Abstract
Aberrant cholangiocyte reactions in response to inflammatory stimuli are important pathogenic factors for the persistent biliary inflammation in patients with cholangiopathies. Overexpression of intercellular cell adhesion molecule-1 (ICAM-1) in cholangiocytes is a common pathological feature in inflammatory cholangiopathies and can promote cholangiocyte interactions with effector lymphocytes in the portal region. In this study, we tested the involvement of miRNA-mediated posttranscriptional regulation in IFN-γ-induced ICAM-1 expression in cholangiocytes. Using both immortalized and nonimmortalized human cholangiocyte cell lines, we found that IFN-γ activated ICAM-1 transcription and increased ICAM-1 protein expression. Inhibition of ICAM-1 transcription could only partially block IFN-γ-induced ICAM-1 expression at the protein level. In silico target prediction analysis revealed complementarity of miR-221 to the 3′-untranslated region of ICAM-1 mRNA. Targeting of ICAM-1 3′-untranslated region by miR-221 resulted in translational repression in cholangiocytes but not ICAM-1 mRNA degradation. Functional inhibition of miR-221 with anti-miR-221 induced ICAM-1 protein expression. Moreover, IFN-γ stimulation decreased miR-221 expression in cholangiocytes in a signal transducer and activator of transcription 1-dependent manner. Transfection of miR-221 precursor abolished IFN-γ-stimulated ICAM-1 protein expression. In addition, miR-221-mediated expression of ICAM-1 on cholangiocytes showed a significant influence on the adherence of cocultured T cells. These findings indicate that both transcriptional and miRNA-mediated posttranscriptional mechanisms are involved in IFN-γ-induced ICAM-1 expression in human cholangiocytes, suggesting an important role for miRNAs in the regulation of cholangiocyte inflammatory responses.
Keywords: epithelium, biliary, microRNAs, inflammation
cholangiocytes are the primary target cells in an important group of genetic and acquired biliary disorders affecting both the adult and pediatric populations, collectively called “cholangiopathies” (12, 35). Despite their heterogeneity, the inflammatory reaction, characterized by a significant amount of inflammatory infiltrate in the portal region, is central to most manifestations of cholangiopathies. Although persistent biliary inflammation is the pathological hallmark, there is usually no evidence supporting a mechanistic link to any specific pathogens or pathogen-specific gene products (1, 27). Aberrant cholangiocyte responses to inflammatory stimuli may be the real pathogenic factors for the chronicity of inflammation in patients with the diseases (23, 37).
A local environment rich in proinflammatory cytokines, such as interferon (IFN)-γ and tumor necrosis factor (TNF)-α, has been implicated in most cholangiopathies including primary biliary cirrhosis, biliary atresia, and primary sclerosing cholangitis (3, 5, 42). IFN-γ is a dimeric glycoprotein, mainly synthesized by NK cells and CD4 and CD8 T cells (39). The cell surface receptors for IFN-γ are IFN-γR1 and IFN-γR2. The major signaling pathway activated by IFN-γ involves sequential phosphorylation of the Janus-activated kinase and signal transducer and activator of transcription (STAT) proteins. Cholangiocytes express several receptors for cytokines and chemokines including IFN-γ, interleukin-4 (IL-4), IL-6, and TNF-α (46, 48).
Evidence suggests that cholangiocytes are involved in the intensification and localization of inflammatory responses via expression of adhesion molecules to recruit lymphocytes to the portal region. A group of adhesion molecules, such as intercellular adhesion molecule 1 (ICAM-1), human leukocyte antigen class I (HLA-I), and lymphocyte function-related antigen 3, is constitutively expressed in human cholangiocytes (14, 34). Upregulation of ICAM-1 and HLA-I in cultured human cholangiocytes was evident after stimulation with IFN-γ, TNF-α, and IL-1 (10). Clinically, overexpression of ICAM-1, vascular cell adhesion molecule-1 (VCAM-1), and HLA antigens is a common pathological feature in chronic cholangiopathies and can promote cholangiocyte interactions with effector lymphocytes in the portal area (10, 28).
MicroRNAs are a newly identified class of endogenous small regulatory RNAs (2, 6). These molecules target mRNAs on the basis of the complementarity between the miRNAs and the 3′-untranslated regions (3′UTRs) of target mRNAs. This causes either mRNA cleavage or translational suppression, resulting in gene suppression (6). Over 700 miRNAs have been identified in humans, and it has been predicted that miRNAs control 20–30% of human genes (6, 7). Because miRNAs appear to provide quantitative regulation of genes, rather than on-off decisions, they can be seen as a fine tuning for the cellular responses to external influences (26). MicroRNAs are involved in regulating Toll-like receptor signaling and ensuing cytokine response (e.g., miR-146) and have been implicated in viral immune escape and antiviral defense (e.g., miR-196) (37). Recent evidence showing altered miRNA expression in chronic inflammatory diseases (miR-203 and miR-146) suggests their involvement in immune-mediated diseases (37). Indeed, miR-126 targets VCAM-1 and regulates endothelial expression of VCAM-1 induced by TNF-α (20). We also recently identified that a cellular miRNA, let-7i, is involved in translational regulation of Toll-like receptor 4 in human cholangiocytes (11). miR-98 and let-7 are involved in translational regulation of the cytokine-inducible Src homology 2 protein in human cholangiocytes in response to microbial challenge (22).
In this study, we report that IFN-γ induces ICAM-1 expression in human cholangiocytes. This induced expression of ICAM-1 at the protein level involves a relief of miRNA-mediated translation repression by miR-221. Furthermore, ICAM-1 expression confers the adherence of T cells to cholangiocytes in vitro. Thus a miRNA-mediated regulatory mechanism of ICAM-1 expression has been identified in cholangiocytes, a process that may be relevant to the regulation of cholangiocyte-T cell interactions during portal inflammation in liver diseases.
MATERIALS AND METHODS
Cells and reagents.
H69 cells (a gift from Dr. D. Jefferson, Tufts University,) are SV40-transformed normal human cholangiocytes originally derived from normal liver harvested for transplant. These cells continue to express biliary epithelial cell markers, including cytokeratin 19, γ-glutamyl transpeptidase, and ion transporters consistent with biliary function and have been extensively characterized (43). Human intrahepatic biliary epithelial (HIBEpiC) cells are nonimmortalized isolated human cholangiocytes commercially available from ScienCell Research Laboratories (Carlsbad, CA). HIBEpiC cells were grown on poly-L-lysine-coated dishes and cultured using instructions and medium provided by the supplier (22, 24). Because of a very limited number of HIBEpiC cells available, they were only applied to selected key experiments. Jurkat cells were purchased from the American Type Culture Collection. Recombinant human IFN-γ and actinomycin D were purchased from R & D Systems (Minneapolis, MN) and Thermo Fisher Scientific (Rockford, IL), respectively.
Western blot.
Whole cell lysates were obtained from cells with mammalian protein extraction reagent (Pierce, Rockford, IL). Cell lysates were then loaded at each line (a total of 40 μg lysate proteins) in 4–12% SDS-PAGE gel to separate proteins and transferred to nitrocellulose membrane. Antibodies to ICAM-1 (Cell Signaling, Danvers, MA) and actin (Sigma-Aldrich, St. Louis, MO) were used. Densitometric levels of ICAM-1 signals were quantified and expressed as their ratio to actin.
Real-time PCR.
For quantitative analysis of mRNA expression, total cellular RNA was isolated from cells using the TRIzol reagent (Invitrogen, Carlsbad, CA). RNAs were treated with DNA-free Kit (Ambion, Austin, TX) to remove any remaining DNA. Comparative real-time PCR was performed with the use of the SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA). The sequences for the amplification of human ICAM-1 were: 5- CGCAAGGTGACCGTGAATGT -3 (forward) and 5- CGTGGCTTGTGTGTTCGGTT -3 (reverse). The primer sequences for the amplification of GAPDH were as follows: 5- TGCACCACCAACTGCTTAGC -3 (forward); 5- GGCATGGACTGTGGTCATGAG -3 (reverse).
For analysis of miR-221, total RNA was isolated from cells with the mirVana miRNA Isolation kit (Ambion). Comparative real-time PCR was performed by using the TaqMan Universal PCR Master Mix (Applied Biosystems). Specific primers and probes for mature miR-221 and snRNA RNU6B were obtained from Applied Biosystems. All reactions were run in triplicate. The amount of miR-221 was obtained by normalizing to snRNA RNU6B and relative to control (untreated cell) as previously reported (11, 19, 22).
Northern blot.
Total RNAs harvested as above were run on a 15% Tris/Borate/EDTA [90 mM Tris/64.6 mM boric acid/2.5 mM EDTA (pH 8.3)] urea gel (Invitrogen) and transferred to a Nytran nylon transfer membrane (Ambion). A locked nucleic acid digoxigenin probe for miRNA-221 (Exiqon, Vedbaek, Denmark) was hybridized using UltraHyb reagents (Ambion) according to the manufacturer's instructions with blotted snRNA RNU6B as a control.
Anti-miR and precursor to miR-221.
To manipulate cellular function of miR-221 in H69 cells, we utilized an antisense approach to inhibit miR-221 function and transfection of cells with miR-221 precursor to increase miR-221 expression. For experiments, H69 cells were grown to 70% confluent and treated with anti-miR-221 (antisense 2-methoxy oligonucleotide to miR-221, Ambion) or the miR-221 precursor (Ambion) using the lipofectamine 2000 reagent (Invitrogen).
Luciferase reporter constructs and luciferase assay.
Complementary 40 bp DNA oligonucleotides containing the putative miR-221 target site within 3′UTR of human ICAM-1 were synthesized with flanking SpeI and HindIII restriction enzyme digestion sites (sense: 5′-ctagGAAGTGGCCCTCCATAGACATGTGTAGCATCAAAAC-3′; antisense: 5′-agctGTTTTGATGCTACACATGTCTATGGAGGGCCACTTC-3′) and cloned into the multiple cloning sites of the pMIR-REPORT Luciferase vector (Ambion). The sense and antisense strands of the oligonucleotides were annealed by adding 2 μg of each oligonucleotide to 46 μl of annealing solution [100 mM potassium acetate, 30 mM 4-(2-hydroxyethyl) piperazine-1-ethanesulfonic acid potassium salt N-(2-hydroxyethyl) piperazine-N′-(2-ethanesulfonic acid) potassium salt, pH 7.4, and 2 mM magnesium acetate] and incubated at 90°C for 5 min and then at 37°C for 1 h. The annealed oligonucleotides were digested with SpeI and HindIII and ligated into the multiple cloning sites of the pMIR-REPORT Luciferase vector (Ambion). In this vector, the posttranscriptional regulation of luciferase was potentially regulated by miRNA interactions with the ICAM-1 3′UTR. Another pMIR-REPORT luciferase construct containing mutant 3′UTR (TGTAGC to ACATCG) was also generated as a control. We then transfected H69 cells with each reporter construct, as well as anti-miR-221 or miR-221 precursor, followed by assessment of luciferase activity 24 h after transfection. Luciferase activity was then measured and normalized to the control β-galactosidase level (22).
Cell adhesion assay.
A fluorescence labeling approach was used to assess epithelial cell-mediated adhesion of T cells as previously reported (8, 18). Briefly, Jurkat cells were first activated with phorbol 12-myristate 13-acetate (20 nM) for 48 h and then labeled with the fluorogenic dye calcein acetoxymethyl ester (calcein AM, 1 μg/ml, Molecular Probes). Labeled Jurkat cells (2 × 105) were then added and incubated with pretreated H69 cells (1 × 105) in 24-well plates at 37°C for 15 min. Nonadhered cells were washed away by gentle shaking twice each for 15 min at room temperature with the adhesion medium (RPMI with 0.1% BSA and 20 mM HEPES, pH 7.4). Fluorescence-positive cells in the plates, reflecting these adhered and labeled Jurkat cells, were counted under the fluorescence microscope and presented as percentage of control (36). Representative images were taken under a Carl Zeiss fluorescent microscope. Blocking experiments were conducted by incubating IFN-γ-treated H69 cells with a neutralizing antibody to ICAM-1 (10 μg/ml, R & D Systems) for 60 min at 37°C before performing the adhesion assay. Nonspecific IgG isotype (R & D Systems) was used as a control.
RESULTS
IFN-γ induces ICAM-1 expression in cholangiocytes.
We first assessed ICAM-1 expression both at the message RNA and protein levels in cholangiocytes in response to IFN-γ stimulation. When H69 cells were exposed to various doses of IFN-γ for 8 h, a significant increase of ICAM-1 at the mRNA level was detected (Fig. 1A). A dose-dependent increase of ICAM-1 at the protein level was also found in H69 cells after exposure to IFN-γ for 24 h (Fig. 1B). Consistently, when H69 cells were exposed to IFN-γ (10 ng/ml) for up to 24 h (for ICAM-1 mRNA measurement) or up to 48 h (for ICAM-1 protein detection), a time-dependent increase of ICAM-1 at both the message and protein levels was detected (Fig. 1, C and D). Increase of ICAM-1 mRNA, as well as ICAM-1 protein, was also confirmed in HIBEpiC after IFN-γ stimulation (Fig. 1, E and F).
Fig. 1.
Interferon (IFN)-γ induces intercellular adhesion molecule (ICAM)-1 expression in cultured human cholangiocyte. A and B: dose-dependent expression of ICAM-1 at the message and the protein levels in H69 cells following IFN-γ stimulation. H69 cells were exposed to the culture medium with various doses of IFN-γ (0, 0.1, 1.0, 10, and 25 ng/ml) followed by real-time PCR (after incubation for 8 h) or Western blotting analysis for ICAM-1 (after incubation for 24 h). C and D: time-dependent expression of ICAM-1 expression in H69 cells induced by IFN-γ. Cells were exposed to IFN-γ (10 ng/ml) followed by real-time PCR (incubation for up to 24 h) or Western blot (incubation for up to 48 h). E and F: IFN-γ-induced expression of ICAM-1 in human intrahepatic biliary epithelial (HIBEpiC) cells. HIBEpiC cells were exposed to culture medium with or without IFN-γ (10 ng/ml) followed by real-time PCR (incubation for 8 h) or Western blot (incubation for 24 h). Bars represent the means ± SD from 3 independent experiments. *P < 0.05 t-test vs. the non-IFN-γ-stimulated control.
Posttranscriptional regulation is involved in IFN-γ-induced ICAM-1 protein expression.
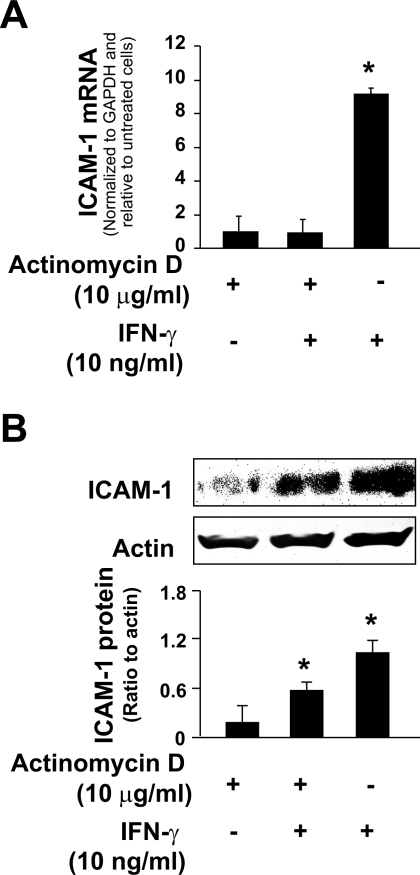
Recent studies suggest that posttranscriptional regulation plays a critical role in the regulation of cytokines and adhesion molecules in cells in response to inflammatory stimuli (19, 22). To test whether posttranscriptional regulation is involved in IFN-γ-induced ICAM-1 expression in cholangiocytes, we tested the expression of ICAM-1 proteins in H69 cells after IFN-γ stimulation in the presence of a transcription inhibitor, actinomycin D (46). H69 cells were treated with actinomycin D (10 μg/ml) for 90 min and exposed to IFN-γ (10 ng/ml) in the presence of actinomycin D for additional 12 h (for ICAM-1 mRNA measurement) or 24 h (for ICAM-1 protein detection). Treatment with actinomycin D significantly decreased the viability of H69 cells after 36 h of incubation (data not shown). We found a complete inhibition of IFN-γ-induced expression of ICAM-1 mRNA in cells treated with actinomycin D (Fig. 2A). Inconsistent to the message expression, a significant upregulation of ICAM-1 protein was detected in IFN-γ-stimulated cells after treatment with actinomycin D (Fig. 2B). The level of ICAM-1 protein in cells treated with actinomycin D and IFN-γ was ∼50% of ICAM-1 level in cells treated with IFN-γ alone. Taken together, the above data suggest that both transcriptional and posttranscriptional mechanisms are involved in IFN-γ-induced ICAM-1 expression in cholangiocytes.
Fig. 2.
Posttranscriptional regulation is involved in IFN-γ-induced ICAM-1 protein expression in cholangiocytes. Expression of ICAM-1 at the message (A) and protein (B) levels in H69 cells following IFN-γ stimulation in the presence or absence of actinomycin D. H69 cells were exposed to culture medium with actinomycin D (10 μg/ml) for 90 min and then exposed to IFN-γ (10 ng/ml) following by real-time PCR for ICAM-1 (after incubation for 12 h) or Western blot (after incubation for 24 h). Data are representative of 3 independent experiments. *P < 0.05 t-test vs. non-IFN-γ-stimulated control.
miR-221 targets ICAM-1 3′UTR and causes posttranscriptional suppression.
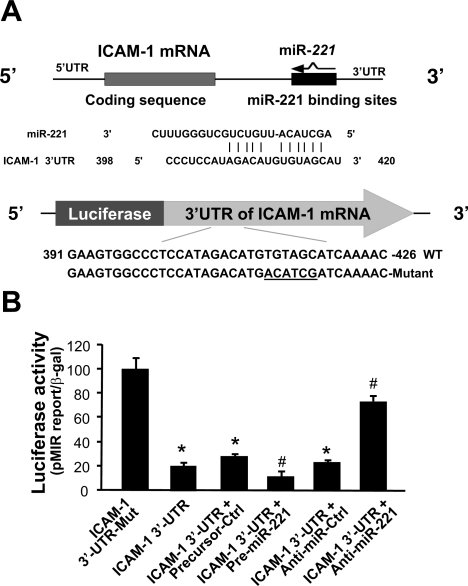
ICAM-1 is a target for miR-222 and miR-339 in colorectal cancer cells (41). miR-221 and miR-222, encoded in tandem on the chromosome X, belong to a conserved miRNA family with a significant homology between them (31). Both miR-221 and miR-222 are expressed in H69 and HIBEpiC cells, and miR-339 was not detected in H69 cells (19, 24). Nevertheless, expression level of miR-221 is much higher than miR-222 (24). miR-221 is one of the most abundant miRNAs expressed in human cholangiocytes, second only to miR-21 (24). To test whether miR-221 targets ICAM-1 3′UTR, we used the MicroRNA.org (http://www.microrna.org) (7). We found that miR-221 is complementary to ICAM-1 3′UTR, extending between 398 and 420 (Fig. 3A).
Fig. 3.
miR-221 targets ICAM-1 3′-untranslated region (UTR) and causes posttranscriptional suppression. A: human ICAM-1 mRNA shows a potential binding site in the 3′UTR for miR-221. B: targeting of ICAM-1 3′UTR by miR-221 resulted in transcriptional suppression. The luciferase reporter constructs containing the potential binding site for miR-221 in ICAM-1 3′UTR or the mutant (Mut) sequence (TGTAGC to ACATCG) were generated. H69 cells were transiently cotransfected with the reporter construct and the miR-221 precursor or anti-miR-221 for 24 h. Luciferase activities were measured and normalized to the control (Ctrl) β-galactosidase (β-gal) level. A nonspecific precursor (precursor-Ctrl) and anti-miR (anti-miR-Ctrl) were used as the controls. Bars represent the means ± SD from 3 independent experiments. *P < 0.05 t-test vs. 3′UTR mutant; #P < 0.05 t-test vs. ICAM-1 3′UTR reporter construct.
To test the potential targeting of ICAM-1 mRNA by miR-221, we generated a pMIR-REPORT luciferase construct containing the ICAM-1 3′UTR with the putative miR-221 binding site (Fig. 3A). In addition, a construct with the TGTAGC to ACATCG mutation at the putative binding site was also generated as control (Fig. 3A). We then transfected H69 cells with these reporter constructs followed by assessment of luciferase activity 24 h after transfection. As shown in Fig. 3B, a significant decrease of luciferase activity was detected in cells transfected with the ICAM-1 3′UTR construct containing the potential binding site compared with the mutant control vector, suggesting endogenous translational repression of the construct with the ICAM-1 3′UTR. miR-221 precursor further significantly decreased the luciferase reporter translation (Fig. 3B). In contrast, anti-miR-221 markedly increased ICAM-1 3′UTR-associated luciferase reporter translation (Fig. 3B). Taken together, the above data suggest that miR-221 targets ICAM-1 3′UTR, resulting in posttranscriptional suppression in cholangiocytes.
Functional inhibition of miR-221 increases ICAM-1 protein expression but does not alter ICAM-1 expression at the message RNA level.
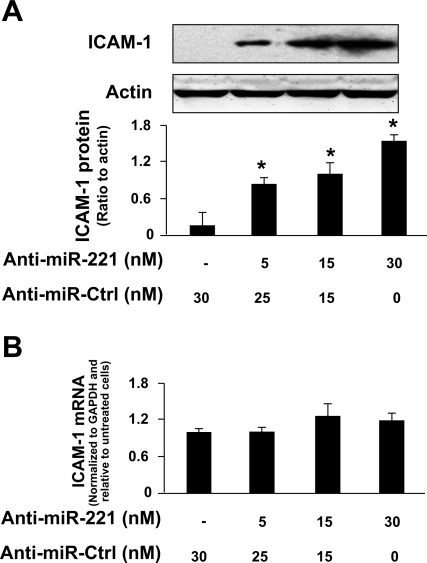
To test whether miRNA-mediated translational repression of ICAM-1 is directly relevant to ICAM-1 protein expression in cholangiocytes, we treated H69 cells with anti-miR-221 for 72 h and then measured ICAM-1 protein expression by Western blot. Transfection of H69 cells with the anti-miR-221 caused a dose-dependent increase of ICAM-1 protein content (Fig. 4A). Moreover, no significant change in ICAM-1 mRNA levels was found between the control cells and cells treated with anti-miR-221 (Fig. 4B), suggesting that miR-221 does not induce ICAM-1 mRNA degradation.
Fig. 4.
Functional inhibition of miR-221 increases ICAM-1 protein expression but does not alter ICAM-1 mRNA level. A: anti-miR-221 increased ICAM-1 protein expression in cholangiocytes. H69 cells were treated with various doses of anti-miR-221 for 24 h followed by Western blot for ICAM-1. B: anti-miR-221 did not alter ICAM-1 expression at the message level in cholangiocytes. H69 cells were treated with various doses of anti-miR-221 for 24 h followed by real-time PCR for ICAM-1. Bars represent the means ± SD from 3 independent experiments. Anti-miR-Ctrl, nonspecific anti-miR control; *P < 0.05 t-test vs. non-anti-miR-221-treated cells.
IFN-γ decreases miR-221 expression in a STAT1-dependent manner.
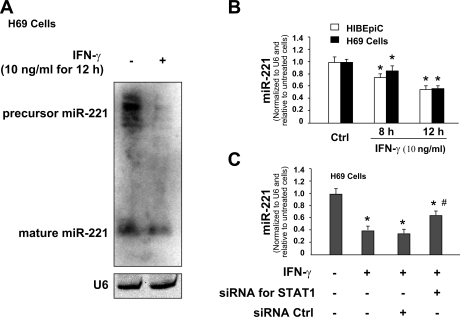
Results of our previous studies using the microarray analysis demonstrate a tendency of decline for miR-221 (0.07 > P > 0.05) in H69 cells after exposure to IFN-γ for 8 h (19). Using Northern hybridization, we detected a significant decrease in the expression levels of miR-221 precursor and mature miR-221 in H69 cells following IFN-γ stimulation for 12 h (Fig. 5A). Furthermore, a significant decrease in miR-221 expression was confirmed in H69 and HIBEpiC cells following IFN-γ stimulation for 8 h and 12 h by real-time PCR analysis with the use of specific probes and primers for mature miR-221 (Fig. 5B).
Fig. 5.
IFN-γ decreases miR-221 expression in a signal transducer and activator of transcription (STAT)1-dependent manner. A and B: IFN-γ stimulation decreased miR-221 expression in cholangiocytes. H69 and HIBEpiC cells were exposed to IFN-γ (10 ng/ml) for 8 h or 12 h followed by Northern blot for miR-221 (in H69 cells in A) and real-time PCR (in H69 and HIBEpiC cells in B). RNU6B (U6) was used as the control. C: knockdown of STAT1 blocked IFN-γ-induced decrease of miR-221 expression. H69 cells were treated with either the STAT1 siRNA or the scrambled control siRNA for 48 h and then exposed to IFN-γ (10 ng/ml) for additional 8 h. Total RNA was isolated, and the expression of miR-221 was quantified by real-time PCR. Data are representative of 3 independent experiments. Bars represent the means ± SD from 3 independent experiments. *P < 0.05 t-test vs. non-IFN-γ-stimulated cells in B and C; #P < 0.05 t-test vs. the scrambled siRNA control in C.
STAT1 is one of key elements for IFN-γ signaling pathways. In our previous studies, we demonstrated that IFN-γ stimulation induced a significant increase of STAT1 tyrosine phosphorylation in H69 cells (19). To test whether STAT1 is involved in IFN-γ-altered miRNA expression, we used a specific siRNA (Cell Signaling) to knock down STAT1 and assessed its effects on IFN-γ-associated expression of miR-221. Cells were transfected with STAT1 siRNA for 48 h followed by an additional incubation for 8 h in the presence or absence of IFN-γ (10 ng/ml). A significant decrease of miR-221 level was detected in the non-siRNA-treated H69 cells after exposure to IFN-γ for 8 h as assessed by real-time PCR (Fig. 5C). H69 cells treated with the scrambled siRNA showed a similar decrease of miR-221 expression following IFN-γ stimulation. However, STAT1 siRNA partially relieved the inhibition of miR-221 expression in H69 cells induced by IFN-γ (Fig. 5C).
IFN-γ induces ICAM-1 expression through downregulation of miR-221.
Because miR-221 targets ICAM-1 3′UTR and induces translational suppression, we expect that IFN-γ stimulation can induce a relief of miR-221-suppressed ICAM-1 translation through downregulation of miR-221. To test this possibility, we transfected H69 cells with the pMIR-REPORT luciferase construct containing the ICAM-1 3′UTR with the putative binding sites for miR-221. Cells simultaneously exposed to IFN-γ for 24 h reversed the decrease of ICAM-1 3′UTR-associated luciferase activity compared with the non-IFN-γ-treated control. No significant change of luciferase activity was found in IFN-γ-stimulated cells transfected with mutant and empty vector control (Fig. 6A).
Fig. 6.
IFN-γ induces ICAM-1 expression through downregulation of miR-221. A: IFN-γ increased the luciferase reporter translational activity in H69 cells transfected with the construct with ICAM-1 3′UTR encoding miR-221 binding site. Cells were transfected with the pMIR-REPORT luciferase construct containing the ICAM-1 3′UTR with the putative miR-221 binding site and then exposed to IFN-γ for 24 h. Luciferase activity in cells after exposure IFN-γ was then measured and normalized to β-gal. B: miR-221 precursor blocked IFN-γ-induced ICAM-1 protein expression. H69 cells were transfected with the miR-221 precursor or a control nonspecific precursor for 48 h and then exposed to IFN-γ (10 ng/ml) for 24 h followed by Western blot for ICAM-1. C: miR-221 precursor does not alter IFN-γ-induced ICAM-1 expression at the message level. H69 cells were transfected with the miR-221 precursor for 24 h and then exposed to IFN-γ (10 ng/ml) for 8 h followed by real-time PCR analysis for ICAM-1 mRNA. Data are representative of 3 independent experiments. Precursor-Ctrl, nonspecific precursor control. *P < 0.05 t-test vs. the empty vector control or 3′UTR mutant (in A) or non-IFN-γ-stimulated cells (in B and C); #P < 0.05 t-test vs. ICAM-1 3′UTR (in A) or non-miR-221 precursor-treated cells (in B).
To test whether relief of miR-221-mediated ICAM-1 translational repression is involved in IFN-γ-induced ICAM-1 protein expression in cholangiocytes, we transfected H69 cells with various doses of miR-221 precursor for 48 h and then exposed those cells to IFN-γ for 24 h followed by Western blot for ICAM-1 protein. As shown in Fig. 6B, miR-221 precursor significantly blocked IFN-γ-induced ICAM-1 protein expression in H69 cells in a dose-dependent manner. A control RNA sequence did not show any effects. Moreover, miR-221 precursor transfection does not decrease IFN-γ-induced ICAM-1 transcription compared with IFN-γ-stimulated cells treated with the control precursor (Fig. 6C). Coupled with the downregulation of cellular miR-221 in response to IFN-γ, the above data suggest that relief of miR-221-mediated translational repression of ICAM-1 is involved in IFN-γ-induced ICAM-1 protein expression in cholangiocytes.
Upregulation of ICAM-1 in cholangiocytes induced by IFN-γ affects T cell adherence.
As an adhesion molecule, increased expression of ICAM-1 on epithelial cells contributes to an enhanced adherence of neutrophils and lymphocytes to epithelial cell surfaces (4, 18). To determine the functional relevance of miR-221-mediated expression of ICAM-1 in cholangiocytes, we performed adhesion assay to assess the adherence of cholangiocytes by the cocultured Jurkat cells. As shown in Fig. 7A, H69 cells transfected with the miR-221 precursor showed a significant decrease of Jurkat cell adherence. A neutralizing antibody to ICAM-1 also decreased adherence by cocultured Jurkat cells (Fig. 7A). In contrast, inhibition of miRNA-221 in H69 cells with the anti-miR-221 significantly enhanced Jurkat cell adherence compared with cells treated with the anti-miR control (Fig. 7B). Moreover, H69 cells with an enhanced ICAM-1 expression attributable to the IFN-γ stimulation showed a significant increased adherence to Jurkat cells compared with control non-IFN-γ-stimulated cells. Similarly, blockage of ICAM-1 on the IFN-γ-stimulated or -nontreated H69 cells with anti-ICAM-1 mAb (10 μg/ml), as well as transfection of cells with miR-221 precursor, significantly decreased the adherence of cocultured Jurkat cells (P < 0.05, Fig. 7C). Effects of miR-221-mediated ICAM-1 expression in cholangiocytes induced by IFN-γ on adherence of cocultured T cells were further confirmed by immunofluorescent microscopy (Fig. 7D). These data suggest that miR-221-mediated ICAM-1 expression in cholangiocytes influences adherence of cocultured Jurkat cells.
Fig. 7.
Upregulation of ICAM-1 in cholangiocytes in response to IFN-γ affects adherence of cocultured T cells. A and B: functional manipulation of miR-221 influenced adherence of H69 by cocultured Jurkat cells in an ICAM-1-dependent manner. H69 cells were first treated with miR-221 precursor (A) or anti-miR-221 (B) for 72 h. After being washed, cells were incubated with the medium containing the IgG isotype control (Ctrl) or anti-ICAM-1 mAb for 1 h. Activated Jurkat cells were labeled with the calcein acetoxymethyl ester (calcein AM) and then incubated with H69 cells. Adherence of Jurkat cells was measured under the fluorescence microscope and presented as percentage of control. C: IFN-γ stimulation increased adherence of H69 cells by cocultured Jurkat cells. H69 cells were exposed to IFN-γ for 24 h followed by treatment with the anti-ICAM-1 mAb or IgG isotype for 1 h. H69 cells were treated with miR-221 precursor for 72 h before exposure to IFN-γ, and adherence of H69 cells by Jurkat cells was then determined. Data are representative of 3 independent experiments. Precursor-Ctrl, nonspecific precursor control. *P < 0.05 t-test vs. IgG isotype control (in A and B) or non-IFN-γ-stimulated cells (in C); #P < 0.05 t-test vs. precursor-Ctrl or IgG isotype control. D: effects of upregulation of ICAM-1 in H69 cells in response to IFN-γ on adherence of cocultured T cells as assessed by immunofluorescent microscopy. Calcein AM-labeled Jurkat cells following adherence to H69 cells were shown. Bars = 50 μm.
DISCUSSION
The key findings in this report are that 1) targeting of ICAM-1 3′UTR by miR-221 suppresses ICAM-1 translation in human cholangiocytes, 2) IFN-γ downregulates miR-221 expression in cultured cholangiocytes through activation of the STAT signaling, 3) IFN-γ-induced expression of ICAM-1 in cholangiocytes involves downregulation of miR-221, and 4) expression of ICAM-1 on cholangiocytes regulated by miR-221 influences the adherence of cocultured T cells. These data suggest that miR-221 is involved in IFN-γ-induced expression of ICAM-1 and further regulates inflammatory responses in cholangiopathies.
Overexpression of ICAM-1, VCAM-1, and HLA antigens is a common pathological feature in inflammatory cholangiopathies and can promote cholangiocyte interactions with effector lymphocytes in the portal area (9, 10). Expression of ICAM-1 in cholangiocytes is finely controlled in response to inflammatory stimuli. Molecular mechanisms underlying the transcriptional regulation of ICAM-1 gene have been well investigated. Activation of intracellular signal pathways and nuclear translocation of transcription factors, including NF-κB and activator protein-1, have been demonstrated to be critical to transactivate ICAM-1 gene (13, 16). Here, we provided evidence that both transcriptional and miRNA-mediated posttranscriptional mechanisms are involved in IFN-γ-induced ICAM-1 expression in human cholangiocytes. First, we identified that miR-221 targets 3′UTR of ICAM-1, resulting in translational suppression in cultured human cholangiocytes. A significant decrease of the luciferase reporter translation was detected in the miR-221 precursor-treated cells using a luciferase reporter plasmid containing ICAM-1 3′UTR with the putative miR-221 binding site. Additionally, anti-miR-221 markedly increased luciferase reporter translation, and a mutation in the binding sequence blocked miR-221 precursor-induced decrease of luciferase reporter translation. Whereas no significant difference of ICAM-1 mRNA levels was found in cells transfected with the anti-miR-221, an increase of ICAM-1 protein was detected in the anti-miR-221-treated cells. Second, we detected a significant decrease of miR-221 expression in cells after IFN-γ stimulation, resulting in relief of translational suppression associated with ICAM-1 3′UTR containing the miR-221 binding site. Moreover, treatment of cells with miR-221 precursor abolished IFN-γ-induced ICAM-1 protein expression. Because a control precursor showed no inhibitory effect, we speculate that inhibition of ICAM-1 protein induction by miR-221 precursor is attributable to enhanced translational repression via targeting of miR-221 to the ICAM-1 3′UTR. Similarly, miR-126 has recently been reported to regulate VCAM-1 expression in endothelial cells (20), and ICAM-1 is a target for miR-222 and miR-339 in colorectal cancer cells (41). Coupled with our observation of complete inhibition of ICAM-1 transcription but only partial blockage of ICAM-1 protein expression by actinomycin D in IFN-γ-treated cells, these results suggest that IFN-γ induces ICAM-1 protein expression in cholangiocytes through both increased transcription of ICAM-1 and relief of miR-221-mediated translational repression.
The importance of miR-221-mediated translational suppression of ICAM-1 is beyond simply determining the rate of ICAM-1 translation at the posttranscriptional level. It may be critical to the stimulated expression of ICAM-1 in cholangiocytes in response to inflammatory stimuli. Using a nontransformed normal human cholangiocyte cell line (HIBEpiC) and several cholangiocarcinoma cell lines, Kawahigashi et al. (24) recently reported the cloning profiles of miRNAs in human cholangiocytes. Highly cloned miRNAs identified in human cholangiocytes include miR-221, representing about 10% of all miRNA clones, second only to miR-21 (24). The abundance of miR-221 in cholangiocytes may assure the low basal expression of ICAM-1 protein at a physiological condition. Therefore, it is plausible that relief of miR-221-mediated translational suppression may be necessary for the stimulated expression of ICAM-1 during inflammation. Indeed, transfection of cholangiocytes with miR-221 precursor could attenuate IFN-γ-induced ICAM-1 expression at its protein level; even a high level of ICAM mRNA still persisted after miR-221 precursor treatment. Although the expression level of miR-221 in other cell types is unclear, our data suggest a critical role for miR-221 in the coordinated regulation of ICAM-1 expression in cholangiocytes in response to IFN-γ.
Most of human miRNA genes are transcribed by polymerase II and can be classified as class II genes (25, 29, 33). Therefore, expression of miRNAs can be elaborately controlled through various regulatory mechanisms including transactivation and transrepression by nuclear transcription factors (17, 32, 40, 49), as well as activation of the microRNA-generating complex at the posttranscriptional level (15, 21, 44, 47). IFN-γ triggers both gene transactivation and transsuppression in stimulated cells. Gene regulation at the posttranscriptional level has also been reported in cells following IFN-γ stimulation (45). Indeed, significant decrease of precursor miR-221 and mature miR-221 was detected in cells following IFN-γ stimulation. Because knockdown of STAT1 via siRNA silencing can block IFN-γ-induced decrease of miR-221 expression, it is likely that IFN-γ decreases miRNA-221 expression through STAT1. Nevertheless, how IFN-γ suppresses miR-221 gene transcription and whether STAT1 can inhibit miRNA-221 maturation at the posttranscriptional level are still unclear and merit further investigation.
The expression of adhesion molecules on the surface of cholangiocytes modulates their interaction with other cell types in the liver. Adhesion molecules expressed on the cholangiocyte surface permit adhesion and recognition of lymphocytes and, subsequently, activation of cytotoxic effector lymphocytes. Upregulation of ICAM-1 and HLA-I in cultured human cholangiocytes was evident after stimulation with IFN-γ, TNF-α, and IL-1 (1). In this study, we confirmed that ICAM-1 expressed on the surface of human cholangiocytes following IFN-γ stimulation increased the attachment of cocultured Jurkat cells to cholangiocytes. An antibody to ICAM-1 completely blocked the attachment of cocultured Jurkat cells. Functional manipulation of miR-221 in cholangiocytes could also influence associated attachment of cocultured Jurkat cells. Thus ICAM-1 may have important regulatory functions to ensure a controlled and balanced inflammatory reaction in the portal region during biliary inflammation. It can be speculated that miR-221-mediated expression of ICAM-1 on cholangiocytes will influence cholangiocyte-T cell interactions in vivo. Infiltration of inflammatory lymphoid cells to the portal region has been demonstrated in the biliary tract in various hepatobiliary diseases (30, 38).
In conclusion, our data indicate that IFN-γ induces ICAM-1 expression in human cholangiocytes via relief of miR-221-mediated translational suppression of ICAM-1. Moreover, miR-221-mediated expression of ICAM-1 in cholangiocytes is involved in cholangiocyte-T cell interactions. It will be of interest to determine the mechanisms by which IFN-γ decreases miR-221 expression and the role of miRNAs in the regulation of biliary inflammatory reactions in vivo.
GRANTS
This work was supported by National Institutes of Health Grant AI071321 and by the Nebraska Tobacco Settlement Biomedical Research Program (LB692) and the Creighton Health Future Foundation (HFF) (to X.-M. Chen), and the Creighton Cancer and Smoking Development Award (LB595) (to X-M. Chen and C. Deng).
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1.Alpini G, McGill JM, LaRusso NF. The pathobiology of biliary epithelia. Hepatology 35: 1256–1268, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Ambros V. The functions of animal microRNAs. Nature 431: 350–355, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Aoki CA, Bowlus CL, Gershwin ME. The immunobiology of primary sclerosing cholangitis. Autoimmun Rev 4: 137–143, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Arnold R, Konig W. Respiratory syncytial virus infection of human lung endothelial cells enhances selectively intercellular adhesion molecule-1 expression. J Immunol 174: 7359–7367, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Aron JH, Bowlus CL. The immunobiology of primary sclerosing cholangitis. Semin Immunopathol 31: 383–397, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116: 281–297, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Betel D, Wilson M, Gabow A, Marks DS, Sander C. The microRNA.org resource: targets and expression. Nucleic Acids Res 36: D149–D153, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bito T, Roy S, Sen CK, Packer L. Pine bark extract pycnogenol downregulates IFN-gamma-induced adhesion of T cells to human keratinocytes by inhibiting inducible ICAM-1 expression. Free Radic Biol Med 28: 219–227, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Bloom S, Fleming K, Chapman R. Adhesion molecule expression in primary sclerosing cholangitis and primary biliary cirrhosis. Gut 36: 604–609, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen XM, O'Hara SP, LaRusso NF. The immunobiology of cholangiocytes. Immunol Cell Biol 86: 497–505, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen XM, Splinter PL, O'Hara SP, LaRusso NF. A cellular micro-RNA, let-7i, regulates Toll-like receptor 4 expression and contributes to cholangiocyte immune responses against Cryptosporidium parvum infection. J Biol Chem 282: 28929–28938, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chuang YH, Lan RY, Gershwin ME. The immunopathology of human biliary cell epithelium. Semin Immunopathol 31: 323–331, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Collins T, Read MA, Neish AS, Whitley MZ, Thanos D, Maniatis T. Transcriptional regulation of endothelial cell adhesion molecules: NF-κB and cytokine-inducible enhancers. FASEB J 9: 899–909, 1995 [PubMed] [Google Scholar]
- 14.Cruickshank SM, Southgate J, Selby PJ, Trejdosiewicz LK. Expression and cytokine regulation of immune recognition elements by normal human biliary epithelial and established liver cell lines in vitro. J Hepatol 29: 550–558, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Dangi-Garimella S, Yun J, Eves EM, Newman M, Erkeland SJ, Hammond SM, Minn AJ, Rosner MR. Raf kinase inhibitory protein suppresses a metastasis signalling cascade involving LIN28 and let-7. EMBO J 28: 347–358, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farina AR, Cappabianca L, Mackay AR, Tiberio A, Tacconelli A, Tessitore A, Frati L, Martinotti S, Gulino A. Transcriptional regulation of intercellular adhesion molecule 1 by phorbol ester in human neuroblastoma cell line SK-N-SH involves jun- and fos-containing activator protein 1 site binding complex(es). Cell Growth Differ 8: 789–800, 1997 [PubMed] [Google Scholar]
- 17.Fazi F, Rosa A, Fatica A, Gelmetti V, De Marchis ML, Nervi C, Bozzoni I. A minicircuitry comprised of microRNA-223 and transcription factors NFI-A and C/EBPalpha regulates human granulopoiesis. Cell 123: 819–831, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Frick AG, Joseph TD, Pang L, Rabe AM, St Geme JW, 3rd, Look DC. Haemophilus influenzae stimulates ICAM-1 expression on respiratory epithelial cells. J Immunol 164: 4185–4196, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Gong AY, Zhou R, Hu G, Li X, Splinter PL, O'Hara SP, LaRusso NF, Soukup GA, Dong H, Chen XM. MicroRNA-513 regulates B7-H1 translation and is involved in IFN-gamma-induced B7-H1 expression in cholangiocytes. J Immunol 182: 1325–1333, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris TA, Yamakuchi M, Ferlito M, Mendell JT, Lowenstein CJ. MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proc Natl Acad Sci USA 105: 1516–1521, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heo I, Joo C, Cho J, Ha M, Han J, Kim VN. Lin28 mediates the terminal uridylation of let-7 precursor MicroRNA. Mol Cell 32: 276–284, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Hu G, Zhou R, Liu J, Gong AY, Eischeid AN, Dittman JW, Chen XM. MicroRNA-98 and let-7 confer cholangiocyte expression of cytokine-inducible Src homology 2-containing protein in response to microbial challenge. J Immunol 183: 1617–1624, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu W, Blazar BR, Manivel JC, Paradis K, Sharp HL. Phenotypical and functional characterization of intrahepatic bile duct cells from common duct ligated mice. J Lab Clin Med 128: 536–544, 1996 [DOI] [PubMed] [Google Scholar]
- 24.Kawahigashi Y, Mishima T, Mizuguchi Y, Arima Y, Yokomuro S, Kanda T, Ishibashi O, Yoshida H, Tajiri T, Takizawa T. MicroRNA profiling of human intrahepatic cholangiocarcinoma cell lines reveals biliary epithelial cell-specific microRNAs. J Nippon Med Sch 76: 188–197, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Kim YK, Kim VN. Processing of intronic microRNAs. EMBO J 26: 775–783, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kloosterman WP, Plasterk RH. The diverse functions of microRNAs in animal development and disease. Dev Cell 11: 441–450, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Kumar H, Kawai T, Akira S. Pathogen recognition in the innate immune response. Biochem J 420: 1–16, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Lazaridis KN, Strazzabosco M, LaRusso NF. The cholangiopathies: disorders of biliary epithelia. Gastroenterology 127: 1565–1577, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Lee Y, Kim M, Han J, Yeom KH, Lee S, Baek SH, Kim VN. MicroRNA genes are transcribed by RNA polymerase II. EMBO J 23: 4051–4060, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu BR, Mack CL. Inflammation and biliary tract injury. Curr Opin Gastroenterol 25: 260–264, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Lukiw WJ, Cui JG, Li YY, Culicchia F. Up-regulation of micro-RNA-221 (miRNA-221; chr Xp11.3) and caspase-3 accompanies down-regulation of the survivin-1 homolog BIRC1 (NAIP) in glioblastoma multiforme (GBM). J Neurooncol 91: 27–32, 2009 [DOI] [PubMed] [Google Scholar]
- 32.O'Hara SP, Splinter PL, Gajdos GB, Trussoni CE, Fernandez-Zapico ME, Chen XM, LaRusso NF. NFkappaB p50-CCAAT-enhancer binding protein beta (C/EBPbeta)-mediated transcriptional repression of microRNA let-7i following microbial infection. J Biol Chem 285: 216–25, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ozsolak F, Poling LL, Wang Z, Liu H, Liu XS, Roeder RG, Zhang X, Song JS, Fisher DE. Chromatin structure analyses identify miRNA promoters. Genes Dev 22: 3172–3183, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ponnuraj EM, Hayward AR. Requirement for TNF-Tnfrsf1 signalling for sclerosing cholangitis in mice chronically infected by Cryptosporidium parvum. Clin Exp Immunol 128: 416–420, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shivakumar P, Sabla GE, Whitington P, Chougnet CA, Bezerra JA. Neonatal NK cells target the mouse duct epithelium via Nkg2d and drive tissue-specific injury in experimental biliary atresia. J Clin Invest 119: 2281–2290, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simpson MA, Reiland J, Burger SR, Furcht LT, Spicer AP, Oegema TR, Jr, McCarthy JB. Hyaluronan synthase elevation in metastatic prostate carcinoma cells correlates with hyaluronan surface retention, a prerequisite for rapid adhesion to bone marrow endothelial cells. J Biol Chem 276: 17949–17957, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Sonkoly E, Stahle M, Pivarcsi A. MicroRNAs and immunity: novel players in the regulation of normal immune function and inflammation. Semin Cancer Biol 18: 131–140, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Stephens J, Cosyns M, Jones M, Hayward A. Liver and bile duct pathology following Cryptosporidium parvum infection of immunodeficient mice. Hepatology 30: 27–35, 1999 [DOI] [PubMed] [Google Scholar]
- 39.Sugie T, Kubota H, Sato M, Nakamura E, Imamura M, Minato N. NK 1+ CD4- CD8- alphabeta T cells in the peritoneal cavity: specific T cell receptor-mediated cytotoxicity and selective IFN-gamma production against B cell leukemia and myeloma cells. J Immunol 157: 3925–3935, 1996 [PubMed] [Google Scholar]
- 40.Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci USA 103: 12481–12486, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ueda R, Kohanbash G, Sasaki K, Fujita M, Zhu X, Kastenhuber ER, McDonald HA, Potter DM, Hamilton RL, Lotze MT, Khan SA, Sobol RW, Okada H. Dicer-regulated microRNAs 222 and 339 promote resistance of cancer cells to cytotoxic T-lymphocytes by down-regulation of ICAM-1. Proc Natl Acad Sci USA 106: 10746–10751, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ueno Y, Phillips JO, Ludwig J, Lichtman SN, LaRusso NF. Development and characterization of a rodent model of immune-mediated cholangitis. Proc Natl Acad Sci USA 93: 216–220, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verdon R, Keusch GT, Tzipori S, Grubman SA, Jefferson DM, Ward HD. An in vitro model of infection of human biliary epithelial cells by Cryptosporidium parvum. J Infect Dis 175: 1268–1272, 1997 [DOI] [PubMed] [Google Scholar]
- 44.Viswanathan SR, Daley GQ, Gregory RI. Selective blockade of microRNA processing by Lin28. Science 320: 97–100, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vyas K, Chaudhuri S, Leaman DW, Komar AA, Musiyenko A, Barik S, Mazumder B. Genome-wide polysome profiling reveals an inflammation-responsive posttranscriptional operon in gamma interferon-activated monocytes. Mol Cell Biol 29: 458–470, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Whiteman SC, Bianco A, Knight RA, Spiteri MA. Human rhinovirus selectively modulates membranous and soluble forms of its intercellular adhesion molecule-1 (ICAM-1) receptor to promote epithelial cell infectivity. J Biol Chem 278: 11954–11961, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Yamagata K, Fujiyama S, Ito S, Ueda T, Murata T, Naitou M, Takeyama K, Minami Y, O'Malley BW, Kato S. Maturation of microRNA is hormonally regulated by a nuclear receptor. Mol Cell 36: 340–347, 2009 [DOI] [PubMed] [Google Scholar]
- 48.Yu J, Sheung N, Soliman EM, Spirli C, Dranoff JA. Transcriptional regulation of IL-6 in bile duct epithelia by extracellular ATP. Am J Physiol Gastrointest Liver Physiol 296: G563–G571, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou R, Hu G, Liu J, Gong AY, Drescher KM, Chen XM. NF-kappaB p65-dependent transactivation of miRNA genes following Cryptosporidium parvum infection stimulates epithelial cell immune responses. PLoS Pathog 5: e1000681, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]