Abstract
The hypothalamic paraventricular nucleus is a key integrative area in the brain involved in influencing sympathetic nerve activity and in the release of hormones or releasing factors that contribute to regulating body fluid homeostasis and endocrine function. The endocrine and hormonal regulatory function of the paraventricular nucleus is well studied, but the regulation of sympathetic nerve activity and blood flow by this region is less clear. Here we review the critical role of the paraventricular nucleus in regulating renal blood blow during hyperthermia and the evidence pointing to an important pathophysiological role of the paraventricular nucleus in the elevated renal sympathetic nerve activity that is a characteristic of heart failure.
Keywords: paraventricular nucleus
the hypothalamic paraventricular nucleus (PVN) is located adjacent to the third ventricle in the forebrain and is an important integrative site involved in hormonal, endocrine, and neural control. The PVN is composed of different neuronal subgroups. Magnocellular neurons project to the posterior pituitary and release vasopressin or oxytocin. Parvocellular neurons, which can be subdivided further into different subgroups based on projections, location within the PVN, and morphology, project intrahypothalamically as well as to extrahypothalamic regions in the forebrain, midbrain, brain stem, and spinal cord (3, 64). There are numerous potential neurotransmitters present within the PVN and contained in the efferent-projecting neurons of the PVN (22, 59, 64). Some are present in specific projections, but others are present in more than one neuronal type; for example, vasopressin is present in the magnocellular neurons, and in parvocellular neurons projecting to the spinal cord and may act to influence renal sympathetic nerve activity (59). Thus vasopressin may act directly in the kidney and indirectly via neural mechanisms in the regulation of body fluid homeostasis.
Some parvocellular neurons can directly and indirectly influence sympathetic nerve activity. There are parvocellular neurons in the PVN that project to regions of the spinal cord where sympathetic preganglionic motor neurons are located and thereby can directly influence sympathetic activity (7, 61, 65). Other parvocellular subgroups project to the pressor region of the rostral ventrolateral medulla and thereby indirectly influence sympathetic nerve activity (61). Some PVN parvocellular neurons send collaterals to both autonomic regions and therefore are capable of both direct and indirect influences on sympathetic nerve activity (61).
Activation of the PVN can markedly alter blood pressure, sympathetic nerve activity, and hemodynamic sequelae. Excitation of the PVN with excitatory amino acids, or with bicuculline to inhibit GABA-mediated inhibition of the PVN, and thereby allowing activation of the PVN via endogenous excitatory inputs, can elicit increases or decreases in renal sympathetic nerve activity and renal blood flow (13, 27, 35), suggesting that both sympathoinhibitory and sympathoexcitatory outflows emanate from the PVN. The functional relevance of the effects on renal sympathetic nerve activity induced by activation of the PVN includes the reflex response to volume expansion where there is strong evidence that the PVN is essential for the reflex reduction in renal sympathetic nerve activity that occurs when blood volume is elevated (26, 47, 52). Evidence has also accumulated to show that the PVN is an important site in the regulation of renal function in heart failure and thermoregulation. This brief review focuses on these conditions and examines the recent evidence suggesting an important functional role played by the PVN in renal blood flow regulation during hyperthermia and in the regulation of renal sympathetic nerve activity in chronic heart failure.
Role of the PVN in Regulation of Renal Blood Flow Induced by Hyperthermia
Evidence that the PVN may contribute to the circulatory responses induced by a temperature challenge comes from several sources, which include the observations that the PVN contains 1) thermosensitive neurons (30); and 2) neurons that project to the spinal cord and the pressor region of the rostral ventrolateral medulla and influence sympathetic nerve activity to important thermoregulatory effector organs such as the brown adipose tissue and the vasculature of the rat tail, salivary gland, as well as kidney and gut (28, 53, 62). Furthermore, increases in body core temperature activate neurons within the PVN, including those that project to the spinal cord and medulla (2, 6, 10).
An increase in body core temperature elicits reflex responses designed to reduce heat production and to dissipate heat so as to restore the body core temperature back to normal. The cardiovascular responses that are evoked are important in these thermoregulatory adjustments and involve the redistribution of blood from the hot internal environment (i.e., the viscera) to regions where it can be in close contact with the cooler external environment (i.e., the skin vasculature). Thus vasoconstriction of the blood vessels supplying visceral organs, primarily the mesenteric and renal vasculature, is important in redistributing blood flow and counteracting the increase in body temperature and the potentially fatal outcome of heat stroke.
Recent studies have highlighted the essential role of the PVN in the reduction in mesenteric and renal blood flows induced by hyperthermia (9, 11). Inhibition of neuronal function within the PVN using the GABAA agonist muscimol prevented the normal reduction in mesenteric blood flow, and the effect was specific to the PVN (11). These observations are in agreement with a report in which midbrain transections reduced the increase in splanchnic nerve activity (38). There is a marked reduction in renal blood flow when body core temperature is elevated, and this too can be prevented when neuronal activity in the PVN is inhibited (9). However, this contrasts with a report in which midbrain transection did not appear to affect the increase in renal sympathetic nerve activity elicited by heating (38). The report suggested the renal vasoconstriction was primarily driven by medullary brain regions. Interestingly, the same laboratory subsequently reported that lesions of the PVN markedly attenuated the renal vasoconstriction elicited by an increased body core temperature in the coronary artery ligation model of heart failure in the rat (37), suggesting the hypothalamic PVN plays a critical role in the renal vasoconstriction. The reasons for the contrasting findings are not clear. Perhaps, the transections used in the previous work damaged descending pathways that contribute to opposing the role of the PVN.
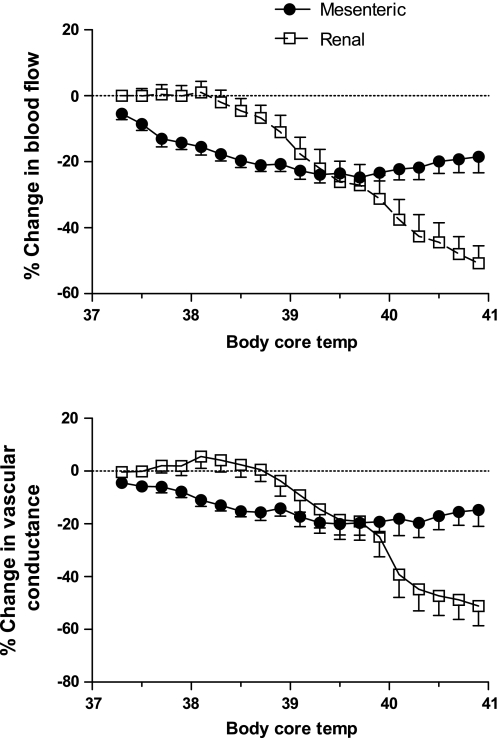
In studies conducted using similar protocols as described previously (9, 11), an examination of the changes in renal and mesenteric blood flows and the respective vascular conductances elicited by an increase in body core temperature in the anesthetized rat highlights some interesting observations. In these experiments, a flow probe (Transonic Systems, Ithaca, NY) was positioned around the right renal artery or the mesenteric artery (separate animals for each organ) and body core temperature was raised at a rate of ∼0.1°C every 2 min by using a water jacket. The first interesting point to note is that renal blood flow remains steady initially as body core temperature increases, and begins to fall when the body core temperature rises above ∼38°C and then continues to fall dramatically (Fig. 1). A similar change in renal vascular conductance was also observed (Fig. 1). By contrast, mesenteric blood flow begins to fall immediately as body core temperature rises from 37°C to reach a maximum plateau by ∼38.5°C (Fig. 1). The reduction in mesenteric vascular conductance followed a similar profile (Fig. 1). The second point to note is that the maximum reduction in renal blood flow peaked at approximately −50% from a resting level of 12.0 ± 1.1 ml/min (Fig. 1). The average resting blood pressure was 110 ± 4 mmHg. The maximum reduction in mesenteric blood flow peaked at approximately −20% from a resting level of 16.9 ± 1.5 ml/min (Fig. 1). In those rats, the average resting blood pressure was 105 ± 4 mmHg. Blood pressures were not markedly affected in these studies.
Fig. 1.
Percent changes in mesenteric (n = 11) and renal (n = 11) blood flows and vascular conductances in response to elevations in body core temperature in urethane-anesthetized rats. Separate animals were used for the renal and mesenteric studies. The body core temperature was raised at a rate of ∼0.1°C every 2 min by using a water jacket as described previously (9, 11).
The results suggest that as body core temperature rises, the mesenteric vasculature responds by vasoconstriction and this appears sufficient in the early stages of hyperthermia to redirect enough blood to the skin to appropriately dissipate the heat. As body core temperature continues to rise, the reduction in mesenteric blood flow peaks and renal blood flow falls dramatically in an attempt to redirect more blood to the cooler skin vasculature. Increases in body core temperature above 41°C can result in a dramatic collapse of the mesenteric vasoconstriction and the subsequent reduction in cardiac output that characterizes heat stroke (39). This suggests that during hyperthermia, limiting the reduction in mesenteric blood flow is critical, and the decrease in renal blood flow may help by continuing to fall as body core temperature rises.
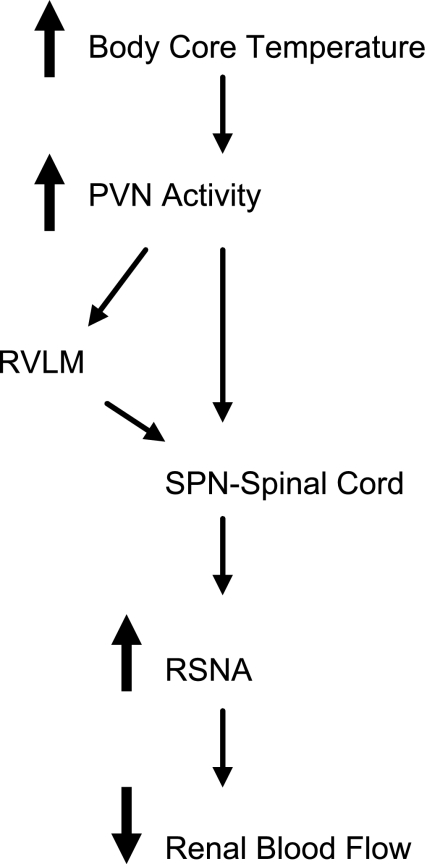
The efferent pathways that contribute to the PVN's involvement in the renal vasoconstriction may include the spinal projecting neurons and/or those that project to the pressor region of the rostral ventrolateral medulla (48, 61, 66). Through these anatomic routes, the PVN can directly and indirectly influence sympathetic nerve activity. Previous studies have shown that PVN neurons are activated by elevations in body core temperature (2, 6, 10), and ∼22% of those activated project to the spinal cord (10) and 8% project to the rostral ventrolateral medulla (8). These latter neurons may be a subset of the activated spinal-projecting neurons since some PVN neurons that project to the lower thoracic spinal cord, where the renal sympathetic preganglionic motor neurons are concentrated, send collaterals to the rostral ventrolateral medulla (61). As shown in Fig. 2, we suggest that an increase in body core temperature activates the PVN to elicit renal vasoconstriction. Influencing neuronal activity within the PVN is known to affect renal function, and activation of the PVN elicits reductions in renal blood flow primarily via the renal nerves; vasopressin makes a smaller contribution (27). We hypothesize that the PVN neurons that project to the spinal cord and/or the rostral ventrolateral medulla contribute to the central pathways mediating the renal and mesenteric vasoconstriction elicited by hyperthermia (Fig. 2). We acknowledge that vasopressin released from the PVN may also contribute to the visceral vasoconstriction.
Fig. 2.
Schema summarizing the effect of raising body core temperature and activation of neurons in the hypothalamic paraventricular nucleus (PVN) that include neurons projecting to the sympathetic preganglionic motor neurons (SPN) in the spinal cord or to the pressor region of rostral ventrolateral medulla (RVLM). The result is an increase in renal sympathetic nerve activity (RSNA) and a reduction in renal blood flow.
The observations that the PVN is essential in the reductions in renal and mesenteric blood flows indicate that the PVN may be a critical integrative site for the cardiovascular responses elicited by hyperthermia. Additionally, magnocellular neurons could be stimulated to release vasopressin systemically, which would also help maintain blood pressure and maximize water retention. Given that the kidneys and mesentery receive a considerable proportion of the cardiac output, vasoconstriction of these vascular beds as body core temperature rises is important in maintaining blood pressure in the face of vasodilation of the large skin vasculature. Thus we hypothesize that any dysfunction within the PVN that prevents the normal vasoconstriction of the kidney and mesenteric vasculature induced by the increased body core temperature could predispose an individual to heat stroke.
The role of the PVN in the regulation of renal and mesenteric nerve activity and the respective blood flows in response to hypothermia would be expected to be the opposite to hyperthermia. This has not been investigated directly; indeed, the role of the PVN in the cardiovascular responses elicited by hypothermia is in need of considerable investigation. The PVN contains neurons that are activated by decreases in body core temperature and these, presumably, are distinct from the neurons that are activated by increases in body core temperature (6, 10). The PVN is involved in regulation of the body's metabolism (41), and some of the neurons in the PVN that contribute to the central pathways involved in increasing metabolic rate and energy expenditure could be activated by hypothermia. A detailed analysis of this is beyond the scope of this review.
Role of the PVN in Regulation of Renal Sympathetic Nerve Activity in Heart Failure
Heart failure is a general term used to describe a complex pathophysiological condition in which the heart is unable to pump sufficient blood to adequately meet the body's needs. Heart failure is the manifestation of ventricular dysfunction that results from disorders that include coronary heart disease, myocardial infarction, hypertension, and genetic abnormalities (29). Symptomatic heart failure is believed to affect ∼0.4–2% of the general population in industrialized nations (29). The incidence of heart failure rises markedly with age such that in the elderly population heart failure (usually congestive heart failure) affects between 3 and 5% of those aged over 65 and 10% of those over 75 (29). Advanced heart failure has a poor prognosis and severely reduces the quality of life.
Congestive heart failure is characterized by neurohumoral activation that results in effects such as salt and fluid retention, resulting in volume expansion and an elevation in sympathetic nerve activity to the heart and kidneys (54). There is also marked attenuation of cardiovascular reflexes including the cardiopulmonary mechanoreceptor vasomotor reflex (i.e., the reflex reduction in renal sympathetic nerve activity which normally occurs in response to increases in blood volume) and the arterial baroreceptor vasomotor reflex (54). The restraining influences on sympathetic nerve activity of these reflexes are therefore diminished in congestive heart failure and may contribute to the elevation in sympathetic nerve activity seen in this condition. The neurohumoral activation seen in congestive heart failure can be mimicked in animal models in which chronic heart failure is induced by myocardial infarction (55).
Sympathetic nerves to the kidney innervate the afferent and efferent arterioles, renal tubules, as well as the renin-containing juxtaglomerular granular cells, and each effector may be innervated selectively by separate nerves as well as sequentially by a single nerve fiber (14). Thus changes in renal sympathetic nerve activity can alter renal function by influencing renal blood flow, changes in urine output, sodium excretion, renin release, and autoregulatory function of the kidney (18). The activation of renal efferent nerves can elicit marked renal vasoconstriction, resulting in decreased renal blood flow and may alter the glomerular filtration rate, although this is dependent on the relative vasoconstriction of the afferent and efferent arterioles and indirect effects mediated by paracrine regulators (17). Low-frequency stimulation of renal sympathetic nerves can increase renin release and reduce sodium and water excretion, and this can occur independently of measurable changes in renal blood flow and glomerular filtration rate (5, 15, 40). Inhibition of renal sympathetic nerve activity induces the opposite effects. For example, an increase in blood volume of ∼10–30% reflexly induces a reduction in renal sympathetic nerve activity, increases urine flow and sodium excretion, while blood pressure and renal blood flow responses vary (12, 20, 26, 49, 51). The renal sympathetic nerve response induced by an increase in blood volume is mediated by cardiopulmonary mechanoreceptors (4). Blood volume expansion or stimulation of cardiopulmonary mechanoreceptors using a small balloon positioned at the atriovenous junction in the conscious rat elicits activation of neurons in the PVN (36). Activation of the PVN in anesthetized rabbits can elicit cardiovascular responses that are similar to those induced by blood volume expansion (13), and inhibition of neuronal activity in the PVN prevents the reduction in renal sympathetic nerve activity induced by increases in blood volume (52). Similar effects are observed in anesthetized rats (26). The results suggest the PVN is a key central nucleus contributing to the reflex reduction in renal sympathetic nerve activity in response to blood volume expansion. In congestive heart failure and in chronic heart failure induced by myocardial infarction, this cardiopulmonary mechanoreceptor vasomotor reflex response is attenuated (1, 19, 54). Thus the neurons in the PVN that mediate the reflex reduction in renal sympathetic nerve activity following blood volume expansion may contribute to the dysfunctional cardiopulmonary vasomotor reflex response in chronic heart failure and thereby may contribute to the elevation in renal sympathetic nerve activity observed in that condition. However, the role of the PVN in regulating renal sympathetic nerve activity in chronic heart failure is more complex. Neuronal activity in the PVN is increased in heart failure induced by myocardial infarction as shown by increased hexokinase activity and by increased numbers of neurons positively stained for Fos, a protein marker of increased neuronal activity (56, 57). It should also be noted that the cardiac remodeling that occurs in chronic heart failure may reduce the responsiveness of the cardiopulmonary mechanoreceptors to changes in blood volume, and this may also contribute to the reduced cardiopulmonary mechanoreceptor vasomotor reflex.
Neurochemical Mediators of Altered Neuronal Activity in the PVN in Heart Failure
The neuropharmacological basis for the activation of neuronal activity in the PVN in chronic heart failure involves changes in the functional role of several known neurotransmitters and neuromodulators. Nitric oxide can tonically inhibit neurons in the PVN that are capable of regulating renal sympathetic nerve activity (63). In heart failure induced by myocardial infarction, the effects on renal sympathetic nerve activity of nitric oxide donors and of inhibitors of nitric oxide synthase in the PVN are markedly attenuated, suggesting nitric oxide function in the PVN is blunted (58, 71). This view is supported by findings that show the number of neurons in the PVN staining for neuronal nitric oxide synthase, the enzyme involved in the synthesis of nitric oxide, is reduced in heart failure (74).
The effects of nitric oxide in the PVN may be mediated by the inhibitory neurotransmitter GABA, since the renal sympathoinhibitory effects of nitric oxide donors microinjected into the PVN can be prevented by the GABAA receptor antagonist bicuculline administered into the PVN (73). The renal sympathetic nerve responses elicited by stimulation or blockade of GABA receptors are also reduced in heart failure, suggesting that the renal sympathoinhibitory actions of GABA are attenuated in chronic heart failure (72), further supporting the view that GABA may mediate the responses of nitric oxide in the PVN. Thus in chronic heart failure, the elevated renal sympathetic nerve activity observed may be due in part to the reduced ability of the inhibitory influences of nitric oxide and GABA in the PVN. The nature of the interactions between nitric oxide and GABA are not entirely clear but may involve a presynaptic effect by nitric oxide that enhances GABA's actions on PVN neurons (44).
Attenuated inhibitory actions of nitric oxide and GABA in the PVN may allow excitatory neurotransmitters and neuromodulators to have a greater influence. Glutamate and angiotensin II are two examples whose effects in the PVN on renal sympathetic nerve activity are enhanced in chronic heart failure. Activation of NMDA receptors in the PVN elicits increases in renal sympathetic nerve activity that are greater in chronic heart failure (45) Conversely, antagonism of NMDA receptors in the PVN elicits reductions in renal sympathetic nerve activity. These responses, too, are greater in chronic heart failure than in sham controls (45). These findings suggest that there is an enhanced influence of glutamatergic excitatory effects in the PVN in chronic heart failure. This may be due to an increased expression of NMDA receptors in the PVN and an increased glutamate release, although the latter is controversial (45, 46).
Angiotensin II is increased in the circulation of rats in chronic heart failure induced by a myocardial infarction. This peptide is a powerful vasoconstrictor and increases mean arterial pressure via sympathetic nerve activation. Similarly, administration of angiotensin II into the lateral brain ventricles elicits pressor responses and increases in sympathetic nerve activity, although of longer duration. There is a high concentration of angiotensin II receptors in the PVN, predominantly the type I receptor, and the expression of this receptor is increased in the PVN in chronic heart failure (75). Angiotensin II into the PVN increases renal sympathetic activity, and the effect is greater in rats with chronic heart failure compared with sham animals. Similarly, the effects on renal sympathetic nerve activity following inhibition of the angiotensin II type I receptor are also enhanced in chronic heart failure (16, 75). Taken together, the results suggest that there is overactivity of angiotensin II function within the PVN in chronic heart failure, although the mechanisms that are responsible are not clear. A recent report has suggested that angiotensin II elicits the release of nitric oxide in the PVN, and it antagonizes the sympathoexcitatory response to angiotensin II (43). Thus, in chronic heart failure, there is a decrease in nitrergic function, which would mean a reduction in its ability to counteract the excitatory actions of angiotensin II.
Inflammation in the PVN and Its Role in Regulating Renal Sympathetic Nerve Activity in Heart Failure
Following a myocardial infarction, there is activation of the immune system, and proinflammatory cytokines such as TNF-α, IL-6, and IL-1β are increased in the heart (42). This contributes to the increased circulating levels of the proinflammatory cytokines observed in plasma following damage to the heart. A myocardial infarction is a common cause of heart failure, and the inflammatory response that occurs after an infarction is detrimental to this condition since the plasma levels of cytokines have been shown to correlate with the degree of heart failure and increased mortality in this condition (60, 67, 68). In addition to the elevation of proinflammatory cytokine levels in the periphery in heart failure, there is evidence now emerging to suggest that they are also increased in the hypothalamic PVN (21, 24). Following a myocardial infarction, the increase in cytokine levels occurs within 24 h and they are elevated at the time heart failure is established, ∼6–8 wk after an infarction in the rat (24). This suggests that the cytokines may remain elevated during the development of heart failure, but this remains to be determined. The increased level of cytokines in the hypothalamus involves local production since mRNA levels of proinflammatory cytokines are elevated within the hypothalamic PVN (24).
Microinjection of proinflammatory cytokines, e.g., TNF-α, into the PVN elicits increases in renal sympathetic nerve activity (21, 32), most likely through the activation of PVN neurons that project to the spinal cord and the rostral ventrolateral medulla. It has been shown that TNF-α increased PVN neuronal activity via a PGE2-dependent mechanism (34). Similarly, IL-1β increased the activity of identified preautonomic parvocellular PVN neurons by a PGE2-dependent mechanism and involved attenuating GABAergic input (23). These sympathoexcitatory effects of proinflammatory cytokines may contribute to the abnormally elevated renal sympathetic nerve activity seen in heart failure.
The actions of TNF-α involves different intracellular signaling pathways, including NF-κB, P38-MAPK, and JNK. In the PVN of rats with heart failure induced by myocardial infarction, mRNA for NF-κB is increased (70). Intravenous and intracerebroventricular administration of etanercept, an antagonist of TNF-α receptors, to rats in heart failure attenuated the increases in proinflammatory cytokines, and the increase in mRNA for NF-κB within the PVN and also reduced renal sympathetic nerve activity (31, 34). Together, the results suggest that activation of NF-κB by TNF-α within the PVN may be an important contributor to the mechanisms ultimately responsible for the activation of neuronal activity in the PVN and the elevated renal sympathetic nerve activity seen in heart failure induced by myocardial infarction.
In heart failure induced by myocardial infarction, reactive oxygen species in the PVN may also play a role in the elevated renal sympathetic nerve activity observed in heart failure. The mRNA for NADPH oxidase isoforms 1, 2, and 4, the enzymes responsible for the generation of superoxide, have been found to be elevated in heart failure, concomitant with the increase in renal sympathetic nerve activity (25). Pentoxifylline, an inhibitor of phosphodiesterase and of the actions of TNF-α, administered systemically attenuated the increases in reactive oxygen species, NADPH oxidase, and attenuated the elevation in renal sympathetic nerve activity normally observed in heart failure induced by myocardial infarction (25). Scavengers of superoxide microinjected into the lateral brain ventricle elicits similar effects to peripherally administered pentoxifylline (32), which supports the suggestion that the increased levels of reactive oxygen species in heart failure may play a role in the elevated renal sympathetic nerve activity observed.
Proinflammatory cytokines, such as TNF-α and IL-1β, induce the production of reactive oxygen species, raising the possibility that reactive oxygen species may mediate some of the inflammatory action of TNF-α and IL-1β in the PVN in heart failure induced by myocardial infarction. The observation that administration of an antagonist of NF-κB into the lateral brain ventricles of rats with heart failure attenuates the increase in NADPH oxidase supports this (32). Although it should be noted that central administration of tempol, the superoxide scavenging agent, resulted in similar observations and also reduced the levels of proinflammatory cytokines in the hypothalamus of rats with heart failure, suggesting that interrupting the inflammatory process in the hypothalamus at an intermediary step may result in downregulation of all intermediaries (32).
Angiotensin II is proinflammatory and increases cytokines such as TNF-α and IL-1β in the plasma and induces the production of reactive oxygen species. Following myocardial infarction, angiotensin contributes to cardiac remodeling and hypertrophy, an action that involves the production of TNF-α (50). Circulating angiotensin II can also influence central inflammatory processes. Recent observations have shown that in the PVN the levels of cytokines, NF-κB, reactive oxygen species, and NADPH oxidase are increased by intravenous infusion of angiotensin II (33). These effects are centrally mediated since they are attenuated by the angiotensin II receptor antagonist losartan administered into the lateral brain ventricle (33). Furthermore, tempol and antagonism of NF-κB in the brain had similar effects, suggesting that reactive oxygen species and NF-κB contribute to the inflammatory process in the PVN induced by angiotensin II (33). Whether this occurs in heart failure is not known; however, there is a high concentration of angiotensin II receptors in the PVN, and this is increased in rats with heart failure induced by myocardial infarction (69). Furthermore, intracerebroventricular losartan reduced renal sympathetic nerve activity more in heart failure rats compared with the responses in sham animals and also restored toward normal the reflex renal sympathetic nerve responses elicited by stimulation of arterial baroreceptors and the cardiac sympathetic afferents (16, 69).
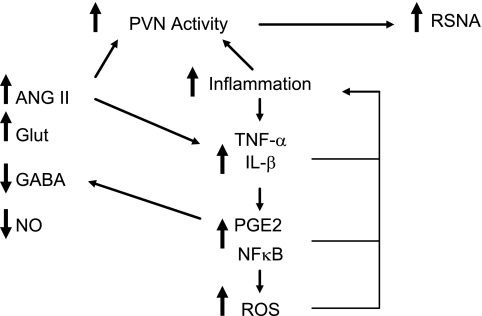
In conclusion, the hypothalamic PVN is an important regulator of renal sympathetic nerve activity and renal blood flow in response to disturbances in body temperature and in conditions in which body fluid and electrolyte homeostasis is disturbed such as in heart failure. The PVN is a key integrative region in the brain since it is able to integrate sympathetic as well as hormonal regulation of renal function. In regulating the renal cardiovascular responses elicited by hyperthermia, the PVN appears to be a critical central nervous system nucleus, and we hypothesize that dysfunction in this region could have important consequences for the susceptibility to heat stroke. In heart failure, there is an elevation in renal sympathetic nerve activity and the neuronal activity in the PVN is increased. As shown in Fig. 3, emerging evidence suggests that proinflammatory cytokines produced locally within the PVN play a role. The mechanisms contributing to cytokine activation of renal sympathetic nerve activity mediated through the PVN are likely to involve a PGE2-dependent inhibition of GABAergic neurons in the hypothalamus that results in disinhibition of neurons in the PVN that influence renal sympathetic nerve activity. The intracellular transduction pathways utilized include NF-κB. Angiotensin II acting locally within the PVN may also contribute to the increase in renal sympathetic nerve activity in heart failure, and this may be mediated, in part, by proinflammatory cytokines and reactive oxygen species. The issue of whether inflammation within the PVN is a key factor in the induction of elevated renal sympathetic nerve activity in heart failure induced by myocardial infarction is yet to be answered.
Fig. 3.
Schema summarizing the potential mechanisms that contribute to an increase in neuronal activity in the hypothalamic PVN and an elevation in RSNA in heart failure. The actions of excitatory neurotransmitters such as ANG II and glutamate (Glut) are enhanced while the inhibitory influences of GABA and nitric oxide (NO) are reduced. There is also an increase in proinflammatory cytrokines such as TNF-α and IL-1β. The actions of these may involve PGE2, the transcription factor NF-κB, and reactive oxygen species (ROS). These can act to further promote the inflammatory process. PGE2 may also contribute to the reduced GABA function. ANG II may act directly to increase neuronal activity as well as acting to increase proinflammatory cytokines.
GRANTS
This work was supported by the National Health and Medical Research Council and the National Heart Foundation of Australia.
DISCLOSURES
No conflicts of interest are declared by the author.
REFERENCES
- 1.Akama H, Mcgrath BP, Badoer E. Volume expansion fails to normally activate neural pathways in the brain of conscious rabbits with heart failure. J Autonom Nerv Syst 73: 54–62, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Bachtell RK, Tsivkovskaia NO, Ryabinin AE. Identification of temperature-sensitive neural circuits in mice using c-Fos expression mapping. Brain Res 960: 157–164, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Badoer E. Cardiovascular role of parvocellular neurones in the paraventricular nucleus of the hypothalamus. News Physiol Sci 11: 43–47, 1996 [Google Scholar]
- 4.Badoer E, Moguilevski V, Mcgrath BP. Cardiac afferents play the dominant role in renal nerve inhibition elicited by volume expansion in the rabbit. Am J Physiol Regul Integr Comp Physiol 274: R383–R388, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Bello-Reuss E, Trevino DL, Gottschalk CW. Effect of renal sympathetic nerve stimulation on proximal water and sodium reabsorption. J Clin Invest 57: 1104–1107, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bratincsak A, Palkovits M. Activation of brain areas in rat following warm and cold ambient exposure. Neuroscience 127: 385–397, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Cechetto D, Saper CB. Neurochemical organisation of the hypothalamic projection to the spinal cord in the rat. J Comp Neurol 272: 579–604, 1988 [DOI] [PubMed] [Google Scholar]
- 8.Cham J, Badoer E. Exposure to a hot environment can activate rostral ventrolateral medulla-projecting neurones in the hypothalamic paraventricular nucleus in conscious rats. Exp Physiol 93: 64–74, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Cham J, Badoer E. Hypothalamic paraventricular nucleus is critical for renal vasoconstriction elicited by elevations in body temperature. Am J Physiol Renal Physiol 294: F309–F315, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Cham JL, Klein R, Owens NC, Mathai ML, McKinley MJ, Badoer E. Activation of spinally projecting and nitrergic neurons in the PVN following heat exposure. Am J Physiol Regul Integr Comp Physiol 291: R91–R101, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Chen F, Dworak M, Wang Y, Cham J, Badoer E. Role of the hypothalamic PVN in the reflex reduction in mesenteric blood flow elicited by hyperthermia. Am J Physiol Regul Integr Comp Physiol 295: R1874–R1881, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Clement DL, Pelletier CL, Shepherd JT. Role of vagal afferents in the control of renal sympathetic nerve activity in the rabbit. Circ Res 31: 824–830, 1972 [DOI] [PubMed] [Google Scholar]
- 13.Deering J, Coote JH. Paraventricular neurons elicit a volume expansion-like change in sympathetic nerves to the heart and kidney in the rabbit. Exp Physiol 85: 177–186, 2000 [PubMed] [Google Scholar]
- 14.DiBona GF. Functionally specific renal sympathetic nerve fibers: role in cardiovascular regulation. Am J Hypertens 14: 163S–170S, 2001 [DOI] [PubMed] [Google Scholar]
- 15.DiBona GF. Neurogenic regulation of renal tubular sodium reabsorption. Am J Physiol Renal Fluid Electrolyte Physiol 233: F71–F81, 1977 [DOI] [PubMed] [Google Scholar]
- 16.DiBona GF, Jones SY, Brooks VL. ANG II receptor blockade and arterial baroreflex regulation of renal nerve activity in cardiac failure. Am J Physiol Regul Integr Comp Physiol 269: R1189–R1196, 1995 [DOI] [PubMed] [Google Scholar]
- 17.DiBona GF, Kopp UC. Neural control of renal function. Physiol Rev 77: 75–197, 1997 [DOI] [PubMed] [Google Scholar]
- 18.DiBona GF, Sawin LL. Effect of renal denervation on dynamic autoregulation of renal blood flow. Am J Physiol Renal Physiol 286: F1209–F1218, 2004 [DOI] [PubMed] [Google Scholar]
- 19.DiBona GF, Sawin LL. Increased renal nerve activity in cardiac failure arterial vs. cardiac baroreflex impairment. Am J Physiol Regul Integr Comp Physiol 268: R112–R116, 1995 [DOI] [PubMed] [Google Scholar]
- 20.DiBona GF, Sawin LL. Renal nerve activity in conscious rats during volume expansion and depletion. Am J Physiol Renal Fluid Electrolyte Physiol 248: F15–F23, 1985 [DOI] [PubMed] [Google Scholar]
- 21.Felder RB, Francis J, Zhang ZH, Wei SG, Weiss RM, Johnson AK. Heart failure and the brain: new perspectives. Am J Physiol Regul Integr Comp Physiol 284: R259–R276, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Ferguson AV, Latchford KJ, Samson WK. The paraventricular nucleus of the hypothalamus—a potential target for integrative treatment of autonomic dysfunction. Expert Opin Ther Targets 12: 717–727, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferri CC, Ferguson AV. Prostaglandin E2 mediates cellular effects of interleukin-1β on parvocellular neurones in the paraventricular nucleus of the hypothalamus. J Neuroendocrinol 17: 498–508, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Francis J, Chu Y, Johnson AK, Weiss RM, Felder RB. Acute myocardial infarction induces hypothalamic cytokine synthesis. Am J Physiol Heart Circ Physiol 286: H2264–H2271, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Guggilam A, Haque M, Kerut EK, McIlwain E, Lucchesi P, Seghal I, Francis J. TNF-α blockade decreases oxidative stress in the paraventricular nucleus and attenuates sympathoexcitation in heart failure rats. Am J Physiol Heart Circ Physiol 293: H599–H609, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Haselton JR, Goering J, Patel KP. Parvocellular neurons of the paraventricular nucleus are involved in the reduction in renal nerve discharge during isotonic volume expansion. J Autonom Nerv Syst 50: 1–11, 1994 [DOI] [PubMed] [Google Scholar]
- 27.Haselton JR, Vari RC. Neuronal cell bodies in paraventricular nucleus affect renal hemodynamics and excretion via the renal nerves. Am J Physiol Regul Integr Comp Physiol 275: R1334–R1342, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Hubschle T, Mathai M, McKinley M, Oldfield B. Multisynaptic neuronal pathways from the submandibular and sublingual glands to the lamina terminalis in the rat: a model for the role of the lamina terminalis in the control of osmo- and thermoregulatory behaviour. Clinl Exper Pharmacol Physiol 28: 558–569, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration With the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation 112: e154–e235, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Inenaga K, Osaka T, Yamashita H. Thermosensitivity of neurons in the paraventricular nucleus of the rat slice preparation. Brain Res 424: 126–132, 1987 [DOI] [PubMed] [Google Scholar]
- 31.Kang YM, He RL, Yang LM, Qin DN, Guggilam A, Elks C, Yan N, Guo Z, Francis J. Brain tumour necrosis factor-α modulates neurotransmitters in hypothalamic paraventricular nucleus in heart failure. Cardiovasc Res 83: 737–746, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kang YM, Ma Y, Elks C, Zheng JP, Yang ZM, Francis J. Cross-talk between cytokines and renin-angiotensin in hypothalamic paraventricular nucleus in heart failure: role of nuclear factor-κB. Cardiovasc Res 79: 671–678, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kang YM, Ma Y, Zheng JP, Elks C, Sriramula S, Yang ZM, Francis J. Brain nuclear factor-kappa B activation contributes to neurohumoral excitation in angiotensin II-induced hypertension. Cardiovasc Res 82: 503–512, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kang Y, Zhang ZH, Johnson R, Yu Y, Beltz T, Johnson AK, Weiss RM, Felder RB. Novel effect of mineralocorticoid receptor antagonism to reduce proinflammatory cytokines and hypothalamic activation in rats with ischemia-induced heart failure. Circ Res 99: 758–66, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Kannan H, Hayashida Y, Yamashita H. Increase in sympathetic outflow by paraventricular nucleus stimulation in awake rats. Am J Physiol Regul Integr Comp Physiol 256: R1325–R1330, 1989 [DOI] [PubMed] [Google Scholar]
- 36.Kantzides A, Owens NC, De Matteo R, Badoer E. Right atrial stretch activates neurons in the autonomic brain regions that project to the rostral ventrolateral medulla. Neuroscience 133: 775–786, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Kenney MJ, Musch TI, Weiss ML. Renal sympathetic nerve regulation to heating is altered in rats with heart failure. Am J Physiol Heart Circ Physiol 280: H2868–H2875, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Kenney MJ, Pickar JG, Weiss M, Saindon CS, Fels RJ. Effects of midbrain and spinal cord transections on sympathetic nerve responses to heating. Am J Physiol Regul Integr Comp Physiol 278: R1329–R1338, 2000 [DOI] [PubMed] [Google Scholar]
- 39.Kregel K, Wall P, Gisolfi C. Peripheral vascular responses to hyperthermia in the rat. J Appl Physiol 64: 2582–2588, 1988 [DOI] [PubMed] [Google Scholar]
- 40.La Grange RG, Sloop CH, Schmid HE. Selective stimulation of renal nerves in the anaesthetised dog. Circ Res 33: 704–712, 1973 [DOI] [PubMed] [Google Scholar]
- 41.Lechan RM, Fekete C. The TRH neuron: a hypothalamic integrator of energy metabolism. Prog Brain Res 153: 209–235, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Levine B, Kalman J, Mayer L, Fillit HM, Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med 323: 236–241, 1990 [DOI] [PubMed] [Google Scholar]
- 43.Li Y, Wang W, Mayhan WG, Patel KP. Angiotensin-mediated increase in renal sympathetic nerve discharge within the PVN: role of nitric oxide. Am J Physiol Regul Integr Comp Physiol 290: R1035–R1043, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Li Y, Zhang W, Stern JE. Nitric oxide inhibits the firing activity of hypothalamic paraventricular neurons that innervate the medulla oblongata: role of GABA. Neuroscience 118: 585–601, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Li YF, Cornish KG, Patel KP. Alteration of NMDA NR1 receptors within the paraventricular nucleus of hypothalamus in rats with heart failure. Circ Res 93: 990–997, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Li YF, Jackson KL, Stern JE, Rabeler B, Patel KP. Interaction between glutamate and GABA systems in the integration of sympathetic outflow by the paraventricular nucleus of the hypothalamus. Am J Physiol Heart Circ Physiol 291: H2847–H2856, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Lovick TA, Malpas S, Mahony MT. Renal vasodilatation in response to acute volume load is attenuated following lesions of parvocellular neurones in the paraventricular nucleus in rats. J Autonom Nerv Syst 43: 247–255, 1993 [DOI] [PubMed] [Google Scholar]
- 48.Malpas SC, Coote JH. Role of vasopressin in sympathetic response to paraventricular nucleus stimulation in anesthetized rats. Am J Physiol Regul Integr Comp Physiol 266: R228–R236, 1994 [DOI] [PubMed] [Google Scholar]
- 49.Mancia G, Shepherd JT, Donald DE. Role of cardiac pulmonary and carotid mechanoreceptors in the control of hindlimb and renal circulation in dogs. Circ Res 37: 200–208, 1975 [DOI] [PubMed] [Google Scholar]
- 50.Mann DL. Angiotensin II as an inflammatory mediator: Evolving concepts in the role of the renin angiotensin system in the failing heart. Cardiovasc Drugs Ther 16: 7–9, 2002 [DOI] [PubMed] [Google Scholar]
- 51.Morita H, Vatner SF. Effects of volume expansion on renal nerve activity, renal blood flow, and sodium and water excretion in conscious dogs. Am J Physiol Renal Fluid Electrolyte Physiol 249: F680–F687, 1985 [DOI] [PubMed] [Google Scholar]
- 52.Ng CW, De Matteo R, Badoer E. Effect of muscimol and l-NAME in the PVN on the RSNA response to volume expansion in conscious rabbits. Am J Physiol Renal Physiol 287: F739–F746, 2004 [DOI] [PubMed] [Google Scholar]
- 53.Oldfield B, Giles ME, Watson A, Anderson CR, Colvill L, McKinley MJ. The neurochemical characterisation of hypothalamic pathways projecting to brown adipose tissue in the rat. Neuroscience 110: 515–526, 2002 [DOI] [PubMed] [Google Scholar]
- 54.Packer M. Neurohormonal interactions and adaptations in congestive heart failure. Circulation 77: 721–730, 1988 [DOI] [PubMed] [Google Scholar]
- 55.Patel KP. Role of paraventricular nucleus in mediating sympathetic outflow in heart failure. Heart Fail Rev 5: 73–86, 2000 [DOI] [PubMed] [Google Scholar]
- 56.Patel KP, Zhang PI, Krukoff TL. Alterations in brain hexokinase activity associated with heart failure in rats. Am J Physiol Regul Integr Comp Physiol 265: R923–R928, 1993 [DOI] [PubMed] [Google Scholar]
- 57.Patel KP, Zhang K, Kenney MJ, Weiss M, Mayhan WG. Neuronal expression of Fos protein in the hypothalamus of rats with heart failure. Brain Res 865: 27–34, 2000 [DOI] [PubMed] [Google Scholar]
- 58.Patel KP, Zhang K, Zucker IH, Krukoff TL. Decreased gene expression of neuronal nitric oxide synthase in hypothalamus and brainstem of rats in heart failure. Brain Res 734: 109–115, 1996 [PubMed] [Google Scholar]
- 59.Pyner S. Neurochemistry of the paraventricular nucleus of the hypothalamus: implications for cardiovascular regulation. J Chem Neuroanat 38: 197–208, 2009 [DOI] [PubMed] [Google Scholar]
- 60.Rauchhaus M, Doehner W, Francis DP, Davos C, Kemp M, Liebenthal C, Niebauer J, Hooper J, Volk HD, Coats AJ, Anker SD. Plasma cytokine parameters and mortality in patients with chronic heart failure. Circulation 102: 3060–3067, 2000 [DOI] [PubMed] [Google Scholar]
- 61.Shafton AD, Ryan AR, Badoer E. Neurons in the hypothalamic paraventricular nucleus send collaterals to the spinal cord and to the rostral ventrolateral medulla in the rat. Brain Res 801: 239–243, 1998 [DOI] [PubMed] [Google Scholar]
- 62.Smith JE, Jansen AS, Gilbey MP, Loewy AD. CNS cell groups projecting to sympathetic outflow of tail artery: neural circuits involved in heat loss in the rat. Brain Res 786: 153–164, 1998 [DOI] [PubMed] [Google Scholar]
- 63.Stern JE, Li Y, Zhang W. Nitric oxide: a local signalling molecule controlling the activity of pre-autonomic neurones in the paraventricular nucleus of the hypothalamus. Acta Physiol Scand 177: 37–42, 2003 [DOI] [PubMed] [Google Scholar]
- 64.Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci 6: 269–324, 1983 [DOI] [PubMed] [Google Scholar]
- 65.Swanson LW, Sawchenko PE, Wiegand SJ, Price JL. Separate neurons in the paraventricular nucleus project to the median eminence and to the medulla or spinal cord. Brain Res 198: 190–195, 1980 [DOI] [PubMed] [Google Scholar]
- 66.Tagawa T, Dampney RA. AT1 receptors mediate excitatory inputs to rostral ventrolateral medulla pressor neurons from hypothalamus. Hypertension 34: 1301–1307, 1999 [DOI] [PubMed] [Google Scholar]
- 67.Torre-Amione G, Kapadia S, Benedict C, Oral H, Young JB, Mann DL. Proinflammatory cytokine levels in patients with depressed left ventricular ejection fraction: a report from the Studies of Left Ventricular Dysfunction (SOLVD). J Am Coll Cardiol 27: 1201–1206, 1996 [DOI] [PubMed] [Google Scholar]
- 68.Torre-Amione G, Kapadia S, Lee J, Durand JB, Bies RD, Young JB, Mann DL. Tumor necrosis factor-alpha and tumor necrosis factor receptors in the failing human heart. Circulation 93: 704–711, 1996 [DOI] [PubMed] [Google Scholar]
- 69.Wang HJ, Zhang F, Zhang Y, Gao XY, Zhu GQ. AT1 receptor in paraventricular nucleus mediates the enhanced cardiac sympathetic afferent reflex in rats with chronic heart failure. Autonom Neurosci 121: 56–63, 2005 [DOI] [PubMed] [Google Scholar]
- 70.Yu Y, Kang YM, Zhang ZH, Wei SG, Chu Y, Weiss RM, Felder RB. Increased cyclooxygenase-2 expression in hypothalamic paraventricular nucleus in rats with heart failure: role of nuclear factor κB. Hypertension 49: 511–518, 2007 [DOI] [PubMed] [Google Scholar]
- 71.Zhang K, Li YF, Patel KP. Blunted nitric oxide-mediated inhibition of renal nerve discharge within PVN of rats with heart failure. Am J Physiol Heart Circ Physiol 281: H995–H1004, 2001 [DOI] [PubMed] [Google Scholar]
- 72.Zhang K, Li YF, Patel KP. Reduced endogenous GABA-mediated inhibition in the PVN of renal nerve discharge in rats with heart failure. Am J Physiol Regul Integr Comp Physiol 282: R1006–R1015, 2002 [DOI] [PubMed] [Google Scholar]
- 73.Zhang K, Patel KP. Effect of nitric oxide within the paraventricular nucleus on renal sympathetic nerve discharge: role of GABA. Am J Physiol Regul Integr Comp Physiol 275: R728–R734, 1998 [DOI] [PubMed] [Google Scholar]
- 74.Zhang K, Zucker IH, Patel KP. Altered number of diaphorase (NOS) positive neurons in the hypothalamus of rats with heart failure. Brain Res 786: 219–225, 1998 [DOI] [PubMed] [Google Scholar]
- 75.Zheng H, LIYF , Wang W, Patel KP. Enhanced angiotensin-mediated excitation of renal sympathetic nerve activity within the paraventricular nucleus of anesthetized rats with heart failure. Am J Physiol Regul Integr Comp Physiol 297: R1364–R1374, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]