Abstract
The action of vasopressin in rodent collecting ducts to regulate water permeability depends in part on increases in phosphorylation of the water channel aquaporin-2 (AQP2) at three sites: Ser256, Ser264, and Ser269. Previous studies of AQP2 phosphorylation have depended largely on qualitative data using protein mass spectrometry and phospho-specific antibodies. Here, we use a new method employing phospho-specific antibodies to determine the percentage of total AQP2 phosphorylated at each site in the presence and absence of the V2-receptor-selective vasopressin analog dDAVP in rat renal inner medullary collecting duct (IMCD) and cultured mpkCCD cells. Phosphorylation of Ser269, a site previously implicated in plasma membrane retention, was found to increase from 3 to 26% of total AQP2 in rat IMCD cells following dDAVP. Quantification of immunogold labeling of the opposite kidneys from the same rats estimated that 11% of total AQP2 is present in the apical plasma membrane (APM) without injection of dDAVP and 25% is present in the APM after dDAVP. Surprisingly, the baseline level of Ser256 phosphorylation was constitutively high, and there was no increase with dDAVP (confirmed in 2 more sets of rats). In general, Ser264 phosphorylation remained below 5% of total. The pattern of response was similar in cultured mpkCCD cells (large increase in Ser269 phosphorylation following dDAVP, but constitutively high levels of Ser256 phosphorylation). We suggest from these studies that Ser269 phosphorylation may be a more consistent indicator of vasopressin action and AQP2 membrane abundance than is Ser256 phosphorylation.
Keywords: vasopressin, trafficking, quantitative immunoblot analysis, immunogold electron microscopy
renal water excretion is regulated in response to changes in the circulating level of the neurohypophysial hormone vasopressin as part of a feedback mechanism that maintains plasma osmolality within a tight range of 290–294 mosmol/kgH2O. To a large extent, the regulation of water excretion is achieved through vasopressin's effects on the collecting duct water channel aquaporin-2 (AQP2) (18).
There are at least two modes of regulation of AQP2: 1) rapid regulation that results from membrane trafficking which moves AQP2 into and out of the plasma membrane; and 2) long-term regulation that results from control of Aqp2 gene transcription. The former process is thought to involve phosphorylation and/or dephosphorylation of AQP2 at four serines in the COOH-terminal tail, viz. Ser256, Ser261, Ser264, and Ser269. Ser256 was the initial site to be recognized. It was inferred to be phosphorylated by mutational analysis (4, 10) and was eventually confirmed to be phosphorylated through development and use of a phospho-specific antibody (20). The three other phosphorylation sites were recently identified by mass spectrometry (5, 7). All four sites are regulated by vasopressin. Ser256, Ser264, and Ser269 increase in phosphorylation (5), while Ser261 phosphorylation decreases in response to vasopressin (6, 7). Ser256 phosphorylation is most likely mediated by protein kinase A (4, 5, 10), while the kinases acting at the other sites have not been reported. Ser269-phosphorylated AQP2 was found to be exclusively localized to the apical plasma membrane of collecting duct cells, leading to the proposal that this site is involved in retention of AQP2 in the plasma membrane, i.e., by inhibiting endocytosis (5, 13, 14). In contrast, phosphorylation of AQP2 at Ser256 has been proposed to be involved in regulation of AQP2 exocytosis (9, 21).
The major observations that are the basis of the above conclusions are largely qualitative in nature. Application of quantitative approaches has the potential of refining and clarifying our understanding of the processes involved. Consequently, in this paper, we developed an immunoblotting-based approach to carry out relative quantification of phosphorylation at each of the known sites in the presence and absence of vasopressin. In addition, we performed immunogold electron microscopy (EM) of native inner medullary collecting duct cells from the same rat models and image quantification to determine what percentage of AQP2 is present in the apical plasma membrane in the absence and presence of vasopressin.
METHODS
Animal Models
Pathogen-free male Sprague-Dawley rats (Taconic Farm, Germantown, NY) were maintained on an autoclaved pelleted rodent chow (413110–75-56, Zeigler Bros., Gardners, PA). All experiments were conducted in accord with an animal protocol approved by the Animal Care and Use Committee of the National Heart, Lung, and Blood Institute (ACUC protocol number H-0110) or the boards of the Institute of Anatomy and Institute of Clinical Medicine, University of Aarhus, according to the licenses for use of experimental animals issued by the Danish Ministry of Justice.
Study 1.
Four rats were treated with intravenous injection of 1 ng of dDAVP in 200 μl of saline/animal, and four saline-injected rats served as controls. After 60 min, the rats were anesthetized, and the kidneys were perfusion-fixed. Between injection of dDAVP and fixation of the kidney, animals had free access to water but not food. Tissue was processed for immunogold EM and immunoblotting (see pertinent subsection below).
Study 2.
Three control rats were injected with 500 μl vehicle intramuscularly in the hind leg. Three experimental rats were injected with 2 nmol of dDAVP. After 60 min, animals were processed for tissue isolation (see pertinent subsection below).
Study 3.
Rats had free access to 200 mM sucrose water for 16 h before experimentation. Three control rats were injected with saline solution intramuscularly in the hind leg, and three experimental rats were injected with 2 nmol of dDAVP. Following injection, the rats had free access to 200 mM sucrose water. After 60 min, animals were processed for tissue isolation (see pertinent subsection below).
Urine Osmolality Measurements
Urine samples from the control and experimental rats before injection and after injection were collected, and urine osmolality (mosmol/kgH2O) was measured using a vapor pressure osmometer (Wescor Vapro 5520, St. Michaels, MD).
Inner Medulla Isolation
Rats were euthanized, kidneys were removed, and the whole inner medulla (IM) was dissected. The whole IM was homogenized in ice-cold isolation solution (250 mM sucrose, 10 mM triethanolamine, pH 7.6, containing 1 mg/ml leupeptin, 0.1 mg/ml phenylmethylsulfonyl fluoride, and 1× HALT phosphatase inhibitor cocktail, Pierce, Rockland, IL) using a tissue homogenizer (Omni 1000 fitted with a micro-sawtooth generator) at maximum speed for three 15-s intervals. Total protein concentrations were measured (BCA kit; Pierce Chemical, Rockford, IL), and the samples were solubilized in Laemmli sample Buffer (5×; 7.5% SDS, 30% glycerol, 50 mM Tris, pH 6.8, bromphenol blue, 25 mM dithiothreitol) at 60°C for 15 min.
Measurement of Percentage of AQP2 Phosphorylated at Specific Sites Using Phosphopeptide Standards
SDS-PAGE was performed on 12% polyacrylamide gels. In addition to the IM samples, each gel was loaded with phosphopeptide standards over a range of quantities (MW of peptide is 4,200 g/mol). The sequence of each synthetic peptide spanned amino acids 241–271 of the COOH-terminal tail of AQP2 and was phosphorylated at the appropriate residue according to the antibody being used (see below). Each standard was detectable by the appropriate phospho-specific antibody and by the total AQP2 antibody; thus the same standards were used to calibrate the blot with a given phospho-specific antibody and the blot with the total AQP2 antibody. The range of standards was optimized in each case such that the signal from the immunoblot for either total AQP2 or the specific phosphoform in the IM samples would fall within a standard curve generated using the phosphopeptides. For each phosphorylation site that needed to be quantified, two immunoblots were produced. These immunoblots were identical in format and produced on the same day. No stripping of immunoblots was performed. The first immunoblot was probed using a phospho-specific antibody (targeted against Ser256, Ser261, Ser264, or Ser269.) The second immunoblot was probed for total AQP2 using an antibody recognizing total AQP2 independently of its phosphorylation status (LKEM Ab K007 targeted to amino acids 237–255 of rat AQP2) (5). The band densities for samples and standards were quantified allowing the calculation of the percentage of total AQP2 phosphorylated at the targeted site. The steps were as follows: 1) standard curves were generated using the molar concentrations of the peptides; 2) absolute amounts of AQP2 and phospho-AQP2 in each sample lane were calculated from the standard curves; and 3) the percentage of AQP2 phosphorylated at the targeted site was calculated by dividing the absolute amount of the phospho-specific form by the absolute amount of the total.
Immunogold EM of IM Tissue
The right kidney was clamped, removed, and processed for IM isolation (see above). The left kidney was fixed by retrograde perfusion via the abdominal aorta. Kidneys were initially perfused with room temperature 0.01 M PBS (pH 7.4) for 10 s, followed by cold 3% paraformaldehyde in 0.1 M cacodylate buffer (pH 7.4) for 3 min. The kidney was removed, and the IM base dissected out, and infiltrated with 2.3 M sucrose for 60 min before freezing in liquid nitrogen. Thin (70 nm) cryosections were prepared from the frozen tissue on a Reichert Ultracut S cryo-ultramicrotome (Leica). The cryosections were blocked by incubation in PBS containing 0.05 M glycine and 0.1% skim milk powder. Sections were incubated overnight at 4°C with AQP2 K5007. After washing, labeling was visualized with goat anti-rabbit IgG conjugated to 10-nm colloidal gold particles. Cryosections were stained with 0.3% uranyl acetate in 1.8% methylcellulose for 10 min.
Image Processing of Immunogold EM Images
Individual images from principal cells of either treated or control animals were obtained using a FEI Morgagni electron microscope and processed for analysis. Whole principal cell images, each from an individual tubule, were analyzed. Each image was enhanced by manually selecting each gold particle representing AQP2. The edge of each gold particle in the images were “edge detected” using the Sobel operator edge detection method (Matlab Image Processing Toolbox). A threshold was then applied to each image to produce binary images. After production of a binary gradient mask, the mask was dilated, holes were filled, and the gold particles were outlined. The numbers of gold particles were determined by automatically counting the number of boundaries determined by the image processing method. The region of interest, corresponding to the apical plasma membrane, was user defined. The number of particles in the region of interest (corresponding to all apical gold) was divided by the total number of gold particles in the entire inner medullary collecting duct (IMCD) cell to determine the fraction of AQP2 on the plasma membrane compared with total AQP2 abundance.
RESULTS
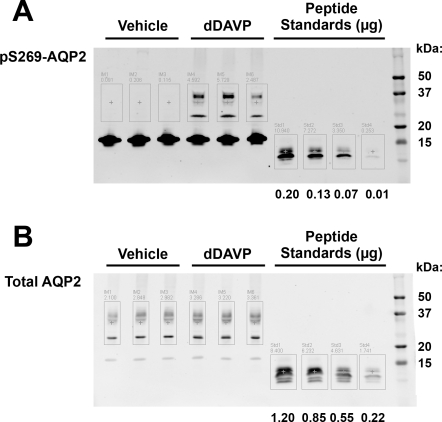
The method for determination of the percentage of AQP2 phosphorylated at a given site is illustrated in Fig. 1, showing quantification of pSer269-AQP2 in Sprague-Dawley rat IMs. Figure 1A shows samples from vehicle- and dDAVP-treated rats (60 min) on the left. The combined band densities for the glycosylated and nonglycosylated bands are determined computationally as illustrated by the boxes surrounding the bands. The protein bands below the boxed-in areas are believed to represent a histone that is recognized by COOH-terminal AQP2 antibodies (8). The band densities of peptide standards, containing the pSer269 phosphorylation, are determined in a similar manner (right). As shown in this representative example, pSer269-AQP2 was markedly increased by acute dDAVP treatment. Figure 1B shows the analogous immunoblot probed for total AQP2. Here, the peptide standards are the same as in Fig. 1A. Since the epitope for the total AQP2 antibody used for this blot is upstream from the phosphorylation sites (5), the presence of a phosphorylated serine at any site will not affect antibody binding. In preliminary studies (not shown), the peptide standard range was determined so that samples will lie within the bounds of the standard curve constructed. The percentage of AQP2 phosphorylated can be calculated by combining the information from the two blots, taking into account differences in the amounts of each sample loaded (see methods).
Fig. 1.
A: representative immunoblotting of vehicle and dDAVP-treated inner medulla samples probed for pSer269-aquaporin-2 (AQP2). B: identical immunoblot using same samples for total AQP2.
In the first study, we treated Sprague-Dawley rats with dDAVP or vehicle for 60 min and determined the percentage of AQP2 phosphorylated at Ser256, Ser264, and Ser269 (Table 1). (We had insufficient material for Ser261 immunoblotting in this sample set.) Ser269 phosphorylation underwent a large increase as seen previously (5), from under 3% to almost 26% in the presence of dDAVP. Ser264 phosphorylation trended toward an increase, but the percentage of phosphorylation at this site was far lower than that at Ser269 in the presence of dDAVP. Interestingly, despite the strong increase in pSer269, phosphorylation at Ser256 did not increase in response to dDAVP. Nevertheless, there was a high percentage of Ser256 phosphorylation in both the presence and absence of dDAVP.
Table 1.
Percent phosphorylation of AQP2 at Ser256, Ser264, and Ser269: effect of acute dDAVP in inner medullas of Sprague-Dawley rats
| Percent Phosphorylation |
||
|---|---|---|
| Phosphorylation Site | Vehicle (n = 4) | dDAVP (n = 4) |
| Ser256 | 49.1 ± 2.6 | 34.3 ± 1.0* |
| Ser264 | 1.9 ± 0.3 | 3.4 ± 0.6 |
| Ser269 | 2.6 ± 1.5 | 25.9 ± 3.8* |
Values are means ± SE. AQP2, aquaporin-2.
P < 0.05 compared with vehicle-treated group, 2-tailed test, unpaired t-test with Welch correction.
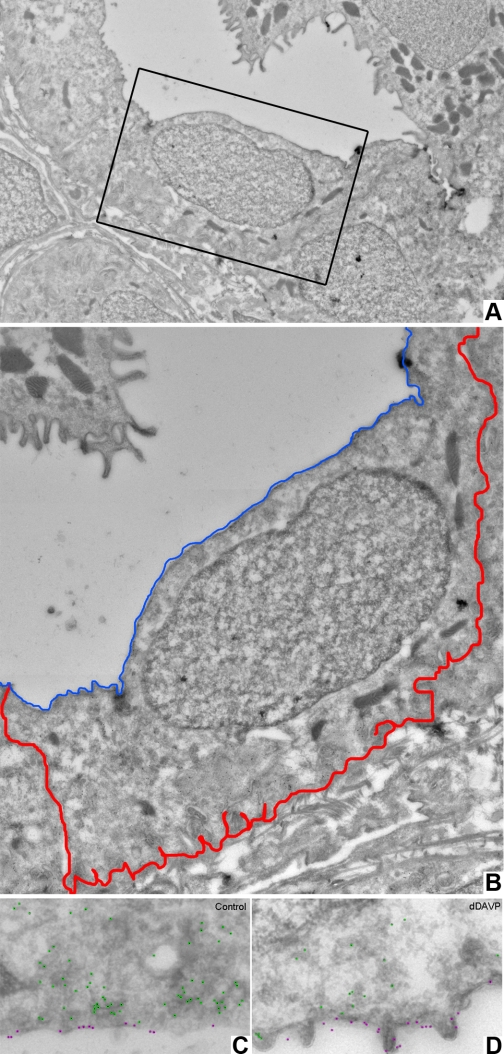
These results offer an opportunity to test again the role of Ser269 vs. Ser256 phosphorylation in AQP2 trafficking, since Ser269 phosphorylation increased but Ser256 phosphorylated did not. To address this, we carried out immunogold labeling of the opposite kidney used for immunoblotting from each rat (same rats as Table 1) to quantify the percentage of AQP2 in the apical plasma membrane, using a semiautomated approach as described in methods. Figure 2 shows an example of an EM image and the counting procedure. Images of the principal cell were obtained at a magnification and resolution that allowed individual gold particles to be observed. If individual gold particles were not apparent, several higher magnification images were obtained and manually “sewn” together (Fig. 2A). The magnification and resolution of these images were sufficient to analyze a whole principal cell at once (Fig. 2B). High-magnification EM images exemplifying the immunogold counting technique using image enhancement are depicted in Fig. 2, C and D. Gold particles within three diameters of the apical surface (i.e., within 30 nm) were counted as in the apical plasma membrane, while gold particles anywhere else in the cell were counted as not in the apical plasma membrane. There were no gold particles over nuclei or in intercalated cells (not shown), indicating that there is little background labeling. The results of the gold particle counting, including the total number of gold particles counted for each rat under each condition, are shown in Table 2. As seen, there was a demonstrable increase in the fraction of the total number of gold particles in the apical plasma membrane, indicating an increase in AQP2 in the apical plasma membrane in response to dDAVP, despite a lack of an increase in pSer256-AQP2. This finding emphasizes the importance of Ser269 phosphorylation in vasopressin-mediated AQP2 accumulation in the apical plasma membrane.
Fig. 2.
Effect of dDAVP on AQP2 distribution. A: low-magnification overview image of a inner medullary collecting duct (IMCD) from the inner medulla base obtained from 6 high-resolution images “fused” together. The boxed area represents the high-resolution image depicted in B. When the same resolution image is magnified to highlight a single principal cell, the gold particles become visible. Red and blue represent the basolateral and apical cell borders, respectively. C: high-magnification immunogold-labeled electron microscopic (EM) image of kidney inner medulla sections from vehicle-treated rats. Apical AQP2 gold particles are labeled in purple, and intracellular particles are labeled in green. D: high-magnification immunogold-labeled EM image of kidney inner medulla sections from dDAVP-treated rats.
Table 2.
Percentage of AQP2 at the apical plasma membrane: effect of acute dDAVP on inner medullas of Sprague-Dawley rats
| Control Group | |||||
|---|---|---|---|---|---|
| Rat no. | Total cells counted | Total number of gold | Intracellular gold | Apical gold | Apical/total, % |
| 1 | 5 | 2,693 | 2,360 | 333 | 12.4 |
| 2 | 5 | 2,303 | 2,167 | 136 | 5.9 |
| 3 | 4 | 1,501 | 1,185 | 256 | 17.1 |
| 4 | 5 | 3,157 | 2,833 | 324 | 10.3 |
| Mean | 11.4 | ||||
| SE | 2.3 | ||||
| dDAVP group | |||||
| 1 | 5 | 2,083 | 1,570 | 533 | 25.6 |
| 2 | 5 | 2,386 | 1,554 | 832 | 34.9 |
| 3 | 5 | 3,356 | 2,679 | 707 | 21.1 |
| 4 | 5 | 3,176 | 2,522 | 654 | 20.6 |
| Mean | 25.5 | ||||
| SE | 3.3 | ||||
| P value | 0.015 | ||||
Each cell counted was from a different inner medullary collecting duct. Total number of gold reflects the number of gold particles counted in all cells for a particular rat. P value was obtained from 2-tailed test, unpaired t-test with Welch correction.
Since phosphorylation of Ser256 was not greatly increased by dDAVP contrary to expectations (Table 1), we repeated the entire immunoblotting protocol in another set of Sprague-Dawley rats (Table 3). Similarly to our previous observations, there was no significant increase in pSer256-AQP2, and the baseline (unstimulated) abundance was high (22% of the total). Again, phosphorylation at Ser269 increased substantially from levels that were virtually undetectable to ∼5% of the total AQP2, while Ser264 phosphorylation was moderately increased. As shown in our previous studies (6, 7), pSer261-AQP2 underwent a significant decrease in phosphorylation. Urinary osmolalities before and 60 min after dDAVP (or vehicle) injection are shown in Table 4.
Table 3.
Percent phosphorylation of AQP2 at Ser256, Ser261, Ser264, and Ser269: effect of acute dDAVP on inner medullas of Sprague-Dawley rats (repeat study)
| Percent Phosphorylation |
||
|---|---|---|
| Phosphorylation Site | Vehicle (n = 9) | dDAVP (n = 9) |
| Ser256 | 22.4 ± 3.3 | 25.6 ± 2.6 |
| Ser261 | 17.7 ± 2.2 | 1.8 ± 0.2† |
| Ser264 | 1.2 ± 0.2 | 2.0 ± 0.3* |
| Ser269 | 0.1 ± 0.1 | 5.1 ± 0.5† |
Values are means ± SE.
P < 0.05,
P < 0.0001 compared with vehicle-treated group, 2-tailed test, unpaired t-test with Welch correction.
Table 4.
Urine osmolality before and after 60 min of vehicle or dDAVP treatment
| Urine Osmolality, mosmol/kgH2O |
||
|---|---|---|
| Sample | Start (0 min) | End (60 min) |
| Vehicle (n = 9) | 637 ± 113 | 1,029 ± 174 |
| dDAVP (n = 9) | 826 ± 130 | 1,994 ± 73* |
Values are means ± SE.
P < 0.0001 compared with vehicle-treated group, 2-tailed test, unpaired t-test with Welch correction.
To test whether water-loading rats for 16 h could suppress the baseline level of Ser256 phosphorylated AQP2, we did another study in which Sprague-Dawley rats were given drinking water containing 200 mM sucrose to elicit an increase in water intake (12). Urine osmolalities before and 60 min after dDAVP (or vehicle) treatments are shown in Table 5. As shown, the baseline urine samples were dilute relative to blood osmolality, i.e., <300 mosmol/kgH2O. The percentage of the total AQP2 that was phosphorylated at Ser256 in these rats was ∼22% in vehicle-treated rats and ∼50% in dDAVP-treated rats (Table 6). Thus, despite a vigorous water diuresis, Ser256 phosphorylation was not suppressed to zero. Again, however, Ser269 phosphorylation was markedly increased by dDAVP treatment.
Table 5.
Urine osmolality before and after 60 min of vehicle or dDAVP treatment (water loaded)
| Urine Osmolality, mosmol/kgH2O |
||
|---|---|---|
| Sample | Start (0 min) | End (60 min) |
| Vehicle (n = 9) | 240 ± 31 | 245 ± 65 |
| dDAVP (n = 9) | 164 ± 9 | 1,399 ± 118* |
Values are means ± SE.
P < 0.01 compared with vehicle-treated group, 2-tailed test, unpaired t-test with Welch correction.
Table 6.
Percent phosphorylation of AQP2 at Ser256 and Ser269: effect of acute dDAVP on inner medullas of water-loaded Sprague-Dawley rats
| Percent Phosphorylation |
||
|---|---|---|
| Phosphorylation Site | Vehicle (n = 3) | dDAVP (n = 3) |
| Ser256 | 22.9 ± 1.5 | 52.2 ± 11.8 |
| Ser269 | 0.3 ± 0.2 | 5.7 ± 0.4* |
Values are means ± SE.
P < 0.05 compared with vehicle-treated group, 2-tailed test, unpaired t-test with Welch correction.
Finally, we measured the percentage of AQP2 phosphorylated at all four sites in cultured mouse collecting duct cells (mpkCCD cells recloned to express high levels of AQP2) (22) in the presence and absence of dDAVP. In these experiments, all cultured cells were initially grown in 1 nM dDAVP to increase the absolute level of AQP2 in the cells. Then, the dDAVP was withdrawn from the cells for 6 h before rechallenge with 1 nM dDAVP or vehicle for 60 min. As shown in Table 7, the responses at Ser269 and Ser256 were qualitatively the same as seen in native IMCD cells. For Ser269 phosphorylation, there was almost no signal in the absence of vasopressin and a very large percentage increase. For Ser256 phosphorylation, the baseline was again high and, although there was a trend to increase, there was no significant change in response to vasopressin.
Table 7.
Percent phosphorylation of AQP2 at Ser256, Ser261, Ser264, and Ser269: effect of acute dDAVP on mpkCCD cells
| Percent Phosphorylation |
||
|---|---|---|
| Phosphorylation Site | Vehicle (n = 3) | dDAVP (n = 3) |
| Ser256 | 53.5 ± 3.7 | 49.1 ± 7.0 |
| Ser261 | 4.3 ± 0.8 | 4.0 ± 0.3 |
| Ser264 | 1.2 ± 0.3 | 2.1 ± 0.3 |
| Ser269 | 0.6 ± 0.3 | 7.1 ± 1.1* |
Values are means ± SE.
P < 0.05 compared with vehicle-treated group, 2-tailed test, unpaired t-test with Welch correction.
DISCUSSION
The purpose of this study was to provide a relative quantification of AQP2 phosphorylation at each of the known phosphorylation sites in the COOH-terminal tail of the protein, both under control conditions or following acute dDAVP treatment. Studies were performed in both native rat IM tissue and in cultured mpkCCD cells. To do this, we developed a method that employs synthetic phosphopeptide standards to determine the amounts of phosphorylated AQP2 relative to total AQP2, as illustrated in Fig. 1. The same standards can be used for both determinations because the peptide-directed total AQP2 antibody, which recognizes the AQP2 peptides upstream from the COOH-terminal phosphorylation sites, recognizes each of the four phosphopeptide standards at equal affinity, and phosphorylation does not affect the ability of the antibody to bind.
The original intent of the study was to quantify phosphorylation at the Ser269 site, which is the third from last amino acid (-S*KA) in AQP2. Our focus on this site was because of our recent finding that Ser269-phosphorylated AQP2 is localized exclusively in the apical plasma membrane, with no detectable pSer269-AQP2 anywhere else in collecting duct principal cells (5, 14). This observation and the associated mutational studies in Madin-Darby canine kidney cells (5) led us to hypothesize that phosphorylation at this site inhibits internalization of AQP2, and recent studies have confirmed that Ser269 phosphorylation partly modulates AQP2 endocytosis (13).Thus vasopressin-induced increases in Ser269 phosphorylation appear to be integrally involved in the well-known role of vasopressin to regulate the distribution of AQP2 between the plasma membrane and endosomal compartments. This idea is in line with previous views (1, 11, 19) that vasopressin regulates apical plasma membrane AQP2 abundance by controlling both AQP2 exocytosis and AQP2 endocytosis. Regulation of AQP2 exocytosis is believed to be dependent on Ser256 phosphorylation (9, 10). In contrast, both prostaglandin E2 and dopamine can induce AQP2 internalization independently of the phosphorylation state of Ser256-AQP2 (16).
In the current studies, the vasopressin analog dDAVP produced a consistent multifold increase in Ser269 phosphorylation in inner medullas from three independent sets of experiments in Sprague-Dawley rats as well as in cultured mpkCCD cells. In contrast, despite this large increase in Ser269 phosphorylation, no significant increase in pSer256-AQP2 was observed in study 1 (Table 1). We took advantage of this observation to address whether AQP2 redistribution can be observed in the renal inner medullary collecting duct without an increase in Ser256 phosphorylation. Immunogold labeling of fixed tissue from the opposite kidney showed that AQP2 was indeed redistributed to the apical plasma membrane in response to dDAVP. However, the increase in plasma membrane abundance was not as great as has been found in previous studies (17) or as great as the increase in water permeability normally seen in isolated, perfused collecting ducts (19). As previously emphasized (1, 18), the steady-state level of AQP2 in the apical plasma membrane depends on a balance of exocytosis and endocytosis, and the overall redistribution appears to involve regulation of both processes. Thus, if the usual regulation of exocytosis by vasopressin did not occur in the first set of experiments described above (as suggested by the lack of an increase in Ser256 phosphorylation), the overall steady-state redistribution of AQP2 would be attenuated as observed. In this study, acute vasopressin treatment may have resulted in a reduction in the intrinsic rate of AQP2 endocytosis related to the increase in Ser269 phosphorylation, but the increase in AQP2 redistribution may be attenuated owing to a minimal increase in Ser256 phosphorylation. These observations fit well with studies in Madin-Darby canine kidney cells that determined that mimicking phosphorylation of AQP2 at S269 (by a Ser-to-Asp mutation resulting in a fixed negative charge) resulted in a reduced rate of AQP2 internalization from the apical plasma membrane (13).
In all of the studies shown in this paper, there was a high level of AQP2 phosphorylation even in the control period without vasopressin addition, and the phosphorylation did not suppress with oral water loading even though urinary osmolality was markedly decreased. Thus we conclude that, at the time points examined in our studies, a high level of Ser256 phosphorylation does not necessarily require a high vasopressin level. Consistent with the results in this paper, using a Ser256 phospho-specific antibody it has previously been shown that 2 h after dDAVP administration to either Wistar rats or Brattleboro rats there is no increase in the abundance of pSer256-AQP2, whereas apical plasma membrane pSer256-AQP2 increased 10-fold (3). Furthermore, the same studies demonstrated that there is a high level of pSer256-AQP2 in intracellular vesicles in kidneys from untreated Brattleboro rats, indicating that Ser256 was phosphorylated, even in the absence of vasopressin. Previous studies, of course, have demonstrated that under some conditions Ser256 phosphorylation does increase with vasopressin administration (15, 20). The findings imply that whereas Ser256 phosphorylation of AQP2 depends somewhat on vasopressin, it also depends on other unknown factors, which may under some circumstances overshadow the effects of vasopressin. The findings may provide an explanation for observations by Lankford et al. (12) that baseline water permeability in isolated, perfused inner medullary collecting ducts can be very high independently of vasopressin's short-term action. One possibility is that Ser256 can be phosphorylated by kinases other than PKA as suggested by Brown (2). Such kinases may be controlled by other than the canonical pathways implicated in vasopressin signaling.
Our previous studies indicated that phosphorylation of Ser256 is necessary for phosphorylation at Ser264 and Ser269 (5). Thus at first glance this conclusion may appear to be at odds with the present results establishing independence of the two phosphorylation events. However, a likely explanation is that an increase in pSer256 is not necessary for vasopressin to increase Ser269 phosphorylation if Ser256 phosphorylation is already high without vasopressin. Thus we propose that a high baseline level of Ser256 phosphorylation is sufficient to allow Ser269 phosphorylation, independently of whether the level is changed by vasopressin. In general, the current findings are consistent with our previous observations that vasopressin regulates Ser269 phosphorylation (5, 14).
Similar conclusions may derive from observations in another biological model exploited in this paper, viz. mpkCCD cells. The fact that increased phosphorylation of AQP2 at Ser256 in these cells is not necessary for vasopressin to increase Ser269 phosphorylation in AQP2 is consistent with previous findings in mpkCCD cells (22). In fact, that study, like the present one, showed that vasopressin-mediated apical redistribution of AQP2 can occur without a change in Ser256 phosphorylation. Overall, we suggest from these studies that Ser269 phosphorylation may be a more consistent indicator of vasopressin action than Ser256 phosphorylation.
One advantage of the methodology described is the ability to calculate absolute numbers of AQP2 molecules per inner medulla, or indeed per IMCD cell. A sample order of magnitude calculation (see Supplementary file; supplementary material for this article is available on the journal web site) estimated there to be ∼5.4–8.4 nmol of AQP2/rat inner medulla, equating to ∼4.4–6.7 × 108 AQP2 molecules/IMCD cell. In principle, application of a similar technique would allow absolute quantification of any protein of interest in the inner medulla.
GRANTS
Funding to M. A. Knepper was provided by the Intramural Budget of the National Heart, Lung, and Blood Institute (National Institutes of Health Project ZO1-HL001285). Funding to R. A. Fenton was provided by the Danish Medical Research Council, the Novo Nordisk Fond, the Carlsberg Foundation (Carlsbergfondet), and the Lundbeck Foundation. The Water and Salt Research Center at the University of Aarhus is established and supported by the Danish National Research Foundation (Danmarks Grundforskningsfond).
DISCLOSURES
No conflicts of interest are declared by the authors.
Supplementary Material
ACKNOWLEDGMENTS
We thank Else-Merete Løcke for expert technical assistance and Kelli Luginbuhl for assistance with the quantification of the images.
REFERENCES
- 1.Brown D. The ins and outs of aquaporin-2 trafficking. Am J Physiol Renal Physiol 284: F893–F901, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Brown D, Hasler U, Nunes P, Bouley R, Lu HA. Phosphorylation events and the modulation of aquaporin 2 cell surface expression. Curr Opin Nephrol Hypertens 17: 491–498, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christensen BM, Zelenina M, Aperia A, Nielsen S. Localization and regulation of PKA-phosphorylated AQP2 in response to V2-receptor agonist/antagonist treatment. Am J Physiol Renal Physiol 278: F29–F42, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Fushimi K, Sasaki S, Marumo F. Phosphorylation of serine 256 is required for cAMP-dependent regulatory exocytosis of the aquaporin-2 water channel. J Biol Chem 272: 14800–14804, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Hoffert JD, Fenton RA, Moeller HB, Simons B, Tchapyjnikov D, McDill BW, Yu MJ, Pisitkun T, Chen F, Knepper MA. Vasopressin-stimulated increase in phosphorylation at Ser269 potentiates plasma membrane retention of aquaporin-2. J Biol Chem 283: 24617–24627, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffert JD, Nielsen J, Yu MJ, Pisitkun T, Schleicher SM, Nielsen S, Knepper MA. Dynamics of aquaporin-2 serine-261 phosphorylation in response to short-term vasopressin treatment in collecting duct. Am J Physiol Renal Physiol 292: F691–F700, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Hoffert JD, Pisitkun T, Wang G, Shen RF, Knepper MA. Quantitative phosphoproteomics of vasopressin-sensitive renal cells: regulation of aquaporin-2 phosphorylation at two sites. Proc Natl Acad Sci USA 103: 7159–7164, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jo I, Nielsen S, Harris HW. The 17 kDa band identified by multiple anti-aquaporin 2 antisera in rat kidney medulla is a histone. Biochim Biophys Acta 1324: 91–101, 1997 [DOI] [PubMed] [Google Scholar]
- 9.Kamsteeg EJ, Heijnen I, van Os CH, Deen PM. The subcellular localization of an aquaporin-2 tetramer depends on the stoichiometry of phosphorylated and nonphosphorylated monomers. J Cell Biol 151: 919–930, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katsura T, Gustafson CE, Ausiello DA, Brown D. Protein kinase A phosphorylation is involved in regulated exocytosis of aquaporin-2 in transfected LLC-PK1 cells. Am J Physiol Renal Physiol 272: F817–F822, 1997 [PubMed] [Google Scholar]
- 11.Knepper MA, Nielsen S. Kinetic model of water and urea permeability regulation by vasopressin in collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol 265: F214–F224, 1993 [DOI] [PubMed] [Google Scholar]
- 12.Lankford SP, Chou CL, Terada Y, Wall SM, Wade JB, Knepper MA. Regulation of collecting duct water permeability independent of cAMP-mediated AVP response. Am J Physiol Renal Fluid Electrolyte Physiol 261: F554–F566, 1991 [DOI] [PubMed] [Google Scholar]
- 13.Moeller HB, Praetorius J, Rutzler MR, Fenton RA. Phosphorylation of aquaporin-2 regulates its endocytosis and protein-protein interactions. Proc Natl Acad Sci USA 107: 424–429, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moeller HB, Knepper MA, Fenton RA. Serine 269 phosphorylated aquaporin-2 is targeted to the apical membrane of collecting duct principal cells. Kidney Int 75: 295–303, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moeller HB, MacAulay N, Knepper MA, Fenton RA. Role of multiple phosphorylation sites in the COOH-terminal tail of aquaporin-2 for water transport: evidence against channel gating. Am J Physiol Renal Physiol 296: F649–F657, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nejsum LN, Zelenina M, Aperia A, Frøkiær J, Nielsen S. Bidirectional regulation of AQP2 trafficking and recycling: involvement of AQP2-S256 phosphorylation. Am J Physiol Renal Physiol 288: F930–F938, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Nielsen S, Chou CL, Marples D, Christensen EI, Kishore BK, Knepper MA. Vasopressin increases water permeability of kidney collecting duct by inducing translocation of aquaporin-CD water channels to plasma membrane. Proc Natl Acad Sci USA 92: 1013–1017, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nielsen S, Frokiaer J, Marples D, Kwon TH, Agre P, Knepper MA. Aquaporins in the kidney: from molecules to medicine. Physiol Rev 82: 205–244, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Nielsen S, Knepper MA. Vasopressin activates collecting duct urea transporters and water channels by distinct physical processes. Am J Physiol Renal Fluid Electrolyte Physiol 265: F204–F213, 1993 [DOI] [PubMed] [Google Scholar]
- 20.Nishimoto G, Zelenina M, Li D, Yasui M, Aperia A, Nielsen S, Nairn AC. Arginine vasopressin stimulates phosphorylation of aquaporin-2 in rat renal tissue. Am J Physiol Renal Physiol 276: F254–F259, 1999 [DOI] [PubMed] [Google Scholar]
- 21.van Balkom BW, Savelkoul PJ, Markovich D, Hofman E, Nielsen S, van der Sluijs P, Deen PM. The role of putative phosphorylation sites in the targeting and shuttling of the aquaporin-2 water channel. J Biol Chem 277: 41473–41479, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Yu MJ, Miller RL, Uawithya P, Rinschen MM, Khositseth S, Braucht DW, Chou CL, Pisitkun T, Nelson RD, Knepper MA. Systems-level analysis of cell-specific AQP2 gene expression in renal collecting duct. Proc Natl Acad Sci USA 106: 2441–2446, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.