Abstract
We previously showed that the inhalational anesthetic isoflurane protects against renal proximal tubule necrosis via isoflurane-mediated stimulation and translocation of sphingosine kinase-1 (SK1) with subsequent synthesis of sphingosine-1-phosphate (S1P) in renal proximal tubule cells (Kim M, Kim M, Kim N, D'Agati VD, Emala CW Sr, Lee HT. Am J Physiol Renal Physiol 293: F1827–F1835, 2007). We also demonstrated that the anti-necrotic and anti-inflammatory effect of isoflurane is due in part to phosphatidylserine (PS) externalization and subsequent release of transforming growth factor-β1 (TGF-β1) (Lee HT, Kim M, Kim J, Kim N, Emala CW. Am J Nephrol 27: 416–424, 2007). In this study, we tested the hypothesis that isoflurane, via TGF-β1 release, increases caveolae formation in the buoyant fraction of the cell membrane of human renal proximal tubule (HK-2) cells to organize SK1 and S1P signaling. To detect SK1 protein in the caveolae/caveolin fractions, we overexpressed human SK1 in HK-2 cells (SK1-HK-2). SK1-HK-2 cells exposed to isoflurane increased caveolae/caveolin formation in the buoyant membrane fractions which contained key signaling intermediates involved in isoflurane-mediated renal tubule protection, including S1P, SK1, ERK MAPK, and TGF-β1 receptors. Furthermore, treating SK1-HK-2 cells with recombinant TGF-β1 or PS liposome mixture increased caveolae formation, mimicking the effects of isoflurane. Conversely, TGF-β1-neutralizing antibody blocked the increase in caveolae formation induced by isoflurane in SK1-HK-2 cells. The increase in SK1 activity in the caveolae-enriched fractions from isoflurane-treated nonlentivirus-infected HK-2 cells, while smaller in magnitude, was qualitatively similar to that found in the SK1-HK-2 cell line. Finally, isoflurane also increased caveolae formation in the kidneys of TGF-β1 +/+ mice but not in TGF-β1 +/− mice (mice with reduced levels of TGF-β1). Our study demonstrates that isoflurane organizes several key cytoprotective signaling intermediates including TGF-β1 receptors, SK1 and ERK, within the caveolae fraction of the plasma membrane. Our findings may help to unravel the cellular signaling pathways of volatile anesthetic-mediated renal protection and lead to new therapeutic applications of inhalational anesthetics during the perioperative period.
Keywords: acute kidney injury, acute renal failure, caveolin-1, volatile anesthetics
perioperative acute renal failure (ARF) is a frequent and devastating event that lacks effective therapy (11). There are many causes of perioperative ARF including renal ischemia and reperfusion (IR) injury, sepsis, rhabdomyolysis, and radiocontrast nephropathy (11). Development of perioperative ARF leads to high mortality and is frequently complicated by many other life-threatening conditions including respiratory failure, sepsis, and multiorgan dysfunction syndrome (1). Unfortunately, the mortality and morbidity rate from perioperative ARF has changed little over the past 50 years (11).
Volatile anesthetics are widely used during the perioperative period, as virtually all patients subjected to general anesthesia are anesthetized with these drugs. Volatile anesthetics, in addition to their analgesic and anesthetic effects, have nonanesthetic properties in many organ systems including the kidney. We demonstrated previously that volatile anesthetics, including isoflurane [2-chloro-2-(difluoromethoxy)-1,1,1-trifluoro-ethane], protected against renal injury in vivo by reducing necrosis and inflammation (18, 21, 23). We also demonstrated that volatile anesthetics produced anti-necrotic and anti-inflammatory effects in vitro via activation of cytoprotective kinases (ERK and Akt) and the induction of HSP70 (19). Subsequently, we showed that volatile anesthetics increased the release of transforming growth factor-β1 (TGF-β1) in human proximal tubule (HK-2) cells via externalization of plasma membrane phosphatidylserine (PS) and that this increase in TGF-β1 in turn activated cytoprotective proteins (i.e., ERK, Akt, and heat shock protein 70) (20, 22). Finally, we recently demonstrated that the volatile anesthetic isoflurane activates and translocates sphingosine kinase-1 (SK1) to the plasma membrane of renal tubule cells and initiates sphingosine 1-phosphate (S1P)→S1P1 receptor signaling to mediate the renal protective effects (12). However, the mechanism of SK1 activation and translocation with isoflurane treatment and how TGF-β1 released with isoflurane interacts with SK1→S1P signaling remain to be elucidated.
Lipid rafts or cholesterol and sphingolipid-rich compartments within the cell membrane play important roles in colocalization and compartmentalization of related signaling molecules. Caveolae (little caves) lipid rafts are nonclathrin-coated, small (50–100 nm), flask-like invaginations that are supported by caveolin scaffolding proteins and create signaling microdomains in the plasma membrane (30, 31, 36, 37). Caveolin proteins function as scaffolds for signaling molecules inside and outside the caveolae, and the caveolae/caveolin complex provides temporal and spatial regulation of signal transduction. Several studies implicated a variety of signaling molecule interactions within these caveolae lipid rafts (7, 8). In this study, we tested the hypothesis that PS externalization and TGF-β1 release by isoflurane directly stimulate SK1 activity via enhancing the colocalization of SK1 and TGF-β1 receptors in caveolae lipid rafts in human renal proximal tubules cells. Our study demonstrates that isoflurane via TGF-β1 release increases caveolae formation in the buoyant membrane fractions of HK-2 cells, and these caveloae fractions contain several key signaling intermediates of isoflurane-mediated renal protection, including TGF-β1 receptors, ERK MAPK, SK1, and increased concentrations of S1P.
METHODS
HK-2 cell culture and generation of SK1-overexpressing HK-2 cells.
HK-2 cells (immortalized human proximal tubular cell line, American Type Culture Collection, Manassas, VA) were grown and passed in culture medium (50:50 mixture of DMEM low glucose and F12 plus 5% serum) and antibiotics (100 U/ml penicillin G, 100 μg/ml streptomycin, and 0.25 μg/ml amphotericin B) at 37°C in a 100% humidified atmosphere of 5% CO2-95% air.
Our preliminary studies demonstrated that commercially available antibodies failed to detect endogenous levels of SK1 protein in unmodified HK-2 cell caveolae fractions. To detect the translocation of SK1 protein in and out of the caveolae layers, we generated HK-2 cells overexpressing wild-type human SK1 (from cDNA designed by Dr. S. Pitson, Hanson Institute, Australia) (29) by infecting HK-2 cells with SK1-encoding lentivirus. Lentivirus-encoding wild-type SK1 were produced by subcloning the SK1 cDNA into a modified shuttle vector CMV-pLL3.7 where the insert expression is driven by a CMV promoter followed by an IRES-enhanced green fluorescent protein (EGFP) sequence for simultaneous coexpression of the EGFP reporter gene and hemagglutinin (HA) tag. When expressed, this vector yields two independent proteins, EGFP and the human SK1 protein fused with the HA tag. Lentivirus was produced by a triple transfection of CMV-SK1-pLL3.7 (10 μg), pVSVG (Vesicular stomatitis virus G, Invitrogen; 7 μg), and pΔ8.9 (from Dr. V. Parjs, MIT, MA; 5 μg) as previously described (26). In brief, CMV-SK1-pLL3.7 and the two packaging vectors were cotransfected into 80–90% confluent HEK-293FT cells in 10-cm tissue culture plates using 20 μl of Lipofectamine 2000 (Invitrogen, Carlsbad, CA) in serum-free OptiMEM medium according to the manufacturer's recommendations. Supernatant was collected after 48 h and passed thorough a 0.45-μm filter to remove cells and debris. An approximate viral titer was determined by infecting HEK293 cells with serial dilutions of the final virus suspension and counting the number of fluorescent cells 48 h after infection. We typically obtained titers of 2–5 × 106 infectious U/ml starting from one 10-cm plate of HEK293FT cells. Cell culture medium was removed from HK-2 cells growing on six-well plates and replaced with 1.5 ml of lentivirus medium and incubated at 37°C. After 48 h, the lentivirus medium was replaced with cell culture medium and infected HK-2 cells were propagated and used for immunoblotting studies.
However, we were able to detect SK1 enzyme activity in nonlentivirus-infected (unmodified) HK-2 cells as well as in SK1-overexpressing HK-2 (SK1-HK-2) cells as described previously (12).
Exposure of HK-2 cells to isoflurane.
For isoflurane treatment, HK-2 cells in 10-cm plates were placed in an air-tight, 37°C, humidified modular incubator chamber (Billups-Rothenberg, Del Mar, CA) with inflow and outflow connectors as described previously (19, 22). The inlet port was connected to an in-line calibrated agent-specific vaporizer (Datex-Ohmeda) to deliver varying concentrations of isoflurane [0–2.5% or 0–2 minimum alveolar concentration (MAC): defined as the percent concentration in the alveolus of an inhaled anesthetic agent required to prevent 50% of subjects from moving in response to a painful stimulus when used as the sole anesthetic] mixed with carrier gas (95% air-5% CO2) at 10 l/min. The outlet port was connected to a Datex-Ohmeda 5250 RGM gas analyzer that measured volatile anesthetic concentrations. Since we previously demonstrated that clinically relevant concentrations of volatile anesthetics (0.5 to 2 MAC) treatment for 4 to 14 h provided significant cytoprotection in HK-2 cells (19), we exposed HK-2 cells to 0.6–2.5% isoflurane (0.5–2 MAC) for 2, 6, or 14 h at 37°C for this study as well. Control cells were exposed to carrier gas in an identical modular incubator chamber. In preliminary experiments, we determined that the cellular protein concentration between carrier gas and isoflurane-treated plates was equivalent by performing protein assays.
Exposure of HK-2 cells to recombinant TGF-β1, TGF-β1-neutralizing antibody, or PS liposome.
We previously demonstrated that treatment of HK-2 cells with volatile anesthetics, including isoflurane, led to the release of TGF-β1 via externalization of PS to the outer leaflet of the cell membrane (20, 22). Some HK-2 cells were treated with recombinant TGF-β1 (1 ng/ml, 14 h; R&D Systems, Minneapolis, MN) in lieu of isoflurane treatment. To block the effects of isoflurane-induced TGF-β1 release, some HK-2 cells were pretreated with neutralizing TGF-β1 antibody (MAB2401, 1 μg/ml; R&D Systems) for 30 min before isoflurane treatment. To mimic PS exposure after isoflurane treatment, small unilamellar liposomes containing a 50-to-50 molar ratio of PS (derived from brain; Avanti Polar Lipids, Alabaster, AL) to phosphatidylcholine (PC; derived from bovine liver; Avanti Polar Lipids) were made as described previously (22). Briefly, the individual phospholipids, stored in chloroform-methanol (90:10), were added to glass tubes and dried under nitrogen. PBS was added, and the lipid mix was vortexed and then sonicated for 3 min. The PC-PS liposomes were used at a concentration of 10 μM total lipids per well (in a 6-well plate) and treated for 14 h. We previously demonstrated that these regimens of TGF-β1-neutralizing antibody or PS liposome blocked and mimicked, respectively, the cellular effects (HSP70 induction, ERK phosphorylation, and cytoprotection) of volatile anesthetics in HK-2 cells (22). We also used nonneutralizing control isotype antibody (mouse IgG) to test the specificity of the neutralizing TGF-β1 antibody (BD Biosciences, San Jose, CA). Moreover, we also tested the nonspecific effects of control liposomal preparations [PC liposome (from bovine liver; Avanti Polar Lipids) and N-(2,3-dioleoyloxy-1-propyl)trimethylammonium methyl sulfate liposome (DOTAP from bovine liver; Sigma)] to establish the specificity of PS and to exclude nonspecific alterations on plasmalemmal integrity, structure, and/or fluidity.
Mice.
All animal protocols were approved by the Institutional Animal Care and Use Committee of Columbia University (New York, NY). Breeder pairs of TGF-β1 heterozygous mice were obtained from Dr. T. Doetschman (University of Cincinnati, OH) to produce wild-type (+/+) and heterozygous (+/−) mice (34). These mice are mixed in genetic background (50% 129SV and 50% CF-1) and TGF-β1 +/− mice synthesize less than 50% of TGF-β1 compared with TGF-β1 +/+ mice. TGF-β1 knockout mice are not viable to adulthood. The genotypes of mice were determined by PCR analysis on tail DNA obtained from 4-wk-old animals and all experiments were performed with littermates to ensure identical genetic backgrounds.
Exposure of mice to isoflurane.
Male TGF-β1 +/+ or TGF-β1 +/− mice (25–30 g) were anesthetized with intraperitoneal pentobarbital sodium (50 mg/kg body wt, or to effect) or with inhaled isoflurane (∼1.2% or 1 MAC) as described previously (18, 21). Pentobarbital sodium-anesthetized mice were allowed to breathe room air spontaneously, whereas isoflurane-anesthetized mice breathed spontaneously while receiving ∼1 MAC isoflurane in room air. To anesthetize the mice with isoflurane, they were placed in an airtight 2-liter chamber with inflow and outflow outlets (Braintree Scientific, Braintree, MA). One MAC isoflurane was delivered in room air at 5 l/min using an agent-specific Datex-Ohmeda vaporizer. The isoflurane concentration was monitored by an infrared analyzer that sampled gas at the outflow hose. All experiments were performed on an electric heating pad under a warming light. Body temperature was maintained at 37°C. Each animal was anesthetized for 4 h.
Caveolae extraction.
SK1-HK-2 cells grown to confluence in 10-cm plates were lysed with ice-cold 1% Triton X-100 buffer. To extract caveolae fractions from mouse kidney, mice anesthetized with pentobarbital sodium or isoflurane (1.2%) for 4 h were killed, and their renal cortices were dissected and homogenized in lysis buffer {100 mM sucrose, 1 mM EGTA, 20 mM MOPS, pH 7.4, 5% Percoll, 0.01% digitonin, protease [4-(2-aminoethyl)-benzenesulfonyl fluoride, aprotinin, bestatin, E-64, leupeptin, pepstatin A (Calbiochem, San Diego, CA)]} and phosphatase [Na3VO4, Na2MoO4, sodium tartrate, imidazole (Sigma, St. Louis, MO)] inhibitors on ice. All reagents were prechilled to 4°C. Caveolae-enriched fractions were obtained by centrifuging triton-extracted cell lysates on a discontinuous OptiPrep (D1556, Sigma) density gradient and allowing the buoyant triton-resistant caveolae to separate from the remaining cellular components. Care was taken to ensure that the temperature of triton-exposed caveolae did not rise above 4°C. One milliliter of HK-2 (from a 10-cm plate) or mouse kidney lysate (1 kidney) was carefully layered on four layers (35, 40, 45, 50%, top to bottom) of varying Optiprep/Lysis Buffer mixtures (2 ml) in 10-ml polyallomer tubes (#355640, BD Biosciences). Preparations were centrifuged at 200,000 g for 4 h at 4°C. Fractions 1 to 6 (1 ml, top to bottom, buoyant fractions) were individually obtained and denatured in 4× Laemmli's buffer for immunoblot analysis. To quantify isolated caveolae, caveolin-1 immunoblotting was performed for layers 1–6 as described below and band intensities from layers 2–4 were obtained for some of the experiments. Fractions 2–4 were pooled and analyzed by HPLC to measure the S1P levels. After caveolae fractions were extracted from renal cortices of TGF-β1 wild-type (+/+) mice and TGF-β1 heterozygous (+/−) mice, we pooled layers 2–4 and performed caveolin-1 immunoblotting in these pooled fractions. In preliminary experiments, fractions 1–8 were analyzed for the distribution of raft (Ganglioside Asialo GM1 in addition to caveolin-1) and nonraft markers (transferrin receptor). We determined that GM1 and caveolin-1 immunoreactivity were most abundant in the buoyant, raft fractions, whereas the transferrin receptors were localized in the heavy membrane fraction layers (layers 6–7; data not shown).
HPLC detection of S1P.
HPLC to measure S1P levels in caveolae fractions was performed as described by Min et al. (27) with two steps of sample pretreatment: enzymatic dephosphorylation of S1P by alkaline phosphatase (100 U/sample; Sigma) and subsequent analysis of o-phthalaldehyde derivatives of the liberated sphingosine bases by HPLC. By introducing C17 S1P (Avanti Polar Lipids) as an internal standard, S1P present in a sample can be quantified on a C18 reversed-phase column with a simple mobile phase of acetonitrile:deionized distilled water (90:10 vol/vol).
Immunoblot analyses.
Immunoblotting analyses of caveolae preparations were performed as described previously (16, 17). We also performed immunoblotting in total cellular lysates to determine whether protein expression changed with isoflurane treatment. The primary antibodies for ERK, TGF-β1 receptor type I, TGF-β1 type II, and HA were from Santa Cruz Biotechnologies (Santa Cruz, CA). The primary antibody for AKT1 was from Cell Signaling Technologies (Danvers, MA). The primary antibody for SK1 was from ECM Biosciences (Versailles, KY). The primary antibodies for caveolin-1, caveolin-2, and caveolin-3 were from Cell BD Biosciences. The primary antibodies for Transferrin receptor and GM-1 were from Abcam (Cambridge, MA) and WAKO Chemicals USA (Richmond, VA), respectively. The secondary antibody (goat anti-rabbit or anti-mouse IgG conjugated to horseradish peroxidase at 1:5,000 dilution) was detected with enhanced chemiluminescence immunoblotting detection reagents (Amersham), with subsequent exposure to a CCD camera coupled to a UVP Bio-Imaging System (Upland, CA) and a personal computer. The band intensities of the immunoblots were within the linear range of exposure for all experiments.
Reverse transcription-polymerase chain reactions for caveolin isoforms (1–3), SK1, and GAPDH.
We detected mRNA expression of caveolin-1, caveolin-2, and caveolin-3 in HK-2 cells with semiquantitative RT-PCR as described previously (24). We also detected expression of SK1 mRNA in HK-2 cells after lentivirus infection and sorting. Caveolin-1–3 and SK1 primers were designed based on published GenBank sequences specific for human genes (Table 1) and to amplify a genomic region that spans one or two introns to eliminate the confounding effect of amplifying contaminating genomic DNA. RT-PCR was performed using the Access RT-PCR System (Promega) as described previously (20). For each experiment, we also performed semiquantitative RT-PCR under conditions yielding linear results for GAPDH (Table 1) to confirm equal RNA input.
Table 1.
Primer sequences
| Primers | Species | Size, bp | Sequence (Sense/Antisense) | Annealing °C/Cycle # |
|---|---|---|---|---|
| Caveolin-1 | Human | 359 | 5′-ACAAGATCTTCCTTCCTCAG-3′ | 60/28 |
| 5′-AGTGAAGGTGGTGAAGCTG-3′ | ||||
| Caveolin-2 | Human | 403 (isomer 1) | 5′-ACGACTCCTACAGCCACCAC-3′ | 62/17 |
| 215 (isomer 2) | 5′-CGTCCTACGCTCGTACACAA-3′ | |||
| Caveolin-3 | Human | 381 | 5′-GATGATGGCAGAAGAGCACA-3′ | 64/32 |
| 5′-GTGCGGATGCAGAGTGAGTA-3′ | ||||
| SK1 | Human | 330 | 5′-ATCTCCTTCACGCTGATGC-3′ | 66/26 |
| 5′-GTGCAGAGACAGCAGGTTCA-3′ | ||||
| 5′-ACCACAGTCCATGCCATCAC-3′ | 65/15 | |||
| GAPDH | Human | 450 | 5′-CACCACCCTGTTGCTGTAGCC-3′ |
SK1, sphingosine kinase-1.
Statistical analysis.
The data were analyzed with Student's t-test when comparing means between two groups or with one-way ANOVA plus Bonferroni post hoc multiple comparison test to compare mean values across multiple treatment groups. In all cases, a probability statistic <0.05 was taken to indicate significance. All data are expressed throughout the text as means ± SE.
Protein determination.
Protein content was determined with the Pierce Chemical (Rockford, IL) bicinchoninic acid protein assay reagent with BSA as a standard.
Materials.
All drugs were in saline and all chemicals were obtained from Sigma unless otherwise specified.
RESULTS
Generation of SK1-overexpressing human renal proximal tubule cells in vitro.
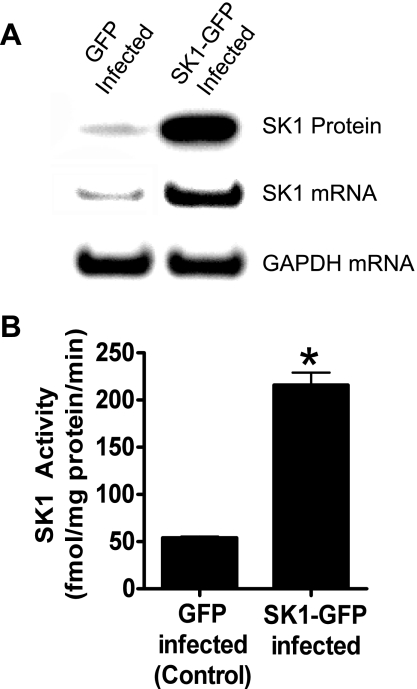
Figure 1 shows increased protein and mRNA expression as well as SK1 activity in HK-2 cells overexpressing the SK1 enzyme (SK1-HK-2 cells).
Fig. 1.
Human renal proximal tubule (HK-2) cells infected with sphingosine kinase-1 (SK1)-encoding lentivirus (SK1-HK-2 cells) for 2 days show increased SK1 protein and mRNA expression (A) and increased SK1 activity [B; n = 4, *P < 0.05 vs. green fluorescent protein (GFP) control].
Caveolin subtypes in HK-2 cells.
With RT-PCR, we detected expression of all three subtypes of caveolin mRNA in HK-2 cells (data not shown). However, in HK-2 cells we detected caveolin-1 and caveolin-2 but not caveolin-3 (muscle-specific caveolin) by immunoblotting.
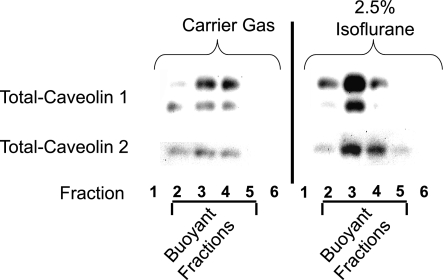
Isoflurane increases caveolin protein content in the buoyant fraction of the plasma membrane in SK1-HK-2 cells.
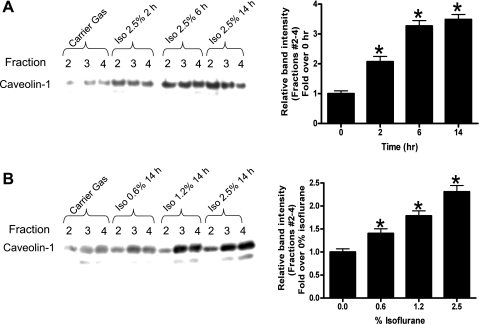
We initially probed for the expression of caveolin-1 and caveolin-2 as markers of caveolae in SK1-HK-2 cells. Isoflurane treatment resulted in an enrichment of total caveolin-1 and caveolin-2 protein in the buoyant membrane microdomain fractions isolated with differential density gradients (Fig. 2). Caveolin-1 immunoblotting showed two isoforms (Fig. 2). In subsequent studies, we performed caveolin-1 immunoblotting as a marker of caveolae isolated from the buoyant fractions. We also showed that isoflurane treatment caused time (2.5% for 2–14 h; A)- and dose (0–2.5% for 14 h; B)-dependent increases in caveolin-1 expression in SK1-HK-2 cells (Fig. 3). However, isoflurane treatment did not increase the mRNA expression of caveolin-1 or caveolin-2 (data not shown). Isoflurane treatment also did not change the total caveolin protein in HK-2 cells (data not shown).
Fig. 2.
Isoflurane (2.5% for 14 h) increases caveolin-1 and caveolin-2 protein in the buoyant fractions of SK1-HK-2 cells. Confluent SK1-HK-2 cells grown in 10-cm plate were processed for each experiment. Control cells were treated with carrier gas (95% air-5% CO2) for 14 h. Caveolin immunoblotting was used to detect the caveolae microdomain fraction. Caveolin-1 immunoblotting showed 2 isoforms. Similar results obtained from 4 independent experiments.
Fig. 3.
Isoflurane treatment causes time (2.5% for 2–14 h; A)- and dose (0–2.5% for 14 h; B)-dependent increases in caveolin-1 protein expression in SK1-HK-2 cells. Confluent SK1-HK-2 cells grown in 10-cm plate were processed for each experiment. Similar results obtained from 4 independent experiments. Densitometric quantifications of relative band intensities from buoyant fractions (layers 2–4) are also shown (n = 4, right of each panel). *P < 0.05 vs. carrier gas. Means ± SE.
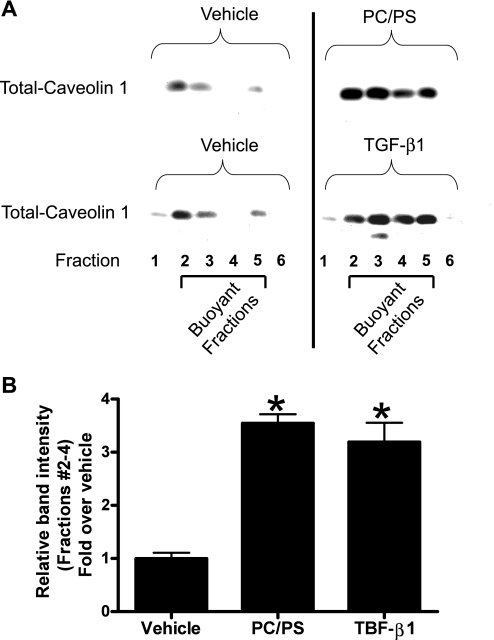
Exogenous TGF-β1 or PS/PC liposome mimics and TGF-β1-neutralizing antibody blocks isoflurane-induced increase in HK-2 cell caveolae lipid rafts.
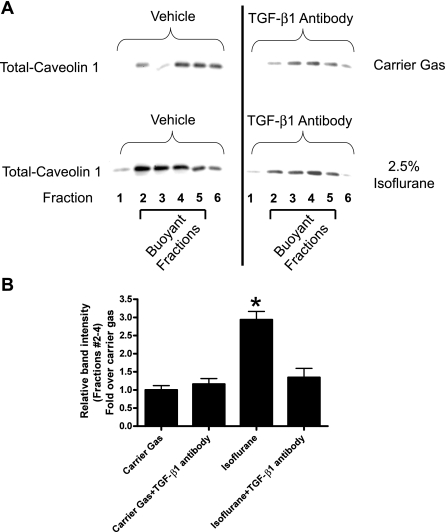
We tested whether modulation of TGF-β1 signaling similarly modulates the caveolae/caveolin microdomain organization in HK-2 cells. HK-2 cells treated for 14 h with 1 ng/ml TGF-β1 or with a PS/PC liposome (10 μM) mixture showed increased caveolin-1 protein and caveolae lipid rafts in the buoyant fractions (Fig. 4). Conversely, pretreatment with neutralizing TGF-β1 antibody (1 μg/ml) prevented the isoflurane-induced increase in caveolin-1 protein in the buoyant fractions (Fig. 5).
Fig. 4.
A: treatment with phosphotidylcholine/phosphatidylserine (PC/PS) mixture (100 μM) or transforming growth factor-β1 (TGF-β1; 1 ng/ml) for 14 h increases caveolin-1 protein in the buoyant fractions of SK1-HK-2 cells. B: densitometric quantifications of relative band intensities from buoyant fractions (layers 2–4) are shown (n = 3). *P < 0.05 vs. vehicle. Means ± SE. Confluent SK1-HK-2 cells grown in 10-cm plate were processed for each experiment.
Fig. 5.
A: pretreatment with neutralizing TGF-β1 antibody (1 μg/ml) prevents the isoflurane (2.5% for 14 h)-induced increase in caveolin-1 protein in the buoyant fractions. Confluent SK1-HK-2 cells grown in 10-cm plate were processed for each experiment. Similar results obtained from 3 independent experiments. B: densitometric quantifications of relative band intensities from buoyant fractions (layers 2–4) are shown (n = 3). *P < 0.05 vs. vehicle. Means ± SE.
Caveolae fraction in plasma membrane increases with isoflurane and associates with key signaling intermediates of TGF-β1 and S1P signaling.
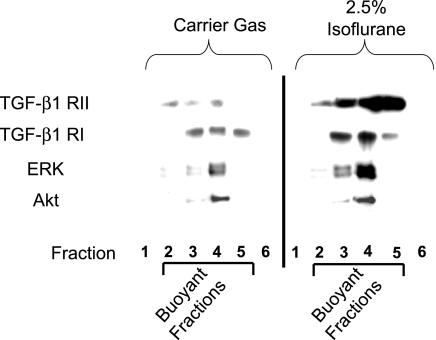
We tested whether isoflurane treatment results in increased association of TGF-β1 signaling protein components in the caveolae fraction (i.e., TGF-β1 receptors). We also probed for the presence of ERK, Akt, and SK1 (key intermediates involved in isoflurane signaling in HK-2 cells). Figure 6 shows that TGF-β1 receptor types I and II indeed increased in gradient-separated caveolae-containing fractions with isoflurane treatment (2.5% for 14 h). Moreover, we detected increased ERK MAPK but not Akt within the caveolae fractions of SK1-HK-2 cells. We also observed increased phospho-ERK but not phospho-Akt within the caveolae fractions after isoflurane treatment (data not shown). Isoflurane treatment did not change the total ERK or TGF-β1 receptor types I and II protein expression in HK-2 cells (data not shown).
Fig. 6.
Isoflurane (2.5% for 14 h) treatment increases the localization of TGF-β1 receptors type I and II and ERK MAPK to the caveolae/caveolin fraction from SK1-HK-2 cells. Confluent SK1-HK-2 cells grown in 10-cm plates were processed for each experiment. Similar results obtained from 4 independent experiments.
Isoflurane treatment increases the SK1 activity and S1P formation in the caveolae/caveolin fraction from HK-2 cells.
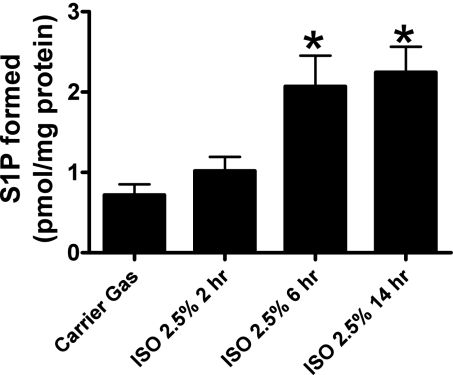
The SK1 activity in the isoflurane-treated group (2.5% for 6 h) increased (1.2 ± 0.2 pmol/min S1P formed, n = 4) compared with the carrier gas-treated group (0.6 ± 0.07 pmol/min S1P formed, n = 4, P < 0.05) in SK1-HK-2 cells. We then utilized HPLC to measure the formation of S1P (pmol/mg protein) after carrier gas or isoflurane (2.5% for 2, 6, or 14 h) within the caveolae fraction of SK1-HK-2 cells and demonstrated an increase in S1P in the isoflurane-treated group at 6 h (2.1 ± 0.4 pmol/mg protein, n = 6) and 14 h (2.3 ± 0.3 pmol/mg protein, n = 6) compared with the carrier gas-treated group (0.72 ± 0.13 pmol/mg protein, n = 6; Fig. 7). The SK1 activity in nonlentivirus-infected HK-2 cells was significantly lower than that of SK1-HK-2 cells but nonetheless also increased with isoflurane treatment (0.054 ± 0.004 pmol/min S1P formed, n = 4) compared with the carrier gas treatment (0.031 ± 0.003 pmol/min S1P formed, n = 4, P < 0.05).
Fig. 7.
Isoflurane (2.5% for 6 or 14 h) treatment increases sphingosine-1-phosphate (S1P) formation in the caveolae/caveolin fraction from SK1-HK-2 cells (n = 6). Confluent SK1-HK-2 cells grown in 10-cm plates were processed for each experiment. *P < 0.05 vs. carrier gas.
Exogenous TGF-β1 or PS/PC liposome mimics and recombinant TGF-β1 antibody blocks isoflurane-induced increases in S1P within the caveolae/caveolin fraction from HK-2 cells.
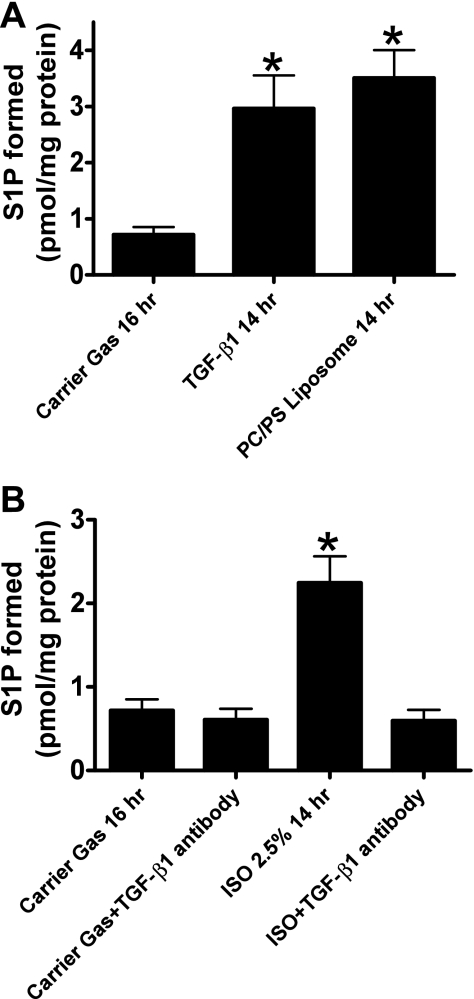
SK1-HK-2 cells treated for 14 h with 1 ng/ml TGF-β1 or with a PS/PC liposome (10 μM) mixture showed increased S1P content in the buoyant fractions (Fig. 8A). Conversely, pretreatment with neutralizing TGF-β1 antibody (1 μg/ml) prevented the increase in S1P in the buoyant fractions (Fig. 8B).
Fig. 8.
A: treatment with PC/PS mixture (100 μM) or TGF-β1 (1 ng/ml) for 14 h increases S1P formation in the buoyant fractions of SK1-HK-2 cells (n = 6). *P < 0.05 vs. carrier gas. B: pretreatment with neutralizing TGF-β1 antibody (1 μg/ml) prevents the isoflurane (2.5% for 14 h)-induced increase in S1P formation in the buoyant fractions (n = 6). Confluent SK1-HK-2 cells grown in 10-cm plates were processed for each experiment. *P < 0.05 vs. carrier gas.
TGF-β1-deficient mice do not increase S1P in caveolae microdomains with isoflurane treatment.
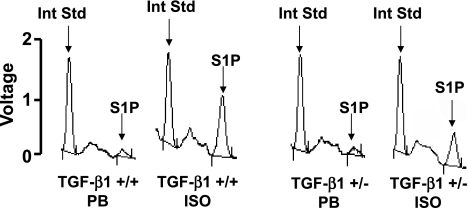
To determine the role of TGF-β1 in isoflurane-mediated increases in S1P, we extracted caveolae fractions from renal cortices of TGF-β1 wild-type (+/+) mice and TGF-β1 heterozygous (+/−) mice. We showed previously that TGF-β1 +/− mice produce significantly (∼60%) less TGF-β1 compared with TGF-β1 +/+ mice (15). Immunoblotting for caveolin-1 showed that isoflurane anesthesia increased caveolin-1 protein more in the buoyant caveolae fractions from TGF-β1 +/+ mice compared with fractions from TGF-β1 +/− mice (data not shown). We measured S1P in these caveolae fractions with HPLC after either 4-h anesthesia with isoflurane (1.2%) or pentobarbital sodium. S1P levels from renal cortical caveolae fractions increased in TGF-β1 +/+ mice after isoflurane anesthesia (1.6 ± 0.3 pmol/mg protein, n = 3; Fig. 9) compared with pentobarbital sodium anesthesia (0.4 ± 0.2 pmol/mg protein, P < 0.05 vs. isoflurane group, n = 3). In contrast, the increase in S1P with isoflurane anesthesia was less in TGF-β1 +/− mice (0.7 ± 0.2 pmol/mg protein, P < 0.05 vs. TGF-β1 +/+ mice treated with isoflurane, n = 3; Fig. 9).
Fig. 9.
HPLC analysis for S1P levels in caveolae fractions isolated from murine renal cortices. TGF-β1 wild-type (+/+) or heterozygous (+/−) mice were either anesthetized with pentobarbital sodium (PB) or with isoflurane (ISO; 1.2%) for 4 h. Representative data from 3 independent experiments. By introducing C17-S1P as an internal standard (Int Std), we can quantify the amount of S1P present in the original sample.
SK1 colocalizes within the caveolae fractions.
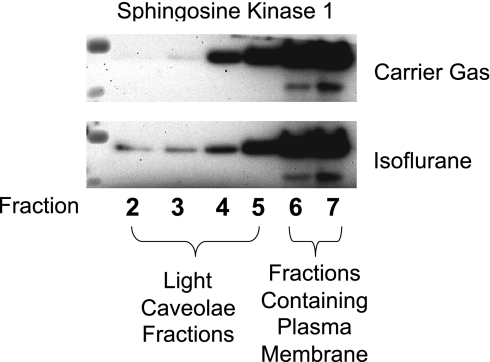
We performed immunoblotting for SK1 in buoyant caveolae fractions after carrier gas or isoflurane (2.5% for 14 h) treatment in HK-2 cells. We show in Fig. 10 that the majority of SK1 is located in the heavy plasma membrane fraction after carrier gas treatment. However, isoflurane treatment caused translocation of SK1 into the light caveolae fractions of HK-2 cells (Fig. 10). We also treated SK1-HK-2 cells with TGF-1 or PC/PS liposome and demonstrated that SK1 also translocated from heavy to light caveolae fractions (data not shown).
Fig. 10.
Isoflurane treatment causes translocation of SK1 from heavy (layers 4–7) to buoyant caveolae fractions (layers 2–3) in HK-2 cells. Representative of 3 independent experiments.
DISCUSSION
The major findings of this study are that isoflurane treatment causes increased caveolae/caveolin expression in the buoyant fractions of HK-2 cells as well as in mouse kidneys. The caveolae fractions from HK-2 cells contained increased signaling intermediates implicated in volatile anesthetic-mediated renal protection including ERK MAPK, SK1, and receptors for TGF-β1 and also showed increased SK1 activity as well as S1P content after isoflurane treatment. The increase in the caveolae fraction after isoflurane treatment is associated with the externalization of PS and subsequent release of TGF-β1, as PS/PC liposome mixture or recombinant TGF-β1 mimicked the isoflurane-mediated increase in caveolae formation. Furthermore, TGF-β1-neutralizing antibody blocked the isoflurane-mediated increase in caveolae formation in HK-2 cells. These data strongly support the hypothesis that isoflurane increases the caveolin protein content of the buoyant caveolae microdomains and the colocalization/interactions of proteins (ERK, SK1, TGF-β1 receptors) as well as lipids (PS) involved in TGF-β1 signaling in these microdomains.
Caveolae are specialized lipid raft signaling microdomains of the plasma membrane, rich in cholesterol and sphingolipids, and supported by caveolin-scaffolding proteins (30, 31, 36, 37). The membrane of caveolae has been characterized as belonging to lipid raft domains, based on its composition and tendency to float higher in density gradient centrifugations than most other cell membrane fractions. Caveolin proteins function as scaffolds for signaling molecules inside and outside the caveolae and the caveolae/caveolin complex provides temporal and spatial regulation of signal transduction. Caveolin-1-supported caveolae are present in most mammalian cells, whereas caveolin-2 and caveolin-3 are preferentially expressed in adipose tissue and muscle cells, respectively (30, 31, 36, 37). We determined in our study that HK-2 cells contain caveolin-1 and caveolin-2 isoforms. We also show increased caveolae/caveolin-scaffolding microdomains in the detergent-resistant and buoyant fraction of cell membranes after isoflurane treatment. These caveolae fractions contained increased SK1 protein as well as SK1 enzyme activity with isoflurane treatment (Fig. 10). SK1 is a conserved lipid kinase that catalyzes the formation of S1P from the sphingolipid precursor, sphingosine. The caveolae fraction is enriched with sphingosine, a direct precursor of S1P (8, 31). Sphingolipid metabolites, such as ceramide, sphingosine, and S1P, are lipid second messengers involved in diverse cellular processes. We showed in our previous study that isoflurane stimulated SK1 to increase S1P in renal tubules to protect against renal IR injury in vivo (12). We also previously showed that isoflurane treatment stimulated SK1 activity leading to the membrane translocation of SK1 in HK-2 cells (12). Our data support the hypothesis that isoflurane increases caveolae-mediated organization of SK1→S1P signaling within the sphingolipid-rich environment. We propose that isoflurane treatment increases the interactions between caveolin-1 and SK1 in caveolae scaffold rafts and causes translocation of SK1 from plasma membrane to the buoyant caveolae fractions.
We previously demonstrated that volatile anesthetics including isoflurane externalized PS on the renal tubule cell plasma membrane that led to increased release of TGF-β1 to mediate the anti-necrotic and anti-inflammatory effects against renal IR injury (20, 22). Moreover, we showed that volatile anesthetics produced dose- and time-dependent phosphorylation of ERK MAPK in renal tubule cells (19, 22). In this study, we show that isoflurane-mediated increases in the caveolae-scaffolding complex increased colocalization of TGF-β1 receptors and ERK MAPK. It remains to be determined in future studies whether the caveolae complex integrates the isoflurane, PS, and sphingolipid interaction via TGF-β1-mediated phosphorylation of ERK MAPK and TGF-β1-mediated stimulation of SK1 to produce S1P.
We show in this study that recombinant TGF-β1 as well as PS/PC liposome mimic isoflurane-mediated increases in sarcolemmal caveolae. Moreover, neutralizing TGF-β1 antibody blocked the isoflurane-mediated increases in caveolae microdomains. Recent evidence supports an important role of TGF-β1 in stimulating the SK1 activity that modulates S1P signaling (32, 38). For example, TGF-β1 markedly upregulated SK1 mRNA and protein and increased SK activity in dermal fibroblasts (38). TGF-β1 strongly enhanced SK activity in mesoangioblasts and protected against Staurosporine-induced cell death (5). Furthermore, TGF-β1 receptors have been shown to associate/localize within the caveolae lipid rafts to regulate nitric oxide synthase activity (33) and SMAD (key transcription factors involved in TGF-β1 signaling; named after the drosophila protein, mothers against decapentaplegic and the Caenorhabditis elegans protein SMA) activation and receptor turnover (4). Stahelin et al. (35) demonstrated that human SK1 selectively bound PS over other anionic phospholipids and strongly preferred the plasma membrane-mimicking membrane to other cellular membrane mimetics. We hypothesize that PS externalization and TGF-β1 released by isoflurane modulate the SK1 enzyme in renal tubule cells and these interactions occur within the caveolae fraction of the sarcolemma.
It is becoming increasingly clear that lipid rafts organize signaling molecules and facilitate effective and coordinated signal transduction (2, 31). The caveolae/caveolin microdomain in the plasma membrane is involved in effectively organizing as well as facilitating the coordination of diverse signaling systems (i.e., eNOS, ERK, Src tyrosine kinase) (9, 10, 25, 37). Caveolins in particular function as scaffolds for signaling molecules providing temporal and spatial regulation of signal transduction (30, 31, 37). Lipid rafts, including caveolae, have been implicated in mediating protective effects of myocardial ischemic preconditioning (3), adenosine receptor-mediated cardiac signal transduction (13, 14), as well as isoflurane-mediated myocyte protection (28) against IR injury. The precise mechanisms of these diverse protective maneuvers converging within the lipid raft/caveolae microdomians remain to be elucidated. Patel et al. (28) demonstrated that caveolin-1 was essential in mediating isoflurane-mediated cardiac protection as mice lacking caveolin-1 were not protected against IR injury with isoflurane treatment. Moreover, they noted that isoflurane increased organization of caveolin-1 within the caveolae layer. Der et al. (3) previously demonstrated that IR of isolated hearts resulted in breakdown of sphingomyelin with corresponding accumulation of ceramide and sphingosine. They also noted that hearts preconditioned with prior ischemia contained significantly higher amounts of S1P. The role of sphingolipid interaction with caveolae microdomains in the kidney has never been reported. However, our current study, as well as previous studies, supports the hypothesis that preconditioning stimuli (ischemia, isoflurane, or adenosine) lead to the orchestrated breakdown of sphingolipids within the caveolae fractions to initiate S1P signaling (3, 13, 14, 28). We propose for the first time that isoflurane-mediated PS externalization and TGF-β1 release are critical for the initiation of SK1 and S1P signaling in the kidney.
We previously showed that isoflurane increases Akt and ERK phosphorylation in HK-2 cells and phosphorylation of these cytoprotective kinases was responsible for protection against necrosis in renal tubule cells (19). However, we only saw localization of ERK but not Akt in the caveolae fraction. Therefore, the results of this study indicate that the mechanisms of Akt and ERK regulation by isoflurane are different.
We show increased SK1 activity as well as protein levels in SK1-HK-2 cells treated with isoflurane. One of the limitations of the current study is that we used SK1-overexpressing HK-2 cells for part of our study. We tested several SK1 antibodies obtained from Dr. S. Pitson (Molecular Signalling Laboratory, Hanson Institute and Division of Human Immunology, Institute of Medical and Veterinary Science, Australia) and from several commercial sources [Abgent (San Diego, CA), ECM Biosciences, and Santa Cruz Biotechnology]. We found that some (antibodies from Dr. Pitson and from ECM) detected SK1 in whole cell HK-2 lysates. However, we performed extensive studies that demonstrated that none of the antibodies were able to detect the endogenous levels of SK1 protein in HK-2 cell caveolae fractions presumably due to very low SK1 protein concentration in these fractions or low antibody affinity for SK1 protein. Therefore, we used the SK1-overexpressing HK-2 cells in our studies to detect the scant expression of SK1 proteins in the caveolae fractions. We recognize the inherent limitations in studying a SK1-overexpressing HK-2 cell line. Artificial overexpression may create a signaling cascade that may not occur in the natural setting. Future studies with more sensitive antibodies for SK1 protein will be necessary to confirm our studies from the overexpression system. However, we were able to demonstrate increased SK1 activity in the caveolae fractions extracted from nonlentivirus-infected HK-2 cells after isoflurane treatment that supports our findings in the SK1-overexpressing HK-2 cells. Moreover, the increases in SK1 activity after isoflurane treatment were similar in magnitude (∼2-fold) in both nonlentivirus-infected and lentivirus-infected HK-2 cells. Therefore, although there was the need for SK1 overexpression to detect protein expression within caveolae/caveolin fractions, we were able to demonstrate a qualitatively similar increase in SK1 activity in both nonlentivirus-infected HK-2 as well as lentivirus-infected SK1-HK-2 cells.
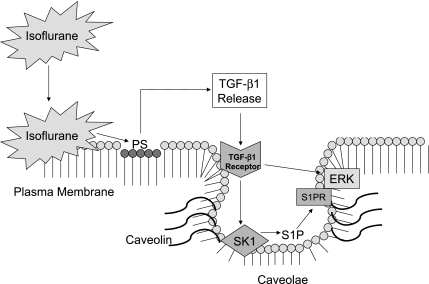
In summary, our current study shows that isoflurane treatment of renal cells causes an increase in caveolae/caveolin lipid rafts in the buoyant fractions of the plasma membranes. Our study is the first to demonstrate an isoflurane-mediated increase in caveolae sequestration of several signaling intermediates important in renal protection (SK1, ERK MAPK, TGF-β1 receptors, and S1P). Initiation of these events is mediated by isoflurane-induced PS externalization and TGF-β1 release. We hypothesize that volatile anesthetics, via interaction with the plasma membrane and caveolae microdomains, increase the signaling/interaction of TGF-β1, TGF-β1 receptor, and SK1 (Fig. 11). The exact biophysical mechanisms of isoflurane-mediated increase in buoyant caveolae microdomains in renal tubule cells remain to be determined. Moreover, the detailed physical interactions between caveolae/caveolin-1 and isoflurane need to be further investigated.
Fig. 11.
Isoflurane treatment of renal cells causes an increase in caveolae/caveolin lipid rafts in the buoyant fractions of the plasma membranes. We hypothesize that volatile anesthetics via interaction with the plasma membrane and caveolae microdomains increase the signaling/interaction of TGF-β1, TGF-β1 receptor, ERK MAPK, and SK1. Stimulation of SK1 produces S1P which stimulates ERK via S1P receptor (S1PR) activation. Initiation of these events is mediated by isoflurane-induced PS externalization and TGF-β1 release.
GRANTS
This work was funded by the National Institutes of Health Grant RO1-GM-067081.
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1.Bonventre JV, Weinberg JM. Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol 14: 2199–2210, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Cohen AW, Hnasko R, Schubert W, Lisanti MP. Role of caveolae and caveolins in health and disease. Physiol Rev 84: 1341–1379, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Der P, Cui J, Das DK. Role of lipid rafts in ceramide and nitric oxide signaling in the ischemic and preconditioned hearts. J Mol Cell Cardiol 40: 313–320, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Di Guglielmo GM, Le RC, Goodfellow AF, Wrana JL. Distinct endocytic pathways regulate TGF-beta receptor signalling and turnover. Nat Cell Biol 5: 410–421, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Donati C, Cencetti F, De PC, Rapizzi E, Brunelli S, Cossu G, Clementi E, Bruni P. TGFbeta protects mesoangioblasts from apoptosis via sphingosine kinase-1 regulation. Cell Signal 21: 228–236, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Emala CW, Aryana A, Levine MA, Yasuda RP, Satkus SA, Wolfe BB, Hirshman CA. Basenji-greyhound dog: increased m2 muscarinic receptor expression in trachealis muscle. Am J Physiol Lung Cell Mol Physiol 268: L935–L940, 1995 [DOI] [PubMed] [Google Scholar]
- 7.Frank PG, Woodman SE, Park DS, Lisanti MP. Caveolin, caveolae, and endothelial cell function. Arterioscler Thromb Vasc Biol 23: 1161–1168, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Galbiati F, Razani B, Lisanti MP. Emerging themes in lipid rafts and caveolae. Cell 106: 403–411, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Insel PA, Head BP, Ostrom RS, Patel HH, Swaney JS, Tang CM, Roth DM. Caveolae and lipid rafts: G protein-coupled receptor signaling microdomains in cardiac myocytes. Ann NY Acad Sci 1047: 166–172, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Insel PA, Head BP, Patel HH, Roth DM, Bundey RA, Swaney JS. Compartmentation of G-protein-coupled receptors and their signalling components in lipid rafts and caveolae. Biochem Soc Trans 33: 1131–1134, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Jones DR, Lee HT. Perioperative renal protection. Best Pract Res Clin Anaesthesiol 22: 193–208, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Kim M, Kim M, Kim N, D'Agati VD, Emala CW, Sr, Lee HT. Isoflurane mediates protection from renal ischemia-reperfusion injury via sphingosine kinase and sphingosine-1-phosphate-dependent pathways. Am J Physiol Renal Physiol 293: F1827–F1835, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Lasley RD, Narayan P, Uittenbogaard A, Smart EJ. Activated cardiac adenosine A(1) receptors translocate out of caveolae. J Biol Chem 275: 4417–4421, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Lasley RD, Smart EJ. Cardiac myocyte adenosine receptors and caveolae. Trends Cardiovasc Med 11: 259–263, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Lee HT, Chen SW, Doetschman TC, Deng C, D'Agati VD, Kim M. Sevoflurane protects against renal ischemia and reperfusion injury in mice via the transforming growth factor-beta1 pathway. Am J Physiol Renal Physiol 295: F128–F136, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee HT, Emala CW. Adenosine attenuates oxidant injury in human proximal tubular cells via A(1) and A(2a) adenosine receptors. Am J Physiol Renal Physiol 282: F844–F852, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Lee HT, Emala CW. Characterization of adenosine receptors in human kidney proximal tubule (HK-2) cells. Exp Nephrol 10: 383–392, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Lee HT, Emala CW, Joo JD, Kim M. Isoflurane improves survival and protects against renal and hepatic injury in murine septic peritonitis. Shock 27: 373–379, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Lee HT, Kim M, Jan M, Emala CW. Anti-inflammatory and anti-necrotic effects of the volatile anesthetic sevoflurane in kidney proximal tubule cells. Am J Physiol Renal Physiol 291: F67–F78, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Lee HT, Kim M, Kim J, Kim N, Emala CW. TGF-beta1 release by volatile anesthetics mediates protection against renal proximal tubule cell necrosis. Am J Nephrol 27: 416–424, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Lee HT, Kim M, Kim M, Kim N, Billings FT, 4th, D'Agati VD, Emala CW., Sr Isoflurane protects against renal ischemia and reperfusion injury and modulates leukocyte infiltration in mice. Am J Physiol Renal Physiol 293: F713–F722, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Lee HT, Kim M, Song JH, Chen SWC, Gubitosa G, Emala CW. Sevoflurane mediated TGF-β1 signaling in renal proximal tubule cells. Am J Physiol Renal Physiol 294: F371–F378, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Lee HT, Ota-Setlik A, Fu Y, Nasr SH, Emala CW. Differential protective effects of volatile anesthetics against renal ischemia-reperfusion injury in vivo. Anesthesiology 101: 1313–1324, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Lee HT, Xu H, Nasr SH, Schnermann J, Emala CW. A1 adenosine receptor knockout mice exhibit increased renal injury following ischemia and reperfusion. Am J Physiol Renal Physiol 286: F298–F306, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Lisanti MP, Tang Z, Scherer PE, Kubler E, Koleske AJ, Sargiacomo M. Caveolae, transmembrane signalling and cellular transformation. Mol Membr Biol 12: 121–124, 1995 [DOI] [PubMed] [Google Scholar]
- 26.Mikami M, Goubaeva F, Song JH, Lee HT, Yang J. β-Adrenoceptor blockers protect against staurosporine-induced apoptosis in SH-SY5Y neuroblastoma cells. Eur J Pharmacol 589: 14–21, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Min JK, Yoo HS, Lee EY, Lee WJ, Lee YM. Simultaneous quantitative analysis of sphingoid base 1-phosphates in biological samples by o-phthalaldehyde precolumn derivatization after dephosphorylation with alkaline phosphatase. Anal Biochem 303: 167–175, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Patel HH, Tsutsumi YM, Head BP, Niesman IR, Jennings M, Horikawa Y, Huang D, Moreno AL, Patel PM, Insel PA, Roth DM. Mechanisms of cardiac protection from ischemia/reperfusion injury: a role for caveolae and caveolin-1. FASEB J 21: 1565–1574, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Pitson SM, D'Andrea RJ, Vandeleur L, Moretti PA, Xia P, Gamble JR, Vadas MA, Wattenberg BW. Human sphingosine kinase: purification, molecular cloning and characterization of the native and recombinant enzymes. Biochem J 350: 429–441, 2000 [PMC free article] [PubMed] [Google Scholar]
- 30.Razani B, Lisanti MP. Caveolins and caveolae: molecular and functional relationships. Exp Cell Res 271: 36–44, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Razani B, Woodman SE, Lisanti MP. Caveolae: from cell biology to animal physiology. Pharmacol Rev 54: 431–467, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Sato M, Markiewicz M, Yamanaka M, Bielawska A, Mao C, Obeid LM, Hannun YA, Trojanowska M. Modulation of transforming growth factor-beta (TGF-beta) signaling by endogenous sphingolipid mediators. J Biol Chem 278: 9276–9282, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Schwartz EA, Reaven E, Topper JN, Tsao PS. Transforming growth factor-beta receptors localize to caveolae and regulate endothelial nitric oxide synthase in normal human endothelial cells. Biochem J 390: 199–206, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shull MM, Doetschman T. Transforming growth factor-beta 1 in reproduction and development. Mol Reprod Dev 39: 239–246, 1994 [DOI] [PubMed] [Google Scholar]
- 35.Stahelin RV, Hwang JH, Kim JH, Park ZY, Johnson KR, Obeid LM, Cho W. The mechanism of membrane targeting of human sphingosine kinase 1. J Biol Chem 280: 43030–43038, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Williams TM, Lisanti MP. The Caveolin genes: from cell biology to medicine. Ann Med 36: 584–595, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Williams TM, Lisanti MP. The caveolin proteins. Genome Biol 5: 214, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamanaka M, Shegogue D, Pei H, Bu S, Bielawska A, Bielawski J, Pettus B, Hannun YA, Obeid L, Trojanowska M. Sphingosine kinase 1 (SPHK1) is induced by transforming growth factor-beta and mediates TIMP-1 upregulation. J Biol Chem 279: 53994–54001, 2004. [DOI] [PubMed] [Google Scholar]