Abstract
Progastrin and insulin-like growth factors (IGFs) stimulate hyperproliferation of intestinal epithelial cells (IECs) via endocrine/paracrine routes; hyperproliferation is a known risk factor for colon carcinogenesis. In the present study, inhibitory potency of curcumin in the presence or absence of progastrin and/or IGF-II was examined. Progastrin and IGF-II significantly increased proliferation of an immortalized IEC cell line, IEC-18, whereas curcumin decreased the proliferation in a dose-dependent manner. IGF-II was significantly more effective than progastrin in reversing antiproliferative effects of curcumin and reversed proapoptotic effects of curcumin by >80%; progastrin was relatively ineffective toward reversing proapoptotic effects of curcumin. IEC-18 clones were generated to overexpress either progastrin (IEC-PG) or hIGF-II (IEC-IGF). Proliferation of IEC-PG and IEC-IGF clones was increased, compared with that of control clones. Curcumin significantly reduced proliferation of IEC-PG, but not IEC-IGF, clones. Similarly, a human colon cancer cell line, Caco-2 (which expresses autocrine IGF-II), was relatively resistant to inhibitory effects of curcumin. However, Caco-2 cells treated with anti-IGF-II-antibodies were rendered sensitive to inhibitory effects of curcumin. Significant differences in inhibitory potency of curcumin against PG- vs. IGF-II-stimulated growth of IEC-18 cells were not reflected by differences in curcumin-mediated inhibition of activated (phosphorylated) ERKs/IKKα/β/p65NF-κB and c-Src in wild-type (wt)IEC-18 cells, in response to the two growth factors. Surprisingly, curcumin was almost ineffective in reducing IGF-II-stimulated activation of p38MAPK but significantly reduced progastrin-stimulated phosphorylation of p38. Treatment with a p38MAPK inhibitor resulted in loss of protective effects of IGF-II against inhibitory effects of curcumin. These novel findings suggest that growth factor profile of patients and tumors may dictate inhibitory potency of curcumin and that combination of curcumin + p38MAPK inhibitor may be required for reducing hyperproliferative or tumorigenic response of IECs to endocrine and autocrine IGFs.
Keywords: NF-κB, c-Src, IEC-18 cells, Caco-2 cells
colorectal cancers (CRCs) are one of the most common forms of cancers in men and women in the US and are one of the leading causes of death. Although genetic instability plays a dominant role in familial cancers, hyperproliferation, in response to aberrant growth factor signaling, is believed to play a permissive role in initiation and progression of sporadic cancers. Several growth factors, including progastrins (PG) and insulin-like growth factors (IGFs), exert potent proliferative effects on normal and cancerous intestinal cells in vitro (8, 31, 39). Studies with mutant mouse models suggest that PG and IGFs function as cocarcinogens and increase the risk of colon carcinogenesis or tumorigenesis (5, 7, 40, 51). Experimental evidence further suggests that PG and IGFs exert growth promoting effects during all phases of colon carcinogenesis via endocrine/paracrine/autocrine routes (5, 7, 8, 39, 40, 51).
The full-length, 80-amino-acid PG peptide is normally expressed in G enteroendocrine cells in the stomach and processed by endopeptidases into G-Gly; subsequent amidation at carboxy terminus generates amidated gastrins (G34, G17). Under physiological conditions, only amidated gastrins are present in circulation and play a role in meal-stimulated acid responses. However, in patients with hypergastrinemia and CRCs, significant levels of circulating PG and G-Gly are detected (reviewed in Ref. 31). Azoxymethane induced colonic tumors in rats, and a significant percent of human colon tumors express gastrin gene (31). However, colon cancer cells lack the ability to process PGs (46), and thus elevated levels of circulating PG are measured in rats and humans bearing colonic cancers (4, 31, 35). Importantly, autocrine gastrins (mainly PG) are required for maintaining tumorigenic potential of gastrin-dependent colon cancers (13, 38).
Epidemiological and experimental studies suggest a correlation between circulating levels of free IGF-I and relative risk for developing colon, breast, prostate, and lung cancer (22, 33). Autocrine IGFs (mainly IGF-II) play an equally important role in conferring a growth advantage to many cancer cells (8, 36, 39). IGF-II expression is 10- to 40-fold higher in CRC compared with that in normal colonocytes (8, 39). Thus both circulating and autocrine/paracrine IGFs and PG stimulate hyperproliferation of colonic crypt cells and exert potent cocarcinogenic effects at all phases of colon carcinogenesis.
Curcumin [diferuloylmethane; 1,7-bis-(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione] is the major pigment in turmeric powder and has anti-inflammatory and antioxidant properties (12, 19). Curcumin inhibits chemically induced carcinogenesis during initiation and/or postinitiation phases (27). Chemopreventative activity of curcumin was also demonstrated during the promotion and progression phases of colon carcinogenesis (17). Mechanisms by which dietary curcumin exerts chemopreventative effects at different phases of colon carcinogenesis was recently reviewed (34). Although antiproliferative effects of curcumin have been observed on most colon cancer cell lines, apoptotic effects of curcumin have not always been observed (3, 10). These differences may reflect growth factor profiles of CRC cells. Overexpression of specific antiapoptotic factors and signaling molecules such as Hsp70 (29), Bcl-xL, and ku70 (28) reduce inhibitory efficacy of curcumin on cancer cells. Thus elevated levels of endocrine/autocrine IGF-II and PG (relevant to colon cancer disease) can be expected to potentially impact chemopreventative efficacy of curcumin. To examine inhibitory effectiveness of curcumin in the presence of either PG or IGF-II, it was critical that we use a cell line that is responsive to IGF-II or PG but does not express the two growth factors so that the results are compared within an isogenic background. Almost all colon cancer cells express either the gastrin gene (PG) and/or IGF-II; for example, HCT-116 cells express gastrin gene and Caco-2 cells express autocrine IGF-II. Although HCT-116 cells are responsive to exogenous IGF-II, they do not respond to exogenous PG. Caco-2 cells become generally nonresponsive by day 9 and undergo spontaneous differentiation in culture by days 9–12. It is thus difficult to develop an isogenic background of cancer cells that are equally responsive to endocrine/autocrine IGF-II AND PG. In the present studies, we examined inhibitory efficacy of curcumin on an immortalized intestinal cell line (IEC-18), which is responsive to both growth factors and does not express IGF-II AND PG. Our hypothesis is that if hyperproliferative effects of growth factors are attenuated in the presence of dietary agents, such as curcumin, then the risk associated with hyperproliferation toward neoplastic transformation of colonic crypts cells, in the presence of initiating agents (such as DNA-damaging agents), can be significantly reduced. Thus, to address our major hypothesis, we chose the nontransformed IEC-18 cell line, which reflects the phenotype of colonic crypt cells in the proliferative zone.
Inhibitory potency of curcumin was also examined on Caco-2 cells, which expresses autocrine IGF-II in a density-dependent manner, from days 3–9 of cell culture (36), and represents an ideal cancer cell model for examining role of autocrine IGF-II. We report for the first time that proapoptotic potency of curcumin was almost completely reversed by IGF-II, whereas PG was much less effective, suggesting that elevated endocrine/autocrine IGF-II in cancer patients will likely impart a resistant phenotype to curcumin treatment. To examine mechanisms contributing to observed differences in protective effects of IGF-II vs. PG, phosphorylation (activation) of specific kinases and transcription factors in response to curcumin ± PG and/or IGF-II was examined. Our studies suggest that increased phosphorylation or activation of p38MAPK may contribute to significant differences in protective potency of IGF-II vs. PG against proapoptotic effects of curcumin. These novel findings can be expected to impact clinical use of curcumin in either preventing the transformation and neoplastic growth of colonic crypt cells and/or treating CRCs (and perhaps other epithelial cancers).
MATERIALS AND METHODS
Materials.
Leupeptin, aprotinin, benzamidine, phenylmethylsulfonyl fluoride (PMSF), sodium orthovanadate, ethylenediaminetetraacetic acid (EDTA), Nonidet P-40, octyl-d-glucoside (ODG), β-mercaptoethanol, Tris(hydroxymethyl)-aminomethane, HEPES, sodium chloride, sodium fluoride, glycerol, and camptothecin were obtained from Sigma Chemical (St. Louis, MO). Polyclonal anti-active caspase 3 and anti-caspase 9 antibodies were purchased from BD Pharmingen (San Diego, CA); polyclonal anti-β-actin was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Monoclonal anti-phospho-p65 NF-κB (Ser536), anti-phospho-IκBα (Ser32/36), anti-IKKα/β (Ser176/180), anti-phospho-p44/42 MAP kinase, anti-phospho-P38 MAP kinase, antibodies were from Cell Signaling Technology (Beverly, MA). Anti-v-Src mouse monoclonal antibody was purchased from Calbiochem (La Jolla, CA). IGF-II was purchased from Biosource (San Jose, CA), and rhPG was generated and purified in our laboratory as described (37). Specific anti-PG-Abs were generated in our laboratory as described (5, 32). NF-κB DNA binding assay kit was purchased from Active Motif (Carlsbad, CA). Anti-IGF-II-antibody was purchased either from Santa Cruz (SC1415) or from Abcam (ab63984).
Cell culture.
IEC-18 cells, a nontransformed intestinal crypt cell line derived from rat ileum (American Type Culture Collection, Rockville, MD) was propagated in DMEM (GIBCO-BRL, Grand Island, NY), supplemented with 10% heat-inactivated fetal calf serum (FCS, Hyclone, Logan, UT), 4 μM l-glutamine, 0.1 μM nonessential amino acids, 1 μM sodium pyruvate, 100 units/ml penicillin G sodium, and 100 mg/ml streptomycin sulfate in an atmosphere of 95% air-5% CO2 at 37°C as described previously (37). Caco-2 cells, a human colon cancer cell line, originally obtained from Dr. Jing Yu, Tufts School of Medicine (Boston, MA), has been maintained in our laboratory at early passages (16–35) for several years. Caco-2 cells were maintained in cell culture as described previously (36). The cell lines were regularly monitored for the absence of mycoplasma, by using a Mycoplasma Detection Kit (Boehringer Mannheim), and confirmed to be positive for E-cadherin. Stock cultures of cells were subcultured at appropriate intervals to maintain the cells at subconfluent densities. For cell counting and subculturing, the cells were dispersed with a solution of 0.05% trypsin and 0.02% EDTA.
Generation of IEC-18 clones overexpressing either hPG or hIGF-II.
Eukaryotic expression plasmids were created for expression of full-length coding sequences either for triple-double mutant (3×) hGAS genes (R57A-R58A, K74A-K75A, R94A-R95A) (as previously described; Ref. 5) or for human insulin-like growth factor II (IGF-II) gene, using a modified pcDNA3.1 vector (Invitrogen). An ATF, two serine, and six histidine codons were inserted 3′ to the vector's HindIII site. The histidine sequence is followed by an XhoI and a BamHI restriction site. Target inserts for 3× mutant hGAS and the hIGF-II open reading frame (accession no. J03242.1) were PCR amplified with primers that introduced a 5′ XhoI site and a 3′ BamHI site (post the termination codon) into the amplified DNA; the previously described Fabp-mut hGAS plasmid (5) was used as a template for amplifying mut hGAS cDNA. hIGF-II cDNA from a human colon cancer cell line (Caco-2) was used as a template for amplifying the IGF-II transcript. Final constructs were confirmed by DNA sequence analysis. The plasmids were transfected into the IEC-18 cells to create stably expressing clones that expressed either IGF-II (IEC-IGF-II) or PG (IEC-PG) by our published methods (38, 41). The vector-transfected clones were used as a control (IEC-C). The clones were confirmed for the expression of PG and IGF-II by Western blot and dot-blot analysis (data not shown), as described previously (5, 36). The concentration of PG/IGF-II secreted by the clones into the conditioned medium of the cells/24 h was determined as described previously (36, 38) to be ∼100–150 pg/10 ml/1 × 107 cells (which is equivalent to 1–2 nM peptide).
In vitro growth assays.
The effect of increasing concentrations of curcumin, in the presence or absence of PG or IGF-II, on the growth response of IEC-18 cells was measured either in a cell count assay or in a 3(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay as previously described (37). Briefly, for cell count assay, an optimal number of cells (3.5 × 105 cells) were plated in 35-mm dishes in 2 ml of growth medium containing 10% FCS. After 24 h, medium was changed to serum-free medium (SFM) for 72 h, followed by treatment of the cells with increasing concentrations of growth factors for 24 h in SFM, after which increasing concentrations of curcumin were added (∼10–100 μM) and incubated for 24–48 h. At the end of the treatment, cells were treated with trypsin-EDTA solution and counted via a Multisizer3 Coulter electronic counter (Beckman Coulter, Fullerton, CA). To measure the effect of growth factors on the antiproliferative effects of curcumin, MTT assay was also used as described previously (37). Briefly, an optimal number of cells (4.5 × 103) were plated in 96-well plates in 200 μl of growth medium containing 10% FCS. After 24 h, the medium was changed to SFM for 72 h, followed by addition of PG or IGF-II for 48 h; curcumin was then added and incubated for additional 24–48 h. Cells were then processed for the MTT assay (37). Inhibitory efficacy of curcumin was also examined on the growth of Caco-2 cells in culture, in the presence or absence of anti-IGF-II-antibodies as described in the legend of Fig. 2B.
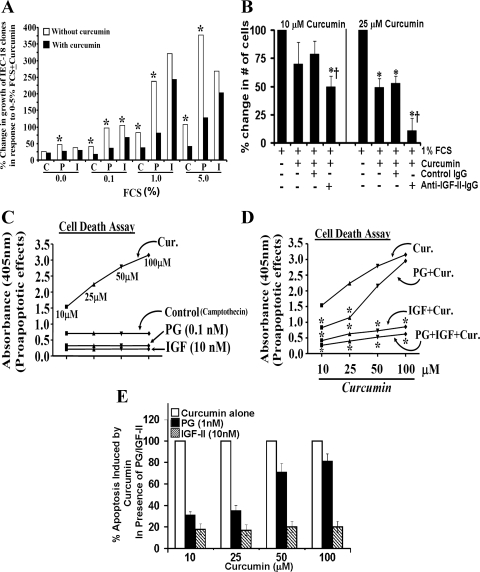
Fig. 2.
A: percent change in growth of IEC-18 clones in response to 0–5% FCS. IEC-18 clones, overexpressing either hPG (P) or hIGF-II (I), were used. Clones expressing vector alone served as control (C). Data (mean values) are derived from Table 1. Open bars, growth in response to fetal calf serum (FCS); solid bars, growth in response to FCS + curcumin (25 μM). Growth of control clones in response to 5% FCS was arbitrarily assigned 100% value; growth of all other clones is presented as a % of it. *P < 0.5 vs. filled (+curcumin) values. B: effect of anti-IGF-II-antibody treatment on the sensitivity of Caco-2 cells to inhibitory effects of curcumin. Caco-2 cells were plated in 35-mm cell culture dishes, as described previously (36). Since Caco-2 cells are extremely sensitive to loss of growth factors, the cells were maintained in 1% FCS from day 5 onward. On day 5 of cell culture, Caco-2 cells were treated with or without 10–25 μM (as shown) for 48 h, in the presence or absence of either the control (nonimmune) or specific anti-IGF-II-IgG (1:1,000 dilution). In each case the IgG was added 2 h before the addition of curcumin. At the end of the treatment, total number of cells/dish was counted by a cell count assay, as described under materials and methods. The % change in the number of cells is shown, wherein the total number of cells in the absence of curcumin treatment was arbitrarily assigned a 100% value. Each bar is mean ± SE of 6 dishes/2 experiments. *P < 0.05 vs. control (non-curcumin-treated values). †P < 0.05 vs. the corresponding control IgG values (3rd vs. 4th bar for the indicated curcumin-treatment). C and D: apoptotic death of wt IEC-18 cells (in a cell death assay) in response to camptothecin (control), in presence of curcumin ± growth factors. Apoptotic death was measured in terms of absorbance at 405 nm (y-axis). Increasing absorbance reflects increasing death. In C, absorbance of control or PG/IGF-II treated cells, are presented as flat lines, in relation to absorbance of curcumin-treated cells. In D, absorbance of samples treated with curcumin ± PG/IGF-II is presented. Each point = mean of 3 values from a representative of 3 experiments. Variation between values was ∼5–10%. *P < 0.05 vs. the corresponding value for curcumin alone. E: % apoptosis of cells induced by curcumin, in the presence of PG/IGF-II. Data presented are based on the data shown in D. Values measured in the presence of indicated concentration of curcumin alone were arbitrarily assigned 100% (open bar); apoptosis in the presence of factor alone was assigned 0%. Values presented for growth factor + curcumin are a % of cell death measured in response to curcumin alone. All values for IGF-II + curcumin-treated samples were significantly different (P < 0.05) from PG + curcumin-treated values.
Treatment of IEC-18 cells for measuring surrogate markers of apoptosis.
Cells were cultured in either 35-mm dishes or T-175 flasks (4 × 106), essentially as described above. For measuring surrogate markers of apoptosis (relative levels of activated caspases 3 and 9), cells were additionally treated for 4 h with the proapoptotic agent (camptothecin), as previously described (49). At the end of the treatment, cells were washed twice with ice-cold PBS, followed by cellular lysis with either ODG buffer (50 μM Tris·HCl, pH 8.0, 1 μM EDTA, 0.25 M NaCl, 20 μM NaF, 1 μM Na3VO4, 1% Nonidet P-40, 2% octyl-d-glucoside, 5 μM, β-mercaptoethanol, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 1 μM PMSF, 5% glycerol) or M-PER mammalian protein extraction reagent (Pierce) with protease inhibitor cocktail (Sigma). Relative levels of activated caspases 3 and 9 were measured by Western immunoblot analysis as described previously (49).
Western blot analysis.
Cellular lysates and cytoplasmic/nuclear extracts were prepared from control or treated IEC-18 cells as described above, followed by boiling in Laemmli buffer, as described previously (32, 49). Samples were processed for Western immunoblot analysis by our published methods (32). Briefly, equal amounts of protein (40–60 μg) were separated by electrophoresis on either 0.1% SDS, 10% polyacrylamide gels or NuPAGE 4–12% Bis-Tris Gel (Invitrogen). Protein was transferred electrophoretically to Hybond-ECL nitrocellulose membranes (Amersham Biosciences) and blocked in 10 mM Tris-Cl buffer pH 8 containing 150 μM NaCl, 0.1% Tween 20, and 5% (wt/vol) nonfat dry milk for 2 h at room temperature. Complete transfer of proteins was confirmed by GelCode Blue staining (Pierce). Membranes were processed by Western immunoblot analysis, using one or more of the indicated specific primary antibodies for 1 h at 4°C at a dilution of 1:1,000. Membranes were washed 4× in Tris-buffered saline containing 0.1% Tween 20, followed by incubation with the appropriate peroxidase-conjugated antibody. The antigen-antibody complexes were detected using chemiluminescence reagent kit (Amersham Pharmacia Biotech) according to the manufacturer's recommendations. To confirm equivalent loading, membranes were stripped with either 2 M glycine pH 2.8 for 30 min or with strip buffer (Pierce) and were reprobed with either anti-β-actin Ab or an Ab that detects the total amount of the indicated kinase, as internal controls. The relative density of the bands was densitometrically analyzed with the Documentation Analysis System (Model AlphaImager 2,000, Alpha Innotech, San Leandro, CA). The data were calculated as a ratio of the densitometric readings for the protein of interest vs. the appropriate control protein. The ratios obtained for control (no peptide or Ab treatment) samples were arbitrarily assigned a 100% value. The ratios obtained with all other treatments were expressed as a percent of the control values.
Cell death assay to measure loss of proapoptotic effect of curcumin in the presence of growth factors.
A cell death detection ELISAPLUS kit (Roche, Basel, Switzerland) was used for these studies, as described previously (32, 49). IEC-18 cells were seeded in 35-mm dishes (0.5 × 104 cells) and incubated at 37°C for 24 h followed by growth in SFM for 72 h. Growth factors were added for 24 h followed by an additional incubation with curcumin for 24 h, and cell death was analyzed as described (32).
DNA binding assay for measuring relative levels of activated NF-κB.
Activation of NF-κB was determined by using the TransAM p65 NF-κB transcription factor assay kit, as per instructions of the manufacturer, as recently described (32, 44). Briefly, 10 μg of nuclear extract samples were incubated in binding buffer with oligos containing NF-κB consensus binding sites, immobilized in the wells, for 2 h at room temperature. The bound complex was washed followed by incubation with anti-p65 NF-κB-Ab for 1 h at room temperature. Wells were washed and incubated with horseradish peroxidase-coupled second antibody for an additional 1 h. Wells were thoroughly washed and color was developed by adding 100 μl of developing solution provided in the kit. The assay was terminated by adding 100 μl of stop solution from the kit and color intensity was determined in a plate reader at 450 nm.
Statistical analysis.
Data are presented as means ± SE of values obtained from four to eight samples from two to three experiments. To test for significant differences between means, nonparametric Mann-Whitney test was employed using Statview 4.1 (Abacus Concepts, Berkeley, CA); P values were considered to be statistically significant if less than 0.05.
RESULTS
Effect of curcumin ± IGF-II and PG on growth of IEC-18 cells.
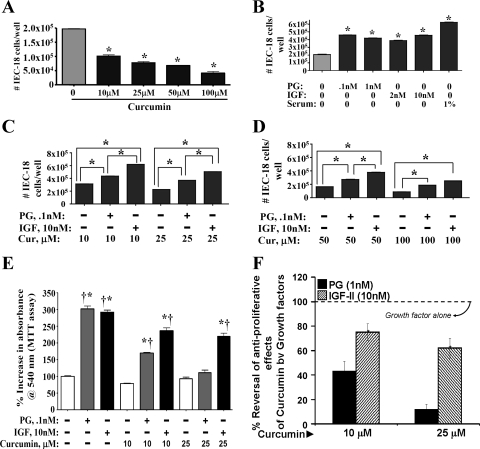
Inhibitory effects of curcumin have been reported on several transformed and neoplastic cell lines. However, inhibitory effects of curcumin, if any, on nontransformed IEC-18 cells have not been reported. Relatively low doses of curcumin (10 μM) inhibited growth of serum-stimulated IEC-18 cells by ∼50% (Fig. 1A). Dose-dependent inhibitory effects of curcumin were measured (Fig. 1A). Both PG and IGF-II increased growth of IEC-18 cells by ∼2.0- to 2.5-fold, in response to optimally effective doses of PG (0.1–1 nM) and IGF-II (2–10 nM) (Fig. 1B); at lower doses, PG and IGF-II were less effective (data not shown). For all further experiments, maximally effective doses of PG (1.0 nM) and IGF-II (10 nM) were used. Both PG and IGF-II significantly reversed growth-inhibitory effects of curcumin, at all doses examined; IGF-II was significantly more effective than PG (Fig. 1, C and D). In an MTT assay, relative protective efficacy of IGF-II vs. PG, against inhibitory effects of curcumin, was further amplified (Fig. 1, E and F). Although the pattern of response measured in a cell count (Fig. 1, C and D) and an MTT (Fig. 1E) assay was similar, the MTT assay was more sensitive.
Fig. 1.
Effect of curcumin (Cur) ± IGF-II or progastrin (PG) on growth of IEC-18 cells (cell count assay). IEC-18 cells were treated with curcumin ± IGF-II or PG as described in materials and methods, and the effect was assessed in a cell count assay. Data are presented as means ± SE of 4 values from a representative of 3 experiments. *P < 0.05 vs. control values (no treatment) in A and B; *P < 0.05 vs. the indicated bars in C and D. E: effect of curcumin ± GF-II/PG on growth of IEC-18 cells (MTT assay). Absorbance measured in control wells (no treatment) was arbitrarily assigned a 100% value. Readings from all other wells are expressed as a % change from control values. Each bar represents mean ± SE of data from 6–8 wells from a representative of 2 experiments. *P < 0.05 vs. control; †P < 0.05 vs. curcumin alone. F: percent reversal of antiproliferative effects of curcumin, in the presence of growth factors. The % reversal of antiproliferative effects of curcumin by growth factors in an MTT assay (E), to levels measured in the presence of growth factors alone are shown. Values measured in the presence of growth factors alone were arbitrarily assigned 100%. Values presented for growth factor + curcumin are presented as a % of growth factor alone. *P < 0.05 vs. the corresponding values for PG + curcumin.
Inhibitory effects of curcumin on growth of IEC-18 clones, overexpressing either hPG or hIGF-II.
IEC-18 clones overexpressing either triple mutant hGAS or wild-type (wt) hIGF-II transcripts were generated and confirmed as described under materials and methods. Progastrin, transcribed from mutant hGAS transcript, lacks dibasic sites required for processing (5), and full-length PG (80 amino acids) is expressed by IEC-PG clones. All clones were confirmed at RNA and protein levels, as described in materials and methods. Growth response of clones to increasing concentrations of FCS, in presence or absence of 25 μM curcumin, was examined in an MTT assay. Growth response of IEC-C clones to 5% FCS was arbitrarily assigned a 100% value to normalize values from different experiments. Curcumin significantly inhibited growth of IEC-C clones in response to increasing concentration of FCS; in the absence of FCS, no significant effects were measured (Fig. 2A, Table 1). Growth of IEC-PG and IEC-IGF-II clones increased significantly in response to FCS in a dose-dependent manner (Fig. 2A, Table 1). Curcumin effectively inhibited growth effects of autocrine PG (IEC-PG clones), at all doses of FCS; however, curcumin was not as effective in inhibiting growth effects of autocrine IGF-II (Fig. 2A, Table 1). These results provide strong evidence that curcumin is significantly more effective in inhibiting growth effects of autocrine PG than autocrine IGF-II, similar to results obtained with exogenous growth factors (Fig. 1).
Table 1.
% change in growth of IEC-18 clones in response to 0.0–5% FCS ± 25 μM curcumin
| 0.0% FCS | 0.1% FCS | 1.0% FCS | 5.0% FCS | |||||
|---|---|---|---|---|---|---|---|---|
| FCS | + Cur | FCS | + Cur | FCS | + Cur | FCS | + Cur | |
| IEC-18-C | (100%) | (85 ± 7) | (100%) | (43 ± 6) | (100%) | (45 ± 5) | (100%) | (41 ± 7) |
| 25 ± 6% | 21 ± 3% | 42 ± 7% | 18 ± 5%* | 83 ± 4% | 38 ± 7%* | 100% | 41 ± 7%* | |
| IEC-18- | (100%) | (57 ± 8) | (100%) | (37 ± 7) | (100%) | (34 ± 29) | (100%) | (34 ± 51) |
| PG | 47 ± 8% | 27 ± 4%* | 97 ± 8% | 36 ± 5%* | 238 ± 32% | 82 ± 7%* | 377 ± 58% | 127 ± 23%* |
| IEC-18- | (100%) | (78 ± 8) | (100%) | (69 ± 7) | (100%) | (76 ± 39) | (100%) | (76 ± 40) |
| IGF-II | 37 ± 9% | 29 ± 6% | 105 ± 8% | 73 ± 4%* | 321 ± 28% | 243 ± 47% | 268 ± 57% | 203 ± 41% |
IEC-18 clones, overexpressing either progastrin (PG) or IGF-II, were generated and confirmed as described in materials and methods. Clones expressing vector alone were used as control (C). Growth response of clones to increasing concentrations of FCS, in the presence or absence of 25 μM curcumin, was examined in a mean transit time assay. The growth response of IEC-C clones to 5% FCS was arbitrarily assigned a 100% value, and growth measured in response to all other treatments is expressed as a % of the response of IEC-18-C clones to 5% FCS. Each data point represents mean ± SE of 4–8 measurements from 2 separate experiments. In each case, % response of clones treated with FCS alone was further arbitrarily assigned a 100% value and is shown in parentheses. The loss in response in the presence of curcumin (+Cur) is presented as a % of the growth in the presence of FCS alone (shown in parentheses).
P < 0.05 vs. corresponding FCS-alone values. Mean values obtained in the presence of various treatments are also presented as bar graphs in Fig 2A.
Inhibitory effects of curcumin on the growth of Caco-2 cells, in the presence or absence of anti-IGF-II antibody.
We and others have previously reported that Caco-2 cells express high concentrations of autocrine IGF-II under subconfluent conditions from days 2–5 of cell culture and that the expression or secretion of IGF-II is progressively decreased to negligible levels by day 9 of cell culture, as the cells become confluent (36). In preliminary studies we further reported that subconfluent Caco-2 cells on days 3–5 of cell culture (expressing relatively high levels of IGF-II) were resistant to proapoptotic and growth inhibitory effects of curcumin, whereas Caco-2 cells on days 7–9 of cell culture (expressing relatively low levels of IGF-II) were sensitive to inhibitory effects of curcumin (26). In the present studies we further examined the role of autocrine IGF-II in impacting the sensitivity of Caco-2 cells to the inhibitory effects of curcumin. Day 5 Caco-2 cells were treated with either nonimmune (control) IgG or specific anti-IGF-II-IgG for 2 h, followed by curcumin treatment as described in the legend of Fig. 2B. Anti-IGF-II-Abs, by itself, reduced the growth of Caco-2 cells (data not shown) as reported previously (36), confirming an important role of endogenous IGF-II in maintaining proliferation of Caco-2 cells. The growth of Caco-2 cells, growing in response to 1% FCS, was inhibited by ∼50%, on treatment with 25 μM curcumin (Fig. 2B); curcumin may have primarily inhibited growth in response FCS. Importantly, Caco-2 cells treated with anti-IGF-II-IgG were further sensitized to the inhibitory effects of curcumin by ∼30% (10 μM) to ∼70% (25 μM), resulting in almost complete attenuation of growth (25 μM) (Fig. 2B). Growth of cells treated with nonimmune control IgG remained similar to that of cells treated with curcumin alone (Fig. 2B). Thus results with Caco-2 cells resemble the results with IEC-IGF-II clones (as described above) and further confirm the novel paradigm that autocrine IGF-II expression imparts a resistant phenotype to inhibitory effects of curcumin.
Effect of curcumin ± growth factors on apoptotic response of IEC-18 cells to camptothecin.
Curcumin significantly increased apoptotic response of IEC-18 cells to camptothecin, in a dose-dependent manner; antiapoptotic effects of PG and IGF-II were confirmed (Fig. 2C). PG and IGF-II reduced camptothecin-induced apoptosis of IEC-18 cells by ∼50% (PG) and 60–70% (IGF-II) (Fig. 2C). Both PG and IGF-II significantly reversed (reduced) proapoptotic effects of 10–25 μM curcumin (Fig. 2, D and E); PG was relatively ineffective against 50–100 μM curcumin, whereas IGF-II remained significantly effective (Fig. 2F). In summary, IGF-II was significantly more effective than PG in reversing antiproliferative and proapoptotic effects of curcumin (Fig. 1, F and E).
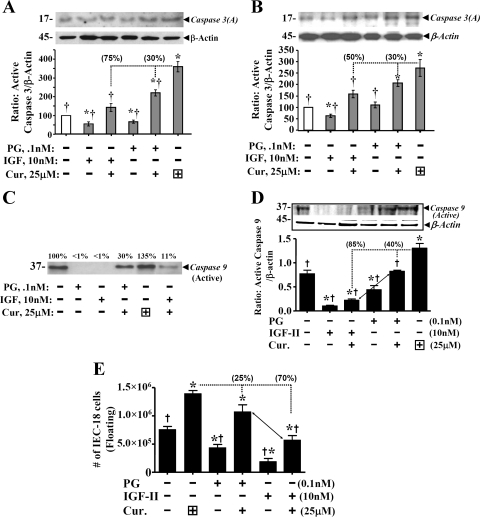
Curcumin significantly increased activation of caspase 3 (Fig. 3A). Sensitivity of cells to proapoptotic effects of curcumin were enhanced in cells that were 50% (Fig. 3A) vs. 70% (Fig. 3B) confluent. Thus confluency further impacts degree of response of cells. Protein yield from 50% confluent cells (Fig. 3C) was low and lysates from several experiments had to be pooled. Pattern of response, however, was similar in 50% (Fig. 3, A and C) and ∼70% (Fig. 3, B and D) confluent cells. IGF-II was ∼50–300% more effective than PG in reversing activation of caspase 3 (Fig. 3, A and B) and caspase 9 (Fig. 3, C and D), in response to curcumin. Results with caspase 9 activation (Fig. 3, C and D) agreed with cell death assay results in Fig. 2D. Differences in the effects on activation of caspases 3 vs. 9 may reflect differences in sensitivity of the two antibodies. Cells undergoing apoptosis detach from extracellular matrix and float in cell culture medium, as an indirect reflection of cell death (49). Numbers of floating cells significantly increased in response to curcumin and significantly decreased in response to PG/IGF-II; IGF-II was significantly more effective than PG in reversing apoptotic effects of PG in the floating assay (Fig. 3E). Average percentage(s) by which growth factors attenuated proapoptotic effects of curcumin are shown in parenthesis above the relevant bar graphs; proapoptotic effects of curcumin were reversed by ∼70–85% by IGF-II and by only 25–30% by PG (Fig. 3).
Fig. 3.
IGF-II is more effective than PG in reversing proapoptotic effects of curcumin on IEC-18 cells. Proapoptotic effects of curcumin were measured in terms of either activation of caspase 3 (A and B) or caspase 9 (C and D), or number of floating cells (E). Representative Western blots (of 4 blots from 2 experiments) are presented in top panels. Relative levels of β-actin in the samples are shown. Ratio of activated caspases 3 or 9/β-actin was calculated, and control (nontreated) ratios were assigned 100% value. Ratios for all other treatments are presented as % of control values. In E, each bar = mean ± SE of floating cells, measured in six culture dishes/treatment. *P < 0.05 vs. control values; †P < 0.5 vs. curcumin alone. Horizontal dotted lines = curcumin-alone values. Dropdown vertical line = loss in proapoptotic effects in the presence of growth factor; % reversal of proapoptotic effects is presented numerically in parentheses on top of the indicated bar.
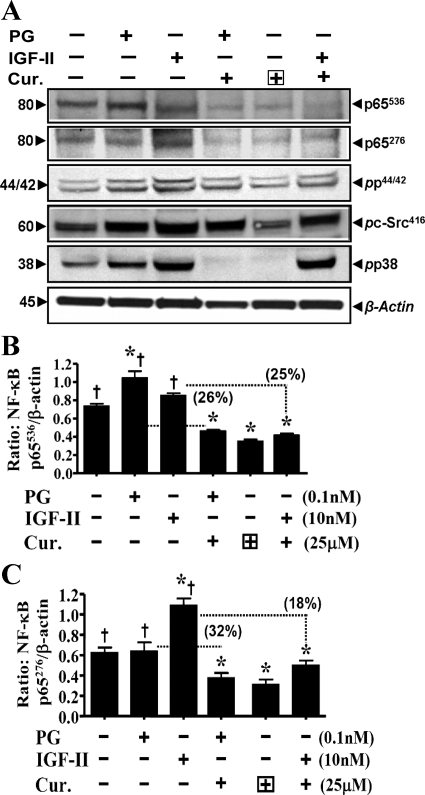
Effect of curcumin ± growth factors on phosphorylation of IKKα/β/NF-κBp65.
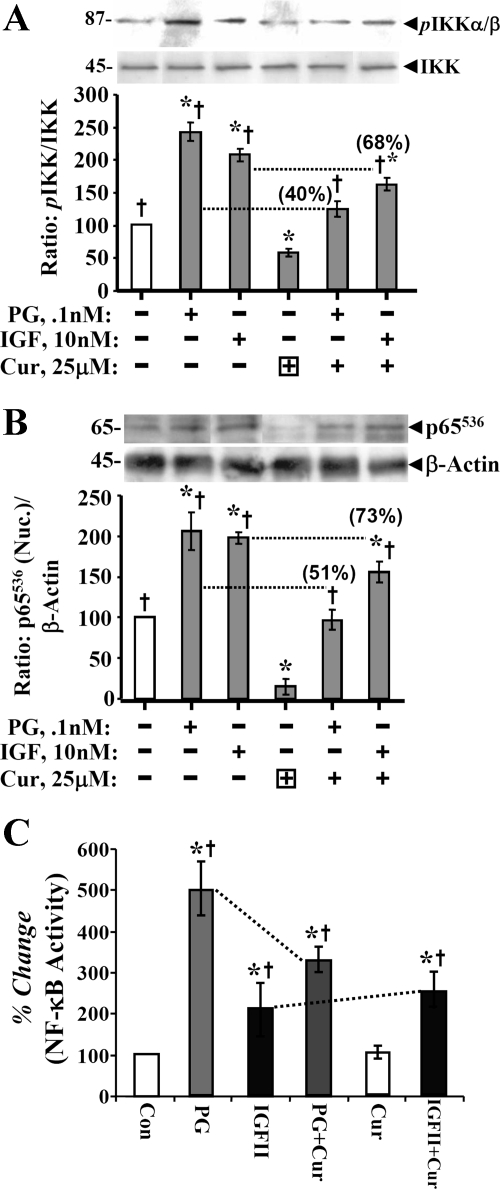
An optimal dose of curcumin (25 μM) was chosen, since at this dose both PG and IGF-II demonstrated significant protective effects; at higher doses only IGF-II was protective (Fig. 2D). Representative Western blot data are presented in top panels of Fig. 4, A and B. Bar graphs represent means ± SE of data from four blots from two separate experiments, and are a ratio of the indicated phospho (p) form of kinase molecule to levels of total kinase (Fig. 4A)/β-actin (Fig. 4B) in the sample. Data in Fig. 4, A and B, are from ∼50% confluent cells. Curcumin treatment resulted in significant loss in relative levels of pIKKα/β (Fig. 4A) and pNF-κBp65536 (Fig. 4B); both PG and IGF-II significantly increased levels of pIKKα/β and p65536. Both PG and IGF-II significantly reversed inhibitory effects of curcumin on phosphorylation of p65536; IGF-II was more effective (Fig. 4B). To confirm functional relevance of the observed changes in phosphorylation status of p65, activation of NF-κB was measured in a DNA binding assay, as described previously (32, 44). Change in NF-κB activity was calculated as a percent change from control (nonstimulated) values, wherein NF-κB activity in control samples was assigned a 100% value. Surprisingly PG was more effective than IGF-II in increasing DNA binding (activation) of NF-κB (Fig. 4C). Reason(s) for the apparent discrepancy in relative potency of the two peptides toward increasing relative levels of pp65 vs. increasing DNA binding of pp65 (in an in vitro assay) is not clear; possible reasons are discussed below. Based on results presented in Fig. 4, it appears that both IGF-II and PG were effective in reversing inhibitory effects of curcumin on phosphorylation and activation of IKKα/β and NF-κBp65. Thus the vast difference in protective effects of IGF-II vs. PG against antiproliferative/proapoptotic effects of curcumin on IEC-18 cells, is not likely mediated via the NF-κB pathway alone. Average percentage by which growth factors reversed inhibitory effects of curcumin on phosphorylation/activation of IKKα/β and NF-κBp65 are presented in parentheses on top of the relevant bar graphs (Fig. 4, A and B).
Fig. 4.
Inhibitory effect of curcumin on phosphorylation and activation of kinases in response to growth factors. A and B: phosphorylated kinase values for IKKα/β and pp65 NF-κB p65536 were calculated as ratio of total kinase/β-actin. Ratios of control (untreated) samples were assigned 100% value, and ratios of all other samples were calculated as a % of control. Data in each bar = means ± SE of 4 measurements from 2 experiments. Horizontal dotted lines = loss in levels of activated kinase in the presence of curcumin. The % loss of kinase activation, in response to IGF/PG, in the presence of curcumin is presented numerically in parentheses on top of the relevant bar. C: relative levels of activated NF-κB from a DNA binding assay. Each bar graph = mean ± SE of 3 measurements from 2 experiments. *P < 0.05 vs. control (untreated) samples; †P < 0.05 vs. curcumin alone.
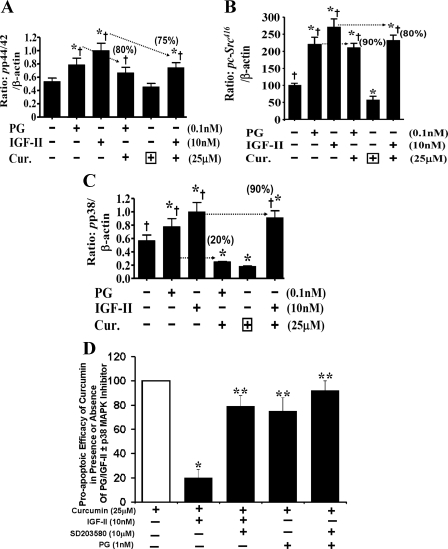
Effect of curcumin ± growth factors on phosphorylation (activation) of p65 and other kinases (p44,42ERKs/c-Src/p38MAPK). As discussed above, to further examine the underlying mechanisms responsible for the significant differences in the proapoptotic effects of curcumin against PG- vs. IGF-II-mediated growth of IEC-18 cells, large-scale studies were conducted to analyze relative levels of several additional kinases within one experiment itself (Fig. 5A). In the large-scale studies, T75 flasks of IEC-18 cells were treated with curcumin ± growth factors. Rather than growing the cells to only 50% confluence (Figs. 4, A–C), cells were grown to 70% confluence (Fig. 5A), to obtain larger amounts of cellular lysate protein. A representative Western blot, demonstrating relative levels of pp65536, pp65276, ppERK, pc-Src, and pp38MAPK is presented from one of two similar experiments; relative levels of β-actin in the corresponding samples from a representative β-actin blot are shown in Fig. 5A. The ratio of the indicated phosphorylated kinase to β-actin levels, calculated as a percent of control (as described in the legends of Figs. 4, A and B), from a total of four blots from two experiments, are presented in Figs. 5, B and C, and 6, A–C. Once again the degree of inhibitory (curcumin) and stimulatory (growth factors) responses of IEC-18 cells were significantly enhanced in cells treated at 50% (Fig. 4, A and B) vs. ∼70% (Fig. 5, A–C) confluence. In other words, protective effects of growth factors were blunted in 70% confluent cells (Fig. 5A).
Fig. 5.
A: representative Western blots for activated kinases in response to curcumin ± PG/IGF-II. Data presented is from one of 4 blots from 2 separate experiments. Relative levels of β-actin measured in a representative blot from corresponding samples is shown. B and C: inhibitory effects of curcumin on activation of pNF-κBp65536 (B) and, pNF-κBp65276 (C) in response to growth factors. Western blot data, as shown in A, were used to calculated the ratios as described in Fig. 4. Briefly, phosphorylated kinase values were calculated at a ratio of β-actin. Ratios of control (untreated) samples were assigned 100% values, and ratios of all other samples were calculated as a % of control. Data in each bar = means ± SE of 4 measurements from 2 experiments. Dotted horizontal lines = inhibitory effects of curcumin on activation of indicated kinases, as described in Fig. 4. Percent activation of kinase by PG/IGF-II was assigned 100% values; numerical values in parentheses = % loss in kinase activation in the presence of curcumin, compared with that in the presence of PG/IGF-II alone. *P < 0.05 vs. control; †P < 0.05 vs. curcumin alone.
Fig. 6.
A–C: inhibitory effects of curcumin and activation of ERKs (A), c-Src (B), and p38MAPK (C), in response to growth factors. Western blot data, as shown in Fig. 5A, was used to calculate the ratios as described above for Fig. 5, B and C. Numerical values in parentheses are % loss in kinase activation in the presence of curcumin, compared with that in the presence of PG/IGF-II alone. *P < 0.05 vs. control; †P < 0.05 vs. curcumin alone. D: proapoptotic efficacy of curcumin in the presence or absence of PG/IGF-II ± p38MAPK inhibitor (measured in a cell death assay). On a scale of 0–100, cell death measured in the presence of curcumin alone was arbitrarily assigned 100%, whereas cell death in wild-type IEC-18 cells in the presence of growth factors alone was assigned 0%. Data represent means ± SE of 8 separate measurements from 2 experiments. *P < 0.05 vs. corresponding growth factor + curcumin values. p38 inhibitor (p38-I) (SB203580, 10 μM) was added 30 min before addition of growth factor ± curcumin, as previously described (32). Addition of p38 inhibitor alone did not significantly change levels of cell death measured (data not shown). *P < 0.05 vs. curcumin alone; **P < 0.05 vs. IGF-II + curcumin (second bar).
Surprisingly, levels of pNF-κBp65276 were also significantly increased by IGF-II, but not PG; IGF-II slightly reversed inhibitory effects of curcumin on pNF-κBp65276 (Fig. 5C). Since IGF-II increased phosphorylation of NF-κB at both Ser536 and Ser276 (Fig. 5, A–C), it may have caused spatial hindrance in an in vitro DNA binding assay (Fig. 4C) and may thus represent an artifact of the in vitro assay, resulting in the observed difference in the DNA binding of PG- vs. IGF-II-stimulated samples. However, both PG and IGF-II significantly reversed inhibitory effects of curcumin on DNA binding of pNF-κB (Fig. 4C).
In the absence of FCS, curcumin did not significantly reduce activation of ERKs (Figs. 5A and 6A) but significantly reduced levels of pSrc416 and pp38MAPK (Figs. 5A and 6, B and C). Both PG and IGF-II significantly increased phosphorylation of ERKs/c-Src416/p38MAPK (Figs. 5A and 6, A–C); IGF-II was generally more potent than PG, but the differences were not statistically significant. Both PG and IGF-II significantly reversed inhibitory effects of curcumin on phosphorylation of ERKs/c-Src, with almost equal potency (Figs. 5A and 6, A and B). Numbers in parentheses, on top of the bar graphs, represent average percentages by which IGF-II AND PG reversed inhibitory effects of curcumin on the indicated kinase(s). The most interesting finding was that although IGF-II overwhelmingly reversed inhibitory effects of curcumin on pp38MAPK (by ≃90%), PG was almost ineffective (Figs. 5A and 6C). Thus the stark difference in protective effects of IGF-II vs. PG on phosphorylation of p38MAPK may have contributed to differential effects of IGF-II vs. PG in overcoming proapoptotic effects of curcumin (Fig. 2D). To test this novel possibility, we used a specific p38MAPK inhibitor (SB203580) (Fig. 6D). Inhibition of p38 overcame protective effects of IGF-II against inhibitory effects of curcumin (Fig. 6D), providing further evidence that IGF-II mediated activation of p38MAPK likely contributed to potent protective effects of IGF-II.
DISCUSSION
The present studies have led us to discover several new paradigms that can be expected to impact clinical use of curcumin as a chemopreventive agent against intestinal hyperproliferation and tumorigenesis. Our studies strongly suggest that inhibitory efficacy of curcumin will be significantly reduced in the presence of endocrine/autocrine growth factors, especially IGFs. Protective potency of the two physiologically relevant endocrine/autocrine growth factors against inhibitory effects of curcumin was growth factor specific. Protective potency of exogenous/autocrine IGF-II was two- to threefold higher than that of PG against inhibitory effects of curcumin on proliferation/survival of IEC-18 cells in vitro. Similarly, a human colon cancer cell line (Caco-2, which expresses significant levels of autocrine IGF-II on days 3–5 of cell culture; Ref. 36) was resistant to inhibitory effects of curcumin; however, day 5 Caco-2 cells were sensitized to inhibitory effects of curcumin in the presence of anti-IGF-II-antibodies (Fig. 2B).
Results with IEC-18 cells suggested a possible difference in inhibitory potency of curcumin against signaling pathways required for mediating proliferative and antiapoptotic effects of the two growth factors. In the case of PG, NF-κB activation by the canonical pathway (activation of IKK and degradation of IκBα), downstream of p38MAPK/ERK activation, is critically required for measuring growth effects of PG in vitro (32) and on colonic crypts in vivo (44). NEMO peptide (selective inhibitor of IKKβ) abrogated antiapoptotic effects of PG in vitro (32) and in vivo (44), suggesting an important role of IKKβ in mediating PG effects. A critical role of NF-κB in mediating antiapoptotic effects of PG was confirmed in experiments with NF-κBp65 siRNA (32). Nuclear translocation/activation of β-catenin were also measured (in vitro) and in vivo in IECs and colonic crypts of mice, in response to PG (45); parallel findings were reported in cancer cells, downregulated for PG expression (24). Our recent findings suggest that β-catenin activation in response to PG is downstream of NF-κB activation (45); thus activation of NF-κB remains critical for measuring proliferative and antiapoptotic effects of PG. Besides PG, other gastrointestinal hormones, such as neurotensin (47) and amidated gastrins (43), also upregulate NF-κB-mediated regulatory molecules such as IL-8. Thus growth-promoting and antiapoptotic effects of gastrointestinal hormones, via their cognate receptors, can be potentially mediated by the target gene products of activated NF-κB pathways.
In the case of IGFs, activation of IGF-I receptor (IGF-I-R) results in recruitment and phosphorylation of adaptor proteins insulin receptor substrate (IRS-I) and SHC (33), which serve as docking sites for other signaling molecules, resulting in activation of several downstream pathways, including c-Src, PI3K/Akt, and p38MAPK/ERKs (33). IGF activation of colon cancer cell lines was shown to protect them from apoptosis by potentiating TNF-α/MAPK/NF-κB signaling pathways (30). Constitutive activation of IGF-I-R in immortalized mammary epithelial cells resulted in overexpression of activated NF-κB and was associated with transformation of the cells (18). Activation of NF-κBp65 was essential for measuring IGF-I-mediated stimulation of metatarsal growth and growth plate chondrogenesis (50). Increased expression of allelic Igf-II increased elongation of intestinal crypts and adenoma growth (11). Results of present studies additionally suggest that IGF-II activates NF-κB in IEC-18 cells (Figs. 4 and 5). Increase in cellular levels of reactive oxygen species (ROS) results in activation of NF-κB, and curcumin reduces cellular levels of ROS (reviewed in Ref. 21). Relative levels of ROS are high in cancer cells but are barely detectable in normal cells. Even though IEC-18 cells are immortalized and can be propagated in the presence of FCS and/or growth factors, the cell line is nontumorigenic. IEC-18 cells have relatively low levels of ROS (unpublished data from our laboratory) and express low levels of activated NF-κB (present study). However, in the presence of PG/IGF-II, significant increases in levels of activated NF-κB were measured (Figs. 4, 5).
Activation of NF-κB is a crucial event both in inflammation and cancer (16). Inhibition of NF-κB plays an important role in curcumin induced apoptosis (reviewed in Ref. 19, 21, 34). Inhibitory effects of curcumin are mediated through IκB/NF-κB pathway in transformed IEC and colon cancer cells (15). Curcumin inhibits cell migration of human colon cancer cells through inhibition of NF-κB/p65, COX-2, and MMP-2 expression (42). Constitutively active NF-κB in melanoma cells plays a central role in cell survival and growth; curcumin selectively inhibited growth of melanoma cells but not normal melanocytes (23). Similarly, constitutive activation of NF-κB has been observed in colorectal cancer cells but not in normal colorectal epithelial cells (discussed in Ref. 20). Radiation resistance of tumor cells, due to activation of NF-κB in response to radiation therapy, was reversed by curcumin (20). In summary, curcumin suppresses NF-κB activation and downregulates expression of NF-κB-regulated gene products involved in survival (Bcl2, Bcl-xL, XIAP, and cIAP-1), proliferation (COX2, cyclin D1, and c-myc), angiogenesis (VEGF and IL-8), invasion (MMP-9), and metastasis (ICAM-1, VCAM-1, and ELAM-1) (discussed in Ref. 20). Therefore, in the present studies, we focused on examining whether curcumin differentially inhibits activation of NF-κB in response to IGF-II vs. PG as a means of understanding significant differences in inhibitory potency of curcumin against the two growth factors. Since NF-κB activation is critically required for mediating PG effects, it was expected that curcumin will likely inhibit PG-mediated NF-κB activation and hence growth. In the case of IGFs, inhibitory effects of curcumin on IGF-I-R and IGF-stimulated pathways was recently reported on cancer cells (25, 52). However, anticancer effects of curcumin, independent of IGF-I-R inhibition, have also been reported (2). At the same time, an increase in IGF-I levels has been reported in response to curcumin in diabetic rats (14). Thus inhibitory vs. stimulatory effects of curcumin on IGF system may be specific to the cell or system.
In the present studies, both PG/IGF-II significantly increased phosphorylation/activation of NF-κB (Figs. 4 and 5). However, the upstream pathways mediating increase in phosphorylation of NF-κB were probably different, since PG only stimulated an increase in p65Ser536, whereas IGF-II increased both p65Ser276 and p65Ser536. Despite these differences, increases in relative levels of pNF-κBp65 in response to PG/IGF-II were significantly reduced in the presence of curcumin. Differences in relative inhibitory potency of curcumin against IGF-II- vs. PG-stimulated activation of NF-κB was only ∼20–30%; this difference, in itself, probably does not account for the much larger difference in protective potency of IGF-II vs. PG against antiproliferative and antiapoptotic effects of curcumin (Figs. 1F and 2D).
Unlike unitargeted or multitargeted drugs, dietary agents such as curcumin have been reported to downregulate activation of several signaling pathways and a large array of unrelated membrane proteins (12, 19). Besides NF-κB, pathways known to be targeted directly or indirectly by curcumin, include ERKs, p38MAPK, and c-Src. An important role of p38MAPK activity was demonstrated in experiments with cisplatin-resistant human ovarian carcinoma cells, wherein curcumin was reported to induce apoptosis of resistant cells by significantly reducing levels of p38MAPK activity (48). Radiosensitivity of cancer cells was significantly reduced by either inhibiting p38MAPK or IGF-I-R (6), further suggesting an important role of p38 in mediating biological responses to both PG (32, 44) and ionizing radiation or IGFs (6). Curcumin significantly inhibits pp60c-Src tyrosine kinase (discussed in Ref. 19). Both PG and IGF-II significantly upregulated activation of p44/42 ERKs, p38MAPK, and c-Src (Figs. 5A and 6, A–C). An interesting finding was that curcumin was not very potent in reversing activation of ERKs/c-Src in response to PG/IGF-II. On the other hand, curcumin was extremely potent in inhibiting activation of p38MAPK in response to PG, but not IGF-II (Figs. 5A and 6C). These findings were unexpected. As described above, γ-irradiation of non-small lung cancer cells activates IGF-I-R, resulting in complex formation with p38MAPK and signals for DNA damage response, independent of PI3K (6). Although in the present study, cells were not irradiated, it is possible that ligand-stimulated IGF-I-R also forms a complex with p38MAPK, which is perhaps resistant to proapoptotic effects of curcumin; this intriguing possibility needs to be examined in future studies.
To confirm a possible critical role p38MAPK in mediating resistance of IGF-II-stimulated cells to inhibitory effects of curcumin, activation of p38 was pharmacologically inhibited by a specific inhibitor of p38MAPK. IEC-18 cells, inhibited for p38MAPK activation, were resensitized to proapoptotic effects of curcumin, in the presence of IGF-II (Fig. 6D). Results with IEC-PG clones, and with day 5 Caco-2 cells, suggest for the first time that cells growing in response to endocrine/autocrine IGFs are likely to be resistant to proapoptotic effects of curcumin and that cells may be resensitized to inhibitory effects of curcumin by either inhibiting the activation of p38MAPK or by negating autocrine IGF-II.
Besides the signaling pathways examined in this study, several other pathways are also known to be directly or indirectly impacted by curcumin, including the EGF receptor pathway. In vitro experiments have shown that short-term treatment with curcumin inhibits EGFR kinase activity and EGF-induced tyrosine phosphorylation of EGFR in A431 cells and depletes the cells of HER2/neu protein (reviewed in Ref. 12). Curcumin is a potent inhibitor of ligand-induced activation of EGF and inhibits phosphorylation of EGFR, which occurs extensively in established cancers (reviewed in Ref. 12). Combined treatment with curcumin + oxaliplatin significantly inhibited growth of colon cancer cell lines, associated with decreased expression and activation of EGFR/HER-2/HER-3 (25). At the same time, PG/gastrin peptides upregulate EGFR (reviewed in Ref. 31); it is possible that some of the growth effects measured in response to PG are indirectly mediated via upregulation of EGFR. Potent inhibitory effects of curcumin on PG-stimulated growth may therefore be additionally mediated via inhibition of the EGFR-mediated pathways. The latter possibility may further explain the significant difference in the inhibitory potency of curcumin against PG- vs. IGF-II-stimulated growth of cells.
Curcumin may potentially regulate action of membrane proteins by changing physical properties of the membranes (1). Curcumin physically interacts with several proteins including c-Src (reviewed in Refs. 12, 19). In preliminary studies we measured significant inhibition of PG binding with its cognate receptor, annexin2; however, curcumin did not inhibit binding of IGFs to IGF-I-R. The possibility that curcumin may inhibit interaction of PG with annexin2 may also contribute to curcumin-mediated attenuation of elevated pp38MAPK in response to PG (Fig. 6C).
Cancer cells exposed to curcumin for 24 h or longer undergo apoptosis (21). In the present studies we measured significant antiproliferative effects of curcumin on IEC-18 cells, growing in the presence of PG or IGF-II (Figs. 1 and 2). Stark differences were measured in proapoptotic potency of curcumin in the presence of IGF-II vs. PG; cells growing in response to IGF-II were relatively resistant to apoptotic effects of curcumin, whereas cells growing in response to PG were much less resistant (Fig. 2D). Similarly, Gautam et al. (16) reported that curcumin induced inhibition of cell proliferation was not specific to cancer cells and was not always associated with apoptosis. It is possible that resistance to proapoptotic effects of curcumin may reflect presence of protective growth factors such as IGF-II. In preliminary studies, we had reported that Caco-2 cells, which express high concentrations of IGF-II on days 3–5 of cell culture (36), are almost completely resistant to proapoptotic effects of curcumin at early time points (26); the latter observation with Caco-2 cells was confirmed in the present studies with clones of IEC-18 cells overexpressing IGF-II. Clones expressing hIGF-II were manyfold more resistant to inhibitory effects of curcumin than clones overexpressing PG (Fig. 2A, Table 1). Role of autocrine IGF-II was further confirmed in the background of colon cancer cells; anti-IGF-II antibodies effectively resensitized Caco-2 cells to inhibitory effects of curcumin (Fig. 2B). We have additionally measured significant inhibitory effects of dietary curcumin (2–4%) against development of preneoplastic lesions (aberrant crypt foci) in transgenic mice overexpressing PG in the intestines (Fabp-PG mice), to levels measured in wild-type FVB/N mice, in response to azoxymethane (unpublished data from our laboratory). In preliminary studies, we have also examined lengths of isolated colonic crypts (by our published methods; Refs. 44, 45) from mice fed control or 2% dietary curcumin ± 10 nM PG/IGF-II injections, twice daily, for 7 days. Colonic crypt lengths measured in the various groups of mice were in the order of control diet+IGF-II ≥ control-diet+PG ≥ curcumin-diet+IGF-II > *curcumin-diet+PG ≅ curcumin-diet+saline ≅ control-diet+saline. Thus the present in vitro studies with wt IEC-18/IEC-PG vs. IEC-IGF-II/Caco-2 cells and the unpublished in vivo studies (described above) provide strong evidence that growth of intestinal epithelial cells, colonic crypt cells, and colon cancer cells, in the presence of endocrine/autocrine IGF-II, is likely to be resistant to inhibitory effects of curcumin.
Rationally designed personalized strategies will likely be required for treating diseases such as cancer. Results of the present studies heighten the need to examine inhibitory efficacy of dietary agents, in the presence of physiologically or pathologically relevant endocrine and autocrine growth factors. Our studies strongly suggest that patients positive for high levels of endocrine growth factors, especially IGFs, are likely to be less responsive to inhibitory effects of curcumin; tumors positive for expression of autocrine growth factors, especially IGF-II, can also be extrapolated to be resistant to inhibitory effects of curcumin and may require combinatorial treatments for reducing intracellular p38 and perhaps other signaling molecules.
GRANTS
These studies were supported by National Cancer Institute Grants R01CA114264 and R01CA097959 to P. Singh and R01CA131413 to S. Umar.
DISCLOSURES
None of the authors have any conflict of interest with any entity for any part of these studies.
ACKNOWLEDGEMENTS
The technical assistance provided by Hai Wu, research associate, and C. S. Pichot, graduate assistant, for generating some of the data is acknowledged. The excellent secretarial support of Cheryl Simmons for typing this manuscript is also acknowledged.
REFERENCES
- 1.Barry J, Fritz M, Brender JR, Smith PE, Lee DK, Ramamoorthy A. Determining the effects of lipophilic drugs on membrane structure by solid-state NMR spectroscopy: the case of the antioxidant curcumin. J Am Chem Soc 131: 4490–44988, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beevers CS, Chen L, Liu L, Luo Y, Webster NJ, Huang S. Curcumin disrupts the Mammalian target of rapamycin-raptor complex. Cancer Res 69: 1000–1008, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bordonaro M, Mariadason JM, Aslam F, Heerdt BG, Augenlicht LH. Butyrate-induced apoptotic cascade in colonic carcinoma cells: modulation of the beta-catenin-Tcf pathway and concordance with effects of sulindac and trichostatin A, but not curcumin. Cell Growth Differ 10: 713–720, 1999 [PubMed] [Google Scholar]
- 4.Ciccotosto GD, McLeish A, Hardy KJ, Shulkes A. Expression, processing, and secretion of gastrin in patients with colorectal carcinoma. Gastroenterology 109: 1142–1153, 1995 [DOI] [PubMed] [Google Scholar]
- 5.Cobb S, Wood T, Ceci J, Varro A, Velasco M, Singh P. Intestinal expression of progastrin significantly increases colon carcinogenesis in transgenic mice in response to AOM. Cancer 100: 1311–1323, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Cosaceanu D, Budiu RA, Carapancea M, Castro J, Lewensohn R, Dricu A. Ionizing radiation activates IGF-1R triggering a cytoprotective signaling by interfering with Ku-DNA binding and by modulating Ku86 expression via a p38 kinase-dependent mechanism. Oncogene 26: 2423–2434, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Diehl D, Oesterle D, Elmlinger MW, Hoeflich A, Wolf E, Lahm H. IGF-II transgenic mice display increased aberrant colon crypt multiplicity and tumor volume after 1,2-dimethylhydrazine treatment. J Carcinog 5: 24, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frasca F, Pandini G, Sciacca L, Pezzino V, Squatrito S, Belfiore A, Vigneri R. The role of insulin receptors and IGF-I receptors in cancer and other diseases. Arch Physiol Biochem 114: 23–37, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Gautam SC, Xu YX, Pindolia KR, Janakiraman N, Chapman RA. Nonselective inhibition of proliferation of transformed and nontransformed cells by the anticancer agent curcumin (diferuloylmethane). Biochem Pharmacol 55: 1333–1337, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Hanif R, Qiao L, Shiff SJ, Rigas B. Curcumin, a natural plant phenolic food additive, inhibits cell proliferation and induces cell cycle changes in colon adenocarcinoma cell lines by a prostaglandin-independent pathway. J Lab Clin Med 130: 576–584, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Harper J, Burns JL, Foulstone EJ, Pignatelli M, Zaina S, Hassan AB. Soluble IGF2 receptor rescues Apc(Min/+) intestinal adenoma progression induced by IGF2 loss of imprinting. Cancer Res 66: 1940–1948, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Hatcher H, Planalp R, Cho J, Torti FM, Torti SV. Curcumin: from ancient medicine to current clinical trials. Cell Mol Life Sci 65: 1631–1652, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hollande F, Imdahl A, Mantamadiotis T, Ciccotosto GD, Shulkes A, Baldwin GS. Glycine-extended gastrin acts as an autocrine growth factor in a nontransformed colon cell line. Gastroenterology 113: 1576–1588, 1997 [DOI] [PubMed] [Google Scholar]
- 14.Isik AT, Celik T, Ulusoy G, Ongoru O, Elibol B, Doruk H, Bozoglu E, Kayir H, Mas MR, Akman S. Curcumin ameliorates impaired insulin/IGF signalling and memory deficit in a streptozotocin-treated rat model. Age (Dordr) 31: 39–49, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jobin C, Bradham CA, Russo MP, Juma B, Narula AS, Brenner DA, Sartor RB. Curcumin blocks cytokine-mediated NF-kappa B activation and proinflammatory gene expression by inhibiting inhibitory factor I-kappa B kinase activity. J Immunol 163: 3474–3483, 1999 [PubMed] [Google Scholar]
- 16.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature 441: 431–436, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Kawamori T, Lubet R, Steele VE, Kelloff GJ, Kaskey RB, Rao CV, Reddy BS. Chemopreventive effect of curcumin, a naturally occurring anti-inflammatory agent, during the promotion/progression stages of colon cancer. Cancer Res 59: 597–601, 1999 [PubMed] [Google Scholar]
- 18.Kim HJ, Litzenburger BC, Cui X, Delgado DA, Grabiner BC, Lin X, Lewis MT, Gottardis MM, Wong TW, Attar RM, Carboni JM, Lee AV. Constitutively active type 1 insulin-like growth factor receptor causes transformation and xenograft growth of immortalized mammary epithelial cells and is accompanied by an epithelial-to-mesenchymal transition mediated by NF-kappaB and snail. Mol Cell Biol 27: 3165–3175, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kunnumakkara AB, Anand P, Aggarwal BB. Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett 269: 199–225, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Kunnumakkara AB, Diagaradjane P, Guha S, Deorukhkar A, Shentu S, Aggarwal BB, Krishnan S. Curcumin sensitizes human colorectal cancer xenografts in nude mice to gamma-radiation by targeting nuclear factor-kappaB-regulated gene products. Clin Cancer Res 14: 2128–2136, 2008 [DOI] [PubMed] [Google Scholar]
- 21.López-Lázaro M. Anticancer and carcinogenic properties of curcumin: considerations for its clinical development as a cancer chemopreventive and chemotherapeutic agent. Mol Nutr Food Res 52: S103–S127, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Ma J, Pollak MN, Giovannucci E, Chan JM, Tao Y, Hennekens CH, Stampfer MJ. Prospective study of colorectal cancer risk in men and plasma levels of insulin-like growth factor (IGF)-I and IGF-binding protein-3. J Natl Cancer Inst 91: 620–625, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Marín YE, Wall BA, Wang S, Namkoong J, Martino JJ, Suh J, Lee HJ, Rabson AB, Yang CS, Chen S, Ryu JH. Curcumin downregulates the constitutive activity of NF-kappaB and induces apoptosis in novel mouse melanoma cells. Melanoma Res 17: 274–283, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Pannequin J, Delaunay N, Buchert M, Surrel F, Bourgaux JF, Ryan J, Boireau S, Coelho J, Pélegrin A, Singh P, Shulkes A, Yim M, Baldwin GS, Pignodel C, Lambeau G, Jay P, Joubert D, Hollande F. Beta-catenin/Tcf-4 inhibition after progastrin targeting reduces growth and drives differentiation of intestinal tumors. Gastroenterology 133: 1554–1568, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Patel BB, Sengupta R, Qazi S, Vachhani H, Yu Y, Rishi AK, Majumdar AP. Curcumin enhances the effects of 5-fluorouracil and oxaliplatin in mediating growth inhibition of colon cancer cells by modulating EGFR and IGF-1R. Int J Cancer 122: 267–273, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Pichot CS, Rengifo-Cam W, Singh A, Owlia A, Singh P. Exogenous and autocrine growth factors significantly reduce the anti-proliferative and proapoptotic effects of curcumin on normal and cancerous epithelial cells. Am Assn Cancer Res 96th Ann Mtg, Anaheim, CA, April 16–20, 2005. [Google Scholar]
- 27.Rao CV, Rivenson A, Simi B, Reddy BS. Chemoprevention of colon carcinogenesis by dietary curcumin, a naturally occurring plant phenolic compound. Cancer Res 55: 259–266, 1995 [PubMed] [Google Scholar]
- 28.Rashmi R, Kumar S, Karunagaran D. Ectopic expression of Bcl-XL or Ku70 protects human colon cancer cells (SW480) against curcumin-induced apoptosis while their down-regulation potentiates it. Carcinogenesis 25: 1867–1877, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Rashmi R, Kumar S, Karunagaran D. Ectopic expression of Hsp70 confers resistance and silencing its expression sensitizes human colon cancer cells to curcumin-induced apoptosis. Carcinogenesis 25: 179–187, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Remacle-Bonnet MM, Garrouste FL, Heller S, Andre F, Marvaldi JL, Pommier GJ. Insulin-like growth factor-I protects colon cancer cells from death factor-induced apoptosis by potentiating tumour necrosis factor α-induced mitogen-activated protein kinase and nuclear factor κB signaling pathways. Cancer Res 60: 2007–2017, 2000 [PubMed] [Google Scholar]
- 31.Rengifo-Cam W, Singh P. Role of progastrins and gastrins and their receptors in GI and pancreatic cancers: targets for treatment. Curr Pharm Des 10: 2345–2358, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Rengifo-Cam W, Umar S, Sarkar S, Singh P. Anti-apoptotic effects of progastrin on pancreatic cancer cells are mediated by sustained activation of p65. Cancer Res 67: 7266–7274, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Sachdev D, Yee D. Disrupting insulin-like growth factor signaling as a potential cancer therapy. Mol Cancer Ther 6: 1–12, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Sarkar FH, Li Y. Cell signaling pathways altered by natural chemopreventive agents. Mutat Res 555: 53–64, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Siddheshwar RK, Gray JC, Kelly SB. Plasma levels of progastrin but not amidated gastrin or glycine extended gastrin are elevated in patients with colorectal carcinoma. Gut 48: 47–52, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh P, Dai B, Yallampalli U, Lu X, Schroy PC. Proliferation and differentiation of a human colon cancer cell line (CaCo2) is associated with significant changes in the expression and secretion of insulin-like growth factor (IGF) IGF-II and IGF binding protein-4: role of IGF-II. Endocrinology 137: 1764–1774, 1996 [DOI] [PubMed] [Google Scholar]
- 37.Singh P, Lu X, Cobb S, Miller BT, Tarasova N, Varro A, Owlia A. Progastrin 1–80 stimulates growth of intestinal epithelial cells in vitro via high-affinity binding sites. Am J Physiol Gastrointest Liver Physiol 284: G328–G339, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Singh P, Owlia A, Varro A, Dai B, Rajaraman S, Wood T. Gastrin gene expression is required for the proliferation and tumorigenicity of human colon cancer cells. Cancer Res 119: 4111–4115, 1996 [PubMed] [Google Scholar]
- 39.Singh P, Rubin N. Insulin like growth factors and binding proteins in colon cancer. Gastroenterology 105: 1218–1237, 1993 [DOI] [PubMed] [Google Scholar]
- 40.Singh P, Velasco M, Given R, Varro A, Wang TC. Progastrin expression predisposes mice to colon carcinomas and adenomas in response to a chemical carcinogen. Gastroenterology 119: 162–171, 2000 [DOI] [PubMed] [Google Scholar]
- 41.Singh P, Wu H, Clark C, Owlia A. Annexin II binds progastrin and gastrin like peptides, and mediates growth factor effects of autocrine and exogenous gastrins on colon cancer and intestinal epithelial cells. Oncogene 26: 425–440, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Su CC, Chen GW, Lin JG, Wu LT, Chung JG. Curcumin inhibits cell migration of human colon cancer colo 205 cells through the inhibition of nuclear factor kappa B/p65 and down-regulates cyclooxygenase-2 and matrix metalloproteinase-2 expressions. Anticancer Res 26: 1281–1288, 2006 [PubMed] [Google Scholar]
- 43.Subramaniam D, Ramalingam S, May R, Dieckgraefe BK, Berg DE, Pothoulakis C, Houchen CW, Wang TC, Anant S. Gastrin-mediated interleukin-8 and cyclooxygenase-2 gene expression: differential transcriptional and posttranscriptional mechanisms. Gastroenterology 134: 1070–1082, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Umar S, Sarkar S, Cowey S, Singh P. Activation of NF-kappaB is required for mediating proliferative and antiapoptotic effects of progastrin on proximal colonic crypts of mice, in vivo. Oncogene 5599–5611, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Umar S, Sarkar S, Wang Y, Singh P. Functional cross-talk between β-catenin and NF-κB signaling pathways in colonic crypts of mice in response to progastrin. J Biol Chem 84: 22274–22284, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Solinge WW, Nielsen FC, Friis-Hansen L, Falkmer UG, Rehfeld JF. Expression but incomplete maturation of progastrin in colorectal carcinomas. Gastroenterology 104: 1099–1107, 1993 [DOI] [PubMed] [Google Scholar]
- 47.Wang X, Wang Q, Ives KL, Evers BM. Curcumin inhibits neurotensin-mediated interleukin-8 production and migration of HCT116 human colon cancer cells. Clin Cancer Res 12: 5346–5355, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weir NM, Selvendiran K, Kutala VK, Tong L, Vishwanath S, Rajaram M, Tridandapani S, Anant S, Kuppusamy P. Curcumin induces G2/M arrest and apoptosis in cisplatin-resistant human ovarian cancer cells by modulating Akt and p38 MAPK. Cancer Biol Ther 6: 178–184, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu H, Owlia A, Singh P. Precursor peptide progastrin1–80 reduces apoptosis of intestinal epithelial cells and upregulates cytochrome. c ATP. Am J Physiol Gastrointest Liver Physiol 285: G1097–G1110, 2003. doi: 10.1152/ajpgi.00216.2003. [DOI] [PubMed] [Google Scholar]
- 50.Wu S, Fadoju D, Rezvani G, De Luca F. The stimulatory effects of insulin-like growth factor-1 on growth plate chondrogenesis are mediated by nuclear factor-kBp65. J Biol Chem 283: 34037–34044, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu Y, Yakar S, Zhao L, Hennighausen L, LeRoith D. Circulating insulin-like growth factor-I levels regulate colon cancer growth and metastasis. Cancer Res 62: 1030–1035, 2002 [PubMed] [Google Scholar]
- 52.Xia Y, Jin L, Zhang B, Xue H, Li Q, Xu Y. The potentiation of curcumin on insulin-like growth factor-1 action in MCF-7 human breast carcinoma cells. Life Sci 80: 2161–2169, 2007. [DOI] [PubMed] [Google Scholar]