Abstract
Levels of tissue kallikrein (TK) are significantly lower in the urine of patients with kidney failure, and TK expression is specifically diminished in rat kidney after recovery from ischemia-reperfusion injury. In this study, we investigated the functional consequence of blocking endogenous TK activity in a rat model of chronic kidney disease. Inhibition of endogenous TK levels for 10 days by neutralizing TK antibody injection in DOCA-salt rats caused a significant increase in blood urea nitrogen and urinary protein levels, and a decrease in creatinine clearance. Kidney sections from anti-TK antibody-treated rats displayed a marked rise in tubular dilation and protein cast accumulation as well as glomerular sclerosis and size. TK blockade also increased inflammatory cell infiltration, myofibroblast and collagen accumulation, and collagen fraction volume. Elevated renal inflammation and fibrosis by anti-TK antibody were associated with increased expression of tumor necrosis factor-α, intercellular adhesion molecule-1, tissue inhibitor of metalloproteinase-2 (TIMP-2), and plasminogen activator inhibitor-1 (PAI-1). Moreover, the detrimental effect of TK blockade resulted in reduced nitric oxide (NO) levels as well as increased serum lipid peroxidation, renal NADH oxidase activity, and superoxide formation. In cultured proximal tubular cells, TK inhibited angiotensin II-induced superoxide production and NADH oxidase activity via NO formation. In addition, TK markedly increased matrix metalloproteinase-2 activity with a parallel reduction of TIMP-2 and PAI-1 synthesis. These findings indicate that endogenous TK has the propensity to preserve kidney structure and function in rats with chronic renal disease by inhibiting oxidative stress and activating matrix degradation pathways.
Keywords: chronic kidney disease, inflammation, fibrosis
tissue kallikrein (TK), a serine proteinase synthesized in many organs, specifically processes low-molecular-weight kininogen to produce potent vasoactive peptides known as kinins (25). Kinins are especially active on the vascular endothelium where they stimulate kinin B2 receptors, which in turn, trigger the release of nitric oxide (NO) and other endothelial mediators to promote vascular dilation and inhibit platelet adhesion and aggregation. TK has also been shown to directly activate the kinin B2 receptor, independent of kinin formation (16). TK is synthesized in large amounts in the kidney, released in the peritubular interstitium, and excreted in the urine (27). It has been reported that renal TK excretion is significantly lower in patients with mild chronic renal disease and more markedly reduced in patients with severe renal failure (8, 20). Conversely, restriction of dietary sodium intake in humans leads to increased kallikrein excretion in the urine (1). Reduced urinary kallikrein levels have also been described in hypertensive rat models, including Dahl salt-sensitive and spontaneously hypertensive rats (9, 33). In addition, TK expression was specifically diminished in the rat kidney after recovery from ischemia-reperfusion injury (2). Interestingly, the use of the potent TK inhibitor aprotinin in cardiac surgery has been shown to be associated with increased renal failure and mortality (29). These combined findings suggest that endogenous TK plays an important role in preserving renal function and that expression of the kallikrein gene may serve as a powerful marker for linkage analysis in populations with salt-sensitive hypertension and renal disease. Therefore, the purpose of this study was to determine the role of endogenous TK in chronic renal injury in a rat model of salt-induced hypertension.
MATERIALS AND METHODS
TK purification and antibody generation.
TK was purified using DEAE-cellulose and aprotinin-affinity column chromatography as previously described (6, 30). Polyclonal antibody to TK was raised in rabbits and purified with a protein A-affinity column. Neutralizing ability of anti-TK antibody was verified by an enzymatic activity assay using the chromogenic substrate S-2266 (diaPharma, West Chester, OH).
Animal treatment.
All procedures complied with the standards for care and use of animal subjects as stated in the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Resources, National Academy of Sciences, Bethesda, MD). The protocol for our animal studies was approved by the Institutional Animal Care and Use Committee at the Medical University of South Carolina. Male Wistar rats (Harlan Sprague-Dawley, Indianapolis, IN) weighing 200–220 g were anesthetized with an intraperitoneal injection of pentobarbital sodium (50 mg/kg) before undergoing left unilateral nephrectomy. One week after surgery, rats in the sham group (n = 6) received weekly subcutaneous injections of sesame oil and were provided with tap water. Experimental animals received weekly subcutaneous injections of DOCA (25 mg/kg body wt; Sigma, St. Louis, MO) suspended in sesame oil and were provided with 1% NaCl drinking water. Ten days after surgery, DOCA-salt rats received daily intravenous injections of either 0.5 mg of polyclonal anti-rat TK antibody (DOCA/α-TK; n = 8) or 0.5 mg of normal rabbit IgG (DOCA/IgG; n = 6). Eleven days after initial antibody treatment (i.e., 3 wk after surgery), rats were anesthetized with pentobarbital sodium (50 mg/kg) and kidneys were removed for morphological, histological, and biochemical analyses.
Blood pressure and renal function measurements.
On the day of death, rats were anesthetized with pentobarbital sodium (50 mg/kg) and a 2F micromanometer-tipped catheter (Millar Instruments) was inserted into the right carotid artery. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured and mean arterial pressure (MAP) was calculated according to the following formula: MAP = 2/3 DBP + 1/3 SBP. Serum was collected by cardiac puncture. Twenty-four-hour urine was collected from rats in metabolic cages 2 days before death. To eliminate contamination of urine samples, animals received only water during the 24-h collection period. Blood urea nitrogen, urinary protein levels, and creatinine clearance were calculated as previously described (3).
Morphological and histological analyses.
Kidneys were fixed in 4% formaldehyde, dehydrated, and paraffin-embedded. Four-micrometer-thick sections were subjected to periodic acid Schiff (PAS), silver, and Sirius red staining. Light microscopic morphological evaluation of glomeruli was conducted in a blinded fashion as previously reported (17). At least 30 glomeruli per section were examined for the evaluation of glomerular lesions and hypertrophy using PAS- and silver-stained slides, respectively. The severity of glomerulosclerosis and glomerular size was calculated semiquantitatively using a 0 to 3 scale (0, normal or almost normal; 1, mild; 2, moderate; 3, severe) for each glomerulus. Cortical areas of kidney sections stained with Sirius red, which stains collagen fibers red, were analyzed for collagen fraction volume with an image analysis system (12). Twenty fields without large vessels were randomly selected from each kidney section at a magnification of ×200. Collagen fraction volume was then calculated as percentage of stained area within a field using National Institutes of Health (NIH) image software.
Immunohistochemical staining.
Immunohistochemistry was performed using the Vectastain Universal Elite ABC Kit (Vector Laboratories, Burlingame, CA) following the supplied instructions. Kidney sections from paraffin-embedded tissue were incubated at 4°C overnight with primary antibodies against the monocyte/macrophage marker ED-1 (Chemicon, Temecula, CA) and the myofibroblast marker α-smooth muscle actin (α-SMA; Sigma). After development, tissue sections were moderately counterstained with hematoxylin. The number of monocytes/macrophages in the interstitium and within glomeruli was counted as positive staining for ED-1 in a blinded manner from 10 different fields of each section at ×200 magnification. Positive staining for α-SMA was quantified using NIH image software from 10 different fields of each section.
Detection of reactive oxygen species formation in human proximal tubular cells.
Immortalized human proximal tubular cells (HKCs) were kindly provided by Dr. L. Racusen of Johns Hopkins University (Baltimore, MD) and maintained in DMEM/F12 medium supplemented with 5% fetal bovine serum (Invitrogen, Carlsbad, CA). Intracellular production of reactive oxygen species (ROS) was detected by oxidation of the cell-permeable dye 2′,7′-dichlorofluorescein diacetate (DCF-DA; Molecular Probes, Carlsbad, CA) to fluorescent DCF by H2O2 (11). HKCs were grown to 80% confluence in six-well plates and incubated for 30 min with DCF-DA (10 nmol/l) in the presence or absence of Nω-nitro-l-arginine methyl ester (l-NAME; 200 μmol/l), an inhibitor of NO synthase (NOS). The cells were then pretreated with TK (0.2 μmol/l) for 30 min before addition of ANG II (100 nmol/l) for 1 h. After incubation, cells were washed twice with PBS and imaged using fluorescence microscopy. To quantitate intracellular ROS levels, cells were seeded on a 96-well plate and treated as described above. Relative fluorescence was measured using a fluorescence plate reader Victor3 (Perkinelmer Life Science, Waltham, MA) at excitation and emission wavelengths of 485 and 528 nm, respectively.
Transforming growth factor-β treatment of HKCs.
Cells were seeded on six-well plates to ∼60–70% confluence in complete medium containing 5% fetal bovine serum for 16 h and then changed to serum-free medium after being washed twice with PBS. Cells were then incubated with transforming growth factor-β (TGF-β1; 3 ng/ml) in the presence or absence of TK (0.2 μmol/l). After 24 h, cells and conditioned media were harvested for measurement of matrix metalloproteinase-2 (MMP-2) activity as well as tissue inhibitor of metalloproteinase-2 (TIMP-2) and plasminogen activator inhibitor-1 (PAI-1) expression.
Biochemical assays.
Serum lipid peroxidation, an indicator of oxidative stress, was determined by measuring circulating thiobarbituric acid-reactive substances (TBARS) levels (21). Renal tissue or cultured HKCs were homogenized in lysis buffer (10 mmol/l Tris, pH 7.4, 100 mmol/l NaCl, 1 mmol/l EDTA, 20 mmol/l Na4P2O7, 2 mmol/l Na3VO4, and 1% Triton X-100) containing 1:100 protease inhibitor cocktail (Sigma). Renal myeloperoxidase activity was measured as previously described (31). NADH oxidase activity in the renal extracts or cell lysates was measured in the presence of NADH substrate (100 μmol/l) and lucigenin (75 μmol/l) by a chemiluminescence assay (13). Renal superoxide production was measured using a ferricytochrome c reduction assay according to a modified previous protocol (10). Levels of nitrate/nitrite (NOx), an indicator of NO formation, in renal extracts and urine were measured by a fluorometric assay as previously described (24). TK levels in kidney extracts were determined by a specific enzyme-linked immunosorbent assay for rat TK using methods similar to previously described procedures (32).
Quantitative RT-PCR.
Total RNA was extracted from kidney and cultured cells using TRIzol reagent (Invitrogen). cDNA was transcribed from 2 μg of RNA using a high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster, CA) following the manufacturer's instructions. PCR was carried out using the following Taqman Gene Expression Assays: Rn00562055_ml for tumor necrosis factor-α (TNF-α), Rn00564227_ml for intercellular adhesion molecule-1 (ICAM-1), Rn00573232_ml or Hs00234278_ml for TIMP-2, Rn00561717_ml or Hs00167155_ml for PAI-1, Rn00586945_ml for p47, Rn00576710-ml for gp91, Hs99999901_sl for the housekeeping gene of 18S RNA, and a detection kit for GAPDH on a 7300 RT-PCR system (Applied Biosystems). Quantification was determined by Relative Quantification Software (Applied Biosystems).
Gelatin zymography and Western blot analysis.
Conditioned media from HKCs were subjected to gelatin zymography to detect MMP-2 activity as previously described (23). Western blot analysis of HKC lysates was performed using antibodies against TIMP-2 (Calbiochem) and PAI-1 (Santa Cruz Biotechnology, Santa Cruz, CA).
Statistical analysis.
Data were analyzed using standard statistical methods and ANOVA followed by Fisher's paired least significant difference. Group data are expressed as means ± SE. Values of all parameters were considered significantly different at a value of P < 0.05.
RESULTS
Inhibitory effect of anti-TK antibody on TK activity in vitro.
Incubation of TK with various concentrations of anti-TK antibody resulted in a dose-dependent reduction in TK activity, as determined by cleavage of the chromogenic substrate S-2266. Incubation of TK with half-fold, equimolar, and twofold amounts of anti-TK antibody reduced TK activity by 34, 81, and 83%, respectively. This indicates that the anti-TK antibody effectively neutralizes TK activity.
Blockade of endogenous TK in vivo promotes renal dysfunction.
To study the effect of endogenous TK on kidney function in chronic renal disease, DOCA-salt rats were injected with polyclonal anti-TK antibody to neutralize endogenous TK activity. Eleven days after the start of antibody administration, endogenous TK levels in the kidney were markedly lower in DOCA-salt rats receiving anti-TK antibody compared with control rats injected with control IgG (15.98 ± 1.33 vs. 26.07 ± 2.86 ng/mg protein, n = 6, P < 0.05). Depletion of endogenous TK was accompanied by impairment of renal function, as indicated by increased urinary protein levels and blood urea nitrogen, and a dramatic decrease in creatinine clearance (Table 1). No significant difference in MAP was observed between DOCA-salt rats receiving control IgG and anti-TK antibody, although MAP was higher in these rats compared with the sham group (Table 1).
Table 1.
Morphological and renal function parameters
| Sham | DOCA/IgG | DOCA/α-TK | |
|---|---|---|---|
| Urinary protein, mg·day−1·100 g BW−1 | 19.06 ± 1.43 | 29.08 ± 3.35* | 47.30 ± 9.08† |
| Blood urea nitrogen, mg/dl | 17.45 ± 0.53 | 18.94 ± 0.34* | 23.98 ± 2.10† |
| Creatinine clearance, ml/min | 0.64 ± 0.06 | 0.42 ± 0.02* | 0.33 ± 0.01† |
| BW, g | 357.2 ± 6.5 | 276.4 ± 9.9* | 271.3 ± 8.4* |
| KW, g | 1.67 ± 0.10 | 2.62 ± 0.11* | 2.82 ± 0.06* |
| KW:BW ratio, g/kg | 4.7 ± 0.3 | 9.6 ± 0.5* | 10.4 ± 0.3* |
| MAP, mmHg | 93.5 ± 6.7 | 132.5 ± 7.7* | 126.3 ± 5.7* |
Data are expressed as means ± SE. α-TK, anti-tissue kallikrein antibody; BW, body weight; KW, kidney weight; MAP, mean arterial pressure. Metabolic data were analyzed 2 days before death. MAP was measured on the day of death.
P < 0.05 vs. sham.
P < 0.05 vs. DOCA/IgG.
Blockade of endogenous TK in vivo aggravates renal injury and inflammatory responses.
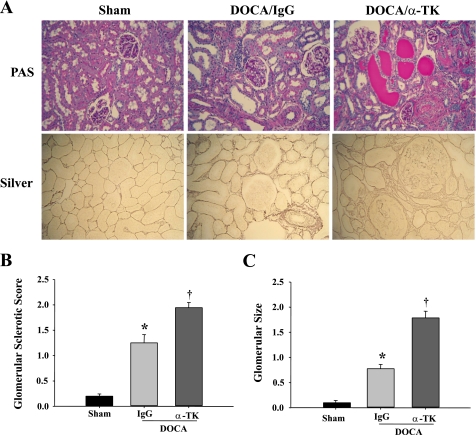
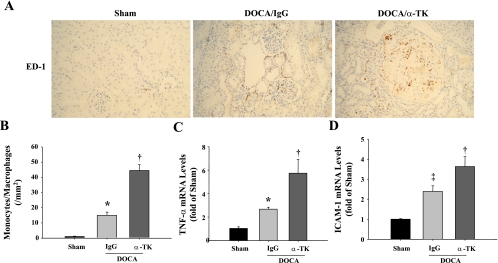
The morphology of kidney damage induced by DOCA-salt was evaluated by PAS and silver staining. Kidneys of DOCA-salt rats developed glomerular enlargement, glomerular sclerosis, tubular dilation, brush-border loss, and cast formation (Fig. 1A). The severity of glomerular and tubulointerstitial lesions was much more enhanced in anti-TK antibody-treated rats than in those receiving control IgG. Semiquantitative analysis indicated that glomerular sclerosis and glomerular size were significantly greater in DOCA/α-TK rats compared with DOCA-salt rats given control IgG (Fig. 1, B and C). Both DOCA-salt groups had lower body weights and higher kidney weights than sham rats. However, no significant differences were observed between the DOCA-salt groups in regards to kidney weight, body weight, and kidney weight-to-body weight ratio (Table 1). Moreover, TK blockade further increased the number of monocytes/macrophages in the interstitium and glomeruli compared with IgG treatment (Fig. 2, A and B). In addition, anti-TK antibody injection markedly increased both TNF-α and ICAM-1 mRNA levels in the kidney above that of rats given control IgG (Fig. 2, C and D).
Fig. 1.
Blockade of endogenous tissue kallikrein (TK) by neutralizing anti-TK antibody worsens renal injury in DOCA-salt rats, as demonstrated by exacerbated tubular dilation, brush-border loss, protein cast formation, glomerulosclerosis, and glomerular size. A: representative images of periodic acid Schiff (PAS) and silver staining in kidney sections. Original magnification is ×200. B: semiquantitative glomerular sclerotic score. C: semiquantitative analysis of glomerular size. Values are expressed as means ± SE (n = 6). *P < 0.05 vs. sham. †P < 0.05 vs. DOCA/IgG.
Fig. 2.
Blockade of endogenous TK by neutralizing anti-TK antibody promotes the accumulation of monocytes/macrophages and elevates tumor necrosis factor-α (TNF-α) and intercellular adhesion molecule-1 (ICAM-1) expression in the kidney of DOCA-salt rats. A: representative images of cells positive for ED-1, a marker of monocytes/macrophages, in the renal cortex. Original magnification is ×200. B: quantification of monocytes/macrophages in the renal cortex. TNF-α (C) and ICAM-1 (D) mRNA levels determined by quantitative RT-PCR. Values are expressed as means ± SE (n = 6). *P < 0.05 and ‡P < 0.01 vs. sham. †P < 0.05 vs. DOCA/IgG.
Blockade of endogenous TK in vivo aggravates renal fibrosis.
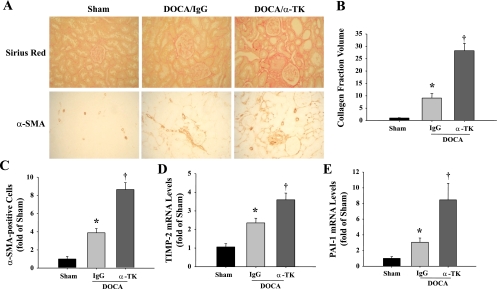
Significant collagen accumulation, identified by Sirius red staining, was observed in the kidneys of rats that received anti-TK antibody compared with the DOCA/IgG group (Fig. 3A). Collagen fraction volume was calculated to confirm this result (Fig. 3B). Myofibroblasts were identified by positive immunohistochemical staining for α-SMA. These cells exhibit upregulated expression of extracellular matrix (ECM) proteins, particularly collagen. Significant expression of α-SMA was detected in glomeruli and the tubulointerstitium of anti-TK antibody-treated rats compared with those given control IgG (Fig. 3A). The number of α-SMA-positive cells was determined to verify these observations (Fig. 3C). Furthermore, TK blockade dramatically increased both TIMP-2 and PAI-1 mRNA levels in the kidney (Fig. 3, D and E). These results suggest that blocking endogenous TK activity augments renal fibrosis in chronic renal injury.
Fig. 3.
Blockade of endogenous TK by neutralizing anti-TK antibody exacerbates DOCA-salt-induced renal fibrosis and profibrotic gene expression. A: representative images of histochemical staining with Sirius red, which stains collagen fibers red, and immunohistochemical staining for α-smooth muscle actin (α-SMA), a marker of myofibroblasts. Original magnification is ×200. B: quantification of collagen fraction volume. C: quantification of α-SMA-positive cells. TIMP-2 (D) and PAI-1 (E) mRNA levels in the kidney determined by quantitative RT-PCR. Values are expressed as means ± SE (n = 6). *P < 0.05 vs. sham. †P < 0.05 vs. DOCA/IgG.
Blockade of endogenous TK in vivo decreases NOx levels and enhances oxidative stress.
A significant increase in circulating TBARS levels was observed in DOCA-salt rats and was further elevated after anti-TK antibody treatment (Table 2). TK blockade also markedly enhanced renal myeloperoxidase activity, NADH oxidase activity, and superoxide formation as well as expression of the NADH oxidase subunits p47 and gp91 compared with control IgG-treated rats (Table 2). Conversely, a significant decrease in NOx levels in the kidney and urine was detected in DOCA/α-TK rats compared with those given control IgG (Table 2). Taken together, these results indicate that endogenous TK is essential in maintaining NO levels and suppressing oxidative stress in chronic renal injury.
Table 2.
Oxidative stress parameters and nitric oxide measurements
| Sham | DOCA/IgG | DOCA/α-TK | |
|---|---|---|---|
| TBARS, μmol/l | 19.77 ± 1.90 | 26.73 ± 1.16* | 34.14 ± 2.61† |
| MPO activity, ΔA655·min−1·100 mg protein−1 | 2.13 ± 0.04 | 2.69 ± 0.12* | 3.48 ± 0.17† |
| NADH oxidase activity, rlu·min−1·mg protein−1 | 11.04 ± 1.48 | 16.00 ± 2.66* | 23.06 ± 2.85† |
| Relative p47 mRNA levels | 0.95 ± 0.14 | 2.01 ± 0.09* | 2.94 ± 0.32† |
| Relative gp91 mRNA levels | 1.01 ± 0.06 | 2.05 ± 0.08* | 3.12 ± 0.53† |
| Superoxide, nmol·min−1·mg protein−1 | 50.17 ± 9.45 | 192.95 ± 19.02* | 292.99 ± 36.20† |
| Renal NOx, mmol/mg protein | 86.26 ± 0.84 | 75.48 ± 0.98* | 71.35 ± 1.18† |
| Urinary NOx, μmol·day−1·100 g BW−1 | 1.32 ± 0.06 | 1.07 ± 0.01* | 0.83 ± 0.02† |
Data are expressed as means ± SE. TBARS, thiobarbituric acid-reactive substances; MPO, myeloperoxidase; rlu, relative light units; NOx, nitrate/nitrite. All biochemical parameters were measured using kidney extracts, except for TBARS and urinary NOx.
P < 0.05 vs. sham.
P < 0.05 vs. DOCA/IgG.
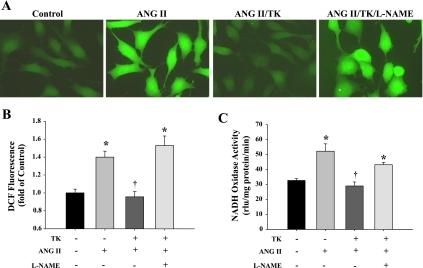
TK inhibits ANG II-induced ROS formation and NADH oxidase activity in HKCs.
ANG II treatment significantly increased ROS formation in HKCs, as determined by DCF fluorescence (Fig. 4, A and B). Pretreatment with TK completely blocked ANG II-induced ROS production, whereas coincubation with l-NAME reversed the protective effect of TK. TK administration also abolished the rise in NADH oxidase activity induced by ANG II, and TK's effect was blocked by l-NAME (Fig. 4C). These results indicate that TK inhibits oxidative stress via NO formation.
Fig. 4.
TK reduces ANG II-induced reactive oxygen species (ROS) generation and NADH oxidase activity in human proximal tubular cells (HKCs) via nitric oxide (NO) formation. A: representative images of ANG II-induced DCF fluorescence. DCF fluorescence results from the oxidation of 2′,7′-dichlorofluorescein diacetate (DCF-DA) by H2O2. Original magnification is ×400. B: quantification of ROS production as determined by DCF-DA fluorescence. C: NADH oxidase activity in HKCs as determined by chemiluminescence assay. Control, no treatment; rlu, relative light units. Values are expressed as means ± SE (n = 4). *P < 0.05 vs. control. †P < 0.05 vs. ANG II.
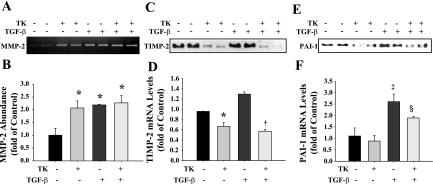
TK enhances MMP activity and suppresses TIMP-2 and PAI-1 expression in HKCs.
ECM degradation is primarily a result of the action of a group of proteolytic enzymes known as MMPs. However, the enzymatic activity of MMPs can be inhibited by a family of proteins known as TIMPs. Activation of MMPs can also be blocked by PAI-1. Gelatin zymographic analysis of HKC-conditioned media indicated that TK treatment promoted MMP-2 activity with or without TGF-β administration (Fig. 5, A and B). TK treatment significantly decreased TGF-β-induced TIMP-2 and PAI-1 protein and mRNA levels as determined by Western blot and quantitative RT-PCR (Fig. 5, C–F). These data indicate that TK promotes ECM degradation by stimulating MMP activity and decreasing MMP inhibitor synthesis.
Fig. 5.
TK exerts anti-fibrotic actions in HKCs stimulated with transforming growth factor-β (TGF-β). Gelatin zymography (A) and densitometric analysis (B) of matrix metalloproteinase-2 (MMP-2) activity of HKC-conditioned media. Western blot (C) and quantitative RT-PCR analysis (D) of tissue inhibitor of metalloproteinase-2 (TIMP-2) expression. Western blot (E) and quantitative RT-PCR analysis (F) of plasminogen activator inhibitor-1 (PAI-1) expression. Control, no treatment. Values are expressed as means ± SE (n = 4). *P < 0.05 and ‡P < 0.01 vs. control. †P < 0.01 and §P < 0.05 vs. TGF-β.
DISCUSSION
In this study, we demonstrated that blocking endogenous TK activity with a specific neutralizing antibody accelerated the onset and progression of renal damage in salt-induced hypertensive rats. Blockade of TK led to an enhancement of renal oxidative stress and inflammatory and fibrotic responses, suggesting that TK is beneficial in the preservation of kidney structure and function. This finding is consistent with previous studies that TK not only promotes tissue repair and regeneration following acute injury (7, 15), but it also modulates the development and progression of chronic diseases (34, 35). Moreover, experiments with cultured proximal tubular cells indicated that TK significantly blocks ANG II-induced ROS production and NADH oxidase activity through NO formation and promotes matrix degradation by augmenting the activity of MMP-2 and inhibiting TIMP-2 and PAI-1 expression. This is the first study to reveal a role of endogenous TK in protection of chronic renal damage and fibrosis by inhibiting oxidative stress and enhancing matrix degradation pathways in vivo and in cultured renal cells.
Endogenous TK levels in the kidney were measured at 11 days after the start of antibody administration to determine the inhibitory efficacy of the anti-TK antibody in vivo. Our results showed that anti-TK antibody treatment significantly decreased renal kallikrein levels compared with the control group injected with the same dose of normal IgG in DOCA-salt hypertensive rats (Table 1). Moreover, using a chromogenic substrate, we showed that enzymatic activity of purified TK was inhibited more than 80% by equimolar or higher amounts of neutralizing anti-TK antibody. These in vivo and in vitro results indicate that anti-TK antibody is effective in reducing TK levels in the damaged kidney. Depletion of endogenous TK by anti-TK antibody administration worsened renal function parameters in DOCA-salt rats compared with those receiving control IgG. Indeed, enhanced renal damage by anti-TK antibody injection was associated with a slight decline in creatinine clearance. We also detected an increase in blood pressure in DOCA-salt rats after 2 wk of salt loading. However, a further induction of blood pressure was not observed by anti-TK antibody injection. The marginal reduction in creatinine clearance in the anti-TK antibody group compared with the IgG-treated rats may explain the absence of a blood pressure rise. Moreover, TK blockade failed to elevate blood pressure in the hypertensive DOCA-salt rats possibly because a high blood pressure state had already been established. Furthermore, 11 days of anti-TK antibody injection may not provide sufficient time to observe a further increase in blood pressure.
Previous reports indicated that the beneficial effects of TK are mediated via activation of NO signaling pathways, which lead to a decrease in oxidative stress in animal models (4, 35). The current study was in agreement with these observations, as blocking endogenous TK with neutralizing antibody aggravated renal injury by enhancing oxidative stress and reducing NO production. Oxidative stress in the kidney contributes to progressive renal disease by virtue of modulating renal hemodynamic actions, altering glomerular permeability, and promoting acute and chronic inflammatory responses. It has been reported that ROS are markedly elevated in DOCA-salt hypertensive animals (22, 36). In the present study, we found that DOCA-salt treatment induced a notable rise in oxidative stress, as evidenced by increased circulating TBARS levels, renal myeloperoxidase, and NADH oxidase activities, and superoxide production, compared with rats in the sham group, while blocking TK activity further increased these oxidative stress parameters. The role of TK in regulating oxidative stress was confirmed by the observation that exogenous administration of TK significantly prevented ANG II-induced ROS production in cultured proximal tubular cells and that this effect was blocked by NOS inhibition. NO, a potent antioxidant, is capable of inhibiting neutrophil superoxide anion production via a direct action on the membrane components of NADH oxidase and the assembly of NADH oxidase subunits (26). In this study, a marked decrease in NO levels in the kidney was detected in rats receiving anti-TK antibody treatment. The combination of reduced NO and increased oxidative stress on diminished endogenous TK levels may contribute to renal injury progression in the form of glomerulosclerosis, inflammation, and fibrosis. Therefore, endogenous TK may function to preserve the normal structure and function of the kidney in part by inhibiting oxidative stress.
It is well-known that NADH oxidase is a major source of ROS in the vessel wall. NADH oxidase contributes to oxidative stress not only by producing superoxide but also by initiating tetrahydrobiopterin oxidation, leading to endothelial NOS uncoupling and subsequent reduction of NO levels (14, 22). The vasculature, interstitium, juxtaglomerular apparatus, and the distal nephron in the kidney have a rich expression of NADH oxidase that generates the superoxide anion. In this study, we found that TK blockade significantly increased renal NADH oxidase activity and expression of the NADH oxidase subunits p47 and gp91. Compelling evidence showed that ANG II can stimulate NADH oxidase (28). Although DOCA-salt hypertension is considered a low-renin state, it is possible that the local ANG II stimulates ROS production via activation of NADH oxidase. Indeed, a previous report demonstrated that local ANG II contributed to renal injury associated with DOCA-salt hypertension (19). Consistent with this view, we found that pretreatment with TK significantly decreased NADH oxidase activity in ANG II-treated proximal tubular cells in vitro. It is also possible that infiltrating leukocytes contribute to increased NADH oxidase activity in the kidney.
The development of renal fibrosis has been reported to be partly due to the accumulation of myofibroblasts, a cell type characterized by excessive secretion of ECM proteins (18). These cells can arise by epithelial-mesenchymal transition of tubular epithelial cells and are identified by positive staining for α-SMA. In our study, we found that DOCA-salt rats receiving anti-TK antibody had elevated amounts of α-SMA-positive cells and increased collagen deposition in the kidney compared with DOCA-salt rats given control IgG. One explanation for the adverse effect of renal fibrosis on TK blockade is suggested by the examination of various molecules that participate in matrix degradation. ECM degradation is primarily mediated by MMPs, a family of matrix-degrading enzymes that play a pivotal role in ECM remodeling. Ultimately, proteolytic activity depends on the final balance between active MMPs and their inhibitors, such as TIMPs (5). TIMP-2 can counteract MMP-2, thus resulting in a shift in MMP/TIMP balance. When TK was added to this system in cultured proximal tubular cells, MMP-2 activity was increased and TIMP-2 expression was significantly reduced. As a result, the balance of MMP/TIMP shifted to MMP activity, allowing TK to exert an anti-fibrotic action. We also found that TK treatment significantly decreased the expression of PAI-1 induced by TGF-β. Consistent with the in vitro results, the in vivo experiments showed that blocking endogenous TK significantly increased both TIMP-2 and PAI-1 expression. These findings indicate that the anti-fibrotic effect of TK is partly mediated by enhancing matrix degradation.
GRANTS
This work was supported by the National Institutes of Health Grants HL-29397 and DK-066350 and Grant C06-RR-015455 from the Extramural Research Facilities Program of the National Center for Research Resources.
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1.Azizi M, Emanueli C, Peyrard S, Maddedu P, Alhenc-Gelas F, Campbell DJ. Genetic and dietary control of plasma tissue kallikrein secretion and urinary kinins exretion in man. J Hypertens 26: 714–720, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Basile DP, Fredrich K, Alausa M, Vio CP, Liang M, Rieder MR, Greene AS, Cowley AW., Jr Identification of persistently altered gene expression in the kidney after functional recovery from ischemic acute renal failure. Am J Physiol Renal Physiol 288: F953–F963, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Bledsoe G, Crickman S, Mao J, Xia CF, Murakami H, Chao L, Chao J. Kallikrein/kinin protects against gentamicin-induced nephrotoxicity by inhibition of inflammation and apoptosis. Nephrol Dial Transplant 21: 624–633, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Bledsoe G, Shen B, Yao Y, Zhang JJ, Chao L, Chao J. Reversal of renal fibrosis, inflammation, and glomerular hypertrophy by kallikrein gene delivery. Hum Gene Ther 17: 545–555, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Catania JM, Chen G, Parrish AR. Role of matrix metalloproteinases in renal pathophysiologies. Am J Physiol Renal Physiol 292: F905–F911, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Chao J, Margolius HS. Isozymes of rat urinary kallikrein. Biochem Pharmacol 28: 2071–2079, 1979. [DOI] [PubMed] [Google Scholar]
- 7.Chao J, Yin H, Gao L, Hagiwara M, Shen B, Yang ZR, Chao L. Tissue kallikrein elicits cardioprotection by direct kinin B2 receptor activation independent of kinin formation. Hypertension 52: 715–720, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiang WC, Lin SL, Chen YM, Wu KD, Tsai TJ. Urinary kallikrein excretion is related to renal function change and inflammatory status in chronic kidney disease patients receiving angiotensin II receptor blocker treatment. Nephrology 13: 198–203, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Churchill PC, Churchill MC, Bidani AK, Rabito SF. Kallikrein excretion in Dahl salt-sensitive and salt-resistant rats with native and transplanted kidneys. Am J Physiol Renal Fluid Electrolyte Physiol 269: F710–F717, 1995 [DOI] [PubMed] [Google Scholar]
- 10.De Cavanagh EM, Fraga CG, Ferder L, Inserra F. Enalapril and captopril enhance antioxidant defenses in mouse tissues. Am J Physiol Regul Integr Comp Physiol 272: R514–R518, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Ding G, Zhang A, Huang S, Pan X, Zhen G, Chen R, Yang T. ANG II induces c-Jun NH2-terminal kinase activation and proliferation of human mesangial cells via redox-sensitive transactivation of the EGFR. Am J Physiol Renal Physiol 293: F1889–F1897, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Fujisawa Y, Nagai Y, Miyatake A, Takei Y, Miura K, Shoukouji T, Nishiyama A, Kimura S, Abe Y. Renal effects of a new member of adrenomedullin family, adrenomedullin2, in rats. Eur J Pharmacol 497: 75–80, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res 74: 1141–1148, 1994 [DOI] [PubMed] [Google Scholar]
- 14.Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res 86: 494–501, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Hagiwara M, Shen B, Chao L, Chao J. Kallikrein-modified mesenchymal stem cell implantation provides enhanced protection against acute ischemic kidney injury by inhibiting apoptosis and inflammation. Hum Gene Ther 19: 807–819, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hecquet C, Tan F, Marcic BM, Erdos EG. Human bradykinin B(2) receptor is activated by kallikrein and other serine proteases. Mol Pharmacol 58: 828–836, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Jin L, Beswick RA, Yamamoto T, Palmer T, Taylor TA, Pollock JS, Pollock DM, Brands MW, Webb RC. Increased reactive oxygen species contributes to kidney injury in mineralocorticoid hypertensive rats. J Physiol Pharmacol 57: 343–357, 2006 [PubMed] [Google Scholar]
- 18.Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest 112: 1776–1784, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim S, Ohta K, Hamaguchi A, Omura T, Yukimura T, Miura K, Inada Y, Wada T, Ishimura Y, Chatani F. Role of angiotensin II in renal injury of deoxycorticosterone acetate-salt hypertensive rats. Hypertension 24: 195–204, 1994. [DOI] [PubMed] [Google Scholar]
- 20.Kimura K, Onodera K, Oike Y, Yamabe H, Numahata H, Kikuchi K, Hanada S. A clinical study on urinary kallikrein in patients with renal diseases. Adv Exp Med Biol 120B: 549–559, 1979. [PubMed] [Google Scholar]
- 21.Kumar KV, Shifow AA, Naidu MUR, Ratnakar KS. Carvedilol: a beta blocker with antioxidant property protects against gentamicin-induced nephrotoxicity in rats. Life Sci 66: 2603–2611, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest 111: 1201–1209, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y, Rajur K, Tolbert E, Dworkin LD. Endogenous hepatocyte growth factor ameliorates chronic renal injury by activating matrix degradation pathways. Kidney Int 58: 2028–2043, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Misko TP, Schilling RJ, Salvemini D, Moore WM, Currie MG. A fluorometric assay for the measurement of nitrite in biological samples. Anal Biochem 214: 11–16, 1993 [DOI] [PubMed] [Google Scholar]
- 25.Moreau ME, Garbacki N, Molinaro G, Brown NJ, Marceau F, Adam A. The kallikrein-kinin system: current and future pharmacological targets. J Pharm Sci 99: 6–38, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Paul SM, Christopher SW, Shakil A. Nitric oxide, oxidative stress, and progression of chronic renal failure. Semin Nephrol 24: 354–365, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Pizard A, Richer C, Bouby N, Picard N, Meneton P, Azizi M, Alhenc-Gelas F. Genetic deficiency in tissue kallikrein activity in mouse and man: effect on arteries, heart and kidney. Biol Chem 389: 701–706, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Rajiv A, Ruth CC, David GW. Oxidative stress in hypertension and chronic kidney disease: role of angiotensin II. Semin Nephrol 24: 101–114, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Schneeweiss S, Seeger JD, Landon J, Walker AM. Aprotinin during coronary-artery bypass grafting and risk of death. N Engl J Med 358: 771–783, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Shimamoto K, Chao J, Margolius HS. The radioimmunoassay of human urinary kallikrein and comparisons with kallikrein activity measurements. J Clin Endocrinol Metab 51: 840–848, 1980 [DOI] [PubMed] [Google Scholar]
- 31.Suzuki K, Ota H, Sasagawa S, Sakatani T, Fujikura T. Assay method for myeloperoxidase in human polymorphonuclear leukocytes. Anal Biochem 132: 345–352, 1983 [DOI] [PubMed] [Google Scholar]
- 32.Wang C, Chao L, Chao J. Direct gene delivery of human tissue kallikrein reduces blood pressure in spontaneously hypertensive rats. J Clin Invest 95: 1710–1716, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang C, Chen YP, Chao L, Chao J. Regulatory elements in the promoter region of the renal kallikrein gene in normotensive vs hypertensive rats. Biochem Biophys Res Commun 217: 113–122, 1995 [DOI] [PubMed] [Google Scholar]
- 34.Xia CF, Bledsoe G, Chao L, Chao J. Kallikrein gene transfer reduces renal fibrosis, hypertrophy, and proliferation in DOCA-salt hypertensive rats. Am J Physiol Renal Physiol 289: F622–F631, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Zhang JJ, Bledsoe G, Kato K, Chao L, Chao J. Tissue kallikrein attenuates salt-induced renal fibrosis by inhibition of oxidative stress. Kidney Int 66: 722–732, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Zheng JS, Yang XQ, Lookingland KJ, Fink GD, Hesslinger C, Kapatos G, Kovesdi I, Chen AF. Gene transfer of human guanosine 5′-triphosphate cyclohydrolase I restores vascular tetrahydrobiopterin level and endothelial function in low renin hypertension. Circulation 108: 1238–1245, 2003. [DOI] [PubMed] [Google Scholar]