Abstract
Little is known about collecting duct adenylyl cyclase (AC) isoforms or regulation in the mouse. We performed RT-PCR for AC isoforms 1–9 in microdissected cortical (CCD) and outer medullary (OMCD) and acutely isolated inner medullary (IMCD) collecting duct. All collecting duct regions contained AC3, AC4, and AC6 mRNA, while CCD and OMCD, but not IMCD, also contained AC5 mRNA. Acutely isolated IMCD expressed AC3, AC4, and AC6 proteins by Western blot analysis. The mIMCD3 cell line expressed AC2, AC3, AC4, AC5, and AC6 mRNA; M-1 CCD cells expressed AC2, 3, 4, and 6, while mpkCCD cell lines contained AC3, AC4, and AC6 mRNA. AVP stimulated cAMP accumulation in acutely isolated mouse IMCD; this was reduced by chelation of extracellular calcium (EGTA) and almost completely abolished by blockade of calmodulin (W-7). Blockade of calmodulin kinase with KN-93 or endoplasmic reticulum calcium ATPase (thapsigargin) also reduced the AVP response. A similar inhibitory effect of W-7, KN-93, and thapsigargin was seen on forskolin-stimulated cAMP content in acutely isolated mouse IMCD. These three agents had the same pattern of blockade of AVP- or forskolin-stimulated AC activity in acutely isolated rat IMCD. AVP responsiveness in primary cultures of mouse IMCD was also reduced by W-7, KN-93, and thapsigargin. Small interfering RNA (siRNA) designed to knock down AC3 or AC6 in primary cultured mouse IMCD significantly reduced AVP-stimulated cAMP accumulation. Together, these data are consistent with a role of AC3 and AC6 in the activation of mouse collecting duct by AVP.
Keywords: calcium, cAMP
adenylyl cyclases (ACs) are key regulators of arginine vasopressin (AVP) action in the collecting duct, including modulation of water and sodium reabsorption, chloride secretion, and cell proliferation (21, 43, 45, 57). While a host of other factors have been reported to stimulate collecting duct cAMP production, including aldosterone (46), PGI2 (56), PGE2 [via EP4 (35)], β-adrenergic agonists (58, 62), oxytocin (61), adenosine (26), angiotensin II (30), and glucagon (1, 24), relatively (compared to AVP) few studies have examined these pathways or determined their physiological significance. Inhibitors of collecting duct cAMP production have almost exclusively been studied in the context of AVP stimulation; such negative regulators of AVP-stimulated cAMP accumulation include PGE2 [likely via EP3 (10)], dopamine (44), ATP (29, 41), adenosine (42), endothelin-1 (ET-1) (36), and others.
Despite such extensive studies on cAMP modulation of AVP action in the collecting duct, relatively little is known about which AC isoforms are involved. There are nine membrane-bound and one cytoplasmic AC (6). The nine membrane-bound AC isoforms are uniquely regulated by Gα and Gβγ G protein subunits, divalent cations, small molecules, posttranslational modification, and subcellular localization (6). There have been some studies on AC isoform expression in the collecting duct, albeit these have been almost exclusively confined to the rat. In general, investigators have failed to detect AC1, AC7, or AC8 mRNA or protein anywhere in the collecting duct (cortex through inner medulla) (7, 27). AC2 mRNA has been detected in cortical collecting duct (CCD) and inner medullary collecting duct (IMCD), albeit quite faintly (7, 27). AC3 mRNA and protein were found in IMCD by one group (27), but no AC3 mRNA was detected by another (7). A mouse CCD cell line, mpkCCDc14, was found to express AC3 mRNA (11). AC4 mRNA has been reported throughout the collecting duct, albeit perhaps highest in cortical regions (7, 27, 47). AC5 mRNA was found in CCD and outer medullary collecting duct (OMCD) but not IMCD (7, 13); one group localized AC5 mRNA to intercalated, but not principal, cells (24). Interestingly, soluble AC appears to localize to intercalated but not principal cells (38). AC6 mRNA and protein are found throughout the collecting duct and appear to be relatively highly expressed in the entire nephron (7, 13, 27, 47). AC9 mRNA was detected in the entire nephron, albeit faintly in CCD (7) and IMCD (27). Thus, at least in the rat, the collecting duct may express the membrane-bound AC isoforms 2, 3, 4, 5, 6, and 9; however, this is in need of clarification. Virtually nothing is known about collecting duct AC isoform expression in the mouse—identification of such expression would be particularly useful in helping to design rational genetic engineering studies that could examine the functional significance of the various isoforms. Consequently, the first part of the present study examined membrane-bound AC isoform expression in the mouse collecting duct.
The role of specific AC isoforms in mediating AVP-stimulated cAMP production in the collecting duct has not been extensively studied. Hoffert et al. (27) found that inhibitors of calmodulin (CaM) markedly decreased AVP responsiveness in rat IMCD. On the basis of the assumption that the only CaM-regulated ACs are 1, 3, and 8, and that rat IMCD did not express AC1 or AC8, they concluded that AC3 is a major regulator of AVP-induced cAMP accumulation. Given this observation, one might predict that AC3-knockout mice would have an inappropriate diuresis; however, such animals were reported not to have significant alterations in urine volume (although they did have a reduced glomerular filtration rate) (40). In addition, recent findings have challenged the traditional concept of CaM regulating only the classic “calcium-stimulated” AC isoforms (AC1, 3, and 8). Indeed, CaM inhibition has now been shown to decrease carbachol activation of transfected AC6 in HEK-293 cells (5). How CaM is involved in AC6 regulation is uncertain, but new findings have helped develop a hypothesis about this system. Depletion of endoplasmic reticulum calcium stores causes release of calcium influx factor, which displaces CaM from CaM kinase (CaMK)II as well as from so-called calcium-independent (a misnomer in this case) phospholipase A2 (iPLA2) (4). A binding site responsible for calcium-dependent iPLA2 regulation by CaM has been identified; displacement of CaM from iPLA2 can accelerate iPLA2 activity (3). iPLA2 produces lysophospholipids that can open store-operated calcium channels, leading to inhibition of AC6 (4, 48). Thus CaM inhibition might lead to reduced AC6 activity. Consequently, the present study examines the role of key components of this proposed pathway in modulating AVP-stimulated cAMP content in the collecting duct. We report that both AC3 and AC6 are likely to modulate AVP-induced AC activity and further confirm a role for AC3 and AC6 by examining AVP responsiveness in cells in which AC isoform expression has been knocked down.
METHODS
Materials.
KN-93, W-7, and thapsigargin were obtained from Calbiochem (San Diego, CA). All antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). All other reagents were obtained from Sigma Chemical (St. Louis, MO) unless specifically stated otherwise.
Acutely isolated IMCD preparations.
Primary isolates of mouse (C57BL/6, 25 g) or rat (Sprague-Dawley, 200–250 g) IMCD cells were obtained with a modification of previously described procedures (50, 51). Institutional Animal Care and Use Committee approval was obtained for the use of mice and rats for these purposes. Mouse renal inner medullas were minced and incubated at 37°C in 0.1% collagenase (type I; Worthington, Freehold, NJ) containing 0.01% DNase (type I) in Hanks’ balanced salt solution (HBSS) supplemented with 15 mM HEPES (pH 7.4). When a suspension containing predominantly single cells and individual tubules was obtained (∼45 min), the digest was filtered through a 74-μm-mesh screen to remove any residual tissue. The tubule suspension was centrifuged at 1,500 rpm for 5 min, and the cell pellet was resuspended in 10% bovine serum albumin in HBSS, followed by an additional two centrifuge/washes with HBSS. The final pellet containing primarily tubules was resuspended in HBSS-HEPES. This procedure has previously been shown to yield predominantly collecting ducts (50, 51).
Cell culture.
Freshly isolated mouse IMCD were grown in primary culture as previously described (51). The cell suspension was plated in 24-well plates in Renal Epithelial Cell Growth Medium (REGM, Cambrex, Walkersville, MD) containing epidermal growth factor, insulin, hydrocortisone, gentamicin, amphotericin, 0.5% fetal bovine serum, epinephrine, tri-iodothyronine, and transferrin (concentrations are proprietary). Once cells reached confluence, the medium was changed to DMEM-F-12 alone for 16–24 h before study.
cAMP studies.
Freshly isolated rat or mouse or cultured mouse IMCD were preincubated in HBSS-HEPES buffer (pH 7.4) containing 1 mM 3-isobutyl-1-methylxanthine (IBMX) for 30 min at 37°C, followed by addition of AVP or forskolin for 5 min. In studies involving additional treatments (inhibitors), theses agents were added for 30 min after IBMX and before addition of AVP or forskolin. The concentrations of W-7, KN-93, and thapsigargin were based on previous reports of the compounds’ IC50 and specificity (25, 39, 54). At the end of the incubations, cellular cAMP and total protein were determined. cAMP was extracted from cells by addition of 70% ethanol overnight at −20°C. The sample was then centrifuged and the ethanol removed from the cell pellet. The ethanol was evaporated, the residual material was resuspended in assay buffer and cAMP was assayed with a commercial enzyme immunoassay kit (Assay Design, Ann Arbor, MI). The cell pellet (precipitated in ethanol) was redissolved in 0.1 N NaOH for determination of total cellular protein by the Bradford assay.
Western blot analysis of adenylyl cyclases.
Acutely isolated mouse IMCD were prepared as above and homogenized in 300 μM sucrose, 10 mM triethanolamine, 1 mM EDTA, and Roche Complete protease inhibitors pH 7.2. The homogenized samples were centrifuged at 4,000 g for 10 min to pellet the unhomogenized fraction and nuclei. The supernatant was removed to a new tube. The pellet was rehomogenized and centrifuged as before, and the supernatants were combined. The membrane fraction was isolated by centrifugation at 17,000 g for 60 min. The pellet was suspended in phosphate-buffered saline. Protein concentration was determined by the Bradford method. For analysis, protein was solubilized in Laemmli buffer containing 0.5% lithium dodecyl sulfate. Samples were run on a denaturing NUPAGE 4–12% Bis-Tris minigel using a MOPS buffer system (Invitrogen, Carlsbad, CA). Proteins were transferred to a polyvinylidene difluoride (PVDF) plus nylon membrane by electroelution and visualized with the Advance ECL system (GE Healthcare, Piscataway, NJ). Densitometry was performed with a Bio-Rad gel documentation system (Hercules, CA).
Anti-AC antibodies used for immunoblotting were incubated with membranes in blocking buffer [5% milk-Tris-buffered saline-Tween 20 (TBST)] overnight at 4°C at concentrations from 1:25,000 to 1:100,000. The primary antibodies used were rabbit polyclonal anti-AC3, 4, 5/6, or 7 and goat polyclonal anti-AC9. Secondary horseradish peroxidase-conjugated antibodies (either goat anti-rabbit or donkey anti-goat) were incubated at room temperature in blocking buffer. After visualization, blots were stripped and incubated with anti-β-actin antibody (Abcam, Cambridge, MA) at 1:10,000 in blocking buffer (to confirm adequate loading) and then exposed to secondary antibody as above. Mouse brain was used as a positive control.
RT-PCR of adenylyl cyclase mRNA.
Total RNA was isolated from primary IMCD cultures and collecting duct cell lines derived from CCD or IMCD (M-1, mIMCD3, and mpkCCD). Cells were grown under the same conditions as described above for primary IMCD cultures. RNA was also obtained from microdissected mouse CCD and OMCD, as well as acutely isolated mouse IMCD. RNA was isolated with acid phenol, and potentially contaminating genomic DNA was removed by incubation with RNase-free DNase I (Promega, Madison, WI). Reverse transcription was performed on 3 μg of total RNA with oligo(dT)12–18 and SuperScript II according to the manufacturer's protocol (Invitrogen). PCR primers were designed from mouse cDNA sequences to span an intron in order to distinguish genomic contamination. PCR amplification was performed with Taq polymerase (Invitrogen). All products were sequenced to ensure fidelity of amplification. Primers used are described in Table 1. Gels were stained with ethidium bromide. Brain was used as a positive control.
Table 1.
Primers used for PCR of adenylyl cyclases
| RNA | Forward Primer | Reverse Primer | Product Size, bp | Exons Spanned |
|---|---|---|---|---|
| AC1 | gctgttcgtggtcaccaatgtccg | gagcgctctgtcaagatccgcacg | 401 | 1–2 |
| AC2 | caacactgtcaacgtcgctagtag | gagcacgtacgtaatcaagacgaag | 311 | 24–25 |
| AC3 | agccagtcttatgacgagattgg | tcggtctcttctaccacctgga | 526 | 18–22 |
| AC4 | caccatggtggaatttgcagtggc | gaggatcttcgaagaggggagctc | 387 | 24–26 |
| AC5 | caatacagtgaatgtggccagccg | cagcaaaggcagaagttgcttctg | 363 | 20–22 |
| AC6 | ggttccggcagctggagaagatca | gtccactcagtgcccaccttggtc | 494 | 5–7 |
| AC7 | gcacgtgcacatcggagtcttggt | cttgaaacttggcagtgtctgtac | 355 | 26–27 |
| AC8 | gcatacaagagatcaacaagcattc | ctggtccttcaggataaggtaggt | 211 | 17–18 |
| AC9 | caccatgctcacgggctctgcagt | gatgttccgtagcagccagtcggc | 423 | 9–11 |
All primers are shown in the 5′-3′ orientation. AC, adenylyl cyclase.
Small interfering RNA studies.
Acutely isolated mouse IMCD cells were plated on 96-well plates and grown to >90% confluence. The medium was replaced with Accell medium containing 1 μM small interfering RNA (siRNA) directed against AC3, AC4, or AC6 or a nontargeting control siRNA (Dharmacon, Chicago, IL). The cells were incubated with the siRNA for 3 days. For AVP studies, at 72 h after transduction the medium was replaced with HBSS containing 1 mM IBMX and returned to the incubator for 30 min, followed by a 5-min incubation with 1 nM AVP, and cAMP content was determined as described above. For AC isoform and GAPDH mRNA determination, RNA was isolated from transduced cells and mRNA was determined by real-time PCR (Smart Cycler, Cephid, Sunnyvale, CA).
Statistics.
Comparisons of cAMP accumulation between the different conditions was done with one-way analysis of variance with the Bonferroni correction. P < 0.05 was taken as significant. Data are expressed as means ± SE.
RESULTS
Expression of adenylyl cyclases by collecting duct.
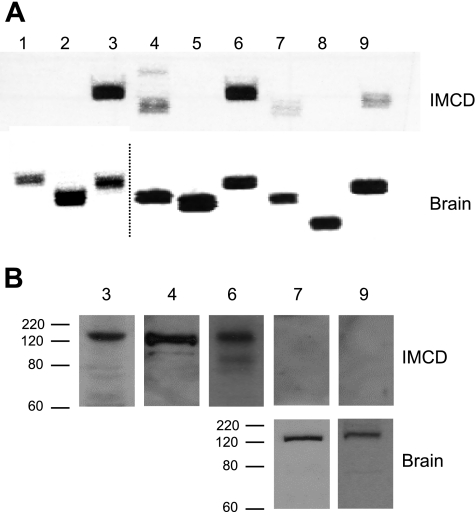
The presence of mRNA for each of the membrane-bound AC (AC1–9) isoforms was determined in mouse collecting duct. Microdissected CCD and OMCD and acutely isolated (by digestion and centrifugation) IMCD were studied. As described in Table 2, AC3, AC4, and AC6 mRNA were consistently detected and yielded relatively strong signals in all three regions of the collecting duct. AC2, AC7, and AC9 mRNA were inconsistently and weakly detected throughout the collecting duct. AC5 mRNA was consistently and relatively strongly detected in CCD and OMCD but was never found in IMCD. A representative gel demonstrating the pattern of AC isoform mRNA expression in mouse IMCD and brain (as a control) is shown in Fig. 1A.
Table 2.
PCR signal intensity in mouse collecting duct for AC isoforms
| Adenylyl Cyclase Isoform |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| M-1 cell line | − | + | + | + | − | + | ± | − | − |
| mIMCD3 cell line | − | + | + | + | + | + | ± | − | − |
| mpkCCD cell line | − | − | + | + | − | + | ± | − | ± |
| IMCD primary culture | − | ± | + | + | − | + | ± | − | ± |
| IMCD acutely isolated | − | ± | + | + | − | + | ± | − | ± |
| OMCD microdissected | − | ± | + | + | + | + | ± | − | − |
| CCD microdissected | − | ± | + | + | + | + | ± | − | − |
−, None detected; ±, inconsistent or weak signal; +, strong consistent signal; n =5 for each data point. IMCD, inner medullary collecting duct; OMCD, outer medullary collecting duct; CCD, cortical collecting duct.
Fig. 1.
RNA and protein expression of adenylyl cyclases (ACs) in acutely isolated mouse inner medullary collecting duct (IMCD). A: PCR of mRNA of the 9 membrane-associated AC isoforms from a representative gel (n = 5 total experiments) in IMCD and brain (the latter as a positive control). Note that the dashed line in the brain PCR samples indicates where 2 different PCR gels are shown; they are placed adjacent to one another to facilitate viewing. B: representative Western analysis for AC3, AC4, AC6, AC7, and AC9 in IMCD (n = 4 total experiments) and AC7 and AC9 in brain. The antibody for AC6 also recognizes AC5; since no AC5 mRNA was detected in acutely isolated mouse IMCD, it is assumed that the band represents AC6. Western blot analysis was not done for AC isoforms lacking mRNA in the IMCD. The approximate expected sizes of AC3, AC4, AC6, AC7, and AC9 based on amino acid sequence without including possible posttranslational modification are 137, 129, 140, 132, and 162 kDa, respectively. Size indications in kDa are shown.
Since mRNA expression does not necessarily equate to the presence of protein, AC expression was examined by Western blot analysis. AC protein content could not be determined in microdissected CCD or OMCD because of insufficient sample size (tubules could only be obtained by microdissection); however, adequate tissue could be obtained from acutely isolated IMCD. AC3 and AC4 protein were readily detected (Fig. 1B); an antibody that did not discriminate between AC5 and AC6 consistently showed a strong signal. Commercially available antibodies purportedly specific for AC6 were tested; however, no signal, in brain, whole kidney, or IMCD, was observed. We could not find antibodies specific for AC5. Antibodies to AC7 and AC9 (faint signals were seen on PCR) gave no signal in acutely isolated IMCD; however, they did yield detectable bands with mouse brain homogenates as a control. Taken together, the mRNA and protein expression data indicate that the only AC isoforms expressed by IMCD are AC3, AC4, and AC6. CCD and OMCD may also express AC5.
Biochemical characterization of AVP-stimulated collecting duct adenylyl cyclases.
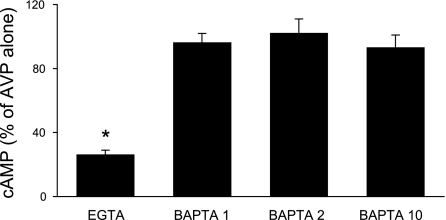
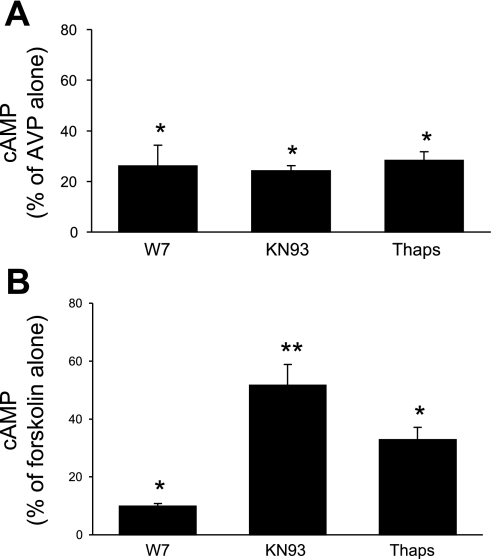
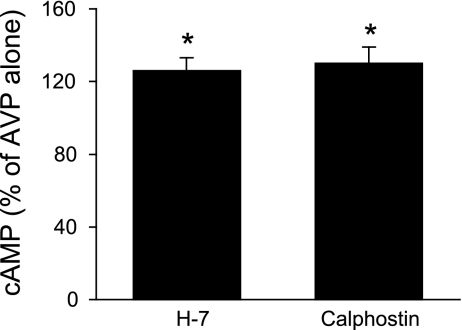
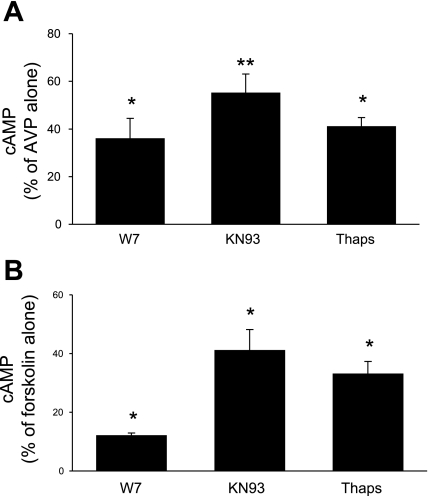
IMCD were used in these studies since this is the only region of the mouse collecting duct from which adequate tissue could be obtained for biochemical characterization experiments. Numerous factors affecting collecting duct AC exist; however, these studies focused primarily on the major regulator, AVP. Since IMCD expressed only AC3, AC4, and AC6, attempts were made to discriminate between these isoforms in terms of which ones were involved in AVP-dependent cAMP accumulation. In the first set of studies, a dose response for 0.01–100 nM AVP was determined (in the presence of phosphodiesterase inhibition). Maximal responsiveness was observed with 1 nM AVP; furthermore, the difference between unstimulated and AVP-treated cells was most evident after 5-min exposure to AVP. Consequently, 5-min exposure to 1 nM AVP was used for all subsequent studies. Unstimulated (no AVP) cells had virtually undetectable levels of cAMP; hence all subsequent data represent the effect solely of AVP ± added agents. The calcium sensitivity of AVP-stimulated cAMP accumulation was tested with 5 mM EGTA; EGTA reduced AVP-stimulated cAMP to 26 ± 3% of values seen with AVP alone (Fig. 2). These data suggested that a large part of the AVP response was calcium dependent and that calcium stimulated part of the AVP-induced cAMP accumulation. Such characteristics are typical of AC3 (17) but not AC4 (calcium independent) or AC6 [generally believed to be inhibited by calcium (49), although as discussed below, the calcium response of AC6 may be more complex]. To further explore calcium dependence, the effect of chelation of intracellular calcium with BAPTA was examined. BAPTA, at 1, 2, or 10 μM, had no effect on AVP-sensitive cAMP content (Fig. 2), suggesting that that the AVP response was primarily dependent on extracellular calcium entry. To more clearly define the role for calcium-dependent pathways, the effect of CaM inhibition with W-7 was determined (Fig. 3A). W-7 (25 μM) markedly reduced AVP-induced cAMP, indicating that the AVP response is dependent on calcium-regulated CaM. To further define such regulation, the effect of inhibition of CaMKII was determined with KN-93. KN-93 (10 μM) also substantially inhibited AVP-dependent cAMP accumulation; similar results were obtained with 1 μM KN-93 (data not shown). Since CaMKII has been reported to inhibit AC3 (19, 59) and may stimulate AC6 activity (4), these data suggest that AC6 may also play a role in AVP-induced cAMP accumulation. Thapsigargin, a blocker of the endoplasmic reticulum calcium ATPase, has been reported not to affect AC3 (20) but to inhibit AC6 activity [via stimulation of capacitative (store operated) calcium entry (CCE)] (6, 18, 52). Thapsigargin (5 μM) inhibited AVP-induced cAMP content in IMCD (Fig. 3), again suggesting a role for AC6. Finally, the effect of PKC inhibition was examined since PKC has been shown to increase AC3 (52) while decreasing AC6 (6) activity. PKC blockade with 10 μM H-7 caused a 26 ± 7% increase in AVP-stimulated cAMP content (P < 0.05 vs. AVP alone; n = 12), while PKC inhibition with 100 nM calphostin C caused a comparable response (Fig. 4). These data also support a role for AC6 in mediating the AVP response.
Fig. 2.
Cellular cAMP accumulation in acutely isolated mouse IMCD. Cells were preincubated with 1 mM 3-isobutyl-1-methylxanthine (IBMX) for 30 min and then incubated with medium alone, 5 mM EGTA, or BAPTA (1, 2, and 10 μM) for 30 min, followed by addition of 1 nM arginine vasopressin (AVP) for 5 min (n = 6 each data point). *P < 0.001 vs. AVP alone.
Fig. 3.
Cellular cAMP accumulation in acutely isolated mouse IMCD. Tubules were preincubated with 1 mM IBMX for 30 min and then incubated with medium alone, 25 μM W-7, 10 μM KN-93, or 5 μM thapsigargin (Thaps) for 30 min, followed by addition of 1 nM AVP (A) or 1 μM forskolin (B) for 5 min (n = 6 each data point). *P < 0.001 vs. AVP or forskolin alone; **P < 0.01 vs. forskolin alone and vs. W-7 + forskolin.
Fig. 4.
Cellular cAMP accumulation in acutely isolated mouse IMCD. Cells were preincubated with 1 mM IBMX for 30 min and then incubated with medium alone, 10 μM H-7, or 500 nM calphostin C for 30 min, followed by addition of 1 nM AVP for 5 min (n = 12 each data point). *P < 0.05 vs. AVP alone.
All IMCD ACs were activated with forskolin in the presence of W-7, KN-93, or thapsigargin for comparison with the AVP response. As shown in Fig. 3B, W-7 markedly reduced (by 90%) forskolin-stimulated cAMP accumulation, indicating that the vast majority of membrane-bound AC activity in the IMCD is CaM regulated (i.e., likely AC3 and AC6). KN-93 also reduced forskolin-induced cAMP, although this was to a lesser extent than with W-7. Thapsigargin also reduced forskolin-stimulated AC activity. Overall, the effect of these agents on the forskolin response was qualitatively similar to that seen with AVP. Together, these data indicate that the primary ACs in IMCD that mediate signaling are AC3 and AC6 (although a role for AC4 is not ruled out).
Previous studies characterizing AC activity used the rat. To facilitate comparison with this data, we examined AVP and forskolin responsiveness in acutely isolated rat IMCD (Fig. 5). Although not quantitatively identical, the pattern of responses to W-7-, KN-93-, and thapsigargin-mediated inhibition of AVP- or forskolin-stimulated cAMP accumulation was very similar to that seen in the mouse. On the basis of these criteria, AVP regulation of mouse and rat ACs in the IMCD has substantial commonality.
Fig. 5.
Cellular cAMP accumulation in acutely isolated rat IMCD. Tubules were preincubated with 1 mM IBMX for 30 min and then incubated with medium alone, 25 μM W-7, 10 μM KN-93, or 5 μM thapsigargin for 30 min, followed by addition of 1 nM AVP (A) or 1 μM forskolin (B) for 5 min (n = 6 each data point). *P < 0.001 vs. AVP or forskolin alone; **P < 0.01 vs. AVP alone.
Characterization of adenylyl cyclases in cultured collecting duct and effect of AC isoform knockdown.
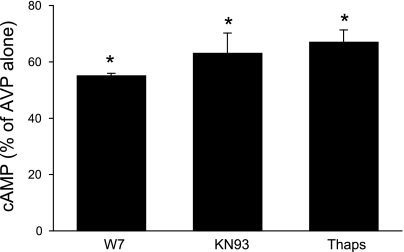
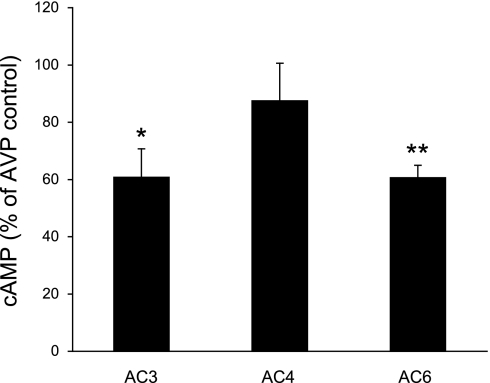
The above studies suggest that AC3 and AC6 are involved in AVP-stimulated cAMP accumulation in the collecting duct. While AC3 has been purportedly implicated in such a role [at least in the rat (27)], AC6 modulation of AVP response has not been clearly identified. To more definitively (as opposed to biochemical characterization) investigate such a role for AC isoforms, studies were untaken in which AVP responsiveness was assessed when AC3, AC4, or AC6 expression was inhibited. Since cultured mouse collecting duct was required for these studies, we first investigated whether cultured mouse collecting duct cells expressed ACs and responded to AVP in a manner similar to acutely isolated IMCD. Primary cultures of IMCD had the same AC mRNA expression pattern as acutely isolated IMCD (Table 2). Several mouse cell lines were then studied. The CCD-derived cell lines (M-1 and mpkCCD) expressed AC3, AC4, and AC6 mRNA but, unlike microdissected CCD, did not express AC5 mRNA. mIMCD3 cells, derived from mouse IMCD, also contained AC3, AC4, and AC6 mRNA but, unlike acutely isolated IMCD, also expressed AC5 mRNA. On the basis of mRNA expression pattern, primary cultures of mouse IMCD were deemed the best cell culture model to further examine AVP responsiveness. AVP- and forskolin-stimulated cAMP accumulation in primary cultured mouse IMCD cells was inhibited by W-7, KN-9, and thapsigargin (Fig. 6). These responses followed the same pattern as seen in acutely isolated IMCD, although the magnitude of each response in cell culture was not identical to that seen in freshly isolated cells. In particular, the overall CaM responsiveness in the cultured cells appeared to be less than in freshly isolated IMCD. On the basis of this data, primary cultures of mouse IMCD seemed a reasonable, albeit not perfect, model to examine the role of AC isoforms in mediating AVP-regulated cAMP levels. To accomplish this, cells were treated with siRNA directed against AC3, AC4, or AC6, with a random sequence as a control (Fig. 7). The siRNA against AC3 and AC6 caused an ∼40% decrease in AVP-stimulated cAMP accumulation, while the AC4 siRNA had no significant effect. Despite these decreases in AVP responsiveness, we were unable to detect significant decreases in AC3, AC4, or AC6 mRNA after targeted siRNA treatment. Part of the problem related to the high intrinsic variability in the amounts of AC isoform mRNA (despite measurement of 15–18 different samples of cells treated with a given AC isoform siRNA), making detection of a significant reduction in mRNA problematic.
Fig. 6.
Cellular cAMP accumulation in primary cultures of mouse IMCD. Cells were preincubated with 1 mM IBMX for 30 min and then incubated with medium alone, 25 μM W-7, 10 μM KN-93, or 5 μM thapsigargin for 30 min, followed by addition of 1 nM AVP for 5 min (n = 6 each data point). *P < 0.001 vs. AVP alone.
Fig. 7.
Effect of AC3, AC4, and AC6 small interfering RNA (siRNA) on AVP-stimulated cAMP accumulation in primary cultures of mouse IMCD. Cells were treated with AC3, AC4, or AC6 siRNA as described in methods. For cAMP studies, control cells (treated with irrelevant random sequence RNA) or cells treated with AC isoform-specific siRNA were preincubated with 1 mM IBMX for 30 min, followed by addition of 1 nM AVP for 5 min (n = 16–18 each data point). *P < 0.01 vs. control; **P < 0.001 vs. control.
DISCUSSION
The goal of the present study was to 1) identify the membrane-bound AC isoforms expressed by the mouse collecting duct and 2) identify their role in mediating AVP-stimulated cAMP accumulation. With regard to the first goal, our data indicate that cells from all regions of the mouse collecting duct are likely to express AC3, AC4, and AC6. This conclusion is based on the findings that CCD, OMCD, and IMCD express AC3, AC4, and AC6 mRNA while IMCD contains AC3, AC4, and AC6 protein. It was also noted that AC5 mRNA was strongly detected in CCD and OMCD but not in IMCD. Taken together, these findings agree with previous studies in the rat in that they support the notion that AC3, AC4, and AC6 are the major AC isoforms expressed by the collecting duct while AC5 is expressed exclusively by intercalated cells (7, 13, 24). Our studies do not permit determination of which cell type(s) (i.e., principal vs. intercalated) in the CCD and OMCD express AC3, AC4, and AC6. Attempts were made at immunostaining renal sections with AC antibodies; however, we were not successful in obtaining clear isoform discrimination with this methodology. Finally, it is notable that we detected AC5 in mIMCD3 cells; this suggests that caution should be used when interpreting studies examining cAMP responses with this cell line.
While the physical presence of specific AC isoforms in a given nephron segment can be reasonably well ascertained, determination of their role in mediating AVP-induced cAMP production is more challenging. Part of the difficulty stems from the absence of isoform-specific inhibitors. The current inhibitors include 1) P site inhibitors (adenosine analogs that inhibit ACs by binding to the active site; a newer series of P site inhibitors has been developed but remains unproven with regard to AC isoform specificity; 2) 2′(3′)-O-(N-methylanthraniloyl) (MANT) nucleotides or various ATP analogs that inhibit ACs but are not isoform specific; 3) forskolin analogs (both agonists and antagonists) in development; and 4) a variety of other nonspecific inhibitors (6, 28). An additional issue is that AC isoform function may be largely influenced by subcellular localization, thereby complicating interpretation of global cell function. In support of this notion is the finding that some ACs colocalize subcellularly with specific G protein-coupled receptors, phosphodiesterase 4, protein kinase A (PKA), gravin, and A-kinase anchoring proteins (16). It should also be noted that many studies examining AC isoform specificity have utilized overexpression strategies that may lead to spurious results. As stated above, it is becoming increasingly evident that AC specificity resides partially in their subcellular compartmentalization; this distribution may be altered in heterologous transgene expression approaches. For example, AC6 is expressed in caveolae in aortic smooth muscle cells, but when overexpressed it is not found in caveolae (37). Finally, opposing results have been reported on regulation of a given AC isoform by different laboratories, typically when using different cell types or expression conditions. Given these limitations, the current state of the art for defining the role of particular endogenous AC isoforms in mediating agonist-stimulated cAMP production largely involves the use of pharmacological agents that alter signaling pathways purportedly involved in the unique regulation of individual, or a subset of, AC isoforms. Toward this end, the present study primarily focused on pharmacological manipulation of largely calcium-regulated ACs since 1) AC3 and AC6 are both calcium regulated and 2) initial experiments indicated that the vast majority of AC activity in the mouse collecting duct was dependent on calcium-regulated pathways.
The present study found that AVP- and forskolin-stimulated cAMP accumulation was markedly calcium dependent in mouse collecting duct. Furthermore, similar observations were made in rat collecting duct. These findings are in agreement with other studies in rat IMCD (27) and suggest that AC3, the major known AC isoform to be stimulated by calcium, is a key regulator of AVP action. AC3 appears to localize to caveolin-rich fractions in lipid rafts, at least in some cell types (37). Interestingly, AC3 localizes to primary cilia in brain (8)—there is no information on AC3 subcellular localization in collecting duct. While a major characteristic of AC3 is its regulation by calcium, the manner of such regulation is controversial. Calcium has been reported to activate AC3 via CaM (17, 27, 52); however, since AC3 does not have a consensus CaM binding site, how this occurs is uncertain. It is notable that in heterologous expression systems CaM activation of AC3 requires 10-fold greater concentrations of CaM compared with those required to activate AC isoforms with CaM consensus binding sites (AC1 and AC8) (17). A confusing aspect of AC3 is that it is also inhibited by submicromolar concentrations of calcium; this effect is exerted through activation of CaMKII, which can directly phosphorylate AC3 (19, 59). A key question is where the calcium comes from that inhibits AC3 through CaMKII and stimulates AC3 through CaM. This is largely unresolved but has been theorized to relate to highly compartmentalized calcium microenvironments influencing AC3 (59). One concept is that cAMP-stimulated PKA activation causes phosphorylation of endoplasmic reticulum inositol trisphosphate (IP3) receptors, leading to localized release of calcium from intracellular pools, and ultimate activation of CaMKII (59). However, others have reported that AC3 may not be affected by calcium release from IP3-sensitive stores but is instead regulated by extracellular calcium entry though calcium channels (52). The nature of such extracellular calcium entry is not fully ascertained but does not involve thapsigargin-sensitive CCE (20). Consideration of these findings indicates that, while AC3 is undoubtedly involved in mediating AVP signaling, it is unlikely to be fully responsible for all of AVP-stimulated cAMP accumulation. We observed that CaMKII inhibition reduced AVP-induced cAMP production; if only AC3 were involved, CaMKII blockade should have increased AVP responsiveness. Furthermore, we observed that thapsigargin reduced AVP-stimulated cAMP accumulation, an effect that has been reported to be independent of AC3. We also found that PKC inhibition increased AVP-induced cAMP production; such inhibition would have been predicted, if anything, to reduce AC3 activity (15, 19, 52). Finally, we did observe that AC3 siRNA significantly reduced AVP-enhanced cAMP content. While the magnitude of the AC3 knockdown could not be ascertained, these data did clearly indicate that AC3 is indeed involved in mediating AVP actions in the IMCD.
The vast majority of AVP-stimulated AC activity was found to be calcium dependent; however, a small fraction (∼10–20%) was not affected by calcium or calcium-regulated pathways. Such activity may relate to AC4; however, relatively little is known about this AC isoform. It is widely expressed and is activated by G protein-coupled receptor stimulation via Gαs or inhibited by Gαi (17, 19); however, it has not been linked to stimulation of any specific receptor. PKC effects are somewhat controversial, being mainly reported to stimulate, but on one occasion to inhibit, AC4 (52). We were unable to detect an effect of AC4 siRNA on AVP-stimulated cAMP levels, suggesting that this isoform is not critically involved in AVP signaling. However, this conclusion has the caveat that AC4 activity may not have been completely abolished by the siRNA treatment. Further studies are needed to clarify the role of AC4 in mediating AVP- or other agonist-stimulated cAMP accumulation.
AC6 regulation has been extensively studied; its regulation is quite complex. Several factors or protein kinases can inhibit AC6 activity, including nitric oxide (22, 32), PKA (6), receptor tyrosine kinase activation/tyrosine phosphorylation (6, 17), and PKC (6). Indeed, several of these factors have been shown to inhibit AVP-stimulated cAMP accumulation in the collecting duct (9); the finding in the present study that PKC blockade increased AVP-stimulated cAMP production supports, albeit does not prove, a role for AC6. A unique feature of AC6 (and AC5) is inhibition by submicromolar calcium concentrations (49). As discussed above, the responsible mechanism(s) are incompletely understood; however, CCE may be primarily involved (6, 18, 52). Further, and unlike AC3, CaM/CaMKII can stimulate AC6 activity (5, 6). The findings that thapsigargin-activated CCE and CaMKII blockade inhibited AVP-stimulated AC activity further support the notion that AC6 is involved in the AVP response. The most convincing evidence for a role for AC6 comes from our observation that AC6 siRNA reduces AVP-stimulated cAMP production in IMCD cells. As for the AC3 studies, we can definitely say that AC6 is involved in mediating AVP actions in the IMCD; however, the relative contribution of AC6 remains to be determined.
Identification of specific AC isoforms that mediate AVP responsiveness may be of significant physiological and pathophysiological relevance. AVP has multiple effects on the collecting duct that conceivably could be due to activation of different AC isoforms. In addition to stimulation of osmotic water permeability, AVP increases epithelial sodium channel activity in CCD via stimulation of cAMP (45); cAMP increases epithelial sodium channel expression in the apical membrane of collecting duct cells (12, 23, 34). Both AVP and cAMP stimulate urea transport in acutely isolated collecting duct segments (43). Interestingly, AVP-stimulated IMCD urea permeability is reduced by CaM blockade, suggesting involvement of AC3 or AC6 (27). In addition, ET-1 inhibits AVP-stimulated collecting duct water, but not urea, permeability (36); since ET-1 inhibits cAMP synthesis, this suggests that AVP-induced cAMP pools regulating urea and water transport may derive from different AC isoforms, or at least ACs in different subcellular compartments. Finally, collecting duct cells secrete chloride in response to AVP, forskolin, or cAMP (14, 57). These effects of AVP and cAMP on collecting duct water, sodium, urea, and chloride transport could be mediated by one or more AC isoforms; clarification of these pathways would yield important physiological insights. However, perhaps the greatest significance relates to the role of AVP in cyst fluid accumulation and cell proliferation in polycystic kidney disease (PKD). A recent study found that crossing Brattleboro rats with rats with autosomal recessive PKD (Pkhd1 knockout) resulted in almost complete inhibition of cystogenesis, while exogenous AVP restored the full cystic phenotype (60). This cystic effect of AVP is thought to be due to stimulation of cAMP (21). When the COOH-terminal tail of mouse polycystin-1 is overexpressed in collecting duct cells cAMP agonism stimulates cell growth, while in normal cells cAMP inhibits cell growth (53). Embryonic renal tubules from PKD1 kidneys respond to cAMP with CFTR-dependent cystic dilation (31). Most importantly, clinical trials have been initiated examining the effectiveness of AVP receptor antagonism in ameliorating kidney disease in patients with PKD (55). A significant potential side effect of such V2 blockade is inappropriate diuresis; clearly, if the proliferative and/or chloride secretory effects of AVP are mediated by different AC isoforms than the water-retaining effects, then impetus would be given to the development of strategies to target specific AC isoforms.
In summary, the present study identified AC3, AC4, and AC6 as the predominant AC isoforms throughout the mouse collecting duct; AC5 may also be present, most likely in intercalated cells. AVP-induced cAMP accumulation is mediated by AC3 and AC6, while a role for AC4 cannot be fully excluded. These studies on the initial characterization of mouse collecting duct ACs and AVP responsiveness will pave the way for subsequent investigations into the physiological and pathophysiological roles of the individual collecting duct AC isoforms. Ultimately, mice with collecting duct-specific knockout of the key AC isoforms identified in this study (starting with AC3 and AC6) should be highly informative. Such studies, in which principal (2) or intercalated (33) cells can be specifically targeted, are clearly the future direction for unraveling our understanding of the biological functions of this complex AC system in the collecting duct.
GRANTS
This research was funded in part by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-96392 and a Merit Review Award from the Department of Veterans Affairs (both to D. E. Kohan).
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1.Aarab L, Siaume-Perez S, Chabardes D. Cell-specific coupling of PGE2 to different transduction pathways in arginine vasopressin- and glucagon-sensitive segments of the rat renal tubule. Br J Pharmacol 126: 1041–1049, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahn D, Ge Y, Stricklett PK, Gill P, Taylor D, Hughes AK, Yanagisawa M, Miller L, Nelson RD, Kohan DE. Collecting duct-specific knockout of endothelin-1 causes hypertension and sodium retention. J Clin Invest 114: 504–511, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akiba S, Sato T. Cellular function of calcium-independent phospholipase A2. Biol Pharm Bull 27: 1174–1178, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Albert AP, Saleh SN, Peppiatt-Wildman CM, Large WA. Multiple activation mechanisms of store-operated TRPC channels in smooth muscle cells. J Physiol 583: 25–36, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beazely MA, Watts VJ. Galphaq-coupled receptor signaling enhances adenylate cyclase type 6 activation. Biochem Pharmacol 70: 113–120, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Beazely MA, Watts VJ. Regulatory properties of adenylate cyclases type 5 and 6: a progress report. Eur J Pharmacol 535: 1–12, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Bek MJ, Zheng S, Xu J, Yamaguchi I, Asico LD, Sun XG, Jose PA. Differential expression of adenylyl cyclases in the rat nephron. Kidney Int 60: 890–899, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Bishop GA, Berbari NF, Lewis J, Mykytyn K. Type III adenylyl cyclase localizes to primary cilia throughout the adult mouse brain. J Comp Neurol 505: 562–571, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Breyer MD, Ando Y. Hormonal signaling and regulation of salt and water transport in the collecting duct. Annu Rev Physiol 56: 711–739, 1994 [DOI] [PubMed] [Google Scholar]
- 10.Breyer MD, Zhang Y, Guan YF, Hao CM, Hebert RL, Breyer RM. Regulation of renal function by prostaglandin E receptors. Kidney Int Suppl 67: S88–S94, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Bustamante M, Hasler U, Leroy V, de Seigneux S, Dimitrov M, Mordasini D, Rousselot M, Martin PY, Feraille E. Calcium-sensing receptor attenuates AVP-induced aquaporin-2 expression via a calmodulin-dependent mechanism. J Am Soc Nephrol 19: 109–116, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butterworth MB, Edinger RS, Johnson JP, Frizzell RA. Acute ENaC stimulation by cAMP in a kidney cell line is mediated by exocytic insertion from a recycling channel pool. J Gen Physiol 125: 81–101, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chabardes D, Firsov D, Aarab L, Clabecq A, Bellanger AC, Siaume-Perez S, Elalouf JM. Localization of mRNAs encoding Ca2+-inhibitable adenylyl cyclases along the renal tubule. Functional consequences for regulation of the cAMP content. J Biol Chem 271: 19264–19271, 1996 [DOI] [PubMed] [Google Scholar]
- 14.Chang CT, Bens M, Hummler E, Boulkroun S, Schild L, Teulon J, Rossier BC, Vandewalle A. Vasopressin-stimulated CFTR Cl− currents are increased in the renal collecting duct cells of a mouse model of Liddle's syndrome. J Physiol 562: 271–284, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi EJ, Wong ST, Dittman AH, Storm DR. Phorbol ester stimulation of the type I and type III adenylyl cyclases in whole cells. Biochemistry 32: 1891–1894, 1993 [DOI] [PubMed] [Google Scholar]
- 16.Cooper DM. Compartmentalization of adenylate cyclase and cAMP signalling. Biochem Soc Trans 33: 1319–1322, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Cooper DM. Regulation and organization of adenylyl cyclases and cAMP. Biochem J 375: 517–529, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cooper DM, Mons N, Karpen JW. Adenylyl cyclases and the interaction between calcium and cAMP signalling. Nature 374: 421–424, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Defer N, Best-Belpomme M, Hanoune J. Tissue specificity and physiological relevance of various isoforms of adenylyl cyclase. Am J Physiol Renal Physiol 279: F400–F416, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Fagan KA, Mahey R, Cooper DM. Functional co-localization of transfected Ca2+-stimulable adenylyl cyclases with capacitative Ca2+ entry sites. J Biol Chem 271: 12438–12444, 1996 [DOI] [PubMed] [Google Scholar]
- 21.Gattone VH, 2nd, Wang X, Harris PC, Torres VE. Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat Med 9: 1323–1326, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Goldstein J, Silberstein C, Ibarra C. Adenylyl cyclase types I and VI but not II and V are selectively inhibited by nitric oxide. Braz J Med Biol Res 35: 145–151, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Gonin S, Deschenes G, Roger F, Bens M, Martin PY, Carpentier JL, Vandewalle A, Doucet A, Feraille E. Cyclic AMP increases cell surface expression of functional Na,K-ATPase units in mammalian cortical collecting duct principal cells. Mol Biol Cell 12: 255–264, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Helies-Toussaint C, Aarab L, Gasc JM, Verbavatz JM, Chabardes D. Cellular localization of type 5 and type 6 ACs in collecting duct and regulation of cAMP synthesis. Am J Physiol Renal Physiol 279: F185–F194, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Hidaka H, Sasaki Y, Tanaka T, Endo T, Ohno S, Fujii Y, Nagata T. N-(6-aminohexyl)-5-chloro-1-naphthalenesulfonamide, a calmodulin antagonist, inhibits cell proliferation. Proc Natl Acad Sci USA 78: 4354–4357, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoenderop JG, Hartog A, Willems PH, Bindels RJ. Adenosine-stimulated Ca2+ reabsorption is mediated by apical A1 receptors in rabbit cortical collecting system. Am J Physiol Renal Physiol 274: F736–F743, 1998 [DOI] [PubMed] [Google Scholar]
- 27.Hoffert JD, Chou CL, Fenton RA, Knepper MA. Calmodulin is required for vasopressin-stimulated increase in cyclic AMP production in inner medullary collecting duct. J Biol Chem 280: 13624–13630, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kamenetsky M, Middelhaufe S, Bank EM, Levin LR, Buck J, Steegborn C. Molecular details of cAMP generation in mammalian cells: a tale of two systems. J Mol Biol 362: 623–639, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kishore BK, Chou CL, Knepper MA. Extracellular nucleotide receptor inhibits AVP-stimulated water permeability in inner medullary collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol 269: F863–F869, 1995 [DOI] [PubMed] [Google Scholar]
- 30.Lee YJ, Song IK, Jang KJ, Nielsen J, Frokiaer J, Nielsen S, Kwon TH. Increased AQP2 targeting in primary cultured IMCD cells in response to angiotensin II through AT1 receptor. Am J Physiol Renal Physiol 292: F340–F350, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Magenheimer BS, St John PL, Isom KS, Abrahamson DR, De Lisle RC, Wallace DP, Maser RL, Grantham JJ, Calvet JP. Early embryonic renal tubules of wild-type and polycystic kidney disease kidneys respond to cAMP stimulation with cystic fibrosis transmembrane conductance regulator/Na+,K+,2Cl− co-transporter-dependent cystic dilation. J Am Soc Nephrol 17: 3424–3437, 2006 [DOI] [PubMed] [Google Scholar]
- 32.McVey M, Hill J, Howlett A, Klein C. Adenylyl cyclase, a coincidence detector for nitric oxide. J Biol Chem 274: 18887–18892, 1999 [DOI] [PubMed] [Google Scholar]
- 33.Miller RL, Lucero OM, Riemondy KA, Baumgartner BK, Brown D, Breton S, Nelson RD. The V-ATPase B1-subunit promoter drives expression of Cre recombinase in intercalated cells of the kidney. Kidney Int 75: 435–439, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morris R, Schafer J. cAMP increase sensitivity of ENaC subunits in the apical membrane of MDCK cells in direct proportion to amiloride-sensitive Na+ transport. J Gen Physiol 120: 71–85, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nasrallah R, Laneuville O, Ferguson S, Hebert RL. Effect of COX-2 inhibitor NS-398 on expression of PGE2 receptor subtypes in M-1 mouse CCD cells. Am J Physiol Renal Physiol 281: F123–F132, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Oishi R, Nonoguchi H, Tomita K, Marumo F. Endothelin-1 inhibits AVP-stimulated osmotic water permeability in rat medullary collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol 261: F951–F956, 1991 [DOI] [PubMed] [Google Scholar]
- 37.Ostrom RS, Liu X, Head BP, Gregorian C, Seasholtz TM, Insel PA. Localization of adenylyl cyclase isoforms and G protein-coupled receptors in vascular smooth muscle cells: expression in caveolin-rich and noncaveolin domains. Mol Pharmacol 62: 983–992, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Paunescu TG, Da Silva N, Russo LM, McKee M, Lu HA, Breton S, Brown D. Association of soluble adenylyl cyclase with the V-ATPase in renal epithelial cells. Am J Physiol Renal Physiol 294: F130–F138, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Pezzi V, Clark BJ, Ando S, Stocco DM, Rainey WE. Role of calmodulin-dependent protein kinase II in the acute stimulation of aldosterone production. J Steroid Biochem Mol Biol 58: 417–424, 1996 [DOI] [PubMed] [Google Scholar]
- 40.Pluznick JL, Zou DJ, Zhang X, Yan Q, Rodriguez-Gil DJ, Eisner C, Wells E, Greer CA, Wang T, Firestein S, Schnermann J, Caplan MJ. Functional expression of the olfactory signaling system in the kidney. Proc Natl Acad Sci USA 106: 2059–2064, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rieg T, Bundey RA, Chen Y, Deschenes G, Junger W, Insel PA, Vallon V. Mice lacking P2Y2 receptors have salt-resistant hypertension and facilitated renal Na+ and water reabsorption. FASEB J 21: 3717–3726, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Rieg T, Pothula K, Schroth J, Satriano J, Osswald H, Schnermann J, Insel PA, Bundey RA, Vallon V. Vasopressin regulation of inner medullary collecting ducts and compensatory changes in mice lacking adenosine A1 receptors. Am J Physiol Renal Physiol 294: F638–F644, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Sands JM, Nonoguchi H, Knepper MA. Vasopressin effects on urea and H2O transport in inner medullary collecting duct subsegments. Am J Physiol Renal Fluid Electrolyte Physiol 253: F823–F832, 1987 [DOI] [PubMed] [Google Scholar]
- 44.Schafer JA, Li L, Sun D. The collecting duct, dopamine and vasopressin-dependent hypertension. Acta Physiol Scand 168: 239–244, 2000 [DOI] [PubMed] [Google Scholar]
- 45.Schafer JA, Troutman SL. cAMP mediates the increase in apical membrane Na+ conductance produced in rat CCD by vasopressin. Am J Physiol Renal Fluid Electrolyte Physiol 259: F823–F831, 1990 [DOI] [PubMed] [Google Scholar]
- 46.Sheader EA, Wargent ET, Ashton N, Balment RJ. Rapid stimulation of cyclic AMP production by aldosterone in rat inner medullary collecting ducts. J Endocrinol 175: 343–347, 2002 [DOI] [PubMed] [Google Scholar]
- 47.Shen T, Suzuki Y, Poyard M, Miyamoto N, Defer N, Hanoune J. Expression of adenylyl cyclase mRNAs in the adult, in developing, and in the Brattleboro rat kidney. Am J Physiol Cell Physiol 273: C323–C330, 1997 [DOI] [PubMed] [Google Scholar]
- 48.Smani T, Zakharov SI, Leno E, Csutora P, Trepakova ES, Bolotina VM. Ca2+-independent phospholipase A2 is a novel determinant of store-operated Ca2+ entry. J Biol Chem 278: 11909–11915, 2003 [DOI] [PubMed] [Google Scholar]
- 49.Stevens T, Nakahashi Y, Cornfield DN, McMurtry IF, Cooper DM, Rodman DM. Ca2+-inhibitable adenylyl cyclase modulates pulmonary artery endothelial cell cAMP content and barrier function. Proc Natl Acad Sci USA 92: 2696–2700, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Strait KA, Stricklett PK, Kohan DE. Altered collecting duct adenylyl cyclase content in collecting duct endothelin-1 knockout mice. BMC Nephrol 8: 8, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Strait KA, Stricklett PK, Kohan JL, Miller MB, Kohan DE. Calcium regulation of endothelin-1 synthesis in rat inner medullary collecting duct. Am J Physiol Renal Physiol 293: F601–F606, 2007 [DOI] [PubMed] [Google Scholar]
- 52.Sunahara RK, Taussig R. Isoforms of mammalian adenylyl cyclase: multiplicities of signaling. Mol Interv 2: 168–184, 2002 [DOI] [PubMed] [Google Scholar]
- 53.Sutters M, Yamaguchi T, Maser RL, Magenheimer BS, St John PL, Abrahamson DR, Grantham JJ, Calvet JP. Polycystin-1 transforms the cAMP growth-responsive phenotype of M-1 cells. Kidney Int 60: 484–494, 2001 [DOI] [PubMed] [Google Scholar]
- 54.Thastrup O, Dawson AP, Scharff O, Foder B, Cullen PJ, Drobak BK, Bjerrum PJ, Christensen SB, Hanley MR. Thapsigargin, a novel molecular probe for studying intracellular calcium release and storage. Agents Actions 27: 17–23, 1989 [DOI] [PubMed] [Google Scholar]
- 55.Torres VE. Vasopressin antagonists in polycystic kidney disease. Semin Nephrol 28: 306–317, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Veis JH, Dillingham MA, Berl T. Effects of prostacyclin on the cAMP system in cultured rat inner medullary collecting duct cells. Am J Physiol Renal Fluid Electrolyte Physiol 258: F1218–F1223, 1990 [DOI] [PubMed] [Google Scholar]
- 57.Wallace DP, Christensen M, Reif G, Belibi F, Thrasher B, Herrell D, Grantham JJ. Electrolyte and fluid secretion by cultured human inner medullary collecting duct cells. Am J Physiol Renal Physiol 283: F1337–F1350, 2002 [DOI] [PubMed] [Google Scholar]
- 58.Wallace DP, Reif G, Hedge AM, Thrasher JB, Pietrow P. Adrenergic regulation of salt and fluid secretion in human medullary collecting duct cells. Am J Physiol Renal Physiol 287: F639–F648, 2004 [DOI] [PubMed] [Google Scholar]
- 59.Wang H, Storm DR. Calmodulin-regulated adenylyl cyclases: cross-talk and plasticity in the central nervous system. Mol Pharmacol 63: 463–468, 2003 [DOI] [PubMed] [Google Scholar]
- 60.Wang X, Wu Y, Ward CJ, Harris PC, Torres VE. Vasopressin directly regulates cyst growth in polycystic kidney disease. J Am Soc Nephrol 19: 102–108, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wargent ET, Burgess WJ, Laycock JF, Balment RJ. Separate receptors mediate oxytocin and vasopressin stimulation of cAMP in rat inner medullary collecting duct cells. Exp Physiol 84: 17–25, 1999 [DOI] [PubMed] [Google Scholar]
- 62.Yasuda G, Umemura S, Jeffries WB. Effect of epinephrine on cAMP accumulation in cultured rat inner medullary collecting duct cells. Am J Physiol Renal Physiol 272: F192–F197, 1997 [DOI] [PubMed] [Google Scholar]