Abstract
We have shown that 20-hydroxyeicosatetraenoic acid (20-HETE) increases both superoxide and nitric oxide (NO) production in bovine pulmonary artery endothelial cells (BPAECs). The current study was designed to determine mechanisms underlying 20-HETE-stimulated NO release, and particularly the role of NADPH oxidase, reactive oxygen species, and PI3-kinase in stimulated NO release. Intracellular hydrogen peroxide (H2O2) and NO production were detected by dichlorofluorescein or dihydrorhodamine and diaminofluorescein fluorescence, respectively. Activation of endothelial nitric oxide synthase (eNOS) (Ser1179) and Akt (Ser473) was assessed by comparing the ratio of phosphorylated to total protein expression by Western blotting. Addition of 20-HETE to BPAECs caused an increase in superoxide and hydrogen peroxide, but not peroxynitrite. 20-HETE-evoked activation of Akt and eNOS, as well as enhanced NO release, are dependent on H2O2 as opposed to superoxide in that these endpoints are blocked by PEG-catalase and not PEG-superoxide dismutase. Similarly, 20-HETE-stimulated NO production in BPAECs is blocked by NADPH oxidase inhibitors apocynin or gp91 blocking peptide, and by PI3-kinase/Akt blockers wortmannin, LY-294002, or Akt inhibitor, implicating NADPH oxidase, PI3-kinase, and Akt signaling pathways, respectively, in this process. Together, these data suggest the following scheme: 20-HETE stimulates NADPH oxidase-dependent formation of superoxide. Superoxide is rapidly dismutated to hydrogen peroxide, which then mediates activation of PI3-kinase/Akt, phosphorylation of eNOS, and enhanced release of NO from eNOS in response to 20-HETE in BPAECs.
Keywords: pulmonary endothelium, superoxide, hydrogen peroxide, endothelial nitric oxide synthase
we have previously demonstrated that 20-HETE, a product of arachidonic acid catalyzed by CYP4, induces activation of nitric oxide synthase (NOS) from pulmonary artery endothelial cells in a [Ca2+]i-dependent manner (9, 23, 36). Furthermore, 20-HETE releases NO, which contributes to endothelium-dependent vasodilation in pulmonary arteries (9, 19). Growing evidence from our own work as well as that from others indicates that 20-HETE activates the vascular NADPH oxidase, leading to increased production of superoxide anion (O2•−) and hydrogen peroxide (2, 23, 32). Superoxide serves as a source of other reactive oxygen species including hydrogen peroxide, which may contribute to vascular disease, and/or, in some cases, may have specific signaling properties (15). In particular, it is known that H2O2 potently induces endothelial nitric oxide synthase (eNOS) activation via a Ca2+/calmodulin-dependent protein kinase II/Janus-kinase 2-dependent pathway in bovine aortic endothelial cells (6, 12). Furthermore, it has been shown that exogenous H2O2 in micromolar concentrations activates eNOS from porcine aortic endothelial cells to cause endothelial NO release (7, 28). Superoxide may also react with NO in a diffusion-limited fashion to form peroxynitrite. This species is believed to result in the loss of many of the beneficial effects of NO, including vasodilation.
The present studies were undertaken to explore the relationship between 20-HETE-stimulated superoxide, hydrogen peroxide, or ONOO− production and eNOS activation or NO release in bovine pulmonary artery endothelial cells (BPAECs). Because 20-HETE activates NADPH oxidase and PI3-kinase (PI3K), we designed experiments to examine the role of these two signaling pathways in 20-HETE-induced NO production. Specifically, we tested the following hypothesis: stimulated release of NO from BPAECs in response to 20-HETE is mediated by 1) activation of NADPH oxidase, 2) generation of superoxide, which is rapidly dismutated to hydrogen peroxide, 3) hydrogen peroxide-mediated activation of PI3K/Akt, and 4) activated Akt-dependent phosphorylation of eNOS with stimulated NO release. We found that 20-HETE significantly stimulates both superoxide and hydrogen peroxide but does not generate peroxynitrite. We further show that 20-HETE-induced hydrogen peroxide, and not superoxide, plays an essential role in subsequent NO production at least in part by modulating the signaling pathways of PI3K/Akt.
MATERIALS AND METHODS
Materials.
Wortmannin (cat. no. 681675), LY-294002 (cat. no. 440202), and Akt inhibitor (cat. no. 124017) were obtained from EMD Chemicals, Gibbstown, NJ. Apocynin (cat. no. 178385) was obtained from Calbiochem, Gibbstown, NJ. Polyethylene-glycolated superoxide dismutase (PEG-SOD; S-9549; 685 U/mg solid, 1 unit inhibits rate of reduction of cytochrome c by 50% in a coupled system with xanthine and xanthine oxidase at pH 7.8 at 25°C in a 3-ml reaction volume), PEG-CAT (C-4963; 17,600 U/mg solid, 1 unit decomposes 1 μmol H2O2/min at pH 7.0 at 25°C, whereas the H2O2 concentration falls from 10.3 to 9.2 mM), H2O2 (cat. no. 216763), DMSO (cat. no. D2650), ethanol (cat. no. 270741), l-arginine HCl (cat. no. 01-6440), and BSA (cat. no. A3156) were procured from Sigma-Aldrich, St. Louis, MO. H2DCF-DA (2′,7′-dichlorodihydrofluorescein diacetate, D-399), dihydrorhodamine 123 (DHR123; D-632), 4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate (DAF-FM DA; D-23844), and 3-morpholinosydnonimine hydrochloride (SIN-1; M-7891) were obtained from Invitrogen, Carlsbad, CA. Peroxynitrite (cat. no. 20-107) and degraded peroxynitrite (cat. no. 20-247) were purchased from Upstate Biotechnology. Diethylenetriamine NONOate (DETANONOate; cat. no. 82120) was from Cayman, Ann Arbor, MI. RPMI medium (cat. no. 11875-093), FBS (cat. no. 16000-044), penicillin-streptomycin (cat. no. 15140-122), and PBS (cat. no. 14190-144) were obtained from Gibco, Grand Island, NY. NOS inhibitor NG-nitro-l-arginine methyl ester HCl (l-NAME HCl; ALX-105-003) was from Alexis Biochemicals, San Diego, CA. A protease inhibitor cocktail was obtained from Roche, Mannheim, Germany (cat. no. 1836 170). Protein determination kit was obtained from Bio-Rad, Hercules, CA (cat. no. 500-0006). 20-HETE and 20-hydroxy-eicosa-5(Z),14(Z)-dienoic acid, termed 20–5,14-HEDE in this work, were synthesized in the laboratory of J. R. Falck (1). Phospho-eNOS (Ser1177, cat. no. 9571S), eNOS (cat. no. 9572), phospho-Akt (Ser473, cat. no. 9271), and Akt (cat. no. 9272) antibodies were obtained from Cell Signaling, Beverly, MA. Goat anti-rabbit IgG (H+L) conjugate (cat. no. 172-1019) was from Bio-Rad, Hercules, CA. Mouse monoclonal anti-nitrotyrosine antibody, clone 1A6 (cat. no. 05-233) was from Upstate Biotech, Lake Placid, NY. ECL Plus detection reagent was from Amersham Biosciences (cat. no. RPN 2133). The blots were scanned using an AlphaImage 220 Analysis System (Alpha Innotech, San Leandro, CA). Precast ready gels were from Bio-Rad (cat. no. 161-1101).
A chimeric peptide that inhibits association of p47phox with gp91 in NADPH oxidase was synthesized by The Protein and Nucleic Acid Core at the Medical College of Wisconsin as described before (23) to test the contribution of NADPH oxidase to ROS production. The sequence of this peptide is [H]-R-K-K-R-R-Q-R-R-R-C-S-T-R-I-R-R-Q-L-NH2. The sequence of the scrambled peptide is R-R-Q-R-R-R-C-L-R-I-T-R-Q-S-R-NH2. Treatments included 1 μM 20-HETE, 1 μM 20–5,14-HEDE, 1 μM apocynin, 100 U/ml−1 PEG-SOD, 500 U/ml −1 PEG-CAT, 50 μM gp91phox ds tat or scr gp91phox ds tat (peptide-based inhibitor of NADPH oxidase), and 0.1 mM l-NAME.
Cell culture.
BPAECs were isolated from small pulmonary arteries (<5-mm diameter) (37) and cultured in RPMI medium containing 10% FBS and 1% penicillin-streptomycin. Tissues from which these cells were isolated were obtained from a local abattoir with all protocols reviewed and approved by the Medical College of Wisconsin. BPAECs were grown to 80% confluence and made quiescent by removal of serum for 6 h before stimulation with 20-HETE for imaging and Western analysis. BSA (0.1%) was added as a lipid carrier. All cells were used between passages 2 and 5. Control and treated cells were matched in each experiment for isolation and passage number, and time to monolayer confluence.
Detection of ROS by fluorescence microscopy.
BPAECs were cultured and imaged in 35-mm dishes to ∼80% confluence. Inhibitors of ROS were applied 30 min before loading with the fluorescent dye H2DCF-DA at a final concentration of 5 μM for monitoring intracellular H2O2 formation (23). H2DCF-DA is deacetylated by intracellular esterases forming H2DCF, which, in the presence of intracellular hydrogen peroxide, is oxidized in the cytoplasm to a highly fluorescent compound, 2′,7′-dichlorofluorescein (DCF) (λex = 485 nm; λem = 530 nm). Three to four images were acquired for each dish with a Nikon Eclipse TE200 microscope equipped with a TE-FM epifluorescence attachment (Lambda DG-4 from Sutter Instrument) and captured using a Hamamatsu digital camera C4742-95. Approximately 20 cells were randomly selected in each field, and average fluorescent intensity within operator-defined cell borders was recorded using Metamorph version 6.2 as previously described (23). Alternatively, DHR123 oxidation was used to detect intracellular hydrogen peroxide as described before (35). In brief, cell monolayers were incubated with 10 μM DHR123 for 30 min at 37°C, washed with PBS, and resuspended in 0.5 ml of PBS. Rhodamine 123 fluorescence intensity resulting from DHR123 oxidation was visualized under the fluorescent microscope (λex = 488 nm; λem = 530 nm).
DCF and DHR123 fluorescence is elicited not only by hydrogen peroxide. These dyes are known to be oxidized by peroxynitrite as well (4). Therefore, we tested 20-HETE-induced fluorescence using these indicators along with membrane-permeant superoxide dismutase and catalase to determine the contribution of hydrogen peroxide and peroxynitrite, respectively, to the signals.
Determination of intracellular NO by fluorescence microscopy.
Intracellular NO was monitored with 4-amino-5-methylamino-2′,7′-difluorofluorescein (DAF-FM) diacetate that emits increased fluorescence following reaction with an active intermediate of NO formed during the spontaneous oxidation of NO to NO2− (20). BPAECs were incubated at 37°C for 30 min with vehicle or inhibitors (PEG-SOD, PEG-catalase, apocynin, etc.) in serum-free RPMI followed by the addition of a low concentration (1 μM) of DAF-FM diacetate. This low concentration of DAF-FM significantly reduced the background autofluorescence and improved the signal-to-noise ratio of NO detection (22). After loading, cells were rinsed three times with RPMI and then placed on the stage of a Nikon Eclipse TE200 microscope equipped with a TE-FM epifluorescence attachment (Lambda DG-4 from Sutter Instrument) and captured using a Hamamatsu digital camera C4742-95. NO fluorescence was measured using λex = 488 nm, λem = 520 nm. Because NOS generates superoxide instead of NO in the absence of l-arginine (34), excess of l-arginine (3 mM) was added to all solutions used for NO measurements except for the treatments with l-NAME. As with detection of ROS using fluorescent probes, average fluorescent intensity within operator-defined cell borders was recorded using Metamorph version 6.2 (23).
Determination of 3-nitrotyrosine formation in BPAECs.
At ∼90% confluence, BPAECs were washed with PBS. The cells were then exposed to ethanol (control), 20-HETE (1 μM), or SIN-1 (10 μM) in serum-free RPMI with incubation at 37°C for 30 min. Detection of 3-nitrotyrosine-modified proteins in cell extracts was accomplished using a specific mouse monoclonal anti-3-nitrotyrosine antibody and slot blot immunoassay. Briefly, 3 μg of protein was applied to a Bio-Dot apparatus (Bio-Rad) to nitrocellulose membrane. The membrane was incubated with anti-nitrotyrosine antibody overnight at 4°C, followed by goat anti-mouse IgG-horseradish peroxidase conjugate and visualized with enhanced chemiluminescence. In separate experiments, the protein extract (50 μg/lane) was subjected to SDS-PAGE followed by Western blotting to visualize the extent of nitration of proteins in BPAECs treated with ethanol, 20-HETE, and SIN-1.
Western analysis.
After stimulation with vehicle or test agents, cells were chilled on ice and washed three times with cold PBS. They were resuspended by scraping in the presence of 0.5 ml of RIPA buffer (cat. no. 20-188; Upstate, Temecula, CA) supplemented with protease inhibitor cocktail. The mixture was kept on ice for 15 min, after which lysates were centrifuged for 10 min at 20,000 g and the supernatants were used for determining protein concentration via the Bio-Rad protein assay kit. Equal amounts of protein (50 μg/lane) were boiled for 5 min in Laemmli sample buffer (161-0737, Bio-Rad) supplemented with 2-mercaptoethanol, resolved on a 10% Tris-HCl SDS polyacrylamide gel (Bio-Rad), and transferred to nitrocellulose membranes as described previously (18, 19). The blots were developed with appropriate primary and matched secondary antibodies conjugated to horseradish peroxidase and visualized using ECL Plus detection reagent. Blots were first probed with a phosphospecific antibody (phospho-eNOS or phospho-Akt), stripped, and reprobed with the corresponding antibody (eNOS or Akt, respectively). They were scanned with an Alpha Image 220 Analysis System, and the relative densities were determined.
Statistical analysis.
20-HETE-stimulated superoxide, H2O2, or NO production, in the presence of vehicle or pharmacological inhibitors, was measured in a minimum of 60 cells/group from three independent culture preparations unless indicated otherwise for each condition. The analysis was performed by an individual blinded to the treatment groups. Fluorescence intensity of test cells imaged in the same time frame and manner as test groups was normalized to the mean intensity for vehicle-treated control groups so that experiments from different days and groups of cells might be compared. For Western blots, a minimum of four to five experiments using cells from three independent culture preparations were performed. The ratio of phosphorylated to total protein in the control group was set as 100%, and other groups were normalized to control. Comparisons among groups for all experiments were performed using one-way analysis of variance. When differences were indicated, a Holm-Sidak post hoc test was employed. Statistical significance was assumed for P < 0.05. All grouped data shown in the figures are presented as means ± SD.
RESULTS
20-HETE stimulates intracellular hydrogen peroxide formation and not peroxynitrite in pulmonary artery endothelial cells.
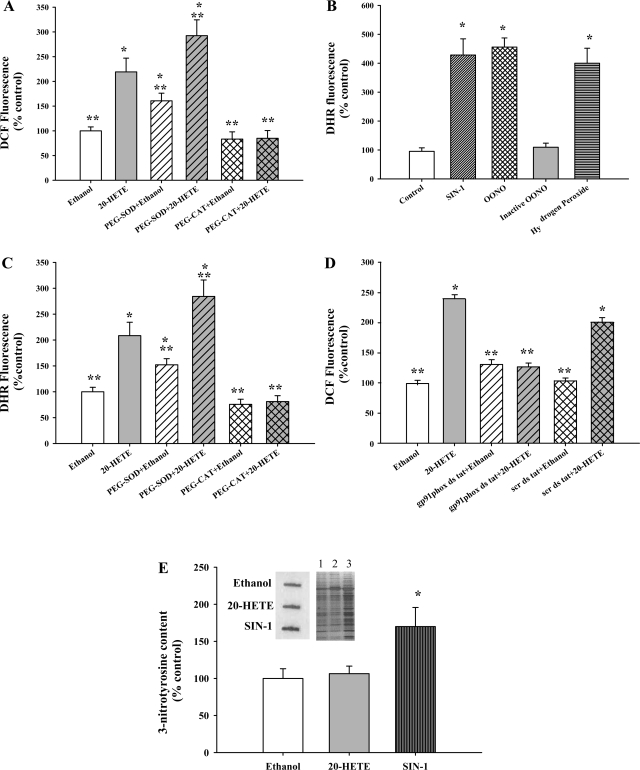
Our previous studies have shown that 20-HETE stimulates superoxide, hydrogen peroxide, and nitric oxide production in BPAECs (9, 23). To further characterize the nature of ROS generated on 20-HETE stimulation, we used DCF fluorescence as an indicator of ROS, in conjunction with PEG-SOD and PEG-CAT. Figure 1A shows changes in intracellular DCF fluorescence as a measure of ROS production 10 min after exposure of BPAECs to 20-HETE (1 μM). There was a significant increase in DCF fluorescence upon 20-HETE stimulation compared with vehicle (ethanol) treatment. Pretreatment with PEG-SOD further increased the DCF fluorescence with 20-HETE. In contrast, PEG-CAT lowered the DCF fluorescence to basal levels, indicating that H2O2 is the major ROS detected with DCF.
Fig. 1.
20-HETE increases dichlorofluorescein (DCF) and dihydrorhodamine (DHR) fluorescence in bovine pulmonary artery endothelial cells (BPAECs). A: 20-HETE (gray bar) increased the DCF fluorescence compared with vehicle (open bar). PEG-SOD pretreatment (gray hatched bar) enhanced 20-HETE-induced DCF fluorescence signal, indicating facilitated formation of hydrogen peroxide. PEG-CAT treatment (gray cross-hatched bar) effectively lowered 20-HETE-induced DCF fluorescence. Signal remaining after treatment with PEG-CAT represents background, whereas the difference in DCF fluorescence in cells treated with vehicle and 20-HETE reflects hydrogen peroxide. B: an increase in DHR signal was observed with either authentic ONOO− (100 μM) or ONOO− derived from SIN-1 (10 μM), but not with pH-inactivated ONOO−. Hydrogen peroxide (100 μM) also increased the DHR fluorescence. *P < 0.05 relative to control (no treatment). C: BPAECs incubated with DHR123 were imaged for quantitation of fluorescent signal 10 min after treatment with vehicle (open bars) or 20-HETE (gray bars), pretreatment with PEG-SOD (gray hatched bars) or PEG-CAT (gray cross-hatched bars). The same pattern of increased fluorescence signal after PEG-SOD and decreased signal after PEG-CAT treatment as was observed with DCF was detected with DHR123. These data support a major contribution of hydrogen peroxide to 20-HETE-evoked DHR fluorescence signal in BPAECs. D: 20-HETE (gray bar) increased the DCF fluorescence compared with vehicle (open bar). gp91phox ds tat peptide pretreatment (gray hatched bar) effectively lowered 20-HETE-induced DCF fluorescence signal, but not the scr ds tat peptide (gray cross-hatched bar) indicating that NADPH oxidase inhibition lowers 20-HETE-induced production of H2O2. A–D: data are composite of at least 60 cells for each experimental condition and 3 independent culture preparations. *P < 0.05 relative to vehicle control (ethanol); **P < 0.05 relative to 20-HETE. E: BPAECs were treated with ethanol, 20-HETE (1 μM), or SIN-1 (10 μM) for 60 min, and whole cell extracts (3 μg of protein) were used for analysis of tyrosine nitration by slot blot analysis. A representative slot blot image and Western blot are shown in inset. Lanes 1–3 are ethanol, 20-HETE, and SIN-1, respectively. Densitometric quantitation of relative abundance of 3-nitrotyrosine from the slot blots is shown (n = 4 for each group). SIN-1 treatment (dark gray vertical bar) induced an increase in the 3-nitrotyrosine compared with both the vehicle (open bar) and 20-HETE (gray bar). *P < 0.05 relative to both vehicle and 20-HETE.
We also probed cells with DHR123 as an alternate indicator of ROS levels. During the intracellular release of ONOO− or H2O2, reduced DHR is irreversibly oxidized and converted to the red fluorescent compound rhodamine 123 (25). We first validated changes in dihydrorhodamine 123 (DHR) signal in response to H2O2 and ONOO− treatments in our endothelial cells. An increase in oxidation of DHR in BPAECs was observed in cells treated with either authentic ONOO− (100 μM) or ONOO− derived from SIN-1 (10 μM), but not with pH-inactivated ONOO−. Hydrogen peroxide (100 μM) also increased the DHR fluorescence (Fig. 1B). Next we measured the DHR fluorescence stimulated by 20-HETE in the presence of vehicle, PEG-SOD, or PEG-CAT to distinguish H2O2 from peroxynitrite. Similar to DCF, PEG-SOD heightened the DHR123 fluorescence in response to 20-HETE, and PEG-CAT attenuated the fluorescence (Fig. 1C). Finally, we tested the effect of gp91phox peptide inhibitor on enhanced DCF fluorescence evoked by 20-HETE (Fig. 1D). The NADPH oxidase blocking peptide, but not the scrambled version, blocked 20-HETE-evoked increased DCF fluorescence, consistent with a role of NADPH oxidase in enhanced ROS production.
Tyrosine nitration of proteins is believed to represent a molecular footprint of peroxynitrite formation. To further explore the nature of ROS produced in BPAECs by 20-HETE, we evaluated the effect of 20-HETE on the formation of 3-nitrotyrosine. Slot blot analysis using an antibody specific for 3-nitrotyrosine revealed no significant differences in intensity between vehicle and 20-HETE-treated endothelial cell protein extracts (Fig. 1E). However, SIN-1 application resulted in an appreciable increase in intensity. These results provide further support for the conclusion that 20-HETE stimulation of BPAECs results in the formation of hydrogen peroxide without increase in the formation of peroxynitrite.
20-HETE-induced Akt activation is dependent on H2O2 formation and NADPH oxidase.
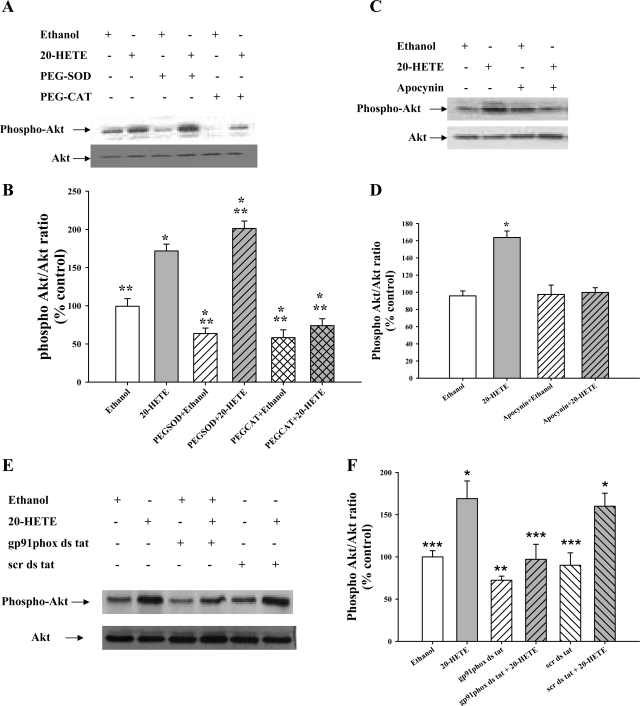
We have previously shown that 20-HETE increases phosphorylation of Akt at Ser473 (9). To determine the role of superoxide and hydrogen peroxide on Akt activation in response to 20-HETE in BPAECs, phosphorylation of Akt at Ser473 was studied in the presence of PEG-SOD and PEG-CAT during 20-HETE stimulation. As observed in Fig. 2, A and B, 20-HETE increased Akt phosphorylation compared with control. This increase was further enhanced by PEG-SOD and attenuated by PEG-CAT, indicating a role for H2O2 in 20-HETE-evoked activation of Akt.
Fig. 2.
Activation of Akt in response to 20-HETE is mediated by H2O2 and NADPH oxidase. A: BPAECs were treated with PEG-SOD (100 U/ml) or PEG-CAT (500 U/ml) for 30 min before 20-HETE stimulation (1 μM, 30 min). Cells were then lysed, and the lysates were analyzed for levels of phospho-Akt (Ser473) and total Akt (n = 5 for each group). A representative Western blot of cell lysates probed with phospho-Akt-Ser473 is shown. B: phospho-Akt and Akt band densities were analyzed by densitometry, and the means ± SE from 5 independent experiments from 2 different isolates of BPAECs were used to calculate the ratio of phospho-Akt/Akt. 20-HETE (gray bar) increases phospho-Akt (Ser473) compared with vehicle control, and this increase is further enhanced by pretreatment with PEG-SOD (gray hatched bar). Removal of H2O2 using PEG-CAT (gray cross-hatched bar) lowers the phospho-Akt levels without altering total Akt levels. *P < 0.05 relative to vehicle control (ethanol); **P < 0.05 relative to 20-HETE. C: BPAECs were incubated for 30 min in RPMI serum-free medium containing an inhibitor of NADPH oxidase, apocynin (1 μM), for 30 min before 20-HETE stimulation (1 μM, 30 min). A representative Western blot of cell lysates probed with phospho-Akt-Ser473 is shown (top) (n = 5 for each group). D: 20-HETE (gray bar) increases phospho-Akt (Ser473) compared with vehicle control, and this increase is blunted by pretreatment with apocynin (gray hatched bar), likely due to a block in NADPH-derived H2O2. *P < 0.05 relative to vehicle control (ethanol; n = 5). E: BPAECS were incubated in serum-free medium with the gp91 peptide inhibitor or the scrambled peptide. A representative Western blot is shown. Like apocynin, the gp91 peptide inhibited the increase in phospho-Akt associated with 20-HETE, whereas the scrambled peptide did not. F: 20-HETE (gray bar) increases phospho-Akt compared with control, and this increase is blocked by pretreatment with the gp91phox inhibitory peptide. **P < 0.05 relative to vehicle control (ethanol n = 4).
It is known that 20-HETE activates NADPH oxidase and produces superoxide/hydrogen peroxide in BPAECs (23). Therefore, we explored the potential role of NADPH oxidase in Akt activation upon 20-HETE stimulation. Figure 2, C and D, reveals that apocynin, an inhibitor of NADPH oxidase, abrogated the increase in phospho-Akt seen with 20-HETE. Since apocynin is reported to have antioxidant properties in some endothelial cells distinct from effects on NADPH oxidase (16, 29), we also tested the peptide-based inhibitor in this model. Like apocynin, the peptide-based inhibitor, but not the scrambled peptide, blocked 20-HETE-associated increments in Akt phosphorylation (Fig. 2, E and F). Together, these data suggest that hydrogen peroxide derived from NADPH oxidase is an excellent candidate for 20-HETE-stimulated phosphorylation of Akt.
20-HETE-induced phosphorylation of eNOS is dependent on H2O2.
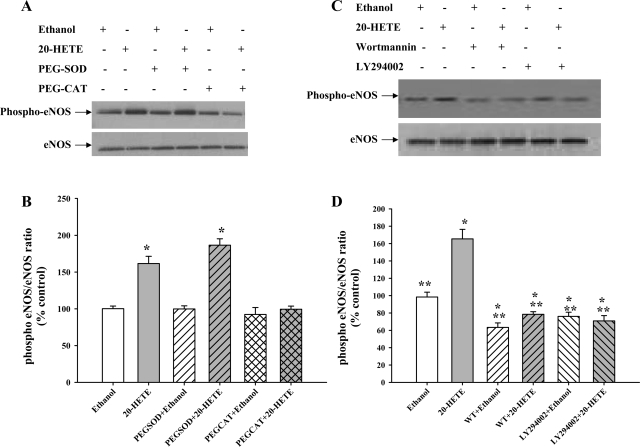
Since it is known that phosphorylation of Akt often results in phosphorylation of eNOS at Ser1179, we investigated eNOS activation following 20-HETE treatment. 20-HETE significantly increased the phosphorylation of eNOS at Ser1179 within 30 min (Fig. 3, A and B). This increase was not blocked by PEG-SOD. In contrast, PEG-CAT decreased 20-HETE-stimulated eNOS phosphorylation. These data indicate that H2O2 and not superoxide control 20-HETE-stimulated eNOS activation.
Fig. 3.
20-HETE-induced activation of eNOS is mediated by H2O2 production and controlled by PI3 kinase (PI3K). A: BPAECs were treated with vehicle, PEG-SOD (100 U/ml), or PEG-CAT (500 U/ml) for 30 min before 20-HETE stimulation (1 μM, 30 min). A representative Western blot of cell lysates probed with phospho-eNOS-Ser1179 is shown (top) (n = 5 for each group). B: 20-HETE (gray bar) increases phospho-eNOS (Ser1179) compared with vehicle control, and this increase is not blocked by pretreatment with PEG-SOD (gray hatched bar; n = 5 each). Facilitated elimination of H2O2 using PEG-CAT (gray cross-hatched bar) reduced the phospho-eNOS levels without altering total eNOS levels. *P < 0.005 relative to vehicle control (ethanol). C: BPAECs were incubated for 30 min with vehicle or inhibitors of PI3K, wortmannin (WT; 200 nM), or LY-294002 (20 μM) before vehicle or 20-HETE (1 μM, 30 min) stimulation. A representative Western blot of cell lysates probed with phospho-eNOS-Ser1179 is shown (top) (n = 5). D: phospho-eNOS and eNOS band densities were analyzed by densitometry, and the means ± SE from 5 independent experiments from 2 different isolates of BPAECs were used to calculate the ratio of phospho-eNOS/eNOS. 20-HETE (gray bar) increases phospho-eNOS (Ser1179) compared with vehicle control, and this increase is abolished by pretreatment with either inhibitor of PI3K, wortmannin or LY-294002 (hatched bars). *P < 0.05 relative to vehicle control (ethanol); **P < 0.05 relative to 20-HETE.
Phospho-eNOS activation is dependent on PI3K/Akt.
Next we studied the role of PI3K/Akt in activation of eNOS in response to 20-HETE. Treatment of BPAECs with inhibitors of PI3K wortmannin or LY-294002 significantly decreased the 20-HETE-induced activation of eNOS (Fig. 3, C and D). These data suggest that PI3K/Akt acts upstream of eNOS activation evoked by 20-HETE.
20-HETE increases phosphorylation of eNOS via NADPH oxidase.
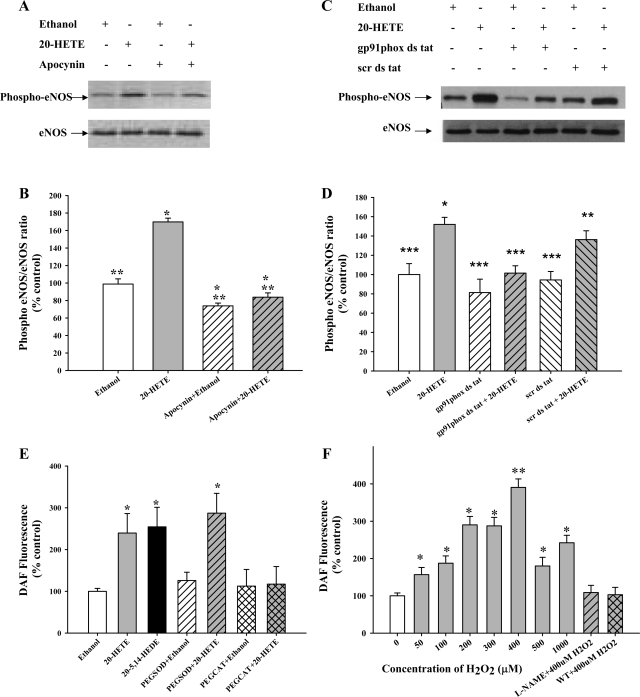
Because Akt activation was dependent on a prior increase in H2O2 and activation of NADPH oxidase, we tested whether eNOS activation also followed a similar trend. 20-HETE-stimulated eNOS activation (phospho-Ser1179) was blocked by pretreatment with apocynin, an inhibitor of NADPH oxidase (Fig. 4, A and B) or the gp91 peptide inhibitor (Fig. 4, C and D).
Fig. 4.
20-HETE-induced eNOS (Ser1179) phosphorylation depends on H2O2. NO generation is mediated by H2O2 and PI3K. A: BPAECs were incubated for 30 min with an inhibitor of NADPH oxidase, apocynin (1 μM), before 20-HETE stimulation (1 μM, 30 min). A representative Western blot of cell lysates probed with phospho-eNOS-Ser1179 is shown (top). The blots were stripped and reprobed with eNOS as shown (bottom) (n = 5). B: phospho-eNOS and eNOS bands were analyzed by densitometry and used to calculate the ratio of phospho-eNOS/eNOS. 20-HETE (gray bar) increases phospho-eNOS (Ser1179) compared with vehicle control, and this increase is attenuated by pretreatment with apocynin (hatched bars). *P < 0.05 relative to vehicle control (ethanol); **P < 0.05 relative to20-HETE. C: BPAECs were incubated with the gp91phox inhibitor or the scrambled peptide and treated with 20-HETE or vehicle. A representative Western blot shows that 20-HETE increased phospho-eNOS/eNOS ratio in cells treated with the scrambled peptide, but not the gp91phox peptide. D: 20-HETE (gray bar) increases phospho-eNOS (Ser1179) compared with vehicle control, and this increase is attenuated by pretreatment with gp91phox peptide (hatched bars), but not the scrambled peptide. *P < 0.05 relative to vehicle control (ethanol); ***P < 0.05 relative to scrambled peptide control. E: 20-HETE (gray bar) increased DAF fluorescence (NO levels) compared with vehicle (open bar). Similar to 20-HETE, a stable analog, 20–5,14-HEDE (1 μM, black bar), increased the DAF fluorescence significantly compared with vehicle. The increase in DAF fluorescence was unaffected by PEG-SOD (gray hatched bar; 100 U/ml) and attenuated by PEG-CAT (gray cross-hatched bar; 500 U/ml). *P < 0.05 relative to vehicle control. A total of >50 cells in 3 randomly chosen fields during each experiment was processed for obtaining average fluorescence intensity. F: BPAECs were treated with vehicle or 50–1,000 μM H2O2 for 20 min and then assessed for DAF fluorescence. Starting at 50 μM, H2O2 had a significant effect on DAF fluorescence. Increasing concentrations of H2O2 (gray bars) elicited a bell-shaped response curve, with a peak observed at 400 μM H2O2. Pretreatment with the NOS inhibitor l-NAME (1 mM, gray hatched bar) or PI3K inhibitor wortmannin (200 nM, gray cross-hatched bar) attenuated the DAF fluorescence seen with 400 μM H2O2, suggesting these changes in DAF fluorescence are mediated by activation of PI3K and NOS. Data represent composite results from at least 60 cells for each experimental condition and 3 independent culture preparations. *P < 0.05 relative to no treatment (control, 0 μM H2O2); **P < 0.05 relative to different concentrations of H2O2 used in the study.
20-HETE-induced increase in NO is dependent on hydrogen peroxide.
To investigate the potential role of intracellular H2O2 in 20-HETE-stimulated NO production, BPAECs were pretreated with PEG-SOD (100 U/ml) or PEG-CAT (500 U/ml) for 30 min before loading with DAF and then exposing to vehicle or 20-HETE. NO release was monitored by the DAF fluorescence after 10 min. As shown in Fig. 4E, DAF fluorescence intensity was consistently increased by 20-HETE or its stable analog, 20–5,14-HEDE, and this response was prevented by PEG-CAT. PEG-SOD had no effect on 20-HETE-stimulated DAF fluorescence. Together, these data suggest that intracellular H2O2 rather than superoxide mediates 20-HETE-stimulated NO stimulation.
H2O2 has bidirectional effect on NO production and is sensitive to PI3K inhibitors.
To mimic the increase in intracellular hydrogen peroxide upon stimulation with 20-HETE in BPAECs, we added single boluses of H2O2 ranging from 0 to 1,000 μM to the external media, and then tracked NO production using DAF fluorescence. Hydrogen peroxide effected a dose-dependent increase in DAF fluorescence in BPAECs treated with 100–400 μM H2O2 for 30 min. However, with higher concentrations of H2O2, there was a decrease in DAF fluorescence (Fig. 4F). Thus, H2O2 shows concentration-dependent effects on NO generation in BPAECs in a manner that peaked at 400 μM final concentration in the external media. As anticipated, treatment with l-NAME decreased DAF fluorescence elicited by 400 μM H2O2 to levels consistent with background. Interestingly, wortmannin to inhibit PI3K also prevented the increase in DAF fluorescence with 400 μM H2O2, suggesting that activation of PI3K/Akt is also required for H2O2-associated NO production.
PI3K/Akt is required for NO production in response to 20-HETE.
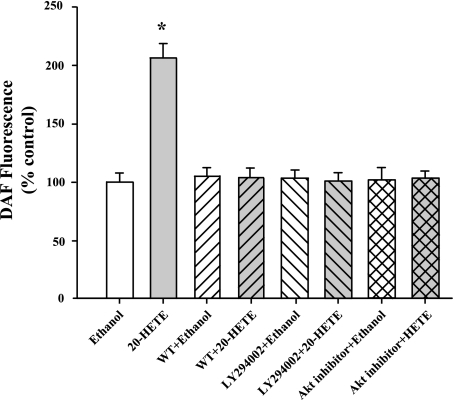
To examine if 20-HETE-induced NO production is mediated by PI3K and Akt, BPAECs were pretreated with wortmannin (200 nM), LY-294002 (20 μM), or Akt inhibitor (10 μM) for 30 min and then loaded with DAF. This treatment was followed by stimulation with vehicle (ethanol) or 20-HETE for another 30 min before assessing changes in intracellular NO formation using DAF fluorescence. 20-HETE failed to increase DAF fluorescence in the presence of these inhibitors, suggesting that PI3K/Akt axis is required for eNOS activation and NO production (Fig. 5).
Fig. 5.
20-HETE-induced NO formation is dependent on PI3K/Akt. 20-HETE resulted in an increase in DAF fluorescence, which was prevented by wortmannin (200 nM), LY-294002 (10 μM), or Akt inhibitor (10 μM), further suggesting that 20-HETE-induced NO formation is modulated by PI3K/Akt. N ≥ 50 cells in each group. *P < 0.05 relative to ethanol.
NADPH oxidase is a candidate source for H2O2 mediating 20-HETE-stimulated NO production.
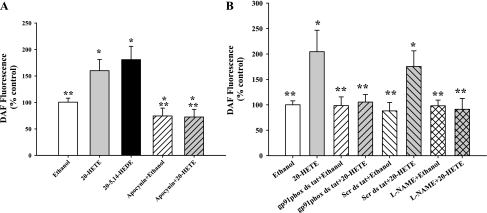
To identify a potential subcellular source of H2O2 that mediates 20-HETE-stimulated NO production, BPAECs were pretreated with apocynin to inhibit NADPH oxidase. BPAECs failed to produce NO in response to 20-HETE when NADPH oxidase was inhibited. We used a second inhibitor of NADPH oxidase, gp91phox ds tat peptide, to block the association of gp91phox subunit with p47phox. Both these inhibitors prevented the increase in NO in response to 20-HETE (Fig. 6, A and B).
Fig. 6.
20-HETE-induced NO formation is mediated by NADPH oxidase. A: 20-HETE and its structural analog, 20–5,14-HEDE, enhanced DAF fluorescence compared with ethanol in a manner that was blunted by apocynin, an inhibitor of NADPH oxidase. N ≥ 50 cells for each experiment. *P < 0.05 relative to ethanol; **P < 0.05 relative to 20-HETE. B: pretreatment with 50 μM gp91phox ds tat peptide for 30 min (hatched bars) effectively blocked both vehicle and 20-HETE-induced DAF fluorescence. In contrast, scrambled peptide inhibitor (hatched bars) did not block the 20-HETE-induced DAF signal relative to scrambled peptide control. N ≥ 60 cells from each group. *P < 0.05 relative to ethanol; **P < 0.05 relative to 20-HETE.
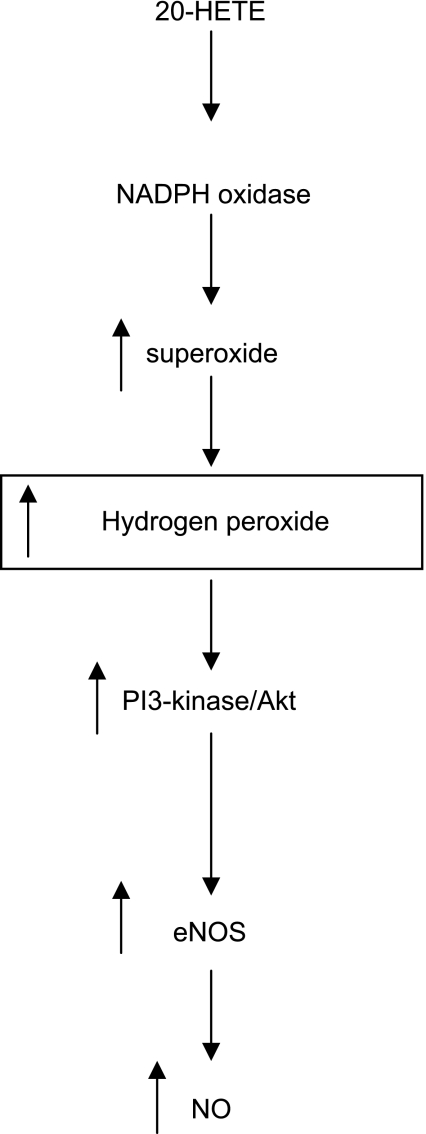
Figure 7 shows a schematic representation of 20-HETE-evoked activation of NO release from BPAECs, which accounts for required activation of NADPH oxidase, H2O2 generation, and activation of PI3K, leading to eNOS activation and stimulated NO release.
Fig. 7.
Hypothesis schematic. A unifying hypothesis depicting the cascade of signaling events in response to 20-HETE that result in the increased production of NO in BPAECs. We speculate that 20-HETE activates NADPH oxidase, which produces superoxide that is rapidly dismutated to H2O2. H2O2 activates PI3K and Akt, which in turn phosphorylates eNOS. Phosphorylation of eNOS at Ser1179 promotes enhanced NO production.
DISCUSSION
20-HETE, the primary arachidonic acid product of CYP4 isoforms, has a number of potent biological effects in a host of cell types (27). Our study adds to the literature regarding 20-HETE signaling in BPAECs with the following new observations: 1) 20-HETE-induced activation of Akt depends on functional NADPH oxidase and H2O2 as opposed to superoxide, 2) 20-HETE-associated phosphorylation of eNOS at Ser1179 and of Akt at Ser473 is driven by H2O2 as opposed to superoxide, 3) 20-HETE-stimulated NO release from BPAECs depends on PI3K/Akt activation, functional NADPH oxidase, and H2O2 as opposed to superoxide, and 4) 20-HETE promotes the formation of H2O2 as well as superoxide, but not ONOO−, in BPAECs. Our previous reports demonstrated 20-HETE induced increases in superoxide production, activation of NADPH oxidase, and enhanced NO release from BPAECs (9, 23). We have also shown that 20-HETE protects against starvation-induced apoptosis in BPAECs and ischemia reoxygenation injury in ex vivo pulmonary arteries in a manner that depends on NADPH oxidase and PI3K and Akt activation (10). Together then, these data suggest that 1) 20-HETE-induced activation of NADPH oxidase promotes the formation of superoxide, which is rapidly dismutated to H2O2, 2) activation of PI3K/Akt by 20-HETE and phosphorylation of eNOS requires H2O2, and finally, 3) stimulated NO release in response to 20-HETE or its structural and stable analog, 20–5,14-HEDE, and/or enhanced survival are the end result of this signaling pathway. This hypothesis schematic is depicted in Fig. 7.
20-HETE-evoked activation of NADPH oxidase has precedent. Regulation of NADPH oxidase in renal arteries by CYP4 was first reported by Wang et al. (32). Overexpression of CYP4A in Sprague-Dawley rats increased synthesis of 20-HETE in renal interlobar arteries, increased generation of superoxide, and increased expression of gp91. In BPAECs, 20-HETE stimulates production of superoxide in a manner that can be blocked by inhibitors of NADPH oxidase, and is accompanied by p47phox translocation and Rac1 activation (23). Activation of NADPH oxidase is required for 20-HETE-associated prosurvival effects in BPAECs (10). The present studies provide new information regarding 20-HETE-induced signaling through NADPH oxidase in that we demonstrate phosphorylation of both eNOS and Akt in BPAECs is suppressed by pretreatment with two mechanistically distinct inhibitors of NADPH oxidase, apocynin or gp91phox ds tat (Fig. 2, C and E, and Fig. 4, A and C). Although gp91phox ds tat is designed on a NOX2 motif, it may lack specificity and inhibit other NOX isoforms in BPAECs. Our data position NADPH oxidase as a mediator of 20-HETE-evoked activation of both proteins.
Our data also show for the first time that 20-HETE-stimulated H2O2 and not superoxide activates Akt and eNOS in BPAECs. This conclusion is based on the fact that 20-HETE-induced phosphorylation of both of these proteins (eNOS at Ser1179 and Akt at Ser473) is blocked by PEG-Cat and not PEG-SOD. These observations are consistent with previous reports of H2O2-induced activation of Akt in three cell lines and rat aortic vascular smooth muscle cells (30, 31, 33), as well as our own reports of Akt activation by 20-HETE in BPAECs (23). Similarly, activation of Akt by NADPH oxidase in porcine coronary artery endothelial cells has been observed (8). Various stimuli, including vascular endothelial growth factor (13) and fluid shear stress (11), activate Akt, resulting in promotion of eNOS activity through increased Ser1179 phosphorylation. Phosphorylation of eNOS is widely recognized as a critical regulatory mechanism controlling eNOS activity in response to physiological stimuli (5, 14, 17, 24). H2O2 is a potent stimulus for NO production and is involved in shear-induced stimulation of the eNOS phosphorylation at Ser1179 and the dephosphorylation of Thr495 in bovine aortic endothelial cells (5). NADPH derived hydrogen peroxide is reported to mediate stimulated NO production in bovine aortic endothelial cells (7). Thus 20-HETE-associated stimulated production of hydrogen peroxide leading to phosphorylation of Akt and eNOS in BPAECs fits well into this background of work.
Exogenous H2O2 is reported to mimic the effect of endogenous receptor-induced production of H2O2 and activation of Akt (21, 31, 33). In this regard, intracellular concentrations of H2O2 after a single bolus of H2O2 to the extracellular media are estimated to be 6- to 10-fold lower in the cytosol and 20-fold lower in peroxisomes (3). To assess the effects of H2O2 on NOS-induced NO production in BPAECs, we exposed cells to a range of concentrations of H2O2 for 30 min and followed changes in DAF fluorescence. We observed a bell-shaped response curve, with significant increases in DAF fluorescence at all concentrations above 50 μM, peaking at 400 μM. Our NO concentration response curve to hydrogen peroxide is very similar to that observed by Cai et al. (7) in bovine aortic endothelial cells, but the x-axis in our experiments is extended to show a decrease in NO release at concentrations above 400 μM. Based on all these data, it can be argued that localized physiological concentrations of H2O2 evoked by 20-HETE may be capable of inducing eNOS activation and NO production.
In addition to phosphorylation of Akt and eNOS, our studies indicate that 20-HETE induced an increase in intracellular NO (based on changes in DAF fluorescence, see Fig. 4E) in a manner that depends on H2O2. These data link 20-HETE-stimulated phosphorylation of eNOS to NO release. The correlation between DAF-FM fluorescence and intracellular NO was confirmed by the data from DETANONOate (a NO donor)-treated BPAECs, which served as a positive control (data not shown), and that of cells treated with l-NAME, which densely blocked 20-HETE-induced DAF signal, which served as the negative control.
Enhanced DAF fluorescence in cells treated with 20-HETE was blocked by NADPH oxidase or PI3K and Akt inhibitors. The observation that PI3K inhibitors wortmannin and LY-294002 blunt 20-HETE-stimulated increase in NO suggests that stimulated eNOS activation is regulated by PI3K/Akt. Inhibition of PI3K also blocked H2O2-stimulated NO production in the same cells. These observations should not be extrapolated to conclusions of direct activation of PI3K or NADPH by 20-HETE as a single mechanism through which enhanced NO is generated in treated cells. Both PI3K and NADPH oxidase are ubiquitous and powerful signaling pathways, such that we expect multiple effects of activation of these signals in BPAECs or other cells for that matter. Very specifically, it is possible that 20-HETE and/or PI3K have distinct effects on several steps in this signaling cascade including NADPH oxidase, PI3K activation, eNOS phosphorylation, or others. Despite these caveats, these data provide critical links of 20-HETE-induced NO release from BPAECs (36).
The fact that formation of H2O2 is required for 20-HETE-induced eNOS activation has important potential implications for pulmonary vascular cell biology and function. First, these data suggest that enhanced H2O2 formation may serve as an important mediator of 20-HETE prosurvival effects (10, 23). This speculation is consistent with reports that NO derived from eNOS protects cells from oxidative stress by an antioxidant mechanism (26). Also, our studies provide a potential mechanistic basis for the vasorelaxation effect of 20-HETE in pulmonary circulation despite an associated increase in superoxide formation (9, 19). We speculate that 20-HETE relaxes small pulmonary arteries via H2O2-dependent eNOS activation. Based on the lack of 20-HETE-stimulated ONOO− within the resolution of our assays, and similar profiles of DCF and DHR fluorescence with PEG-SOD or PEG-Cat in response to 20-HETE, we hypothesize that 20-HETE-stimulated superoxide in BPAECs is rapidly dismutated to hydrogen peroxide. In this case, there may be limited opportunity for superoxide interaction with NO and therefore minimal ONOO− generation. Additional studies to identify mechanisms through which 20-HETE activates NADPH oxidase and promotes survival (NO dependent or independent) are needed.
GRANTS
Financial support was provided by National Institutes of Health Grants HL-49294 (E. R. Jacobs), HL-68627 (E. R. Jacobs), and GM-31278 (J. R. Falck) and the Robert A. Welch Foundation (J. R. Falck).
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
We thank Dr. Vani Nilakantan, Div. of Surgery, Medical College of Wisconsin, Milwaukee, for the generous gift of 3-nitrotyrosine antibody used in this work.
REFERENCES
- 1.Alonso-Galicia M, Falck JR, Reddy KM, Roman RJ. 20-HETE agonists and antagonists in the renal circulation. Am J Physiol Renal Physiol 277: F790–F796, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Amaral SL, Maier KG, Schippers DN, Roman RJ, Greene AS. CYP4A metabolites of arachidonic acid and VEGF are mediators of skeletal muscle angiogenesis. Am J Physiol Heart Circ Physiol 284: H1528–H1535, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Antunes F, Cadenas E. Estimation of H2O2 gradients across biomembranes. FEBS Lett 475: 121–126, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Bilski P, Belanger AG, Chignell CF. Photosensitized oxidation of 2′,7′-dichlorofluorescin: singlet oxygen does not contribute to the formation of fluorescent oxidation product 2′,7′-dichlorofluorescein. Free Radic Biol Med 33: 938–946, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Boo YC, Hwang J, Sykes M, Michell BJ, Kemp BE, Lum H, Jo H. Shear stress stimulates phosphorylation of eNOS at Ser(635) by a protein kinase A-dependent mechanism. Am J Physiol Heart Circ Physiol 283: H1819–H1828, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Cai H, Davis ME, Drummond GR, Harrison DG. Induction of endothelial NO synthase by hydrogen peroxide via a Ca(2+)/calmodulin-dependent protein kinase II/janus kinase 2-dependent pathway. Arterioscler Thromb Vasc Biol 21: 1571–1576, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Cai H, Li Z, Dikalov S, Holland SM, Hwang J, Jo H, Dudley SC, Jr, Harrison DG. NAD(P)H oxidase-derived hydrogen peroxide mediates endothelial nitric oxide production in response to angiotensin II. J Biol Chem 277: 48311–48317, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Chen JX, Zeng H, Tuo QH, Yu H, Meyrick B, Aschner JL. NADPH oxidase modulates myocardial Akt, ERK1/2 activation, and angiogenesis after hypoxia-reoxygenation. Am J Physiol Heart Circ Physiol 292: H1664–H1674, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y, Medhora M, Falck , JR, Pritchard KA, Jr, Jacobs ER. Mechanisms of activation of eNOS by 20-HETE and VEGF in bovine pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol 291: L378–L385, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Dhanasekaran A, Bodiga S, Gruenloh S, Gao Y, Dunn L, Falck JR, Buonaccorsi JN, Medhora M, Jacobs ER. 20-HETE increases survival and decreases apoptosis in pulmonary arteries and pulmonary artery endothelial cells. Am J Physiol Heart Circ Physiol 296: H777–H786, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399: 601–605, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Drummond GR, Cai H, Davis ME, Ramasamy S, Harrison DG. Transcriptional and posttranscriptional regulation of endothelial nitric oxide synthase expression by hydrogen peroxide. Circ Res 86: 347–354, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature 399: 597–601, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallis B, Corthals GL, Goodlett DR, Ueba H, Kim F, Presnell SR, Figeys D, Harrison DG, Berk BC, Aebersold R, Corson MA. Identification of flow-dependent endothelial nitric-oxide synthase phosphorylation sites by mass spectrometry and regulation of phosphorylation and nitric oxide production by the phosphatidylinositol 3-kinase inhibitor LY294002. J Biol Chem 274: 30101–30108, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Griendling KK, Ushio-Fukai M. Reactive oxygen species as mediators of angiotensin II signaling. Regul Pept 91: 21–27, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Heumuller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schroder K, Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension 51: 211–217, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Igarashi J, Michel T. Sphingosine 1-phosphate and isoform-specific activation of phosphoinositide 3-kinase beta. Evidence for divergence and convergence of receptor-regulated endothelial nitric-oxide synthase signaling pathways. J Biol Chem 276: 36281–36288, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Jacobs ER, Effros RM, Falck JR, Reddy KM, Campbell WB, Zhu D. Airway synthesis of 20-hydroxyeicosatetraenoic acid: metabolism by cyclooxygenase to a bronchodilator. Am J Physiol Lung Cell Mol Physiol 276: L280–L288, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Jacobs ER, Zhu D, Gruenloh S, Lopez B, Medhora M. VEGF-induced relaxation of pulmonary arteries is mediated by endothelial cytochrome P-450 hydroxylase. Am J Physiol Lung Cell Mol Physiol 291: L369–L377, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Kojima H, Nakatsubo N, Kikuchi K, Kawahara S, Kirino Y, Nagoshi H, Hirata Y, Nagano T. Detection and imaging of nitric oxide with novel fluorescent indicators: diaminofluoresceins. Anal Chem 70: 2446–2453, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Konishi H, Matsuzaki H, Tanaka M, Takemura Y, Kuroda S, Ono Y, Kikkawa U. Activation of protein kinase B (Akt/RAC-protein kinase) by cellular stress and its association with heat shock protein Hsp27. FEBS Lett 410: 493–498, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Leikert JF, Rathel TR, Muller C, Vollmar AM, Dirsch VM. Reliable in vitro measurement of nitric oxide released from endothelial cells using low concentrations of the fluorescent probe 4,5-diaminofluorescein. FEBS Lett 506: 131–134, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Medhora M, Chen Y, Gruenloh S, Harland D, Bodiga S, Zielonka J, Gebremedhin D, Gao Y, Falck JR, Anjaiah S, Jacobs ER. 20-HETE increases superoxide production and activates NAPDH oxidase in pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol 294: L902–L911, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Michell BJ, Griffiths JE, Mitchelhill KI, Rodriguez-Crespo I, Tiganis T, Bozinovski S, de Montellano PR, Kemp BE, Pearson RB. The Akt kinase signals directly to endothelial nitric oxide synthase. Curr Biol 9: 845–848, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Mohanty JG, Jaffe JS, Schulman ES, Raible DG. A highly sensitive fluorescent micro-assay of H2O2 release from activated human leukocytes using a dihydroxyphenoxazine derivative. J Immunol Methods 202: 133–141, 1997 [DOI] [PubMed] [Google Scholar]
- 26.Paxinou E, Weisse M, Chen Q, Souza JM, Hertkorn C, Selak M, Daikhin E, Yudkoff M, Sowa G, Sessa WC, Ischiropoulos H. Dynamic regulation of metabolism and respiration by endogenously produced nitric oxide protects against oxidative stress. Proc Natl Acad Sci USA 98: 11575–11580, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev 82: 131–185, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Thomas SR, Chen K, Keaney JF., Jr Hydrogen peroxide activates endothelial nitric-oxide synthase through coordinated phosphorylation and dephosphorylation via a phosphoinositide 3-kinase-dependent signaling pathway. J Biol Chem 277: 6017–6024, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Touyz RM. Apocynin, NADPH oxidase, and vascular cells: a complex matter. Hypertension 51: 172–174, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Ushio-Fukai M, Alexander RW. Reactive oxygen species as mediators of angiogenesis signaling: role of NAD(P)H oxidase. Mol Cell Biochem 264: 85–97, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Ushio-Fukai M, Alexander RW, Akers M, Yin Q, Fujio Y, Walsh K, Griendling KK. Reactive oxygen species mediate the activation of Akt/protein kinase B by angiotensin II in vascular smooth muscle cells. J Biol Chem 274: 22699–22704, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Wang JS, Singh H, Zhang F, Ishizuka T, Deng H, Kemp R, Wolin MS, Hintze TH, Abraham NG, Nasjletti A, Laniado-Schwartzman M. Endothelial dysfunction and hypertension in rats transduced with CYP4A2 adenovirus. Circ Res 98: 962–969, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Wang X, McCullough KD, Franke TF, Holbrook NJ. Epidermal growth factor receptor-dependent Akt activation by oxidative stress enhances cell survival. J Biol Chem 275: 14624–14631, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Xia Y, Tsai AL, Berka V, Zweier JL. Superoxide generation from endothelial nitric-oxide synthase. A Ca2+/calmodulin-dependent and tetrahydrobiopterin regulatory process. J Biol Chem 273: 25804–25808, 1998 [DOI] [PubMed] [Google Scholar]
- 35.Yamane Y, Furuichi M, Song R, Van NT, Mulcahy RT, Ishikawa T, Kuo MT. Expression of multidrug resistance protein/GS-X pump and gamma-glutamylcysteine synthetase genes is regulated by oxidative stress. J Biol Chem 273: 31075–31085, 1998 [DOI] [PubMed] [Google Scholar]
- 36.Yu M, McAndrew RP, Al-Saghir R, Maier KG, Medhora M, Roman RJ, Jacobs ER. Nitric oxide contributes to 20-HETE-induced relaxation of pulmonary arteries. J Appl Physiol 93: 1391–1399, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Zhu D, Zhang C, Medhora M, Jacobs ER. CYP4A mRNA, protein, and product in rat lungs: novel localization in vascular endothelium. J Appl Physiol 93: 330–337, 2002. [DOI] [PubMed] [Google Scholar]