Abstract
We used micro-infusions during eyelid conditioning in rabbits to investigate the relative contributions of cerebellar cortex and the underlying deep nuclei (DCN) to the expression of cerebellar learning. These tests were conducted using two forms of cerebellum-dependent eyelid conditioning for which the relative roles of cerebellar cortex and DCN are controversial: delay conditioning, which is largely unaffected by forebrain lesions, and trace conditioning, which involves interactions between forebrain and cerebellum. For rabbits trained with delay conditioning, silencing cerebellar cortex by micro-infusions of the local anesthetic lidocaine unmasked stereotyped short-latency responses. This was also the case after extinction as observed previously with reversible blockade of cerebellar cortex output. Conversely, increasing cerebellar cortex activity by micro-infusions of the GABAA antagonist picrotoxin reversibly abolished conditioned responses. Effective cannula placements were clustered around the primary fissure and deeper in lobules hemispheric lobule IV (HIV) and hemispheric lobule V (HV) of anterior lobe. In well-trained trace conditioned rabbits, silencing this same area of cerebellar cortex or reversibly blocking cerebellar cortex output also unmasked short-latency responses. Because Purkinje cells are the sole output of cerebellar cortex, these results provide evidence that the expression of well-timed conditioned responses requires a well-timed decrease in the activity of Purkinje cells in anterior lobe. The parallels between results from delay and trace conditioning suggest similar contributions of plasticity in cerebellar cortex and DCN in both instances.
INTRODUCTION
The wealth of knowledge about the synaptic organization and physiology of the cerebellum enables the development of relatively specific ideas about its function and the underlying mechanisms. It is no coincidence that Marr's seminal theory of computation and learning in the cerebellum (Marr 1969) followed closely the groundbreaking anatomical and physiological studies of Eccles and colleagues (1967). The ability to test such ideas is enabled by the relatively direct way the cerebellum is engaged by experimentally tractable and well-characterized forms of learning such as adaptation of the vestibular-ocular reflex and Pavlovian eyelid conditioning (Ohyama et al. 2003b; Raymond et al. 1996). From these advantages, the question of whether the cerebellum learns has yielded to the specific mechanistic questions of what the cerebellum computes, how learning contributes, and what cellular and molecular processes are involved (Hansel et al. 2001; Ito 2001; Mauk and Donegan 1997; Ohyama et al. 2003b).
An example of this specificity is found in the hypothesis that cerebellar learning involves at least two sites of plasticity—cerebellar cortex and cerebellar deep nuclei (DCN) (Mauk and Donegan 1997; Medina et al. 2000b; Perrett et al. 1993; Raymond et al. 1996). This hypothesis is based on observations that climbing fiber inputs control the induction of plasticity at granule cell-to-Purkinje cell synapses in cerebellar cortex (Coesmans et al. 2004; Ito and Kano 1982), whereas the induction of plasticity in DCN is controlled by inhibitory input from Purkinje cells (Medina and Mauk 1999; Pugh and Raman 2006, 2008). Furthermore, lesions in cerebellar cortex disrupt the timing of conditioned eyelid responses, unmasking responses with short and relatively fixed onset latencies (Medina et al. 2000a; Perrett and Mauk 1995; Perrett et al. 1993). The abolition of learned timing was proposed to reflect loss of plasticity in cerebellar cortex, and short-latency responses to reflect plasticity in DCN (Ohyama et al. 2006; Perrett et al. 1993; but see Bracha et al. 2009 for an alternative interpretation).
These observations suggest that eyelid conditioning induces long term depression (LTD) at conditioned stimulus (CS) activated granule cell-to-Purkinje cell synapses, promoting decreases in Purkinje cell activity during the CS (typically a tone) that in turn promote the induction of plasticity in DCN. Together these two sites of plasticity control conditioned response expression. In support, Purkinje cells develop transient pauses in activity during the CS (Hesslow and Ivarsson 1994; Jirenhed et al. 2007; Rasmussen et al. 2008) and stimulation of cerebellar cortex inhibits the expression of delay conditioned responses (Hesslow 1994b).
There remain at least two sources of controversy regarding cerebellar cortex contributions to eyelid conditioning. The first relates to observations that lesions or inactivation of lobule HVI abolish conditioned responses (Attwell et al. 1999, 2001; Yeo et al. 1985). This calls into question the region of cerebellar cortex (lobule HVI vs. anterior lobe) that is involved and whether there is a role for plasticity in the DCN. The second relates to observations that genetic manipulations in mice that disrupt the integrity of cerebellar cortex affect delay but not trace conditioned responses (Kishimoto and Kano 2006; Kishimoto et al. 2001a,b; Woodruff-Pak et al. 2006). This calls into question whether forebrain-dependent trace conditioning requires cerebellar cortex (Kalmbach et al. 2009; Kronforst-Collins and Disterhoft 1998; Moyer et al. 1990; Simon et al. 2005; Weible et al. 2000; Woodruff-Pak and Disterhoft 2008).
To address these controversies, we have used regimented infusion protocols in cerebellar cortex and DCN. We find that silencing anterior lobe of cerebellar cortex with the anesthetic lidocaine reversibly unmasks short-latency responses while, presumably, increasing cerebellar cortex output by infusing GABAA receptor antagonists reversibly abolishes conditioned responses. These data support the hypothesis that for both delay and trace eyelid conditioning, adaptively timed conditioned responses involve learned decreases in Purkinje cell activity in anterior lobe and DCN plasticity.
METHODS
Subjects and surgery
Male New Zealand albino rabbits (Oryctolagus cuniculus; Myrtle's Rabbitry, Thompsons Station, TN), each initially weighing 2.5–3.0 kg served as subjects. Rabbits were housed in individual cages, maintained on a fixed daily diet, and given water freely. Treatment of animals and surgical procedures were in accordance with an approved animal welfare protocol.
Before training, animals were surgically prepared with a headstage to hold measurement devices and cannula implanted in the cerebellar cortex or anterior interpositus nuclei of the DCN. Rabbits were preanesthetized with a cocktail of acepromazine (1.5 mg/kg) and ketamine (45 mg/kg). They were then intubated, placed on a stereotaxic device and maintained for the rest of the procedure under isoflurane anesthesia (1∼2% mixed in oxygen). Using sterile surgical procedures, the skull was exposed with a midline incision (∼4 cm), and four holes were drilled to accommodate screws that anchored the head bolt in place. Another hole was then drilled to accommodate the cannula and was covered with bone wax. The animal's head was then positioned with lambda 1.5 mm ventral to bregma, and a 26-gauge stainless steel guide cannula (Plastics One, Roanoke, VA) was placed at stereotaxic coordinates targeting the left cerebellar cortex or the left anterior interpositus nucleus. To avoid penetrating the cerebellar tentorium, an approach tilted 40° caudal to vertical was employed for cerebellar cortex cannula. From this approach, the coordinates most often used were (relative to lambda): 5.9 mm posterior, 6.0 mm left lateral, and 17.8 mm ventral. The coordinates for the DCN were (relative to lambda): 1 mm anterior, 5 mm lateral, and 13.3 mm ventral. The head bolt and cannula were secured with dental acrylic, and the skin was loosely sutured where the skull was exposed. Finally, two stainless steel stimulating electrodes were implanted subdermally superior to the left eye. One was placed rostral and the other caudal to the eye. Rabbits were given analgesics and antibiotics for 2 day after surgery and ≥1 wk of recovery before training began.
Apparatus and training procedures
Custom-designed dual training chambers were used in all experiments. The internal dimensions of each wooden chamber were 89 × 64 × 49 cm (width × length × height). A 25-cm-high wooden divider separated the chamber into left and right compartments, each accommodating a plastic restrainer in which the rabbits were placed. The chambers were equipped with infrared emitter/detectors, each of which could be attached to the headbolt of an individual rabbit and directed at the eye. These detectors measure eyelid position by measuring the amount of infrared light reflected back to the detector, which increases as the eyelid closes. These signals are amplified and produce a voltage that is linearly related to eyelid position (within 5%). At the start of each daily training/test session, immediately after placement in the chamber, the detector was calibrated by delivery of the unconditioned stimulus (US) to elicit maximum eyelid closure. Assuming full eyelid closure to be 6.0 mm for each animal, the amplification of the signal was adjusted to yield a peak response amplitude of ∼6 V. Each chamber was also equipped with a speaker connected to an audio source module (Coulbourn Instruments model No. V85-05; Allentown, PA) to generate tones. The CS was either a 1 or 9.5 kHz sinusoidal tone (85 dB), which was ramped at onset and offset with a 5 ms time constant to avoid clicks. For the US, isolated pulse stimulators (A-M Systems model No. 2100; Carlsborg, WA) were used to deliver electrical pulses through the implanted periorbital electrodes. This stimulation involved trains of 1 ms current pulses delivered at 100 Hz for 50 ms. The US stimulation was adjusted for each animal to an intensity that was just suprathreshold for a full-amplitude unconditioned response. At this intensity, typically 1.0 to 3 mA, the animals show no overt signs of discomfort.
Stimulus presentation was controlled by custom-designed software operated on a computer adjacent to the two chambers. For paired delay conditioning sessions, the CS was a 550 ms, 1 kHz tone, and the US was timed to co-terminate with the CS to yield an interstimulus interval (ISI) of 500 ms. Subjects trained in delay conditioning were given five sessions. These subjects were also given five extinction sessions in which the same CS was delivered and the US was omitted. For trace conditioning sessions, the CS was a 500 ms, 1 kHz tone, and the US was delivered 500 ms later to yield a trace interval of 500 ms. Subjects trained in trace conditioning were given 10 sessions. For dual delay/trace conditioning procedures, a delay trial followed every two trace trials. The delay and trace trials were as described in the preceding text, except that a 1 kHz tone signaled one type of trial and a 9.5 kHz tone signaled the other type of trial (counterbalanced across subjects). Subjects trained in dual delay/trace conditioning were given 10–15 sessions. Each training session consisted of twelve nine-trial blocks (108 total trials), with each block including eight paired CS-US trials and one CS-alone trial. The mean intertrial interval was 30 s (range = 20 – 40 s).
Drugs and infusions
After initial training sessions, either lidocaine (4%), the GABAA antagonist picrotoxin (500 μM; Sigma), the GABAA receptor antagonist SR-95531 (gabazine; 200 μM; Sigma), or artificial cerebrospinal fluid (ACSF) was infused into cerebellar cortex. In separate animals, gabazine (20 μM) or ACSF was infused into DCN. Infusions were conducted during a test session that was otherwise identical to training sessions. All compounds were dissolved in a solution adapted from a standard ACSF used for brain slice experiments. To improve pH buffering, the bicarbonate buffer was augmented with 10 mM HEPES to yield the following components [in mM: 124 NaCl, 3.0 KCl, 26.0 NaHCO3,1.3 NaH2PO4–1 H2O, 2.0 MgCl, 10.0 dextrose, 10.0 HEPES (pH 7.35), and 2.0 CaCl2]. For the experiments shown in Figs. 1, 2, 5, 7, and 8, 2.0 μl solution was infused at a rate of 1.0 μl/min after the 36th trial of the test session. For lidocaine and ACSF control infusions, there were two identical infusions, one after the 36th trial and a second after the 54th trial. In early experiments, such as that shown in Fig. 4, a continuous infusion protocol was used to prolong the effects of lidocaine. For this animal, there was an initial infusion of 2 μl (0.5 μl/min) followed 1 min later by a continuous infusion of 0.05 μl/min for 10 more minutes. Training trials resumed during this continuous infusion. For the experiments in Fig. 6, 2 μl gabazine or ACSF was infused at a rate of 0.5 μl/min before the start of the session. At least a day of retraining was given between infusion sessions and the order of infusion was counterbalanced across subjects.
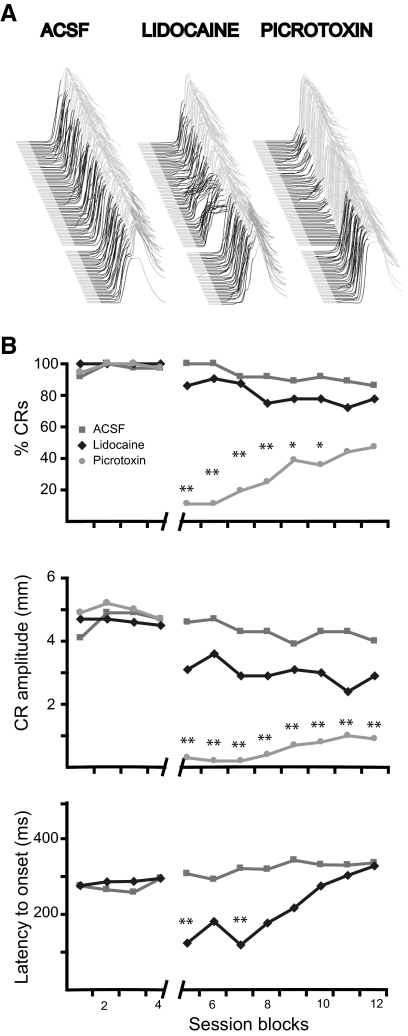
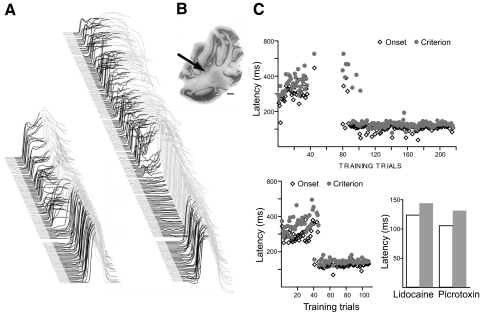
Fig. 1.
The expression of normally timed delay eyelid responses requires a decrease in Purkinje cell activity. A: point-by-point averaging of eyelid position sweeps of 4 subjects during each infusion session. Trials are stacked from 1st on bottom, to last on top with upward deflection indicating eyelid closure. In this and subsequent figures, black shaded regions denote the CS and thus deflections during the black region represent learned responses elicited by the CS and those in the gray region represent reflexive responses to the US. The gap between trials indicates the point in the experiment where the infusion was delivered. B, top: the response rate was reduced by infusions of picrotoxin but not infusions of lidocaine or artificial cerebrospinal fluid (ACSF). Middle: response amplitude was reduced by infusions of picrotoxin and not lidocaine or ACSF. Bottom: the latency to onset of responses decreased after lidocaine infusions but not ACSF infusions. Notice the transient nature of the lidocaine effect. Aterisk, P < 0.05; double asterisk, P < 0.01. Comparisons were made between the last block before infusions and each subsequent postinfusion block using Tukey's test to correct for multiple comparisons.
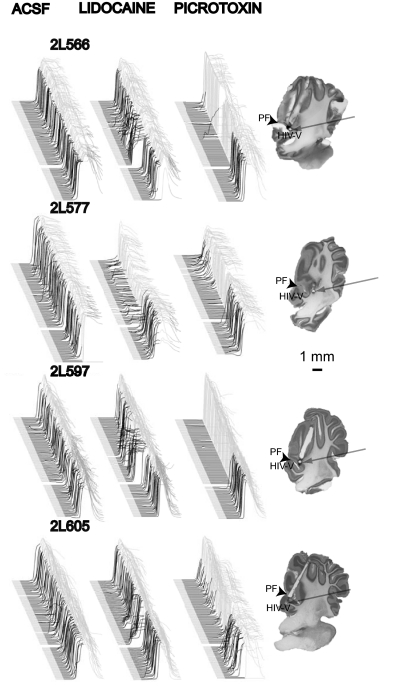
Fig. 2.
Raw data from individual subjects show that gabazine infusions into cerebellar cortex abolish responses while lidocaine infusions unmask short-latency responses. Effective cannula placements clustered around the primary fissure and into lobules HV and HIV of anterior lobe. Behavioral sweeps during each infusion session (left) and cannula placements (right) for each subject are shown. Note the lack of effect of ACSF infusions, the transient effect on the timing of responses of lidocaine infusions and the longer lasting effect on the likelihood of responding of picrotoxin infusions.
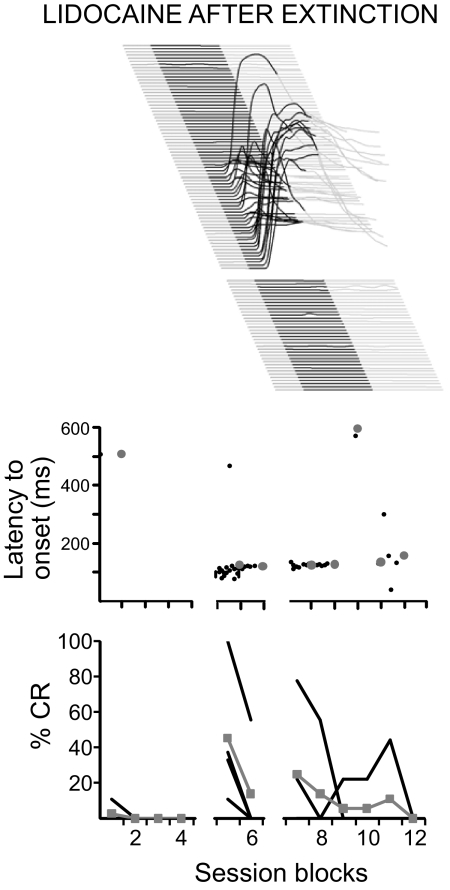
Fig. 5.
Short-latency responses elicited by silencing anterior lobe after extinction. Top: behavioral sweeps from 1 subject during a lidocaine infusion. Note that before the lidocaine infusion (bottom sweeps before the gap) there was no responding and after there was a transient period of responses with a short, fixed latency to onset. Middle: the onset latency of responses as a function of trial number. Black dots, data from individual trials from individual subjects; large gray dots, mean data from all subjects during a 9-trial block. Bottom: response likelihood as a function of trial number. Black lines, data from individual subjects; gray line, grouped mean data.
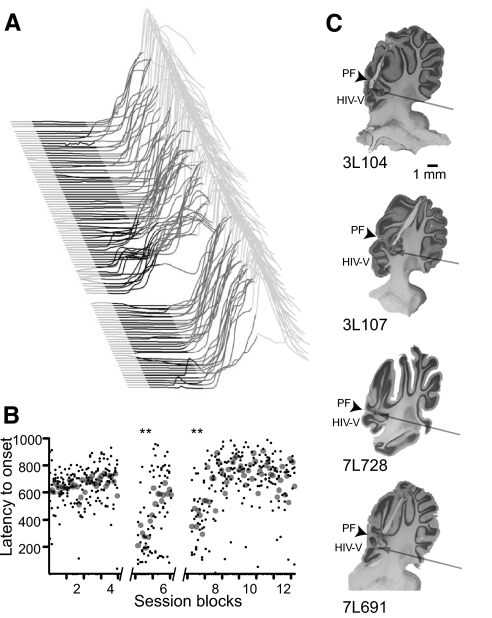
Fig. 7.
Infusing lidocaine into anterior lobe during trace conditioning unmasks short-latency responses. A: point-by-point averages of behavioral sweeps of 5 subjects during a lidocaine infusion. The black and dark gray shaded regions represent the duration of the CS and the stimulus-free trace interval, respectively. The gap between sweeps shows the time at which the infusion was administered. B: the latency to onset of responses is plotted as a function of the trial number. Small black points are data from individual trials from individual subjects and large gray points are mean values from all subjects during a trial. Notice, as with delay responses, the transient nature of the lidocaine effect. Double asterisk, P < 0.01. Comparisons were made between the last block before infusions and each subsequent postinfusion block using Tukey's test to correct for multiple comparisons. C: cannula placements for each animal are shown. The cannula placement for 1 animal (3L107) is not shown because the brain was mistakenly discarded before histological processing.
Fig. 8.
Raw data from 2 subjects administered ACSF, lidocaine, and gabazine infusions after trace conditioning. Gabazine infusions into cerebellar cortex appear to have abolished or suppressed responses while lidocaine infusions unmasked short-latency responses. ACSF infusions produced no measurable changes in responding.
Fig. 4.
Within-animal comparison of the effects of pharmacologically silencing cerebellar cortex versus blocking Purkinje cell input to the cerebellar deep nuclei (DCN). A: a lidocaine infusion unmasked short-latency responses within 10 trials of infusion. Picrotoxin infusion initially abolished responses and then unmasked short-latency responses. B: the location of the cannula was between anterior lobe and the DCN. C: the latency to onset and latency to criterion of responses as a function of trial number. The data shown above are from the picrotoxin infusion and those shown below and to the left are from the lidocaine infusion. Botom right: the mean latency to onset and criterion during the 2 infusions were similar.
Data analysis
For each trial, 2,500 ms of data were collected at 1 kHz and at 12 bit resolution; this involved the 200 ms prior to and 2,300 ms after CS onset. Data were stored to computer disk for subsequent off-line analysis. Response measures calculated for each trial included: peak conditioned response amplitude, peak unconditioned response amplitude, latency to onset, latency to criterion, and percent conditioned responses. Peak amplitude was measured during the interstimulus interval of all trials as was calculated relative to the average of the 200 ms baseline collected prior to CS onset. The criterion for a conditioned response was a movement of the eyelid during the CS that reached ≥0.3 mm prior to US onset during paired trials, and prior to CS offset in CS-alone trials. Trials for which there was >0.3 mm of movement during the baseline were excluded from further analysis. Latency to criterion was the time at which the 0.3 mm criterion was achieved. Latency to onset was determined using an algorithm designed to detect the initial deflection of each response away from the baseline. Statistical analyses involved one- and two-way repeated measures ANOVAs. Because of the transient nature of lidocaine effects, statistical analyses for cortex infusions involved ANOVA followed by post hoc comparisons of responses during the last block before the infusion to each subsequent postinfusion block using Tukey's test to correct for multiple comparisons. Criterion for statistical significance for all comparisons was 0.05 (2-tailed).
To determine the approximate distance from midline of effective infusions, we correlated the effectiveness of each infusion to its medial-lateral position in cerebellar cortex. Infusion effectiveness was quantified by creating an effectiveness scale (range: 1–3). We based the rating solely on the action of lidocaine because GABA antagonists could abolish CRs when infused a small distance away from relevant Purkinje cells by acting on synapses between Golgi and granule cells and lidocaine can presumably only affect the timing of responses by acting directly on Purkinje cells or their axons. In this scale the complete absence of an effect for lidocaine was rated 1, whereas robust effects rated 3. Partial/weak effects were rated 2. This infusion effectiveness rating was determined blind by one experimenter. Individual cannula placements were then ordered from most medial to most lateral by a second experimenter also blind to the animal number and to the infusion rating. The lateral placement of the cannula was determined using local anatomical landmarks within ∼2 mm of the cannula tract. As cannula tracts could be observed in multiple saggital sections, the section with the largest marking lesion was chosen for this analysis. The effectiveness scores were plotted as a function of their relative distance from midline. One brain was accidentally discarded before histological verification of the cannula placement could be made. Data from this animal were not included in this analysis.
Histology
Following experiments, the location of the cannula tip was determined for each animal using standard histological procedures. Briefly, the infusion site was marked by passing DC current (200 μA for 20 s) through a small insulated stainless steel wire cut to the length of the internal cannula and exposed at the tip. Animals were killed with an overdose of sodium pentobarbital and perfused intracardially with 0.9% saline (1.0 l) followed by 10% formalin (1.0 l). The brains were removed and stored in 10% buffered formalin for ≥2 wk. Brains were embedded in an albumin gelatin mixture, and the cerebellum was sectioned using a freezing microtome (80 mm sections). Tissue was mounted and stained with cresyl violet. We used the anatomical nomenclature of Larsell (1970).
RESULTS
Cerebellar cortex and delay conditioning
To silence Purkinje cells, we infused into cerebellar cortex the local anesthetic lidocaine, whereas to increase tonic Purkinje cell activity (and therefore increase tonic inhibition of DCN), we infused the GABAA receptor antagonist picrotoxin. Because Purkinje cells receive strong inhibition from basket and stellate cells, blocking inhibition presumably increases their tonic activity and precludes large phasic decreases in activity. We used lidocaine rather than AMPA receptor antagonists as used in a previous report (Attwell et al. 1999) because Purkinje cells are spontaneously active in the absence of synaptic input (Häusser et al. 2004). Thus infusing AMPA receptor antagonists such as CNQX in cerebellar cortex may not have the desired effect of preventing Purkinje cells from inhibiting DCN but may instead prevent CS-activated granule cells from influencing Purkinje cell activity.
We conducted extensive preliminary experiments to determine the best cannula placements and infusion parameters. We tested a variety of infusion sites and infusion protocols including: single infusions, two infusions per session separated by 10 min, and continuous infusions. An example of a continuous infusion is shown in Fig. 4 and will be discussed later. Although these experiments were informative and produced results in qualitative agreement with the results we report, these variations made group comparisons difficult. We therefore adopted a standardized infusion protocol that was used for the four animals the data of which are shown in Figs. 1–3. In separate test sessions, each of these animals received infusions of lidocaine (4%), picrotoxin (0.5 mM), ACSF control, and a second infusion of lidocaine after conditioned responses were extinguished. For ACSF and lidocaine, there were two infusions per session, one after the 36th trial and another after the 54th trial. For picrotoxin, there was only one infusion following the 36th trial of the test session.
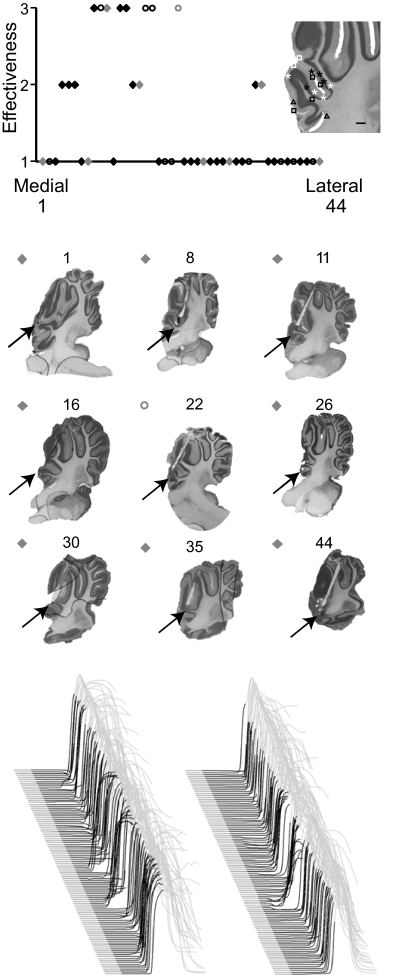
Fig. 3.
Effective cannula placements clustered at the same relative distance from midline. Top: the effectiveness of infusions (ordinate) is correlated to its rank ordered medial-lateral placement in anterior lobe (abscissa). Diamonds, delay conditioned animals and circles represent trace conditioned animals. Shaded symbols correspond to the para-saggital sections shown at middle. Inset: infusion sites from 16 subjects with cannula placements at approximately the effective lateral distance from midline are plotted. Black and white symbols, delay and trace conditioned rabbits, respectively. Asterisks, infusions scored “3;” triangles, infusions scored “2;” squares, infusions scored “1.” Bottom: 2 examples of lidocaine infusions rated 2. Infusion rated 3 can be seen in Figs. 2 and 7. Infusions rated 1 are similar to the ACSF infusions shown in Figs. 2 and 7.
In four animals, we observed robust effects of lidocaine and picrotoxin infusions on the expression of learned responses (Fig. 1). In contrast, ACSF had no measurable effects on response likelihood, amplitude or latency to onset (Fig. 1, A, left, and B; P > 0.05). Lidocaine infusions unmasked short-latency responses similar to those produced after lesions of cerebellar cortex or infusions of the GABAA receptor antagonists picrotoxin or gabazine into the DCN (Bao et al. 2002; Garcia and Mauk 1998; Medina et al. 2000a, 2001; Ohyama et al. 2002, 2003a; Perrett and Mauk 1995; Perrett et al. 1993) (Fig. 1, A, middle, and B, bottom). This effect was relatively short lasting and was unaccompanied by changes in response likelihood or amplitude (P > 0.05). In contrast, picrotoxin infusions abolished the expression of conditioned responses as evidenced by a longer lasting reduction in amplitude and likelihood of conditioned responses (Fig. 1, A, right, and B, top, middle). The traces shown in Fig. 1A are point by point averages of the test sessions for all the animals. The effects of these infusions, along with a parasagittal view of the cannula placement, are shown in Fig. 2 for each animal. These effective cannula placements clustered around the primary fissure and deeper in lobules HIV and V of anterior lobe.
In contrast, picrotoxin and lidocaine infusions in 29 other delay conditioned animals had little to no effect on the expression of learned responses. Similar to effective infusion sites, ineffective infusion sites were around the primary fissure and deeper in lobules HIV and V of anterior lobe (Fig. 3). Because climbing fibers that code for similar stimuli project to similar parasagittal zones of cerebellar cortex (Atkins and Apps 1997; Hesslow 1994a,b), the medial-lateral position of infusions within anterior lobe may contribute to their effectiveness. To test this, we examined the effectiveness of individual infusions to their relative distance from midline (methods). We found that ineffective infusion sites were distributed medial and lateral to a cluster of effective sites (Fig. 3). Within this cluster of effective infusion sites there were a few ineffective infusions, suggesting that medial-lateral placement is just one of many factors contributing to the effectiveness of an infusion. Indeed these ineffective infusions that were clustered among effective ones tended to be placed between lobules or in white matter (Fig. 3, inset).
Last, the results from one experiment seemed especially informative (Fig. 4). During this experiment, the animal was tested with picrotoxin and lidocaine using infusion parameters that differed from the standardized protocol described in the preceding text. The lidocaine (4%) protocol involved an initial infusion of 0.5 μl/min for 2 min after the 36th trial and, on continuation of the training trials, a continuous infusion of 0.05 μl/min for the remainder of the session (2.5 μl total). This infusion unmasked robust short-latency responses that appeared ∼5 min after the onset of the first infusion and that persisted throughout the remainder of the session (Fig. 4, A, left, and C). During a separate test session, we infused an initial bolus of picrotoxin (500 μM) after the 36th trial at a rate of 0.5 μl/min for 2 min followed by a continuous infusion of 0.1 μl/min for another 10 min (2 μl total). For this infusion, there was an initial abolition of conditioned responses, consistent with the effects of picrotoxin in the fixed infusion protocol animals (Fig. 1). However, beginning on the 56th postinfusion trial, ∼23 min after the initial infusion, there was an abrupt change in the effect from abolition to short-latency responses (Fig. 4, A, right, and C). These short-latency responses were eventually as robust as those seen with the lidocaine infusion. This transition may have occurred because of diffusion of picrotoxin to the DCN, where its action of blocking the Purkinje cell to DCN cell synapses should override its action of increasing the output of the cerebellar cortex. This interpretation is supported by the cannula placement, which was offset somewhat from the primary fissure in the direction of the anterior interpositus nucleus of the DCN (Fig. 4B; compare with cannula placements in Fig. 3). Acknowledging the assumptions of this interpretation (for example, because lidocaine infusions did not eventually abolish responses—as would be predicted if lidocaine diffused to the DCN—we must assume that lidocaine does not diffuse as far as picrotoxin), we find these effects interesting in part because they may provide a functional readout of the extent over which such an infusion spreads over tens of minutes. We believe the extent of diffusion to be underestimated in most studies of this kind and that these effects should signal caution in interpreting experiments where the selective action on cerebellar cortex or DCN is conceptually relevant to the interpretation.
This effect also provides an opportunity to make a within-animal comparison of short-latency responses elicited by lidocaine in the cerebellar cortex versus picrotoxin in the DCN. The effects of these two infusions on two measures of response latency (latency to criterion and latency to onset) are shown in Fig. 4C, right. The summary graph depicts averages of these measures over the last 70 trials of each session, where the latency of responding was stable in each case. The responses are quite similar other than a subtle tendency for the latency of the picrotoxin-elicited responses to be slightly shorter than those for lidocaine. These similarities are consistent with the notion that the short-latency responses in each case arise from the same functional manipulation, namely, silencing communication between the Purkinje cells and their downstream targets in the DCN either directly via lidocaine in the cortex or indirectly via the putative diffusion of picrotoxin into the DCN.
Cerebellar cortex and extinction
Previous findings indicate that the extinction of conditioned eyelid responses involves the reestablishment of Purkinje cell activity during the CS (Jirenhed et al. 2007; Mauk and Ohyama 2004; Medina et al. 2001, 2002). For example, learned pauses in Purkinje cell activity that develop during the acquisition of conditioned eyelid responses diminish during extinction (Jirenhed et al. 2007). As Purkinje cells are inhibitory and are the sole output of cerebellar cortex (Ito 1984), this increase in activity would result in extinction by inhibiting the DCN. Because infusions of GABAA antagonists into the DCN reveal short-latency responses well after responses have been extinguished, increases in Purkinje cell activity during extinction appear to leave plasticity in the DCN somewhat intact (Medina et al. 2001). Thus decreasing cerebellar cortex output by infusing lidocaine directly into the cortex after responses are extinguished should reveal residual plasticity in the DCN as evidenced by the appearance of short-latency responses.
To test for short-latency responses after extinction, we extinguished delay responses for five sessions and then infused lidocaine into the cerebellar cortex of four animals with effective cannula placements (Figs. 1 and 2). Five sessions of extinction resulted in full behavioral extinction (data not shown). In all four animals, the subsequent repeat infusion of lidocaine with the same infusion parameters as employed in Fig. 2 unmasked short-latency responses similar to those observed before extinction from a baseline of nonresponding (Fig. 5). These responses are unlikely to be unlearned responses reflecting tonic changes in the eyeblink circuit (Bracha et al. 2009) because previous reports have failed to find short-latency responses in naïve subjects (Ohyama et al. 2006) or in response to an input that has not been paired with the US (Garcia et al. 1999; Ohyama et al. 2003a; but see Poulos et al. 2009). Together with previous reports (Medina et al. 2001), these data show that reversible disconnection of cerebellar cortex from its synaptic targets in DCN unmasks short-latency responses after extinction of conditioned responses. Furthermore, they suggest that extinction involves a learned increase in Purkinje cell activity during the CS, leaving residual plasticity in the DCN (Jirenhed et al. 2007; Mauk and Ohyama 2004; Medina et al. 2001, 2002).
Cerebellar cortex and trace conditioning
Do other types of cerebellar-dependent eyelid conditioning paradigms similarly involve cerebellar cortex? While it is increasingly clear that trace eyelid conditioning involves cerebellar learning to a forebrain-driven input (Clark and Squire 1998; Clark et al. 2002; Kalmbach et al. 2009; Kronforst-Collins and Disterhoft 1998; Moyer et al. 1990; Simon et al. 2005; Weible et al. 2000; Woodruff-Pak et al. 1985), a specific role for cerebellar cortex in trace conditioning has recently been questioned. Many genetic manipulations that affect the integrity of cerebellar cortex impair delay and not trace conditioning (Kishimoto and Kano 2006; Kishimoto et al. 2001a,b; Woodruff-Pak et al. 2006). This has led to the interpretation that trace, unlike delay conditioning, does not require the cerebellar cortex (Woodruff-Pak and Disterhoft 2008).
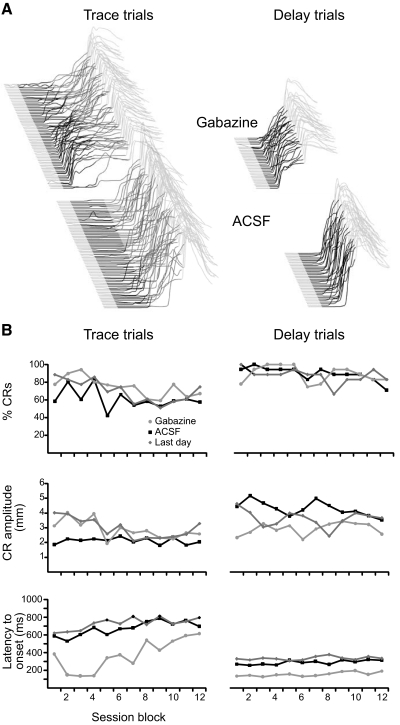
We first addressed this issue by infusing the GABAA receptor antagonist gabazine into the DCN of animals well trained in a dual delay/trace conditioning procedure (Kalmbach et al. 2009). This procedure, which involves alternating delay and trace trials, allowed us to test for short-latency responses during delay and trace conditioning in the same animal and test session. Infusions of gabazine unmasked short-latency responses that began shortly after CS onset in both delay and trace conditioning trials and did not affect response likelihood or amplitude (Fig. 6, A and B; P < 0.01 for both delay and trace; Tukey's post hoc test comparing latency to onset during gabazine infusion session with last day of training). ACSF infusions did not affect response latency, likelihood, or amplitude (P > 0.05, Tukey's post hoc test comparing performance during ACSF infusion session with last day of training). We also found that gabazine infusions into the DCN resulted in similar short-latency responses in three subjects trained in trace conditioning alone, suggesting that the similarity of delay and trace short-latency responses is not an artifact of the dual delay/trace conditioning procedure (data not shown). These data suggest that the expression of trace responses, like delay responses, involves plasticity in cerebellar cortex and DCN.
Fig. 6.
Short-latency responses elicited by infusions of gabazine into the DCN during dual delay/trace conditioning. A: point-by-point averages of trace (left) and delay (right) conditioning behavioral sweeps from 6 subjects during infusions of ACSF and gabazine. Delay and trace trials were interleaved during the experiment but have been separated here for clarity of presentation. The black and dark gray shaded regions mark the CS and the stimulus-free trace interval, respectively. B: trace (left) and delay (right) response likelihood, amplitude, and latency to onset are plotted during the last day of training (dark gray), the ACSF infusion session (black) and the gabazine infusion session (lighter gray) as a function of session block. Only the latency to onset of trace and delay responses during the gabazine infusion differed from the last day of infusion. Note that there are twice as many trace trials as delay trials.
Do trace and delay conditioning rely on similar areas of cerebellar cortex? We addressed this issue by infusing lidocaine into the cerebellar cortex of subjects well-trained in trace conditioning. In five subjects, infusions had no effect on response likelihood or amplitude and a transient large effect on the latency to onset of responses (Fig. 7). In two of these subjects, we also infused gabazine and ACSF in separate test sessions. The raw traces from these infusion sessions are shown in Fig. 8 and show that while ACSF infusions did not affect responses (P > 0.05; blocks effect, 1-way ANOVA) gabazine infusions transiently abolished responses (P < 0.05; blocks effect, 1-way ANOVA). The cannula in these subjects was located around the primary fissure and in lobules HIV-V of anterior lobe at approximately the same lateral distance from midline as effective delay conditioning infusions (Figs. 3 and 7C). In contrast, in seven animals infusions did not affect response expression. Cannula in these subjects were also located around the primary fissure and lobules HIV-V but distributed lateral and medial to effective ones (Figs. 3 and 7C). The similarity in effective cannula placements for subjects trained on delay and trace conditioning suggests that properly timed delay and trace conditioned responses rely on similar areas of cerebellar cortex.
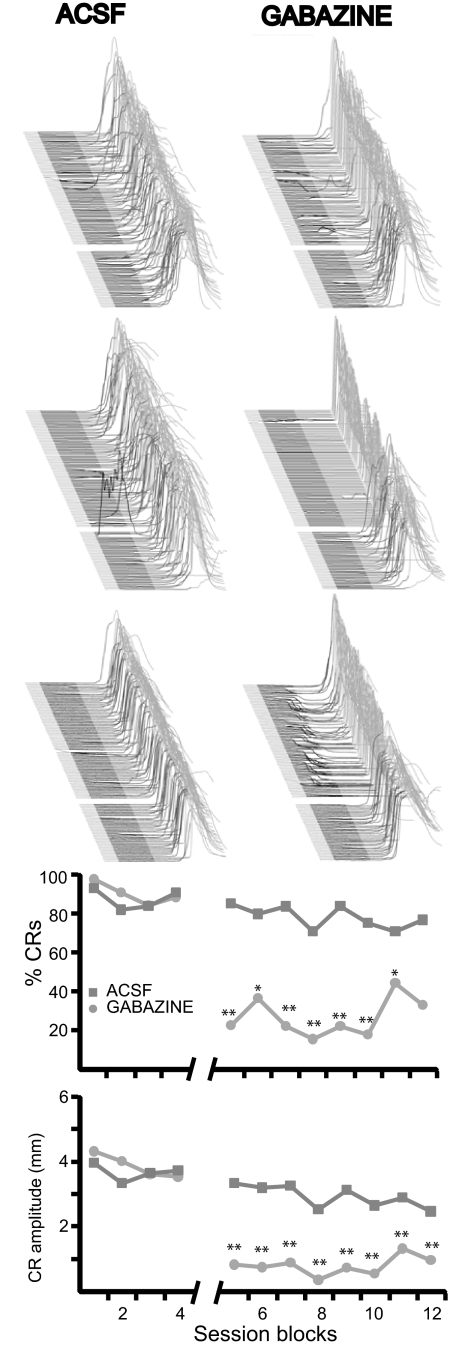
In addition to the two subjects presented in Fig. 8, infusions of gabazine into cerebellar cortex of three other subjects abolished trace conditioned responses (P < 0.05; blocks effect, 1-way ANOVA) while infusions of ACSF had no effect (P > 0.05; blocks effect, 1-way ANOVA; Fig. 9). Because gabazine infusions may abolish responses by acting on synapses that are remote to the Purkinje cells necessary for trace conditioning, these infusions were not used to localize the area of cerebellar cortex necessary for the expression of trace eyelid responses. Nonetheless, these infusions, together with those in Fig. 8, support the hypothesis that cerebellar cortex is necessary for the expression of trace eyelid responses.
Fig. 9.
Gabazine infusions into cerebellar cortex abolish trace eyelid responses. Top: raw data from 3 subjects where gabazine infusions abolished trace eyelid responses and ACSF infusions did not. Below: response likelihood and amplitude from these 3 subjects and the 2 presented in Fig. 8 are plotted as a function of session block. **, P < 0.01; *, P < 0.05. Comparisons were made between the last block before infusions and each subsequent postinfusion block using Tukey's test to correct for multiple comparisons.
DISCUSSION
We have provided evidence that the expression of properly timed conditioned eyelid responses involves a well-timed decrease in Purkinje cell activity in anterior lobe. Increasing the tonic output of anterior lobe by infusing the GABAA receptor antagonist, picrotoxin, reversibly abolished the expression of learned eyelid responses. This suggests that the expression of cerebellar learning requires a decrease in Purkinje cell activity during the CS. Conversely, silencing the same region of anterior lobe by directly infusing lidocaine reversibly abolished the learned timing of both delay and trace eyelid responses, suggesting that decreases in Purkinje cell activity must be well timed to produce properly timed responses.
These data are seemingly inconsistent with previous reports that lesions of cerebellar cortex or infusions of AMPA receptor antagonists in cerebellar cortex abolish conditioned responses but do not unmask short-latency responses (Attwell et al. 1999; Yeo et al. 1985). Two observations may reconcile these reports with ours. First, Purkinje cells are spontaneously active in the absence of synaptic input (Häusser et al. 2004). This suggests that infusions of AMPA receptor antagonists in cerebellar cortex may not have the desired effect of preventing Purkinje cells from inhibiting the DCN. Rather, these infusions may prevent the expression of learned pauses in Purkinje cell activity by preventing CS-activated granule cells from influencing Purkinje cell activity. We used infusions of the local anesthetic lidocaine to block sodium channels, a more certain method to decrease Purkinje cell activity. Second, our infusions were made into anterior lobe, whereas previous manipulations were made in lobule HVI. Thus it is possible that manipulations in different areas of cerebellar cortex have different effects on the expression of eyelid responses. We return to this important issue of localization later in the discussion.
Our data are consistent with the growing body of evidence that cerebellar learning involves a learned decrease in Purkinje cell activity. Granule cell-to-Purkinje cell synapses active coincidently with climbing fiber input undergo LTD (Coesmans et al. 2004; Ito 2001; Ito and Kano 1982; Lev-Ram et al. 2003). Thus subsequent activation of these synapses would tend to decrease Purkinje cell activity and thus increase cerebellar output. Consistent with this interpretation, simple spike Purkinje cell activity is modulated by climbing fiber input on a trial-to-trial basis in several cerebellum-dependent behaviors (Hesslow and Ivarsson 1994; Jirenhed et al. 2007; Medina and Lisberger 2008; Raymond and Lisberger 1998). For example, over many trials of delay eyelid conditioning, Purkinje cell activity develops transient pauses during the CS (Hesslow and Ivarsson 1994; Jirenhed et al. 2007; Rasmussen et al. 2008). The timing of this decrease depends on the relative timing of the CS- and the US-evoked climbing fiber input, such that longer CS-US intervals result in a longer latency decrease in activity (Jirenhed et al. 2007). Stimulating these same areas of cerebellar cortex inhibits the expression of conditioned responses, suggesting that decreases in Purkinje cell activity are necessary for responses expression (Hesslow 1994b). Finally, previous studies show that permanent lesions of cerebellar cortex or temporary blockade of cerebellar cortex communication with its downstream targets in the DCN using the GABAA antagonists picrotoxin or gabazine unmask short-latency responses (Bao et al. 2002; Garcia and Mauk 1998; Medina et al. 2000a; Ohyama et al. 2006; Perrett et al. 1993). Together, these data are consistent with the effects of our lidocaine and picrotoxin infusions and suggest that decreases in Purkinje cell activity must be well timed to produce well-timed learned behaviors.
Our data are also consistent with evidence that the modulation of simple spike Purkinje cell activity by climbing fiber input is bidirectional. Granule cell-to-Purkinje cell synapses that are active in the absence of climbing fiber input undergo LTP that reverses long-term potentiation (Coesmans et al. 2004; Lev-Ram et al. 2003). Thus the activation of synapses that have undergone LTP would tend to decrease cerebellar output by increasing Purkinje cell activity. Consistent with this, the bidirectional modulation of simple spike Purkinje cell activity is under the control of climbing fiber input (Jirenhed et al. 2007; Medina and Lisberger 2008; Rasmussen et al. 2008). For example, during eyelid conditioning, decreases in Purkinje cell activity during the CS reverse during extinction training when the CS is presented without the US (Jirenhed et al. 2007). Because Purkinje cells inhibit the DCN (Ito 1984), the extinction of eyelid responses may largely involve an increase in Purkinje cell activity during the CS. Indeed pharmacologically blocking the Purkinje cell-to-DCN synapse by infusing GABAA receptor antagonists into the DCN reveals short-latency responses after previously learned responses have been extinguished (Medina et al. 2001). Because short-latency responses reflect plasticity at the mossy fiber-to DCN synapses (Ohyama et al. 2006), this suggests that extinction is mediated by an increase in Purkinje cell activity that leaves residual plasticity in the DCN (Medina et al. 2001). That lidocaine infusions into cerebellar cortex after extinction resulted in short-latency responses (Fig. 5) is consistent with this interpretation.
Eyelid conditioning relevant region of cerebellar cortex
Our data have implications regarding the region of cerebellar cortex relevant for eyelid conditioning in rabbit. Purkinje cells within a parasagittal strip receive climbing fiber input that codes for similar stimuli (Atkins and Apps 1997; Hesslow 1994a,b; Ito 1984). Thus the relevant part of cerebellar cortex for eyelid conditioning is likely to be contained within a parasaggital zone that receives input from climbing fibers that code for somatasensory input to the eye. In cat and ferret, areas receiving periorbital-driven climbing fiber input are largely confined to the C3 microzone of lobules HV and HVI have been shown to be important for eyelid conditioning (Hesslow 1994a,b; Hesslow and Ivarsson 1994; Jirenhed et al. 2007; Rasmussen et al. 2008).
Unfortunately, the precise location of eyelid-relevant regions of rabbit cerebellar cortex is less clear. We have previously provided evidence using permanent lesions that anterior lobe is necessary for eyelid conditioning (Garcia et al. 1999; Medina et al. 2000a; Perrett and Mauk 1995; Perrett et al. 1993), while others have provided evidence using permanent lesions and infusions of AMPA receptor antagonists that lobule HVI is necessary (Attwell et al. 1999, 2001, 2002; Yeo et al. 1985). Because sagittal zones often span multiple lobules, the eyelid controlling region in rabbit may not be confined to one lobule. Furthermore, given the close proximity of lobule HVI and anterior lobe, infusions make precise localization difficult, unless as in the present study, there is a specific effect on expression that permits some sense of the latency to action of the infusion. With this, it is possible to make estimates not only of effective placements, but of the relative distance of placements from the site of action. For our infusions, the most robust and shortest latencies to action were observed for placements anterior to the primary fissure and therefore in the anterior lobe (Fig. 3) (Larsell 1970). The delayed infusion effects shown in Fig. 4 were associated with a cannula placement near this region but tending caudally toward the white matter between anterior lobe and the interpositus nucleus. These sites are also a good deal more lateral and rostral than the effective HVI lesion sites reported originally (Yeo et al. 1985). While we cannot rule out the possibility that lobule HVI is required for the expression of properly timed conditioned eyelid responses, our data suggest that anterior lobe of cerebellar cortex is required. This is consistent with evidence in rabbit that periorbital stimulation evokes climbing fiber activity in anterior lobe, Purkinje cell activity in anterior lobe decreases during the CS in well-trained animals, and stimulating anterior lobe evokes EMG activity in the muscles that close the eye (Green and Steinmetz 2005).
How far from midline is the eyelid conditioning relevant region of anterior lobe? Without a detailed stereotaxic atlas of the rabbit cerebellum in sagittal sections, it is difficult to say for certain. However, given the animal-to-animal variability in the skull sutures used to define lambda during sterotaxic surgery, our cannula placements were likely distributed around their typical 6 mm target. The observation that ineffective cannula placements tended to be placed more lateral than effective ones suggests that the eyelid relevant region of cerebellar cortex is slightly medial to 6 mm lateral from midline (assuming an even distribution of placements). Ongoing mapping studies, similar to those made in ferrets and cats (Hesslow 1994a; Hesslow and Ivarsson 1994), may more precisely define the eyelid conditioning relevant microzone in rabbit anterior lobe.
Cerebellar cortex and trace conditioning
Because the effects of infusing lidocaine into cerebellar cortex or gabazine into the DCN during trace conditioning were similar to those during delay conditioning, our data indicate that trace conditioning, like delay conditioning, requires a well-timed decrease in Purkinje cell activity and involves plasticity in DCN. Furthermore, because effective cannula placements for trace conditioned animals were similar to those for delay conditioned animals (Fig. 3), the timing of delay and trace conditioning appears to rely on similar areas of cerebellar cortex. Together, these data suggest that trace conditioning requires similar cerebellar learning mechanisms as delay conditioning.
Therefore our data are seemingly inconsistent with hypotheses that trace eyelid conditioning does not require cerebellar cortex (Woodruff-Pak and Disterhoft 2008). This hypothesis comes largely from evidence that genetic manipulations that affect the integrity of cerebellar cortex in mice fail to affect trace conditioning (Kishimoto and Kano 2006; Kishimoto et al. 2001a,b; Woodruff-Pak et al. 2006). Behavioral studies in mutant mice are often difficult to interpret for a number of reasons (e.g., compensatory mechanisms, differences in circuitry between mutant and wild-type mice, etc). Furthermore, differences in the neural circuitry involved in the acquisition of eyelid responses in mice and rabbits make direct comparisons between these species difficult. This hypothesis is also based on previous reports that lesions of the cerebellar cortex only transiently affect the expression of trace conditioned responses. However, these lesions spared anterior lobe and were largely localized to lobule HVI (Woodruff-Pak et al. 1985). Our infusions into anterior lobe suggest that a well-timed pause in Purkinje cell activity is necessary for the production of well-timed trace conditioned eyelid responses.
GRANTS
This work was supported by National Institute of Mental Health Grants MH-46904, MH-57051, and MH-46904.
ACKNOWLEDGMENTS
We thank N. Taylor and F. Riusech for their assistance with histology and surgery.
REFERENCES
- Atkins MJ, Apps R. Somatotopical organization within the climbing fiber projection to the paramedian lobule and copula pyramidis of the rat cerebellum. J Comp Neurol 389: 249–263, 1997 [PubMed] [Google Scholar]
- Attwell PJ, Cooke SF, Yeo CH. Cerebellar function in consolidation of a motor memory. Neuron 34: 1011–1020, 2002 [DOI] [PubMed] [Google Scholar]
- Attwell PJ, Rahman S, Ivarsson M, Yeo CH. Cerebellar cortical AMPA-kainate receptor blockade prevents performance of classically conditioned nictitating membrane responses. J Neurosci 19: RC45, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell PJ, Rahman S, Yeo CH. Acquisition of eyeblink conditioning is critically dependent on normal function in cerebellar cortical lobule HVI. J Neurosci 21: 5715–5722, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao S, Chen L, Kim JJ, Thompson RF. Cerebellar cortical inhibition and classical eyeblink conditioning. Proc Natl Acad Sci USA 99: 1592–1597, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracha V, Zbarska S, Parker K, Carrel A, Zenitsky G, Bloedel JR. The cerebellum and eye-blink conditioning: learning versus network performance hypotheses. Neuroscience 162: 787–796, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RE, Manns JR, Squire LR. Classical conditioning, awareness, and brain systems. Trends Cogn Sci 6: 524–531, 2002 [DOI] [PubMed] [Google Scholar]
- Clark RE, Squire LR. Classical conditioning and brain systems: the role of awareness. Science 280: 77–81, 1998 [DOI] [PubMed] [Google Scholar]
- Coesmans M, Weber JT, De Zeeuw CI, Hansel C. Bidirectional parallel fiber plasticity in the cerebellum under climbing fiber control. Neuron 44: 691–700, 2004 [DOI] [PubMed] [Google Scholar]
- Eccles JC, Ito M, Szentagothai J. The Cerebellum as a Neuronal Machine. Berlin: Springer-Verlag, 1967 [Google Scholar]
- Garcia KS, Mauk MD. Pharmacological analysis of cerebellar contributions to the timing and expression of conditioned eyelid responses. Neuropharmacology 37: 471–480, 1998 [DOI] [PubMed] [Google Scholar]
- Garcia KS, Steele PM, Mauk MD. Cerebellar cortex lesions prevent acquisition of conditioned eyelid responses. J Neurosci 19: 10940–10947, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JT, Steinmetz JE. Purkinje cell activity in the cerebellar anterior lobe after rabbit eyeblink conditioning. Learn Mem 12: 260–269, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansel C, Linden DJ, D'Angelo E. Beyond parallel fiber LTD: the diversity of synaptic and non-synaptic plasticity in the cerebellum. Nat Neurosci 4: 467–475, 2001 [DOI] [PubMed] [Google Scholar]
- Häusser M, Raman IM, Otis T, Smith SL, Nelson A, du Lac S, Loewenstein Y, Mahon S, Pennartz C, Cohen I, Yarom Y. The beat goes on: spontaneous firing in mammalian neuronal microcircuits. J Neurosci 24: 9215–9219, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesslow G. Correspondence between climbing fiber input and motor output in eyeblink-related areas in cat cerebellar cortex. J Physiol 476: 229–244, 1994a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesslow G. Inhibition of classically conditioned eyeblink responses by stimulation of the cerebellar cortex in the decerebrate cat. J Physiol 476: 245–256, 1994b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesslow G, Ivarsson M. Suppression of cerebellar Purkinje cells during conditioned responses in ferrets. Neuroreport 5: 649–652, 1994 [DOI] [PubMed] [Google Scholar]
- Ito M. Purkinje cells: membrane and synapse functions. In: The Cerebellum and Neural Control New York: Raven, 1984, p. 300–302 [Google Scholar]
- Ito M. Cerebellar long-term depression: characterization, signal transduction, and functional roles. Physiol Rev 81: 1143–1195, 2001 [DOI] [PubMed] [Google Scholar]
- Ito M, Kano M. Long-lasting depression of parallel fiber-Purkinje cell transmission induced by conjunctive stimulation of parallel fibers and climbing fibers in the cerebellar cortex. Neurosci Lett 33: 253–258, 1982 [DOI] [PubMed] [Google Scholar]
- Jirenhed DA, Bengtsson F, Hesslow G. Acquisition, extinction, and reacquisition of a cerebellar cortical memory trace. J Neurosci 27: 2493–2502, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmbach BE, Ohyama T, Kreider JC, Riusech F, Mauk MD. Interactions between prefrontal cortex and cerebellum revealed by trace eyelid conditioning. Learn Mem 16: 86–95, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto Y, Kano M. Endogenous cannabinoid signaling through the CB1 receptor is essential for cerebellum-dependent discrete motor learning. J Neurosci 26: 8829–8837, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto Y, Kawahara S, Fujimichi R, Mori H, Mishina M, Kirino Y. Impairment of eyeblink conditioning in GluRdelta2-mutant mice depends on the temporal overlap between conditioned and unconditioned stimuli. Eur J Neurosci 14: 1515–1521, 2001a [DOI] [PubMed] [Google Scholar]
- Kishimoto Y, Kawahara S, Suzuki M, Mori H, Mishina M, Kirino Y. Classical eyeblink conditioning in glutamate receptor subunit delta 2 mutant mice is impaired in the delay paradigm but not in the trace paradigm. Eur J Neurosci 13: 1249–1253, 2001b [DOI] [PubMed] [Google Scholar]
- Kronforst-Collins MA, Disterhoft JF. Lesions of the caudal area of rabbit medial prefrontal cortex impair trace eyeblink conditioning. Neurobiol Learn Mem 69: 147–162, 1998 [DOI] [PubMed] [Google Scholar]
- Larsell O. Rabbit. Minneapolis, MN: University of Minnesota Press, 1970 [Google Scholar]
- Lev-Ram V, Mehta SB, Kleinfeld D, Tsien RY. Reversing cerebellar long-term depression. Proc Natl Acad Sci USA 100: 15989–15993, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr D. A theory of cerebellar cortex. J Physiol 202: 437–470, 1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauk MD, Donegan NH. A model of Pavlovian eyelid conditioning based on the synaptic organization of the cerebellum. Learn Mem 4: 130–158, 1997 [DOI] [PubMed] [Google Scholar]
- Mauk MD, Ohyama T. Extinction as new learning versus unlearning: considerations from a computer simulation of the cerebellum. Learn Mem 11: 566–571, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina JF, Garcia KS, Mauk MD. A mechanism for savings in the cerebellum. J Neurosci 21: 4081–4089, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina JF, Garcia KS, Nores WL, Taylor NM, Mauk MD. Timing mechanisms in the cerebellum: testing predictions of a large-scale computer simulation. J Neurosci 20: 5516–5525, 2000a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina JF, Lisberger SG. Links from complex spikes to local plasticity and motor learning in the cerebellum of awake-behaving monkeys. Nat Neurosci 11: 1185–1192, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina JF, Mauk MD. Simulations of cerebellar motor learning: computational analysis of plasticity at the mossy fiber to deep nucleus synapse. J Neurosci 19: 7140–7151, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina JF, Nores WL, Mauk MD. Inhibition of climbing fibers is a signal for the extinction of conditioned eyelid responses. Nature 416: 330–333, 2002 [DOI] [PubMed] [Google Scholar]
- Medina JF, Nores WL, Ohyama T, Mauk MD. Mechanisms of cerebellar learning suggested by eyelid conditioning. Curr Opin Neurobiol 10: 717–724, 2000b [DOI] [PubMed] [Google Scholar]
- Moyer JR, Jr, Deyo RA, Disterhoft JF. Hippocampectomy disrupts trace eye-blink conditioning in rabbits. Behav Neurosci 104: 243–252, 1990 [DOI] [PubMed] [Google Scholar]
- Ohyama T, Medina JF, Nores WL, Mauk MD. Trying to understand the cerebellum well enough to build one. Ann NY Acad Sci 978: 425–438, 2002 [DOI] [PubMed] [Google Scholar]
- Ohyama T, Nores WL, Mauk MD. Stimulus generalization of conditioned eyelid responses produced without cerebellar cortex: implications for plasticity in the cerebellar nuclei. Learn Mem 10: 346–354, 2003a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama T, Nores WL, Medina JF, Riusech FA, Mauk MD. Learning-induced plasticity in deep cerebellar nucleus. J Neurosci 26: 12656–12663, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama T, Nores WL, Murphy M, Mauk MD. What the cerebellum computes. Trends Neurosci 26: 222–227, 2003b [DOI] [PubMed] [Google Scholar]
- Perrett SP, Mauk MD. Extinction of conditioned eyelid responses requires the anterior lobe of cerebellar cortex. J Neurosci 15: 2074–2080, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrett SP, Ruiz BP, Mauk MD. Cerebellar cortex lesions disrupt learning-dependent timing of conditioned eyelid responses. J Neurosci 13: 1708–1718, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulos AM, Nobuta H, Thompson RF. Disruption of cerebellar cortical inhibition in the absence of learning promotes sensory-evoked eyeblink responses. Behav Neurosci 123: 694–700, 2009 [DOI] [PubMed] [Google Scholar]
- Pugh JR, Raman IM. Potentiation of mossy fiber EPSCs in the cerebellar nuclei by NMDA receptor activation followed by postinhibitory rebound current. Neuron 51: 113–123, 2006 [DOI] [PubMed] [Google Scholar]
- Pugh JR, Raman IM. Mechanisms of potentiation of mossy fiber EPSCs in the cerebellar nuclei by coincident synaptic excitation and inhibition. J Neurosci 28: 10549–10560, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen A, Jirenhed DA, Hesslow G. Simple and complex spike firing patterns in purkinje cells during classical conditioning. Cerebellum 7: 563–566, 2008 [DOI] [PubMed] [Google Scholar]
- Raymond JL, Lisberger SG. Neural learning rules for the vestibulo-ocular reflex. J Neurosci 18: 9112–9129, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond JL, Lisberger SG, Mauk MD. The cerebellum: a neuronal learning machine? Science 272: 1126–1131, 1996 [DOI] [PubMed] [Google Scholar]
- Simon B, Knuckley B, Churchwell J, Powell DA. Post-training lesions of the medial prefrontal cortex interfere with subsequent performance of trace eyeblink conditioning. J Neurosci 25: 10740–10746, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weible AP, McEchron MD, Disterhoft JF. Cortical involvement in acquisition and extinction of trace eyeblink conditioning. Behav Neurosci 114: 1058–1067, 2000 [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Disterhoft JF. Where is the trace in trace conditioning? Trends Neurosci 31: 105–112, 2008 [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Green JT, Levin SI, Meisler MH. Inactivation of sodium channel Scn8A (Na-sub(v)1.6) in Purkinje neurons impairs learning in Morris water maze and delay but not trace eyeblink classical conditioning. Behav Neurosci 120: 229–240, 2006 [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Lavond DG, Thompson RF. Trace conditioning: abolished by cerebellar nuclear lesions but not lateral cerebellar cortex aspirations. Brain Res 348: 249–260, 1985 [DOI] [PubMed] [Google Scholar]
- Yeo CH, Hardiman MJ, Glickstein M. Classical conditioning of the nictitating membrane response of the rabbit. II. Lesions of the cerebellar cortex. Exp Brain Res 60: 99–113, 1985 [DOI] [PubMed] [Google Scholar]