Abstract
Acute ischemic insults to the kidney are recognized complications of human sickle cell disease (SCD). The present study analyzed in a transgenic SCD murine model the early renal response to acute ischemia. Renal hemodynamics were profoundly impaired following ischemia in sickle mice compared with wild-type mice: glomerular filtration rate, along with renal plasma flow and blood flow rates, were markedly reduced, while renal vascular resistances were increased more than threefold in sickle mice following ischemia. In addition to these changes in renal hemodynamics, there were profound disturbances in renal signaling processes: phosphorylation of members of the MAPK and Akt signaling proteins occurred in the kidney in wild-type mice after ischemia, whereas such phosphorylation did not occur in the kidney in sickle mice after ischemia. ATP content in the postischemic kidney in sickle mice was less than half that observed in wild-type mice. Examination of the expression of candidate genes uncovered changes that may predispose to increased sensitivity of the kidney in sickle mice to ischemia: increased expression of inducible nitric oxide synthase and decreased expression of endothelial nitric oxide synthase, and increased expression of TNF-α. Inducibility of anti-inflammatory, cytoprotective genes, such as heme oxygenase-1 and IL-10, was not impaired in sickle mice after ischemia. We conclude that the kidney in SCD is remarkably vulnerable to acute ischemic insults. We speculate that such sensitivity of the kidney to ischemia in SCD may underlie the occurrence of acute kidney injury in patients with SCD and may set the stage for the emergence of chronic kidney disease in SCD.
Keywords: acute kidney injury, vasoocclusion, MAPK proteins, nitric oxide synthase, heme oxygenase, inflammation
ischemia represents a major pathway contributing to tissue injury and end-organ damage that occur in sickle cell disease (SCD) (9, 22, 26, 36, 38). Such ischemia arises either because of vasoocclusive disease that impairs the perfusion of circulatory beds, or because of altered vascular reactivity characterized by increased vasoconstriction (22, 36). Ischemia, if sufficiently severe and acute, can induce tissue infarction in SCD, and if relatively sustained and low grade, can set the stage for inflammation, fibrosis, and scarring. Understanding the basis for, and the responses elicited by, such ischemic insults is thus an important issue in elucidating the pathogenesis of the complications of SCD.
The kidney is prone to ischemic insults in SCD as evidenced by the frequent occurrence of medullary infarction and papillary necrosis, the latter resulting from the sludging of red blood cells in medullary vessels, impaired medullary perfusion, and attendant ischemia (4, 36, 45). However, ischemic insults are not restricted to the medullary circulation but also afflict the kidney cortex. Some 10% of hospitalized patients with SCD exhibit acute kidney injury (47), and while there are relatively few histological studies of the kidney in such patients, kidney biopsies and autopsy findings in patients with sickle cell disease/trait have demonstrated cortical lesions that include acute congestion of the glomerular microcirculation, cortical ischemia, and cortical infarction (2, 26, 30, 46). Radiographic and other visualization techniques have also documented cortical infarction in sickle cell trait/disease, which, in some instances, has involved the entire kidney (20, 29, 30, 46, 51).
Using a transgenic murine model of SCD, we previously demonstrated that the kidney in SCD exhibited increased sensitivity to ischemic insults, as evidenced by increased blood urea nitrogen and serum creatinine, and worsened histological injury, when assessed 24 h after the insult (34). At this time point, there was also evidence of a prominent systemic inflammatory response and adverse effects in distant organs in sickle mice following localized renal ischemia (34). Adverse systemic and extrarenal effects are recognized complications of localized renal ischemia of sufficient severity (17, 39). Such systemic and extrarenal processes may themselves feed back onto the kidney and thereby contribute to the greater deterioration in renal function and heightened histological injury observed at 24 h after localized renal ischemia.
The present study examined the nature of the renal response shortly after the release of the ischemic clamp to the kidney so as to determine whether there exists an early and intrinsic sensitivity of the kidney to an ischemic insult; examining the kidney shortly after the release of the ischemic clamp would mitigate the extent to which the observed changes in the kidney reflect the adverse effects of systemic and extrarenal phenomena that are elicited by localized renal ischemia. To this end, we undertook studies of renal hemodynamics, the latter enabling not only the determination of glomerular filtration rate (GFR) but also renal blood flow (RBF) and vascular resistances. Along with an assessment of renal hemodynamics, the present study examined cellular responses recognized in the literature as determinants of the severity of acute ischemic injury. Studies were thus undertaken of the expression of the MAPK/Akt signaling proteins and the expression of candidate genes that are known to promote or mitigate postischemic renal injury (3, 11, 14, 19, 27, 33, 48, 52, 54).
METHODS
All studies were approved by the Institutional Animal Care and Use Committee of Mayo Clinic and performed in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The transgenic murine model of SCD used in the current study is on a C57BL/6 background and is homozygous for the murine β-globin deletion and carries two transgenes, αHβS and αHβS-Antilles (23, 34, 35). In our prior studies using this murine model of SCD, an aging phenotype was not observed in the kidney in mice older than 12 mo of age (35). The present studies were performed in age-matched wild-type (C57BL/6) mice and sickle mice 10–12 mo old with similar numbers of male and female mice in each group.
Renal hemodynamics.
Studies of renal hemodynamics were performed as previously described (49, 50). Wild-type and sickle mice were anesthetized with pentobarbital sodium (60 mg/kg body wt ip) and placed on a temperature-regulated table to maintain body temperature at 37°C. Following a midline abdominal incision, both renal pedicles were gently dissected and then occluded with nontraumatic clamps (RS5426, Micro Aneurysm clip, straight, 10 mm, 125-g pressure; Roboz Surgical Instruments, Rockville, MD) for 22.5 min. The trachea was catheterized with PE-160 tubing; PE-10 tubing was used to cannulate the carotid artery, the jugular vein, and the urinary bladder to monitor mean arterial pressure, to infuse fluids, and to collect urine, respectively; mean arterial pressure was continuously recorded throughout the study. A solution of 0.9% saline containing 2.25% bovine serum albumin and 0.75% FITC-inulin (FITC-I; Sigma-Aldrich, St. Louis, MO) was infused into the jugular vein (0.25 μl·min−1·g body wt−1) so as to determine GFR under euvolemic conditions. An electromagnetic flow probe (Transonic Systems, Ithaca, NY) was placed around the left renal artery to determine RBF; RBF was continuously recorded throughout the study using a small animal blood flowmeter (T206; Transonic Systems). One hour and 45 min following the removal of the clamps to the renal pedicles, renal hemodynamic measurements were undertaken. Concentrations of FITC-I in urine and blood were measured using a fluorescence reader (FL 600; Bio-Tek, Winooski, VT), and GFR was determined from the calculated clearance of FITC-I. Renal vascular resistances were determined as the ratio of mean arterial pressure/RBF; renal plasma flow was determined as RBF × (100 − hematocrit)/100; and filtration fraction was calculated as GFR/renal plasma flow.
mRNA expression by quantitative real-time RT-PCR.
For analysis of gene expression, total RNA was extracted from mouse kidney tissue using the TRIzol method (Invitrogen, Carlsbad, CA) with subsequent purification with an RNeasy Mini Kit (Qiagen, Valencia, CA), according to each manufacturer's protocol (41). Two hundred nanograms of purified total RNA were subsequently used in 40-μl reverse transcription reactions (Transcriptor First Strand cDNA Synthesis Kit, Roche Applied Science, Indianapolis, IN) employing random hexamers. The resulting cDNA was used in quantitative real-time PCR analysis as in our earlier study (41). Reactions were performed on an ABI Prism 7900HT (Applied Biosystems, Foster City, CA) using TaqMan Mastermix reagent (part no. 4324020, Applied Biosystems). Probes and primers used for quantification were obtained as assay sets for each target mRNA (TaqMan Gene Expression Assays, Applied Biosystems) and used according to the manufacturer's protocol. Parameters for quantitative PCR were as follows: 10 min at 95°C, followed by 40 cycles of amplification for 15 s at 95°C, and 1 min at 60°C. Values for the expression of each target gene were derived from relative quantification against a standard curve constructed for each mRNA target, and normalized for the expression of 18S rRNA.
Western blotting for MAPK and Akt signaling proteins.
Western blot analysis was performed, as outlined in our prior studies (41), in kidney tissue that was immediately frozen in liquid nitrogen after ischemia and reflow. Briefly, 50 μg of whole organ lysate-protein were separated on 10% Tris·HCl Criterion gels (Bio-Rad) and transferred to polyvinylidene fluoride membranes. After blocking, membranes were incubated overnight at 4°C with primary antibodies followed by room temperature incubation with the appropriate horseradish peroxidase-conjugated secondary antibody. The bands were then visualized using enhanced chemiluminescence (catalog no. RPN-2106, Amersham, Piscataway, NJ). Primary antibodies employed in these studies were obtained from Cell Signaling (Danvers, MA): phospho-ERK1/2 and ERK1/2 (catalog nos. 9101 and 9102, respectively); phospho-p38 and p38 (catalog nos. 9211 and 9212, respectively); phospho-JNK and JNK (catalog nos. 9251 and 9252, respectively); and phospho-Akt and Akt (catalog nos. 9271 and 9272, respectively). Immunoblotting for β-actin (catalog no. 612657; BD Transduction Laboratories, San Diego, CA) was performed to verify equal protein loading.
Measurement of ATP in the kidney.
ATP content was measured employing a luminescence-based assay kit (catalog no. FLAA-1KT, Sigma) in kidney tissue that was immediately frozen in liquid nitrogen after ischemia and reflow. Kidney lysates were prepared by homogenization of tissues in 10 volumes of ice-cold 2.5% TCA and centrifugation at 1,000 g for 10 min at 4°C. The pH of the lysates was adjusted to 7.8 with Tris base, and a second centrifugation was performed to clear precipitation. ATP concentration was determined in these lysates according to the manufacturer's protocol using a TD-20/20 luminometer (Turner Designs, Sunnyvale, CA). The protein content of each TCA-precipitated pellet was determined using the Bradford method following resuspension in 0.5 N NaOH, and these values were used to normalize the measured ATP content for each respective lysate.
Statistical analysis.
Data are expressed as means ± SE. Comparison between two groups was undertaken by Student's t-test for parametric data and the Mann-Whitney test for nonparametric data. Data were considered significant for P < 0.05.
RESULTS
Renal hemodynamics.
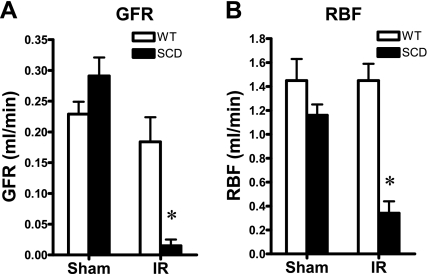
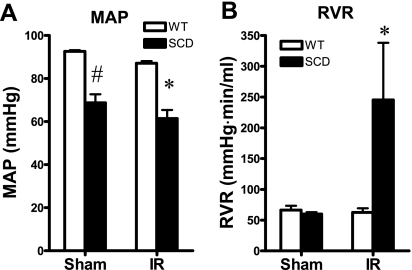
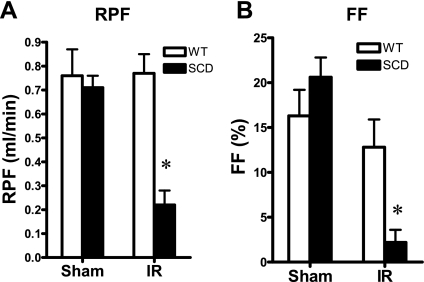
Following the same duration of renal ischemia as imposed on the wild-type mice, and as shown in Fig. 1, sickle mice exhibited a dramatic impairment of GFR; such reduction in GFR in sickle mice subjected to ischemia was contrasted by a tendency toward a higher GFR in sickle mice following sham ischemia. This marked reduction in GFR was accompanied by a pronounced reduction in RBF (Fig. 1). Mean arterial pressure was significantly lower in sickle mice, compared with wild-type mice, after both ischemia and sham ischemia (Fig. 2). The marked reduction in RBF in sickle mice following ischemia was due to heightened renal vascular resistances, the latter increased more than threefold in sickle mice following ischemia (Fig. 2). Renal plasma flow was decreased, and filtration fraction markedly diminished, in sickle mice in response to the ischemic insult, these indices not being significantly different in wild-type and sickle mice following sham ischemia (Fig. 3).
Fig. 1.
Renal hemodynamics: glomerular filtration rate (GFR) and renal blood flow (RBF). A: total GFR was determined by clearance techniques in wild-type (WT) and sickle mice (SCD) following bilateral renal ischemia-reperfusion (IR) for 22.5 min or after sham ischemia (Sham). B: RBF was determined by a flow probe placed on the left renal artery following bilateral renal ischemia for 22.5 min (IR) or after Sham; n = 5 and n = 4–5 for WT and SCD mice subjected to IR, respectively, and n = 4 for both WT and SCD mice subjected to Sham. *P < 0.05 vs. WT mice subjected to IR for that index.
Fig. 2.
Renal hemodynamics: mean arterial pressure (MAP) and renal vascular resistance (RVR). A: MAP was determined during studies of renal hemodynamics undertaken following bilateral renal IR for 22.5 min or after Sham. B: RVR was calculated as MAP/RBF from values of these indices determined during renal hemodynamic studies undertaken following bilateral renal IR for 22.5 min or after Sham; n = 5 and n = 4–5 for WT and SCD mice subjected to IR, and n = 4 for both WT and SCD mice subjected to Sham. *P < 0.05 vs. WT mice subjected to IR for that index. #P < 0.05 vs. WT mice subjected to Sham for that index.
Fig. 3.
Renal hemodynamics: renal plasma flow (RPF) and filtration fraction (FF). A: RPF was calculated as RBF × (100 − hematocrit)/100 from values of these indices determined during studies of renal hemodynamics undertaken following bilateral IR for 22.5 min or after Sham. B: FF of the kidney was calculated as single kidney GFR/RPF from these indices; n = 5 and n = 4 for WT and SCD mice subjected to IR and n = 4 for both WT and SCD mice subjected to Sham. *P < 0.05 vs. WT mice subjected to IR for that index.
Renal histology.
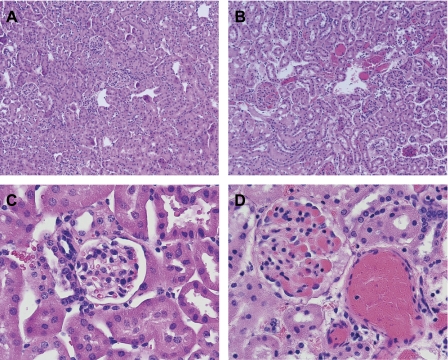
Histological analyses were performed of the kidneys following the renal hemodynamic studies. As shown in Fig. 4, analysis of the kidney in wild-type mice following ischemia showed little histological evidence of injury. The kidney in sickle mice after ischemia showed congestion in small arteries, arterioles, and glomerular capillaries, along with congestion in the medullary microcirculation; at this time point, the tubular epithelium appeared well preserved with little or no evidence of tubular necrosis (Fig. 4). The vasculature and glomeruli in the kidney in sickle mice were variably congested after ischemia (Fig. 4B), and Fig. 4D illustrates severe congestion in the vasculature and glomeruli in sickle mice after ischemia. In sickle mice subjected to sham ischemia, vascular congestion was restricted to the medullary circulation and not observed in the cortex (data not shown).
Fig. 4.
Histological examination of the renal cortex in WT and SCD mice following renal hemodynamic studies. A: renal cortex in WT mice after ischemia (×100). B. renal cortex in SCD mice after ischemia (×100). C: renal cortex in WT mice after ischemia (×400). D: renal cortex in SCD mice after ischemia (×400). All sections were stained with hematoxylin and eosin. As shown in these representative sections, the vasculature and glomeruli were congested in SCD mice (B and D) following ischemia; such congestion was not observed in the kidney in WT mice (A and C). The vasculature and glomeruli in the kidney in SCD mice were variably congested after ischemia (B), and D illustrates severe congestion in the vasculature and glomeruli in SCD mice after ischemia. Renal tubules showed no injury in WT and SCD mice.
MAPK/Akt signaling.
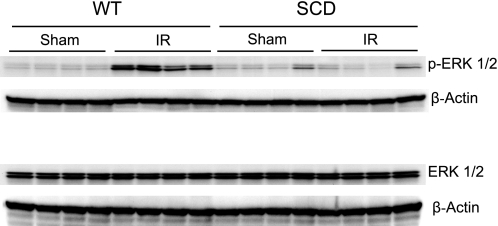
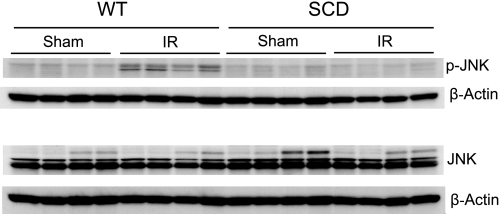
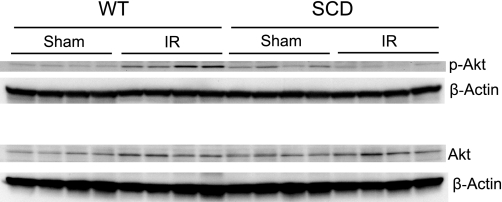
Components of the MAPK/Akt signaling pathways were assessed in the kidney 15 min after the release of the clamps to the renal pedicles. Expression of this signaling system was profoundly impaired in sickle mice after ischemia. Expression of ERK1/2 (p42/44), JNK (p44/46), p38, and Akt was entirely comparable in wild-type and sickle mice following renal ischemia or sham ischemia (Figs. 5, 6, 7, and 8). However, expression of the phosphorylated forms of these signaling proteins was markedly altered in sickle mice, compared with wild-type mice, after ischemia: in wild-type mice, ischemia induced a prompt and prominent phosphorylation in all of these proteins (Figs. 5–8), whereas in sickle mice, ischemia failed to induce phosphorylation of ERK1/2 (Fig. 5), JNK (Fig. 6), and p38 (Fig. 7), and, indeed, the expression of p-Akt in sickle mice decreased following the ischemic insult (Fig. 8).
Fig. 5.
Western blot analysis for the expression of ERK1/2 in WT and SCD mice following IR injury or Sham. Top: expression of phospho-ERK1/2 in the kidney in WT and SCD mice following renal IR or Sham. Bottom: expression of ERK1/2 in the kidney in WT and SCD mice following renal IR or Sham. For each Western blot analysis, each lane represents protein extracted from a single kidney of an individual mouse; evaluation of adequacy of loading was provided by immunoblotting for β-actin.
Fig. 6.
Western blot analysis for the expression of JNK in WT and SCD mice following IR injury or Sham. Top: expression of phospho-JNK in the kidney in WT and SCD mice following renal IR or Sham. Bottom: expression of JNK in the kidney in WT and SCD mice following renal IR or Sham. For each Western blot analysis, each lane represents protein extracted from a single kidney of an individual mouse; evaluation of adequacy of loading was provided by immunoblotting for β-actin.
Fig. 7.
Western blot analysis for the expression of p38 in WT and SCD mice following IR injury or Sham. Shown is expression of phospho-p38 and p38 in the kidney in WT and SCD mice following renal IR or Sham. For each Western blot analysis, each lane represents protein extracted from a single kidney of an individual mouse; evaluation of adequacy of loading was provided by immunoblotting for β-actin.
Fig. 8.
Western blot analysis for the expression of Akt in WT and SCD mice following IR or Sham. Top: expression of phospho-Akt in the kidney in WT and SCD mice following renal IR or Sham. Bottom: expression of Akt in the kidney in WT and SCD mice following renal IR or Sham. For each Western analysis, each lane represents protein extracted from a single kidney of an individual mouse; evaluation of adequacy of loading was provided by immunoblotting for β-actin.
Phosphorylation of proteins requires a supply of ATP, and it is well established that renal content of ATP decreases following ischemia and correlates with the severity of the ischemic insult (37, 53). We thus measured renal content of ATP in the same kidney tissue in which the above Western blot analyses were performed. Kidney ATP content in wild-type and sickle mice following sham ischemia was not significantly different (1.63 ± 0.49 vs. 1.21 ± 0.13 nmol/mg protein, n = 4 in each group, P = not significant). However, the ATP content in the postischemic kidney in sickle mice was <50% of the kidney ATP content in wild-type mice following ischemia (0.36 ± 0.05 vs. 0.17 ± 0.01 nmol/mg protein, P < 0.05). This lower renal ATP content in sickle mice after ischemia is consistent with a heightened sensitivity of the kidney in SCD to an ischemic insult, and such reduction in ATP content may contribute to the impaired phosphorylation of MAPK and Akt proteins in the kidney in sickle mice following ischemia.
Gene expression.
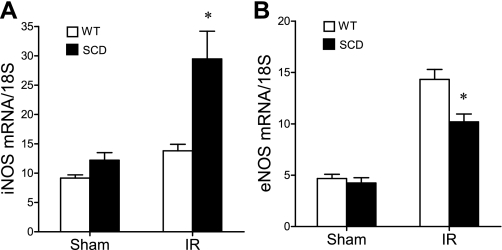
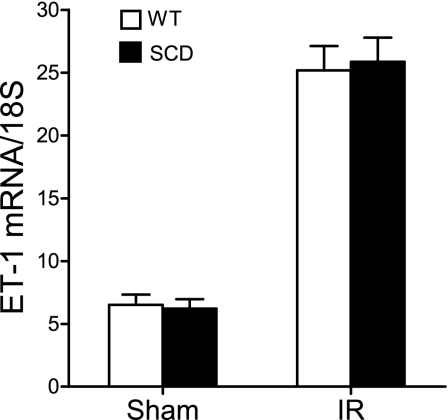
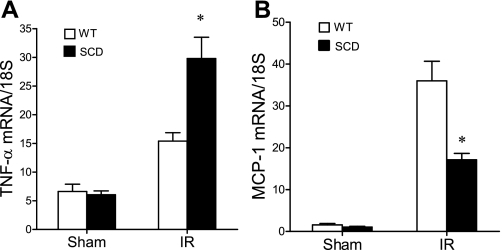
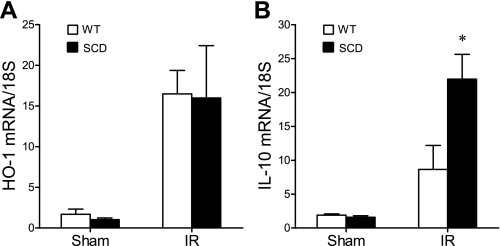
At 6 h after ischemia, we surveyed the expression of representative genes that are determinants of the sensitivity of the kidney to an ischemic insult; the genes surveyed included those of the nitric oxide synthase (NOS) family, and those that are essentially vasoconstricting [such as endothelin-1 (ET-1)], proinflammatory (TNF-α and MCP-1), or cytoprotective [heme oxygenase-1 (HO-1) and IL-10]. Inducible (i)NOS contributes to, and endothelial (e)NOS protects against, postischemic acute kidney injury, and notably, the expression of iNOS was increased, and that of eNOS was decreased, in the postischemic kidney in sickle mice compared with wild-type mice (Fig. 9). Expression of the vasoconstricting cytokine ET-1 was comparable in the postischemic kidney in wild-type and sickle mice (Fig. 10). After ischemia, the kidney in sickle mice exhibited increased expression of TNF-α but decreased expression of MCP-1 (Fig. 11). Expression of cytoprotective genes revealed comparable expression of HO-1 and increased expression of IL-10 in the postischemic kidney in sickle mice compared with wild-type mice (Fig. 12).
Fig. 9.
Expression of inducible nitric oxide synthase (iNOS) and endothelial nitric oxide synthase (eNOS) mRNA following IR or Sham in WT and SCD mice. iNOS mRNA (A) and eNOS mRNA (B) were determined by quantitative real-time RT-PCR in the kidney in WT and SCD mice following renal IR or Sham. For either gene, n = 6 in WT and SCD mice subjected to Sham, and n = 9 and n = 8 in WT and SCD mice, respectively, subjected to IR. *P < 0.05 SCD mice vs. WT mice subjected to IR for that gene.
Fig. 10.
Expression of endothelin-1 (ET-1) mRNA following IR or Sham in WT and SCD mice. ET-1 mRNA was determined by quantitative real-time RT-PCR in the kidney in WT and SCD mice following renal IR or Sham; n = 6 in WT and SCD mice subjected to Sham, and n = 9 and n = 8 in WT and SCD mice, respectively, subjected to IR.
Fig. 11.
Expression of TNF-α and MCP-1 mRNA following IR or Sham in WT and SCD mice. TNF-α mRNA (A) and MCP-1 mRNA (B) were determined by quantitative real-time RT-PCR in the kidney in WT and SCD mice following renal IR or Sham. For either gene, n = 6 in WT and SCD mice following Sham, and n = 9 and n = 8 in WT and SCD mice, respectively, following IR. *P < 0.05 SCD mice vs. WT mice following IR for that gene.
Fig. 12.
Expression of heme oxygenase-1 (HO-1) and IL-10 mRNA following IR or Sham in WT and SCD mice. HO-1 mRNA (A) and IL-10 mRNA (B) were determined by quantitative real-time RT-PCR in the kidney in WT and SCD mice following renal IR or Sham. For either gene, n = 6 in WT and SCD mice after Sham, and n = 9 and n = 8 in WT and SCD mice, respectively, following IR. *P < 0.05 SCD mice vs. WT mice following IR.
DISCUSSION
Abnormalities in the kidney and the renal circulation are widely recognized complications of SCD (4, 36, 45). Up to 70% of patients with SCD exhibit increased rates of urinary albumin excretion, and in a significant subset of these patients, chronic kidney disease develops (42). Other abnormalities include papillary necrosis, a host of tubular defects, and a propensity toward medullary carcinoma (4, 36, 45). Acute kidney injury is also a recognized complication of SCD (21, 47) and, indeed, reductions in creatinine clearances have been documented during sickle crisis, and following such crisis episodes, creatinine clearances improve (1). Over 10% of hospitalized patients with SCD exhibit acute kidney injury (47). While the basis for such renal dysfunction is likely multifactorial, cortical ischemia and infarction are relevant mechanisms occurring in at least a subset of these patients (2, 26, 30, 46); understanding the renal response to ischemia in SCD is thus a clinically relevant issue.
In the current study, we employed a technique traditionally employed in assessing renal function of the mammalian kidney, namely, the study of renal hemodynamics. These techniques, commonly employed in rats but undertaken in fewer published studies in mice, in part, because of the challenging nature of this technique in mice, allow not only the determination of GFR by the most rigorous method currently available (the clearance of inulin), but concomitantly afford an assessment of renal vascular behavior. Studies of renal hemodynamics are of particular utility in assessing the early renal responses to ischemic insults for at least two reasons. First, such studies may detect changes in GFR with greater sensitivity and reliability than can be revealed by filtration markers such as blood urea nitrogen or serum creatinine; the latter, in particular, represents an insensitive marker in the setting of reduced renal function, as creatinine is secreted in the urine in increased amounts in SCD (12). Since the present study was undertaken to address whether the vulnerability of the kidney in SCD appears promptly after ischemia, the study of renal hemodynamics affords an approach that is sufficiently sensitive to reliably detect differences in renal responses in wild-type and sickle mice. Second, in murine models of SCD, studies of regional blood flow and vascular resistances in vivo are quite limited in number, in general, and in the kidney, in particular: the study of renal hemodynamics thus allows an evaluation of the renal circulation in SCD in the unstressed state and following ischemia.
The present findings reveal that there is a prompt impairment of renal hemodynamics in sickle mice following ischemia. A dramatic finding was the severity of the filtration failure that occurred in sickle mice as characterized by a precipitous fall in GFR and in filtration fraction. As renal plasma flow is one of the four determinants of glomerular filtration, the marked fall in GFR can be attributed, at least in part, to the decreased renal plasma flow. This diminution in renal plasma flow and RBF arose, in turn, from increased renal vascular resistances, the latter increasing more than threefold in the kidney in sickle mice. Thus at least one mechanism underlying the marked reduction in GFR in sickle mice following ischemia is the marked augmentation in renal vascular resistances. It should also be noted that, as reflected by the fall in filtration fraction, the GFR was decreased to a greater extent than renal plasma flow rate. Thus it is possible that a decrement in other determinants of GFR, besides renal plasma flow, may contribute to the striking fall in GFR; these include the glomerular ultrafiltration coefficient and the glomerular transcapillary hydraulic pressure gradient.
Histological analyses of the kidney following the hemodynamic study demonstrated little or no injury in the wild-type mice. In contrast, the kidneys in sickle mice showed prominent congestion in the cortical vasculature and glomerular capillaries, along with congestion in the medullary microcirculation; at this time point, the tubular epithelium in the kidney in sickle mice appeared well preserved with no evidence of tubular necrosis. Such congestion of erythrocytes in the vasculature and glomerular microcirculation observed in the present studies was also apparent at a later time point after ischemia in the kidney in sickle mice, as described in our prior studies (34). However, at this later time point, acute tubular necrosis was also present, in all likelihood, reflecting the adverse effects of tubular ischemia due to the marked decrement in RBF documented in the present studies. The prominent vasoocclusive disease observed in the kidney in sickle mice following ischemia may underlie the heightened renal vascular resistances, either because of resistance to blood flow imposed by luminal vasoocclusive disease, or because of the instigation of a vasoconstrictive myogenic reflex in resistor vessels in the kidney.
Increased renal vascular resistances in sickle mice following ischemia may also reflect the possibility that ischemia may preferentially promote the elaboration and effects of vasoconstricting rather than vasorelaxant species. In this regard, we observed that following ischemia, the kidney in sickle mice, compared with wild-type mice, exhibited diminished induction of eNOS and augmented induction of iNOS. Such alterations in the profile of expression of these NOS isoforms would be expected, from the current literature, to predispose to acute ischemic kidney injury: experimental strategies that inhibit iNOS expression or those that augment eNOS expression, are generally protective in ischemic acute kidney injury (19). The lesser induction of eNOS that was observed in the kidney in sickle mice following ischemia may lead to less generation of the vasodilator, nitric oxide. The augmented induction of iNOS may generate increased amounts of nitric oxide which, by interacting with the superoxide anion, would produce the potent oxidizing species, peroxynitrite; peroxynitrite, by oxidizing tetrahydrobiopterin, may uncouple eNOS, with an attendant increased generation of superoxide anion and decreased production of nitric oxide; furthermore, peroxynitrite may also oxidatively inactivate prostacyclin synthase, the enzyme that generates prostacyclin, a vasorelaxant species (19, 25). In view of these considerations, the profile of expression of iNOS and eNOS in sickle mice after ischemia, as observed in the present study, may thereby promote a heightened vasoconstrictive response. It seems unlikely that this vasoconstrictive response involves ET-1 since tissue production of ET-1 is transcriptionally regulated after renal ischemia, and gene expression of ET-1 was comparably increased in the postischemic kidneys in wild-type and sickle mice; however, it is possible that the vascular responsivity to ET-1 may be increased in the sickle kidney after ischemia. Inhibition of endothelin receptors has been reported to reduce vasoconstrictive responses, organ injury, and mortality in response to hypoxia-reoxygenation in a murine model of SCD (44).
The kidney in sickle mice also exhibited a marked alteration in expression of MAPK and Akt signaling proteins. These proteins influence inflammatory responses and cell survival, proliferation, migration, and differentiation following ischemia and thus modulate the nature of the renal response to ischemia; in particular, ERK and Akt represent prosurvival pathways in the kidney and other tissues (3, 14, 52). The kidney in sickle mice exhibited a profoundly altered expression of these proteins after ischemia: while exhibiting levels of expression of these proteins comparable to that observed in wild-type mice, the postischemic kidney in sickle mice failed to exhibit increased expression of the phosphorylated forms of any of these proteins, the latter clearly observed in the postischemic kidney of wild-type mice. As the phosphorylated form of these signaling proteins usually determines the active moiety, these data demonstrate that the recruitment of signaling processes following ischemia is markedly impaired in the kidney in sickle mice. This uniform failure of expression of phosphorylation of MAPK proteins, observed in the present study in response to ischemia, differs from prior observations regarding expression of these proteins in murine SCD in response to hypoxia (28).
One mechanism underlying this failure of phosphorylation in the postischemic kidney in sickle mice may involve the lower amounts of ATP present in these kidneys. This latter finding also underscores the increased sensitivity of the kidney in SCD since, as is well described in the literature, renal ATP content is predictably decreased as ischemia of increasing severity is imposed, and strategies that augment ATP are protective against ischemic acute kidney injury (37, 53).
In an attempt to determine whether altered expression of specific genes may contribute to the increased sensitivity of the kidney to ischemic injury, we assessed the expression of proinflammatory and anti-inflammatory genes, as an exaggerated expression of proinflammatory mediators, or an impaired expression of anti-inflammatory mediators, may contribute to acute ischemic kidney injury (11, 27, 33, 48). Such increased sensitivity of the kidney in sickle mice may reflect increased expression of TNF-α but not MCP-1; nor can such sensitivity be explained by impaired expression of anti-inflammatory, cytoprotective genes (such as HO-1 or IL-10), as expression of these genes was not impaired in the postischemic kidney in sickle mice. These studies of gene expression thus identify TNF-α as an inflammatory cytokine that may contribute to the increased sensitivity of the kidney in sickle mice to ischemia.
The impaired expression of MCP-1 in sickle mice, compared with wild-type mice, following ischemia was quite surprising as MCP-1 is markedly induced by oxidative stress, heme, and iron, all of which would be expected to be increased in the kidney in sickle mice following ischemia (8, 24). It is possible that such impaired induction of MCP-1 may reflect the impairment of signaling processes in the kidney in sickle mice following ischemia: specifically, expression of MCP-1 is dependent on p-ERK-dependent cell signaling. Interestingly, expression of eNOS also relies on p-ERK-dependent cell signaling, and less inducibility of eNOS was observed in the postischemic kidney in sickle mice.
A number of murine models of SCD have been introduced, and their essential features have been previously summarized (31, 32). As assessed by the degree of hemolytic anemia and vasoocclusion, the murine model employed in our present and prior studies provides a phenotype of mild to moderate severity in its unstressed state, and the same is true for other murine SCD models expressing both murine and human globin genes, such as, for example, the NYC1 model (15, 16) and the SAD model (13, 44). Since murine globin chains hinder polymerization of hemoglobin S, murine models of SCD that express only human globin chains (“knockout-transgenic” models) exhibit a severe phenotype as reflected by marked hemolytic anemia and vasoocclusive disease (40, 43). Murine models exhibit nephromegaly, medullary congestion, and decreased concentrating ability, and the kidney in these models has been variably characterized. The model used in the current studies shows increased content of heme, oxidative stress, and a sensitivity to oxidative insults (34, 35); peroxynitrite and nitrotyrosine content is increased in the NYC1 model (5, 6); glomerulosclerosis occurs in transgenic SAD mice (13), while the knockout-transgenic models exhibit features of human sickle cell nephropathy even at a relatively young age (40, 43).
Human SCD exhibits marked variation in the severity of disease and heterogeneity in the clinical phenotype. The availability of murine SCD models that exhibit varying severity thus recapitulates a recognized feature of the human disease (31, 32). Moreover, murine models of varying severity lend themselves to the interrogation of specific issues (31, 32). The knockout-transgenic models, for example, facilitate the study of end-organ damage that can evolve in SCD. The murine models of mild to moderate severity, such as employed in the present study, allow the examination of imposed stress or insults on SCD. In this regard, our present and prior studies employing a murine model of mild to moderate severity have uncovered the increased sensitivity of the kidney in SCD to ischemic insults (34), and the distant effects of localized renal ischemia in SCD (34).
In summary, the kidney in SCD appears remarkably vulnerable to acute ischemic insults. Such sensitivity of the kidney may be relevant not only to the occurrence of acute kidney injury observed in SCD patients during crisis or other acute illnesses but also to chronic kidney disease that may develop in patients with SCD. It is now increasingly recognized that acute kidney injury is not a uniformly reversible process (7, 10, 18) and that inadequate resolution of acute ischemic kidney injury may set the stage for chronic kidney disease (7, 10, 18). It is thus possible that episodes of acute ischemic insults to the kidney, to which the patient with SCD is prone, may leave in their wake, processes that promote the emergence of chronic kidney disease.
GRANTS
These studies are supported by National Institutes of Health Grants HL55552 and DK47060.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
We thank Allan Ackerman for technical expertise and Tammy Engel for secretarial expertise.
REFERENCES
- 1.Adenbigbe A, Arije A, Akinkugbe O. Glomerular function in sickle cell disease patients during crisis. Afr J Med Sci 23: 153–160, 1994 [PubMed] [Google Scholar]
- 2.al Ani O, Baqi N. Clinical quiz. Cortical infarction secondary to intravascular sickling in the renal cortex. Pediatr Nephrol 13: 267–268, 1999 [PubMed] [Google Scholar]
- 3.Arany I, Megyesi JK, Kaneto H, Tanaka S, Safirstein RL. Activation of ERK or inhibition of JNK ameliorates H2O2 cytotoxicity in mouse renal proximal tubule cells. Kidney Int 65: 1231–1239, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Ataga KI, Orringer EP. Renal abnormalities in sickle cell disease. Am J Hematol 63: 205–211, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Bank N, Aynedjian HS, Qiu JH, Osei SY, Ahima RS, Fabry ME, Nagel RL. Renal nitric oxide synthases in transgenic sickle cell mice. Kidney Int 50: 184–189, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Bank N, Kiroycheva M, Singhal PC, Anthony GM, Southan GJ, Szabo C. Inhibition of nitric oxide synthase ameliorates cellular injury in sickle cell mouse kidneys. Kidney Int 58: 82–89, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Basile DP. Rarefaction of peritubular capillaries following ischemic acute renal failure: a potential factor predisposing to progressive nephropathy. Curr Opin Nephrol Hypertens 13: 1–7, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Belcher JD, Beckman JD, Balla G, Balla J, Vercellotti G. Heme degradation and vascular injury. Antioxid Redox Signal 12: 233–248, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belcher JD, Mahaseth H, Welch TE, Vilback AE, Sonbol KM, Kalambur VS, Bowlin PR, Bischof JC, Hebbel RP, Vercellotti GM. Critical role of endothelial cell activation in hypoxia-induced vasoocclusion in transgenic sickle mice. Am J Physiol Heart Circ Physiol 288: H2715–H2725, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Coca SG, Yusuf B, Shlipak MG, Garg AX, Parikh CR. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis 53: 961–973, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curtis LM, Agarwal A. HOpe for contrast-induced acute kidney injury. Kidney Int 72: 907–909, 2007 [DOI] [PubMed] [Google Scholar]
- 12.de Jong PE, de Jong-Van Den Berg TW, Sewrajsingh GS, Schouten H, Donker AJ, Statius van Eps LW. The influence of indomethacin on renal haemodynamics in sickle cell anaemia. Clin Sci (Lond) 59: 245–250, 1980 [DOI] [PubMed] [Google Scholar]
- 13.De Paepe ME, Trudel M. The transgenic SAD mouse: a model of human sickle cell glomerulopathy. Kidney Int 46: 1337–1345, 1994 [DOI] [PubMed] [Google Scholar]
- 14.di Mari JF, Davis R, Safirstein RL. MAPK activation determines renal epithelial cell survival during oxidative injury. Am J Physiol Renal Physiol 277: F195–F203, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Fabry ME, Costantini F, Pachnis A, Suzuka SM, Bank N, Aynedjian HS, Factor SM, Nagel RL. High expression of human beta S- and alpha-globins in transgenic mice: erythrocyte abnormalities, organ damage, and the effect of hypoxia. Proc Natl Acad Sci USA 89: 12155–12159, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fabry ME, Nagel RL, Pachnis A, Suzuka SM, Costantini F. High expression of human beta S- and alpha-globins in transgenic mice: hemoglobin composition and hematological consequences. Proc Natl Acad Sci USA 89: 12150–12154, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feltes CM, Van Eyk J, Rabb H. Distant-organ changes after acute kidney injury. Nephron Physiol 109: p80–p84, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Goldberg R, Dennen P. Long-term outcomes of acute kidney injury. Adv Chronic Kidney Dis 15: 297–307, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Goligorsky MS, Brodsky SV, Noiri E. Nitric oxide in acute renal failure: NOS versus NOS. Kidney Int 61: 855–861, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Granfortuna J, Zamkoff K, Urrutia E. Acute renal infarction in sickle cell disease. Am J Hematol 23: 59–64, 1986 [DOI] [PubMed] [Google Scholar]
- 21.Hassell KL, Eckman JR, Lane PA. Acute multiorgan failure syndrome: a potentially catastrophic complication of severe sickle cell pain episodes. Am J Med 96: 155–162, 1994 [DOI] [PubMed] [Google Scholar]
- 22.Hebbel RP, Vercellotti GM. The endothelial biology of sickle cell disease. J Lab Clin Med 129: 288–293, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Juncos JP, Grande JP, Murali N, Croatt AJ, Juncos LA, Hebbel RP, Katusic ZS, Nath KA. Anomalous renal effects of tin protoporphyrin in a murine model of sickle cell disease. Am J Pathol 169: 21–31, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanakiriya SK, Croatt AJ, Haggard JJ, Ingelfinger JR, Tang SS, Alam J, Nath KA. Heme: a novel inducer of MCP-1 through HO-dependent and HO-independent mechanisms. Am J Physiol Renal Physiol 284: F546–F554, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Katusic ZS, d'Uscio LV, Nath KA. Vascular protection by tetrahydrobiopterin: progress and therapeutic prospects. Trends Pharmacol Sci 30: 48–54, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimmelsteil P. Vascular occlusion and ischemic infarction in sickle cell disease. Am J Med Sci 216: 11–19, 1948. [PubMed] [Google Scholar]
- 27.Kinsey GR, Li L, Okusa MD. Inflammation in acute kidney injury. Nephron Exp Nephrol 109: e102–e107, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kiroycheva M, Ahmed F, Anthony GM, Szabo C, Southan GJ, Bank N. Mitogen-activated protein kinase phosphorylation in kidneys of beta(s) sickle cell mice. J Am Soc Nephrol 11: 1026–1032, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Madani G, Papadopoulou AM, Holloway B, Robins A, Davis J, Murray D. The radiological manifestations of sickle cell disease. Clin Radiol 62: 528–538, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Miller WA, Peck D, Lowman RM. Perirenal hematoma in association with renal infarction in sickle-cell trait. Radiology 92: 351–352, 1969 [DOI] [PubMed] [Google Scholar]
- 31.Nagel RL. A knockout of a transgenic mouse—animal models of sickle cell anemia. N Engl J Med 339: 194–195, 1998 [DOI] [PubMed] [Google Scholar]
- 32.Nagel RL, Fabry ME. The panoply of animal models for sickle cell anaemia. Br J Haematol 112: 19–25, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Nath KA. Heme oxygenase-1: a provenance for cytoprotective pathways in the kidney and other tissues. Kidney Int 70: 432–443, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Nath KA, Grande JP, Croatt AJ, Frank E, Caplice NM, Hebbel RP, Katusic ZS. Transgenic sickle mice are markedly sensitive to renal ischemia-reperfusion injury. Am J Pathol 166: 963–972, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nath KA, Grande JP, Haggard JJ, Croatt AJ, Katusic ZS, Solovey A, Hebbel RP. Oxidative stress and induction of heme oxygenase-1 in the kidney in sickle cell disease. Am J Pathol 158: 893–903, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nath KA, Katusic ZS, Gladwin MT. The perfusion paradox and vascular instability in sickle cell disease. Microcirculation 11: 179–193, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Nath KA, Norby SM. Reactive oxygen species and acute renal failure. Am J Med 109: 665–678, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Osarogiagbon UR, Choong S, Belcher JD, Vercellotti GM, Paller MS, Hebbel RP. Reperfusion injury pathophysiology in sickle transgenic mice. Blood 96: 314–320, 2000 [PubMed] [Google Scholar]
- 39.Paladino JD, Hotchkiss JR, Rabb H. Acute kidney injury and lung dysfunction: a paradigm for remote organ effects of kidney disease? Microvasc Res 77: 8–12, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paszty C, Brion CM, Manci E, Witkowska HE, Stevens ME, Mohandas N, Rubin EM. Transgenic knockout mice with exclusively human sickle hemoglobin and sickle cell disease. Science 278: 876–878, 1997 [DOI] [PubMed] [Google Scholar]
- 41.Pittock ST, Norby SM, Grande JP, Croatt AJ, Bren GD, Badley AD, Caplice NM, Griffin MD, Nath KA. MCP-1 is up-regulated in unstressed and stressed HO-1 knockout mice: Pathophysiologic correlates. Kidney Int 68: 611–622, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Powars DR, Chan LS, Hiti A, Ramicone E, Johnson C. Outcome of sickle cell anemia: a 4-decade observational study of 1056 patients. Medicine (Baltimore) 84: 363–376, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Ryan TM, Ciavatta DJ, Townes TM. Knockout-transgenic mouse model of sickle cell disease. Science 278: 873–876, 1997 [DOI] [PubMed] [Google Scholar]
- 44.Sabaa N, de Franceschi L, Bonnin P, Castier Y, Malpeli G, Debbabi H, Galaup A, Maier-Redelsperger M, Vandermeersch S, Scarpa A, Janin A, Levy B, Girot R, Beuzard Y, Leboeuf C, Henri A, Germain S, Dussaule JC, Tharaux PL. Endothelin receptor antagonism prevents hypoxia-induced mortality and morbidity in a mouse model of sickle-cell disease. J Clin Invest 118: 1924–1933, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scheinman JI. Sickle cell disease and the kidney. Nat Clin Pract Nephrol 5: 78–88, 2009 [DOI] [PubMed] [Google Scholar]
- 46.Sickles EA, Korobkin M. Perirenal hematoma as a complication of renal infarction in sickle-cell trait. A case report. Am J Roentgenol Radium Ther Nucl Med 122: 800–803, 1974 [DOI] [PubMed] [Google Scholar]
- 47.Sklar AH, Perez JC, Harp RJ, Caruana RJ. Acute renal failure in sickle cell anemia. Int J Artif Organs 13: 347–351, 1990 [PubMed] [Google Scholar]
- 48.Sutton TA, Fisher CJ, Molitoris BA. Microvascular endothelial injury and dysfunction during ischemic acute renal failure. Kidney Int 62: 1539–1549, 2002 [DOI] [PubMed] [Google Scholar]
- 49.Tracz MJ, Juncos JP, Croatt AJ, Ackerman AW, Grande JP, Knutson KL, Kane GC, Terzic A, Griffin MD, Nath KA. Deficiency of heme oxygenase-1 impairs renal hemodynamics and exaggerates systemic inflammatory responses to renal ischemia. Kidney Int 72: 1073–1080, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tracz MJ, Juncos JP, Grande JP, Croatt AJ, Ackerman AW, Rajagopalan G, Knutson KL, Badley AD, Griffin MD, Alam J, Nath KA. Renal hemodynamic, inflammatory, and apoptotic responses to lipopolysaccharide in HO-1−/− mice. Am J Pathol 170: 1820–1830, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wong WS, Moss AA, Federle MP, Cochran ST, London SS. Renal infarction: CT diagnosis and correlation between CT findings and etiologies. Radiology 150: 201–205, 1984 [DOI] [PubMed] [Google Scholar]
- 52.Yang CW, Ahn HJ, Jung JY, Kim WY, Li C, Choi BS, Kim HW, Kim YS, Moon IS, Kim J, Bang BK. Preconditioning with cyclosporine A or FK506 differentially regulates mitogen-activated protein kinase expression in rat kidneys with ischemia/reperfusion injury. Transplantation 75: 20–24, 2003 [DOI] [PubMed] [Google Scholar]
- 53.Zager RA. Pathogenetic mechanisms in nephrotoxic acute renal failure. Semin Nephrol 17: 3–14, 1997 [PubMed] [Google Scholar]
- 54.Zager RA, Johnson AC, Lund S, Hanson S. Acute renal failure: determinants and characteristics of the injury-induced hyperinflammatory response. Am J Physiol Renal Physiol 291: F546–F556, 2006. [DOI] [PubMed] [Google Scholar]