Abstract
We showed that luminal flow increases net superoxide (O2−) production via NADPH oxidase in thick ascending limbs. Protein kinase C (PKC) activates NADPH oxidase activity in phagocytes, cardiomyocytes, aortic endothelial cells, vascular smooth muscle cells, and renal mesangial cells. However, the flow-activated pathway that induces NADPH oxidase activity in thick ascending limbs is unclear. We hypothesized that PKC mediates flow-stimulated net O2− production by thick ascending limbs. Initiation of flow (20 nl/min) increased net O2− production from 4 ± 1 to 61 ± 12 AU/s (P < 0.007; n = 5). The NADPH oxidase inhibitor apocynin completely blocked the flow-induced increase in net O2− production (2 ± 1 vs. 1 ± 1 AU/s; P > 0.05; n = 5). Flow-stimulated O2− was also blocked in p47phox-deficient mice. We measured flow-stimulated PKC activity with a fluorescence resonance energy transfer (FRET)-based membrane-targeted PKC activity reporter and found that the FRET ratio increased from 0.87 ± 0.02 to 0.96 ± 0.04 AU (P < 0.05; n = 6). In the absence of flow, the PKC activator phorbol 12-myristate 13-acetate (200 nM) enhanced net O2− production from 5 ± 2 to 92 ± 6 AU/s (P < 0.001; n = 6). The PKC-α- and βI-selective inhibitor Gö 6976 (100 nM) decreased flow-stimulated net O2− production from 54 ± 15 to 2 ± 1 AU/s (P < 0.04; n = 5). Flow-induced net O2− production was inhibited in thick ascending limbs transduced with dominant-negative (dn)PKC-α but not dnPKCβI or LacZ (Δ = 11 ± 3 AU/s for dnPKCα, 55 ± 7 AU/s for dnPKCβI, and 63 ± 7 AU/s for LacZ; P < 0.001; n = 6). We concluded that flow stimulates net O2− production in thick ascending limbs via PKC-α-mediated activation of NADPH oxidase.
Keywords: reactive oxygen species, protein kinases, NADPH oxidase, luminal flow
superoxide (O2−) is an important regulator of kidney function (43, 47). This reactive oxygen species decreases urinary volume (22) and sodium excretion (27, 47) and may be implicated in salt-sensitive hypertension (22, 28, 39). The thick ascending limb of the loop of Henle contributes to inappropriate NaCl retention in salt-sensitive hypertension (19, 21, 36), and O2− increases NaCl transport (20, 31) in this nephron segment. We previously showed that increased luminal flow stimulates net O2− production by thick ascending limbs (10, 16, 17). However, the signaling pathway by which flow activates NADPH oxidase, the source of O2− in the thick ascending limb (16, 24, 25, 47), is unknown.
The protein kinase C (PKC) family is comprised of 12 related serine/threonine kinases (5). PKC has been shown to activate NADPH oxidase in several types of cells, including phagocytes (6), cardiomyocytes (42), aortic endothelial cells (13, 18), vascular smooth muscle cells (12, 37), and renal mesangial cells (41). Various PKC isoforms have been shown to stimulate O2− production, including α (41), β (23), δ (2), ε (42), and ζ (4). In addition, several PKC isoforms are activated in response to cellular stretch (34) and shear stress (35), which are mechanical stimuli associated with increased luminal flow.
The thick ascending limb expresses several PKC isoforms, including α, β, γ, δ, ε, θ, λ, ι, and ζ (38). PKC has been shown to affect several different transporters in the thick ascending limb. This kinase mediates the stimulatory effect of angiotensin II on Na+-K+-2Cl− cotransporter activity (1) and stimulates NaHCO3 absorption (11). In addition, a PKC-dependent pathway mediates inhibition of apical K+ channel activity caused by high concentrations of prostaglandin E2 (26). However, the role of PKC in flow-enhanced O2− production as well as the isoform(s) involved are unknown.
We hypothesized that luminal flow stimulates net O2− production in the thick ascending limb via activation of PKC-α and consequently NADPH oxidase. To test this hypothesis, we examined the effect of acutely increasing luminal flow on net O2− production and PKC activity in isolated thick ascending limbs in the absence and presence of NADPH oxidase and PKC inhibitors. Dominant-negative (dn) mutants of specific PKC isoforms were also utilized. Our findings indicate that PKC-α mediates flow-stimulated net O2− production in the thick ascending limb.
MATERIALS AND METHODS
Chemicals and solutions.
Dihydroethidium was obtained from Molecular Probes (Eugene, OR). Apocynin (acetovanillone), hypoxanthine, xanthine oxidase, N,N′-dimethyl-9,9′-biacridinium dinitrate (lucigenin), 4,5-dihydroxy-1,3-benzenedisulfonic acid (tiron), and phorbol 12-myristate 13-acetate (PMA) were obtained from Sigma (St. Louis, MO). Gö 6976 was purchased from Biomol International (Plymouth Meeting, PA). The physiological saline used to perfuse and bathe the thick ascending limbs contained (in mM) 130 NaCl, 2.5 NaH2PO4, 4 KCl, 1.2 MgSO4, 6 l-alanine, 1 trisodium citrate, 5.5 glucose, 2 calcium dilactate, and 10 HEPES, pH 7.4 at 37°C. The osmolarity of this solution should be 290 ± 3 mosM; slight adjustments (±3 mosM), when necessary, were made by adding either H2O or NaCl.
Isolation and perfusion of thick ascending limbs.
All protocols involving animals were approved by the Institutional Animal Care and Use Committee (IACUC) in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Male Sprague-Dawley rats (Charles River Breeding Laboratories, Wilmington, MA) were maintained on a diet containing 0.22% sodium and 1.1% potassium (Purina, Richmond, IN) for at least 5 days. Male C57BL/6J and p47phox-deficient mice were from the Jackson Laboratory (Bar Harbor, ME). On the day of the experiment, rats weighing 120–150 g or 5- to 6-wk-old mice were anesthetized intraperitoneally with ketamine and xylazine (100 and 20 mg/kg body wt, respectively). The abdominal cavity was opened and the left kidney was bathed in ice-cold saline and removed. Coronal slices were placed in oxygenated physiological saline. Medullary thick ascending limbs were dissected from the outer medulla under a stereomicroscope at 4–10°C. Tubules measuring 0.5–1.0 mm were transferred to a temperature-regulated chamber (37 ± 1°C) and perfused using concentric glass pipettes as described previously (9). We perfused tubules at a physiological flow rate of 20 nl/min using a nanoliter syringe pump (Harvard Apparatus, Holliston, MA).
Adenovirus construction.
MyrPalm-CKAR (Addgene plasmid 14862), a membrane-targeted fluorescence resonance energy transfer (FRET)-based PKC activity reporter developed by Dr. A. Newton at the University of California at San Diego (40), was obtained from Addgene (Cambridge, MA). The dnPKCα and βI plasmids were kindly provided by Dr. J.-W. Soh at the Biomedical Research Center for Signal Transduction Networks, Incheon, Korea (www.pkclab.org). These genes were subcloned into the adenoviral shuttle vector pVQAd5CMV K-NpA, which contains the cytomegalovirus (CMV) promoter, and sent to ViraQuest (North Liberty, IA) for adenovirus production. VQAd5 CMV ntLacZ purchased from ViraQuest served as a negative control.
Adenoviral gene delivery.
Rat medullary thick ascending limbs were transduced in vivo with an adenovirus carrying 1) MyrPalm-CKAR, 2) dnPKCα, 3) dnPKCβI, or 4) lacZ as reported previously (32, 33). Briefly, the left kidney of a rat weighing 85–105 g was exposed via a flank incision, and then the left renal artery and vein were clamped. Four 20-μl injections of virus (2–5 × 1010 PFU/ml) at a rate of 20 μl/min were made by inserting the needle perpendicular to the renal capsule, parallel to the medullary rays and directed toward the medulla. Injection points were along the longitudinal axis of the kidney separated by 2.5 mm. The clamp was released and the kidney was returned to the abdominal cavity. The muscle was sutured and the skin was closed with staples. Medullary thick ascending limbs were dissected 3–5 days after injection.
Measurement of O2− using dihydroethidium.
Isolated thick ascending limbs were loaded with 10 μM dihydroethidium in physiological saline for 15 min and then washed in dye-free solution for 20 min. O2− converts dihydroethidium to oxyethidium (7, 46). Oxyethidium and dihydroethidium were excited using 488- and 365-nm light, respectively. Emitted fluorescence between 520 and 600 nm (oxyethidium) and 400 and 450 nm (dihydroethidium) was imaged digitally with an image intensifier connected a CCD camera and recorded with the Metafluor system (Universal Imaging, West Chester, PA). Fluorescence was measured from regions of interest every 5 s for 12 measurements. Regression analysis of the fluorescence ratios for each measurement period was performed and differences in slopes were evaluated.
O2− concentration drives the reaction between dihydroethidium and O2−. However, O2− concentration is determined by both production and degradation. An increased rate of change of the oxyethidium/dihydroethidium fluorescence ratio is a measure of increased “net O2− production” (production − degradation), although it is commonly referred to as O2− production. Given that net O2− production represents production − degradation, it may increase when degradation is reduced or production is enhanced without indicating whether the former, latter, or both has/have occurred. Therefore, we will refer to O2− measurements in this study as net O2− production.
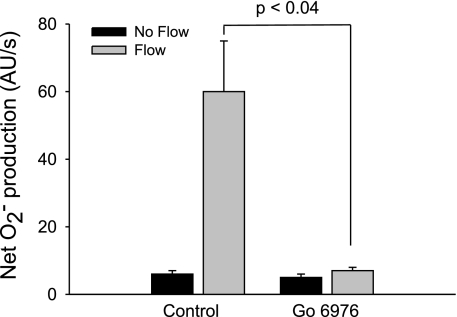
The general format for all protocols was that O2− was measured in a tubule in the absence of luminal flow (period 1) and then again 5 min after luminal flow was initiated (period 2). For Fig. 6, an additional set of measurements was performed. After period 2, flow was stopped. In the same tubule, the PKC-α- and βI-selective inhibitor Gö 6976 was added. After 15 min, O2− measurements were recorded again in the absence and presence of flow.
Fig. 6.
Effect of 100 nM Gö 6976, a PKC-α- and -βI-selective inhibitor, on flow-stimulated net O2− production in thick ascending limbs (n = 5). The flow rate was 20 nl/min.
Measurement of O2− production in a cell-free system.
To generate O2−, a 1-ml solution of xanthine oxidase (10 mU; 0.9 U/mg) and 5 μM lucigenin in physiological saline was placed in a glass tube and incubated for 10 min at 37°C. Hypoxanthine (0.5 mM final concentration) was added, and the solution was incubated for 5 min. The tube was then placed in a luminometer (model FB12/Sirius, Zylux) and maintained at 37°C. Luminescence was measured for a 5-min control period, and then apocynin (10 μM final concentration) was added and measurements were taken for 5 min. Then, the O2− scavenger tiron was added at a final concentration of 10 mM, and the measurements were repeated. The difference in average luminescence between periods with and without tiron was used to calculate the luminescence produced by O2−.
Measurement of PKC activity using MyrPalm-CKAR.
PKC activity was measured using a FRET-based membrane-targeted PKC activity reporter, MyrPalm-CKAR (40), which contains the FRET pair cyano fluorescent protein (CFP) and yellow fluorescent protein (YFP). Isolated tubules from rat kidneys transduced with MyrPalm-CKAR were equilibrated for 20 min. Using a laser-scanning confocal microscope system (VisiTech International), MyrPalm-CKAR was excited at 442 nm (CFP); CFP and YFP emissions were measured at 480 ± 20 and 540 ± 20 nm, respectively, and the CFP/YFP emission ratio was calculated. Data were obtained once every minute for 5 min (control period). Then, flow (20 nl/min) was initiated and measurements were taken once every minute for 15 min (experimental period). The mean of the 5-min control period was compared with that of five consecutive measurements during the peak of response in the experimental period. Increases in CFP/YFP ratio were taken as a measure of increases in PKC activity. Control experiments were performed to show that emissions from YFP were due to FRET (14). Time control experiments were also performed. The same microscope settings (laser intensity, contrast, brightness, resolution, and exposure time) were used for all data.
Statistical analysis.
Results are expressed as means ± SE. Statistical analysis was performed by the Henry Ford Hospital Department of Biostatistics and Epidemiology. Data were evaluated using Student's t-test for paired experiments (see Figs. 1–5), Student's t-test of paired differences (see Fig. 6), or ANOVA of the three differences (see Fig. 7), taking P < 0.05 as significant.
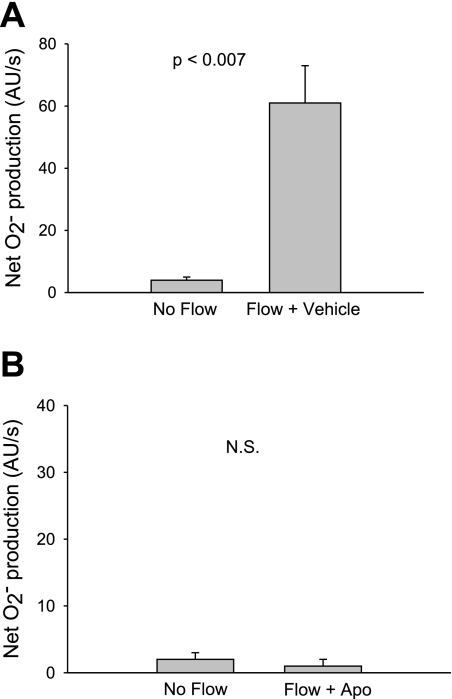
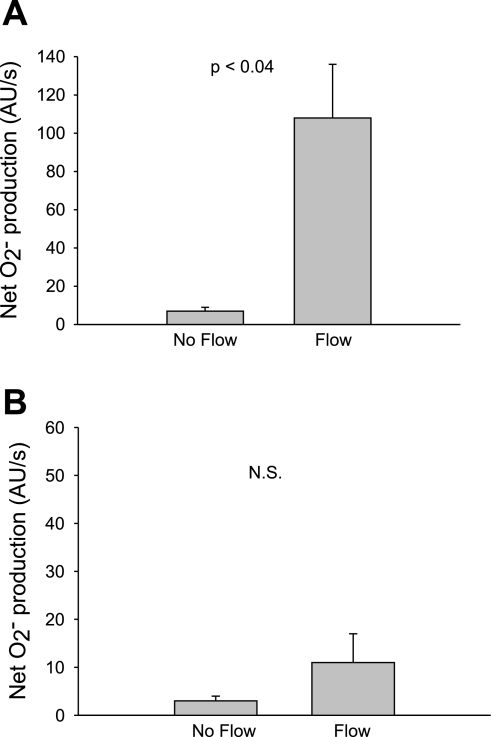
Fig. 1.
Effect of flow on net O2− production by thick ascending limbs in the absence and presence of the NADPH oxidase inhibitor apocynin. A: effect of flow in the absence of apocynin (n = 5). B: effect of flow in the presence of 10 μM apocynin (Apo); n = 5. The flow rate was 20 nl/min.
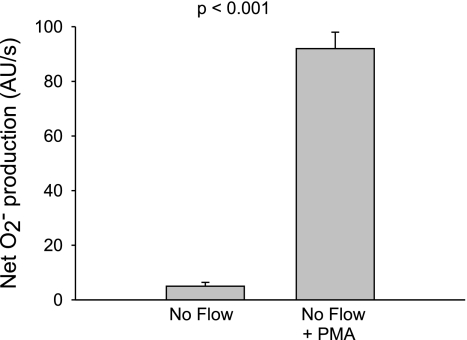
Fig. 5.
Effect of the PKC activator phorbol 12-myristate 13-acetate (PMA; 200 nM) on net O2− production by thick ascending limbs in the absence of flow (n = 5).
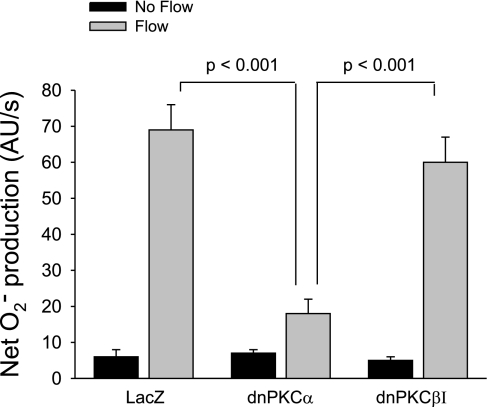
Fig. 7.
Effect of flow on net O2− production in thick ascending limbs transduced with 1) LacZ, 2) dominant-negative (dn)PKCα, and 3) dnPKCβI (n = 6 per group). Tubules were perfused at a rate of 20 nl/min.
RESULTS
To begin to test our hypothesis, we first subjected isolated thick ascending limbs to enhanced luminal flow and measured the net rate of O2− production using the dye dihydroethidium. Figure 1A shows that in tubules in which there was no luminal flow, net O2− production was 4 ± 1 AU/s. Flow (20 nl/min) increased net O2− production to 61 ± 12 AU/s (P < 0.007; n = 5).
To study whether this increase was dependent on activation of NADPH oxidase, we used apocynin (Fig. 1B). In the presence of 10 μM apocynin and in the absence of flow, net O2− production was 2 ± 1 AU/s. After flow was started, it was 1 ± 1 AU/s (P > 0.05; n = 5).
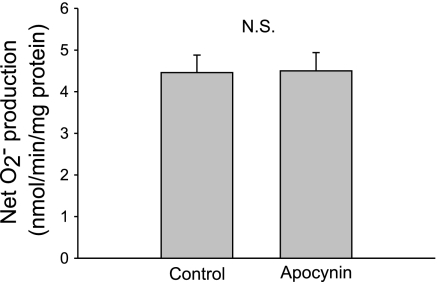
A high concentration of apocynin (1 mM) has been shown to reduce O2− by acting as a scavenger (15). Therefore, the effect of apocynin we observed (Fig. 1B) could be due to scavenging rather than inhibition of NADPH oxidase. To rule out this possibility, we used a lucigenin-based assay to measure O2− generated by xanthine oxidase/hypoxanthine to study the effect of apocynin at the concentration used in our study. In the absence of apocynin, net O2− production was 4.46 ± 0.42 nmol O2−·min−1·mg−1 xanthine oxidase. Net O2− production did not change significantly after 10 μM apocynin was added (4.50 ± 0.44 nmol O2−·min−1·mg−1 xanthine oxidase; P > 0.05; n = 5; Fig. 2).
Fig. 2.
Effect of 10 μM Apo on net O2− generated from xanthine oxidase and hypoxanthine in a cell-free system (n = 5).
To further demonstrate that NADPH oxidase is the source of flow-induced O2−, we used a nonpharmacological approach in which we examined the effect of flow on O2− in wild-type and p47phox-deficient mice. Figure 3A shows that in wild-type C57BL/6J mice, net O2− production in the absence of flow was 7 ± 2 AU/s. An increase in flow caused net O2− production to rise to 108 ± 28 AU/s (P < 0.04; n = 4). In mice deficient in p47phox net O2− production in the absence of flow was 3 ± 1 AU/s, and it did not change significantly when flow was increased (11 ± 6 AU/s; P > 0.05; n = 4; Fig. 3B). Taken together, these data indicate that luminal flow stimulates net O2− production by NADPH oxidase.
Fig. 3.
Effect of flow on net O2− production by thick ascending limbs in wild-type C57BL/6J and p47phox-deficient mice. A: effect of flow in wild-type C57BL/6J mice (n = 4). B: effect of flow in mice deficient in the NADPH oxidase subunit p47phox (n = 4). The flow rate was 20 nl/min.
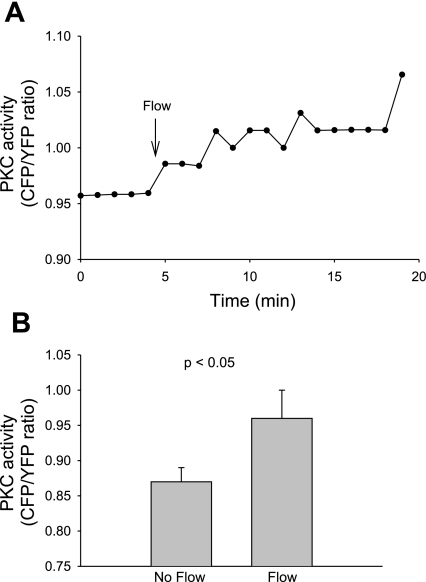
To study whether flow-induced increases in net O2− production in the thick ascending limb involve PKC, we examined the effect of flow on PKC activity in isolated thick ascending limbs from rat kidneys transduced with the membrane-targeted FRET-based PKC activity reporter MyrPalm-CKAR (40). A representative experiment (Fig. 4A) shows that in the absence of flow, basal PKC activity was 0.96 AU. After flow was initiated, it rose to an average of 1.03 AU. Increasing flow raised mean PKC activity from 0.87 ± 0.02 to 0.96 ± 0.04 AU (P < 0.05; n = 6; Fig. 4B).
Fig. 4.
Effect of flow on PKC activity in thick ascending limbs transduced with the fluorescence resonance energy transfer (FRET)-based membrane-targeted PKC activity reporter, MyrPalm-CKAR. A: representative experiment showing the flow-stimulated change in the cyano fluorescent protein (CYP)/yellow fluorescent protein (YFP) emission ratio. Flow was increased from 0 to 20 nl/min as indicated by the arrow. B: mean data (n = 6).
PKC stimulates NADPH oxidase activity and thus O2− production in several types of cells. To study whether activation of PKC alone can stimulate net O2− production in thick ascending limbs, we used the PKC activator PMA in the absence of flow (Fig. 5). Adding 200 nM PMA in the absence of flow caused an increase in net O2− production from 5 ± 2 to 92 ± 6 AU/s (P < 0.001; n = 5). Thus, PKC activation stimulates net O2− production in the thick ascending limb.
Changes in PKC activity measured by MyrPalm-CKAR are primarily due to diacylglycerol-activated and Ca2+-dependent PKCs (the conventional PKCs α, βI, βII, and γ). Therefore, we next examined the specific PKC isoform(s) involved in flow-stimulated O2− production by using the PKC-α, βI-selective inhibitor Gö 6976 (Fig. 6). In the absence of flow, net O2− production was 6 ± 1 AU/s. Initiation of flow caused net O2− production to increase to 60 ± 15 AU/s. In the presence of 100 nM Gö 6976 but no flow, net O2− production was 5 ± 1 AU/s. After flow was started in the presence of 100 nM Gö 6976, net O2− production was 7 ± 1 AU/s. Thus, Gö 6976 blocked flow-induced net O2− production (Δ = 54 ± 15 vs. 2 ± 1 AU/s; P < 0.04; n = 5). These data indicate that virtually all of the net O2− production stimulated by flow is mediated by PKC-α and/or PKC-βI.
To study which of these two isoforms is responsible for flow-induced net O2− production, we transduced tubules with dnPKCα or dnPKCβI. Tubules tranduced with LacZ served as controls and exhibited an increase from 6 ± 2 to 69 ± 7 AU/s in net O2− production after flow was applied (Fig. 7). In contrast, this flow-induced increase was attenuated in dnPKCα-transduced tubules (7 ± 1 AU/s in the absence of flow vs. 18 ± 4 AU/s with flow). Thus, flow-stimulated net O2− production in dnPKCα-transduced tubules decreased by 83% (Δ = 11 ± 3 vs. 63 ± 7 AU/s for LacZ-transduced tubules; P < 0.001; n = 6). However, in dnPKCβI-transduced tubules flow increased net O2− production from 5 ± 1 to 60 ± 7 AU/s (Δ = 55 ± 7 AU/s; P < 0.001; n = 6 vs. dnPKCα; P > 0.05 vs. LacZ). Therefore, flow-induced net O2− production was inhibited in thick ascending limbs transduced with dnPKCα, but not dnPKCβI or LacZ.
DISCUSSION
We hypothesized that flow stimulates net O2− production in thick ascending limbs by activating PKC-α, which in turn activates NADPH oxidase. To test this, we first had to confirm that flow increases net O2− production and that the source of O2− is NADPH oxidase. We found that flow stimulated net O2− production and that apocynin, a NADPH oxidase inhibitor, blocked this effect confirming that the source of flow-induced O2− is NADPH oxidase.
The use of apocynin as an NADPH oxidase inhibitor has come into question, because it has been found to act as a scavenger of reactive oxygen species at concentrations of 1 mM or greater (15). However, we used 10 μM apocynin, a 100 times lower concentration, in the present and previous studies. Thus, it is likely that the effect of apocynin we observed was due to inhibition of the NADPH oxidase rather than scavenging. To directly address this issue, we tested the effect of 10 μM apocynin on O2− produced in vitro by xanthine oxidase from the substrate hypoxanthine. We found that at this concentration, apocynin did not affect O2− production, indicating that it did not act as a scavenger nor an inhibitor of xanthine oxidase.
Additionally, we used a nonpharmacological approach to confirm that NADPH oxidase is involved in flow-stimulated O2− production: p47phox-deficient mice. p47phox is a regulatory subunit of NADPH oxidase and its deletion prevents O2− generation from NADPH oxidase. We found that flow did not change O2− production. Thus, taken together with the apocynin data, we conclude that luminal flow stimulates NADPH oxidase to cause an increase in O2− production.
There are several other sources of O2− in the thick ascending limb, such as mitochondria, xanthine oxidase, and uncoupled nitric oxide synthase (43). However, our data are consistent with our previous study (16) as well as others attributing O2− production in the renal medulla and thick ascending limb to NADPH oxidase (24, 25, 30, 47). We demonstrated that Na-K-2Cl cotransporter activation and mechanical stress stimulated NADPH oxidase-dependent O2− production. Li et al. (25) showed that outward flow of H+ ions activated NADPH oxidase and O2− production, whereas O'Connor et al. (30) observed that NADPH oxidase-dependent O2− production was enhanced in Dahl salt-sensitive rats.
NADPH oxidase can be activated by several pathways, including PKC. To study whether PKC is involved in flow-induced O2− production, we first used the FRET-based reporter MyrPalm-CKAR to measure PKC activity in the absence and presence of flow. We found that flow stimulated PKC activity. Next, we showed that in the absence of flow, the PKC activator PMA stimulated O2− production, indicating a PKC-dependent pathway.
To study the PKC isoform(s) involved in flow-induced O2− production, we utilized the PKC inhibitor Gö 6976 and dnPKCs. It appears that only PKC-α is involved in flow-induced activation of NADPH oxidase and resultant increased O2− production. Although the thick ascending limb expresses several PKC isoforms, we did not test all of them as possible mediators of flow-stimulated O2− production, since we found that we could block virtually all flow-stimulated O2− production with Gö 6976 which is selective for PKC-α and -βI. Moreover, flow-induced O2− production was reduced by 83% in dnPKCα-transduced thick ascending limbs, whereas it was not affected in dnPKCβI-transduced tubules. Taken together, our data indicate that PKC-α is responsible for most if not all flow-stimulated O2− production. To our knowledge, this is the first study to show PKC-mediated O2− production in the thick ascending limb.
Recently, we reported that cellular stretch is involved in flow-stimulated O2− production (10). Others showed that cellular stretch activates PKC. PKC-α, -δ, and -ε are stimulated in cardiac myocytes subjected to various types of stretch (3). PKC-α and -δ mediate Rho GTPase and MAP kinase activation in cyclic stretch-induced hypertrophy (34). In addition, PKC-α is involved in stretch-induced myogenic tone (45) and hyaluronan secretion in fibroblast-like synoviocytes (29). These findings support our data that suggest PKC plays a role in flow-stimulated O2− production.
Our data are consistent with other studies proposing a PKC-α/NADPH oxidase/O2− pathway. In cell-free systems, many PKC isoforms, including PKC-α, phosphorylate p47phox, a regulatory subunit of NADPH oxidase (8). PKC-α-dependent activation of NADPH oxidase via p47phox phosphorylation has been demonstrated in cultured mesangial cells (41). In addition, knockdown of PKC-α with siRNA blocks upregulation of NADPH oxidase in human umbilical vein endothelial cells (44). As far as we are aware, p47phox is the only NADPH oxidase subunit known to be regulated by PKC.
Our findings are also consistent with studies showing that PKC activates NADPH oxidase in other types of cells. PKC-dependent NADPH oxidase-mediated oxidative burst has been characterized in phagocytes (6). A PKC/NADPH oxidase pathway has been implicated in inhibition of the Na+/K+ pump by angiotensin II in cardiomyocytes (42) as well as in the oxidative stress associated with diabetes and hypertension in the vasculature (13, 18).
An increase in net O2− production may be due to enhanced production and/or reduced degradation. Therefore, our data could be interpreted as PKC inhibiting enzymes involved in degradation and a resultant net increase in O2− production. However, given the abundance of data that show that PKC phosphorylates p47phox to activate NADPH oxidase assembly, and no evidence that PKC inhibits degradation enzymes such as superoxide dismutase, it is most likely that the role of PKC in flow-stimulated O2− production is as an activator of NADPH oxidase.
The experiments in the present study were performed in the absence l-arginine, the substrate for nitric oxide synthase. Under these conditions, it is possible that O2− could be produced from uncoupled nitric oxide synthase. However, previously we found that the nitric oxide synthase inhibitor l-NAME alone did not change net O2− production compared with nontreated tubules (17). These data suggest that the source of flow-stimulated O2− is not nitric oxide synthase.
Under physiological conditions, luminal flow stimulates both O2− and nitric oxide production in the thick ascending limb, creating a delicate balance between these opposing factors to maintain proper renal function. We previously showed that nitric oxide reduces flow-induced O2− (17). In conditions of chronic high flow or reduced nitric oxide production, the resultant increase in net O2− production leads to renal dysfunction and damage associated with diseases such as diabetes and hypertension.
In summary, we found that luminal flow stimulates NADPH oxidase-dependent O2− production by the thick ascending limb via PKC-α. Enhanced O2− in the thick ascending limb may be important in the pathogenesis of hypertension and other diseases associated with oxidative stress.
GRANTS
This work was supported by the National Institutes of Health Grants HL-28982, HL-70985, and HL-90550 to J. L. Garvin.
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1.Amlal H, LeGoff C, Vernimmen C, Soleimani M, Paillard M, Bichara M. ANG II controls Na+-K+(NH4+)-2Cl− cotransport via 20-HETE and PKC in medullary thick ascending limb. Am J Physiol Cell Physiol 274: C1047–C1056, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Bey EA, Xu B, Bhattacharjee A, Oldfield CM, Zhao X, Li Q, Subbulakshmi V, Feldman GM, Wientjes FB, Cathcart MK. Protein kinase C delta is required for p47phox phosphorylation and translocation in activated human monocytes. J Immunol 173: 5730–5738, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Bullard TA, Hastings JL, Davis JM, Borg TK, Price RL. Altered PKC expression and phosphorylation in response to the nature, direction, and magnitude of mechanical stretch. Can J Physiol Pharmacol 85: 243–250, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Dang PM, Fontayne A, Hakim J, El Benna J, Perianin A. Protein kinase C zeta phosphorylates a subset of selective sites of the NADPH oxidase component p47phox and participates in formyl peptide-mediated neutrophil respiratory burst. J Immunol 166: 1206–1213, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Dempsey EC, Newton AC, Mochly-Rosen D, Fields AP, Reyland ME, Insel PA, Messing RO. Protein kinase C isozymes and the regulation of diverse cell responses. Am J Physiol Lung Cell Mol Physiol 279: L429–L438, 2000 [DOI] [PubMed] [Google Scholar]
- 6.El Benna J, Dang PM, Gougerot-Pocidalo MA, Marie JC, Braut-Boucher F. p47phox, the phagocyte NADPH oxidase/NOX2 organizer: structure, phosphorylation and implication in diseases. Exp Mol Med 41: 217–225, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fink B, Laude K, McCann L, Doughan A, Harrison DG, Dikalov S. Detection of intracellular superoxide formation in endothelial cells and intact tissues using dihydroethidium and an HPLC-based assay. Am J Physiol Cell Physiol 287: C895–C902, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Fontayne A, Dang PM, Gougerot-Pocidalo MA, El Benna J. Phosphorylation of p47phox sites by PKC alpha, beta II, delta, and zeta: effect on binding to p22phox and on NADPH oxidase activation. Biochemistry (Mosc) 41: 7743–7750, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Garvin JL, Burg MB, Knepper MA. Active NH4+ absorption by the thick ascending limb. Am J Physiol Renal Fluid Electrolyte Physiol 255: F57–F65, 1988 [DOI] [PubMed] [Google Scholar]
- 10.Garvin JL, Hong NJ. Cellular stretch increases superoxide production in the thick ascending limb. Hypertension 51: 488–493, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Good DW. PGE2 reverses AVP inhibition of HCO3− absorption in rat MTAL by activation of protein kinase C. Am J Physiol Renal Fluid Electrolyte Physiol 270: F978–F985, 1996 [DOI] [PubMed] [Google Scholar]
- 12.Gupte SA, Kaminski PM, George S, Kouznestova L, Olson SC, Mathew R, Hintze TH, Wolin MS. Peroxide generation by p47phox-Src activation of Nox2 has a key role in protein kinase C-induced arterial smooth muscle contraction. Am J Physiol Heart Circ Physiol 296: H1048–H1057, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heitzer T, Wenzel U, Hink U, Krollner D, Skatchkov M, Stahl RA, MacHarzina R, Brasen JH, Meinertz T, Munzel T. Increased NAD(P)H oxidase-mediated superoxide production in renovascular hypertension: evidence for an involvement of protein kinase C. Kidney Int 55: 252–260, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Herrera M, Hong NJ, Ortiz PA, Garvin JL. Endothelin-1 inhibits thick ascending limb transport via Akt-stimulated nitric oxide production. J Biol Chem 284: 1454–1460, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heumuller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schroder K, Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension 51: 211–217, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Hong NJ, Garvin JL. Flow increases superoxide production by NADPH oxidase via activation of Na-K-2Cl cotransport and mechanical stress in thick ascending limbs. Am J Physiol Renal Physiol 292: F993–F998, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Hong NJ, Garvin JL. Nitric oxide reduces flow-induced superoxide production via cGMP-dependent protein kinase in thick ascending limbs. Am J Physiol Renal Physiol 296: F1061–F1066, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inoguchi T, Sonta T, Tsubouchi H, Etoh T, Kakimoto M, Sonoda N, Sato N, Sekiguchi N, Kobayashi K, Sumimoto H, Utsumi H, Nawata H. Protein kinase C-dependent increase in reactive oxygen species (ROS) production in vascular tissues of diabetes: role of vascular NAD(P)H oxidase. J Am Soc Nephrol 14: S227–S232, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Ito O, Roman RJ. Role of 20-HETE in elevating chloride transport in the thick ascending limb of Dahl SS/Jr rats. Hypertension 33: 419–423, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Juncos R, Hong NJ, Garvin JL. Differential effects of superoxide on luminal and basolateral Na+/H+ exchange in the thick ascending limb. Am J Physiol Regul Integr Comp Physiol 290: R79–R83, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Kirchner KA, Crosby BA, Patel AR, Granger JP. Segmental chloride transport in the Dahl-S rat kidney during l-arginine administration. J Am Soc Nephrol 5: 1567–1572, 1995 [DOI] [PubMed] [Google Scholar]
- 22.Kopkan L, Majid DS. Superoxide contributes to development of salt sensitivity and hypertension induced by nitric oxide deficiency. Hypertension 46: 1026–1031, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Korchak HM, Kilpatrick LE. Roles for beta II-protein kinase C and RACK1 in positive and negative signaling for superoxide anion generation in differentiated HL60 cells. J Biol Chem 276: 8910–8917, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Li N, Yi FX, Spurrier JL, Bobrowitz CA, Zou AP. Production of superoxide through NADH oxidase in thick ascending limb of Henle's loop in rat kidney. Am J Physiol Renal Physiol 282: F1111–F1119, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Li N, Zhang G, Yi FX, Zou AP, Li PL. Activation of NAD(P)H oxidase by outward movements of H+ ions in renal medullary thick ascending limb of Henle. Am J Physiol Renal Physiol 289: F1048–F1056, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Liu HJ, Wei Y, Ferreri NR, Nasjletti A, Wang WH. Vasopressin and PGE2 regulate activity of apical 70 pS K+ channel in thick ascending limb of rat kidney. Am J Physiol Cell Physiol 278: C905–C913, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Majid DS, Nishiyama A. Nitric oxide blockade enhances renal responses to superoxide dismutase inhibition in dogs. Hypertension 39: 293–297, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Meng S, Cason GW, Gannon AW, Racusen LC, Manning RD., Jr Oxidative stress in Dahl salt-sensitive hypertension. Hypertension 41: 1346–1352, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Momberger TS, Levick JR, Mason RM. Mechanosensitive synoviocytes: a Ca2+-PKCalpha-MAP kinase pathway contributes to stretch-induced hyaluronan synthesis in vitro. Matrix Biol 25: 306–316, 2006 [DOI] [PubMed] [Google Scholar]
- 30.O'Connor PM, Lu L, Schreck C, Cowley AW., Jr Enhanced amiloride-sensitive superoxide production in renal medullary thick ascending limb of Dahl salt-sensitive rats. Am J Physiol Renal Physiol 295: F726–F733, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ortiz PA, Garvin JL. Superoxide stimulates NaCl absorption by the thick ascending limb. Am J Physiol Renal Physiol 283: F957–F962, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Ortiz PA, Hong NJ, Plato CF, Varela M, Garvin JL. An in vivo method for adenovirus-mediated transduction of thick ascending limbs. Kidney Int 63: 1141–1149, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Ortiz PA, Hong NJ, Wang D, Garvin JL. Gene transfer of eNOS to the thick ascending limb of eNOS-KO mice restores the effects of l-arginine on NaCl absorption. Hypertension 42: 674–679, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Pan J, Singh US, Takahashi T, Oka Y, Palm-Leis A, Herbelin BS, Baker KM. PKC mediates cyclic stretch-induced cardiac hypertrophy through Rho family GTPases and mitogen-activated protein kinases in cardiomyocytes. J Cell Physiol 202: 536–553, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Ridge KM, Linz L, Flitney FW, Kuczmarski E, Chou YH, Omary MB, Sznajder JI, Goldman RD. Keratin 8 phosphorylation by PKC delta regulates shear stress-mediated disassembly of keratin intermediate filaments in alveolar epithelial cells. J Biol Chem 280: 30400–30405, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Roman RJ, Kaldunski ML. Enhanced chloride reabsorption in the loop of Henle in Dahl salt-sensitive rats. Hypertension 17: 1018–1024, 1991 [DOI] [PubMed] [Google Scholar]
- 37.Seshiah PN, Weber DS, Rocic P, Valppu L, Taniyama Y, Griendling KK. Angiotensin II stimulation of NAD(P)H oxidase activity: upstream mediators. Circ Res 91: 406–413, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Silva GB, Ortiz PA, Hong NJ, Garvin JL. Superoxide stimulates NaCl absorption in the thick ascending limb via activation of protein kinase C. Hypertension 48: 467–472, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Swei A, Lacy F, DeLano FA, Parks DA, Schmid-Schonbein G. A mechanism of oxygen free radical production in the Dahl hypertensive rat. Microcirculation 6: 179–187, 1999 [PubMed] [Google Scholar]
- 40.Violin JD, Zhang J, Tsien RY, Newton AC. A genetically encoded fluorescent reporter reveals oscillatory phosphorylation by protein kinase C. J Cell Biol 161: 899–909, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wei XF, Zhou QG, Hou FF, Liu BY, Liang M. Advanced oxidation protein products induce mesangial cell perturbation through PKC-dependent activation of NADPH oxidase. Am J Physiol Renal Physiol 296: F427–F437, 2009 [DOI] [PubMed] [Google Scholar]
- 42.White CN, Figtree GA, Liu CC, Garcia A, Hamilton EJ, Chia KK, Rasmussen HH. Angiotensin II inhibits the Na+-K+ pump via PKC-dependent activation of NADPH oxidase. Am J Physiol Cell Physiol 296: C693–C700, 2009 [DOI] [PubMed] [Google Scholar]
- 43.Wilcox CS. Reactive oxygen species: roles in blood pressure and kidney function. Curr Hypertens Rep 4: 160–166, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Xu H, Goettsch C, Xia N, Horke S, Morawietz H, Forstermann U, Li H. Differential roles of PKCalpha and PKCepsilon in controlling the gene expression of Nox4 in human endothelial cells. Free Radic Biol Med 44: 1656–1667, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Yeon DS, Kim JS, Ahn DS, Kwon SC, Kang BS, Morgan KG, Lee YH. Role of protein kinase C- or RhoA-induced Ca2+ sensitization in stretch-induced myogenic tone. Cardiovasc Res 53: 431–438, 2002 [DOI] [PubMed] [Google Scholar]
- 46.Zhao H, Kalivendi S, Zhang H, Joseph J, Nithipatikom K, Vasquez-Vivar J, Kalyanaraman B. Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: potential implications in intracellular fluorescence detection of superoxide. Free Radic Biol Med 34: 1359–1368, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Zou AP, Li N, Cowley AW., Jr Production and actions of superoxide in the renal medulla. Hypertension 37: 547–553, 2001. [DOI] [PubMed] [Google Scholar]