Abstract
Endogenous trypsin inhibitors are synthesized, stored, and secreted by pancreatic acinar cells. It is believed that they play a protective role in the pancreas by inhibiting trypsin within the cell should trypsinogen become prematurely activated. Rodent trypsin inhibitors are highly homologous to human serine protease inhibitor Kazal-type 1 (SPINK1). The mouse has one pancreatic trypsin inhibitor known as SPINK3, and the rat has two trypsin inhibitors commonly known as pancreatic secretory trypsin inhibitors I and II (PSTI-I and -II). Rat PSTI-I is a 61-amino acid protein that shares 65% sequence identity with mouse SPINK3. It was recently demonstrated that mice with genetic deletion of the Spink3 gene (Spink3−/−) do not survive beyond 15 days and lack normal pancreata because of pancreatic autophagy. We have shown that targeted transgenic expression of the rat Psti1 gene to acinar cells in mice [TgN(Psti1)] protects mice against caerulein-induced pancreatitis. To determine whether the autophagic phenotype and lethality in Spink3−/− mice were due to lack of pancreatic trypsin inhibitor, we conducted breeding studies with Spink3+/− heterozygous mice and TgN(Psti1) mice. We observed that, whereas Spink3+/+, Spink3+/−, and Spink3−/−/TgN(Psti1) mice had similar survival rates, no Spink3−/− mice survived longer than 1 wk. The level of expression of SPINK3 protein in acini was reduced in heterozygote mice compared with wild-type mice. Furthermore, endogenous trypsin inhibitor capacity was reduced in the pancreas of heterozygote mice compared with wild-type or knockout mice rescued with the rat Psti1 gene. Surprisingly, the lesser amount of SPINK3 present in the pancreata of heterozygote mice did not predispose animals to increased susceptibility to caerulein-induced acute pancreatitis. We propose that a threshold level of expression is sufficient to protect against pancreatitis.
Keywords: PSTI-I, pancreatitis, autophagy
trypsin is synthesized in the pancreas in the form of inactive trypsinogen and stored in zymogen granules within acinar cells. Pancreatic secretory trypsin inhibitor (PSTI) is an endogenous protein inhibitor of trypsin that is also present in zymogen granules and inhibits prematurely activated trypsin to protect the pancreas from possible damage (11). Serine protease inhibitor Kazal-type 1 (SPINK1) is the human homolog of PSTI and is expressed at high levels in the pancreas (7) representing 0.1–0.6% of total pancreatic protein (8) and 0.1–0.8% of the total protein in pancreatic juice (28). It has been estimated that the amount of trypsinogen exceeds the amount of trypsin inhibitor in the pancreas, and in humans it has been shown that SPINK1 mRNA levels are 1,000-fold lower than cationic trypsinogen (PRSS1) levels (14) raising the possibility that should trypsinogen become active in the pancreas there would be insufficient inhibitor to prevent activation of other zymogens and the subsequent deleterious effects of active enzymes within the pancreas.
SPINK1 is one of three known genes involved in chronic pancreatitis in humans (3, 15, 16, 33); the two other genes being PRSS1 (cationic trypsinogen) (32) and CFTR (cystic fibrosis transmembrane conductance regulator) (5). In contrast to PRSS1, which has been identified as a high-penetrance gene leading to chronic pancreatitis through gain-of-function mutations (2), SPINK1 appears to be an intermediate-penetrance gene in which the most common variant allele (N34S) increases pancreatitis susceptibility (2, 13). Homozygote mutation at position 34 (N34S) confers a high-penetrance effect of SPINK1 on chronic pancreatitis (13, 33).
The mouse homolog of human SPINK1 is SPINK3. SPINK3 is expressed early during embryogenesis as indicated by in situ hybridization and LacZ staining of transgenic Spink3+/lacz mice. It is detected in the foregut, midgut, and hindgut as well as forebrain/midbrain at 9.5 days postcoitus (dpc) (31). SPINK3 appears in the pancreas at 11.5 dpc and is also present in the large intestine at 11.5 dpc, small intestine at 13.5 dpc, and genital system at 13.5 dpc. Interestingly, SPINK3 was first characterized in the ventral prostate of adult male mice and was initially named P12 protein (4, 19). Unlike its expression in the pancreas, SPINK3 expression in the prostate is stimulated by testosterone (20). The human PSTI peptide has also been detected in a number of organs in addition to exocrine pancreas including the stomach, intestine, and urinary tract in both fetus and adult (6) and its mRNA is present in exocrine pancreas, gastrointestinal tract, and breast acini (18).
Recently a Spink3 knockout (Spink3−/−) mouse was engineered (26). The pancreata of these mice developed normally up to 15.5 dpc but at 16.5 dpc a progressive degradation of the exocrine pancreas was observed that was attributed to autophagic cell death. Spink3−/− mice did not survive beyond 15 days postpartum. Furthermore, active trypsin was detectable in acini of Spink3−/− mice 0.5–1.5 days old but was absent in wild-type or heterozygote mice (25).
Two related PSTIs are present in the rat pancreas: PSTI-I (also known as PSTI-61 or monitor peptide) and PSTI-II (also known as PSTI-56) (21). There is evidence that PSTI-I but not PSTI-II stimulates the release of cholecystokinin from neuroendocrine cells of the intestine (1, 10, 17, 21, 22). We had previously designed a transgenic mouse in which a minigene composed of the mouse elastase promoter coupled to the rat PSTI-I gene (Psti1) was inserted into the mouse genome. The rat peptide was detected exclusively in pancreatic acini and increased the total trypsin inhibitor activity of the pancreas. Moreover, the severity of experimental secretagogue-induced acute pancreatitis was significantly reduced in PSTI-I transgenic [TgN(Psti1)] mice (23).
In the present study we sought to determine whether the mortality of Spink3 knockout mice was due to lack of trypsin inhibitor in the pancreas and whether transgenic expression of PSTI-I in Spink3−/− mice could compensate for the absence of SPINK3 and confer a normal phenotype to the animal.
MATERIALS AND METHODS
Animal care.
Transgenic mice expressing the rat PSTI-I gene [TgN(Psti1)] were maintained in a C57BL/6J background. Spink3+/+ and Spink3+/− mice in a C57BL/6J background were generated in the laboratory of Dr. Yamamura. Spink3+/− mice were crossed with TgN(Psti1) to obtain mice expressing the Psti1 transgene in heterozygote Spink3+/−. Spink3+/−/TgN(Psti1) mice were subsequently crossed to obtain mice lacking the endogenous Spink3 gene [Spink3−/−/TgN(Psti1)]. Mice were genotyped for Spink3 and Psti1 genes and the expression of SPINK3 was determined by Western blot analysis. Mice were housed in 12:12-h light-dark cycle and given water and chow ad libitum. Studies were approved by the Institutional Animal Care and Use Committee of Duke University.
Western blot analysis.
Expression of SPINK3 was assessed in Spink3+/+ and Spink3+/− pancreata by Western blot analysis using a rabbit anti-mouse SPINK3 antibody. Pancreata were homogenized in 50 mM Tris buffer pH 7.5 containing 150 mM NaCl, 10 mM EDTA, 1 mM DTT, 0.1% SDS, 1% Nonidet P-40, and 0.5% deoxycholic acid sodium salt. Supernates were loaded on a NuPAGE 4–12% Bis-Tris gel (Invitrogen, Carlsbad, CA) and transferred onto nitrocellulose membrane (0.2 μm Hybond; GE Healthcare, Piscataway, NJ). The membrane was cut in half; one half was incubated with monoclonal mouse anti-α-tubulin (Amersham Biosciences, Piscataway, NJ), and the other half was reacted with a rabbit anti-SPINK3 antibody. Each blot was either incubated with a horseradish peroxidase-conjugated rabbit anti-mouse antibody (Pierce, Eugene, OR) or a horseradish peroxidase-conjugated goat anti-rabbit antibody (Pierce). The antigens were detected via an ECL+ detection system (GE Healthcare). Bands were quantified by use of the Bio-Chemi system (UVP Bioimaging Systems, Upland, CA) according to the manufacturer's instructions.
Inhibition of trypsin activity.
Pancreatic extracts were prepared as previously described (23, 30). Trypsin activity was measured by fluorescence following the method described by Kawabata et al. (12). Pancreatic extracts containing 160 μg/ml of protein were added to 20 pM bovine trypsin (15,000 BAEE units/mg) (Pierce). The reaction was started after the addition of the substrate Boc-Gln-Ala-Arg-MCA at a final concentration of 40 μM (Peptides International, Louisville, KY). The kinetic reaction in the linear range was monitored for 10 min in a Safire plate reader (TECAN, Mannedorf, Switzerland) with an excitation wavelength of 380 nm and emission wavelength of 440 nm. Results were expressed as the percent inhibition of trypsin activity and were determined as the ratio of the rate of reaction by using the formula %inhibition = 100(ΔT−ΔN)/ΔT in which ΔT is the rate of change with trypsin alone and ΔN is the rate of change with trypsin + pancreatic extract (23).
Acute pancreatitis.
Caerulein-induced pancreatitis was induced in mice by injection of supramaximal doses of caerulein (50 μg/kg) intraperitoneally every hour for 12 h (23). Control animals received isotonic saline injections every hour for 12 h. Animals were killed 1 h postinjection and blood was collected for measurement of serum amylase. The pancreas was quickly collected and weighed for determination of pancreas wet weight-to-body weight ratio as a measure of pancreatic edema, and then pancreatic tissues were fixed in 10% formalin, paraffin embedded, and stained with hematoxylin and eosin. Histological assessment of pancreatitis severity was determined by using a scoring system for disease severity (edema, neutrophil infiltration, and necrosis) as previously described (23, 29).
Electron microscopy and histological staining.
Pancreatic tissues were fixed in 10% formalin, embedded in paraffin, and stained with hematoxylin and eosin. Slides were photographed with a ×10 objective. Electron microscopy was performed as described previously (23).
Biochemical measurements.
Serum amylase was measured as previously described (23).
Statistical analyses.
Data for Western blot analysis, trypsin inhibition, amylase, edema, and total histological scoring were analyzed by one-way ANOVA analysis of variance with the Tukey posttest when more than two populations were considered and Student's t-test when two populations were considered (GraphPad Software, San Diego, CA). Results were expressed as means ± SE. Statistical significance was set at P < 0.05.
Survival rates were analyzed by comparison of survival curves with the log-rank (Mantel-Cox) test (GraphPad Software). Results were expressed as means ± SE. Statistical significance was set at P < 0.05.
RESULTS
Sequence comparison of mouse SPINK3 and rat PSTI-I.
Endogenous trypsin inhibitors have been found in the pancreas of several species. In the mouse, the endogenous trypsin inhibitor is SPINK3, which shares sequence similarity with PSTI-I in the rat. Mouse SPINK3 and rat PSTI-I belong to a subfamily of Kazal-type PSTI proteins. The primary sequences of mouse SPINK3 and rat PSTI-I are shown in Fig. 1. The conserved region consists of two subregions, containing 27 amino acids and 9 amino acids separated by a 6-amino acid nonconsensus sequence (www.ncbi.nlm.nih.gov/Structure/cdd/cdd.shtml). These two proteins are highly similar with only two significant amino acids being dissimilar in the 27-amino-acid subdomain (an aspartic acid in the mouse protein is replaced by an asparagine in the rat protein and a valine in the mouse protein is replaced by serine in PSTI-I). The second subdomain shows slightly more divergence with the sequence RKG in the mouse being replaced by QRR in the rat protein. Both proteins possess protease cleavage sites and a protease binding site.
Fig. 1.
Amino acid sequences of serine protease inhibitor Kazal-type 3 (SPINK3) and pancreatic secretory trypsin inhibitor I (PSTI-I) proteins. The Kazal-type PSTI domain is delineated by shaded boxes. Identical sequences are shown in light gray and similar amino acids are shown in dark gray. A protease cleavage site (*) and protease binding sites (#) are indicated.
Survival rates of Spink3+/+, Spink3+/−, Spink3−/−, and Spink3−/−/TgN(Psti1) mice.
Spink3+/+, Spink3+/−, and Spink3−/−/TgN(Psti1) mice were bred in the Duke University vivarium and the survival of newborn pups was monitored. Table 1 indicates that no null mice for Spink3 gene survived past 1 wk of age. Similar results were previously described by Ohmuraya et al. (26). Survival rates for Spink3+/+and Spink3+/− mice were 94.7 ± 3.6 and 85.7 ± 5.9%, respectively, at 3 wk of age. To determine whether lethality in Spink3+/− mice was due to pancreas-specific trypsin inhibitor deficiency, Spink3+/− mice were bred with TgN(Psti1) mice to obtain mice bearing the Spink3−/−/TgN(Psti1) genotype. The presence of PSTI-I and absence of SPINK3 proteins in the pancreata of Spink3−/−/TgN(Psti1) mice were confirmed by Western blot analysis using a specific anti-PSTI-I antibody (23) and anti-SPINK3 antibody, respectively (results not shown). These mice were viable and had a survival rate of 87.2 ± 5.4% at 3 wk of age. The survival rate remained unchanged for these three populations [Spink3+/+, Spink3+/−, and Spink3−/−/TgN(Psti1)] well into adulthood. Moreover, these mice were very similar in size at 1 wk of age (Fig. 2). In contrast, however, Spink3−/− mice were much smaller indicating altered growth and development most likely due to the absence of pancreatic exocrine function. No Spink3−/− mice survived past 1 wk of age.
Table 1.
Survival rate for Spink3−/−, Spink3±, Spink−/−, and Spink3−/−/TgN(Psti1) mice
| Genotype | n | % Survival: Mean ± SE |
|---|---|---|
| Spink3+/+ | 39 | 94.7 ± 3.6 |
| Spink3± | 35 | 85.7 ± 5.9 |
| Spink3−/− | 20 | 0.0 ± 0.0 |
| Spink3−/−/TgN(Psti1) | 39 | 87.2 ± 5.4 |
The survival of newborn mice was monitored daily until 3 wk of age. Afterward no unanticipated deaths were observed in the surviving mice well into adulthood. n, Number of mice born in each group.
Fig. 2.
Photograph of Spink3+/+, Spink3+/−, Spink3−/−, Spink3−/−/TgNPsti1, and TgNPsti1 mice. The Spink3−/− mouse photograph was taken after death (8 days old), whereas all the other mice were killed at 8 days of age.
Pancreatic histology.
The histological appearances of pancreata from wild-type, Spink3+/+, and Spink3−/−/TgN(Psti1) mice at 8 wk of age are shown in Fig. 3. The histological appearances of pancreata from wild-type, Spink3 heterozygote, and Spink3−/−/TgN(Psti1) mice were indistinguishable and there were no features of inflammation or necrosis. The histological appearance is consistent with the normal gross appearance of the pancreata, which were also similar among these genotypes.
Fig. 3.
Photomicrograph of pancreas from Spink3+/+, Spink3+/−, and Spink3−/−/TgN(Psti1) mice. Pancreatic tissues from adult mice were fixed in formalin and stained with hematoxylin and eosin for histological analysis.
Trypsin inhibitor expression in Spink3+/+ and Spink3+/− mice.
Western blot analysis of SPINK3 was performed on pancreas from wild-type and Spink3+/− heterozygote mice (Fig. 4). SPINK3 protein was more highly expressed in wild-type mice (Spink3+/+) than in the heterozygote Spink3+/− mice (Fig. 4A). The level of expression was compared by quantifying the intensity of the SPINK3 band vs. α-tubulin used as an internal control. SPINK3 protein was fivefold greater in wild-type mice compared with heterozygote mice (Fig. 4B).
Fig. 4.
Western blot analysis of SPINK3 and α-tubulin in pancreatic extracts. Pancreatic extracts prepared from individual mice were loaded onto separate lanes and subjected to electrophoresis. Following transfer onto nitrocellulose, the blot was cut in half and the top half was incubated with anti-α-tubulin antibody and the bottom half with anti-SPINK3 antibody (A). The far right lane shows the molecular weight markers. SPINK3 is a 6-kDa protein, whereas α-tubulin runs as a 45-kDa protein. Scanning densitometry was performed for each lane; the intensity of SPINK3 was measured and normalized with the intensity of tubulin for each lane (B). Results were expressed as means ± SE (n = 6). *P < 0.05.
Trypsin inhibitor activity in Spink3+/+, Spink3+/−, and Spink3−/−/TgN(Psti1) mice.
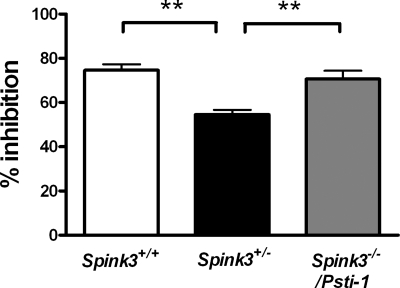
Trypsin inhibitor activity was measured in pancreas from mice and results were expressed relative to inhibition of standard amounts of bovine trypsin (Fig. 5). Pancreatic extracts from Spink3+/− mice had significantly less inhibitory capacity compared with Spink3+/+ or Spink3−/−/TgN(Psti1) extracts. Pancreatic extracts from Spink3+/− mice inhibited 55% of trypsin activity, whereas Spink3+/+ and Spink3−/−/TgN(Psti1) mice had inhibitory capacities of 75 and 71%, respectively (n = 4; P < 0.01). These findings indicate that the transgenic expression of PSTI completely restored trypsin inhibitor activity to that of the normal pancreas.
Fig. 5.
Measurement of trypsin inhibitor activity in pancreatic extracts from Spink3+/+, Spink3+/−, and Spink3−/−/TgN(Psti1) mice. Pancreatic extracts were added to bovine trypsin and the substrate Boc-Gln-Ala-Arg-MCA and the rate of product formation in a 10-min period in the presence of pancreatic extracts was compared with a standard curve of pure trypsin. Results are expressed as means ± SE (n = 4; **P < 0.01).
Electron microscopy.
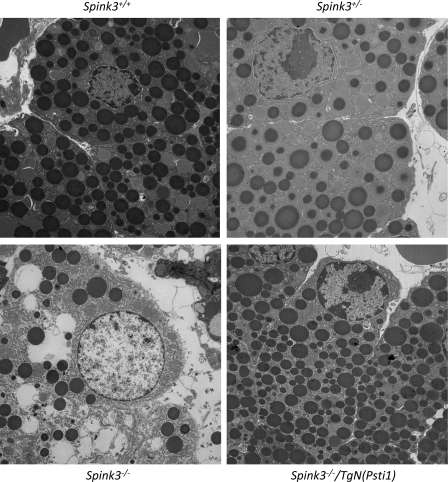
Representative electron microscopic images of pancreatic acinar cells from mouse embryos at 18.5 dpc are shown in Fig. 6. Cellular structures of the transgenic Spink3+/+, Spink3+/−, and Spink3−/−/TgN(Psti1) mouse acinar cells appear normal with a very large number of zymogen granules. In contrast, pancreatic acini of Spink3−/− mice showed evidence of autophagy with large cytoplasmic vacuoles and nuclear chromatin disruption.
Fig. 6.
Electron micrographs of pancreas from Spink3+/+, Spink3+/−, Spink3−/−, and Spink3−/−/TgN(Psti1) mice. Electron micrographs of pancreas from embryos, 18.5 days postcoitus (dpc) are shown at ×3,000 direct magnification. Representative photographs of acinar cells from Spink3+/+ (A), Spink3+/+ (B), Spink3−/− (C), and Spink3−/−/TgN(Psti1) (D) mice are shown.
Acute pancreatitis.
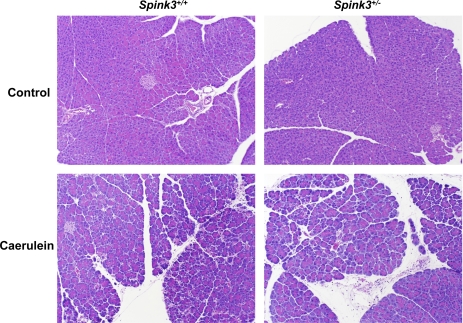
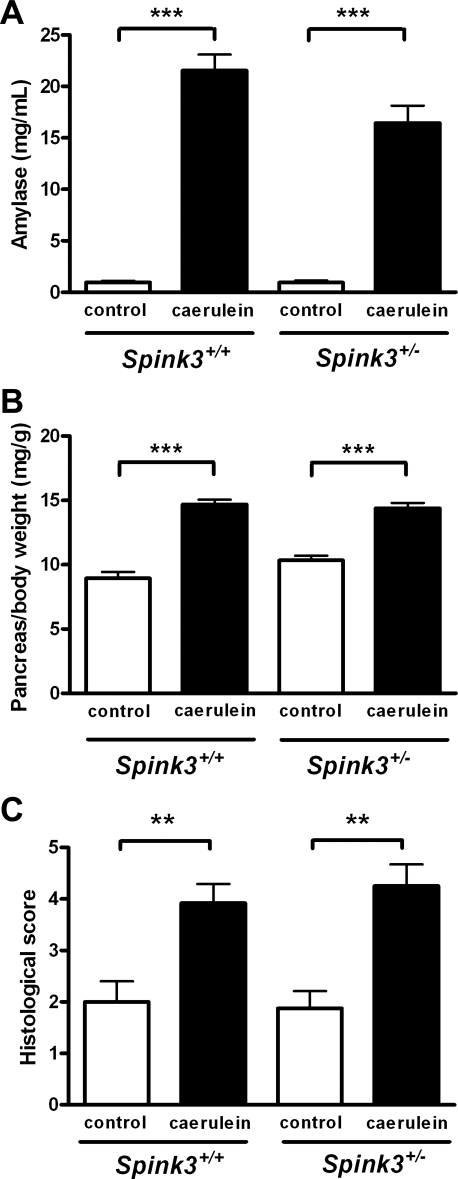
To determine whether lower SPINK3 levels predisposed animals to pancreatitis, we subjected wild-type and heterozygote mice to caerulein-induced pancreatitis. Representative photomicrographs of pancreas from Spink3+/+ or Spink3+/− mice following vehicle (control) or caerulein injection are shown in Fig. 7. Edema, necrosis, and neutrophil infiltration characteristic of acute pancreatitis were evident in both wild-type and heterozygote mice after 12 h of caerulein treatment. Quantitative analyses of blood amylase, edema (measured as pancreas wet weight per body weight) and total histological score (consisting of edema, necrosis, neutrophil infiltration and hemorrhage) are shown in Fig. 8. Despite lower trypsin inhibitor levels in SPINK3+/− mice compared with wild-type mice, the increases in blood amylase, edema, and total histological scores were not significantly different.
Fig. 7.
Effects of repeated caerulein injections on pancreas of Spink3+/+ and Spink3+/− mice. Mice were treated with 12 hourly injections of vehicle (top) or caerulein (bottom). Representative histological sections of pancreas are shown from wild-type (left) or Spink3+/+ (right) mice are shown. Photographs were taken with a ×10 objective. Note the presence of neutrophils, edema, and necrosis in pancreas of caerulein-treated animals.
Fig. 8.
Effects of caerulein treatment on serum amylase, pancreatic edema, and pancreatitis severity score. Caerulein administration caused a significant increase in serum amylase (A), pancreas wet wt/body wt (B), and histological pancreatitis severity score (C) in Spink3+/+ and Spink3+/+ mice. Results are expressed as means ± SE (n = 6); **P < 0.01, ***P < 0.001 caerulein vs. control.
DISCUSSION
SPINK3 is highly expressed in pancreas, where it is thought to prevent activation of trypsinogen and other pancreatic proenzymes should small amounts of trypsin become activated within the pancreas. It has been reported that mutation in the human equivalent Spink1 gene can lead to chronic pancreatitis (13). This report shows that pancreas-specific expression of the rat PSTI-I protein, which is similar but not identical to the mouse SPINK3 protein, was able to compensate for the absence of SPINK3 in Spink3−/− mice and restore viability in Spink3−/−/TgN(Psti1) mice.
Pancreatic extracts from wild-type and Spink3−/−/TgN(Psti1) mice exhibited similar trypsin inhibitory capacities in an in vitro assay. However, Spink3+/− mice inhibited significantly less trypsin activity than either Spink3+/+ or Spink3−/−/TgN(Psti1) mice. Although the trypsin inhibition assay is not a commonly used method to assess SPINK expression, the differences we observed in inhibitor activity are in agreement with the reduced amount of SPINK3 protein (measured by Western blot analysis) in the pancreas of Spink3+/− animals compared with wild-type mice. Interestingly, the reduction in SPINK3 levels in heterozygote mice did not affect the viability of these mice, suggesting that there was a sufficient amount of SPINK3 present in the pancreas to protect the organ against spontaneous trypsin activation and autophagic degradation.
In this study, we demonstrated that mice lacking the Spink3 gene do not grow normally and die prematurely. Within 1 wk of birth all Spink3−/− mice died and, upon examination, the pancreas was markedly abnormal. The pancreata of Spink3−/− mice were small, and in most animals only a small remnant of pancreas was apparent. Electron microscopy revealed autophagic vacuoles within pancreatic acinar cells, consistent with earlier findings (26).
Autophagy is a normal cellular process involved in the bulk degradation and clearance of intracellular proteins. A distinctive morphological feature is the envelopment of lysosomes forming autophagosomes with double membranes. Recent reports suggest that abnormalities in autophagic processing may play a role in the pathogenesis of pancreatitis (26). When the autophagy-related gene (Atg5) was genetically deleted in conditional knockout mice, trypsinogen activation was diminished and mice were protected against caerulein-induced experimental pancreatitis, suggesting that autophagic activation of trypsin may be required for the initiation of pancreatitis (9, 27).
We proposed that trypsin inhibitor may protect the pancreas against pancreatitis by reducing conversion of trypsinogen to active trypsin (23). If this is true, it is possible that reduced trypsin inhibitor levels in the pancreas may increase the susceptibility of the pancreas to pancreatic injury, trypsin activation, and subsequent pancreatitis. To address this possibility we subjected Spink3+/− mice to caerulein-induced injury that causes mild to moderate pancreatitis in wild-type animals. Surprisingly, we observed that even though Spink3+/− mice have trypsin inhibitor levels that are substantially lower than wild-type animals, the severity of pancreatitis was no greater in these mice. However, these findings do not negate the importance of trypsin inhibitor as a protective mechanism in pancreatitis but may suggest that even small amounts of trypsin inhibitor play a protective role in our model of acute pancreatitis. Although we did not demonstrate increased susceptibility of Spink3+/− mice to acute pancreatitis, it is possible that pancreatitis susceptibility would be evident under other conditions in which the severity or timing of pancreatic injury and recovery was different.
It has been estimated that patients heterozygous for a SPINK1 mutation have a 20- to 40-fold increased risk of developing chronic pancreatitis (13, 24). This risk may be as high as 500-fold in individuals with homozygous mutations (13). The mechanism by which SPINK1 mutations predisposes one to chronic pancreatitis is not entirely clear but has been attributed to ultimate loss of function. Therefore, it is likely that impaired interactions between trypsin and trypsin inhibitor occur. Quantitation of SPINK1 and PRSS1 mRNA levels in normal human pancreas by real-time PCR indicated that SPINK1 mRNA levels may be 1,000 times lower than PRSS1 levels (14). Interestingly, however, SPINK1 mRNA levels were increased in patients with pancreatitis with a ratio of SPINK1/PRSS1 as high as 6 to 1. There was no definitive information regarding the respective SPINK1 and trypsinogen protein levels.
In summary, accumulating data indicate an important relationship between endogenous pancreatic trypsin and trypsin inhibitor and the development of pancreatitis. Our findings illustrate the requirement for trypsin inhibitor in normal pancreatic development, but further work is necessary to clarify the pathogenic role of SPINK in pancreatitis.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK 073908 and DK 038626 (R. A. Liddle).
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENT
The authors thank Sara Miller and Phillip Christopher for expert technical assistance.
REFERENCES
- 1.Bouras EP, Misukonis MA, Liddle RA. Role of calcium in monitor peptide-stimulated cholecystokinin release from perifused intestinal cells. Am J Physiol Gastrointest Liver Physiol 262: G791–G796, 1992 [DOI] [PubMed] [Google Scholar]
- 2.Chen JM, Ferec C. Chronic pancreatitis: genetics and pathogenesis. Annu Rev Genomics Hum Genet 10: 63–87, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Chen JM, Mercier B, Audrezet MP, Ferec C. Mutational analysis of the human pancreatic secretory trypsin inhibitor (PSTI) gene in hereditary and sporadic chronic pancreatitis. J Med Genet 37: 67–69, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen LY, Lin YH, Lai ML, Chen YH. Developmental profile of a caltrin-like protease inhibitor, P12, in mouse seminal vesicle and characterization of its binding sites on sperm surface. Biol Reprod 59: 1498–1505, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Cohn JA, Friedman KJ, Noone PG, Knowles MR, Silverman LM, Jowell PS. Relation between mutations of the cystic fibrosis gene and idiopathic pancreatitis. N Engl J Med 339: 653–658, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Fukayama M, Hayashi Y, Koike M, Ogawa M, Kosaki G. Immunohistochemical localization of pancreatic secretory trypsin inhibitor in fetal and adult pancreatic and extrapancreatic tissues. J Histochem Cytochem 34: 227–235, 1986. [DOI] [PubMed] [Google Scholar]
- 7.Graf R, Bimmler D. Biochemistry and biology of SPINK-PSTI and monitor peptide. Endocrinol Metab Clin North Am 35: 333–343, ix, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Greene LJ, Pubols MH, Bartelt DC. Human pancreatic secretory trypsin inhibitor. Methods Enzymol 45: 813–825, 1976 [DOI] [PubMed] [Google Scholar]
- 9.Hashimoto D, Ohmuraya M, Hirota M, Yamamoto A, Suyama K, Ida S, Okumura Y, Takahashi E, Kido H, Araki K, Baba H, Mizushima N, Yamamura K. Involvement of autophagy in trypsinogen activation within the pancreatic acinar cells. J Cell Biol 181: 1065–1072, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herzig KH. Cholecystokinin- and secretin-releasing peptides in the intestine—a new regulatory interendocrine mechanism in the gastrointestinal tract. Regul Pept 73: 89–94, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Hirota M, Ohmuraya M, Baba H. The role of trypsin, trypsin inhibitor, and trypsin receptor in the onset and aggravation of pancreatitis. J Gastroenterol 41: 832–836, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Kawabata S, Miura T, Morita T, Kato H, Fujikawa K, Iwanaga S, Takada K, Kimura T, Sakakibara S. Highly sensitive peptide-4-methylcoumaryl-7-amide substrates for blood-clotting proteases and trypsin. Eur J Biochem 172: 17–25, 1988 [DOI] [PubMed] [Google Scholar]
- 13.Keim V. Role of genetic disorders in acute recurrent pancreatitis. World J Gastroenterol 14: 1011–1015, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khalid A, Finkelstein S, Thompson B, Kelly L, Hanck C, Godfrey TE, Whitcomb DC. A 93 year old man with the PRSS1 R122H mutation, low SPINK1 expression, and no pancreatitis: insights into phenotypic non-penetrance. Gut 55: 728–731, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liddle RA. Pathophysiology of SPINK mutations in pancreatic development and disease. Endocrinol Metab Clin North Am 35: 345–356, x, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Liddle RA. Susceptibility to pancreatitis related to PSTI/SPINK1 expression. Gastroenterol Clin North Am 33: 807–816, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Liddle RA, Misukonis MA, Pacy L, Balber AE. Cholecystokinin cells purified by fluorescence-activated cell sorting respond to monitor peptide with an increase in intracellular calcium. Proc Natl Acad Sci USA 89: 5147–5151, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marchbank T, Chinery R, Hanby AM, Poulsom R, Elia G, Playford RJ. Distribution and expression of pancreatic secretory trypsin inhibitor and its possible role in epithelial restitution. Am J Pathol 148: 715–722, 1996 [PMC free article] [PubMed] [Google Scholar]
- 19.Mills JS, Needham M, Parker MG. Androgen regulated expression of a spermine binding protein gene in mouse ventral prostate. Nucleic Acids Res 15: 7709–7724, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mills JS, Needham M, Parker MG. A secretory protease inhibitor requires androgens for its expression in male sex accessory tissues but is expressed constitutively in pancreas. EMBO J 6: 3711–3717, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyasaka K, Funakoshi A, Nakamura R, Kitani K, Uda K, Murata A, Ogawa M. Differences in stimulatory effects between rat pancreatic secretory trypsin inhibitor-61 and -56 on rat pancreas. Jpn J Physiol 39: 891–899, 1989 [DOI] [PubMed] [Google Scholar]
- 22.Miyasaka K, Guan DF, Liddle RA, Green GM. Feedback regulation by trypsin: evidence for intraluminal CCK-releasing peptide. Am J Physiol Gastrointest Liver Physiol 257: G175–G181, 1989 [DOI] [PubMed] [Google Scholar]
- 23.Nathan JD, Romac J, Peng RY, Peyton M, Macdonald RJ, Liddle RA. Transgenic expression of pancreatic secretory trypsin inhibitor-I ameliorates secretagogue-induced pancreatitis in mice. Gastroenterology 128: 717–727, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Noone PG, Zhou Z, Silverman LM, Jowell PS, Knowles MR, Cohn JA. Cystic fibrosis gene mutations and pancreatitis risk: relation to epithelial ion transport and trypsin inhibitor gene mutations. Gastroenterology 121: 1310–1319, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Ohmuraya M, Hirota M, Araki K, Baba H, Yamamura K. Enhanced trypsin activity in pancreatic acinar cells deficient for serine protease inhibitor kazal type 3. Pancreas 33: 104–106, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Ohmuraya M, Hirota M, Araki M, Mizushima N, Matsui M, Mizumoto T, Haruna K, Kume S, Takeya M, Ogawa M, Araki K, Yamamura K. Autophagic cell death of pancreatic acinar cells in serine protease inhibitor Kazal type 3-deficient mice. Gastroenterology 129: 696–705, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Ohmuraya M, Yamamura K. Autophagy and acute pancreatitis: a novel autophagy theory for trypsinogen activation. Autophagy 4: 1060–1062, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Pubols MH, Bartelt DC, Greene LJ. Trypsin inhibitor from human pancreas and pancreatic juice. J Biol Chem 249: 2235–2242, 1974 [PubMed] [Google Scholar]
- 29.Romac JM, McCall SJ, Humphrey JE, Heo J, Liddle RA. Pharmacologic disruption of TRPV1-expressing primary sensory neurons but not genetic deletion of TRPV1 protects mice against pancreatitis. Pancreas 36: 394–401, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Singh VP, Saluja AK, Bhagat L, van Acker GJ, Song AM, Soltoff SP, Cantley LC, Steer ML. Phosphatidylinositol 3-kinase-dependent activation of trypsinogen modulates the severity of acute pancreatitis. J Clin Invest 108: 1387–1395, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang J, Ohmuraya M, Hirota M, Baba H, Zhao G, Takeya M, Araki K, Yamamura K. Expression pattern of serine protease inhibitor kazal type 3 (Spink3) during mouse embryonic development. Histochem Cell Biol 130: 387–397, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Whitcomb DC, Gorry MC, Preston RA, Furey W, Sossenheimer MJ, Ulrich CD, Martin SP, Gates LK, Jr, Amann ST, Toskes PP, Liddle R, McGrath K, Uomo G, Post JC, Ehrlich GD. Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nat Genet 14: 141–145, 1996 [DOI] [PubMed] [Google Scholar]
- 33.Witt H, Luck W, Hennies HC, Classen M, Kage A, Lass U, Landt O, Becker M. Mutations in the gene encoding the serine protease inhibitor, Kazal type 1 are associated with chronic pancreatitis. Nat Genet 25: 213–216, 2000. [DOI] [PubMed] [Google Scholar]