Abstract
Collagen VII anchoring fibrils in the basement membrane zone (BMZ) are part of a supracellular anchoring network that attaches the epithelium to the BMZ. Sloughing of airway epithelium in asthmatics (creola bodies) is a pathology associated with the supracellular anchoring network. In a rhesus monkey model of house dust mite (HDM)-induced allergic asthma, we found increased deposition of collagen I in the BMZ. In this study, we determine whether HDM also affected deposition of collagen VII in the BMZ. In the developing airway of rhesus monkeys, the width of collagen VII anchoring fibrils in the BMZ was 0.02 ± 0.04 μm at 1 mo of age. At 6 mo the width had increased to 1.28 ± 0.34 μm and at 12 mo 2.15 ± 0.13 μm. In animals treated with HDM, we found a 42.2% reduction in the width of collagen VII layer in the BMZ at 6 mo (0.74 ± 0.15 μm; P < 0.05). During recovery, the rate of collagen VII deposition returned to normal. However, the amount of collagen VII lost was not recovered after 6 mo. We concluded that normal development of the collagen VII attachment between the epithelium and BMZ occurs in coordination with development of the BMZ. However, in HDM-treated animals, the collagen VII attachment with the epithelium was significantly reduced. Such a reduction in collagen VII may weaken the supracellular anchoring network and be associated with sloughing of the epithelium and formation of creola bodies in asthmatics.
Keywords: supracellular anchoring network, intermediate filaments, hemidesmosomes, desmosomes
the airway basement membrane zone (BMZ) is the central component of the epithelial mesenchymal trophic unit (13). The BMZ consists of three anatomic regions, the lamina (l.) lucida, l. densa, and l. reticularis. The structural framework of the l. reticularis consists of types I, III, and V collagen (10). They form heterogeneous fibers that account for the thickness of the lamina reticularis (10). These fibers are not randomly arranged but instead appear as a mat of fibers oriented along the longitudinal axis of the airway that is distinct from the rest of the ECM (14, 20). A function of the BMZ is related to attachment of the epithelium with the ECM.
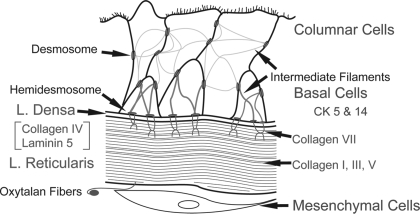
Collagen VII anchoring fibrils are a component of the BMZ. They are an integral part of the supracellular anchoring network that attaches the epithelium to the ECM. This network consists of intermediate filaments of columnar and basal cells connected together with desmosomes. The intermediate filaments of basal cells (cytokeratin 5 and 14) are then attached to hemidesmosomes, which are attached to the l. densa by laminin 5. Collagen VII anchoring fibrils attach to laminin 5 in the l. densa, and collagen I fibers in the l. reticularis to complete the network of epithelial attachment (22, 26). The l. reticularis is attached to the ECM by oxytalan fibers of the elastic lamina interacting with collagen fibers in the l. reticularis (2, 16). Through these junctional connections, adjacent epithelial cells are connected forming a supracellular anchoring network that is connected with the ECM (Fig. 1). In the airways, the amount of junctional adhesion in the supracellular network is related to the mass of the epithelium. In a tall epithelium, there are more desmosomes, hemidesmosomes, and associated intermediate filaments, and the BMZ is wider than in airways with shorter epithelium (6, 7).
Fig. 1.
Illustration of the supracellular anchoring system connecting the epithelium with the basement membrane zone (BMZ) and the ECM. Intermediate filaments of columnar and basal cells are connected together with desmosomes. Intermediate filaments of basal cells [cytokeratins (CK) 5 and 14] connected to desmosomes are also attached to hemidesmosomes. Hemidesmosomes are attached to the lamina (l.) densa by laminin 5. Collagen VII anchoring fibrils attach to laminin 5 in the l. densa, and collagen I fibers in the l. reticularis. The l. reticularis is attached to the ECM by oxytalan fibers of the elastic lamina.
Sloughing of airway epithelium is a pathology associated with the supracellular anchoring network. In asthmatics, the sloughed patches of epithelium are termed creola bodies (15). They are made up of columnar airway cells connected to each other with desmosomes and appear as palisades of cells in the microscope. Formation of creola bodies occurs when the desmosome attachments between columnar and basal cells are broken (17). The cause of epithelial sloughing is thought to be an influx of inflammatory cells (28). An influx of inflammatory cells is common to a number of lung diseases, but the production of creola bodies is unique to asthma (15). This characteristic suggests that lesions within the anchoring system in the airway epithelium of asthmatics may be associated with sloughing of epithelium and formation of creola bodies.
We (9, 21) have been studying development of the BMZ in a rhesus monkey model of allergic airway disease that mimics the asthma phenotype. We (10) found that the l. reticularis of the BMZ develops postnatally in the rhesus monkey. Development appears in the BMZ as an increase in width of the collagen fiber layer and associated proteoglycans from 1 to 12 mo of age. In the experimental groups treated with a regimen of house dust mite (HDM) to induce allergic airway disease, there is an increase in the width of the BMZ due to enhanced production of collagens I, III, and V. When these animals are allowed to recover in air for 6 mo, the BMZ maintains the increase in width, suggesting that developmental change in the collagen framework of the BMZ due to HDM persists. In the present study, we determined whether development of collagen VII anchoring fibrils was also affected by the HDM regimen. We found that development of the collagen VII anchoring fibrils was significantly reduced in the HDM-treated animals. Also, the initial loss of collagen VII fibrils was not replaced after 6 mo of recovery in air.
MATERIALS AND METHODS
The data obtained in this study came from tissue samples of previous studies. These studies concerned development of the BMZ (10), the effects of HDM on development of the BMZ (12), and recovery of the BMZ from the effects of HDM (8). In this paper, we are studying development of the collagen VII anchoring fibrils in the BMZ, the effects of HDM on their development, and ability of collagen VII anchoring fibrils to recover from the effects of HDM. To summarize the previous experimental protocols, the developmental study involved infant rhesus monkeys (30 days old) raised in filtered air (FA). Animals were each euthanized at 1, 3, 6, and 12 mo of age in these studies. The HDM-treated group involved infant rhesus monkeys (30 days old) exposed to a regimen of HDM for 5 mo. After 2 and 5 mo of treatment, animals each were euthanized to determine the effects of HDM on development. In the recovery studies, animals treated with HDM for 5 mo were allowed to recover in FA for 6 mo before euthanization to determine whether the effects were reversible or persisted (8). The exposure protocol involved infant rhesus monkeys (30 days old) exposed to 11 episodes of either FA or HDM (5 days each followed by 9 days of FA). The HDM monkeys were sensitized to HDM before exposure (Dermatophagoides farinae) at age 14 and 28 days by subcutaneous inoculation of HDM in aluminum and intramuscular injection of heat-killed Bordetella pertussis cells. HDM sensitization was confirmed via skin testing with subcutaneous HDM on day 38 of the exposure protocol. Sensitized monkeys were exposed to HDM aerosol for 2 h/day. After the exposure protocol, they were allowed to recover in FA for 6 mo. The HDM group was reexposed to HDM aerosol for 2 h at monthly intervals during recovery to maintain sensitization for pulmonary function testing. At the end of the 6th month, they were challenged with HDM aerosol for 2 h/day for 3 days, followed by pulmonary function testing. It should be noted that this modified recovery protocol is not the same as a true FA recovery protocol.
Monkeys were euthanized with an overdose of pentobarbital after being sedated with Telazol (8 mg/kg im) and anesthetized with Diprivan (0.1–0.2 mg·kg−1·min−1 iv). Monkeys were then necropsied following exsanguination through the abdominal aorta, and the lungs were prepared for analysis as previously described (23). Tracheal samples were sliced into rings perpendicular to the long axis of the airway. Tracheal slices were fixed in 1.0% paraformaldehyde for 1 h and embedded in paraffin. For routine histology, 5-μm sections were stained with hematoxylin and eosin (H&E). For immunohistochemistry, 5-μm sections were deparaffinized in xylene, rehydrated through an ethanol series, and washed in PBS. For collagen VII, sections were treated with pepsin (1.0 mg pepsin/ml 3.0% acetic acid) at 37°C for 2 h, blocked with bovine serum albumin, and incubated with antibody to collagen I (1:250; rabbit anti-human polyclonal antibody; Biogenesis, Kingston, NH) or collagen VII (1:1,000; rabbit anti-human polyclonal antibody; Biogenesis) overnight at 4°C. Following immunohistochemistry, the sections were washed in PBS, treated with the secondary antibody (1:1,000; Alexa Fluor 568; Molecular Probes, Eugene, OR) for 30 min, and washed in PBS, and the coverslip was mounted in fluorescent safe media (GelMount). Fluorescence was visualized on an Olympus BH2 fluorescent microscope. The antibodies for collagens I and VII had negligible cross-reactivity with other collagen or noncollagen matrix proteins (per supplier).
Quantitation.
The width of the collagen VII band in the BMZ was measured morphometrically with the Scion Image program version 4.0.3 (Beta release). In a previous report using human biopsy samples, it was demonstrated that 31–45 measurements at least 20 μm apart and covering 1,000-μm BMZ are necessary to give an accuracy of ±15.0% (24). In this study, 8 micrographs were taken equidistant apart around the circumference of the tracheal preparations per animal. The width of the collagen VII band was measured at 4 points, 50 μm apart, on each micrograph. A total of 1,600 μm of BMZ was sampled in each tracheal preparation in this manner. An observer blinded to the study groups made the measurements. The average width of the collagen VII band was determined from these measurements for each animal. Measurements of tracheal diameters were made from the epithelial surface of the long and short diameters of the tracheal rings stained with H&E, and the average diameter was determined. The height of the epithelium was measured on these same sections. The data are presented as means ± SD. The differences between treatment groups were compared with the Mann-Whitney rank sum test with significance set at P < 0.05.
All monkeys selected for these studies were colony-born rhesus macaques (Macaca mulatta) from the California Regional Primate Research Center at the University of California, Davis. Care and housing of animals before, during, and after treatment complied with the provisions of the Institute of Laboratory Animal Resources and conformed to practices established by the American Association for Accreditation of Laboratory Animal Care. Animal protocols were reviewed by the University of California, Davis, Institutional Animal Use and Care Committee.
RESULTS
Physical characteristics of the growing trachea.
The circumference of the trachea increased from 8.5 ± 0.9 mm at 1 mo of age to 13.3 ± 1.5 mm at 12 mo. The height of the columnar epithelium also increased from 44.0 ± 4.2 μm at 1 mo of age to 54.5 ± 4.2 μm at 12 mo. The measurements in the HDM group were similar (Table 1). Figure 2 is a routine histological preparation of the tracheal epithelium. The epithelium is morphologically differentiated at 1 mo of age. The BMZ appears as an amorphous layer beneath the epithelium (Ref. 10; Fig. 2). Using immunohistochemistry for collagen I, the l. reticularis of the BMZ becomes distinct, appearing as a dense layer under the epithelium (Fig. 3). Collagen VII immunoreactivity appears as a thin layer in the epithelial region of the l. reticularis (Fig. 4). In this study, we measured the width of the collagen VII immunoreactivity to determine changes in development of collagen VII anchoring fibrils (Table 1).
Table 1.
Width of the collagen VII layer of the BMZ
| Treatment | Age, mo | n | Collagen VII, μm | Height, μm | Circumference, mm |
|---|---|---|---|---|---|
| Filtered air (FA) | 1 | 3 | 0.02 ± 0.04 | 44.0 ± 4.2 | 8.5 ± 0.9 |
| 3 | 6 | 0.61 ± 0.12 | 45.0 ± 5.2 | 9.1 ± 0.3 | |
| 6 | 6 | 1.28 ± 0.34* | 49.3 ± 5.2 | 11.3 ± 0.9 | |
| 12 | 4 | 2.15 ± 0.13† | 54.5 ± 4.2 | 13.3 ± 1.5 | |
| House dust mite | 3 | 6 | 0.48 ± 0.20 | 43.6 ± 3.9 | 9.4 ± 0.5 |
| 6 | 6 | 0.74 ± 0.15‡ | 47.3 ± 2.6 | 11.6 ± 0.6 | |
| Recovery in FA | 12 | 4 | 1.68 ± 0.11§ | 58.0 ± 6.6 | 13.2 ± 2.1 |
Values are means ± SD.
Greater than 3 mo, P < 0.05.
Greater than 6 mo, P < 0.05.
Less than 6-mo FA, P < 0.05.
Less than 12-mo FA, P < 0.05. BMZ, basement membrane zone.
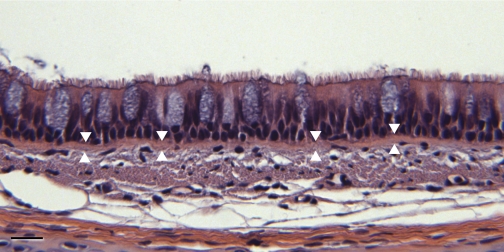
Fig. 2.
Light micrograph of the tracheal epithelium in a 3-mo-old animal. The average height of the epithelium increases as the animals get older. The increases in average height correlates with the increase in the circumference of the trachea in the growing animals up to 12 mo (Table 1). The BMZ is not distinct in standard light microscope preparations stained with hematoxylin and eosin. It is marked with arrowheads. Bar: 10 μm.
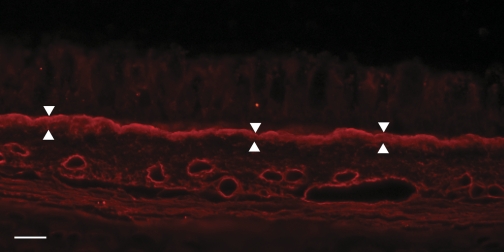
Fig. 3.
Immunohistochemistry of the collagen I in a 3-mo-old animal demonstrating the density and width of the BMZ (arrowheads). The width of the BMZ is related to its role in attaching the epithelium with the ECM. Collagen VII is found in the epithelial surface of the BMZ. Bar: 10 μm.
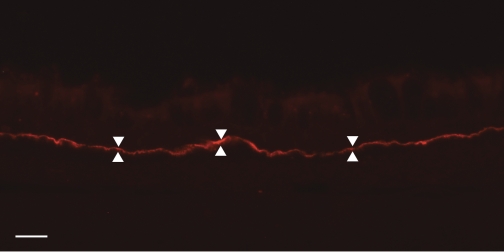
Fig. 4.
Immunohistochemistry of the collagen VII layer of anchoring fibrils on the epithelial interface of the BMZ in a 3-mo-old animal (arrowheads). Preparations such as this were used to measure the width of the collagen VII layer. Bar: 10 μm.
Development of collagen VII anchoring fibrils.
At 1 mo, the BMZ is beginning to develop. At this time, there are focal areas of collagen VII on the epithelial surface of the BMZ. The average width of the collagen VII layer was 0.02 ± 0.04 μm. At 3 mo, there is a continuous layer of collagen VII in the epithelial surface of the BMZ. The average width of the collagen VII layer was 0.61 ± 0.12 μm. At 6 mo, the width of the collagen VII layer had increased to 1.28 ± 0.34 μm, and at 12 mo 2.15 ± 0.13 μm. The absolute change in width was significantly greater between the 3- and 6-mo-old animals, 0.67 μm (P < 0.05), and also between the 6- and 12-mo-old animals, 0.87 μm (P < 0.05). This change represents normal growth of collagen VII anchoring fibrils in the BMZ.
The effect of HDM on development of collagen VII anchoring fibrils.
After 2 mo of the HDM protocol, the width of the collagen VII layer was 0.48 ± 0.20 μm. After 5 mo of the protocol, the width of the collagen VII layer has increased to 0.74 ± 0.15 μm. The change in width of the collagen VII layer during this 3-mo period was 0.26 μm. Compared with the change of 0.67 μm in the FA group, these data indicate that development of the collagen VII layer was reduced during the HDM protocol. This resulted in a significant difference in width of the collagen VII layer at 6 mo between the FA and HDM groups (0.54 μm; P < 0.05).
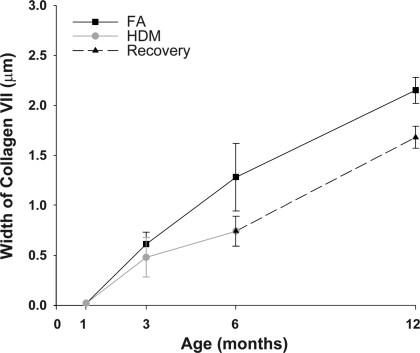
During the recovery period, the collagen VII layer in the HDM group increased from 0.74 ± 0.15 μm at 6 mo to 1.68 ± 0.11 μm at 12 mo (Fig. 5). Comparing the FA and HDM groups shows that during recovery the normal rate of collagen VII anchoring fibril deposition in the BMZ returned in the HDM group. However, the collagen VII layer was still significantly less than the FA group. Compared with the absolute change in width of the collagen VII layer at 6 mo, there was an initial decrease of 0.54 μm on the HDM protocol. After recovery in FA for 6 mo, the absolute width collagen VII layer in the HDM group was still significantly less than the FA group (0.47 μm; P < 0.05). These data indicate that the decreased deposition of collagen VII during the HDM protocol was not replaced during the recovery period of 6 mo (Fig. 5).
Fig. 5.
Comparing the filtered air (FA) and house dust mite (HDM) groups shows that during recovery the rate of collagen VII anchoring fibril growth returned to normal in the HDM group. However, the collagen VII layer was still significantly less than the FA group. Compared with the absolute change in width of the collagen VII layer, there was an initial decrease of 0.54 μm at 6 mo on the HDM protocol. After recovery in FA for 6 mo, the difference in width collagen VII layer was 0.47 μm, indicating that the amount of collagen VII lost during the HDM protocol had not been replaced.
DISCUSSION
The purpose of this study was to determine whether development of the collagen VII anchoring fibril component of the BMZ was affected in a model of allergic asthma. During normal postnatal development of the rhesus monkey, collagen VII anchoring fibrils are just beginning to appear at 1 mo of age. At 3 mo of age, a layer of collagen VII anchoring fibrils is present in the BMZ, and the fibrils continued to increase in width up to 12 mo of age. The increase in width of the collagen VII anchoring fibrils was related to the increase in width of the epithelium (Table 1). This change is related to the role of collagen VII in attachment of the epithelium to the ECM. A similar relationship was demonstrated previously between increasing epithelial width and increases in the supracellular anchoring network (desmosomes, hemidesmosomes, and intermediate filaments) in different animal species and airway levels and in the growing trachea (6, 7). In general, as the volume of the epithelium increases, the amount of junctional adhesion present in the supracellular anchoring network also increases.
In animals treated with HDM, we found that development of collagen VII anchoring fibrils was reduced. In the group treated for 5 mo, there was a significant reduction in the width of collagen VII layer in the BMZ (42.2%). During recovery, the rate of collagen VII deposition returned to normal, however, the amount collagen VII originally lost (0.54 μm) was not replaced (Fig. 5). These results demonstrate a lesion (reduced deposition of collagen VII) in the supracellular anchoring network in this model of allergic asthma.
Reduction of collagen VII is associated with sloughing of epithelium in several diseases. The decrease we found in the layer of collagen VII fibrils is similar to that reported for recessive dystrophic epidermolysis bullosa and epidermolysis bullosa acquisita. Both are subepidermal blistering diseases of the skin specific for collagen VII anchoring fibrils and are reported to also affect mucous membranes in the airways (25, 27). The two diseases are etiologically unrelated. Recessive dystrophic epidermolysis bullosa is a genetic disease involving the gene that encodes collagen VII, and epidermolysis bullosa acquisita is an acquired disease involving autoimmunity toward collagen VII. Both diseases share the common feature of decreased or absent collagen VII anchoring fibrils in the BMZ and sloughing of epithelium from the BMZ (4). In allergic asthma, sloughing of epithelium and the formation of creola bodies is associated with a plane of cleavage between basal cells and columnar cells, not basal cells and the BMZ (17). Thus the reduction in collagen VII we found is not directly related to sloughing of the epithelium. However, it does point out a lesion in the supracellular anchoring network.
Previous studies have shown there is a close relationship between the growth of the epithelium and development of the supracellular anchoring network in the airways (desmosomes, associated intermediate filaments, and hemidesmosomes; Refs. 6, 7, 11). This close relationship suggests that a lesion in one component of the supracellular anchoring network may affect development of the whole network. In human allergic asthmatics, two lesions of this network have been reported. Shahana et al. (23) showed that desmosomes between epithelial columnar and basal cells are significantly smaller in allergic asthmatics, and Amin et al. (1) showed laminin 5 molecules in the BMZ are assembled in an uncoordinated manner. Abnormal assembly of laminin 5 could be the primary lesion in the supracellular anchoring network associated with cell sloughing. Laminin 5 is a nucleation point for attachment with the α6β4-integrin of hemidesmosomes and collagen VII in the BMZ (22). The atypical formation of laminin 5 molecules could limit the attachment sites for α6β4-integrin in hemidesmosomes and collagen VII in the BMZ. In support of this concept, incomplete formation of laminin 5 during development is associated with abnormal development of hemidesmosomes (18). It is not known whether alterations in laminin 5 formation effect development of collagen VII anchoring fibrils. However, it is clear that laminin 5 plays a pivotal role in connecting the epithelium to the BMZ.
In the present study of a primate model of allergic asthma, reduction in collagen VII anchoring fibrils was demonstrated during postnatal development. It is not known whether the reduction in collagen VII persists past 1 yr or whether development of other components in the supracellular anchoring network were effected. The basal cell is central to producing components of the supracellular anchoring network of the airway epithelium. Desmosomal proteins, cytokeratins 5 and 14, hemidesmosomal proteins, laminin 5 and collagen VII, are all produced by basal cells. The transcription factor p63, found only in basal cells (5), regulates production of many molecules making up the supracellular anchoring network (3, 19). Future research directed toward studying regulation of development of the supracellular anchoring network at the molecular level in basal cells may expand on these findings.
In summary, we show that HDM can reduce postnatal development of collagen VII fibrils in the supracellular anchoring network of airway epithelium of the monkey. We also found the reduction in collagen VII anchoring fibrils was not recovered during 6-mo recovery in air. At this point, it is not clear whether or how a reduction in collagen VII is related to epithelial sloughing and the formation of creola bodies. However, it is clear that such a reduction in collagen VII anchoring fibrils would lead to an increased fragility of the epithelial supracellular anchoring network in the airways.
GRANTS
This study was supported by National Institute of Environmental Health Sciences Grants P01-ES-00628, P01-ES-11617, and P01-ES-06700 and National Center for Research Resources Grant RR-000169.
DISCLOSURES
No conflicts of interest are declared by the author(s).
REFERENCES
- 1.Amin K, Janson C, Seveus L, Miyazaki K, Virtanen I, Venge P. Uncoordinated production of Laminin-5 chains in airways epithelium of allergic asthmatics. Respir Res 6: 110, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bock P, Stockinger L. Light and electron microscopic identification of elastic, elaunin and oxytalan fibers in human tracheal and bronchial mucosa. Anat Embryol (Berl) 170: 145–153, 1984 [DOI] [PubMed] [Google Scholar]
- 3.Carroll DK, Brugge JS, Attardi LD. p63, cell adhesion and survival. Cell Cycle 6: 255–261, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Chen M, Doostan A, Bandyopadhyay P, Remington J, Wang X, Hou Y, Liu Z, Woodley DT. The cartilage matrix protein subdomain of type VII collagen is pathogenic for epidermolysis bullosa acquisita. Am J Pathol 170: 2009–2018, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chilosi M, Doglioni C. Constitutive p63 expression in airway basal cells. A molecular target in diffuse lung diseases. Sarcoidosis Vasc Diffuse Lung Dis 18: 23–26, 2001 [PubMed] [Google Scholar]
- 6.Evans MJ, Cox RA, Burke AS, Moller PC. Differentiation of anchoring junctions in tracheal basal cells in the growing rat. Am J Respir Cell Mol Biol 6: 153–157, 1992 [DOI] [PubMed] [Google Scholar]
- 7.Evans MJ, Cox RA, Shami SG, Plopper CG. Junctional adhesion mechanisms in airway basal cells. Am J Respir Cell Mol Biol 3: 341–347, 1990 [DOI] [PubMed] [Google Scholar]
- 8.Evans MJ, Fanucchi MV, Baker GL, Van Winkle LS, Pantle LM, Nishio SJ, Schelegle ES, Gershwin LJ, Miller LA, Hyde DM, Plopper CG. The remodelled tracheal basement membrane zone of infant rhesus monkeys after 6 months of recovery. Clin Exp Allergy 34: 1131–1136, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Evans MJ, Fanucchi MV, Plopper CG. The basement membrane in asthma. Curr Respir Med Rev 2: 331–337, 2006 [Google Scholar]
- 10.Evans MJ, Fanucchi MV, Van Winkle LS, Baker GL, Murphy AE, Nishio SJ, Sannes PL, Plopper CG. Fibroblast growth factor-2 during postnatal development of the tracheal basement membrane zone. Am J Physiol Lung Cell Mol Physiol 283: L1263–L1270, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Evans MJ, Moller PC. Biology of airway basal cells. Exp Lung Res 17: 513–531, 1991 [DOI] [PubMed] [Google Scholar]
- 12.Evans MJ, Van Winkle LS, Fanucchi MV, Baker GL, Murphy AE, Nishio SJ, Schelegle ES, Gershwin LJ, Sannes PL, Plopper CG. Fibroblast growth factor-2 in remodeling of the developing basement membrane zone in the trachea of infant rhesus monkeys sensitized and challenged with allergen. Lab Invest 82: 1747–1754, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Evans MJ, Van Winkle LS, Fanucchi MV, Plopper CG. The attenuated fibroblast sheath of the respiratory tract epithelial-mesenchymal trophic unit. Am J Respir Cell Mol Biol 21: 655–657, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Evans MJ, Van Winkle LS, Fanucchi MV, Toskala E, Luck EC, Sannes PL, Plopper CG. Three-dimensional organization of the lamina reticularis in the rat tracheal basement membrane zone. Am J Respir Cell Mol Biol 22: 393–397, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Laucirica R, Ostrowski ML. Cytology of nonneoplastic occupational and environmental diseases of the lung and pleura. Arch Pathol Lab Med 131: 1700–1708, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Mauad T, Xavier AC, Saldiva PH, Dolhnikoff M. Elastosis and fragmentation of fibers of the elastic system in fatal asthma. Am J Respir Crit Care Med 160: 968–975, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Montefort S, Roberts JA, Beasley R, Holgate ST, Roche WR. The site of disruption of the bronchial epithelium in asthmatic and non-asthmatic subjects. Thorax 47: 499–503, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen NM, Pulkkinen L, Schlueter JA, Meneguzzi G, Uitto J, Senior RM. Lung development in laminin gamma2 deficiency: abnormal tracheal hemidesmosomes with normal branching morphogenesis and epithelial differentiation. Respir Res 7: 28, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Romano RA, Ortt K, Birkaya B, Smalley K, Sinha S. An active role of the DeltaN isoform of p63 in regulating basal keratin genes K5 and K14 and directing epidermal cell fate. PLoS One 4: e5623, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saglani S, Molyneux C, Gong H, Rogers A, Malmstrom K, Pelkonen A, Makela M, Adelroth E, Bush A, Payne DN, Jeffery PK. Ultrastructure of the reticular basement membrane in asthmatic adults, children and infants. Eur Respir J 28: 505–512, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Schelegle ES, Gershwin LJ, Miller LA, Fanucchi MV, Van Winkle LS, Gerriets JP, Walby WF, Omlor AM, Buckpitt AR, Tarkington BK, Wong VJ, Joad JP, Pinkerton KB, Wu R, Evans MJ, Hyde DM, Plopper CG. Allergic asthma induced in rhesus monkeys by house dust mite (Dermatophagoides farinae). Am J Pathol 158: 333–341, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schneider H, Muhle C, Pacho F. Biological function of laminin-5 and pathogenic impact of its deficiency. Eur J Cell Biol 86: 701–717, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Shahana S, Bjornsson E, Ludviksdottir D, Janson C, Nettelbladt O, Venge P, Roomans GM. Ultrastructure of bronchial biopsies from patients with allergic and non-allergic asthma. Respir Med 99: 429–443, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Sullivan P, Stephens D, Ansari T, Costello J, Jeffery P. Variation in the measurements of basement membrane thickness and inflammatory cell number in bronchial biopsies. Eur Respir J 12: 811–815, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Trebing D, Ziemer A. [Acquired epidermolysis bullosa with a highly varied clinical picture and successful treatment with mycophenolate mofetil.] Hautarzt 52: 717–721, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Villone D, Fritsch A, Koch M, Bruckner-Tuderman L, Hansen U, Bruckner P. Supramolecular interactions in the dermo-epidermal junction zone: anchoring fibril-collagen VII tightly binds to banded collagen fibrils. J Biol Chem 283: 24506–24513, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woodley DT, Remington J, Chen M. Autoimmunity to type VII collagen: epidermolysis bullosa acquisita. Clin Rev Allergy Immuno 33: 78–84, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Yoshihara S, Yamada Y, Abe T, Lindén A, Arisaka O. Association of epithelial damage and signs of neutrophil mobilization in the airways during acute exacerbations of paediatric asthma. Clin Exp Immunol 144: 212–216, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]