Abstract
Objective:
To develop and validate a latent model of health outcomes among persons with spinal cord injury.
Methods:
Survey data were collected at a large specialty hospital in the southeastern USA from 1,388 adult participants with traumatic spinal cord injury of at least 1 year's duration. Multiple indicators of health outcomes were used, including general health ratings, days adversely affected by poor health and poor mental health, treatments and hospitalizations, depressive symptoms, symptoms of illness or infection (eg, sweats, chills, fever), and multiple individual conditions (eg, pressure ulcers, subsequent injuries, fractures, contractures).
Results:
We performed exploratory factor analysis on half of the sample and confirmatory factor analysis on the other. A 6-factor solution was the best overall solution, because there was an excellent fit with the exploratory factor analysis (root mean square error of approximation = 0.042) and acceptable fit with the confirmatory factor analysis (root mean square error of approximation = 0.065). Four of the factors were types of secondary conditions, including symptoms of illness or infection, orthopedic conditions, pressure ulcers, and subsequent injuries. The 2 remaining factors reflected global health and treatment. Gender, race-ethnicity, age, injury severity, and years of education were all significantly related to at least 1 factor dimension, indicating variations in health outcomes related to these characteristics.
Conclusion:
Identification of the 6 factors represents an improvement over the utilization of multiple individual indicators, because composite scores generated from multiple individual indicators provide more informative and stable outcome scores than utilization of single indicators.
Keywords: Spinal cord injuries, Health, Health indicators, Rehabilitation, Disability, Quality of life, Secondary conditions, Pressure ulcers, Fractures, Obesity, Spasticity, Depression
INTRODUCTION
The direct complications of spinal cord injury (SCI) include changes in sensory, motor, and bowel and bladder function. There is also an increased susceptibility to a number of secondary health conditions that are only indirectly related to SCI. Because secondary health conditions are less directly tied to the SCI, the probability of occurrence varies among individuals, even those with similar types and severity of injury. Occurrences are also more likely to be related to health behaviors. For example, loss of sensation and motor function may contribute to the development of pressure ulcers as individuals lose the ability to feel the sore developing and do not naturally shift their weight in response to the pressure. Yet not all individuals get pressure ulcers and those who do generally have behavioral patterns that put them at greater risk (1,2).
In a book commissioned by The Institute of Medicine, Brandt and Pope (3) define a secondary condition as a preventable disease but as an impairment, injury, or disability as conditions occurring at an increased frequency as a result of a primary disabling condition (3). Because secondary conditions are common in persons with disabilities (4–8), persons with severe SCI are more vulnerable to developing them (9,10) Anson and Shepherd (11) reported patterns of secondary complications among 348 outpatients evaluated by a clinical team. Approximately 95% had at least 1 secondary condition and 58% had 3 or more, with the most prevalent being pain (45%), overweight or obesity (40%), spasticity in patients with cervical or thoracic injuries (74%), urinary tract infections (27.2%), and pressure ulcers (22.4%).
There is no single listing of secondary conditions or comprehensive approaches to investigating health after SCI. At the most general level, there are overall health ratings, such as those included in the SF-36 (12), to measure quality of life. Similarly, the Behavioral Risk Factor Surveillance System (BRFSS) is used by the Centers for Disease Control and Prevention to monitor health outcomes in the general population and includes the same general health items as the SF-36, as well as additional items that include the number of days in poor health and the number of days in poor mental health over the previous month.
The secondary condition after SCI that has received the majority of clinical and research focus is pressure ulcer (2,13–15). Similarly, urinary tract infections and other infections have been a common focus (14,16). Both pressure ulcers and infections are related to mortality after SCI (17). Although receiving less attention in the literature, individuals are also prone to subsequent injuries from new events, including falls. Brotherton, Krause, and Nietert (18,19) found that 75% of a sample of participants with incomplete SCI sustained at least one fall during the previous year. Risk of fractures is elevated because the frequency of fractures increases with age and completeness of injury, and this risk is higher in females (20,21). A Veterans Affairs Medical Center study reported that 34% of their male patients had a fracture after SCI (22). It has also been reported that 25% of persons with SCI are at risk for fractures 1 year after injury, and half are at risk by 10 years after injury (23). Amputations are less frequent but have been found to be related to a greater risk of mortality (17).
In contrast with both the indices of general health and reoccurring physical conditions, many of which are related to events, psychosocial conditions are also of concern. Depressive disorders are the most frequent concern after SCI and have significantly affected rehabilitation, community integration, quality of life, health, and health outcomes (24–30). Although rates of depressive disorders vary among persons with SCI, 2 literature reviews indicate that the rates range from 11% to >30% (31,32). A clinical practice guideline published in 1998 noted that 25% of men and 47% of women with SCI experienced some form of depressive disorder (33).
Another set of indicators of health status relates to treatments or interventions. Rates of hospitalization range from 0.55 to 1.85 hospitalizations per person per year in the first year after injury (34) and 0.26 to 0.55 in subsequent years (35,36). As with the general health indicators, these variables may be taken as indicators of severity of the effect of secondary conditions on health. In other words, they indicate that something bad is going on that is leading to diminished health and need for treatment. In a recent study, the most common causes of hospitalization after SCI were found to be conditions associated with the genitourinary system, respiratory complications, and diseases of the skin (34). Krause et al (17) found that the number of hospital bed days over the past year was a significant predictor of increased mortality rates. Because hospitalizations represent a primary indicator of health outcomes (37) and are related to the development of secondary heath conditions, it is important to understand the association of the individuals' behavior and the probability of hospitalization.
Krause (37) developed a theoretical risk model to account for multiple sets of risk factors, morbidity, and mortality after SCI. According to the model, secondary conditions represent the most immediate risk factor for mortality and are themselves related to behavioral, psychologic, and environmental risk factors. Biographic and injury-related variables were the most basic predictors in the model.
To fully understand the risk factors associated with health outcomes, it is first necessary to narrow the number of potential outcomes to manageable domains. The second step is to identify how the domains relate to the most basic and stable characteristics of the individual, including biographic factors (ie, age, gender, and race-ethnicity) and SCI factors (severity of SCI, years since SCI onset).
Purpose
The purpose of this study was to develop a structural model of health outcomes after SCI. The related objectives were to (a) develop a measurement model through both exploratory and confirmatory factor analysis of health domains and (b) develop a latent model linking basic biographic, injury, and educational characteristics with health outcomes.
Research Questions
What are the underlying factors (or common elements) for health and secondary conditions?
Will the underlying structure be stable upon confirmatory analyses?
What will be the relationship between biographic, injury, and educational characteristics and the underlying factor structure?
Hypotheses
Exploratory factor analysis (EFA) using half of a random split of the full participant sample will reveal multiple health dimensions with good fit (root mean square error of approximation [RMSEA] < 0.05).
Confirmatory factor analysis (CFA) applied to the second half of the participant sample will validate the preliminary analysis based on model fit statistics with acceptable fit (RMSEA < 0.08).
METHODS
After obtaining approval from the Institutional Review Board, participants were identified from records of a specialty hospital in the southeastern USA. There were 4 inclusion criteria: (a) traumatic SCI, (b) SCI of at least 1 year's duration, (c) 18 years of age, and (d) some residual impairment from the SCI (those with complete recovery were excluded). All 1,929 potential participants were enrolled in the study in 1997–1998. Letters announcing the study and describing the materials were sent to all prospective participants 4 to 5 weeks prior to sending the survey. A second mailing was initiated for nonrespondents within 2 months of the initial mailing. Those still not responding were contacted by phone, if possible, and an additional packet of materials was sent if requested. If there were large amounts of missing data, typically resulting from either a printing error or pages of the survey sticking together and being inadvertently missed, the missing information was requested either by phone or via mail.
Participants were allowed to selectively skip items. In rare instances, surveys were taken by phone for those who requested this type of assistance. (We did not keep data on the actual percentage, although it was clearly 5% or less.) Participants received a $20 stipend upon completion and return of the survey, and all participants who completed the survey were included in drawings totaling $1,500.
Measures
Several existing instruments were used to develop our final survey questionnaire, which included (a) a subset of 3 items from the BRFSS (38), (b) a subset of 3 items from the Life Situation Questionnaire (revised version) (39,40), (c) the Older Adult Health and Mood Questionnaire (22 items) (41), and (d) 18 items from the Spinal Cord Injury Health Survey that was developed for the study. If the existing instrument was designed to combine items into a scale to measure a particular outcome, then the full scale was used in our analyses. However, some individual items reflect outcomes without being part of a larger scale, and these were used in our analyses as individual outcomes. For instance, the 22-item Older Adult Health and Mood Questionnaire was used to measure the depression outcome represented by a single score, whereas a single item from the BRFSS was used to measure global health (a 5-choice item reflecting overall health).
Measures were selected from the BRFSS (38), a standardized instrument that has been used with more than 100,000 individuals by the Centers for Disease Control and Prevention. It is used to monitor relevant basic health behaviors and outcomes in the general population. Items selected for this study have been used extensively by the Centers for Disease Control and Prevention with existing data available for normative purposes (42). The BRFSS contains 3 health-related items: self-rated health, poor physical health days in past month, and poor mental health days in past month. These items were used as indicators of general health. In reliability studies, the demographic and risk factor sections of the BRFSS have been found to be highly consistent within households (43).
The Life Situation Questionnaire (revised version) is used to measure multiple outcomes, including those related to recent treatments and hospitalizations (39,40). Three treatment variables were used from this questionnaire. Each of these items requires participants to indicate over the previous 12 months the (a) number of nonroutine physician office visits, (b) number of hospitalizations, and (c) number of days hospitalized. Although not used in the current manuscript, 7 Life Situation Questionnaire (revised version) factor scales were found to be stable over a 15-month period (average test-retest stability of 0.81) and internally consistent, with an average alpha coefficient of 0.86 and a range of 0.79 to 0.92 (39).
The 22-item Older Adult Health and Mood Questionnaire (41) was used to measure depressive symptoms. It was developed to measure depression in older adults and among people with physical disabilities by including few items that reflect physical or vegetative symptomatology. These types of items may inflate scores when used with people who are in poor health or aging or who have a disability. Scores are used to generate the following diagnoses: nondepressed [0–5], clinically significant symptomatology [6–10], and probable major depression [11–22]. The test developers (41) found a test-retest coefficient of 0.87 (P < 0.001), and for the full scale, the standardized-item alpha was 0.93 (P < 0.001). In terms of validity, sensitivity and specificity were reported to be 0.93 and 0.87, respectively (41). The Older Adult Health and Mood Questionnaire was also compared with 2 other depression scales with established validity and reliability: the Geriatric Depression Scale and the Symptom Checklist-90-R. Correlation for the Older Adult Health and Mood Questionnaire and both measures was 0.70 (P < 0.001) (41).
The Spinal Cord Injury Health Survey was developed for the study to measure multiple health factors, including secondary conditions. The items were phrased so as to clearly describe each condition or event in order to allow accurate recall and reporting. Some items reflect recurring conditions, such as pressure ulcers and subsequent injuries, whereas others reflect critical events, including amputations and fractures. Some conditions, such as urinary tract infections, require diagnosis from a physician, and in these cases items were generated that reflected the symptoms of infections and illness. There were no comparisons with medical charts. Although such methods of validation are enlightening when care is reliably documented in charts, the charts themselves become unreliable when dealing with secondary conditions in community settings as individuals disperse geographically and receive different types of care in varying settings (ie, it is not possible to obtain all the verifying information). Indirect information on item validity comes from identification of patterns of response consistent with expectations, such as the demonstration of relationships between particular risk behaviors and outcomes, such as pressure ulcers (2) and subsequent injuries (44).
Because the items were to measure secondary conditions occurring in community settings, it was not possible to compare self-report responses with information from secondary sources, such as medical charts.
There were several item sets covering a wide range of secondary conditions and health outcomes. Pressure ulcers were measured by several items that focused on asking participants for objective information on their pressure ulcer history. Pressure ulcers were described as “open sores in pressure areas, such as your tailbone, ischium, heel, and elbows. They are usually caused by pressure but may also be caused by friction or shearing (rubbing), moisture, burns, or falls” (2). Items included the presence of a current pressure ulcer, the number of pressure ulcers within the past year, days in which sitting was adversely affected by pressure ulcers during the past year, and the total number of surgeries to repair pressure ulcers since SCI onset.
Items to measure subsequent injuries were developed in collaboration with the National Center for Injury Prevention of the Centers for Disease Control and Prevention. We defined subsequent injuries in the following manner: “The following questions relate to INJURIES, including broken bones, burns, or lacerations (cuts). Injuries happen as the result of some type of mishap or event, such as a fall, collision, motor vehicle wreck, or act of violence” (44). Participants were asked the number of times in the past year they had been injured “…seriously enough to receive medical care in a clinic, emergency room, or hospital.” They were also asked the total number of injuries since SCI onset and the number of injury-related hospitalizations since SCI onset. Injuries were reported in 19% of participants over a 12-month period, with 27% of those participants reporting at least one injury also reporting one or more injury-related hospitalization over that timeframe (44).
A third set of items asked participants to indicate the frequency of various SCI-related health events over the past 12 months. A multiple choice grouped frequency was used: 0, 1–2, 3–6, 7–12, and 13 or more. The items included stomach pain and distention, bowel accidents, rectal bleeding, urine leaking and accidents, fevers, sweats or chills, and urinary tract infections.
Participants were also asked whether they had ever had any of the following orthopedic conditions: amputation of lower extremity (leg, foot), amputation of upper extremity (hand, arm), curvature of the spine (more difficulty sitting up straight), broken bone in lower extremity (leg, foot), broken bone in upper extremity (hand, arm), contracture of lower extremity (hip, knee, ankle), and contracture of upper extremity (elbow, wrist). These conditions tend to be generally irreversible (amputations), cumulative (contractures), and nonrecurring. However, some additional events could occur, such as repeated new fractures and amputations. Because many of these are of low incidence, some were combined for data analysis. Specifically, we created 3 variables that combined upper and lower extremity occurrences: amputation, fracture, and contracture, each of which reflected upper and/or lower extremity involvement (ie, upper or lower extremity amputation, upper or lower extremity fracture, contracture of an upper or lower extremity).
Analyses
Respondents and nonrespondents were compared on 6 characteristics: gender, race-ethnicity, injury level, age at injury, age at the study, and years since injury using the chi-square statistic for categorical variables and t tests for metric variables. SPSS was used for the analyses.
All SEM analyses were conducted using Mplus (45). Mplus offers specialized software for a wide range of structural equation models, diverse selection of models, estimators, and algorithms and has explicit features for missing data, complex survey data, and multilevel data. Mplus has special features for performing factor analysis on items that are in different metrics, and we used the Mplus categorical option that allows for skewness and kurtosis (45), because some of the data were categorical.
Full structural models are composed of 2 components: a measurement model and a latent model. Prior to initiating the first stage of the analysis, the sample was randomly split into 2 groups of approximately equal sizes. We used a combination of EFA and CFA to define the measurement model, performing the EFA on the first half of the sample and the CFA on the second half of the sample.
In the first step, an EFA using a weighted least-square parameter was used to initially evaluate the factor structure of the outcome variables (46). The chi-square and root mean square error of approximation (RMSEA) were used to evaluate the fit of the model with varying numbers of factors. Root mean square error of approximation is determined by the discrepancy per degrees of freedom and is said to correct for model complexity. A RMSEA of less than 0.05 represents an excellent fit, and 0.05 to 0.08 represents good fit (47). Factor rotation was carried out on these common factors using a Promax approach (Varimax followed by Procrustean targets), so the resultant factors could have a simple structure and be correlated (48). The solution with the lowest RMSEA that maintained a minimum of 3 items loading for each factor was used. A minimum of 3 items is necessary to produce stable factors that can be evaluated for their internal consistency.
In the second step, CFA with rigid constraints (defined by the EFA) was then used to cross-validate the factor structure using the second half of the sample. With CFA, the number of factors and the items that load with each factor are specified a priori. All items that loaded a minimum of 0.30 during the EFA were included with the factor in the CFA. If an item loaded with more than one factor, it was grouped with the factor with which it had the highest loading. From the CFA, the minimum loading retained in the final model was 0.40.
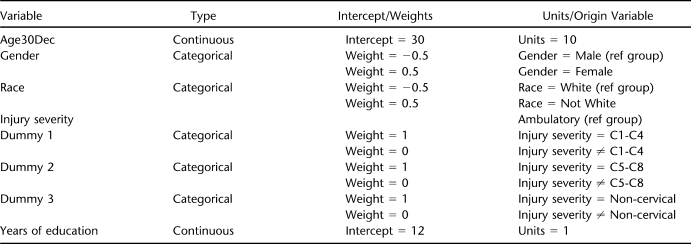
The structural equation model approach allows for relationships of exogenous (observable) variables with the endogenous factors defined from the measurement model (the factors derived from factor analysis) (46,49). In the final step, the full structural model was developed linking the latent variables to the measurement model. The following biographic, injury, and educational variables were used: (a) gender, (b) race-ethnicity, (c) chronologic age, (d) injury severity, and (e) years of education. We developed a table (Appendix) that summarizes the weights for these variables. The reference groups for gender and race-ethnicity were male and white (all nonwhites were grouped together). There were 4 groups for injury severity based on a combination of injury level and ambulatory status. The ambulatory group served as the reference group and included all ambulatory participants regardless of neurologic level. The other 3 nonambulatory groups were broken down according to level as follows: C1–C4, C5–C8, noncervical. Age and years of education were continuous variables.
RESULTS
Responders and Nonresponders
Of the 1,929 contacted, 1,388 participated (72% response rate). A greater percentage of women (79.6%) responded than men (70.4%), chi-square (1, n = 1,916) = 14.5, P ≤ 0.001. No differences were observed for the other biographic and injury variables.
Participants
Seventy-four percent of the participants were male, and 75% were white. The overall breakdown by gender and race-ethnicity was 54.6% white men, 20.2% white women, 19.4% minority men, and 5.9% minority women. Of the minority participants, 88% were African American. Average age at time of injury was 31.8 years and 41.6 years at the time of reporting. Participants had been injured an average of 9.7 years. Average number of years of education was 13.1 years.
The primary etiology was vehicular crashes (51%), followed by falls/flying objects (17.2%), acts of violence (12.7%), sports (11.9%), and other (7.2%). Fifty-five percent reported cervical injuries. The cases were unevenly distributed as a function of injury severity: 21.1% were ambulatory, 35.1% had noncervical injuries (35.1%), 30.6% had C5–C8 level injuries, and 13.2% had C1–C4 injuries (the latter 3 groups were nonambulatory).
Exploratory Factor Analysis
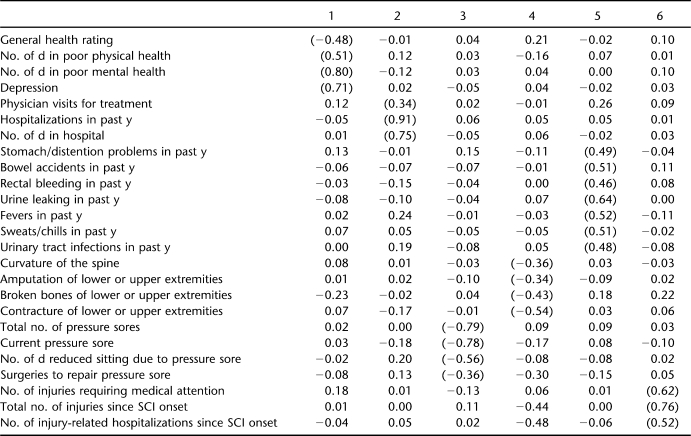
Table 1 summarizes the rotated factor loadings using the first half of the sample. Six factors were derived using EFA with an excellent fit (RMSEA = 0.042). One factor included items that appear to reflect global health and included general health ratings, days affected by poor health and poor mental health, and depressive symptoms. The second factor was composed of items related to treatments, including physician visits, hospitalizations in the last year, and days spent in the hospital. The 4 remaining factors appear to reflect a specific type of secondary condition domain. These included symptoms of illness or infection (stomach problems, bowel accidents, rectal bleeding, urine leaking, fevers, and sweats/chills), orthopedic conditions (curvature of the spine, amputations, broken bones, and contracture), pressure ulcers (total number of sores, current sore, reduced days of sitting due to sore, and surgeries to repair a sore), and subsequent injuries (injuries requiring medical attention, total number of injuries since SCI onset, and hospitalizations for injury).
Table 1.
Promax Rotated Loadings of Health Outcomes From Exploratory Factor Analysis of Split Sample (Root Mean Square Error of Approximation = 0.042)
Confirmatory Factor Analysis
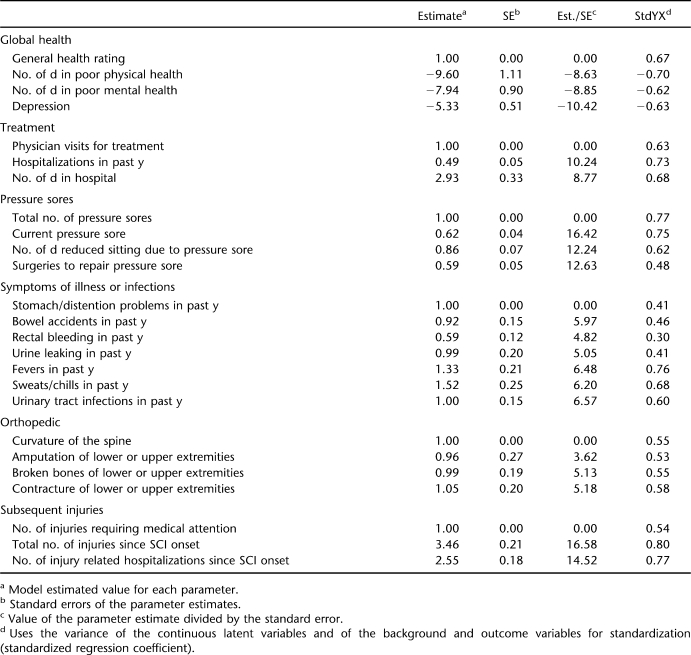
Based on the standardized regression coefficients in the EFA, 6 factors were created for the CFA. Table 2 summarizes the subsequent restricted confirmatory solution. The RMSEA for the CFA indicated an acceptable fit (RMSEA = 0.065). Rectal bleeding dropped out of the confirmatory analysis, because the standardized regression coefficient was less than the minimum of 0.40. All other items maintained coefficients of 0.40 or higher.
Table 2.
Confirmatory Factor Analysis of Split Sample (Root Mean Square Error of Approximation = 0.065)
Development of the Latent Model
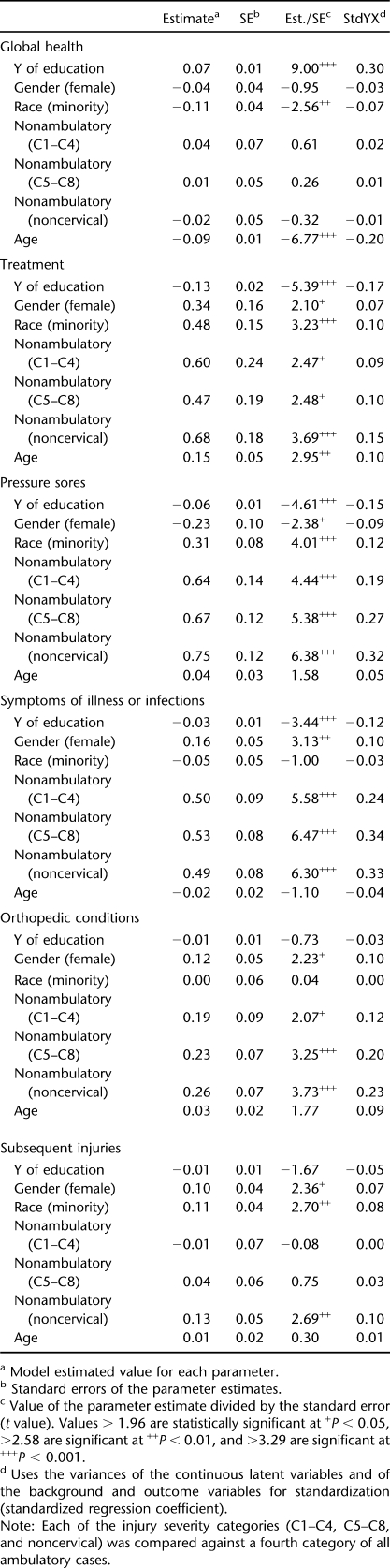
To develop the latent model, the factors from the CFA were converted to z-scores and the model was generated identifying the relationships with biographic, injury, and educational characteristics (RMSEA = 0.059; chi-square [162, n = 1,374] = 927.9, P < 0.001). Table 3 summarizes these relationships. The standardized regression coefficients represent the best indicator of the strength of the relationship of the exogenous variable (eg, gender, race-ethnicity, age) with the observed dimension.
Table 3.
Latent Model Identifying the Relationships Between the 6 Factors and Biographic, Injury, and Educational Characteristics (Root Mean Square Error of Approximation = 0.059)
Gender was significantly associated with several dimensions. Women scored lower on the pressure ulcer dimension but higher on treatment, symptoms of illness or infection, orthopedic conditions, and injuries. This indicates that women were less likely to report pressure ulcers. However, they reported more treatments, symptoms of illness or infection, orthopedic conditions, and subsequent injuries.
Race-ethnicity was significantly associated with 4 dimensions. Nonwhites scored lower on the general health dimension (indicating poor general health) and higher on the treatment, pressure ulcer, and injury dimensions (suggesting greater risk of these outcomes).
Injury severity was also significantly related to all dimensions, except general health, in which there were no significant differences. With the exception of the injury dimension, in every case in which significant differences were observed, nonambulatory participants reported higher scores (indicative of worse outcomes) than the ambulatory reference group. For injuries, only the noncervical, nonambulatory group reported worse scores than the ambulatory reference group. Years of education was significantly related to all but orthopedic conditions and injuries. Those with more education reported higher scores on the general health dimension and lower scores for treatment, symptoms of illness or infection, and pressure ulcers (indicating a lower risk of these adverse outcomes).
Age was only significantly related to global health and treatments. Global health declined with age, whereas treatments significantly increased with age. None of the other dimensions was significantly related with age.
DISCUSSION
Results of this study help to define domains of health outcomes after SCI. One of the major findings was the 4 secondary conditions domains and 2 health impact domains (global health, treatment). Identification of domains represents an improvement over the utilization of multiple single indicators, because composite scores generated from multiple individual indicators allow for the measurement of common variance between multiple outcomes.
The domains were relatively clearly defined, although examination of the loading patterns indicated that some items loaded heavily with more than 1 factor. This is not surprising, because factor analysis rarely produces truly orthogonal factors and one would not expect different health outcomes to be fully independent. Rather, we would anticipate that some secondary conditions would be highly correlated (in this case, the variables that made up the factors) and that the clusters of secondary conditions (factors) would be moderately intercorrelated. Also, because 2 of the factors appear to reflect the impact of secondary conditions (ie, global health and treatments), it is not surprising that some of the secondary condition factors are more highly correlated with these impact factors.
In addition to defining the health domains, important relationships were identified between participant characteristics and the health outcomes. No participant characteristic was associated with better health in all areas. However, having a higher education and being ambulatory were correlated with better health in several areas. Whereas higher education has been consistently associated with better outcomes after SCI, particularly in vocational areas (50–52), ambulatory status has been associated with a mixed pattern of favorable and unfavorable outcomes (53,54).
Both gender and race-ethnicity appear to be important predictors of health outcomes. It is somewhat surprising that women reported higher scores on the injury dimension, because these are typically related with high-risk behaviors (44). However, the definition of injuries used in this study required that the individuals seek treatment. Therefore, we cannot determine whether women were at greater risk for the actual events leading to injury or whether they were simply more likely to seek treatment once an injury had occurred. It is also possible that this is related to a greater risk of osteoporosis among women, such that similar events may have more likely resulted in fractures.
Nonwhite participants reported poorer health outcomes in several areas. This raises questions as to such factors as access to care or health insurance. Disparities in health care coverage are widespread in the general population (55,56), so it is not surprising to identify them among people with SCI. However, it is noteworthy that nonwhites were found to report fewer high-risk health behaviors in previous research (57), so there are likely multiple contributory factors to the observed findings.
Limitations
There are several noteworthy limitations. First, all data were self-reported and subject to recall bias. This may be of greatest concern with variables that require recall of earlier events, such as injuries, pressure ulcers, and hospitalizations. The selected timeframe is consistent with the type of information requested in order to limit recall bias. For instance, items such as amputations, fractures, and pressure ulcer surgeries were asked over the entire time since injury, but more specific symptoms (eg, sweats, chills) were restricted to a much narrower timeframe. Although it would be preferable to have some benchmark for which to compare at least a portion of the responses, because we utilized a community sample, there was no straightforward source of benchmarking, such as hospital or medical charts (participants were dispersed and no doubt utilized multiple sources of care). The major issue with recall bias is that it adds error to measurement, which limits our ability to identify true relationships between variables.
Second, all data were cross-sectional. Therefore, we cannot assume that observed relationships between age-related variables, such as age and years since injury, with health outcomes reflect age related changes over time. They may also reflect cohort effects, and longitudinal research would be required to identify how health outcomes change with these variables over time.
Third, although we have identified 6 domain areas that appear to be relatively stable upon confirmation using a split sample, this does not suggest that we have tapped all health outcome domains. Rather, this study has laid the foundation by identifying several key domains and should be augmented by future research with additional domains, including those that have received a substantial amount of attention in rehabilitation research and practice (eg, pain, fatigue, spasticity).
Fourth, although our response rate was relatively high (72%), nonresponse is always a concern in self-report studies. It is of less concern in studies that do not include identifying the true incidence and prevalence of a condition but rather the relationships between conditions (such as the current study).
Lastly, there have been significant changes in rehabilitation practices since the initial data collection. This could have some unknown effects on the results. The findings have laid the foundation for confirmatory factor analysis with newer and expanded data (this research is currently under way).
Future Research
Additional research is required to continue to build a predictive model of health outcomes (we will continue with this endeavor). Specifically, whereas this study identified 6 domains, inclusion of a broader number of health variables is required. Given that one of the key findings in this study was the identification of 4 independent secondary condition dimensions and 2 impact dimensions (ie, global health, treatments), more attention is needed to identify the circumstances under which secondary conditions lead to declines in global health and increased need for treatment.
In future extensions of this study, we will measure more diverse secondary conditions, including chronic conditions. Additional research is needed that encompasses a broader set of secondary conditions inclusive of pain, obesity, and spasticity. Whereas some of these conditions are quantifiable and have large subjective components, it is also important to identify other conditions, including more fundamental health conditions commonly observed in the general population (eg, diabetes, cardiovascular disease). Some conditions will require measurement of biomarkers and assignment to discreet categories (with condition, without condition). Genetic indicators of health, such as telomere length and its relationship with stress after SCI, is also an important consideration for further research (58).
We are conducting longitudinal research to identify how secondary conditions and health change over time; the sequence in which secondary conditions, health impact, and biomarkers of health unfold; and the risk and protective factors that predict future health outcomes.
CONCLUSION
Six domains of health outcomes were identified. There were several significant relationships between these and biographic, injury, and educational status. Identification of these 6 factors represents an improvement over the utilization of multiple individual indicators, because composite scores generated from multiple individual indicators provide more informative and stable outcome scores than does utilization of single indicators.
Acknowledgments
We thank the following people whose contributions made the completion of this article possible: Richard Aust, Jennifer Coker, Philip Edles, Kristian Manley, Dr Lee L. Saunders, Sean Twohig, and Yusheng Zhai and Sarah Lottes and Christina McCleery of the Shepherd Center.
APPENDIX
Table.
Biographic and Injury Variables That Comprise the Latent Model
Footnotes
This research was supported by funding from the National Institutes of Health grant #1R01 NS 48117–01; the US Department of Education, National Institute on Disability and Rehabilitation Research grant #H133G050165; and the Model Spinal Cord Injury Systems grant #H133N000005. The opinions here are those of the grantee and do not necessarily reflect those of the funding agencies.
References
- Krause JS, Vines CL, Farley TL, Sniezek J, Coker J. An exploratory study of pressure ulcers after spinal cord injury: relationship to protective behaviors and risk factors. Arch Phys Med Rehabil. 2001;82(1):107–113. doi: 10.1053/apmr.2001.18050. [DOI] [PubMed] [Google Scholar]
- Krause JS, Broderick L. Patterns of recurrent pressure ulcers after spinal cord injury: identification of risk and protective factors 5 or more years after onset. Arch Phys Med Rehabil. 2004;85(8):1257–1264. doi: 10.1016/j.apmr.2003.08.108. [DOI] [PubMed] [Google Scholar]
- Brandt EN, Pope AM. Enabling America: Assessing the Role of Rehabilitation Science and Engineering. Washington, DC: North Academy Press; 1997. [PubMed] [Google Scholar]
- Campbell M, Sheets D, Strong PS. Secondary health conditions among middle-aged individuals with chronic physical disabilities: implications for unmet needs for services. Assist Technol. 1999;11(2):105–122. doi: 10.1080/10400435.1999.10131995. [DOI] [PubMed] [Google Scholar]
- Coyle CP, Santiago MC, Shank JW, Ma GX, Boyd R. Secondary conditions and women with physical disabilities: a descriptive study. Arch Phys Med Rehabil. 2000;81(10):1380–1387. doi: 10.1053/apmr.2000.9169. [DOI] [PubMed] [Google Scholar]
- Gajdosik C, Cicirello N. Secondary conditions of the musculoskeletal system in adolescents and adults with cerebral palsy. Phys Occup Ther Pediatr. 2001;21(4):49–68. doi: 10.1300/j006v21n04_04. [DOI] [PubMed] [Google Scholar]
- Rimmer JH. Health promotion for people with disabilities: the emerging paradigm shift from disability prevention to prevention of secondary conditions. Phys Ther. 1999;79(5):495–502. [PubMed] [Google Scholar]
- Wilber N, Mitra M, Walker DK, Allen D, Meyers AR, Tupper P. Disability as a public health issue: findings and reflections from the Massachusetts survey of secondary conditions. Milbank Q. 2002;80(2):393–421. doi: 10.1111/1468-0009.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinley WO, Jackson AB, Cardenas DD, DeVivo MJ. Long-term medical complications after traumatic spinal cord injury: a regional model systems analysis. Arch Phys Med Rehabil. 1999;80(11):1402–1410. doi: 10.1016/s0003-9993(99)90251-4. [DOI] [PubMed] [Google Scholar]
- DeLisa J, Kirshblum S. A review: frustrations and needs in clinical care of spinal cord injury patients. J Spinal Cord Med. 1997;20(4):384–390. doi: 10.1080/10790268.1997.11719494. [DOI] [PubMed] [Google Scholar]
- Anson C, Shepherd C. Incidence of secondary complications in spinal cord injury. Int J Rehabil Res. 1996;19(1):55–66. doi: 10.1097/00004356-199603000-00006. [DOI] [PubMed] [Google Scholar]
- Brazier JE, Harper R, Jones NMB, et al. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ. 1992;305(6846):160–164. doi: 10.1136/bmj.305.6846.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saladin LS, Krause JS, Adkins RH. Pressure ulcer prevalence and barriers to treatment after spinal cord injury: comparisons of 4 groups based on race-ethnicity. NeuroRehabilitation. 2009;24(1):57–66. doi: 10.3233/NRE-2009-0454. [DOI] [PubMed] [Google Scholar]
- Kroll T, Neri MT, Ho PS. Secondary conditions in spinal cord injury: results from a prospective survey. Disabil Rehabil. 2007;29(15):1229–1237. doi: 10.1080/09638280600950603. [DOI] [PubMed] [Google Scholar]
- Raghavan P, Raza WA, Ahmed YS, Chamberlain MA. Prevalence of pressure sores in a community sample of spinal injury patients. Clin Rehabil. 2003;17(8):879–884. doi: 10.1191/0269215503cr692oa. [DOI] [PubMed] [Google Scholar]
- Hess MJ, Hess PE, Sullivan MR, Nee M, Yalla SV. Evaluation of cranberry tablets for the prevention of urinary tract infections in spinal cord injured patients with neurogenic bladder. Spinal Cord. 2008;46(9):622–626. doi: 10.1038/sc.2008.25. [DOI] [PubMed] [Google Scholar]
- Krause JS, Carter RE, Pickelsimer E, Wilson D. A prospective study of health and risk of mortality after spinal cord injury. Arch Phys Med Rehabil. 2008;89(8):1482–1491. doi: 10.1016/j.apmr.2007.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotherton SS, Krause JS, Nietert PJ. A pilot study of factors associated with falls in individuals with incomplete spinal cord injury. J Spinal Cord Med. 2007;30(3):243–250. doi: 10.1080/10790268.2007.11753932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotherton SS, Krause JS, Nietert PJ. Falls in individuals with incomplete spinal cord injury. Spinal Cord. 2007;45(1):37–40. doi: 10.1038/sj.sc.3101909. [DOI] [PubMed] [Google Scholar]
- Krause JS. Aging after spinal cord injury: an exploratory study. Spinal Cord. 2000;38(2):77–83. doi: 10.1038/sj.sc.3100961. [DOI] [PubMed] [Google Scholar]
- Maynard FM, Karunas RS, Adkins RH, Richards JS, Waring WP. Management of the neuromuscular system. In: Stover SL, DeLisa JA, Whiteneck GG, editors. Spinal Cord Injury: Clinical Outcomes of the Model Systems. Gaithersburg, MD: Aspen; 1995. pp. 163–169. [Google Scholar]
- Lazo MG, Shirazi P, Sam M, Giobbie-Hurder A, Blacconiere MJ, Muppidi M. Osteoporosis and risk of fracture in men with spinal cord injury. Spinal Cord. 2001;39(4):208–214. doi: 10.1038/sj.sc.3101139. [DOI] [PubMed] [Google Scholar]
- SCI Forum Reports. Common musculoskeletal problems after SCI: contractures, osteoporosis, fractures, and shoulder pain. SCI Forum. 2002. Available at: http://sci.washington.edu/info/forums/reports/musculoskeletal.asp. Accessed April 23, 2007.
- Anderson CJ, Vogel LC, Chlan KM, Betz RR, McDonald CM. Depression in adults who sustained spinal cord injuries as children or adolescents. J Spinal Cord Med. 2007;30(suppl 1):S76–S82. doi: 10.1080/10790268.2007.11754609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryden DM, Saunders LD, Rowe BH, et al. Depression following traumatic spinal cord injury. Neuroepidemiology. 2005;25(2):55–61. doi: 10.1159/000086284. [DOI] [PubMed] [Google Scholar]
- Charlifue S, Gerhart K. Community integration in spinal cord injury of long duration. Neurorehabilitation. 2004;19(2):91–101. [PubMed] [Google Scholar]
- Price GL, Kendall M, Amsters DI, Pershouse KJ. Perceived causes of change in function and quality of life for people with long duration spinal cord injury. Clin Rehabil. 2004;18(2):164–171. doi: 10.1191/0269215504cr714oa. [DOI] [PubMed] [Google Scholar]
- Tate DG, Kalpakjian CZ, Forchheimer MB. Quality of life issues in individuals with spinal cord injury. Arch Phys Med Rehabil. 2002;83(12 suppl 2):S18–S25. doi: 10.1053/apmr.2002.36835. [DOI] [PubMed] [Google Scholar]
- Kemp BJ, Krause JS. Depression and life satisfaction among people aging with post-polio and spinal cord injury. Disabil Rehabil. 1999;21(5–6):241–249. doi: 10.1080/096382899297666. [DOI] [PubMed] [Google Scholar]
- Krause JS, Kemp B, Coker JL. Depression after spinal cord injury: relation to gender, ethnicity, aging, and socioeconomic indicators. Arch Phys Med Rehabil. 2000;81(8):1099–1109. doi: 10.1053/apmr.2000.7167. [DOI] [PubMed] [Google Scholar]
- Elliott TR, Frank RG. Depression following spinal cord injury. Arch Phys Med Rehabil. 1996;77(8):816–823. doi: 10.1016/s0003-9993(96)90263-4. [DOI] [PubMed] [Google Scholar]
- Frank RG, Elliott TR, Corcoran JR, Wonderlich SA. Depression after spinal cord injury: is it necessary. Clin Psychol Rev. 1987;7(6):611–630. [Google Scholar]
- Consortium for Spinal Cord Medicine. Depression Following Spinal Cord Injury: A Clinical Practice Guideline for Primary Care Physicians. Washington, DC: Paralyzed Veterans of America; 1998. [Google Scholar]
- Cardenas D, Hoffman J, Kirshblum S, McKinley W. Etiology and incidence of rehospitalization after traumatic spinal cord injury: a multicenter analysis. Arch Phys Med Rehabil. 2004;85(11):1757–1763. doi: 10.1016/j.apmr.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Charlifue S, Lammertse DP, Adkins RH. Aging with spinal cord injury: changes in selected health indices and life satisfaction. Arch Phys Med Rehabil. 2004;85(11):1848–1853. doi: 10.1016/j.apmr.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Pagliacci MC, Franceschini M, Di Clemente B, Agosti M, Spizzichino L. A multicentre follow-up of clinical aspects of traumatic spinal cord injury. Spinal Cord. 2007;45(6):404–410. doi: 10.1038/sj.sc.3101991. [DOI] [PubMed] [Google Scholar]
- Krause JS. Secondary conditions and spinal cord injury: a model for prediction and prevention. Top Spinal Cord Inj Rehabil. 1996;2(2):217–227. [Google Scholar]
- CDC. Behavioral Risk Factor Surveillance System Survey Questionnaire. 2006. Available at: http://www.cdc.gov/brfss/questionnaires/pdf-ques/2006brfss.pdf. Accessed July 16, 2009.
- Krause JS. Dimensions of subjective well-being after spinal cord injury: an empirical analysis by gender and race/ethnicity. Arch Phys Med Rehabil. 1998;79(8):900–909. doi: 10.1016/s0003-9993(98)90085-5. [DOI] [PubMed] [Google Scholar]
- Krause JS. Intercorrelations between secondary conditions and life adjustment among people with spinal cord injuries. SCI Psychosoc Process. 1998;11:3–7. [Google Scholar]
- Kemp BJ, Adams BM. The Older Adult Health and Mood Questionnaire: a measure of geriatric depressive disorder. J Geriatric Psychiatry Neurol. 1995;8(3):162–167. doi: 10.1177/089198879500800304. [DOI] [PubMed] [Google Scholar]
- Krause JS, Coker J, Charlifue S, Whiteneck GG. Health behaviors among American Indians with spinal cord injury: comparison with data from the 1996 Behavioral Risk Factor Surveillance System. Arch Phys Med Rehabil. 1999;80(11):1435–1440. doi: 10.1016/s0003-9993(99)90255-1. [DOI] [PubMed] [Google Scholar]
- Stein AD, Courval JM, Lederman RI, Shea S. Reproducibility of responses to telephone interviews: demographic predictors of discordance in risk factor status. Am J Epidemiol. 1995;141(11):1097–1105. doi: 10.1093/oxfordjournals.aje.a117375. [DOI] [PubMed] [Google Scholar]
- Krause JS. Factors associated with risk for subsequent injuries after the onset of traumatic spinal cord injury. Arch Phys Med Rehabil. 2004;85(9):1503–1508. doi: 10.1016/j.apmr.2004.01.017. [DOI] [PubMed] [Google Scholar]
- Muthen L, Muthen B. Mplus, The Comprehensive Modeling Program for Applied Researchers User's Guide. 4th ed. Los Angeles, CA: Muthen & Muthen; 2006. [Google Scholar]
- McDonald R. Factor Analysis and Related Methods. Mahwah, NJ: Lawrence Erlbaum Associates; 1985. [Google Scholar]
- Browne M, Cudeck R. Alternative ways of assessing model fit. In: Bollen K, Long S, editors. Testing Structural Equation Models. Beverly Hills, CA: Sage; 1993. pp. 136–162. [Google Scholar]
- Browne MW. An overview of analytic rotation in exploratory factor analysis. Multivariate Behavioral Res. 2001;36(1):111–150. [Google Scholar]
- McArdle JJ, Prescott CA. Age-based construct validation using structural equation models. Exp Aging Res. 1992;18(3–4):87–115. doi: 10.1080/03610739208253915. [DOI] [PubMed] [Google Scholar]
- Krause JS, Reed KS. Obtaining employment after spinal cord injury: relationship with pre- and post-injury education. Rehabil Couns Bull. 2009;53(1):27–33. [Google Scholar]
- Schonherr MC, Croothoff JW, Mulder GA, Schoppen T, Eisma WH. Vocational reintegration following spinal cord injury: expectations, participation and interventions. Spinal Cord. 2004;42(3):177–184. doi: 10.1038/sj.sc.3101581. [DOI] [PubMed] [Google Scholar]
- Hess DW, Ripley DL, McKinley WO, Tewksbury M. Predictors for return to work after spinal cord injury: a 3-year multicenter analysis. Arch Phys Med Rehabil. 2000;81(3):359–363. doi: 10.1016/s0003-9993(00)90084-4. [DOI] [PubMed] [Google Scholar]
- Krause JS. Is the ability to ambulate associated with better employment outcomes in participants with traumatic spinal cord injury. Rehabil Couns Bull. 2008 Dec 29. E-pub ahead of print: doi:10.1177/0034355208329442.
- Krause JS, Carter RE, Brotherton SS. Association of mode of locomotion and independence in locomotion with long-term outcomes after spinal cord injury. J Spinal Cord Med. 2009;32(3):237–248. doi: 10.1080/10790268.2009.11760778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahluwalia IB, Bolen J. Lack of health insurance coverage among working-age adults, evidence from the Behavioral Risk Factor Surveillance System, 1993–2006. J Community Health. 2008;33(5):293–296. doi: 10.1007/s10900-008-9106-8. [DOI] [PubMed] [Google Scholar]
- Guendelman S, Angulo V, Oman D. Access to health care for children and adolescents in working poor families: recent findings from California. Med Care. 2005;43(1):68–78. [PubMed] [Google Scholar]
- Krause JS, McArdle JJ, Pickelsimer E, Reed KS. A latent variable structural path model of health behaviors after spinal cord injury. J Spinal Cord Med. 2009;32(2):162–174. doi: 10.1080/10790268.2009.11760768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause JS, Newman S. Clinical outcomes after spinal cord injury. In: Lajtha A, editor. Handbook of Neurochemistry and Molecular Neurobiology: Central Nervous System Injuries and Disorders. Vol 24. 3rd ed. New York: Springer; 2008. pp. 615–632. [Google Scholar]