Abstract
Antenatal corticosteroids may have long-term effects on renal development which have not been clearly defined. Our objective was to compare the responses to intrarenal infusions of ANG II in two groups of year-old, male sheep: one group exposed to a clinically relevant dose of betamethasone before birth and one not exposed. We wished to test the hypothesis that antenatal steroid exposure would enhance renal responses to ANG II in adult life. Six pairs of male sheep underwent unilateral nephrectomy and renal artery catheter placement. The sheep were infused for 24 h with ANG II or with ANG II accompanied by blockade of the angiotensin type 1 (AT1) or type 2 (AT2) receptor. Baseline mean arterial blood pressure among betamethasone-exposed sheep was higher than in control animals (85.8 ± 2.2 and 78.3 ± 1.0 mmHg, respectively, P = 0.003). Intrarenal infusion of ANG II did not increase systemic blood pressure (P ≥ 0.05) but significantly decreased effective renal plasma flow and increased renal artery resistance (P < 0.05). The decrease in flow and increase in resistance were significantly greater in betamethasone- compared with vehicle-exposed sheep (betamethasone P < 0.05, vehicle P ≥ 0.05). This effect appeared to be mediated by a heightened sensitivity to the AT1 receptor among betamethasone-exposed sheep. Sodium excretion initially decreased in both groups during ANG II infusion; however, a rebound was observed after 24 h. AT1 blockade was followed by a significant rebound after 24 h in both groups. AT2 blockade blunted the 24-h rebound effect among the vehicle-exposed sheep compared with the betamethasone-exposed sheep. In conclusion, antenatal corticosteroid exposure appears to modify renal responsiveness to ANG II by increasing AT1- and decreasing AT2 receptor-mediated actions particularly as related to renal blood flow and sodium excretion.
Keywords: angiotensin receptor, renal plasma flow, glomerular filtration rate, corticosteroids
the 1994 national institutes of health consensus statement recommended the use of antenatal betamethasone for the prevention of prematurity-related complications including a decreased incidence of respiratory distress, intraventricular hemorrhage grade III and IV, and necrotizing enterocolitis (37). This recommendation was recently ratified in a Cochrane review (46). However, recent evidence suggests that antenatal glucocorticoid exposure has unexpected effects on other organ systems. For example, glucocorticoid administration during pregnancy in animal models appears to have marked effects on the fetal renin-angiotensin system (RAS) and on nephron number, renal function and blood pressure in adulthood (12–14, 17, 32, 35, 36, 56).
ANG II is considered the most biologically active product of the RAS. Its actions include vascular constriction, vasopressin and aldosterone release, cellular growth and migration, and fibrosis, glomerular filtration rate (GFR) alterations, and stimulation of tubular sodium reabsorption and bicarbonate transport (6). Most of these actions are mediated through angiotensin type I (AT1) receptors although the angiotensin type II (AT2) receptor may modulate these effects (7, 11, 39, 43).
The prenatal use of glucocorticosteroids for prevention of prematurity-related complications has been demonstrated to cause a decrease in number of nephrons (13, 14, 17). In the sheep model, exposure to corticosteroids at a gestational age and with a dose similar to that at which betamethasone is given in human pregnancy to prevent prematurity related complications, is associated with a 25% decrease in the number of functional glomeruli (17, 56), independently of any effect on renal weight or birth weight (17).
The information regarding the long-term effect of antenatal glucocorticoid exposure on the renin-angiotensin system (RAS) is limited. This is complicated by the several levels at which RAS functions. Not only is there a systemic RAS effect as documented by fluctuations in plasma renin or angiotensin levels, but there are also organ-specific differences as well as intracellular differences within a given organ. The acute changes related to antenatal corticosteroid exposure shortly after birth include increased plasma renin and ANG II levels and increased expression of renal AT1 and AT2 receptor mRNA (32, 35). The long-term phenotypic changes in the sheep model include increased angiotensin-converting enzyme (ACE) to ACE2 activity and upregulation of the AT1 receptor in the kidney (21, 47).
There is no evidence regarding the effect of ANG II on renal vascular tone and sodium excretion in adult sheep previously exposed to antenatal corticosteroids. Therefore, the objective of this study was to evaluate the impact of antenatal betamethasone exposure during nephrogenesis on renal responses to direct intrarenal infusion of ANG II. We wanted to establish whether steroid exposure alters renal functional responses to the peptide. We hypothesized that antenatal betamethasone at 80–81 days (0.6) of gestation would increase responsiveness to ANG II in adult rams.
MATERIALS AND METHODS
Animal Preparation
All procedures were approved by the Institutional Animal Care and Use Committee of Wake Forest University School of Medicine. Time-dated pregnant ewes were randomly assigned to receive either two 0.17 mg/kg intramuscular injections of a 1:1 mixture of betamethasone acetate and betamethasone phosphate (Celestone Soluspan, Schering, Kenilworth, NJ) or vehicle alone, which contained 3.4 mg of monobasic sodium phosphate, 7.1 mg of dibasic sodium phosphate, 0.1 mg of sodium EDTA, and 0.2 mg of benzalkonium chlorine/ml given 24 h apart at days 80 and 81 of gestation The ewes were allowed to deliver naturally at term (term is ∼145 days in our flock).
Six pairs of male offspring were transferred to the laboratory at 12–18 mo of age. The research team was blinded to the sheep allocation. The temperature in the laboratory facilities and in the sheep pens was kept stable at 20 ± 2°C, and the humidity at ∼35–40%. Sheep were fed a standard commercial diet (PMI Rumilab, Brentwood, MO) containing 0.75% NaCl and had ad libitum access to clean tap water. Throughout the experiment, 12:12-h day-night cycles were kept constantly.
Surgical Procedure
A midline suprapubic laparotomy was made in the lower abdomen to expose the bladder. A 14 French silicone Foley catheter (Bard Medical, Covington, GA) was placed through the left flank. One polyvinyl catheter was placed in the left carotid artery, and two catheters were inserted into the left external jugular vein. These were tunneled subcutaneously and emerged through a left anterior neck incision. Bilateral groin incisions were made, and the femoral vessels were exposed. Each animal had one right femoral artery, one left femoral artery, and two left femoral vein polyvinyl catheters placed. All vascular catheters were fastened to their corresponding vessels, and the distal vessels were all ligated. The femoral catheters were tunneled subcutaneously to the left flank region.
A paravertebral left (n = 10) or right (n = 2) flank incision was made to expose the corresponding renal artery. A modified, Teflon-coated, 22-gauge endovascular catheter, 25 mm long and with a 0.9-mm external diameter, was placed into the right renal artery. The catheter was tunneled a short distance to emerge adjacent to the spine. We then removed the contralateral kidney.
All the animals received daily doses of ampicillin (1,000 mg), gentamicin (80 mg), and ketorolac (100 mg iv) until 48 h after surgery. All vascular catheters were flushed with the heparin solution on a daily basis to confirm patency. During the postoperative period and throughout the experimental period, each pair of animals was housed together in individual carts with free access to food, alfalfa hay, and water.
Experiments
After 5 days were allowed for the sheep to recover, they underwent a series of experiments designed to evaluate the response to a 24-h infusion of ANG II alone or in combination with specific antagonists. The sequence of infused substances was 1) ANG II, 2) ANG II and candesartan (AT1 receptor antagonist), and 3) ANG II and PD 123319 (AT2 receptor antagonist). There were 48–72 h between experiments to allow for adequate clearance of previously infused drugs. The experiments were designed to evaluate the effect of each infusion on blood pressure and renal function over a 24-h period.
Blood samples and urine samples were serially obtained before initiation of all infusions, subsequent to initiation of infusions and over the course of the following 24 h. All animals underwent continuous arterial blood pressure monitoring by means of an intravascular arterial line and continuous monitoring of urinary output.
Lithium, PAH, and inulin infusions were started 1 h before the antagonist infusion and 2 h before the angiotensin infusion as indicated. An initial bolus or loading dose was given, followed by a continuous infusion to maintain a stable state concentration as described below. ANG II was infused directly into the renal artery during 24 h starting 2 h after the lithium, PAH, and inulin infusion.
PD 123319 was infused continuously through the renal artery catheter beginning 1 h before the angiotensin infusion. Candesartan was given as an intra-arterial dose 1 h before the ANG II infusion was begun. Intravenous infusions of inulin and PAH were discontinued overnight and restarted on day 2 with a bolus followed by a maintenance dose.
To guarantee adequate hydration status, 2,000 ml of normal saline was given intravenously on the afternoon before each experiment began. Blood and urine sampling was performed 1 h before infusion on the first day and at 1, 4, and 24 h after the start of angiotensin infusion. Samples were used to measure inulin, PAH, lithium, sodium, potassium, chlorine, creatinine, angiotensin peptides, and renin plasma activity. The blood samples for plasma electrolytes, inulin, and PAH were collected in heparinized tubes. The blood samples for angiotensin peptide and renin plasma activity were collected in tubes with EDTA (25 μl/ml blood) and the proteinase inhibitor bestatin (20 μl/ml blood). Urine samples were collected in plain tubes. All blood and urine samples were centrifuged at 3,200 rpm, 4°C, for 10 min immediately after collection. The hematocrit was also measured.
After all experiments had concluded, the animals were euthanized and organ harvesting was performed.
Blood Pressure Measurement
Arterial blood pressure was monitored continuously during experiments by a Cobe transducer connected to a DigiMed analyzer, which digitized the pressure signal that was then recorded by a computer. Pressure was sampled at 100 Hz and averaged over 1-min intervals. The reported blood pressure values are the averaged value over 1 h before the time point reported.
Preparation Infusates
PAH.
PAH was obtained from Sigma (St. Louis, MO). A priming dose of PAH using 225 mg dissolved in 40 ml of normal saline was given over 1 min, followed by 7.66 mg/min (0.23 ml/min of a 3.33% solution) that was infused continuously.
Inulin.
Inulin was provided by Sigma. A priming dose of inulin using 850 mg dissolved in 40 ml of normal saline was given over 1 min, followed by 6.96 mg/min (0.23 ml/min of a 3.03% solution) that was infused continuously.
Lithium.
Lithium chloride was obtained from Sigma. On the day of the study, a priming dose of lithium using 580 mg dissolved in 40 ml of normal saline was given over 1 min, followed by 278 μg/min (0.23 ml/min of a 0.17% solution) that was infused continuously.
ANG II.
ANG II was provided by Bachem BioScience (H-1705.0025, Torrance, CA). The weight-based dosage for ANG II was 1 ng·kg−1·min−1. The solution was infused at the rate of 2.08 ml/h.
Candesartan.
Candesartan (CV11974) was generously provided as a salt from AstraZeneca (Möindal, Sweden). The dosage for candesartan was 0.3 mg/kg given as a single dose once each day of the experiment, given its extended half-life of 5–10 h.
PD 123319.
PD 123319 was provided by Sigma-Aldrich (P186). The AT2 antagonist came as a powder. PD 123319 stock was diluted in sterile isotonic saline, and an initial loading bolus of 500 μg was followed by an infusion at 10 ng·kg−1·min−1. The solution was infused at the rate of 2.08 ml/h.
Renal Function Tests
Plasma inulin.
Plasma inulin was measured using an anthrone method and a colorimetric assay for determining the concentration of inulin as previously described (9, 25). Renal inulin excretion was used to derive the glomerular filtration rate (GFR) according to the following formula: GFR = rate of inulin infusion mg/min × (plasma inulin mg/ml) − 1 = GFR ml/min.
Plasma PAH assay.
Plasma PAH was measured using a previously described colorimetric assay (14). Renal PAH excretion was used to derive effective plasma flow rate (EPFR) according to the following formula: ERPF = rate of PAH infusion mg/min × (plasma PAH mg/ml) − 1 = ERPF ml/min.
Plasma and urinary electrolytes.
Plasma and urinary concentration of electrolytes were determined using a Medical Easylyte instrument (Bedford, MA). The Easylyte margin of error for plasma electrolytes is 2% and for urinary electrolytes, 5%. All results were reported as millimoles per liter. The analyzer was used to measure Na, K, Cl, and Li in plasma, and Na, K, and Cl in urine.
Sodium excretion.
Sodium excretion was the product of the urine sample Na concentration multiplied by the urine volume obtained during the hour before the sampling time (Na meq·lt−1·1,000−1 × urinary volume 1 ml/h = Na meq). These values were indexed to the sheep weight: meq·h−1·kg−1.
Plasma ANG II.
We used a commercially available kit (Bühlmann Angiotensin II radioimmunoassay) to measure immunoreactive ANG II. Extracted EDTA plasma samples, calibrators, and controls is first preincubated for 16 h with an anti-ANG II antibody. ANG II-I125 is added and competes with ANG II present in samples, calibrators, and controls for the same antibody binding sites in a second 6-h incubation step. After this incubation, a solid-phase second antibody is added to the mixture. The antibody-bound fraction is precipitated and counted in a gamma counter.
Renal artery resistance.
Hemodynamically, renal artery resistance (RAR) would be a ratio of the difference between the renal mean arterial pressure (MAP) and the renal venous pressure to the renal blood flow. Renal artery resistance can be roughly approximated by the ratio of the mean arterial pressure to the renal blood flow according to the following formula: mean arterial pressure/RBP = RAR mmHg·min·ml−1.
Statistical Analysis
The study data were tabulated and graphed using Graph Prism software. Statistical analysis was accomplished with the use of a paired Student's t-test and two-way analysis of variance for repeated measurements. Means between the betamethasone- and vehicle-exposed sheep were compared with a two-sample t-test followed by Bonferroni's post hoc test for multiple t-tests. A P value of <0.05 was considered statistically significant. The data are reported as means ± SE.
RESULTS
MAP
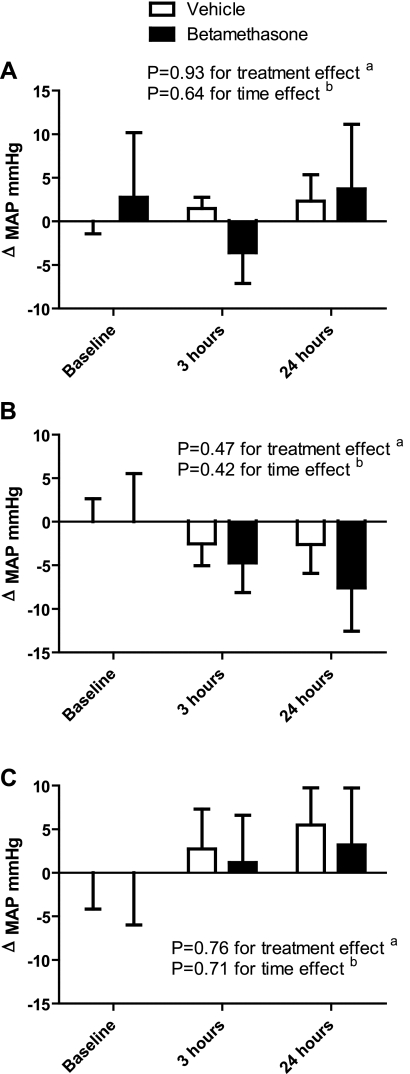
Baseline MAP was higher in the betamethasone- compared with the vehicle-exposed sheep (85.8 ± 2.2 and 78.3 ± 1.0 mmHg, respectively, P = 0.003). The intrarenal infusion of ANG II did not significantly change systemic MAP over time. Furthermore, the combined infusions of the antagonists and ANG II did not cause significant changes in blood pressure (Fig. 1).
Fig. 1.
A: effect of angiotensin II infusion on mean arterial pressure changes (ΔMAP; in mmHg). a: ANOVA between the vehicle- and betamethasone-exposed sheep over the 3 time points. b: ANOVA between the 3 time points within each treatment group. B: effect of angiotensin II infusion and angiotensin receptor 1 (AT1) blockade with candesartan on ΔMAP. C: effect of angiotensin II infusion and angiotensin receptor 2 (AT2) blockade with PD 123319 on ΔMAP.
GFR
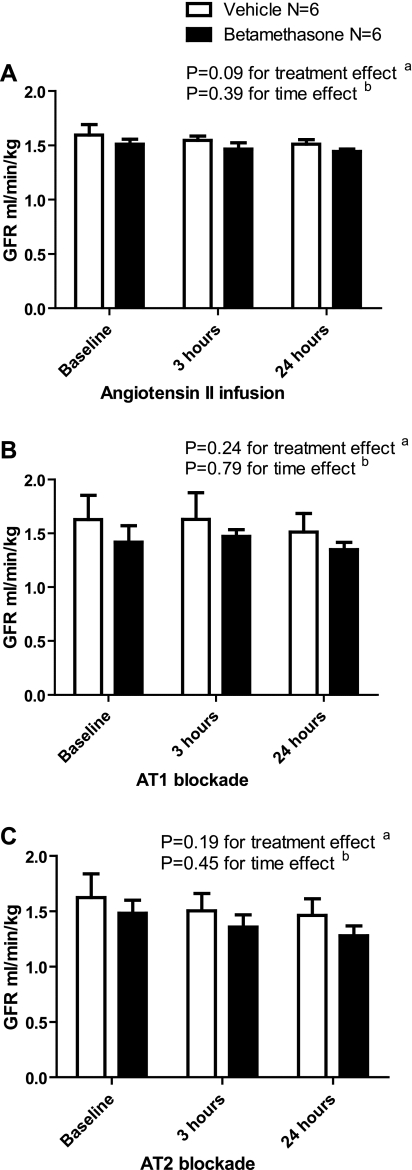
GFR at baseline was not significantly different between betamethasone- and vehicle-treated sheep (1.51 ± 0.05 and 1.59 ± 0.1 ml·min−1·kg−1, respectively, P = 0.39), and this did not change after 24 h of ANG II infusion. Concurrent AT1 or AT2 receptor blockade during ANG II infusion did not produce any difference in the GFR over time regardless of the treatment group (Fig. 2).
Fig. 2.
A: effect of angiotensin II infusion on glomerular filtration rate (GFR; ml·min−1·kg−1). B: effect of angiotensin II infusion and AT1 blockade with candesartan on GFR. C: effect of angiotensin II infusion and angiotensin receptor 2 (AT2) blockade with PD 123319 on GFR.
ERPF
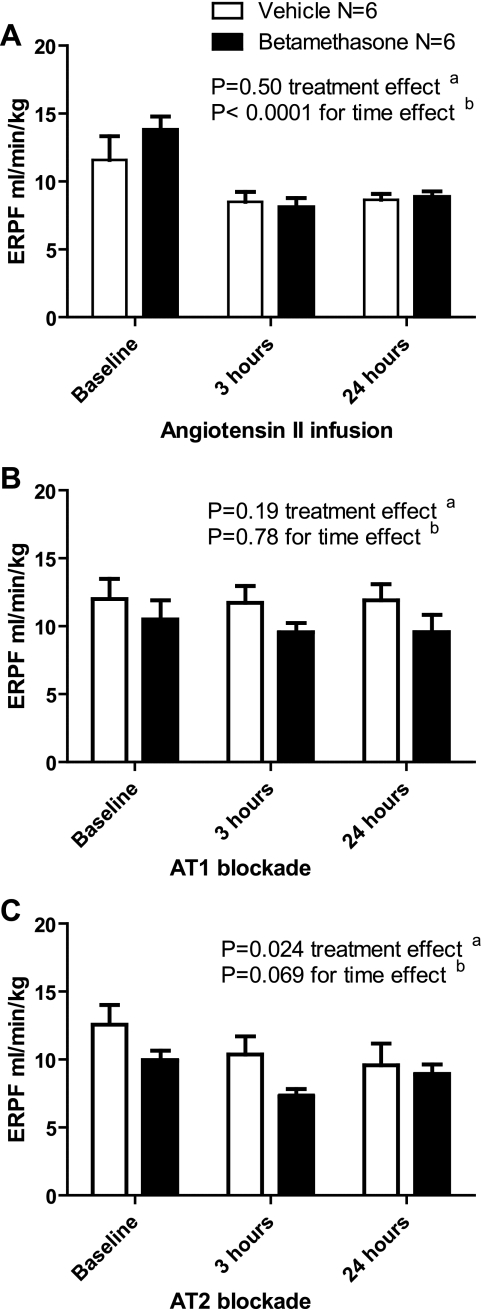
The ERPF decreased by the third hour and continued throughout the 24 h of ANG II infusion in both the vehicle- and betamethasone-treated sheep, with a decrease observed by 3 and 24 h compared with baseline (P < 0.05 for both comparisons), but no difference between the treatment groups (P > 0.05) (Fig. 3A). The decrease in the ERPF secondary to ANG II infusion was abrogated during AT1 receptor blockade, with no difference in the ERPF between the treatment groups over time (P > 0.05) (Fig. 3B). AT2 receptor blockade led to a significant decrease in the ERPF over 24 h among betamethasone (2-way ANOVA: F = 4.12, P = 0.04, baseline compared with 3 h: P < 0.05, and 3 h compared with 24 h: P < 0.05)- but not the vehicle-exposed sheep (2-way ANOVA: F = 1.09, P = 0.37) (Fig. 3C).
Fig. 3.
A: effect of angiotensin II infusion on effective renal plasma flow (ERPF: ml·min−1·kg−1). B: effect of angiotensin II infusion and AT1 blockade with candesartan on ERPF. C: effect of angiotensin II infusion and AT2 blockade with PD 123319 on ERPF.
RAR
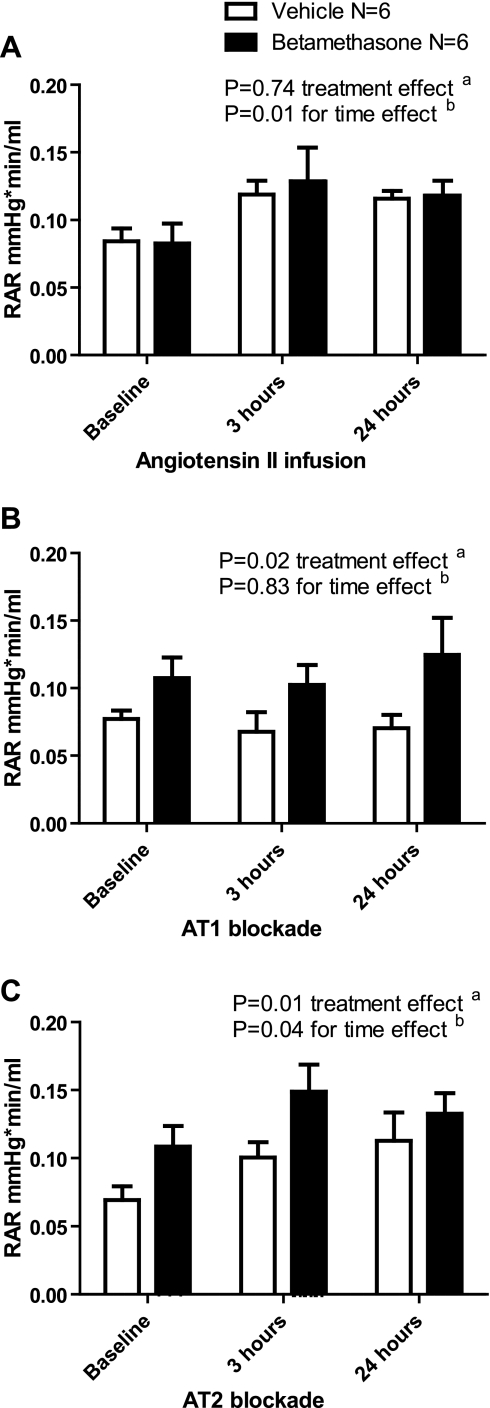
ANG II infusion raised the RAR in both groups within 3 h of the infusion, and this persisted during the 24 h of infusion (Fig. 4A). The increased RAR was only significant in the betamethasone group (baseline compared with 3 h: P < 0.05 and 24 h: P < 0.05). The ANG II-induced increase in RAR was blocked by the AT1 receptor antagonist candesartan (Fig. 4B). AT2 receptor blockade was followed by an increase in RAR in both groups, with a greater response in the betamethasone group where the RAR continued to increased over time (betamethasone baseline compared with 3 h: P < 0.05, 3 compared with 24 h: P < 0.05, vehicle baseline compared with 3 h: P < 0.05, and 3 compared with 24 h: P > 0.05) (Fig. 4C).
Fig. 4.
A: effect of angiotensin II infusion on renal artery resistance (RAR: mmHg·min−1·ml). B: effect of angiotensin II infusion and AT1 blockade with candesartan on RAR. C: effect of angiotensin II infusion and AT2 blockade with PD 123319 on RAR.
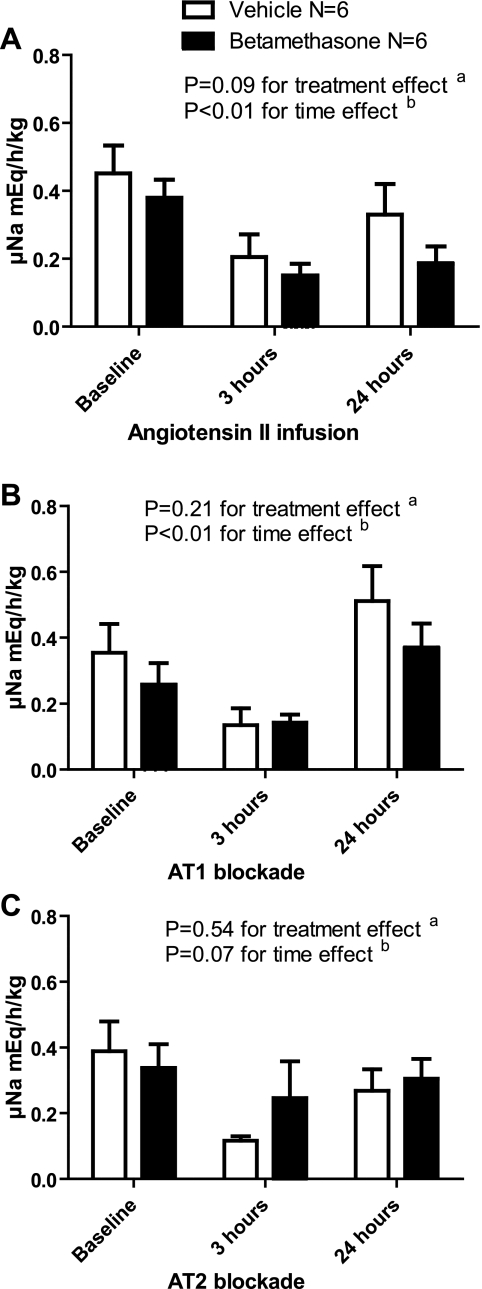
Urinary Sodium Excretion
The continuous infusion of ANG II led to a significant decrease in urinary sodium excretion that at 24 h remained markedly suppressed in the betamethasone animals (baseline compared with 3 h: P < 0.05, and baseline compared with 24 h: P < 0.05) but not in the vehicle animals (baseline compared with 3 h: P < 0.05, and baseline compared with 24 h: P > 0.05). There was a tendency for sodium excretion to be less in the betamethasone sheep (Fig. 5A). With AT1 receptor blockade, there was an initial suppression of sodium excretion followed by an increase in sodium excretion after 24 h of ANG II infusion in both groups (3 compared with 24 h: P < 0.05 in both betamethasone- and vehicle-exposed sheep) (Fig. 5B). When ANG II was infused with AT2 blockade, sodium excretion decreased after 3 h and was significant in the vehicle (baseline compared with 3 h: P < 0.05)- but not in the betamethasone (baseline compared with 3 h: P > 0.05)-exposed animals (betamethasone: F = 0.29, P = 0.75, vehicle: F = 4.38, P = 0.03). In both groups, excretion returned to baseline after 24 h of infusion with AT2 blockade (Fig. 5C).
Fig. 5.
A: effect of angiotensin II infusion on urinary sodium excretion (uNa; meq·h−1·kg−1). B: effect of angiotensin II infusion and AT1 blockade with candesartan on uNa. C: effect of angiotensin II infusion and AT2 blockade with PD 123319 on uNa.
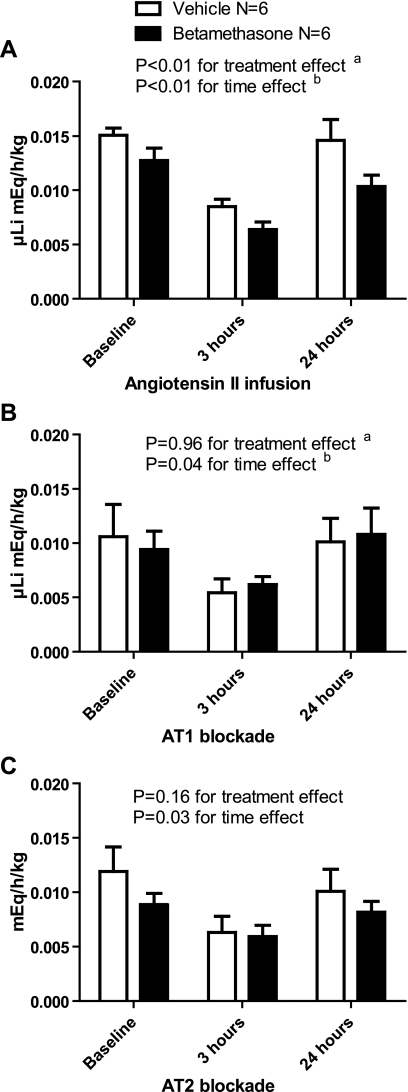
Urinary Lithium Excretion
Urinary excretion of lithium followed a pattern similar to that observed for sodium excretion (Fig. 6A). There was lower lithium excretion in the betamethasone compared with the vehicle sheep, which was most noticeable after 3 h (baseline compared with 3 h: P < 0.05 for both groups) of infusion followed by a rebound in lithium excretion by 24 h of infusion (3 compared with 24 h: P < 0.05) especially in the vehicle-exposed sheep (vehicle- compared with betamethasone-exposed sheep at 24 h: P < 0.05). This temporal pattern in the lithium excretion was also observed during AT1 and AT2 receptor blockade. AT1 receptor blockade abrogated the suppressive effect of betamethasone exposure on lithium excretion at 24 h with no difference in excretion compared with the vehicle sheep (P > 0.05) (Fig. 6B). AT2 receptor blockade did not affect the excretion pattern observed with ANG II (Fig. 6C).
Fig. 6.
A: effect of angiotensin II infusion on urinary lithium excretion (uLi; meq·h−1·kg−1). B: effect of angiotensin II infusion and AT1 blockade with candesartan on uLi. C: effect of angiotensin II infusion and AT2 blockade with PD 123319 on uLi.
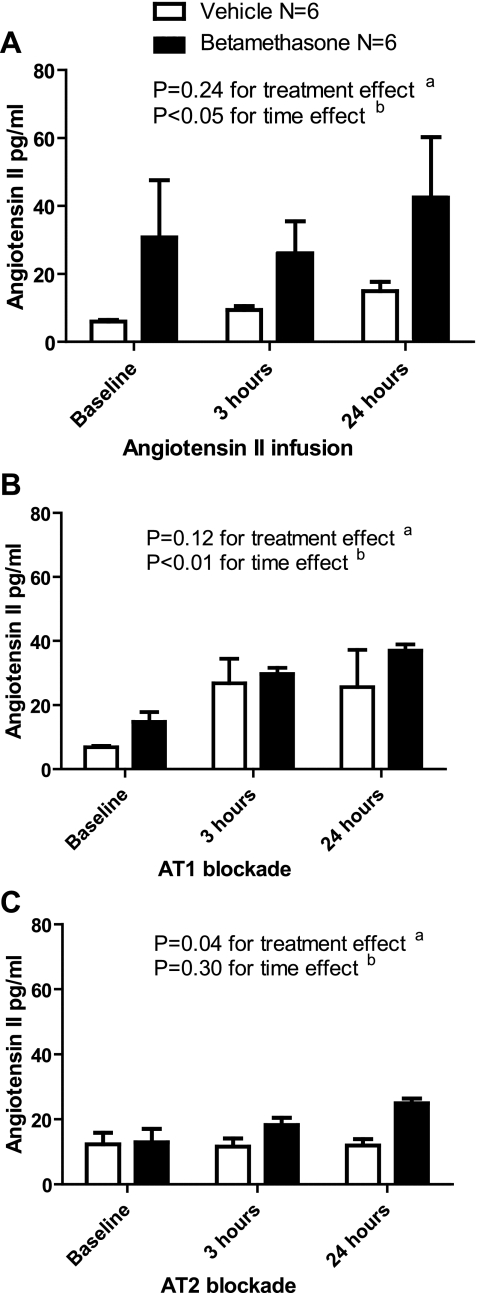
Plasma ANG II
The plasma concentration of ANG II increased in vehicle compared with the betamethasone sheep during the ANG II infusion (1-way ANOVA for betamethasone: F = 0.31, P = 0.74 and for vehicle: F = 6.56, P = 0.03, t-test of baseline compared with 24-h levels in betamethasone sheep: P > 0.05 and vehicle sheep: P < 0.05) (Fig. 7A). There was an increase in the ANG II concentration over time in both groups during AT1 blockade and peptide infusion (Fig. 7B). The increase over time was not observed during AT2 receptor blockade, but there was a treatment effect evident at 24 h of infusion (t-test of mean ANG II plasma concentration between betamethasone and vehicle sheep at 24 h: P < 0.05) (Fig. 7C).
Fig. 7.
A: effect of angiotensin II infusion on plasma angiotensin II concentrations. B: effect of angiotensin II infusion and AT1 blockade with candesartan on plasma ANG II concentrations. C: effect of angiotensin II infusion and AT2 blockade with PD 123319 on plasma ANG II concentrations.
Kidney Weights
The mean weights of kidneys at the time of uninephrectomy in the vehicle and betamethasone sheep were similar (88.8 ± 5.2 and 89.4 ± 4.1 g for vehicle- and betamethasone-exposed sheep, respectively, P = 0.93). After completing all experiments, the sheep were euthanized and the remaining kidney was harvested for further analysis. There was a significant increase in the size of the remaining kidney. The mean renal weight was 142 ± 5.1 and 142.6 ± 6.5 g for the vehicle and betamethasone sheep, respectively (P = 0.94). The mean increase in renal weight was 53.1 ± 7.6 (38%) and 53.2 ± 7.4 g (37%) for the vehicle and betamethasone groups, respectively. This increase was significant in both the vehicle (P < 0.01) and betamethasone (P < 0.01) groups but not between the two groups (P = 0.94).
DISCUSSION
The purpose of these studies was to determine whether antenatal betamethasone exposure altered renal responses to intrarenal infusions of ANG II. Our working hypothesis was that responses mediated by activation of the AT1 receptor would be enhanced in adults that were exposed to clinically relevant doses of betamethasone during fetal life. We chose to use intrarenal infusions in an attempt to avoid some of the systemic effects of elevations in plasma levels of ANG II that can affect the kidney. Our data suggest that antenatal steroid exposure does upregulate some of the AT1 receptor-mediated renal responses to ANG II.
Intrarenal ANG II infusion decreased ERPF and increased RAR. The changes observed secondary to antenatal betamethasone exposure appeared to be mediated by increased AT1 activity in the betamethasone-exposed compared with the vehicle-exposed sheep and were evident within 3 h after the infusion was started.
Under normal conditions, GFR and ERPF are autoregulated through a mechanism that controls afferent and efferent arterial tone. Increased perfusion pressure and GFR lead to a decreased efferent artery vascular tone and increased renal blood flow, with subsequent decrease in the filtration fraction. Conversely, a decrease in the GFR causes efferent artery vasoconstriction. This will increase perfusion pressure, filtration fraction and decrease renal blood flow (5, 20).
We did not observe a significant decrease in GFR associated with betamethasone exposure under basal conditions or during the ANG II infusions. The latter is consistent with a previous report of ANG II infusion in fetal sheep that reported no change in GFR after 5 days of infusion, but does not agree with earlier studies in dogs (31, 50). The explanation for this difference is not clear but could be related to species differences in responses to the peptide. Long-term studies in adolescent individuals who were antenatally exposed to betamethasone documented a lower GFR (18). Tang et al. (51) have demonstrated a decrease in GFR and sodium excretion by 18 mo of age among male but not female sheep antenatally exposed to betamethasone. This difference may be due to our experimental design using uninephrectomized animals with its associated renal hypertrophy.
Although decreased renal blood flow with ERPF suppression mediated by glomerular mesangial cell contraction has been described after ANG II infusion, the most likely mechanism would appear to be efferent artery vasoconstriction (4, 24). Our finding of increased RAR without modifying the GFR is consistent with an effect on both efferent and afferent arterial vascular tone, which has been reported in other species (29).
During AT1 receptor blockade, the effect of ANG II infusion on ERPF in both groups was abrogated, suggesting that the effect on ERPF is mediated through the AT1 receptor. Candesartan, which was used for the AT1 receptor blockade, can also induce changes in ERPF through an antioxidant effect. This is supported by evidence that bioinactivation of nitric oxide by free oxygen radicals in the kidneys of spontaneously hypertensive rats can be blunted by candesartan (55).
In contrast, ERPF decreased significantly during ANG II infusion with AT2 blockade in the betamethasone- but not the vehicle-exposed sheep. This is probably due to unopposed AT1 activation by ANG II with increased vasoconstriction among the betamethasone sheep.
In terms of RAR, betamethasone-exposed sheep demonstrated higher RAR with ANG II infusion in combination with AT1 blockade compared with vehicle-exposed sheep. This is demonstrated by the treatment (group) effect seen with AT1 blockade in vehicle- compared with betamethasone-exposed sheep. This might be a consequence of increased expression of AT1 receptors in the betamethasone animals (21).
With AT2 blockade, an increase in RAR was observed in both groups, supporting the concept of AT2 activation counteracting AT1 activation. Several mechanisms for AT2 function have been postulated, including AT1/AT2 dimerization with downregulation of AT1, and AT2/AT2 dimerization with subsequent production of nitric oxide, a known vasodilator. (16, 34, 45) Furthermore, ANG III is derived from ANG II, which activates the AT2 receptor and can increase nitric oxide production. Other potential pathways of AT2 activation include bradykinin, prostaglandin, and cGMP release, all of which can impact renal vascular resistance (48, 49). Betamethasone could possibly induce changes in any of the above mechanisms, through decreased AT1 or AT2 dimerization, decreased nitric oxide availability, or changes in the cytokine milieu. The enhanced response among betamethasone-exposed sheep compared with vehicle sheep supports our suggestion regarding a heightened response to AT1 activation in betamethasone- compared with vehicle-exposed sheep.
The MAP in betamethasone-exposed sheep is significantly higher than in vehicle-exposed sheep. The mean difference of 7.5 ± 2.4 mmHg is similar to that previously reported for betamethasone-exposed sheep (17) and would not be accounted for by unilateral nephrectomy.
These results are compatible with our hypothesis that antenatal corticosteroids administrated to sheep at a time and dose similar to that used during human pregnancies for prevention of prematurity related complications can program renal and cardiovascular function (17, 19, 30).
The betamethasone sheep had consistently higher blood pressure, but there was no significant increase in systemic MAP during the ANG II infusion regardless of the use of AT1 or AT2 blockade. Previous studies in a sheep model (50) did not observe any difference in systemic MAP with a short infusion of considerably higher doses of ANG II into the renal artery, suggesting that there is little spillover into the systemic circulation. This is consistent with observations that the kidney clears a large proportion of ANG II (33, 40, 54) and with the very modest increase in plasma levels of ANG II during the peptide infusions (discussed below).
ANG II infusion led to a decrease in sodium excretion. This decrease persisted at 24 h and correlates with the increase in RAR observed during ANG II infusion. AT1 blockade interfered with the suppression of sodium excretion, which was low at 3 h of infusion and rebounded significantly by 24 h in both treatment groups. Candesartan is highly selective for AT1 receptors, with tight binding and slow dissociation (9). Evidence derived from ANG II infusions in a rat model has shown that the natriuretic response observed after candesartan-induced AT1 blockade is due to conversion of ANG II into ANG III with direct stimulation of the AT2 receptor by ANG III (42–44). The process would require time for the upregulation and translocation of AT2 receptors to occur and thus could account for the time-dependent effects of blockade that we observed.
During AT2 blockade, no rebound effect was seen by 24 h, suggesting that it is the AT2 receptor that upregulates sodium excretion. AT2 blockade led to a decreased sodium excretion at 3 h in the vehicle- but not in the betamethasone-exposed sheep. This suggests that the steroid exposure reduces the counterbalancing effects of AT2 receptor activation on AT1 receptor-mediated responses. The decrease in AT2 activity in betamethasone-exposed sheep could be secondary to decreased conversion of ANG II to ANG III, decreased AT2 expression, or decreased transfer of AT2 to the apical tubular cell membrane (42, 43).
The biphasic responses observed for both lithium and sodium excretion appear to be mediated by an initial response to increased RAR and decreased ERPF, followed by early activation of AT1 receptors and delayed activation of AT2 receptors in the tubular epithelium.
Lithium is filtered by the glomeruli, and 80% is reabsorbed in the proximal tubule (52, 53). Lithium reabsorption is relatively stable during short-term infusions in ovine models (8). Although a low salt intake resulting in low tubular concentration of sodium will increase lithium reabsorption, it has been previously reported that renal lithium excretion does not vary with changes in renal perfusion pressure (22).
During the ANG II infusion, we observed a significant decrease in lithium excretion at 3 h and a return to preinfusion levels by 24 h. The same pattern was observed during AT1 blockade, analogous to that observed with urinary sodium excretion, suggesting that AT2 receptor activity may be involved in lithium excretion by decreasing absorption from the proximal tubule. During AT2 blockade, we also observed a decrease in lithium excretion by 3 h of infusion. The rebound in lithium excretion observed after 24 h during AT1 blockade was only seen in the vehicle sheep during AT2 blockade. Lithium excretion remained suppressed in the betamethasone sheep after 24 h of AT2 blockade, similar to what was seen for sodium excretion. This is consistent with increased AT2 receptor activity in the proximal tubule compared with the distal tubule and collecting duct of vehicle- compared with betamethasone-exposed sheep.
The effects of ANG II infusion and subsequent AT1 and AT2 blockade on lithium excretion closely paralleled the changes observed in sodium excretion. This would suggest that the initial suppression is secondary to decreased ERPF and increased RAR, that the effects on renal sodium and lithium excretion are both regulated at the level of the proximal tubule through AT1 and AT2, and that the AT2 receptor appears to be less active in betamethasone- compared with vehicle-exposed sheep. The functional assessment of renal function among the betamethasone- compared with the vehicle-exposed sheep is consistent with our measurement of AT1 and AT2 receptors in the cortex and medulla of betamethasone-exposed sheep, which showed an increased AT1/AT2 ratio in both nuclear and plasma membrane of cortical renal tissue (21).
Plasma concentrations of ANG II increased slightly during the infusion of the peptide alone; however, ANG II concentrations essentially doubled during the infusion and AT1 blockade. Elevated ANG II levels in both groups in the AT1 receptor blockade experiments may be the result of the loss of the normal inhibitory feedback on renin production induced by ANG II (23). The plasma concentrations are the result of an increase in endogenous levels and a small contribution from the infused peptide. Alternatively, if the AT1 receptor is important for clearance of the peptide, then its blockade would allow larger amounts of ANG II to appear in the peripheral circulation. During AT2 blockade, although the concentration of ANG II did not increase over time, betamethasone-exposed sheep had consistently higher ANG II concentrations. Increased plasma ANG II levels are consistent with our previously published data reporting decreased plasma and renal cortical tubule concentration of ACE2 among betamethasone-exposed sheep (47).
In conclusion, our findings support the notion of fetal renal programming secondary to antenatal betamethasone exposure, leading to long-term changes in renal function of adult male sheep that not only affect renal vascular resistance but affect renal sodium excretion.
Intrarenal ANG II exerts its effects through activation of AT1 and AT2 receptors within the renal vasculature and cortical tubular epithelium. Our findings suggest that although ANG II exerts a stimulatory effect on RAR with a reduction in ERPF', it does not appear to alter GFR secondary to compensatory mechanisms including afferent arteriolar vasoconstriction. The decreased ERPF with increased RAR seems to be under AT1 receptor control and is stronger in betamethasone-exposed sheep, supporting the concept of an increased AT1-to-AT2 activity ratio in betamethasone sheep compared with vehicle sheep.
The increased MAP we have documented among the betamethasone-exposed sheep is consistent with previous reports and demonstrates that unilateral nephrectomy does not abolish the hypertensive effect of antenatal steroid exposure, at least in the short term. Our findings suggest there is a shift in the balance of AT1 to AT2 receptor activity among adult male sheep exposed antenatally to betamethasone compared with the vehicle-exposed sheep. The shift tends to favor AT1- over AT2-mediated responses.
GRANTS
This work was supported by National Institutes of Health Grants HD-47584, HL-68728, and HD-17644.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
Nancy Vallego was the laboratory manager and helped with sample collection. Xiurong Sun participated in sample collection and ANG II measurements. Lijun Tang participated in sample collection and animal care. David Jones and Eric Lesane, who provided invaluable support in caring for the sheep, assisted with preparing materials for surgery, and anesthesia during surgery as well as during immediate preoperative and postoperative animal care.
REFERENCES
- 1.Barker DJ. Fetal origins of coronary heart disease. BMJ 311: 171–174, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barker DJP, Eriksson JG, Forsen T, Osmond C. Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol 31: 1235–1239, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Blantz RC. The glomerular and tubular actions of angiotensin II. Am J Kidney Dis 10, Suppl 1: 2–6, 1987 [PubMed] [Google Scholar]
- 4.Brenner BM, Chertow GM. Congenital oligonephropathy: an inborn cause of adult hypertension, and progressive renal injury? Curr Opin Nephrol Hypertens 2: 691–695, 1993 [PubMed] [Google Scholar]
- 5.Burnier M. Blockade of the renin-angiotensin system for renal protection: from history to future perspectives. J Renin Angiotensin Aldosterone Syst 8: 208–211, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Carey RM, Siragy HM. Newly recognized components of the renin-angiotensin system: potential roles in cardiovascular and renal regulation. Endocr Rev 24: 261–271, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Carey RM. Update on the role of the AT2 receptor. Curr Opin Nephrol Hypertens 14: 67–71, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Cha SC, Aberdeen GW, Mukaddam-Daher S, Quillen EW, Jr, Nuwayhid BS. Tubular handling of fluid and electrolytes during ovine pregnancy. Am J Physiol Renal Fluid Electrolyte Physiol 265: F278–F284, 1993 [DOI] [PubMed] [Google Scholar]
- 9.Chung O, Csikós T, Unger T. Angiotensin II receptor pharmacology and AT1-receptor blockers. J Hum Hypertens 13, Suppl 1: S11–S20, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Davidson WD, Sackner MA. Simplification of the anthrone method for the determination of inulin in clearance studies. J Lab Clin Med 62: 351–356, 1963 [PubMed] [Google Scholar]
- 11.de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev 52: 415–472, 2000 [PubMed] [Google Scholar]
- 12.Dodic M, May CN, Wintour EM, Coghlan JP. An early prenatal exposure to excess glucocorticoid leads to hypertensive offspring in sheep. Clin Sci (Lond) 94: 149–155, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Dodic M, Hantzis V, Duncan J, Rees S, Koukoulas I, Johnson K, Wintour EM, Moritz K. Programming effects of short prenatal exposure to cortisol. FASEB J 16: 1017–1026, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Dodic M, Abouantoun T, O'Connor A, Wintour EM, Moritz KM. Programming effects of short prenatal exposure to dexamethasone in sheep. Hypertension 40: 729–734, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Dubovsky EV, Russell CD. Quantitation of renal function with glomerular and tubular agents. Semin Nucl Med 12: 308–329, 1982 [DOI] [PubMed] [Google Scholar]
- 16.Eskild-Jensen A, Thomsen K, Rungø C, Ferreira LS, Paulsen LF, Rawashdeh YF, Nyengaard JR, Nielsen S, Djurhuus JC, Frøkiær J. Glomerular and tubular function during AT1 receptor blockade in pigs with neonatal induced partial ureteropelvic obstruction. Am J Physiol Renal Physiol 292: F921–F929, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Figueroa JP, Rose JC, Massmann GA, Zhang J, Acuña G. Alterations in fetal kidney development and elevations in arterial blood pressure in young adult sheep after clinical doses of antenatal glucocorticoids. Pediatr Res 58: 510–515, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Finken MJ, Keijzer-Veen MG, Dekker FW, Frölich M, Walther FJ, Romijn JA, van der Heijden BJ, Wit JM; Dutch POPS19 Collaborative Study Antenatal glucocorticoid treatment is not associated with long-term metabolic risks in individuals born before 32 weeks of gestation. Arch Dis Child Fetal Neonatal Ed 93: F442–F447, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Gardner DS, Jackson AA, Langley-Evans SC. Maintenance of maternal diet-induced hypertension in the rat is dependent on glucocorticoids. Hypertension 30: 1525–1530, 1997 [DOI] [PubMed] [Google Scholar]
- 20.Guyton A. (Editor). Textbook of Medical Physiology Philadelphia, PA: Saunders, 1986, p. 410–413 [Google Scholar]
- 21.Gwathmey TM, Shaltout HA, Diz DI, Figueroa JP, Rose JC, Chappell MC. Steroid-induced fetal programming alters angiotensin II receptor subtype expression in the sheep kidney. FASEB J 22: 735.15, 2008 [Google Scholar]
- 22.Haas JA, Granger JP, Knox FG. Effect of renal perfusion pressure on sodium reabsorption from proximal tubules of superficial and deep nephrons. Am J Physiol Renal Fluid Electrolyte Physiol 250: F425–F429, 1986 [DOI] [PubMed] [Google Scholar]
- 23.Hackenthal E, Paul M, Ganten D, Taugner R. Morphology, physiology, and molecular biology of renin secretion. Physiol Rev 70: 1067–1116, 1990 [DOI] [PubMed] [Google Scholar]
- 24.Hall JE, Guyton AC, Jackson TE, Coleman TG, Lohmeier TE, Trippodo NC. Control of glomerular filtration rate by renin-angiotensin system. Am J Physiol Renal Fluid Electrolyte Physiol 233: F366–F372, 1977 [DOI] [PubMed] [Google Scholar]
- 25.Jelinek J, Hackenthal E, Hackenthal R. Role of the renin-angiotensin system in the adaptation to high salt intake in immature rats. J Dev Physiol 14: 89–94, 1990 [PubMed] [Google Scholar]
- 26.Jung K, Klotzek S, Schulze BD. Refinements of assays for low concentrations of inulin in serum. Nephron 54: 360–361, 1990 [DOI] [PubMed] [Google Scholar]
- 27.Keller G, Zimmer G, Mall G, Ritz E, Amann K. Nephron number in patients with primary hypertension. N Engl J Med 348: 101–108, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Kim HS, Krege JH, Kluckman KD, Hagaman JR, Hodgin JB, Best CF, Jennette JC, Coffman TM, Maeda N, Smithies O. Genetic control of blood pressure and the angiotensinogen locus. Proc Natl Acad Sci USA 92: 2735–2739, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev 59: 251–287, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Langley-Evans SC. Hypertension induced by foetal exposure to a maternal low-protein diet, in the rat, is prevented by pharmacological blockade of maternal glucocorticoid synthesis. J Hypertens 15: 537–544, 1997 [DOI] [PubMed] [Google Scholar]
- 31.Lohmeier TE, Cowley AW., Jr Hypertensive and renal effects of chronic low level intrarenal angiotensin infusion in the dog. Circ Res 44: 154–155, 1979 [DOI] [PubMed] [Google Scholar]
- 32.Massmann GA, Zhang J, Rose JC, Figueroa JP. Acute and long-term effects of clinical doses of antenatal glucocorticoids in the developing fetal sheep kidney. J Soc Gynecol Investig 13: 174–180, 2006 [DOI] [PubMed] [Google Scholar]
- 33.May CN. Prolonged systemic and regional haemodynamic effects of intracerebroventricular angiotensin II in conscious sheep. Clin Exp Pharmacol Physiol 10: 878–884, 1996 [DOI] [PubMed] [Google Scholar]
- 34.Mogi M, Iwai M, Horiuchi M. Emerging concepts of regulation of angiotensin II receptors: new players and targets for traditional receptors. Arterioscler Thromb Vasc Biol 27: 2532–2539, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Moritz KM, Johnson K, Douglas-Denton R, Wintour EM, Dodic M. Maternal glucocorticoid treatment programs alterations in the renin-angiotensin system of the ovine fetal kidney. Endocrinology 143: 4455–4463, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Moritz KM, Dodic M, Wintour EM. Kidney development and the fetal programming of adult disease. Bioessays 25: 212–220, 2003 [DOI] [PubMed] [Google Scholar]
- 37.National Institutes of Health The Effect of Antenatal Steroids for Fetal Maturation on Perinatal Outcomes Bethesda, MD: National Institutes of Health, 1994, p. 1–24 [PubMed] [Google Scholar]
- 38.Niimura F, Labosky PA, Kakuchi J, Okubo S, Yoshida H, Oikawa T, Ichiki T, Naftilan AJ, Fogo A, Inagami T, Hogan BL. Gene targeting in mice reveals a requirement for angiotensin in the development and maintenance of kidney morphology and growth factor regulation. J Clin Invest 96: 2947–2954, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nishimura H, Yerkes E, Hohenfellner K, Miyazaki Y, Ma J, Hunley TE, Yoshida H, Ichiki T, Threadgill D, Phillips JA, 3rd, Hogan BM, Fogo A, Brock JW, 3rd, Inagami T, Ichikawa I. Role of the angiotensin type 2 receptor gene in congenital anomalies of the kidney and urinary tract, CAKUT, of mice and men. Mol Cell 3: 1–10, 1999 [DOI] [PubMed] [Google Scholar]
- 40.Oparil S, Bailie MD. Mechanism of renal handling of angiotensin II in the dog. Circ Res 33: 500–507, 1973 [DOI] [PubMed] [Google Scholar]
- 41.Ortiz LA, Quan A, Weinberg A, Baum M. Effect of prenatal dexamethasone on rat renal development. Kidney Int 59: 1663–1669, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Padia SH, Howell NL, Siragy HM, Carey RM. Renal angiotensin type 2 receptors mediate natriuresis via angiotensin III in the angiotensin II type 1 receptor-blocked rat. Hypertension 47: 537–544, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Padia SH, Kemp BA, Howell NL, Fournie-Zaluski MC, Roques BP, Carey RM. Conversion of renal angiotensin II to angiotensin III is critical for AT2 receptor-mediated natriuresis in rats. Hypertension 51: 460–465, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Padia SH, Kemp BA, Howell NL, Gildea JJ, Keller SR, Carey RM. Intrarenal angiotensin III infusion induces natriuresis and angiotensin type 2 receptor translocation in Wistar-Kyoto but not in spontaneously hypertensive rats. Hypertension 53: 338–343, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Palm F, Connors SG, Mendonca M, Welch WJ, Wilcox CS. Angiotensin II type 2 receptors and nitric oxide sustain oxygenation in the clipped kidney of early Goldblatt hypertensive rats. Hypertension 51: 345–351, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Roberts D, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev 3: CD004454, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Shaltout HA, Figueroa JP, Rose JC, Diz DI, Chappell MC. Alterations in circulatory and renal angiotensin-converting enzyme and angiotensin-converting enzyme 2 in fetal programmed hypertension. Hypertension 53: 404–408, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Siragy HM, Carey RM. The subtype 2 (AT2) angiotensin receptor mediates renal production of nitric oxide in conscious rats. J Clin Invest 100: 264–269, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Siragy HM, Inagami T, Ichiki T, Carey RM. Sustained hypersensitivity to angiotensin II and its mechanism in mice lacking the subtype-2(AT2) angiotensin receptor. Proc Natl Acad Sci USA 96: 6506–6510, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stevenson KM, Lumbers ER. Effects of angiotensin II in fetal sheep and modification of its actions by indomethacin. J Physiol 487: 147–158, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tang L, Carey LC, Bi J, Valego N, Sun X, Deibel P, Perrott J, Figueroa JP, Chappell MC, Rose JC. Gender differences in the effects of antenatal betamethasone exposure on renal function in adult sheep. Am J Physiol Regul Integr Comp Physiol 296: R309–R317, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thomsen K, Schou M. Renal lithium excretion in man. Am J Physiol 215: 823–827, 1968 [DOI] [PubMed] [Google Scholar]
- 53.Thomsen K. Lithium clearance: a new method for determining proximal and distal tubular reabsorption of sodium and water. Nephron 37: 217–223, 1984 [DOI] [PubMed] [Google Scholar]
- 54.Vågnes ØB, Iversen BM, Arendshorst WJ. Short-term ANG II produces renal vasoconstriction independent of TP receptor activation and TxA2/isoprostane production. Am J Physiol Renal Physiol 293: F860–F867, 2007 [DOI] [PubMed] [Google Scholar]
- 55.Wilcox CS. Oxidative stress and nitric oxide deficiency in the kidney: a critical link to hypertension? Am J Physiol Regul Integr Comp Physiol 289: R913–R935, 2005 [DOI] [PubMed] [Google Scholar]
- 56.Wintour EM, Moritz KM, Johnson K, Ricardo S, Samuel CS, Dodic M. Reduced nephron number in adult sheep, hypertensive as a result of prenatal glucocorticoid treatment. J Physiol 549: 929–935, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Woods LL. Maternal glucocorticoids and prenatal programming of hypertension. Am J Physiol Regul Integr Comp Physiol 291: R1069–R1075, 2006 [DOI] [PubMed] [Google Scholar]
- 58.Zohdi V, Moritz KM, Bubb KJ, Cock ML, Wreford N, Harding R, Black MJ. Nephrogenesis and the renal renin-angiotensin system in fetal sheep: effects of intrauterine growth restriction during late gestation. Am J Physiol Regul Integr Comp Physiol 293: R1267–R1273, 2007 [DOI] [PubMed] [Google Scholar]