Abstract
Objective:
To develop a new, clinically relevant large animal model of pediatric spinal cord injury (SCI) and compare the clinical and experimental features of pediatric SCI.
Methods:
Infant piglets (3–5 weeks old) underwent contusive SCI by controlled cortical impactor at T7. Severe complete SCI was induced in 6 piglets, defined as SCI with no spontaneous return of sensorimotor function. Eight piglets received incomplete SCI, which was followed by partial recovery. Somatosensory evoked potentials, magnetic resonance imaging, neurobehavioral function, and histopathology were measured during a 28-day survival period.
Results:
Mean SCI volume (defined as volume of necrotic tissue) was larger after complete compared with incomplete SCI (387 ± 29 vs 77 ± 38 mm3, respectively, P < 0.001). No functional recovery occurred after complete SCI. After incomplete SCI, piglets initially had an absence of lower extremity sensorimotor function, urinary and stool retention, and little to no rectal tone. Sensory responses recovered first (1–2 days after injury), followed by spontaneous voiding, lower extremity motor responses, regular bowel movements, and repetitive flexion-extension of the lower extremities when crawling. No piglet recovered spontaneous walking, although 4 of 8 animals with incomplete injuries were able to bear weight by 28 days. In vivo magnetic resonance imaging was performed safely, yielded high-resolution images of tissue injury, and correlated closely with injury volume seen on histopathology, which included intramedullary hemorrhage, cellular inflammation, necrosis, and apoptosis.
Conclusion:
Piglets performed well as a reproducible model of traumatic pediatric SCI in a large animal with chronic survival and utilizing multiple outcome measures, including evoked potentials, magnetic resonance imaging, functional outcome scores, and histopathology.
Keywords: Spinal cord injuries; Animal model, piglet; Children; Evoked potential; Magnetic resonance imaging; Functional outcome; Contusion; Trauma; Paralysis; Paraplegia
INTRODUCTION
Spinal cord injury (SCI) exacts enormous emotional, psychological, and financial costs on affected children and their families and places great burdens on schools, medical facilities, and society as a whole. Although the incidence is low, the prevalence of the disease is relatively high due to long median survival, with an estimated 250,000 people currently living with SCI in the US alone. SCI in children is a relatively uncommon disease, affecting 1.99/100,000 children in the US per year, or 1,500 to 2,000 children younger than age 18 years (1). This represents 15 to 20% of all US cases of SCI annually. Several clinical trials have been conducted in adults with SCI; however, there has never been a trial of acute interventions for SCI enrolling children younger than age 14 years.
Similarly, in the preclinical setting, injury to the immature spinal cord has received little attention in basic science research. The pediatric spinal cord and spinal column have anatomic and biomechanical differences compared with adults that predispose children to different mechanisms of injury and result in a unique injury profile (2–5). A small number of pediatric animal models of SCI have been established and have been used primarily to investigate mechanisms of injury, repair, and regeneration. Although a substantial amount of excellent work has been done with spinalized kittens (6–11), a larger animal model of pediatric SCI would be advantageous for translational investigations. Clinically relevant large animal models of pediatric SCI are needed to provide preclinical data regarding efficacy, safety, and tolerability of therapeutic interventions before clinical trials.
We recently developed a large animal model of SCI in 3- to 5-week-old infant piglets using the controlled cortical impactor to cause a spinal cord contusion at T7 through a posterior laminectomy. In this paper, we describe our early results from this model and review the relevant literature on experimental pediatric SCI.
METHODS
General Preparation
All of the following procedures were approved by the Institutional Animal Care and Use Committee of the University of Miami School of Medicine. Female infant piglets (Yorkshire or “domestic” strain) were used for this study; they weighed 5 to 7 kg and were 3 to 5 weeks old. All animals were prepped with a general chlorhexidine gluconate scrub followed by Betadine application to sterile sites before surgery. Anesthesia was induced with ketamine 45 mg/kg, xylazine 4 mg/kg, and acepromazine 0.4 mg/kg intramuscularly (IM). As soon as intravascular access was achieved, fentanyl 50 µg/kg was given intravenously (IV) followed by a continuous infusion of 10 µg/kg/h IV. Additionally, propofol was given as intermittent boluses 2 to 4 mg/kg or by continuous infusion 50 to 100 µg/kg/min for sedation and reduction of anxiety. Additional doses of fentanyl 10 to 20 µg/kg were given as needed for breakthrough pain and agitation. Piglets were ventilated through an endotracheal tube to achieve normal arterial blood gases. Normothermia and adequate hydration were maintained throughout the study using heating blankets and normal saline, respectively.
All animals received antibiotic prophylaxis with cefazolin 30 mg/kg IV every 8 hours beginning at the start of surgery and continuing for up to 24 hours postoperatively to prevent infection. A strict sterile technique was applied for all surgical procedures. Arterial blood gases, plasma glucose, and electrolytes were measured every 30 to 60 minutes during surgery and magnetic resonance imaging (MRI) and again 30 minutes after extubation. Catheters were placed in the external jugular vein and the superficial femoral artery for delivery of fluid and drugs, measurement of vascular pressures, and blood sampling. The central venous catheter was tunneled from the external jugular entrance site to the nape of the neck for long-term maintenance. Catheter patency was maintained with heparin-saline locks. At the end of the study, piglets were euthanatized with an overdose of propofol 10 mg/kg IV and KCl 4 mEq/kg IV and processed for histopathologic assessment of SCI.
Controlled Cortical Impact SCI Through a Posterior Laminectomy
After induction of general anesthesia and surgical preparation, an 8- to 10-cm posterior midline incision was made over the thoracic vertebra centering on T7, which was marked under fluoroscopy. After careful exposure of the spinous processes of T6–T8, the T7 spinous process was removed and shaved down with rongeurs to the lamina, which was then carefully removed. Small portions of the T6 and T8 spinous processes were also removed to provide better exposure and allow room for the impactor. The spinal cord was exposed after removing the ligamentum flavum and the underlying epidural fat. The wound was then temporarily closed for 30 minutes, after which baseline measurements of vascular pressure, temperature, blood gases, blood chemistries, and somatosensory evoked potentials were performed, followed by induction of SCI. The circular impactor tip used in this study was not beveled and had a diameter of 6 mm, which matched the average width of the thoracic spinal cord at this level (5.5–6.5 mm).
We initially sought to induce complete SCI to test whether this animal model could be used for chronic studies. We defined “complete” SCI as an injury that produces complete sensorimotor deficits below the level of injury that do not resolve spontaneously. In the first animals, the depth and velocity (set as pounds per square inch [psi]) of the impact were set high in an attempt to achieve a complete SCI and were progressively decreased until we observed spontaneous recovery of sensorimotor function after injury. We found that severe complete SCI, defined as SCI with no return of sensorimotor function, was produced with a depth of either 5 or 8 mm and either 60 or 80 psi. In contrast, incomplete SCI occurred consistently with a depth of 3 mm and 30 psi. After impact and adequate hemostasis with Gelfoam or Surgicel, the incision was closed in layers and infiltrated with bupivacaine and cefazolin for long-acting local anesthesia and infection prophylaxis, respectively.
Somatosensory Evoked Potentials
Neurophysiologic measurements of somatosensory evoked potentials (SSEPs) were performed at baseline, 30 minutes after SCI and serially during survival to assess recovery of spinal cord electrophysiological function. The SSEPs measure the competency of the neural circuitry from the peripheral sensory nervous system in the lower extremities through the dorsal columns to the sensory cerebral cortex. This method was available only for the group receiving incomplete SCI, which was studied later in this project.
The SSEP procedure involved bilateral stimulation of the tibial (sensory) nerve at the ankle. Stimuli were applied in an interleaved manner. Square-wave, monophasic pulses of 0.2 millisecond duration and at least 2 times threshold were used. Stimulus-evoked averages of electroencephalography were recorded via subdermal electrodes placed in the scalp over the sensory cortex, and a ground electrode was placed in the shoulder. Electrode impedances were kept below 5 kΩ, and typically 256 responses were averaged for each measurement. The primary end points were the latency and amplitude of the electroencephalographic response, which were quantitated digitally by the monitor. The procedure took approximately 5 minutes. The details of this method were originally developed at our center for clinical use during spine surgical procedures (12,13).
Magnetic Resonance Imaging
In vivo MRI of the spinal cord was performed 1 to 2 hours after SCI to assess tissue injury severity. Piglets undergoing MRIs remained intubated, mechanically ventilated, sedated with propofol 50 to 100 µg/kg/min, and paralyzed for optimal image resolution with pancuronium 0.2 to 0.3 mg/kg IV every 30 to 40 minutes. Close monitoring of cardiorespiratory status, including blood pressure, heart rate, core temperature, depth and rate of respirations, and arterial blood gases, was performed on all animals before, during, and after MRI. Temperature was maintained using a water-filled warming blanket.
MRI datasets were collected on a 4.7-Tesla (200-MHz) 40-cm bore magnet with a Bruker Avance console using an actively shielded gradient set. A homemade quadrature saddle-shaped transmit-receive surface radio frequency coil was used for imaging the injured area to increase sensitivity and reduce field-of-view sizes. A high-resolution T2 rapid acquisition relaxation-enhanced (RARE) scan was collected in the sagittal plane with a slice thickness of 1.25 mm and an in-plane resolution of 391 µm × 398 µm. In addition to the sagittal T2-RARE scan, an axial T2-RARE scan was collected with a slice thickness of 1.50 mm and an in-plane resolution of 375 µm × 357 µm. In addition to the T2-RARE scans, a T1-weighted fluid-attenuated inversion recovery scan was collected in the sagittal plane with a slice thickness of thickness of 2.0 mm and an in-plane resolution of 521 µm × 547 µm.
Ex vivo MRI of the injured spinal cord was performed to obtain diffusion-tensor imaging data sets and high-resolution standard imaging before processing the tissue for histopathology. The spinal cord was placed in fixative (formalin∶acetic acid∶methanol in a ratio of 1∶1∶8) for a minimum of 1 week after removal to ensure adequate fixation. The spinal cord sample included 2 to 3 cm on either side of the injury epicenter. Twenty-four hours before ex vivo MRI, the tissue was washed and placed in phosphate-buffered saline at 4°C until scanning. Tissue was again placed in fresh phosphate-buffered saline solution just before the 24-hour MRI procedure. The diffusion-tensor imaging acquisition was collected in the axial orientation, with a slice thickness of 0.60 mm, an in-plane resolution of 90 µm × 90 µm, a diffusion time of 18 milliseconds, and a b-value of 2000 s/mm2; 7 diffusion directions were employed. In addition to the high-resolution diffusion-tensor imaging data, T2-RARE experiments were collected in 3 orientations (sagittal, coronal, and axial). The coronal and sagittal data sets were collected with a slice thickness of 0.4 mm and an in-plane resolution of 68 µm × 70 µm. The axial T2-RARE data were collected with a 0.6-mm slice thickness and an in-plane resolution of 70 µm × 70 µm.
MRI analysis was accomplished using Paravision software (Bruker, Billerica, MA). The volume of injury was measured on the T2-RARE data sets and was defined as the volume of tissue with abnormal MRI signal plus volume loss due to atrophy. Both serial axial and sagittal data sets were employed separately to calculate the volume of tissue injury and atrophy.
Postinjury Care
General Care
Postinjury care of piglets with SCI is labor intensive. Each animal received 1 to 2 hours of hands-on care every day, including weekends from research and veterinary technicians. When possible, pigs were housed together with SCI littermates to improve social interactions and overall well-being. Side-by-side pens were constructed so that piglets could see their littermates and socialize after injury. Soft rubber mats covered the floor of the pens, and 2 to 3 inches of thin wood shavings were placed over the mats to absorb body fluids. The environment was also enriched with toys to improve and maintain quality of life and also to more closely mimic the human condition.
Infection Control
Routine practice for intake of all pigs included nasal and rectal swabs for bacterial cultures and antibiotic sensitivities. Cultures revealed occasional colonization with gram-negative organisms, such as Klebsiella, and Escherichia coli, and parasites. All personnel interacting with the piglets wore gowns, gloves, and footwear during all aspects of this study. Frequent chlorhexidine gluconate baths were used to keep down bacterial counts on the skin. Chlorhexidine ointment was used on all incisions and wounds. Also, 1 dose of enrofloxacin, a broad-spectrum fluoroquinolone antibiotic, 10 mg/kg IM, was given at the time of surgery. Enrofloxacin is effective against gram-negative bacteria that can cause pneumonia, urinary tract infection, and gastroenteritis.
Rehabilitation
We purchased 2 specially designed wheelchairs that support the lower half of the body, which enabled piglets to walk around the animal facility. A lower-body harness similar to a pair of shorts is attached to the lower half of the body using Velcro. The harness is snapped into place over 2 wheels. The upper extremities are then placed through a chest harness that secures the piglet to the wheelchair. Piglets are then strapped into the wheelchair once daily beginning 2 to 3 days after injury, depending on the overall stability of the animal. In addition, range-of-motion exercises of the lower extremities were carried out for 5 minutes twice daily.
Nutrition
Piglets were examined for general systemic health problems and weighed daily. Their food and water consumption was monitored closely. Fruit and other treats were also given daily. If they refused to eat and drink or lost weight, then they were gavage fed a veterinary milk formulation through an orogastric tube.
Neurogenic Bladder Care
Bladder emptying was monitored closely to prevent bladder disruption and urinary tract infection. The bladder was emptied by the Credé method and/or by sterile suprapubic needle tap of the bladder immediately after the initial MRI and again at 24 hours.
Neurogenic Bowel
Constipation and marked abdominal distension occurred commonly due to neurogenic bowel. Spontaneous bowel movements usually returned 2 to 3 days after incomplete SCI, but intestinal dysmotility can occur anytime during survival. Therefore, stool output was monitored closely and constipation treated with bisacodyl suppositories and cola or Fleet enema. Episodes of abdominal distension became less problematic after limiting food consumption to the recommended daily allowance (14).
Skin Care
Skin was monitored on a daily basis and treated when required with chlorhexidine gluconate ointment, frequent bathing, and routine wound care. The period of highest risk is the first 2 to 3 days after SCI, when the piglets are learning to right themselves and begin to ambulate more in the pen.
Neurologic Assessment and Functional Outcome Measurement
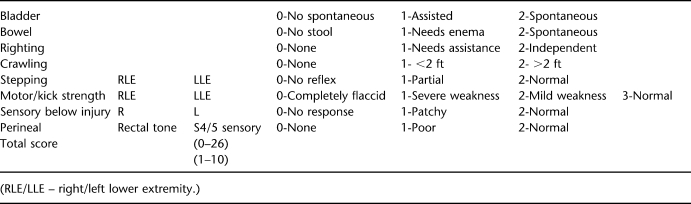
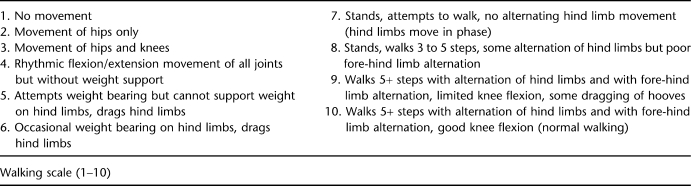
For neurobehavioral analysis, animals were tested before surgery and then daily until they were killed. The general neurologic assessment (see Appendix) was designed to measure overall neurologic status during recovery from SCI, with special emphasis on bowel and bladder functions and sensorimotor function of the lower extremities and perineal region. Assessment of locomotor function was confined to spontaneous movements of the lower extremities. Animals were placed in an open pen (approximately 10 ft × 10 ft) with nonslip floor mats (for better traction) for 20 minutes twice per exam day and were evaluated according to the Porcine Walking Scale (see Appendix), a 10-point walking scale that we modified from the Basso, Beattie, and Bresnahan locomotor rating scale for rodents.
Histopathologic Measurement of Injury
After the piglets were euthanatized with propofol 10 mg/kg and KCl 4 mEq/kg IV, the thoracic spinal cord was removed and placed in fixative (formalin∶acetic acid∶methanol in a ratio of 1∶1∶8) for a minimum of 1 week befpre ex vivo MRI. After ex vivo MRI, the tissue was placed overnight in a tissue processor and then set in paraffin blocks (4–5 cm in length) for later sectioning (10 µ) in the sagittal plane (longitudinally) using a sliding microtome. Sections were mounted on slides and stained with hematoxylin-eosin and Luxol fast blue (myelin stain) to identify the volume of traumatic injury, size of contusion, inflammatory cell infiltrate, and degree of atrophy. An antibody to activated caspase-3 (Cell Signaling Technology, Danvers, MA) was used for immunohistochemical detection of apoptotic cells. A technician blinded to injury severity identified the area of injury by tissue pallor and disruption and the presence of reactive astrocytes, microglia, and macrophages. The percentage of damaged tissue area in each section was quantitatively determined by using computer-aided image analysis (MetaMorph Imaging System, Universal Imaging, Downingtown, PA), and SCI volume of injury (defined as volume of necrotic tissue) was calculated by numerical integration using personal computer software. Histopathologic assessments were made in 2 piglets in each group 6 hours and 3 days after SCI and 28 days after complete (n = 6) and incomplete (n = 8) SCI.
Statistical Analysis
Statistical analysis was performed using statistical software (SSPS). Variables measured serially over time, such as hemodynamic values, temperature, serum chemistries, biomarkers, MRI volume of injury, SSEP and motor evoked potential (latencies, amplitude, threshold values), and functional outcome scores, were analyzed by ANOVA for repeated measures with Dunnett's posthoc correction. Statistical significance was set at P < 0.05. Comparisons between groups were made using 2-way ANOVA with Bonferroni's posthoc correction. Comparisons within each group were made using repeated-measures ANOVA with Dunnett's posthoc correction.
RESULTS
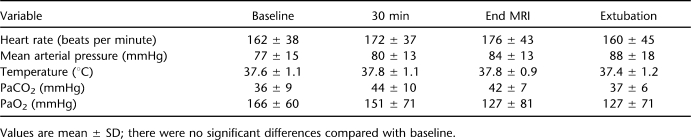
We induced complete (n = 6) and incomplete (n = 8) SCI in female infant piglets and allowed them to survive for up to 28 days. Complete SCI without spontaneous recovery of sensorimotor function occurred when the controlled cortical impactor's depth and pressure settings were at 5 or 8 mm and 60 or 80 psi, respectively. When a 3-mm depth and 30 psi of pressure were used, incomplete SCI was induced in 8 of 8 piglets. The physiologic data from a total of these 14 piglets are shown in Table 1. There were no differences in the cardiorespiratory responses to T7 SCI in complete vs incomplete injury, nor were there significant differences after SCI compared with baseline in any of the physiologic variables (data not shown).
Table 1.
Physiologic Variables Before and After Spinal Cord Injury in Piglets
Acute histopathology of the 2 severities of SCI was examined in 2 piglets in each group 6 hours after injury, and it is displayed in Figure 1. With complete SCI, tissue destruction occurred throughout the thickness of the spinal cord. After incomplete SCI, central hemorrhage, inflammation, and tissue injury occurred along with partial dorsal column injury and much less ventral cord injury acutely. Three days after SCI, high-power views demonstrated areas of severe traumatic injury and tissue loss, patchy but marked inflammatory cell infiltrates, and a moderately high number of apoptotic cells (caspase-3 positive), particularly along the border zone between the injured and normal spinal cord (Figure 2). After chronic survival of 28 days, mean SCI volume was significantly larger after complete compared with incomplete SCI (387 ± 29 vs 77 ± 38 mm3, respectively, P < 0.001). Figure 3 contrasts the difference in severity, thickness, and length of the injury in complete vs incomplete SCI.
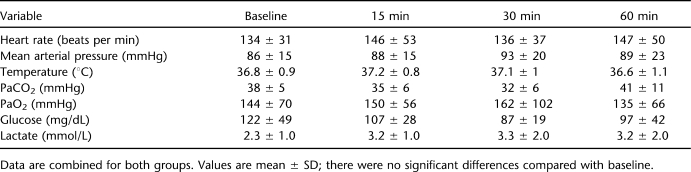
Figure 1.
Acute histopathology 6 hours after complete (A) and incomplete (B and C) spinal cord injury (SCI). High-resolution T2-weighted ex vivo magnetic resonance image is shown in C. Tissue destruction is evident throughout the thickness of the thoracic cord after complete SCI. White matter is considerably more spared after incomplete SCI, with partial dorsal column injury and primarily central zone of injury, inflammation, and hemorrhage.
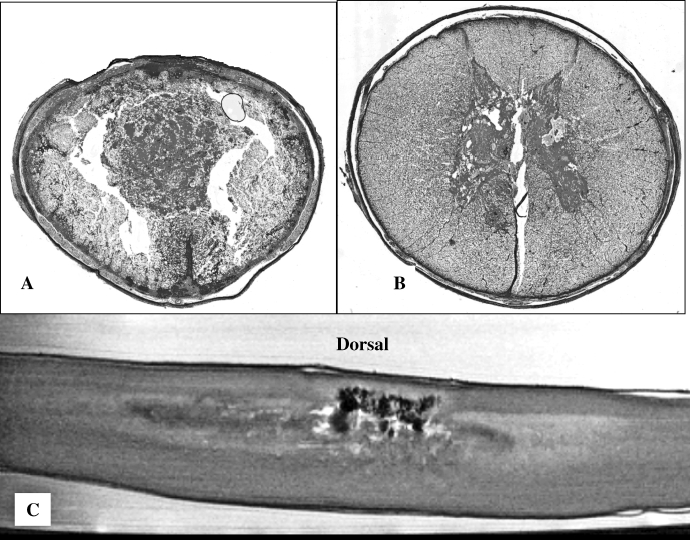
Figure 2.
High-power views of histopathology 3 days after incomplete spinal cord injury in an infant piglet. Marked inflammatory cell infiltrate (A, large arrow) and moderately high number of apoptotic cells (B, small arrows, caspase-3 positive) are seen in the border zone between injury and normal-appearing spinal cord.
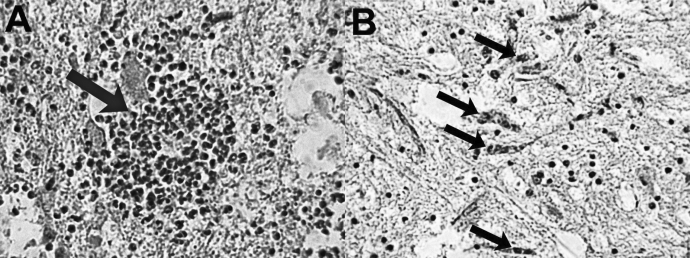
Figure 3.
Representative longitudinal sections of thoracic spinal cord stained with Luxol fast blue 28 days after complete (A–C) and incomplete (D–F) spinal cord injury (SCI). Chronic lesion size is significantly greater in thickness and length after complete compared with incomplete SCI.
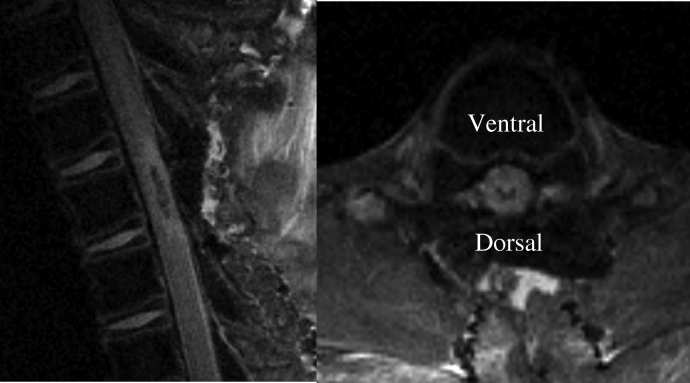
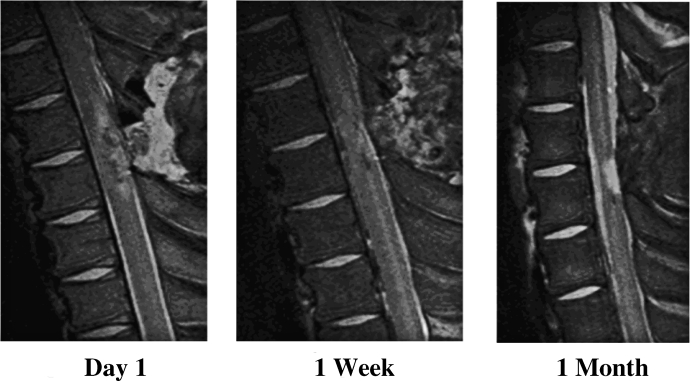
High-resolution in vivo MRI was achieved consistently using the Bruker 4.7-Tesla imaging system (see “Methods”). Physiologic variables were stable throughout the MRI procedure, as shown in Table 2. The area of hypodensity of T1-weighted fluid-attenuated inversion recovery scan images acutely after SCI corresponded to central hemorrhagic contusion seen on histopathology and also correlated with long-term injury volume measured histopathologically. Figure 4 demonstrates the early appearance of the spinal cord lesion 2 hours after injury on MRI in both sagittal and transaxial planes. Serial imaging was also successfully performed in a small number of piglets showing the changes in injury characteristics over the survival period (Figure 5). The final MRI in Figure 5 corresponds closely to the actual tissue when examined histologically (Figure 3).
Table 2.
Physiologic Variables Before, During, and After Magnetic Resonance Imaging (MRI) in Piglets With Incomplete Spinal Cord Injury
Figure 4.
T1-weighted fluid-attenuated inversion recovery magnetic resonance images 2 hours after incomplete spinal cord injury.
Figure 5.
Serial T2-weighted in vivo magnetic resonance image of incomplete spinal cord injury in an infant piglet.
There was no recovery of sensorimotor function in piglets with complete SCI. Hind limbs displayed moderate muscle atrophy and mild-moderate contractures by the end of the 28-day survival period. Regular spontaneous voiding occurred by the end of the first week, although symptoms of neurogenic bowel recurred throughout the entire survival period, necessitating frequent enemas and suppositories to maintain regularity and reduce abdominal distention. One piglet in this group developed severe abdominal distention, died 3 days after SCI, and was found to have a ruptured bladder and peritonitis on postmortem examination.
After incomplete SCI, for the first 24 to 48 hours, piglets had markedly decreased to no lower extremity sensorimotor function, urine and stool retention consistent with neurogenic bladder and bowel, and little to no rectal tone. Sensory response to pin prick was the first sign of spinal cord recovery, usually between 24 and 48 hours after injury. Thereafter, recovery proceeded in the following order: return of regular spontaneous voiding, rhythmic kicking of the lower extremities when the hind legs were pulled, regular bowel movements, and, finally, spontaneous movements of the lower extremities during attempts at ambulation. Piglets, however, required close monitoring and frequent interventions to ensure appropriate weight gain, prevent stool and urinary retention, and maintain good overall health during survival.
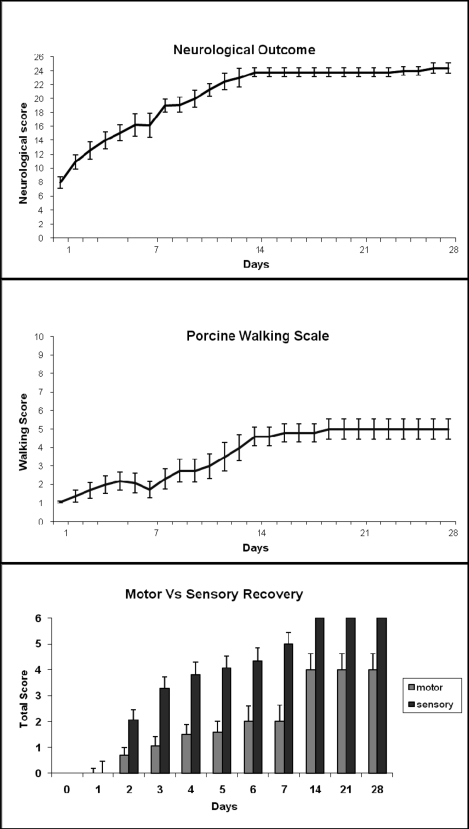
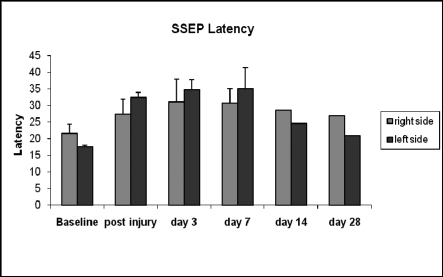
Mean functional recovery improved up to 2 weeks after incomplete SCI and seemed to level off after that (Figure 6). The mean overall neurologic outcome score was 24 out of 26 two weeks after SCI and did not change thereafter. The same plateau at 14 days after injury was found for the walking scale and sensorimotor function. Sensory responses to light touch and pin prick recovered completely, whereas motor recovery was incomplete and no animal was able to take more than 2 to 3 steps with the hind legs 28 days after injury. As shown in Figure 7, incomplete SCI at T7 caused an increase in latency, which peaked between 3 and 7 days after injury, and returned to baseline between 2 and 4 weeks of recovery.
Figure 6.
Functional recovery in piglets (n = 8) after incomplete spinal cord injury (SCI) over 28-day survival period. In most cases, outcome reached a plateau 14 days after SCI. Sensory function recovered completely in all animals but motor recovery did not. (See Appendix for details of the neurologic examination).
Figure 7.
Somatosensory evoked potentials before and after incomplete spinal cord injury.
DISCUSSION
Clinical Features of Pediatric SCI
The spinal cord and spinal column in the pediatric population are anatomically and biomechanically different from those in adults, and these differences predispose children to different mechanisms of injury (2–5). In comparison with their adult counterparts, children with SCI tend to have a higher percentage of upper cervical and thoracic spine injuries (15–18), higher susceptibility to delayed onset of neurologic deficits (19), greater percentage of complete SCIs (20–22), and higher incidence of SCI without radiographic abnormality (SCIWORA) (23). The term SCIWORA was coined prior to the availability of MRIs, when radiographs and computed tomographic scans failed to show spinal column injury, such as fractures, misalignment, and dislocation, despite the presence of a SCI. Pang et al (23) first described the condition and estimated the incidence of SCIWORA to be 30% to 40% of all pediatric cases of SCI. Children have unique features that result in hypermobility of the spinal column, leading to distraction injury after trauma. These include having a large occiput and laxity of the spinal ligaments (24,25). In addition, the intervertebral disks have a high water content, allowing them to stretch considerably without rupture (26). The facet joints are more horizontal and the vertebral bodies are wedged anteriorly, allowing for increased forward slippage, which stretches the spinal cord (23,27). These features make the pediatric spine malleable and, in combination with a large occiput, may result in stretch or distraction injury to the spinal cord after trauma. The prevailing theory is that SCIWORA results from the fact that the spinal column can stretch as much as 5 to 8 times more than the spinal cord (28,29). In contrast, the adult spine is rigid and more likely to fracture and cause damage to the spinal cord via direct tissue trauma. In an updated review of pediatric SCI, Pang et al (28) reviewed the MRI findings in 50 cases of SCIWORA and found that 18 of 50 children had intramedullary hemorrhage, which was either minor or major, or disruption of the spinal cord, and all but 2 of these children had severe or complete deficits 6 months after injury. All 23 SCIWORA children with normal spinal cords on initial MRI made a complete recovery, whereas 9 children with “edema only” on MRI recovered to normal or mild disability. The implication of Pang's findings is that SCIWORA may result from torsional stretch injuries, but in most cases it also includes a significant component of direct tissue injury from contusion or compression forces. These clinical data provide the rationale for selecting the mechanism of injury in the piglet model of pediatric SCI used in this study.
Review of Animal Models
Only a small number of animal models of pediatric SCI have been described, and even fewer are currently in use. The mechanisms of injury in these models include transection, hemitransection, compression with forceps, contusion, and radiation-induced myelopathy. The most extensively studied species in these investigations has been the cat. Using transection and hemisection as the injury mechanism in developing kittens, several laboratories (6–8,11,29) have demonstrated since the early 1980s that “when spinal pathways are damaged in newborn animals and their behavior is examined in adulthood, motor function is superior to that seen in animals in which the same lesion was made in adulthood.” Dr M.E. Goldberger coined the term “infant lesion effect” to describe this phenomenon (30). After low thoracic spinal cord transection in 1-day-old kittens, no axonal growth occurred into or across the lesion (31). However, after hemisection at the same developmental time point and recovery of motor function of the affected hind limb, investigators postulated that the late-developing corticospinal tract axons reached caudal segments of the spinal cord through an aberrant pathway (32). In support of the use of developing kittens for SCI studies are several well-established methods for measuring sensorimotor function in this species (31,33–36), and these need to be adapted to the piglet/pig in future investigations.
Another of the more exciting developmental models of SCI was developed by an Australian group led by Dr Norman Saunders and has been used extensively to study the unique capacity of the immature spinal cord to regenerate after SCI (37). In this model, the neonatal opossum, Monodelphis domestica, undergoes complete spinal cord transection by a microscalpel in the midthoracic region (T4–T6). Opossums are marsupials and give birth to prenatal infants, so this model actually simulates fetal SCI. By measuring functional recovery after SCI induced at different stages of development, Saunders et al (38) found that 1-week-old opossums recovered completely, 2-week-old opossums only partially, and 1-month-old and older opossums did not recover at all. Histopathologic examination in this model has revealed that the ability for axons to grow across a lesion is substantial at 1 week of age but declines by 2 weeks of age and disappears completely by 1 month (39). Using mouse inflammatory gene arrays, these investigators showed differences in levels of expression of transforming growth factor, tumor necrosis factor, cytokine, chemokine, and interleukin gene families, suggesting that at least some of the greater ability to recover from spinal cord transection at P7 compared with P14 in opossums is due to differences in inflammatory cellular and molecular responses (40).
Several investigators have used the “overhemisection” method of SCI in neonatal rat pups of 4 to 7 days of age, in which bilateral dorsal columns and unilateral ventral and lateral funiculi are severed with microscissors (41–44). In another neonatal rat model, McEwen et al (45) used handheld microforceps to compress the thoracic spinal cord in the horizontal plane. With this method, severity of injury can be adjusted by varying the degree and duration of compression, that is, compressing the cord by 90% of its original diameter results in a mild-moderate injury, whereas a 95% compression achieves severe injury; the longer the duration of compression, the greater the injury. Brown et al (46,47) used the weight-drop method in 2-week-old rat pups and found that they recovered faster than their adult counterparts, possibly due to decreases in glutamate receptor subunits (GluR1, GluR2, and GluR4) acutely after injury. Van den Aardweg et al (48) established a model of radiation-induced myelopathy in immature piglets and found that irradiation of mature pigs resulted in the development of frank paralysis, with animals showing combined parenchymal and vascular pathologic changes in their white matter, whereas immature pigs, after receiving comparable doses, only developed transient neurologic changes. The age of the immature pigs in this study was 15 to 23 weeks, which is well outside of the infant-toddler period and more like a prepubertal older child or adolescent. Nevertheless, even at this advanced “pediatric” age and size, the younger pig recovered from radiation-induced SCI better than the mature pig did.
There are several important deficiencies in each of these models. To begin with, the extremely small size of the neonatal rats (10–12 g) and opossums (2–4 g) and relatively small size of neonatal kittens (100 g at birth) precludes monitoring of physiologic variables, such as blood pressure, blood gases, and serum chemistries, and it does not allow for serial blood and cerebrospinal fluid sampling for measurements of biomarkers of injury. Kittens are unable to walk unassisted until the fourth week of life, whereas piglets stand and begin walking within hours after birth. Both rats and opossums at this stage of development are most likely in the third trimester of fetal life compared with humans, a time in humans when SCI is extremely rare. Several investigators have argued that the brain, and presumably the spinal cord, of neonatal rats up to 10 to 14 days of age has many of the characteristics of that of premature human neonates, thereby reducing the applicability of results from these models to the human condition of pediatric SCI (49,50). Furthermore, the method of injury, such as transection, although ensuring a complete severing of axons, leaves a relatively small injury epicenter and therefore a short distance needed for axon growth and regeneration compared with contusion models. The model of radiation-induced SCI in immature pigs appears to be a valid model of that injury mechanism, but it shares little with traumatic SCI in terms of mechanisms of injury and repair. Although the available small animal models of pediatric/neonatal/fetal SCI offer low-cost methods of examining basic mechanisms of injury and repair, they have many shortcomings regarding the ability to translate findings in these models to human infants and children with SCI.
Traumatic SCI in Infant Piglets: A Clinically Relevant Translational Model of Pediatric SCI
Our laboratory has used the Yorkshire infant piglet as a model of several different critical illnesses in children, including cardiac arrest and cardiopulmonary resuscitation (51–53), ischemic stroke (54), traumatic brain injury (55), acute lung injury (56), and, more recently, SCI. This animal species was originally chosen for these studies because its body size, heart, lungs, chest, and brain structures are remarkably similar to those of human infants. Well-established methods used in these studies include systemic hemodynamic measurements with thermodilution and dye dilution methods, regional organ blood flow measurements using radiolabeled microspheres, tissue processing and slide preparation for routine light microscopy for histopathologic assessment of injury, immunohistochemistry using a variety of antibodies, electron microscopy, extraction of proteins (including cytokines for measurement by enzyme-linked immunosorbent assay and Western blot, positron emission tomography, and single photon emission computed tomography brain imaging), and in vivo and ex vivo MRI of brain and spinal cord.
Infant piglets have several unique characteristics that make them well-suited as a large animal model of pediatric SCI. First, unlike lambs, which are a commonly used model for the study of cardiopulmonary and cerebral hemodynamics, piglets are available year-round and do not require special infection control practices, such as lambs and sheep do to prevent outbreaks of Q fever (57). Piglets are relatively less expensive compared with other large animal models. Infant piglets can be weaned from their mothers several days after birth, thereby developing greater independence. Serial sampling of cerebrospinal fluid and serum can be easily accomplished in piglets without untoward side effects, procedures that would be very difficult in neonatal kittens. A final advantage of piglets, particularly for SCI experiments that involve paraplegia and limited mobility, is their relatively thick skin, which reduces the incidence of decubitus ulcers and other skin problems. Our preliminary data demonstrate that piglets serve well as a large animal model of pediatric SCI. However, much additional work remains to be done in piglets similar to that performed previously in developing kittens to establish normal locomotor development for comparison with animals recovering from SCI. Characterization of the anatomic development of the piglet spinal cord and its individual tracts is also needed to further establish this model.
Several investigators have developed porcine models of ischemic SCI in order to mimic the SCI that occurs with cardiothoracic procedures, such as aortic aneurysm repair, coarctation repair, cardiopulmonary bypass, and ischemia from low-output states (58–63). These have been mostly adult models, with one exception (59). Most previous porcine studies of ischemic SCI were terminal experiments utilizing end points (eg, motor and somatosensory evoked potentials (61,63)), neurotransmitter release into cerebrospinal fluid (62), histopathologic scores, or blood flow determinations (64). The few studies in which the pigs were allowed to awaken used vague, nonspecific scales, such the Tarlov scale, and the longest survival period reported after porcine ischemic SCI was only 3 days (61).
Our piglet model of pediatric SCI represents the first attempt to establish a traumatic SCI model in pigs of any age with chronic survival up to 28 days and to utilize multiple outcome measures, including neurophysiology, MRI, 2 functional outcome scores, and histopathology, including immunohistochemistry. From a cardiorespiratory standpoint, piglets tolerated the induction of injury and the anesthesia quite well, even though the latter lasted up to 6 to 7 hours inclusive of the immediate postinjury MRI. Occasionally, piglets required supplemental oxygen overnight, but otherwise their respiratory function was not affected by SCI at T7. Even after complete SCI, piglets survived well for up to 28 days, although they required very labor-intensive postoperative care to prevent secondary complications. The most pressing issues in the first 2 days after injury were (a) neurogenic bladder with urinary retention; (b) generalized weakness, which was most likely secondary to lingering effects of anesthesia; and (c) hydration and nutritional status. The tortuous porcine urethra, even in females, precludes serial catheterizations due to the urethral trauma that can occur. We found that the Credé method works well most of the time, but occasionally the bladder is not emptied completely. For this reason, particularly after discovering a case of bladder rupture, we instituted a policy of routine sterile suprapubic needle aspiration of urine immediately after all procedures on day 1 and again the following morning. We are now using noninvasive ultrasound to assess bladder volume after Credé to determine whether needle aspiration is warranted.
The injury to the spinal cord found in our study in piglets with traumatic SCI is similar to that reported in other animal models of SCI, as well as in individuals with SCI (65–67). The pathology of complete or irreversible SCI invariably involves disruption of fiber tracts and spinal cord tissue through the thickness of the spinal cord, whereas incomplete SCI has intact fiber tracts passing through the injury zone. This was also found to be true in our piglets with SCI of different severities. We measured SCI volume to be nearly 5 times greater after complete compared with incomplete SCI, and the entire width of the spinal cord was clearly severely damaged after complete SCI. Incomplete injury was associated with preserved fiber tracts, particularly in the ventral and lateral aspects of the injury site.
Similarly, the histopathology of traumatic SCI in piglets in this study mimics that reported for both rodents and humans. Early after injury, piglet spinal cord contained marked cellular inflammation, as well as a moderately high number of caspase-3 positive cells, suggesting involvement of apoptosis in the evolution of SCI in this model. Inflammation has long been known to play an important role in the pathogenesis of SCI experimentally, as well as in humans (67–69). This includes the early infiltration of the injured spinal cord with neutrophils followed by microglial activation and persistent lymphocytic involvement chronically (67). Pro- and anti-apoptotic mechanisms are in play after SCI, and therapeutic strategies to regulate or inhibit apoptosis have been shown to improve outcome after experimental SCI (70,71). Inhibition of apoptosis by estrogen was shown to improve functional recovery after SCI in adult male rats through calpain inhibition (72). Although we used only female piglets in the present study, their estrogen levels should not have been elevated above their male counterparts. However, it is well known that during early development apoptotic pathways are readily activated after a variety of insults and experimental conditions, including inhaled and intravenous anesthetics, such as isoflurane, ketamine, and midazolam (73).
In summary, our data suggest that the infant piglet model of traumatic SCI has many desirable features of a clinically relevant translational model of pediatric SCI. With the controlled cortical impactor device used through a posterior laminectomy, both complete and incomplete SCI can be achieved consistently, and animals can be allowed to survive for at least 28 days, and probably much longer, with good overall animal welfare. Clinically important outcome measures, such as neurophysiology, MRI, neurobehavioral outcome scores, and histopathology, contribute to the relevance of this model to the human condition of SCI in children. Literature review of experimental pediatric SCI reveals only a small number of animal models currently being used. Further development of this large animal model will determine its usefulness as a preclinical testing platform for therapeutic interventions before their use in pediatric clinical trials.
Acknowledgments
The authors acknowledge Juan Carlos Buitrago, MD, and Farid Alam, MD, for their technical assistance and Morayma Barreto for her assistance with preparation and submission of the manuscript.
APPENDIX
Table I.
Piglet Spinal Cord Injury–Focused Neurologic Examination
Table II.
Porcine Walking Scale
References
- Vitale MG, Goss JM, Matsumoto H, Roye DP., Jr Epidemiology of pediatric spinal cord injury in the United States: years 1997 and 2000. J Pediatr Orthop. 2006;26(6):745–749. doi: 10.1097/01.bpo.0000235400.49536.83. [DOI] [PubMed] [Google Scholar]
- Birney TJ, Hanley EN., Jr Traumatic cervical spine injuries in childhood and adolescence. Spine. 1989;14(12):1277–1282. doi: 10.1097/00007632-198912000-00001. [DOI] [PubMed] [Google Scholar]
- Dickman CA, Rekate HL, Sonntag VK, Zabramski JM. Pediatric spinal trauma: vertebral column and spinal cord injuries in children. Pediatr Neurosci. 1989;15(5):237–256. doi: 10.1159/000120476. [DOI] [PubMed] [Google Scholar]
- Hadley MN, Zabramski JM, Browner CM, Rekate H, Sonntag VK. Pediatric spinal trauma: review of 122 cases of spinal cord and vertebral column injuries. J Neurosurg. 1988;68(1):18–24. doi: 10.3171/jns.1988.68.1.0018. [DOI] [PubMed] [Google Scholar]
- Ruge JR, Sinson GP, McLone DG, Cerullo LJ. Pediatric spinal injury: the very young. J Neurosurg. 1988;68(1):25–30. doi: 10.3171/jns.1988.68.1.0025. [DOI] [PubMed] [Google Scholar]
- Johnson DJ, Smith LA, Eldred E, Edgerton VR. Exercise-induced changes of biochemical, histochemical and contractile properties of muscle in cordotomized kittens. Exp Neurol. 1982;76(2):414–427. doi: 10.1016/0014-4886(82)90218-7. [DOI] [PubMed] [Google Scholar]
- Bregman BS, Goldberger ME. Anatomical plasticity and sparing of function after spinal cord damage in neonatal cats. Science. 1982;217(4559):553–555. doi: 10.1126/science.7089581. [DOI] [PubMed] [Google Scholar]
- Fish CJ, Blakemore WF. A model of chronic spinal cord compression in the cat. Neuropathol Appl Neurobiol. 1983;9(2):109–119. doi: 10.1111/j.1365-2990.1983.tb00329.x. [DOI] [PubMed] [Google Scholar]
- Bregman BS, Goldberger ME. Infant lesion effect: I. Development of motor behavior following neonatal spinal cord damage in cats. Brain Res. 1983;285(2):103–117. doi: 10.1016/0165-3806(83)90045-7. [DOI] [PubMed] [Google Scholar]
- Howland DR, Bregman BS, Tessler A, Goldberger ME. Transplants enhance locomotion in neonatal kittens whose spinal cords are transected: a behavioral and anatomical study. Exp Neurol. 1995;135(2):123–145. doi: 10.1006/exnr.1995.1072. [DOI] [PubMed] [Google Scholar]
- McKinley PA, Smith JL. Age-dependent differences in reorganization of primary somatosensory cortex following low thoracic (T12) spinal cord transection in cats. J Neurosci. 1990;10(5):1429–1443. doi: 10.1523/JNEUROSCI.10-05-01429.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calancie B, Harris W, Brindle GF, Green BA, Landy HJ. Threshold-level repetitive transcranial electrical stimulation for intraoperative monitoring of central motor conduction. J Neurosurg. 2001;95(suppl 2):161–168. doi: 10.3171/spi.2001.95.2.0161. [DOI] [PubMed] [Google Scholar]
- Calancie B, Harris W, Broton JG, Alexeeva N, Green BA. “Threshold-level” multipulse transcranial electrical stimulation of motor cortex for intraoperative monitoring of spinal motor tracts: description of method and comparison to somatosensory evoked potential monitoring. J Neurosurg. 1998;88(3):457–470. doi: 10.3171/jns.1998.88.3.0457. [DOI] [PubMed] [Google Scholar]
- Swindle M. Swine in the Laboratory. 2nd ed. Boca Raton, FL: CRC Press; 2007. pp. 7–8. [Google Scholar]
- Hasue M, Hoshino R, Omata S, et al. Cervical spine injuries in children. Fukushima J Med Sci. 1974;20(3–4):115–123. [PubMed] [Google Scholar]
- Hill SA, Miller CA, Kosnik EJ, Hunt WE. Pediatric neck injuries: a clinical study. J Neurosurg. 1984;60(4):700–706. doi: 10.3171/jns.1984.60.4.0700. [DOI] [PubMed] [Google Scholar]
- Duhem R, Tonnelle V, Vinchon M, Assaker R, Dhellemmes P. Unstable upper pediatric cervical spine injuries: report of 28 cases and review of the literature. Childs Nerv Syst. 2008;24(3):343–348. doi: 10.1007/s00381-007-0497-0. [DOI] [PubMed] [Google Scholar]
- Polk-Williams A, Carr BG, Blinman TA, Masiakos PT, Wiebe DJ, Nance ML. Cervical spine injury in young children: a National Trauma Data Bank review. J Pediatr Surg. 2008;43(9):1718–1721. doi: 10.1016/j.jpedsurg.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Zike K. Delayed neuropathy after injury to the cervical spine in children. Pediatrics. 1959;24:413–417. [PubMed] [Google Scholar]
- Bresnan MJ, Abroms IF. Neonatal spinal cord transection secondary to intrauterine hyperextension of the neck in breech presentation. J Pediatr. 1974;84(5):734–737. doi: 10.1016/s0022-3476(74)80022-3. [DOI] [PubMed] [Google Scholar]
- Kewalramani LS, Kraus JF, Sterling HM. Acute spinal-cord lesions in a pediatric population: epidemiological and clinical features. Paraplegia. 1980;18(3):206–219. doi: 10.1038/sc.1980.36. [DOI] [PubMed] [Google Scholar]
- Ohry A, Rozin R, Brooks ME. Pediatric spinal cord injuries in Israel. Isr J Med Sci. 1980;21(6):526–528. [PubMed] [Google Scholar]
- Pang D, Willberger JE., Jr Spinal cord injury without radiographic abnormalities in children. J Neurosurg. 1982;57(1):114–129. doi: 10.3171/jns.1982.57.1.0114. [DOI] [PubMed] [Google Scholar]
- Townsend EH, Jr, Rowe ML. Mobility of the upper cervical spine in health and disease. Pediatrics. 1982;10(5):567–573. [PubMed] [Google Scholar]
- Sullivan CR, Bruwer AJ, Harris E. Hypermobility of the cervical spine in children: a pitfall in the diagnosis of cervical dislocation. Am J Surg. 1985;95(4):636–640. doi: 10.1016/0002-9610(58)90445-8. [DOI] [PubMed] [Google Scholar]
- Henrys P, Lyne ED, Lifton C, Salciccioli G. Clinical review of cervical spine injuries in children. Clin Orthop Relat Res. 1977;Nov–Dec(129):172–176. doi: 10.1097/00003086-197711000-00020. [DOI] [PubMed] [Google Scholar]
- Pang D. Spinal cord injury without radiographic abnormality in children, 2 decades later. Neurosurgery. 2004;55(6):1325–1342. doi: 10.1227/01.neu.0000143030.85589.e6. [DOI] [PubMed] [Google Scholar]
- Leventhal HR. Birth injuries of the spinal cord. J Pediatr. 1960;56(4):447–453. doi: 10.1016/s0022-3476(60)80356-3. [DOI] [PubMed] [Google Scholar]
- Chau CW, McKinley PA. Chronological observations of primary somatosensory cortical maps in kittens following low thoracic (T12) spinal cord transection at 2 weeks of age. Somatosens Mot Res. 1991;8(4):355–376. doi: 10.3109/08990229109144758. [DOI] [PubMed] [Google Scholar]
- Goldberger ME. Mechanisms contributing to sparing of function following neonatal damage to spinal pathways. Neurochem Pathol. 1986;5(3):289–307. doi: 10.1007/BF02842940. [DOI] [PubMed] [Google Scholar]
- Howland DR, Bregman BS, Tessler A, Goldberger ME. Development of locomotor behavior in the spinal kitten. Exp Neurol. 1995;135(2):108–122. doi: 10.1006/exnr.1995.1071. [DOI] [PubMed] [Google Scholar]
- Bregman BS, Goldberger ME. Infant lesion effect: III. Anatomical correlates of sparing and recovery of function after spinal cord damage in newborn and adult cats. Brain Res. 1983;285(2):137–154. doi: 10.1016/0165-3806(83)90047-0. [DOI] [PubMed] [Google Scholar]
- Robinson GA, Goldberger ME. The development and recovery of motor function in spinal cats. I. The infant lesion effect. Exp Brain Res. 1986;62(2):373–386. doi: 10.1007/BF00238857. [DOI] [PubMed] [Google Scholar]
- Robinson GA, Goldberger ME. The development and recovery of motor function in spinal cats. II. Pharmacological enhancement of recovery. Exp Brain Res. 1986;62(2):387–400. doi: 10.1007/BF00238858. [DOI] [PubMed] [Google Scholar]
- Howland DR, Bregman BS, Goldberger ME. The development of quadrupedal locomotion in the kitten. Exp Neurol. 1995;135(2):93–107. doi: 10.1006/exnr.1995.1070. [DOI] [PubMed] [Google Scholar]
- Salimi I, Friel K, Martin J. Pyramidal tract stimulation restores normal corticospinal tract connections and visuomotor skill after early postnatal motor cortex activity blockade. J Neurosci. 2008;28(29):7426–7434. doi: 10.1523/JNEUROSCI.1078-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders NR, Deal A, Knott GW, Varga ZM, Nicholls JG. Repair and recovery following spinal cord injury in a neonatal marsupial (Monodelphis domestica) Clin Exp Pharmacol Physiol. 1995;22(8):518–526. doi: 10.1111/j.1440-1681.1995.tb02060.x. [DOI] [PubMed] [Google Scholar]
- Saunders NR, Kitchener P, Knott GW, Nicholls JG, Potter A, Smith TJ. Development of walking, swimming and neuronal connections after complete spinal cord transection in the neonatal opossum, Monodelphis domestica. J Neurosci. 1998;18(1):339–355. doi: 10.1523/JNEUROSCI.18-01-00339.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XM, Basso DM, Terman JR, Bresnahan JC, Martin GF. Adult opossums (Didelphis virginiana) demonstrate near normal locomotion after spinal cord transection as neonates. Exp Neurol. 1998;151(1):50–60. doi: 10.1006/exnr.1998.6795. [DOI] [PubMed] [Google Scholar]
- Lane MA, Truettner JS, Brunschwig JP, et al. Age-related differences in the local cellular and molecular responses to injury in developing spinal cord of the opossum, Monodelphis domestica. Eur J Neurosci. 2007;25(6):1725–1742. doi: 10.1111/j.1460-9568.2007.05439.x. [DOI] [PubMed] [Google Scholar]
- Bernstein DR, Stelzner DJ. Plasticity of the corticospinal tract following midthoracic spinal injury in the postnatal rat. J Comp Neurol. 1983;221(4):382–400. doi: 10.1002/cne.902210403. [DOI] [PubMed] [Google Scholar]
- Kunkel-Bagden E, Dai HN, Bregman BS. Recovery of function after spinal cord hemisection in newborn and adult rats: differential effects on reflex and locomotor function. Exp Neurol. 1992;116(1):40–51. doi: 10.1016/0014-4886(92)90174-o. [DOI] [PubMed] [Google Scholar]
- Kunkel-Bagden E, Dai HN, Bregman BS. Methods to assess the development and recovery of locomotor function after spinal cord injury in rats. Exp Neurol. 1993;119(2):153–164. doi: 10.1006/exnr.1993.1017. [DOI] [PubMed] [Google Scholar]
- Bregman BS, Kunkel-Bagden E, Reier PJ, Dai HN, McAtee M, Gao D. Recovery of function after spinal cord injury: mechanisms underlying transplant-mediated recovery of function differ after spinal cord injury in newborn and adult rats. Exp Neurol. 1993;123(1):3–16. doi: 10.1006/exnr.1993.1136. [DOI] [PubMed] [Google Scholar]
- McEwen ML, Stehouwer DJ. Kinematic analyses of air-stepping of neonatal rats after mid-thoracic spinal cord compression. J Neurotrauma. 2001;18(5):1383–1397. doi: 10.1089/08977150152725678. [DOI] [PubMed] [Google Scholar]
- Brown KM, Wolfe BB, Wrathall JR. Rapid functional recovery after spinal cord injury in young rats. J Neurotrauma. 2005;22(5):559–574. doi: 10.1089/neu.2005.22.559. [DOI] [PubMed] [Google Scholar]
- Brown KM, Wrathall JR, Yasuda RP, Wolfe BB. Glutamate receptor subunit expression after spinal cord injury in young rats. Brain Res Dev Brain Res. 2004;152(1):61–68. doi: 10.1016/j.devbrainres.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Van den Aardweg GJ, Hopewell JW, Whitehouse EM, Calvo W. A new model of radiation-induced myelopathy: a comparison of the response of mature and immature pigs. Int J Radiat Oncol Biol Phys. 2004;29(4):763–770. doi: 10.1016/0360-3016(94)90564-9. [DOI] [PubMed] [Google Scholar]
- Romijn HJ, Hofman MA, Gramsbergen A. At what age is the developing cerebral cortex of the rat comparable to that of the full-term newborn human baby. Early Hum Dev. 1991;26(1):61–67. doi: 10.1016/0378-3782(91)90044-4. [DOI] [PubMed] [Google Scholar]
- Hagberg H, Bona E, Gilland E, Puka-Sundvall M. Hypoxia-ischemia model in the 7-day-old rat: possibilities and shortcomings. Acta Paediatr Suppl. 1997;Jul(422):85–88. doi: 10.1111/j.1651-2227.1997.tb18353.x. [DOI] [PubMed] [Google Scholar]
- Caceres MJ, Schleien CL, Kuluz JW, Gelman B, Dietrich WD. Early endothelial damage and leukocyte accumulation in piglet brains following cardiac arrest. Acta Neuropathol (Berl) 1995;90(6):582–591. doi: 10.1007/BF00318570. [DOI] [PubMed] [Google Scholar]
- Gelman B, Schleien CL, Lohe AS, Kuluz JW. Selective brain cooling in infant piglets after cardiac arrest and resuscitation. Crit Care Med. 1996;24(6):1009–2017. doi: 10.1097/00003246-199606000-00022. [DOI] [PubMed] [Google Scholar]
- Schleien CL, Kuluz JW, Gelman B. Hemodynamic effects of nitric oxide synthase inhibition before and after cardiac arrest in infant piglets. Am J Physiol. 1998;274(4 pt 2):H1378–H1385. doi: 10.1152/ajpheart.1998.274.4.H1378. [DOI] [PubMed] [Google Scholar]
- Kuluz JW, Prado R, He D, Zhao W, Dietrich WD, Watson B. New pediatric model of ischemic stroke in infant piglets by photothrombosis: acute changes in cerebral blood flow, microvasculature, and early histopathology. Stroke. 2007;38(6):1932–1937. doi: 10.1161/STROKEAHA.106.475244. [DOI] [PubMed] [Google Scholar]
- McGoron AJ, Maob X, Georgiou MF, Kuluz J. Computer phantom study of brain PET glucose metabolism imaging using a rotating SPECT/PET camera. Comput Biol Med. 2001;35(6):511–531. doi: 10.1016/j.compbiomed.2004.03.005. [DOI] [PubMed] [Google Scholar]
- McLaughlin GE, Kulatunga S, Kuluz JW, Gelman B, Schleien CL. Cerebral blood flow during partial liquid ventilation in surfactant-deficient lungs under varying ventilation strategies. Pediatr Crit Care Med. 2001;2(1):88–92. doi: 10.1097/00130478-200101000-00017. [DOI] [PubMed] [Google Scholar]
- Kazar J. Coxiella burnetii infection. Ann N Y Acad Sci. 2005;Dec(1063):105–114. doi: 10.1196/annals.1355.018. [DOI] [PubMed] [Google Scholar]
- Papakostas JC, Matsagas MI, Toumpoulis IK, et al. Evolution of spinal cord injury in a porcine model of prolonged aortic occlusion. J Surg Res. 2005;133(2):159–166. doi: 10.1016/j.jss.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Mellgren G, Friberg LG. Spinal cord injury after subclavian flap aortoplasty: an experimental study in piglets. Scand J Thorac Cardiovasc Surg. 1990;24(3):187–189. doi: 10.3109/14017439009098067. [DOI] [PubMed] [Google Scholar]
- Qayumi AK, Janusz MT, Lyster DM, Gillespie KD. Animal model for investigation of spinal cord injury caused by aortic cross-clamping. J Invest Surg. 1997;10(1–2):47–52. doi: 10.3109/08941939709032125. [DOI] [PubMed] [Google Scholar]
- Strauch JT, Lauten A, Spielvogel D, et al. Mild hypothermia protects the spinal cord from ischemic injury in a chronic porcine model. Eur J Cardiothorac Surg. 2004;25(5):708–715. doi: 10.1016/j.ejcts.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Rokkas CK, Cronin CS, Nitta T, et al. Profound systemic hypothermia inhibits the release of neurotransmitter amino acids in spinal cord ischemia. J Thorac Cardiovasc Surg. 1995;110(1):27–35. doi: 10.1016/S0022-5223(05)80006-6. [DOI] [PubMed] [Google Scholar]
- Owen JH, Laschinger J, Bridwell K, et al. Sensitivity and specificity of somatosensory and neurogenic-motor evoked potentials in animals and humans. Spine. 1998;13(10):1111–1118. doi: 10.1097/00007632-198810000-00010. [DOI] [PubMed] [Google Scholar]
- Halstead JC, Wurm M, Etz C, et al. Preservation of spinal cord function after extensive segmental artery sacrifice: regional variations in perfusion. Ann Thorac Surg. 2007;84(3):789–794. doi: 10.1016/j.athoracsur.2007.04.073. [DOI] [PubMed] [Google Scholar]
- Stokes BT, Jakeman LB. Experimental modeling of human spinal cord injury: a model that crosses the species barrier and mimics the spectrum of human cytopathology. Spinal Cord. 2002;40(3):101–109. doi: 10.1038/sj.sc.3101254. [DOI] [PubMed] [Google Scholar]
- Kwon BK, Mann C, Sohn HM, et al. Hypothermia for spinal cord injury. Spine J. 2008;8(6):859–874. doi: 10.1016/j.spinee.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Fleming JC, Norenberg MD, Ramsay DA, et al. The cellular inflammatory response in human spinal cords after injury. Brain. 2006;129(pt 12):3249–3269. doi: 10.1093/brain/awl296. [DOI] [PubMed] [Google Scholar]
- Carlson SL, Parrish ME, Springer JE, Doty K, Dossett L. Acute inflammatory response in spinal cord following impact injury. Exp Neurol. 1998;151(1):77–88. doi: 10.1006/exnr.1998.6785. [DOI] [PubMed] [Google Scholar]
- de Rivero Vaccari JP, Lotocki G, Marcillo AE, Dietrich WD, Keane RW. A molecular platform in neurons regulates inflammation after spinal cord injury. J Neurosci. 2008;28(13):3404–3414. doi: 10.1523/JNEUROSCI.0157-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane RW, Davis AR, Dietrich WD. Inflammatory and apoptotic signaling after spinal cord injury. J Neurotrauma. 2006;23(3–4):335–344. doi: 10.1089/neu.2006.23.335. [DOI] [PubMed] [Google Scholar]
- Ozawa H, Keane RW, Marcillo AE, Diaz PH, Dietrich WD. Therapeutic strategies targeting caspase inhibition following spinal cord injury in rats. Exp Neurol. 2002;177(1):306–313. doi: 10.1006/exnr.2002.7998. [DOI] [PubMed] [Google Scholar]
- Sribnick EA, Matzelle DD, Ray SK, Banik NL. Estrogen treatment of spinal cord injury attenuates calpain activation and apoptosis. J Neurosci Res. 2006;84(5):1064–1075. doi: 10.1002/jnr.21016. [DOI] [PubMed] [Google Scholar]
- Sanders RD, Xu J, Shu Y, Fidalgo A, Ma D, Maze M. General anesthetics induce apoptotic neurodegeneration in the neonatal rat spinal cord. Anesth Analg. 2008;106(6):1708–1711. doi: 10.1213/ane.0b013e3181733fdb. [DOI] [PubMed] [Google Scholar]