Abstract
The severity of asthma, a disease characterized by airway hyperresponsiveness and inflammation, is enhanced in some women during the menstrual cycle and during pregnancy but relieved in others. These clinical findings suggest that sex steroids modulate airway tone. Based on well-known relaxant effects of estrogens on vascular smooth muscle, we hypothesized that estrogens relax airway smooth muscle (ASM), thus facilitating bronchodilation. In ASM tissues from female patients, Western and immunocytochemical analyses confirmed the presence of both estrogen receptor (ER) isoforms, ERα and ERβ. In fura 2-loaded, dissociated ASM cells maintained in culture, acute exposure to physiological concentrations of 17β-estradiol (E2; 100 pM to 10 nM) decreased the intracellular Ca2+ ([Ca2+]i) response to 1 μM histamine, an effect reversed by the ER antagonist ICI-182,780. The ERα-selective agonist (R,R)-THC had a greater reducing effect on [Ca2+]i responses to histamine and 1 μM ACh compared with the ERβ-selective agonist (DPN). The effects of E2 on [Ca2+]i were mediated, at least in part, via decreased Ca2+ influx through l-type channels and store-operated Ca2+ entry but not via Ca2+-activated K+ channels, receptor-operated entry, or sarcoplasmic reticulum reuptake. Overall, these data support our hypothesis that estrogens relax ASM and suggest a potentially novel therapeutic target in airway hyperresponsiveness.
Keywords: sex steroid, lung, asthma, bronchial smooth muscle, estrogen receptor
asthma, a disease characterized by airway hyperresponsiveness and inflammation, is more prevalent among adult women compared with men (11, 32, 45). Clinical data reveal an increased severity and frequency of exacerbations in women (2, 41). Cyclical variations in airway reactivity termed premenstrual asthma have been estimated to affect approximately 40–50 percent of women with preexisting disease (8, 37). Although these clinical data would suggest that sex steroids such as estrogens may have a detrimental effect in asthma, closer examination reveals that asthma exacerbation is actually greater during the late luteal phase when estrogen levels are lowest (8, 16, 18). Furthermore, some postmenopausal women experience decreased airway function (3). Finally, although asthma is the most prevalent airway disease endured by pregnant women (approximately 4–8%; Refs. 29, 51) during the third trimester of pregnancy (when circulating estrogen levels are high and the gravid uterus would be expected to compress the lungs), only one-third of women with preexisting disease will experience exacerbation of symptoms, and another one-third actually experience improvement in asthma symptoms (2, 24). Taken together, these contrasting clinical data suggest that sex steroids modulate airway tone. What is not clear is which sex steroid (estrogen vs. progesterone) and whether these hormones are detrimental or beneficial.
In both normals and asthmatics, airway tone represents a balance between bronchoconstriction and bronchodilation, mediated by airway smooth muscle (ASM) contractility vs. relaxation. ASM contractility is determined by both regulation of intracellular Ca2+ ([Ca2+]i) and force (28, 40). Previous studies have already demonstrated that increased airway tone in diseases such as asthma involve, at least in part, increased [Ca2+]i (25, 43, 50). Therefore, it is possible that sex steroids such as estrogens and progesterone modulate airway tone by altering [Ca2+]i in ASM.
There are currently limited data on the role of sex steroids in regulation of airway tone or their effects on ASM, especially in humans. Measurements of airway resistance in mouse models of asthma have found both downregulation (5, 13, 31) as well as upregulation (6) of airway hyperresponsiveness by estrogens. Other studies in guinea pig have found that progesterone can induce ASM relaxation (44). However, in humans and mouse models, airway function is actually reduced when progesterone levels are high (20, 52). Based on the clinical data that asthma exacerbation is greater when estrogen levels are lower, we focused on estrogens and hypothesized that estrogens facilitate bronchodilation.
There is already a precedent for estrogen-induced relaxation of smooth muscle in the well-recognized vasodilatory effects of estrogens (34, 36). In this regard, as with vascular smooth muscle, estrogen may act via both acute (likely nongenomic) and genomic mechanisms in modulating ASM tone. In vascular smooth muscle, both of these effects are mediated through estrogen receptors (ERs), primarily ERα. Previous studies have shown that the ERα and ERβ isoforms are expressed in human lung (35); however, their functional role is unclear. Although ER expression has been noted in the lung, specific ER expression in ASM or its function have not been established. In the present study, we determined the expression of ERs in normal human ASM and their role in acute (nongenomic) regulation of [Ca2+]i during agonist stimulation, thus setting the foundation for examining the role of estrogens in asthmatic ASM. In this regard, signaling pathways activated by the two ER isoforms are likely different [e.g., in other tissues, ERβ modulates ERα signaling, which, in turn, involves multiple signaling pathways such as mitogen-associated kinases and Src-phosphatidylinositol pathway (19, 30)]; however, we did not specifically examine these differential signaling pathways here.
MATERIALS AND METHODS
Isolation of Human ASM Cells
Studies were conducted using ASM cells enzymatically dissociated from 3rd to 6th generation bronchi of lung samples incidental to patient surgery at Mayo Clinic, Rochester, MN (approved and considered exempt by Mayo Institutional Review Board). This study was limited to tissues obtained from adult female patients, although no attempt was made to determine whether these patients were in menopause or not. Lung samples excised for empyema or other infectious or severe inflammatory causes were excluded. Visibly normal airway, as confirmed by pathological review, was excised and rapidly transferred to the laboratory in ice-cold HBSS. ASM cells were enzymatically dissociated as previously described by our group (46). Briefly, epithelium and connective tissues were removed by blunt dissection, and smooth muscle tissue was carefully minced followed by papain and collagenase dissociation and ovomucoid/albumin separation as per manufacturer's instructions (Worthington Biochemical, Lakewood, NJ). Cell pellets were resuspended and seeded into 75-cm2 tissue culture flasks or eight-well Lab-Tek chambers (Nalge Nunc International, Rochester, NY). Cells were maintained at 37°C (5% CO2-95% air) using phenol red-free DMEM/F-12 medium (phenol red is known to activate ERs, albeit weakly; Invitrogen, Carlsbad, CA) supplemented with 10% FBS until ∼80% confluent. Before experiments, the cells were washed in PBS, and medium was changed to phenol red-free DMEM/F-12 lacking serum for 48 h. All experiments were performed in cells from passages 1 to 3 of subculture. Periodic assessment of smooth muscle myosin heavy chain (or other contractile protein) expression, and lack of fibroblast markers, was performed to rule out dedifferentiation during the cell-processing period. ER expression in passaged cells was compared with expression in ASM tissue from which they were derived and found to be comparable.
Isolation of ASM Cell Fractions
Human ASM cells were obtained as described above and plated on 100-mm plates under the same conditions. Cells were grown to 80% confluence and harvested. These whole cell lysates were then separated into heavy (nuclear/Golgi), cytosolic, and membrane fractions using the Fraction-PREP Cell Fractionation System (BioVision, Mountain View, CA) according to the manufacturer's protocol.
Immunoprecipitation of Proteins from Cell Fractions
The above fractions were subjected to immunoprecipitation for either ERα or ERβ. Primary antibody (2 μg; mouse anti-ERα: cat. no. 2512, Cell Signaling Technology; mouse anti-ERβ: cat. no. SC-53494, Santa Cruz Biotechnology) was used per 200 μl of sample fraction and incubated overnight at 4°C with gentle rotation. Protein G agarose beads (50 μl) were added to the sample and incubated for 4 h at 4°C. Proteins were recovered through centrifugation and eluted from the beads at 100°C for 5 min. These samples were then processed as described below for Western analysis (goat-anti-ERβ SC-6822 was used for overnight incubation for ERβ detection). Purity of cell fractions was confirmed via Western analysis of proteins exclusive to each fraction (heavy: rabbit anti-c-Jun SC-1694; membrane: rabbit anti-Gαi-3 SC-262; cytosol: rabbit anti-ERK42 SC-94).
Western Blot Analysis
Proteins were separated by SDS-PAGE (10% gradient gels; Criterion Gel System; Bio-Rad, Hercules, CA) at 200 V for 1 h and transferred to polyvinylidene difluoride (PVDF) membranes (Bio-Rad) for 60–75 min. Membranes were blocked for 1 h at room temperature with 5% milk in Tris-buffered saline (TBS) containing 0.1% Tween (TBST) and then incubated overnight at 4°C with 1 μg/ml rabbit anti-ERα (SC-542; Santa Cruz Biotechnology) or mouse anti-ERβ (SC-53494; Santa Cruz Biotechnology). GAPDH (cat. no. 2118; Cell Signaling Technology) was used for normalization. Following three washes with TBST, primary antibodies were detected using horseradish peroxidase-conjugated secondary antibodies and signals developed by SuperSignal West Pico Chemiluminescent Substrate (Pierce Chemical, Rockford, IL). Blots were imaged on a Kodak Image Station 4000MM (Carestream Health, New Haven, CT) and quantified using densitometry.
Human ASM samples were processed as described above. Samples of postpartum rat uterus were used as positive controls for ER expression. To determine applicability of the primary antibodies to other human tissues, human pulmonary artery samples were also analyzed.
Immunofluorescence Microscopy
ER expression in ASM cells was determined using immunofluorescence as described for other proteins (47). ASM grown on glass slides to 50% confluence were fixed with 2% paraformaldehyde for 15 min at room temperature and permeabilized with 0.1% Triton X-100 in 0.1 M TBS for 10 s. After washing gently with TBS, cells were blocked in 4% normal donkey serum for 1 h at room temperature and incubated overnight at 4°C with primary antibody (1 μg/ml rabbit anti-ERα SC-542, goat anti-ERβ SC-6822). After thoroughly washing with TBS, cells were incubated with appropriate Cy3 or Alexa Fluor 488 fluorescent secondary antibodies (1:200 dilution; donkey anti-rabbit or anti-goat IgG; Jackson ImmunoResearch) for 1 h at room temperature.
Labeled ASM cells were visualized using an Olympus FluoView laser scanning confocal microscope mounted on an Olympus BX50WI equipped with Ar and Kr lasers and appropriate filters. Cells were imaged at 1,024 × 1,024 pixels and a 0.4-μm optical section thickness using a ×40 oil-immersion lens and different hardware zooms.
Real-Time [Ca2+]i Imaging
The techniques for [Ca2+]i imaging of human ASM cells has been previously described (47). ASM cells were incubated in 5 μM fura 2-AM (Molecular Probes, Eugene, OR) for 45 min at room temperature and visualized using a fluorescence imaging system (MetaFluor; Universal Imaging, Downingtown, PA) on a Nikon Diaphot Inverted Microscope (Fryer Instruments, Edina, MN) equipped with a Photometric Cascade digital camera system (Roper Scientific, Tucson, AZ). [Ca2+]i responses of ∼15 cells per visual field were obtained using individual, software-defined regions of interest (×40 oil-immersion lens, 512 × 512 pixel resolution, 0.33-Hz image acquisition of 510-nm emissions following alternative excitation at 340 vs. 380 nm). Cells were initially perfused with 2.5 mM Ca2+ HBSS, and baseline fluorescence was established. Actual [Ca2+]i levels were estimated using previously described calibration techniques for fura 2 (39, 47).
Materials
(R,R)-5,11-diethyl-5,6,11,12-tetrahydro-2,8-chrysenediol [(R,R)-THC] and diarylpropionitrile (DPN) were obtained from Tocris Biosciences (Ellisville, MO). 17β-Estradiol (E2), ICI-182,780 (ICI), and other chemicals were obtained from Sigma Chemical (St. Louis, MO) unless mentioned otherwise. Tissue culture reagents, including DMEM/F-12 and FBS, were obtained from Invitrogen.
Statistical Analysis
Twelve bronchial samples from different female patients were used to obtain ASM cells. [Ca2+]i experiments were performed in at least 25 cells each for 4 different bronchial (patient) samples, although not all protocols were performed in each sample obtained. In experiments where responses were compared in the presence or absence of specific drugs, paired t-test was used, whereas population studies were compared using unpaired t-test or 1-way ANOVA with repeated measures as appropriate. Bonferroni correction was applied for multiple comparisons. Statistical significance was established at P < 0.05. All values are expressed as means ± SE.
RESULTS
Expression of ERs in Human ASM
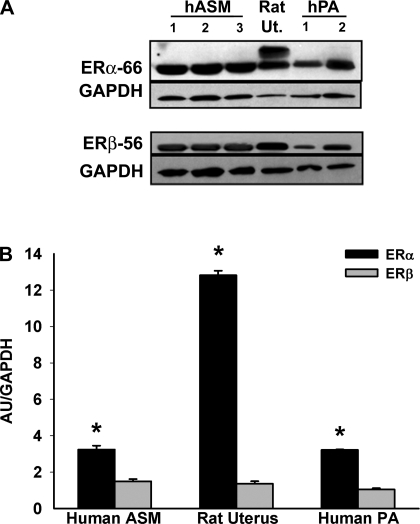
Western blot analysis of ASM (i.e., epithelium denuded, ASM isolated) from bronchial samples of female patients revealed that both full-length ERα and ERβ are expressed to a considerable extent, even compared with tissues known to have these isoforms such as postpartum rat uterus (n = 6; Fig. 1). Furthermore, human pulmonary artery also showed expression of these ER isoforms, albeit to different extents compared with ASM (Fig. 1A). Overall, in human ASM, ERα expression (calculated relative to GAPDH expression) was greater than that of ERβ (Fig. 1B). In other tissues, lower molecular weight ER isoforms (that lack specific domains) have been reported (15, 26). Such isoforms were also noted in human ASM (data not shown).
Fig. 1.
Expression of estrogen receptors (ERs) in human (h) airway smooth muscle (ASM). A: full-length ER isoforms -α (ERα-66) and -β (ERβ-56) are expressed in both ASM and pulmonary artery (PA) from lungs of female patients. Rat uterus (Ut.) was used as a positive control in both cases. B: densitometric analysis of relative ER expression normalized to GAPDH levels shows ERα is more prominent than ERβ in ASM. Values are means ± SE. *Significant difference between ERα and ERβ (P < 0.05). AU, arbitrary units.
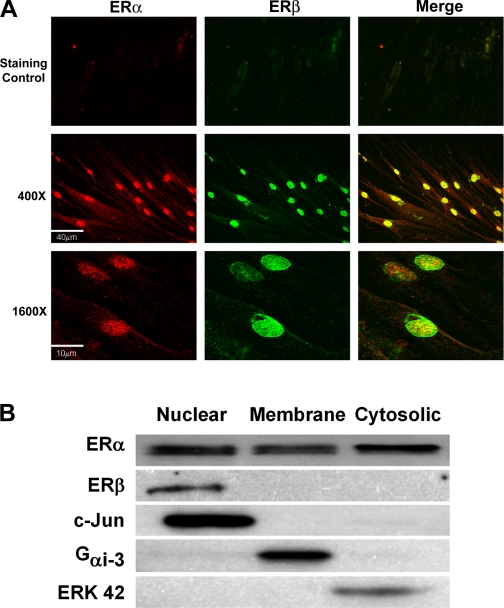
Immunostaining of enzymatically dissociated ASM cells demonstrated plasma membrane, cytosolic, as well as diffuse nuclear expression of ERα. In comparison, ERβ was expressed at the plasma membrane also but to a lesser extent; however, distinct expression of this isoform was detected at the nucleus. Overall, ERα expression was more ubiquitous than ERβ with ERβ preferentially localizing to the nucleus (Fig. 2A). These results were confirmed by Western analysis of cell fractions illustrating ERα expression in heavy (nuclear/Golgi), cytosolic, and membrane fractions, whereas ERβ expression was expressed largely in the heavy fraction (Fig. 2B).
Fig. 2.
Localization of ERs in ASM cells. A: immunocytochemical staining of enzymatically dissociated ASM cells from female patients followed by confocal imaging demonstrated expression of both ERα (Cy3-conjugated secondary antibody; red) and ERβ (Alexa Fluor 488-conjugated secondary antibody; green). Top shows secondary staining controls, whereas middle and bottom shows ERα and ERβ expression (left, middle) as well as colocalization (merged images with yellow) at 2 different magnifications. ERα and ERβ are present at the plasma membrane as well as in the cytosol and nucleus, albeit to different extents. B: ER expression was determined in whole ASM cell lysates separated into heavy (nuclear/Golgi), membrane, and cytosolic fractions. Complementary to immunocytochemical staining, ERα was expressed by all 3 fractions, whereas ERβ expression was limited to the heavy fraction. Purity of the cell fractions was tested by blotting for proteins exclusive to each fraction. B represents original scan contrasts and brightness, which differed between blots.
Effect of E2 on [Ca2+]i Response to Agonist
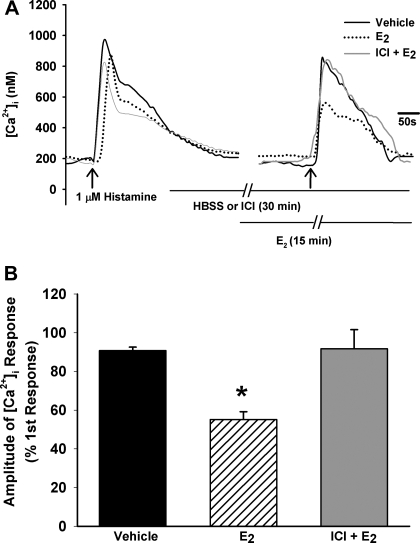
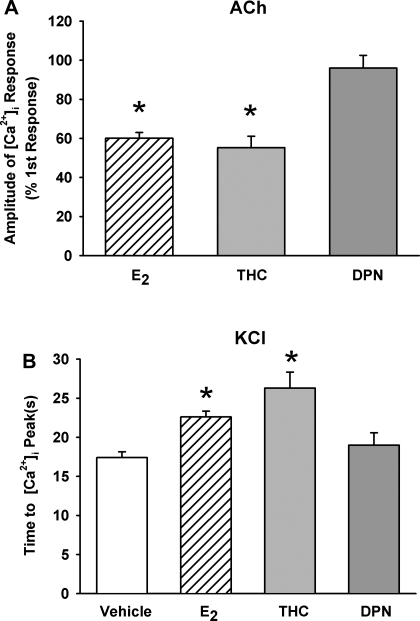
In the first set of studies, fura 2-loaded human ASM cells were exposed to 1 μM histamine, resulting in the typical transient [Ca2+]i elevation to a peak level followed by a lower plateau that was still greater than baseline Ca2+ (Fig. 3A). Baseline [Ca2+]i levels ranged from 100 to 150 nM. With exposure to histamine, the amplitude of the [Ca2+]i response was ∼750 nM. Following a thorough washout of histamine with 2.5 mM Ca2+ HBSS for at least 15 min, cells were exposed to 1 nM E2 for 15 min. This resulted in a small but insignificant rise in [Ca2+]i basal levels. Subsequent exposure to histamine resulted in a significantly smaller [Ca2+]i response compared with vehicle controls (n = 4; P < 0.05; Fig. 3, A and B). To determine whether these results were specific to histamine, the above experiment was repeated in separate sets of cells using ACh (1 μM) as an alternative agonist and KCl (110 mM) as a nonreceptor agonist that changes membrane potential. In general, the inhibitory effects of E2 on [Ca2+]i responses to ACh persisted (n = 4; P < 0.05; Fig. 6A); however, there was no difference in the peak [Ca2+]i response between E2-treated cells and vehicle controls with KCl depolarization (data not shown). Further analysis of [Ca2+]i response to KCl showed that E2 extended the time required to reach peak [Ca2+]i levels (n = 4; P < 0.05; Fig. 6B).
Fig. 3.
Estrogen decreases intracellular calcium ([Ca2+]i) response to histamine in human ASM cells. A: in cells loaded with fura 2 and perfused with 2.5 mM Ca2+ HBSS, 1 μM histamine resulted in a rise in [Ca2+]i (black). In separate cells, pretreatment with 1 nM 17β-estradiol (E2) decreased the response to histamine by ∼40% (dotted). Pretreatment with the isoform-nonspecific ER antagonist ICI-182,780 (ICI; 1 μM; Faslodex) reversed E2 effects (gray), returning peak response to that of vehicle controls. See materials and methods for details of protocol. B: summary of E2 and ICI effects (n = 4). Values are means ± SE. *Significant effect of E2 (P < 0.05).
Fig. 6.
Role of ER isoforms in [Ca2+]i responses to ACh and KCl. A: in human ASM cells, pretreatment with 1 nM E2 or 10 nM (R,R)-THC (but not 10 nM DPN) significantly reduced peak Ca2+ response to 1 μM ACh (n = 4). These effects were qualitatively similar to E2 and (R,R)-THC effects on responses to histamine. B: in human ASM cells depolarized with 110 mM KCl, pretreatment with 1 nM E2 or 10 nM (R,R)-THC prolonged the time required to reach peak [Ca2+]i compared with vehicle controls (n = 4). Values are means ± SE. *Significant drug effect compared with vehicle control (P < 0.05).
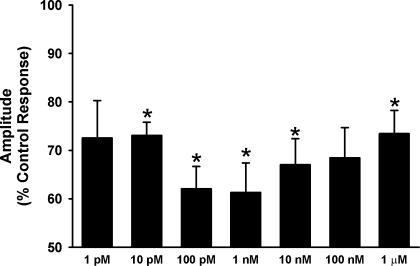
In a subsequent set of studies, seven concentrations of E2 that span the physiological range were used to establish a dose-response relationship for effects on [Ca2+]i response to histamine. Because of known tachyphylaxis with histamine, as well as the number of interventions, these studies had to be performed as comparisons of cell populations. ASM cells were exposed to 0 (vehicle control), 1 pM, 10 pM, 100 pM, 1 nM, 10 nM, 100 nM, and 1 μM E2 for 15 min and then stimulated with 1 μM histamine. Compared with vehicle controls, there was considerable decrease in amplitude of [Ca2+]i responses for all E2 concentrations, forming a biphasic dose-response curve (Fig. 4). All E2 concentrations in the physiological range (100 pM to 10 nM) significantly decreased the [Ca2+]i amplitude, with the maximum reduction in [Ca2+]i occurring at 1 nM E2 (n = 4; P < 0.05). Accordingly, for subsequent studies, we used 1 nM E2 to further explore estrogen signaling.
Fig. 4.
Dose-response of E2 effects on [Ca2+]i response to histamine. In separate cell populations, pretreatment with E2 in concentrations ranging from 1 pM to 1 μM resulted in a consistent, significant decrease in [Ca2+]i in response to histamine. However, maximum reduction in [Ca2+]i was observed at 1 nM, whereas higher concentrations were less effective in reducing [Ca2+]i levels. Overall, E2 effects were most prominent within the physiological range 100 pM to 10 nM. Values are means ± SE. *Significant effect of E2 (P < 0.05).
Effect of ER Inhibition on E2 Effects
To ascertain whether the blunting of [Ca2+]i responses by E2 was mediated by ERs, in an additional set of experiments, 1 μM isoform-nonspecific ER antagonist ICI (Faslodex) was administered 15 min before 1 nM E2 exposure and was continued during the subsequent 15 min in combination with E2 (n = 4). In the presence of ICI, the previously observed blunting of [Ca2+]i response by E2 was abolished, and [Ca2+]i response to histamine approximated that of vehicle controls (n = 4; Fig. 3).
Effect of ER Isoform-Selective Agonists
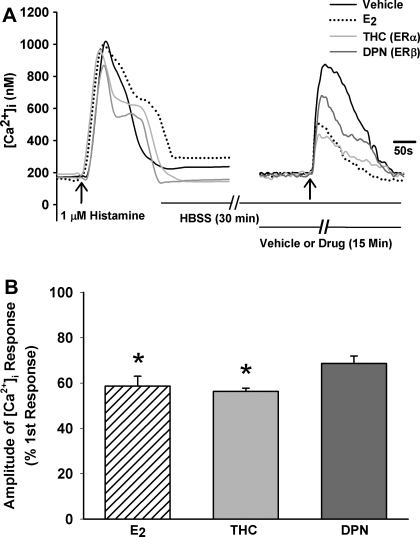
E2 is a nonspecific ER agonist and does not differentiate between ERα and ERβ activation. To determine which receptor subtype is involved in modulation of [Ca2+]i, ERα- and ERβ-selective agonists were used. (R,R)-THC is a nonsteroidal, ERα-selective agonist (EC50 = 10 nM; Ref. 53) but also an ERβ-selective antagonist. DPN is an ERβ-selective agonist (EC50 = 0.85 nM; Ref. 33). The protocol described above to test E2 effects was modified by replacing E2 with either (R,R)-THC or DPN.
Acute exposure of ASM cells to 10 nM (R,R)-THC decreased [Ca2+]i response to ∼50% of vehicle control (n = 4; P < 0.05; Fig. 5). The reduction of [Ca2+]i by (R,R)-THC approximated the observed effects of E2. In contrast, 10 nM DPN decreased the [Ca2+]i response to a much lesser extent than (R,R)-THC and was statistically comparable with vehicle control (Fig. 5; n = 4). The combination of (R,R)-THC and DPN did not appear to have additive effects on [Ca2+]i responses.
Fig. 5.
Role of ER isoforms in estrogen-induced reduction of [Ca2+]i. A: in human ASM cells, pretreatment with 10 nM ERα-specific agonist (R,R)-THC (THC; see results for details) caused ∼45% reduction in peak Ca2+ response to histamine, comparable with the effect of 1 nM E2 alone. Pretreatment with ERβ-specific agonist DPN (10 nM) resulted in a smaller decrement of [Ca2+]i responses to histamine. B: summary of (R,R)-THC and DPN effects on [Ca2+]i responses (n = 4). Values are means ± SE. *Significant drug effect compared with vehicle control (P < 0.05).
These experiments were repeated using ACh and KCl as described previously. In the case of ACh, the results were qualitatively similar to those with histamine. [Ca2+]i was decreased in both cases by (R,R)-THC to ∼50% of control, whereas DPN did not decrease [Ca2+]i significantly (n = 4; P < 0.05; Fig. 6A). There was no difference in the peak [Ca2+]i response between (R,R)-THC- or DPN-treated cells and vehicle controls with KCl depolarization (data not shown). Analysis of [Ca2+]i response to KCl showed that (R,R)-THC further extended the time required to reach peak [Ca2+]i levels compared with vehicle controls and E2 treatment, whereas DPN response approximated that of controls (n = 4; P < 0.05; Fig. 6B). The use of these additional agents illustrates that the E2 effect is not specific to the agonist histamine but rather is likely related to Ca2+ influx triggered by either receptor-activated channels or membrane depolarization.
Mechanisms of E2 Effects on [Ca2+]i Regulation
Ca2+ influx.
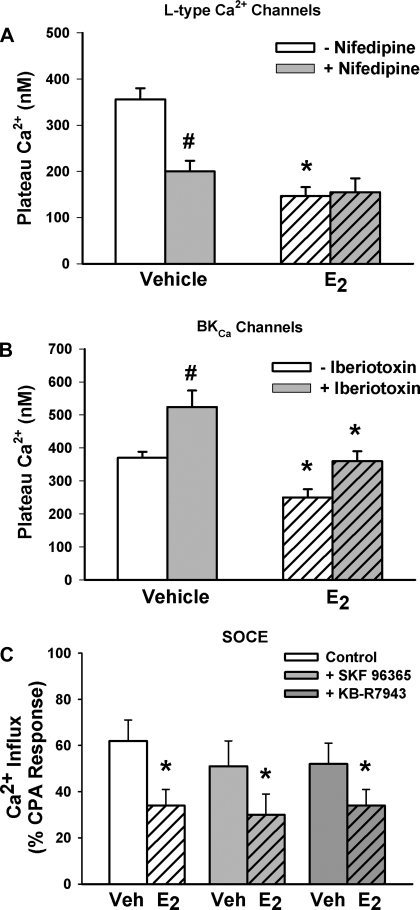
Previous studies (including our own) in vascular smooth muscle have demonstrated that estrogens decrease Ca2+ influx (27, 48). Accordingly, we tested the role of influx mechanisms in estrogen effects in human ASM. The protocol described above for ICI was modified by replacing this compound with the l-type Ca2+ channel inhibitor nifedipine (1 μM). In the presence of nifedipine, we found that 1 nM E2 effects on [Ca2+]i plateau levels to 1 μM histamine were considerably smaller, compared with effects with E2 alone (P < 0.05; n = 4; Fig. 7A). However, when similar studies were conducted in the presence of 100 nM iberiotoxin (to inhibit BKCa channels), E2 effects were largely unaffected (n = 4; P < 0.05; Fig. 7B). Addition of iberiotoxin resulted in a ∼50% increase in plateau Ca2+ levels compared with vehicle controls (likely representing Ca2+ influx in response to membrane depolarization; Fig. 7B). E2 in the presence of iberiotoxin reduced these elevated plateau levels by ∼50% compared with iberiotoxin alone (P < 0.05). The extent of E2-induced decrease in Ca2+ was comparable with E2 effects on plateau Ca2+ in cells not exposed to iberiotoxin.
Fig. 7.
Mechanisms underlying E2 effects on [Ca2+]i regulation in human ASM cells. A: pretreatment with the l-type Ca2+ channel inhibitor nifedipine (1 μM) blunted E2 effects on [Ca2+]i responses to histamine (n = 4), suggesting a partial role for this influx mechanism in E2 effects. B: however, pretreatment with the Ca2+-activated K+ channel inhibitor iberiotoxin did not considerably change E2 effects (but did increase the [Ca2+]i response to histamine as expected due to membrane depolarization; n = 4). C: using previously published protocols (1), assessment of store-operated Ca2+ entry (SOCE) showed that preexposure to E2 significantly decreased the extent of Ca2+ influx by this mechanism [n = 4; cyclopiazonic acid (CPA), inhibitor of sarcoplasmic reticulum (SR) Ca2+ reuptake; see materials and methods for protocol details]. Pretreatment with the receptor-operated channel inhibitor SKF-96365 or the Na+-Ca2+ exchange inhibitor KB-R7943 slightly decreased Ca2+ influx as evaluated using the SOCE protocol. Nonetheless, even in the presence of either inhibitor, E2 significantly blunted Ca2+ influx, indicating a predominant effect on SOCE per se (n = 4). Values are means ± SE. *Significant effect of E2; #significant inhibitor effect (P < 0.05). Veh, vehicle.
In parallel studies, we examined E2 effects on store-operated Ca2+ entry (SOCE). We (1, 39) have previously established the presence of this Ca2+ influx mechanism in human ASM cells. As previously described (1, 39), extracellular Ca2+ was first removed by perfusion with zero Ca2+ HBSS. Calcium influx through voltage-gated channels was then blocked by addition of 1 μM nifedipine, and the membrane was partially depolarized using 10 mM KCl. Sarcoplasmic reticulum (SR) Ca2+ stores were then depleted by exposure to 10 μM cyclopiazonic acid (CPA; inhibitor of SR Ca2+ reuptake). Following stabilization of [Ca2+]i levels, 1 nM E2 (or vehicle) was introduced. In the continued presence of nifedipine, KCl, CPA, and E2 (or vehicle), extracellular Ca2+ was rapidly reintroduced, triggering SOCE. The presence of E2 resulted in significantly smaller SOCE-mediated Ca2+ influx compared with vehicle controls (n = 4; P < 0.05; Fig. 7C).
We (55) have previously demonstrated that SOCE in ASM involves transient receptor potential (TRP) channels. However, Ca2+ influx on reintroduction of extracellular Ca2+ in the protocol used may also be due to other influx mechanisms that are not TRP-dependent, particularly receptor-operated calcium entry (ROC) or influx mode sodium-calcium exchange (NCX). To determine the role of these other mechanisms in E2 effects, the above protocol was modified by introducing either 10 μM SKF-96365 (a potent inhibitor of ROC) or 5 μM KB-R7943 (NCX inhibitor) for 15 min following CPA exposure but before reintroduction of extracellular calcium. E2 (1 nM) was added thereafter. ROC inhibition decreased the observed influx following introduction of extracellular Ca2+; however, even in the presence of SKF-96365, 1 nM E2 inhibited the influx to the same extent as it did without SKF-96365 being present (Fig. 6C; n = 4; P < 0.05). NCX inhibition slightly decreased the extent of influx, and E2 continued to inhibit the influx to the same extent as that without KB-R7943 being present (n = 4; P < 0.05; Fig. 7C).
SR Ca2+ reuptake.
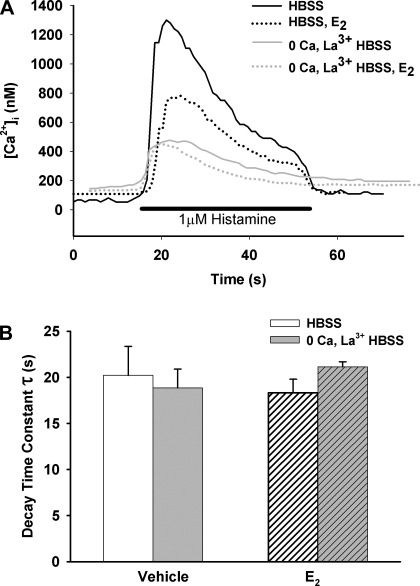
The initial protocol for E2-treated cells (e.g., Fig. 3) was repeated, and the decay time constant of the [Ca2+]i responses with or without 1 nM E2 was evaluated by single exponential decay fit (calculated from peak [Ca2+]i until return to baseline; n = 3; Fig. 8). Although the amplitude of [Ca2+]i response was decreased as reported above, the rate of fall was not significantly affected by E2 (Fig. 8).
Fig. 8.
A and B: role of SR Ca2+ reuptake in E2 effects. The rate of fall of the [Ca2+]i response to histamine (calculated as a decay time constant, τ, using a single exponential fit), representing Ca2+ reuptake as well as efflux, was largely unaffected by preexposure to E2. Absence of plasma membrane Ca2+ fluxes (inhibited by 0 Ca2+ and La3+) did not affect this conclusion, suggesting lack of E2 effect on SR Ca2+ reuptake.
To verify lack of E2 effect on SR Ca2+ reuptake, additional experiments were performed in a separate set of cells in the absence of extracellular Ca2+. Following initial perfusion in HBSS, extracellular Ca2+ was removed by perfusion with zero Ca2+ HBSS, and Ca2+ influx was nonspecifically blocked with 1 mM LaCl3. This resulted in a typical, small decrease in baseline [Ca2+]i levels. Cells were then exposed to 1 μM histamine in the presence or absence of 1 nM E2. Under these conditions, the [Ca2+]i responses were comparable (vehicle 363 ± 40 nM; E2 352 ± 17 nM; n = 3; Fig. 8). In parallel experiments, E2 effects on sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA) reuptake were evaluated. In identical extracellular conditions described above, preexposure to 1 nM E2 did not alter the rate of decline of [Ca2+]i following histamine exposure as calculated from [Ca2+]i peak to baseline value as described above (n = 3; Fig. 8).
DISCUSSION
ASM is the main regulator of airway hyperreactivity and inflammation and has become an important target with the recent increases in asthma diagnoses. Sex disparities in clinical findings as well as changes in airway function with the menstrual cycle, pregnancy, and menopause have raised the question of hormonal influences on ASM. The few studies that have looked at the effects of sex steroids on airway function are conflicting. ERs are expressed in a wide variety of tissues including vascular endothelium and smooth muscle and even the lung; however, few studies have examined ER expression specifically within ASM, especially in humans. We have shown for the first time that both full-length ERα and ERβ are present in ASM of female patients and that activation of these receptors results in reduction of agonist-induced elevations in [Ca2+]i. These novel data set the foundation for examining dysregulation of ER signaling as a potential mechanism underlying sex differences in airway diseases such as asthma.
[Ca2+]i Regulation in ASM
Airway tone reflects a balance between bronchoconstriction and bronchodilation. A key element of airway tone is ASM contractility, which, in turn, is determined to large extent by [Ca2+]i. Elevation of [Ca2+]i in ASM following bronchoconstrictor stimulation involves both SR Ca2+ release and plasma membrane Ca2+ influx (49). Subsequent decrease in [Ca2+]i and maintenance of Ca2+ homeostasis involve SR Ca2+ reuptake and plasma membrane efflux. In this regard, the role of both SR and plasma membrane in [Ca2+]i responses to histamine are well-recognized. Ca2+ influx occurs in ASM through both voltage-gated (57) and receptor-gated (38) channels. Ca2+ influx may also be indirectly modulated via alterations in plasma membrane potential, mediated via mechanisms such as iberiotoxin-sensitive Ca2+-activated K+ channels, although its contribution may be species-dependent (9, 50). In addition, recent studies, including our own, have demonstrated controlled Ca2+ influx in response to SR Ca2+ depletion (SOCE; Refs. 1, 42). SOCE involves members of the TRP channel including TRP canonical (TRPC) 1, 3, 4, 5, and 6 (10, 55). In addition, members of the TRP vanilloid (TRPV) family (ROC) can also contribute to Ca2+ influx in ASM [e.g., with changing osmolarity (12, 23)]. Furthermore, some studies suggest a role for NCX in ASM (21). Accordingly, multiple [Ca2+]i regulatory targets exist in human ASM for estrogens. The results of the present study indicate that E2 indeed inhibits several mechanisms but almost exclusively at the plasma membrane.
Estrogen Signaling
In humans, E2 is the most potent ER agonist. Two full-length ERs of similar estrogen binding affinities are of significance: ERα and ERβ both belonging to the nuclear receptor family of transcription factors (34). The role of “truncated” or shorter ERα isoforms (ERα-46 and ERα-36) that lack activation factor AF-1 is not clear (19). Accordingly, in the present study, we focused on the full-length ERs.
ERs are expressed in a wide variety of tissues such as vascular endothelium and smooth muscle. However, few studies have examined ER expression within the airway, especially in humans. ERα and ERβ are expressed in human bronchial epithelial cells (22), however, expression in ASM or innervation in vivo is not known. Again, based on perimenstrual fluctuations in asthma symptoms, ER expression is likely. In a recent study using gene and protein profiling techniques, Catley et al. (7) detected ERβ in human ASM cells in vitro exposed to the cytokine IL-1β. The present study is the first to directly demonstrate ER expression within ASM of bronchi of female patients. Although we did not limit selection of patients by age, hormone status, or airway disease, our findings of significant expression of both ERα and ERβ along with truncated isoforms suggest that ERs are likely expressed across these different groups. Growing ASM cells for 48–72 h also maintained full-length ER expression, consistent with the findings of Catley et al. (7).
The well-known transcriptional (genomic) response to estrogens is complex and cell-specific, depending (at least) on estrogen concentration and duration of exposure, coregulatory proteins, promoters in estrogen-responsive genes, active genes, and ERβ-ERα interactions (19). Given these complexities and the relative lack of knowledge on estrogen signaling in the airway, we did not address the issue of prolonged estrogen exposure in ASM exposure or genomic signaling.
In other tissues, rapid effects of E2 (seconds to minutes) have been demonstrated (30, 36). It is not entirely clear whether nongenomic ER signaling involves the same intracellular/nuclear receptors as in genomic effects, but the observed effects are dependent on E2 concentration. Accordingly, in the present study, we initially selected a range of E2 concentrations that spans the physiological range (1–10 nM during menses).
Estrogen, ERs, and effects on smooth muscle.
In the vasculature, acute estrogen exposure leads to blunting of agonist-induced [Ca2+]i responses (34). In comparison, relatively little is known about estrogen effects on [Ca2+]i in the airway. ERα-deficient mice exhibit increased airway hyperresponsiveness (6), and hormone replacement in ovariectomized animals attenuates allergen-induced asthma (13), suggesting that estrogens contribute to bronchodilation (and/or decreased inflammation), consistent with our hypothesis. However, the role of estrogen signaling may be species- and model-specific, and the underlying mechanisms need further investigation. A single study in mouse tracheal and bronchial rings sensitized by serum from asthmatic humans demonstrated reductions in carbachol-induced contractions by 30-min exposure to 100 nM E2 (14). Our study demonstrates that even 1 nM E2 can acutely and substantially reduce [Ca2+]i responses of human ASM cells to agonists such as histamine. These novel data strongly support the idea of acute, nongenomic bronchodilatory effects of estrogens in the human airway.
Varying concentrations of E2 produced a nonclassic dose-response curve in human ASM for [Ca2+]i effects. Although the underlying mechanisms are not clear, other studies have noted the antagonizing effects of ERβ activation on estrogen effects, mostly in the context of breast cancer, ERα-dependent transcription, and ERα degradation (19, 56). Given the likely complex role of ERα vs. ERβ signaling, specific signaling mechanisms were not examined. Regardless, what is important to note is that significant reductions in [Ca2+]i were observed in the physiological range of E2 concentrations.
Previous work by Montgomery et al. (36) and Levin (30) in vascular smooth muscle have tested the efficacy of selective ER agonists on relaxation compared with the nonspecific E2. In their work, the ERα agonist 4,4′,4″-(4-propyl-[1H]-pyrazole-1,3,5-triyl) trisphenol (PPT) was used. (R,R)-THC was selected as the ERα-specific agonist in our study because it also acts as an ERβ antagonist and has similar binding affinity for ERα as PPT. We found that even low concentrations of ERα-specific agonist produced substantial reduction in [Ca2+]i, mimicking effects of the nonspecific E2, whereas an ERβ agonist was less effective. These data also complement the findings of Montgomery et al. (36) that PPT was more effective than E2 and DPN at relaxing mesenteric arteries, whereas DPN was less effective than both PPT and E2. Therefore, it is possible that although both isoforms are present in human ASM, nongenomic effects may be mediated via only ERα.
There are very little data on estrogen effects on Ca2+ influx in the airway. The present study found that E2 effects on [Ca2+]i were blunted by inhibiting l-type channels (nifedipine). In isolated mouse tracheal or bronchial rings, Dimitropoulou et al. (14) found that estrogen effects are partly inhibited by blocking BKCa channels (similar to vasculature; Ref. 48). However, in human ASM, we were unable to detect any effect of iberiotoxin on estrogen-induced reduction of [Ca2+]i. There are no data on estrogen modulation of SOCE in the airway. During these studies, we noted that on introduction of E2 when [Ca2+]i levels were elevated by the SERCA inhibitor CPA, there was an abrupt reduction in [Ca2+]i, raising the possibility of accelerated NCX-mediated efflux. This effect was abolished in KB-R7943-treated cells, however, KB-R7943 had little effect on E2 modulation of SOCE-mediated influx per se, suggesting that even if NCX was present in human ASM, estrogens may not specifically inhibit this mechanism.
As mentioned above, SOCE-mediated Ca2+ influx occurs in ASM and involves TRPC channels. Although estrogens do not appear to modulate TRP channel expression (17), there is no information on interactions between ER activation and TRP (specifically TRPC) channels in terms of [Ca2+]i regulation. In the present study, pretreatment with SKF-96365, a potent inhibitor of receptor-operated channels, did not affect the observed E2 effect on SOCE-mediated Ca2+ influx, indicating that the effect of estrogens is specifically on SOCE rather than other influx mechanisms not involving TRPC.
Studies in vascular smooth muscle (27) have found no evidence for estrogen effects on SR Ca2+ reuptake, a potential mechanism for reducing [Ca2+]i. Ovariectomy or prolonged changes in estrogen levels do alter SERCA expression but not activity (4). In the present study, we also did not find any observable impact of acute E2 exposure on SR Ca2+ reuptake.
Physiological and Clinical Relevance
The present study demonstrates that in human ASM, functional ERs exist and that estrogens acutely (likely in a nongenomic fashion) reduce agonist-induced [Ca2+]i levels, akin to the well-known effects in the vasculature. Such estrogen effects appear to involve inhibition of Ca2+ influx via different mechanisms. The relevance of these findings lie in the potential modulation of airway tone by ER signaling, or its dysregulation in airway disease in women. In this regard, further study is required to understand how estrogens inhibit Ca2+ influx. In other tissues, estrogens can increase cAMP (54), which produces bronchodilation by targeting plasma membrane mechanisms including influx channels. Whether estrogens modulate cAMP or other pathways involved in bronchodilation is a potentially clinically relevant question that will be examined in future studies.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL-088029 (Y. S. Prakash) and HL-090595 (C. M. Pabelick) and by the Flight Attendant Medical Research Institute (FAMRI; Y. S. Prakash). E. A. Townsend is a graduate student in Y. S. Prakash's laboratory and is supported by the Mayo Graduate School.
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
We acknowledge the contribution of Dr. Stephen Cassivi (Dept. of Surgery, Mayo Clinic) to this project.
REFERENCES
- 1.Ay B, Prakash YS, Pabelick CM, Sieck GC. Store-operated Ca2+ entry in porcine airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 286: L909–L917, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Becklake MR, Kauffmann F. Gender differences in airway behaviour over the human life span. Thorax 54: 1119–1138, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellia V, Augugliaro G. Asthma and menopause. Monaldi Arch Chest Dis 67: 125–127, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Bupha-Intr T, Wattanapermpool J. Regulatory role of ovarian sex hormones in calcium uptake activity of cardiac sarcoplasmic reticulum. Am J Physiol Heart Circ Physiol 291: H1101–H1108, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Card JW, Carey MA, Bradbury JA, DeGraff LM, Morgan DL, Moorman MP, Flake GP, Zeldin DC. Gender differences in murine airway responsiveness and lipopolysaccharide-induced inflammation. J Immunol 177: 621–630, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carey MA, Card JW, Bradbury JA, Moorman MP, Haykal-Coates N, Gavett SH, Graves JP, Walker VR, Flake GP, Voltz JW, Zhu D, Jacobs ER, Dakhama A, Larsen GL, Loader JE, Gelfand EW, Germolec DR, Korach KS, Zeldin DC. Spontaneous airway hyperresponsiveness in estrogen receptor-alpha-deficient mice. Am J Respir Crit Care Med 175: 126–135, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Catley MC, Birrell MA, Hardaker EL, de Alba J, Farrow S, Haj-Yahia S, Belvisi MG. Estrogen receptor beta: expression profile and possible anti-inflammatory role in disease. J Pharmacol Exp Ther 326: 83–88, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Chhabra SK. Premenstrual asthma. Indian J Chest Dis Allied Sci 47: 109–116, 2005 [PubMed] [Google Scholar]
- 9.Corompt E, Bessard G, Lantuejoul S, Naline E, Advenier C, Devillier P. Inhibitory effects of large Ca2+-activated K+ channel blockers on beta-adrenergic- and NO-donor-mediated relaxations of human and guinea-pig airway smooth muscles. Naunyn Schmiedebergs Arch Pharmacol 357: 77–86, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Corteling RL, Li S, Giddings J, Westwick J, Poll C, Hall IP. Expression of transient receptor potential C6 and related transient receptor potential family members in human airway smooth muscle and lung tissue. Am J Respir Cell Mol Biol 30: 145–154, 2004 [DOI] [PubMed] [Google Scholar]
- 11.de Marco R, Locatelli F, Sunyer J, Burney P. Differences in incidence of reported asthma related to age in men and women. A retrospective analysis of the data of the European Respiratory Health Survey. Am J Respir Crit Care Med 162: 68–74, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Dietrich A, Chubanov V, Kalwa H, Rost BR, Gudermann T. Cation channels of the transient receptor potential superfamily: their role in physiological and pathophysiological processes of smooth muscle cells. Pharmacol Ther 112: 744–760, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Dimitropoulou C, Drakopanagiotakis F, Chatterjee A, Snead C, Catravas JD. Estrogen replacement therapy prevents airway dysfunction in a murine model of allergen-induced asthma. Lung 187: 116–127, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Dimitropoulou C, White RE, Ownby DR, Catravas JD. Estrogen reduces carbachol-induced constriction of asthmatic airways by stimulating large-conductance voltage and calcium-dependent potassium channels. Am J Respir Cell Mol Biol 32: 239–247, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Figtree GA, McDonald D, Watkins H, Channon KM. Truncated estrogen receptor α 46-kDa isoform in human endothelial cells: relationship to acute activation of nitric oxide synthase. Circulation 107: 120–126, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Gibbs CJ, Coutts II, Lock R, Finnegan OC, White RJ. Premenstrual exacerbation of asthma. Thorax 39: 833–836, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guilbert A, Dhennin-Duthille I, Hiani YE, Haren N, Khorsi H, Sevestre H, Ahidouch A, Ouadid-Ahidouch H. Expression of TRPC6 channels in human epithelial breast cancer cells. BMC Cancer 8: 125–135, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanley SP. Asthma variation with menstruation. Br J Dis Chest 75: 306–308, 1981 [DOI] [PubMed] [Google Scholar]
- 19.Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Strom A, Treuter E, Warner M, Gustafsson JA. Estrogen receptors: how do they signal and what are their targets. Physiol Rev 87: 905–931, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Hellings PW, Vandekerckhove P, Claeys R, Billen J, Kasran A, Ceuppens JL. Progesterone increases airway eosinophilia and hyper-responsiveness in a murine model of allergic asthma. Clin Exp Allergy 33: 1457–1463, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Hirota S, Janssen LJ. Store-refilling involves both l-type calcium channels and reverse-mode sodium-calcium exchange in airway smooth muscle. Eur Respir J 30: 269–278, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Ivanova MM, Mazhawidza W, Dougherty SM, Minna JD, Klinge CM. Activity and intracellular location of estrogen receptors alpha and beta in human bronchial epithelial cells. Mol Cell Endocrinol 305: 12–21, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jia Y, Wang X, Varty L, Rizzo CA, Yang R, Correll CC, Phelps PT, Egan RW, Hey JA. Functional TRPV4 channels are expressed in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 287: L272–L278, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Juniper EF, Daniel EE, Roberts RS, Kline PA, Hargreave FE, Newhouse MT. Improvement in airway responsiveness and asthma severity during pregnancy. A prospective study. Am Rev Respir Dis 140: 924–931, 1989 [DOI] [PubMed] [Google Scholar]
- 25.Kellner J, Tantzscher J, Oelmez H, Edelmann M, Fischer R, Huber RM, Bergner A. Mechanisms altering airway smooth muscle cell Ca+ homeostasis in two asthma models. Respiration 76: 205–215, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Kim KH, Moriarty K, Bender JR. Vascular cell signaling by membrane estrogen receptors. Steroids 73: 864–869, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kitazawa T, Hamada E, Kitazawa K, Gaznabi AK. Non-genomic mechanism of 17 beta-oestradiol-induced inhibition of contraction in mammalian vascular smooth muscle. J Physiol 499: 497–511, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kitazawa T, Somlyo AP. Modulation of Ca2+ sensitivity by agonists in smooth muscle. Adv Exp Med Biol 304: 97–109, 1991 [DOI] [PubMed] [Google Scholar]
- 29.Kwon HL, Triche EW, Belanger K, Bracken MB. The epidemiology of asthma during pregnancy: prevalence, diagnosis, and symptoms. Immunol Allergy Clin North Am 26: 29–62, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Levin ER. Cell localization, physiology, and nongenomic actions of estrogen receptors. J Appl Physiol 91: 1860–1867, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Matsubara S, Swasey CH, Loader JE, Dakhama A, Joetham A, Ohnishi H, Balhorn A, Miyahara N, Takeda K, Gelfand EW. Estrogen determines sex differences in airway responsiveness after allergen exposure. Am J Respir Cell Mol Biol 38: 501–508, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Melgert BN, Ray A, Hylkema MN, Timens W, Postma DS. Are there reasons why adult asthma is more common in females? Curr Allergy Asthma Rep 7: 143–150, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Meyers MJ, Sun J, Carlson KE, Marriner GA, Katzenellenbogen BS, Katzenellenbogen JA. Estrogen receptor-beta potency-selective ligands: structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J Med Chem 44: 4230–4251, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Miller VM, Duckles SP. Vascular actions of estrogens: functional implications. Pharmacol Rev 60: 210–241, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mollerup S, Jorgensen K, Berge G, Haugen A. Expression of estrogen receptors alpha and beta in human lung tissue and cell lines. Lung Cancer 37: 153–159, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Montgomery S, Shaw L, Pantelides N, Taggart M, Austin C. Acute effects of oestrogen receptor subtype-specific agonists on vascular contractility. Br J Pharmacol 139: 1249–1253, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murphy VE, Gibson PG. Premenstrual asthma: prevalence, cycle-to-cycle variability and relationship to oral contraceptive use and menstrual symptoms. J Asthma 45: 696–704, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Murray RK, Kotlikoff MI. Receptor-activated calcium influx in human airway smooth muscle cells. J Physiol 435: 123–144, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pabelick CM, Ay B, Prakash YS, Sieck GC. Effects of volatile anesthetics on store-operated Ca2+ influx in airway smooth muscle. Anesthesiology 101: 373–380, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Pabelick CM, Jones KA, Street K, Lorenz RR, Warner DO. Calcium concentration-dependent mechanisms through which ketamine relaxes canine airway smooth muscle. Anesthesiology 86: 1104–1111, 1997 [DOI] [PubMed] [Google Scholar]
- 41.Paoletti P, Carrozzi L, Viegi G, Modena P, Ballerin L, Di Pede F, Grado L, Baldacci S, Pedreschi M, Vellutini M. Distribution of bronchial responsiveness in a general population: effect of sex, age, smoking, and level of pulmonary function. Am J Respir Crit Care Med 151: 1770–1777, 1995 [DOI] [PubMed] [Google Scholar]
- 42.Parekh AB, Penner R. Store depletion and calcium influx. Physiol Rev 77: 901–930, 1997 [DOI] [PubMed] [Google Scholar]
- 43.Pelaia G, Renda T, Gallelli L, Vatrella A, Busceti MT, Agati S, Caputi M, Cazzola M, Maselli R, Marsico SA. Molecular mechanisms underlying airway smooth muscle contraction and proliferation: implications for asthma. Respir Med 102: 1173–1181, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Perusquia M, Hernandez R, Montano LM, Villalon CM, Campos MG. Inhibitory effect of sex steroids on guinea-pig airway smooth muscle contractions. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol 118: 5–10, 1997 [DOI] [PubMed] [Google Scholar]
- 45.Postma DS. Gender differences in asthma development and progression. Gend Med 4: S133–S146, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Prakash YS, Kannan MS, Sieck GC. Regulation of intracellular calcium oscillations in porcine tracheal smooth muscle cells. Am J Physiol Cell Physiol 272: C966–C975, 1997 [DOI] [PubMed] [Google Scholar]
- 47.Prakash YS, Thompson MA, Vaa B, Matabdin I, Peterson TE, He T, Pabelick CM. Caveolins and intracellular calcium regulation in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 293: L1118–L1126, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Salom JB, Burguete MC, Perez-Asensio FJ, Torregrosa G, Alborch E. Relaxant effects of 17-beta-estradiol in cerebral arteries through Ca2+ entry inhibition. J Cereb Blood Flow Metab 21: 422–429, 2001 [DOI] [PubMed] [Google Scholar]
- 49.Sanders KM. Mechanisms of calcium handling in smooth muscles. J Appl Physiol 91: 1438–1449, 2001 [DOI] [PubMed] [Google Scholar]
- 50.Sanderson MJ, Delmotte P, Bai Y, Perez-Zogbhi JF. Regulation of airway smooth muscle cell contractility by Ca2+ signaling and sensitivity. Proc Am Thorac Soc 5: 23–31, 2008 [DOI] [PubMed] [Google Scholar]
- 51.Schatz M, Dombrowski MP. Asthma in pregnancy. N Engl J Med 360: 1862–1869, 2009 [DOI] [PubMed] [Google Scholar]
- 52.Stanford KI, Mickleborough TD, Ray S, Lindley MR, Koceja DM, Stager JM. Influence of menstrual cycle phase on pulmonary function in asthmatic athletes. Eur J Appl Physiol 96: 703–710, 2006 [DOI] [PubMed] [Google Scholar]
- 53.Sun J, Meyers MJ, Fink BE, Rajendran R, Katzenellenbogen JA, Katzenellenbogen BS. Novel ligands that function as selective estrogens or antiestrogens for estrogen receptor-alpha or estrogen receptor-beta. Endocrinology 140: 800–804, 1999 [DOI] [PubMed] [Google Scholar]
- 54.Teoh H, Man RY. Enhanced relaxation of porcine coronary arteries after acute exposure to a physiological level of 17beta-estradiol involves non-genomic mechanisms and the cyclic AMP cascade. Br J Pharmacol 129: 1739–1747, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.White TA, Xue A, Chini EN, Thompson M, Sieck GC, Wylam ME. Role of transient receptor potential C3 in TNF-alpha-enhanced calcium influx in human airway myocytes. Am J Respir Cell Mol Biol 35: 243–251, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Williams C, Edvardsson K, Lewandowski SA, Strom A, Gustafsson JA. A genome-wide study of the repressive effects of estrogen receptor beta on estrogen receptor alpha signaling in breast cancer cells. Oncogene 27: 1019–1032, 2008 [DOI] [PubMed] [Google Scholar]
- 57.Worley JF, 3rd, Kotlikoff MI. Dihydropyridine-sensitive single calcium channels in airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 259: L468–L480, 1990. [DOI] [PubMed] [Google Scholar]