Abstract
Background/Objective:
To examine the differences in intrathecal baclofen management of individuals with spasticity of cortical vs spinal etiologies.
Design:
Retrospective chart review of 57 individuals with the diagnoses of severe cortical and spinal spasticity requiring an intrathecal baclofen pump.
Methods:
Parameters evaluated included daily dosage of medication required, flex vs simple continuous delivery modes, dosing changes, need for other local spasticity treatment, and catheter complications.
Results:
There were no statistically significant differences between individuals with cortical spasticity and spinal spasticity when comparing daily dosage, number of contacts, and mode of delivery. At 6 months, there was a statistically significant difference in dosing between individuals with multiple sclerosis and those without. Within groups, there was a significant difference in average daily dosing over 3 years. A significant difference was found comparing the use of botulinum toxin type A for upper extremity spasticity within the cortical group. Nine individuals had catheter complications.
Conclusions:
Cortical and spinal spasticity appear to parallel each other with no significant differences in daily dosing, dosing changes, and mode of delivery of intrathecal baclofen. This did not hold true at all time points for the multiple sclerosis subgroup. The significant difference noted within groups for daily dosing over the first 3 years challenges the notion of stable dosing over time. Focal injections of Botox/phenol in the upper extremities are an important adjunct therapy for patients with cortical spasticity, even after the placement of an intrathecal baclofen pump. Our complication rate was slightly lower than that reported in the literature.
Keywords: Spinal cord injuries; Multiple sclerosis; Cerebral palsy; Stroke; Brain injury, traumatic; Baclofen, intrathecal; Botulinum toxin type A; Phenol; Spasticity, cortical, spinal; Intrathecal pump
INTRODUCTION
Intrathecal baclofen (ITB) has become the standard of care for the treatment of severe spasticity of both spinal and cortical origin (1–5). Baclofen, a gamma-aminobutyric acid (GABA) agonist, works to inhibit the excitatory activity at the spinal reflexes. Intrathecal baclofen acts at the GABA receptors in the spinal canal, increasing its inhibitory effect and decreasing side effects of fatigue and sedation produced with oral antispasmolytic agents (6,7). Although the use of ITB has increased among patients with severe spasticity of spinal and cortical origin, there has been little published data comparing the 2 groups in terms of ITB pump management, including dosing, mode of dosing, and dosing changes (6,8).
Cortical spasticity usually manifests as dystonic posturing and hemiplegia (9); spinal spasticity typically involves spasticity found in the antigravity muscles, that is, flexor muscles in the arms and extensor muscles in the legs (9–11). Cortical spasticity applies to lesions within the brain; spinal spasticity is indicative of spinal pathology. Individuals with multiple sclerosis (MS) have routinely been placed in the spinal group due to the location of their lesions in the spinal cord, although they may possess brain lesions, decreased cognitive involvement, and more pronounced response to oral baclofen than those individuals with purely cortical spasticity, such as traumatic brain injury, cerebral palsy, and stroke (7,12–14).
Schiess et al (15) performed a retrospective review looking at their adult clinic data from more than 200 patients with spasticity of diverse etiologies, including both cortical and spinal. They found that flex dosing worked significantly better at controlling tone in stroke and was more effective for controlling upper extremity spasticity. A consensus panel for the use of ITB in stroke, convened in 2006, did not find this to be the case, however, and reported no difference in therapy between simple continuous and flex dosing (16). Most research findings reported less upper extremity tone reduction than lower extremity reduction with the use of ITB (17–21).
A review of the recent literature reporting average daily doses for spasticity of cortical etiology including cerebral palsy and stroke showed averages ranging from 263 µg/d to 591 µg/d with a wide variation from 93 µg/d to 2,000 µg/d (17,18,22). Much of this research was based on periods of only 1 year and a few of up to 2 years, making it harder to look at the stability of dosing across time (22). The stroke consensus panel reported that a reasonable time to achieve optimal dosing was 3 months, with longer periods required in some individuals (16,23). Albright et al (24) observed decreased tone up to 5 years out, with stable dosing occurring after the first 2 years.
Coffey et al (7) studied individuals with spinal spasticity for a period of almost 2 years on average, but some as long as 41 months, and reported a need for increased dosing over time. Azouvi et al (20) and Zahavi et al (25) found stable dosing occurring over 6 to 9 months based on a long-term mean follow-up of 38 months. Significant increases were noted in ITB from initial doses of 142 µg/d to 312 µg/d at 6 months, with moderate increases from 6 to 12 months to 339 µg/d (20). Hsieh and Penn (6) surveyed the use of ITB in individuals with spinal spasticity and based on their own experience found that optimal dosing was usually attained in 2 to 6 adjustments over 1 to 6 months, with 1-year mean dosages ranging from 175 µg/d to 477 µg/d.
In a study conducted from 1989 through 1996, Nielsen et al (26) noted a gradual increase in daily ITB dosing during the first 12 to 18 months for both MS and non-MS (mixed-origin spasticity) patients. The dosage continued to increase significantly after this period in the MS group but not in the non-MS group. Akman et al (27) and Ordia et al (14) also noted similar findings, with an increased need for ITB with individuals with MS after the first 12 months. In contrast, this increasing daily dosing trend was not observed in the study by Zahavi et al (25) over 5 years. Boviatsis et al (28) followed participants (MS and spinal cord injury [SCI]) for a mean of 34 months. They noted an increased need for dosing changes in the first 6 months, with dosing changes based on both outcome measures and the subjective feelings of patients. A significant difference between the groups was reported at 2.5 to 3 years. For individuals with MS, the daily dosage at 2.5 to 3 years was 405 µg vs the initial daily dosage of 180 µg. For individuals with SCI, the daily dosage at 2.5 to 3 years was 252 µg vs the initial daily dosage of 160 µg.
In the present study, we examined the differences in the ITB pump management of individuals with spinal vs cortical spasticity. We hypothesized that patients with cortical spasticity would have fewer contacts because of the stable nature of their spasticity, have higher daily dosing because of the nature of their spasticity (ie, dystonia and hemiplegia), and use flex dosing more frequently than patients with spasticity of spinal origin because of increased upper extremity involvement and the nature of their spasticity. We postulated that there would be significant changes when comparing dosing across time in both groups in the first year and less likelihood of differences after that period based on prior research. Similarly, we expected individuals with MS to have a need for increased contacts and dosing increases over time due to previously reported findings and the progressive nature of their disease.
Adjunct therapy would be required more in subjects with spasticity of cortical origin, because they would have more severe upper extremity involvement and dystonic posturing, for which ITB has less therapeutic effect (15). We assumed our complication rate to be slightly lower than the literature of 0.012 complications per month (26), because one experienced neurosurgeon and his team implanted most of our pumps.
METHODS
Subjects
We used the data from 57 individuals seen consecutively in our outpatient spasticity clinic over 3 years. All subjects with MS were noted to have documented spinal lesions with minimal cognitive involvement, although approximately half also had brain lesions. All patients were noted to have severe spasticity and/or inability to tolerate oral medication and reported that spasticity interfered with their function. The patients consisted of 7 individuals with CP, 8 individuals with traumatic brain injury, 4 individuals with stroke, 19 individuals with MS (diagnosed with either primary or secondary progressive forms of MS), 10 individuals with incomplete SCI (7 diagnosed with tetraplegia and 3 with paraplegia), and 9 with complete SCI (3 with tetraplegia from C4–C8 and 6 with paraplegia from T2–T12). For SCI, completeness and level of injury were based on the International Standards for Neurological Classification of Spinal Cord Injury.
We excluded patients for whom the etiology of their spasticity was unknown. Of the 57, 15 were at least household ambulators. All of them had onset of spasticity for greater than 1 year and participated in an ITB trial prior to pump implantation. One person had both morphine and baclofen in his pump. This patient was included in our study, because the intrathecal morphine was used to treat his neuropathic pain and had been added to his intrathecal pump after he had already been using ITB. Spasticity management was not altered by the addition of morphine, that is, the need for baclofen was unchanged and attempts to decrease the dose were not successful.
Ages ranged from 19 to 86 years, with mean age of 47 years. There were 37 men and 20 women. Forty-nine were seen at 3 months, 46 at 6 months, 41 at 1 year, 33 at 2 years, and 22 at 3 years after pump implantation.
Study Design
After the institutional review board gave its approval, we reviewed the charts of 57 individuals. We obtained relevant clinical information and created a database consisting of daily dosage, mode of infusion, and number of contacts occurring at 3 months, 6 months, 1 year, 2 years, and 3 years. We also examined the number of patients utilizing adjunct spasticity management after their pump, including phenol and Botox injections. The need for flex dosing vs simple continuous mode of infusion was determined based on the significance of upper extremity involvement, severity of spasticity, and amount of dystonic posturing and hemiplegia. Decision making with regard to the dosing of ITB and the addition of adjunct treatment was based on clinical assessment, including interval medical and functional history, neurologic examination, modified Ashworth score, and functional evaluation. Lastly, we looked at pump complication rates between the 2 groups. Patients were queried on follow up regarding such symptoms as fever, chills, change in spasticity control, headache, dizziness, and drainage from surgical sites. Any change in spasticity control or lack of response to an increased dosage and/or bolus dose of ITB was evaluated for complications. The algorithm sequence included plain radiography, evaluation of pump volume to ensure dose delivery, rotor study, catheter dye study, and magnetic resonance imaging of the intrathecal catheter.
Outcome Measures
We compared individuals with spinal vs cortical spasticity on daily dosing, number of contacts/dosage adjustment made by health care providers, and mode of delivery (simple continuous or flex dosing). We also compared the 2 groups based on their need for Botox/phenol after ITB pump placement and rate and types of complications. Additionally, we examined the differences between MS and non-MS groups based on daily dosing, number of contacts/dosage adjustment made by health care providers, and mode of delivery.
Statistical Methods
Linear mixed models were used to analyze repeated measures data. These models process data when observations are not independent, correctly modeling errors when tests in the general linear model family (eg, t tests, ANOVA, correlation, regression) usually do not. Compound symmetry was selected as the covariance structure. In this study, “time” refers to 3 months, 6 months, 1 year, 2 years, and 3 years. Dose and number of contacts were the outcomes of interest. Each outcome was modeled by group (cortical vs spinal spasticity) and time. To analyze categorical data, a Fisher exact test was used.
RESULTS
Daily Dosing and Contacts
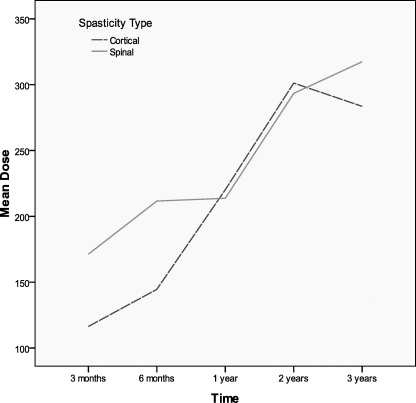
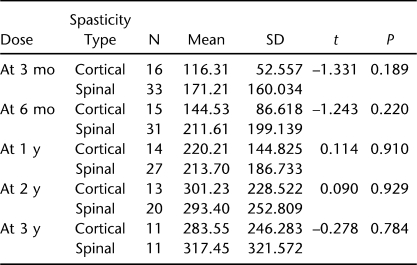
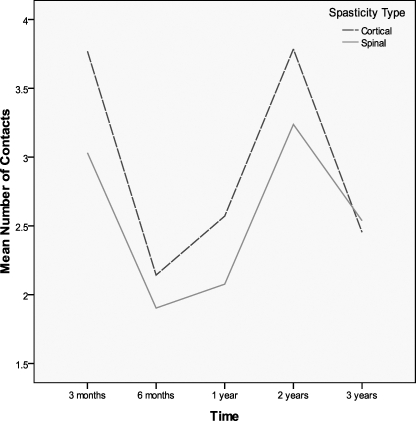
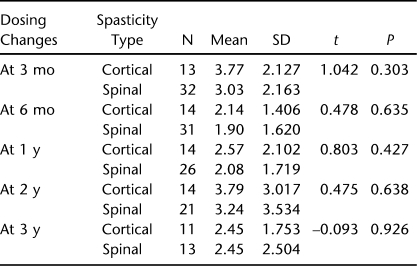
There were no group differences for daily dosing (F = 0.121, P = 0.729), but there was a main effect of time such that daily dosing increased across time (F = 57.98, P ≤ 0.001) (Figure 1, Table 1). For contacts, there was no main effect for group (F = 0.511, P = 0.478) or time (F = 0.345, P = 0.558) (Figure 2, Table 2). The numbers along the x-axis correspond to 3 months, 6 months, 1 year, 2 years, and 3 years, respectively for time after initiation of intrathecal baclofen therapy.
Figure 1.
Daily dosing increased over time.
Table 1.
Daily Dosing
Figure 2.
Mean number of contacts vs time in cortical and spinal spasticity patients.
Table 2.
Patient Contacts for Dose Changes at Time Intervals After Initiation of Intrathecal Baclofen Therapy
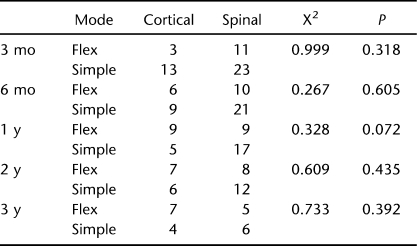
Mode of Dosing (Simple Continuous vs Flex Dosing)
There were no differences in mode of delivery between groups at any time point using a Fischer exact test (Table 3).
Table 3.
Flex vs Simple Dosing at Time Intervals After Initiation of Intrathecal Baclofen Therapy
MS vs Non-MS
We compared our MS cohort with the rest of the non-MS individuals in the spasticity clinic on daily dosing, number of contacts, and delivery modes. We found only one area demonstrating a statistical significance with dosing at 6 months. Using an independent t test with P = 0.020, the mean daily dose for the MS cohort was 227 ± 203 mcg compared with a mean dose for the non-MS cohort of 127 ± 67 mcg.
Adjunct Therapy
Categorical data were analyzed using a Fisher exact test. Of the 14 individuals receiving adjunct therapy to the upper extremities, 11 had cortical spasticity and 3 spinal spasticity (P = 0.005). Thirty-three percent of all subjects needed adjunct therapy after ITB placement; 74% (14) had cortical spasticity and only 26% (5) had spinal spasticity (P = 0.005). Within the spinal spasticity group, adjunct therapy was equally divided between upper and lower extremity.
Pump Complications
With respect to complications, there were no statistically significant differences between groups (P = 0.71), with an overall complication prevalence of 16% over 3 years, or 0.012 per month. Complications were reported in 2 individuals with catheter migration, 6 with fractured catheters, and 1 with a pump infection with meningitis, with subsequent catheter removal and replacement. Complications occurred in 22% of individuals with cortical spasticity and 13% of individuals with spinal spasticity. For 3 of the individuals, complications occurred with the first year.
DISCUSSION
Based on our data, there were no statistically significant differences between cortical and spinal spasticity groups when looking at daily dosing, mode of dosing, and dosing changes. Unlike the findings by Rawlins (8) and our own expectations, we found no differences between dosing for cortical and spinal spasticity. This may signify increased similarity between the 2 groups in terms of ITB management and a more uniform, incremental dosing schedule.
In contrast to the majority of literature reporting the stability of ITB dosing overtime, we noted statistical differences when looking at daily dosing changes over time within both groups over a 3-year period. These findings may be affected by tolerance to drug, disease progression, or other triggering factors, including medical comorbidities and pump complications driving these increases.
The number of contacts for cortical individuals was higher, although not significantly, than spinal for each time frame except the third year, when they were essentially equal. This was contrary to what we expected to find and may denote more fluctuation in cortical spasticity than previously thought.
The mode of dosing was not a contributing factor in our research, similar to that reported by the Stroke Consensus panel (10). Clinicians such as Schiess et al (9), however, have used the flex dosing to improve spasticity control in the upper extremities and in their stroke population. The mechanism of the flex dosing or bolus is not completely understood, but it is thought that a bolus of ITB better reaches the upper spinal canal and, thus, the upper extremities.
In the MS cohort, there was a statistical significance for higher daily dosages at 6 months compared with the non-MS cohort. In our sample, that difference disappeared with time, and there was no difference in contacts between the 2 groups, which is similar to the findings reported by Zahavi et al (25). It should be noted the majority of MS subjects had pumps for less than 2 years, making it difficult to assess differences greater than 1 year out. The trend for individuals with MS to have increased dosing over time that was noted in our research corresponded to other more recent findings (14,26).
Adjunct therapies after ITB were predominantly needed for the subjects with cortical spasticity. This outcome confirmed our hypothesis for the need for adjunct therapies even with the ITB pump in place, especially in those with upper extremity involvement and more so in those with cortical spasticity (although approximately half of the individuals with SCI had tetraplegia and upper extremity involvement). It may be that individuals with spinal spasticity respond better to ITB therapy because it is administered into the spinal canal. In our population, the cortical individuals had more severe upper extremity spasticity, most with dystonic posturing and/or hemiplegia, which may have accounted for the need for more focal spasticity treatment. There is a growing body of literature (17–21) reporting more difficulty in achieving tone reduction in the upper extremities. Hypotheses include the presence of fewer receptors in the thoracic spinal canal and/or suboptimal flow at that level.
Our complication rate was slightly lower than the reported average, which was as we expected. An important part of ITB management is having a surgeon experienced in implanting ITB pumps as part of the team. We had similar types of complications as various other authors, including 2 individuals with catheter migration into the epidural space. We used radiography to identify this in one subject, and the other needed a dye study for confirmation. Two of the catheter fractures were identified using radiography. We had one individual who was diagnosed with meningitis shortly after his pump placement.
Limitations
Attrition occurred within the groups, and there were missing data points, which may have skewed some of the results. In the last 2 years, our clinic population has more than doubled from 25 patients to 57. This should be taken into account when looking at data more than 2 years old, because 75% of patients with pumps implanted within the last 2 years were noted to have spinal spasticity. There were also very small sample sizes. Individuals were patients of the authors, and most of the management was done by one attending physician; however, the older pump patients were managed by one other physician. Lastly, the decision to categorize individuals with MS within the spinal origin category was based on the ITB literature review to date on ITB therapy, which has consistently placed MS within this category. We realize the inherent difficulty in placing individuals with the diagnosis of MS in either category or both, because they may have both brain and spinal lesions.
Future Direction
Further direction with multicenter trials to increase sample size is required to more thoroughly examine such phenomena as steady states of ITB over time within certain groups and subgroups (eg, MS, cerebral palsy, SCI). A variety of components go into the management of ITB, including patient satisfaction, clinical examination, assessment of changes in function, and quality of life. Further research is needed to discover reliable measures to examine function and its ability to predict positive outcomes after ITB placement. Because each individual had varying degrees of spasticity, there was no protocol or standardized means of adequately performing uniform dosing adjustments to compare individuals based on similar dosing. More studies focusing on patients with MS are warranted, because the use of ITB rapidly grows within this population. Comparison between nonambulatory and ambulatory subjects with ITB based on daily dosing, mode of dosing, and dosing contacts may help to further assist in protocols for pump management in the growing number of ambulatory individuals receiving ITB therapy. Lastly, much of the ITB research was not statistically measured, and the results are based more on descriptive data. More rigorous measurements and an improved definition of spasticity are needed.
CONCLUSION
Dosing across the 3 years changed significantly, contrary to expectations based on the majority of the extant literature, which reports stability at 3 to 18 months. Contrary to expectations, contacts over time and daily dosing were similar between groups, suggesting that despite the etiology of spasticity, management of patients is generally similar in this sample. The exception to this that was observed in dosing at the 6-month time frame in the MS subgroup may be due to progression of the disease or tolerance to the drug. Adjunct focal therapies were needed primarily for individuals with cortical spasticity and associated upper extremity involvement and will continue to be used as we search for better ways to optimize ITB in the upper extremities. Interestingly, one of the methods of optimizing ITB at upper extremities has been flex dosing; however, we found no difference in our study sample. Although ITB therapy is highly individualized, the need for ITB management guidelines and consensus is necessary as ITB therapy and research evolves.
Acknowledgments
We thank Dr Claire Kalpakjian and Martin Forchheimer for assistance with statistical analysis.
References
- Vender JR, Hughes M, Hughes BD, Hester S, Holsenback S, Rosson B. Intrathecal baclofen therapy and multiple sclerosis: outcomes and patient satisfaction. Neurosurg Focus. 2006;21(2):e6. doi: 10.3171/foc.2006.21.2.7. [DOI] [PubMed] [Google Scholar]
- Meythaler JM, Guin-Renfroe S, Grabb P, Hadley MN. Long-term continuously infused intrathecal baclofen for spastic-dystonic hypertonia in traumatic brain injury: 1-year experience. Arch Phys Med Rehabil. 1999;80(4):13–19. doi: 10.1016/s0003-9993(99)90301-5. [DOI] [PubMed] [Google Scholar]
- Meythaler JM, Guin-Renfroe S, Hadley MN. Continuously infused intrathecal baclofen for spastic/dystonic hemiplegia: a preliminary report. Am J Phys Med Rehabil. 1999;78(3):247–254. doi: 10.1097/00002060-199905000-00012. [DOI] [PubMed] [Google Scholar]
- Ridley B. Intrathecal baclofen therapy: challenges in patients with multiple sclerosis. Rehabil Nurs. 2006;31(4):158–164. doi: 10.1002/j.2048-7940.2006.tb00381.x. [DOI] [PubMed] [Google Scholar]
- Smail BD, Peskine A, Roche N, Mailhan L, Thiebaut JB, Bussel B. Intrathecal baclofen for treatment of spasticity of multiple sclerosis patients. Mult Scler. 2006;12(1):101–103. doi: 10.1191/1352458506ms1232sr. [DOI] [PubMed] [Google Scholar]
- Hsieh JC, Penn RD. Intrathecal baclofen in the treatment of adult spasticity. Neurosurg Focus. 2006;21(2):e5. doi: 10.3171/foc.2006.21.2.6. [DOI] [PubMed] [Google Scholar]
- Coffey RJ, Cahill D, Steers W, et al. Intrathecal baclofen for intractable spasticity of spinal origin: results of a long-term multicenter study. J Neurosurg. 1993;78(2):226–232. doi: 10.3171/jns.1993.78.2.0226. [DOI] [PubMed] [Google Scholar]
- Rawlins PK. Intrathecal baclofen therapy over 10 years. J Neurosci Nurs. 2004;36(6):322–327. doi: 10.1097/01376517-200412000-00005. [DOI] [PubMed] [Google Scholar]
- Katz RT. Management of spasticity. Am J Phys Med. 1988;67(3):108–116. doi: 10.1097/00002060-198806000-00004. [DOI] [PubMed] [Google Scholar]
- Brown P. Pathophysiology of spasticity. J Neurol Neurosurg Psychiatr. 1994;57(7):773–777. doi: 10.1136/jnnp.57.7.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlquist GI. Evaluation and primary management of spasticity. Nurse Pract. 1987;12(3):27–32. [PubMed] [Google Scholar]
- Knutsson E, Lindblom U, Martensson A. Plasma and cerebrospinal fluid levels of baclofen (Lioresal) at optimal therapeutic responses in spastic paresis. J Neurol Sci. 1974;23(3):473–484. doi: 10.1016/0022-510x(74)90163-4. [DOI] [PubMed] [Google Scholar]
- Penn RD, Savoy SM, Corcos D, et al. Intrathecal baclofen for severe spinal spasticity. N Engl J Med. 1989;320(23):1517–1521. doi: 10.1056/NEJM198906083202303. [DOI] [PubMed] [Google Scholar]
- Ordia JI, Fischer E, Adamski E, Chagnon KG, Spatz EL. Continuous intrathecal baclofen infusion by a programmable pump in 131 consecutive patients with severe spasticity of spinal origin. Neuromodulation. 2002;5(1):16–24. doi: 10.1046/j.1525-1403.2002._2004.x. [DOI] [PubMed] [Google Scholar]
- Schiess M, Furr ES, Fisher S, Acosta F, Richard S, Izor R. Functional motor improvement in stroke related spastic hemiparesis after intrathecal baclofen pump therapy. Neurology. 2006;66:A324–A. [Google Scholar]
- Francisco GE, Yablon SA, Schiess MC, Wiggs L, Cavalier S, Grissom S. Consensus panel guidelines for the use of intrathecal baclofen therapy in poststroke spastic hypertonia. Top Stroke Rehabil. 2006;13(14):74–85. doi: 10.1310/tsr1304-74. [DOI] [PubMed] [Google Scholar]
- Meythaler JM, Guin-Renfroe S, Brunner RC, Hadley MN, Francisco GE. Intrathecal baclofen for spastic hypertonia from stroke. Commentary. Stroke. 2001;32(9):2099–2109. doi: 10.1161/hs0901.095682. [DOI] [PubMed] [Google Scholar]
- Meythaler JM, Guin-Renfroe S, Law C, Grabb P, Hadley MN. Continuously infused intrathecal baclofen over 12 months for spastic hypertonia in adolescents and adults with cerebral palsy. Arch Phys Med Rehabil. 2001;82(2):155–161. doi: 10.1053/apmr.2001.19246. [DOI] [PubMed] [Google Scholar]
- Korenkov AI, Niendorf WR, Darwish N, Glaeser E, Gaab MR. Continuous intrathecal infusion of baclofen in patients with spasticity caused by spinal cord injuries. Neurosurg Rev. 2002;25(4):228–230. doi: 10.1007/s10143-002-0221-1. [DOI] [PubMed] [Google Scholar]
- Azouvi P, Mane M, Thiebaut JB, Denys P, Remy-Neris O, Bussel B. Intrathecal baclofen administration for control of severe spinal spasticity: functional improvement and long-term follow-up. Arch Phys Med Rehabil. 1996;77(1):35–39. doi: 10.1016/s0003-9993(96)90217-8. [DOI] [PubMed] [Google Scholar]
- Francisco GE. Intrathecal baclofen therapy for stroke-related spasticity. Top Stroke Rehabil. 2001;8(1):36–46. doi: 10.1310/14PN-TV5C-AYUB-NDP2. [DOI] [PubMed] [Google Scholar]
- Francisco GE, Hu MM, Boake C, Ivanhoe CB. Efficacy of early use of intrathecal baclofen therapy for treating spastic hypertonia due to acquired brain injury. Brain Inj. 2005;19(5):359–364. doi: 10.1080/02699050400003999. [DOI] [PubMed] [Google Scholar]
- Ivanhoe CB, Francisco GE, McGuire JR, Subramanian T, Gussom SP. Intrathecal baclofen management of poststroke spastic hypertonia: implications for function and quality of life. Arch Phys Med Rehabil. 2006;87(11):1509–1515. doi: 10.1016/j.apmr.2006.08.323. [DOI] [PubMed] [Google Scholar]
- Albright AL, Gilmartin R, Swift D, Krach LE, Ivanhoe CB, McLaughlin JF. Long-term intrathecal baclofen therapy for severe spasticity of cerebral origin. J Neurosurg. 2003;98(2):291–295. doi: 10.3171/jns.2003.98.2.0291. [DOI] [PubMed] [Google Scholar]
- Zahavi A, Geertzen JH, Middel B, Staal M, Rietman JS. Long term effect (more than five years) of intrathecal baclofen on impairment, disability, and quality of life in patients with severe spasticity of spinal origin. J Neurol Neurosurg Psychiatr. 2004;75(11):1553–1557. doi: 10.1136/jnnp.2003.014282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen JF, Hansen HJ, Sunde N, Christensen JJ. Evidence of tolerance to baclofen in treatment of severe spasticity with intrathecal baclofen. Clin Neurol Neurosurg. 2002;104(2):142–145. doi: 10.1016/s0303-8467(02)00009-4. [DOI] [PubMed] [Google Scholar]
- Akman MN, Loubser PG, Donovan WH, O'Neill ME, Rossi CD. Intrathecal baclofen: does tolerance occur. Paraplegia. 1993;31(8):516–520. doi: 10.1038/sc.1993.84. [DOI] [PubMed] [Google Scholar]
- Boviatsis EJ, Kouyialis AT, Korfias S, Sakas DE. Functional outcome of intrathecal baclofen administration for severe spasticity. Clin Neurol Neurosurg. 2005;107(4):289–295. doi: 10.1016/j.clineuro.2004.09.007. [DOI] [PubMed] [Google Scholar]