA. Coupling between somatosensory evoked potentials and hemodynamic response in the rat. NeuroImage 41: 189–203, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraud AL, Lorenzi C, Ashburner J, Wable JY, Johnsrude I, Frackowiak R, Kleinschmidt A. Representation of the temporal envelope of sounds in the human brain. J Neurophysiol 84: 1588–1598, 2000. [DOI] [PubMed] [Google Scholar]
- Glover GH. Deconvolution of impulse response in event-related BOLD fMRI. NeuroImage 9: 416–429, 1999. [DOI] [PubMed] [Google Scholar]
- Gutschalk A, Oxenham AJ, Micheyl C, Wilson EC, Melcher JR. Human cortical activity during streaming without spectral cues suggests a general neural substrate for auditory stream segregation. J Neurosci 27: 13074–13081, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutschalk A, Patterson RD, Rupp A, Uppenkamp S, Scherg M. Sustained magnetic fields reveal separate sites for sound level and temporal regularity in human auditory cortex. NeuroImage 15: 207–216, 2002. [DOI] [PubMed] [Google Scholar]
- Gutschalk A, Patterson RD, Scherg M, Uppenkamp S, Rupp A. Temporal dynamics of pitch in human auditory cortex. NeuroImage 22: 755–766, 2004. [DOI] [PubMed] [Google Scholar]
- Hall DA, Haggard MP, Akeroyd MA, Palmer AR, Summerfield AQ, Elliott MR, Gurney EM, Bowtell RW. “Sparse” temporal sampling in auditory fMRI. Hum Brain Mapp 7: 213–223, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hämäläinen M, Hari R, Ilmoniemi RJ, Knuutila J, Lounasmaa OV. Magnetoencephalography: theory, instrumentation, and applications to noninvasive studies of the working human brain. Rev Mod Phys 65: 413–497, 1993. [Google Scholar]
- Hämäläinen MS, Sarvas J. Realistic conductivity geometry model of the human head for interpretation of neuromagnetic data. IEEE Trans Biomed Eng 36: 165–171, 1989. [DOI] [PubMed] [Google Scholar]
- Hari R, Aittoniemi K, Järvinen ML, Katila T, Varpula T. Auditory evoked transient and sustained magnetic fields of the human brain. Exp Brain Res 40: 237–240, 1980. [DOI] [PubMed] [Google Scholar]
- Hari R, Kaila K, Katila T, Tuomisto T, Varpula T. Interstimulus interval dependence of the auditory vertex response and its magnetic counterpart: implications for their neural generation. Electroencephalogr Clin Neurophysiol 54: 561–569, 1982. [DOI] [PubMed] [Google Scholar]
- Harms MP, Guinan JJ, Jr, Sigalovsky IS, Melcher JR. Short-term sound temporal envelope characteristics determine multisecond time patterns of activity in human auditory cortex as shown by fMRI. J Neurophysiol 93: 210–222, 2004. [DOI] [PubMed] [Google Scholar]
- Harms MP, Melcher JR. Sound repetition rate in the human auditory pathway: representations in the waveshape and amplitude of fMRI activation. J Neurophysiol 88: 1433–1450, 2002. [DOI] [PubMed] [Google Scholar]
- Harms MP, Melcher JR. Detection and quantification of a wide range of fMRI temporal responses using a physiologically-motivated basis set. Hum Brain Mapp 20: 168–183, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imada T, Watanabe M, Mashiko T, Kawakatsu M, Kotani M. The silent period between sounds has a stronger effect than the interstimulus interval on auditory evoked magnetic fields. Electroencephalogr Clin Neurophysiol 102: 37–45, 1997. [DOI] [PubMed] [Google Scholar]
- Jääskeläinen IP, Ahveninen J, Bonmassar G, Dale AM, Ilmoniemi RJ, Levänen S, Lin FH, May P, Melcher J, Stufflebeam S, Tiitinen H, Belliveau JW. Human posterior auditory cortex gates novel sounds to consciousness. Proc Natl Acad Sci USA 101: 6809–6814, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, Steinschneider M, Schroeder CE, Vaughan HG, Jr, Arezzo JC. Detection of stimulus deviance within primate primary auditory cortex: intracortical mechanisms of mismatch negativity (MMN) generation. Brain Res 667: 192–200, 1994. [DOI] [PubMed] [Google Scholar]
- Lammertmann C, Lütkenhöner B. Near-DC magnetic fields following a periodic presentation of long-duration tonebursts. Clin Neurophysiol 112: 499–513, 2001. [DOI] [PubMed] [Google Scholar]
- Lin FH, Witzel T, Hamalainen MS, Dale AM, Belliveau JW, Stufflebeam SM. Spectral spatiotemporal imaging of cortical oscillations and interactions in the human brain. NeuroImage 23: 582–595, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature 412: 150–157, 2001. [DOI] [PubMed] [Google Scholar]
- Lu T, Liang L, Wang X. Temporal and rate representations of time-varying signals in the auditory cortex of awake primates. Nat Neurosci 4: 1131–1138, 2001. [DOI] [PubMed] [Google Scholar]
- Mathiak K, Rapp A, Kirchner TTJ, Grodd W, Hertrich I, Weiskopf N, Lutzenberger W, Ackermann H. Mismatch responses to randomised gradient switching noise as reflected by fMRI and whole-head magnetoencephalography. Hum Brain Mapp 16: 190–195, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nangini C, Hlushchuk Y, Hari R. Predicting stimulus-rate sensitivity of human somatosensory fMRI signals with MEG. Hum Brain Mapp 30: 1824–1832, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nangini C, Tam F, Graham SJ. A novel method for integrating MEG and BOLD fMRI signals with the linear convolution model in human primary somatosensory cortex. Hum Brain Mapp 29: 97–106, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niessing J, Ebisch B, Schmidt KE, Niessing M, Singer W, Galuske RAW. Hemodynamic signals correlate tightly with synchronized gamma oscillations. Science 309: 948–951, 2005. [DOI] [PubMed] [Google Scholar]
- Pantev C, Eulitz C, Hampson S, Ross B, Roberts LE. The auditory evoked “off” response: sources and comparison with the “on” and the “sustained” responses. Ear Hear 17: 255–265, 1996. [DOI] [PubMed] [Google Scholar]
- Pantev C, Okamoto H, Ross B, Stoll W, Ciurlia-Guy E, Kakigi R, Kubo T. Lateral inhibition and habituation of the human auditory cortex. Eur J Neurosci 19: 2337–2344, 2004. [DOI] [PubMed] [Google Scholar]
- Picton TW, Woods DL, Proulx GB. Human auditory sustained potentials. II. Stimulus relationships. Electroencephalogr Clin Neurophysiol 45: 198–210, 1978. [DOI] [PubMed] [Google Scholar]
- Ravicz ME, Melcher JR. Isolating the auditory system from acoustic noise during functional magnetic resonance imaging: examination of noise conduction through the ear canal, head, and body. J Acoust Soc Am 109: 216–231, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson MD, Dorosz JL, Gore JC. Measurement of the temporal fMRI response of the human auditory cortex to trains of tones. NeuroImage 7: 185–198, 1998. [DOI] [PubMed] [Google Scholar]
- Scherg M. Fundamentals of dipole source analysis. In: Auditory Evoked Magnetic Fields and Electric Potentials. Basel: Karger, 1990, p. 40–69. [Google Scholar]
- Schummers J, Yu H, Sur M. Tuned responses of astrocytes and their influence on hemodynamic signals in the visual cortex. Science 320: 1638–1643, 2008. [DOI] [PubMed] [Google Scholar]
- Seifritz E, Esposito F, Hennel F, Mustovic H, Neuhoff JG, Bilecen D, Tedeschi G, Scheffler K, Di Salle F. Spatiotemporal pattern of neural processing in the human auditory cortex. Science 297: 1706–1708, 2002. [DOI] [PubMed] [Google Scholar]
- Steinschneider M, Reser DH, Fishman YI, Schroeder CE, Arezzo JC. Click train encoding in primary auditory cortex of the awake monkey: evidence for two mechanisms subserving pitch perception. J Acoust Soc Am 104: 2935–2955, 1998. [DOI] [PubMed] [Google Scholar]
- Tan AYY, Zhang LI, Merzenich MM, Schreiner CE. Tone-evoked excitatory and inhibitory synaptic conductances of primary auditory cortex neurons. J Neurophysiol 92: 630–643, 2004. [DOI] [PubMed] [Google Scholar]
- Viswanathan A, Freeman RD. Neurometabolic coupling in cerebral cortex reflects synaptic more than spiking activity. Nat Neurosci 10: 1308–1312, 2007. [DOI] [PubMed] [Google Scholar]
- Wang X, Lu T, Snider RK, Liang L. Sustained firing in auditory cortex evoked by preferred stimuli. Nature 435: 341–346, 2005. [DOI] [PubMed] [Google Scholar]
- Wehr M, Zador AM. Balanced inhibition underlies tuning and sharpens spike timing in auditory cortex. Nature 426: 442–446, 2003. [DOI] [PubMed] [Google Scholar]
- Woldorff MG, Tempelmann C, Fell J, Tegeler C, Gaschler-Markefski B, Hinrichs H, Heinze HJ, Scheich H. Lateralized auditory spatial perception and the contralaterality of cortical processing as studied with functional magnetic resonance imaging and magnetoencephalography. Hum Brain Mapp 7: 49–66, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu GK, Arbuckle R, Liu BH, Tao HW, Zhang LI. Lateral sharpening of cortical frequency tuning by approximately balanced inhibition. Neuron 58: 132–143, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.