Abstract
The forearm vasodilator response to mental stress is multifactorial and widely variable among individuals. We evaluated the association between the heart rate and forearm vascular conductance (FVC) responses to a color word test in 101 healthy adults. We found a striking correlation between heart rate and FVC (r = 0.66, p < 0.001), which remained significant when controlling for subject characteristics, blood pressure, and catecholamines. This suggests that the mechanical stimulation is one of the key factors that contribute to the increase in FVC during mental stress.
Synopsis
Our major new finding is that there is a strong correlation between the increase in heart rate and the increase in forearm vascular conductance during mental stress.
Keywords: Mental stress, forearm vascular conductance, heart rate, catecholamines
Introduction
The forearm vasodilator response to mental stress is governed by activation of the autonomic nervous system and complex integration with the mechanisms controlling the forearm vasculature. The forearm vasodilator response is widely variable among individuals. Because of the multifactorial control of forearm blood flow (FBF) during mental stress, reasons behind the interindividual variability are difficult to establish.
The heart rate response to mental stress is mediated by a combination of parasympathetic withdrawal and sympathetic activation. This is relevant to forearm vasodilation because the increase in FBF is blunted by systemic administration of atropine and beta-blockers, and in patients who have undergone adrenalectomy [1, 7]. In this context, the FBF response to mental stress may be largely dependent on the heart rate response. Additionally, the mechanical effects of increased flow on the forearm vasculature might serve to augment vasodilation, demonstrated by an increase in forearm vascular conductance (FVC), which is the reciprocal of forearm vascular resistance (FVR) and proportional to the fourth power of vessel radius.
Taken together, these ideas suggest an overall importance of heart rate on the contribution to forearm vasodilation during mental stress. The purpose of this report was to test the hypothesis that during mental stress, the increase in heart rate would be correlated with the increase in FVC. We also determined whether heart rate is an independent predictor of FVC, controlling for other potential contributors such as the pressor response, catecholamine response, and subject characteristics.
Methods
Subjects
This study is one component of an ongoing comprehensive study evaluating the relationships between blood pressure regulation and adrenergic receptor gene variation [13]. A group of 101 normally active, lean, normotensive, non-smoking healthy adults on no chronic medications participated in the study (ages 18-40 yr, 67 females, 34 males). Means ± SD of age, height, weight, and BMI of the participants were 26.5 years ± 6.6 years, 170.3 cm ± 8.4 cm, 70 kg ± 11 kg, and 24 ± 2.2 kg/m2 respectively. Female subjects were studied during the low hormone phase of the menstrual cycle or oral contraceptive use. All subjects fasted for 6 hours prior to study, which was performed between 7 am and 3 pm in the Mayo Clinical Research Unit. All participants gave their written informed consent to participate in the study. The study protocol was approved by the Mayo Institutional Review Board.
Instrumentation and Measurements
Subjects underwent brachial artery cannulation in the non-dominant arm using aseptic technique after local anesthesia (2% lidocaine) for blood pressure and catecholamine measurements. A 3-lead electrocardiogram was used to measure heart rate, and FBF was measured with venous occlusion plethysmography. A computerized version of the Stroop colored word test was administered to induce mental stress. Heart rate, blood pressure, and FBF were continuously collected throughout the mental stress protocol. Forearm vascular conductance was calculated as FBF/mean arterial pressure (FBF/MAP) X 100 and expressed as arbitrary units, whereas FVR was calculated as (MAP/FBF). Arterial blood samples were obtained for determination of plasma epinephrine and norepinephrine concentrations via high performance liquid chromatography.
Experimental protocol
Following instrumentation, subjects rested quietly for 15 minutes. In the final minute, arterial blood was drawn for catecholamine determination and these values were considered baseline. As part of the larger protocol, subjects underwent head-up tilt and baroreflex testing (modified Oxford) over approximately 1.5 hr (data not shown). Subjects were transferred to a recumbent chair and seated quietly for 25 minutes. All subjects performed the mental stress at the same point in the overall protocol. Following 2 min of resting heart rate, blood pressure, and FBF recording, identical printed instructions were read aloud to each subject lasting 30 sec. The instructions reminded subjects that their best effort was required and that their performance on the test would be compared to the other subjects. The Stroop test lasted for 3 min, and to maximize the stress induced, the computer program voiced distracting colors, while one investigator voiced consistent monologue urging each subject to respond faster and to concentrate fully throughout the test. During the final 30 sec of mental stress, arterial blood was drawn for catecholamines. Hemodynamic and forearm data were averaged over the 3 min mental stress test and compared to averages in the 2 min pre-stress control period. To investigate the influence of perceived stress on physiologic variables, the final 27 participants completed a psychological distress survey immediately after the task, modified from a mental arithmetic stress survey by Reims and colleagues, consisting of 3 questions: 1) Did you feel stressed while performing the color word task? (i.e., “perceived stress”); 2) Was it important for you to obtain a good result on the color word task? 3) How did you experience the task altogether? [14]. In response to each question, subjects marked one of 10 unnumbered boxes between extremes of least to highest degrees of perceived stress, effort, and overall discomfort, respectively.
Statistical analysis
Paired t-tests were used to determine changes in hemodynamics and catecholamines from baseline to mental stress. Simple linear regression analysis was used to obtain the association between the change in FBF or FVC and the change in heart rate, MAP, and catecholamines. Simple linear regression was also used for comparing the change in catecholamines with the change in hemodynamics. Multivariate linear regression was performed to determine the independent variables influencing the change in FVC. A P < 0.05 was accepted as statistically significant. Unless indicated otherwise, data is presented as mean ± SE.
Results
Table 1 displays the averages of measured variables during the pre-stress baseline, and during mental stress. Mental stress elicited significant changes in hemodynamics, catecholamines, and forearm values. There was a robust correlation between the increase in heart rate and the forearm vasodilator response to mental stress. This association was consistent when comparing heart rate to either the increase in FBF (r = 0.70, P < 0.001), or the increase in FVC as shown in Fig. 1 (r = 0.66, P < 0.001). The increase in heart rate was also negatively correlated to the change in FVR (r = -0.39, P < 0.001).
Table 1.
Mean ± SE values at baseline and during mental stress
| Variable | Baseline | Mental Stress |
|---|---|---|
| Heart Rate (bpm) | 64.7 ± 0.9 | 86.5 ± 1.3* |
| Systolic Blood Pressure (mmHg) | 123.3 ± 1.1 | 137.2 ± 1.2* |
| Diastolic Blood Pressure (mmHg) | 60.2 ± 0.7 | 71.6 ± 0.7* |
| Mean Arterial Pressure (mmHg) | 81.9 ± 0.7 | 96.5 ± 0.9* |
| Forearm Blood Flow (ml/dl tissue/min) | 2.4 ± 0.1 | 5.8 ± 0.3* |
| Forearm Vascular Conductance (units) | 2.9 ± 0.1 | 5.9 ± 0.3* |
| Forearm Vasc. Resistance (MAP/FBF) | 42.0 ± 1.6 | 22.7 ± 1.1* |
| Epinephrine (pg/ml) | 34.1 ± 3.7 | 83.6 ± 6.0* |
| Norepinephrine (pg/ml) | 116.6 ± 5.3 | 196.7 ± 6.9* |
P < 0.05 vs. baseline. FVC was calculated as [(FBF/MAP) X 100] and expressed as arbitrary units.
Figure 1.
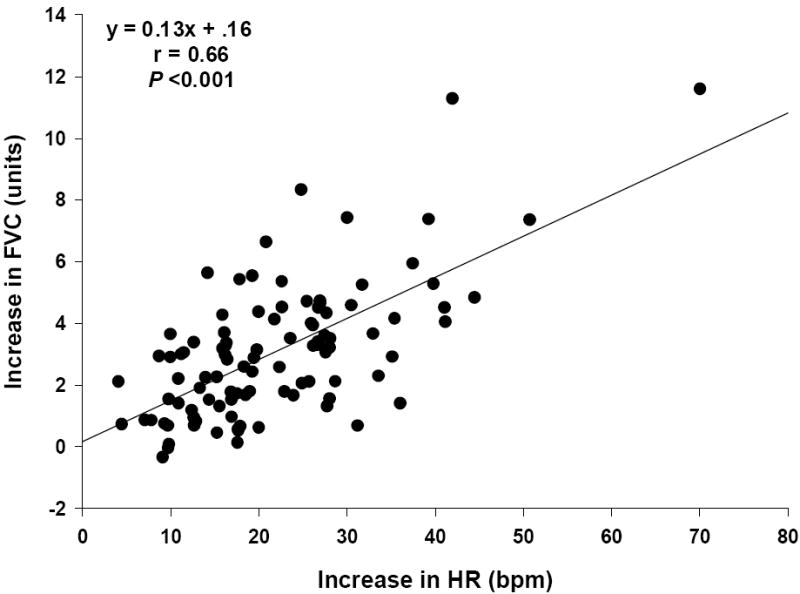
The increase in forearm vascular conductance (FVC) and HR from pre-stress baseline to mental stress. A significant positive correlation was found between delta FVC and delta HR.
With the increase in heart rate and the increase in FVC as continuous variables, all subjects were ranked by their heart rate response to mental stress from lowest to highest, and divided into three equal groups (tertiles), so that approximately 33% of the total heart rate observations were present in each group. Between groups, there were no differences in gender ratio, age, BMI, and resting blood pressure. As shown in Fig. 2, FVC increased by 1.9 ± 0.2, 2.9 ± 0.3, and 4.4 ± 0.4 units in the low, middle, and high responders, respectively (PANOVA < 0.001).
Figure 2.
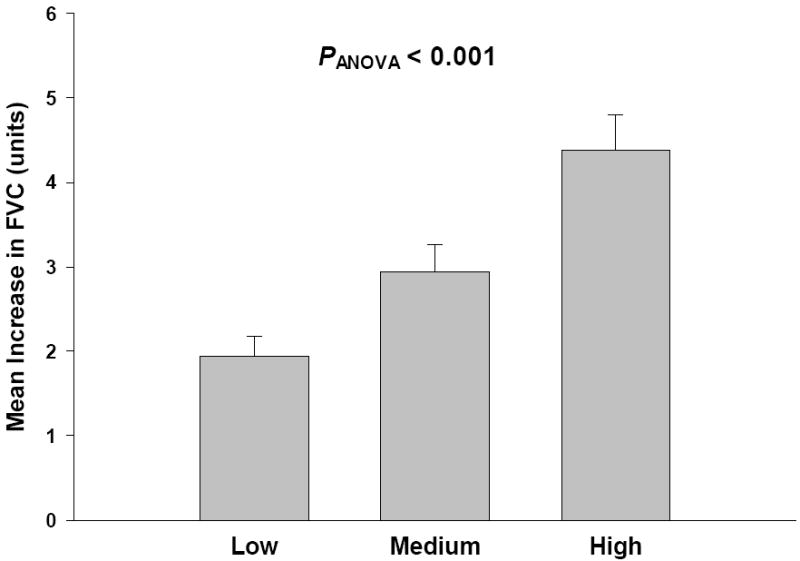
The mean ± SE increase in forearm vascular conductance (FVC) with the study participants grouped according to tertiles based on the ranked HR response to mental stress (low ΔHR < 16.3 bpm, n = 34; medium ΔHR = 16.3 − 26.1 bpm, n = 34; high ΔHR > 26.1 bpm, n = 33; PANOVA < 0.001)). There were no differences in clinical characteristics between groups.
Significant correlations existed between the increase in epinephrine and the increase in FVC (r = 0.42, P < 0.001), and the increase in norepinephrine and the increase in FVC (r = 0.40, P < 0.001). However, in a multiple linear regression model, with the increases in heart rate, epinephrine, norepinephrine, and MAP included as explanatory variables, heart rate was the only variable found to be significantly associated with the increase in FVC (P < 0.001). After adjusting for HR, none of the other explanatory variables were significant (P > 0.25 for all). Additionally, to examine the possible influence of cardiovascular risk factors on the forearm vascular response, a multiple linear regression model was constructed with age, gender, BMI, resting blood pressure, and the increase in heart rate as explanatory variables. The heart rate response was significantly associated with the increase in FVC (P < 0.0001), while gender, BMI, and resting blood pressure were not associated with FVC (P > 0.3 for all). In this model, age had a positive association with the increase in conductance (P = 0.01) but this effect was not significant in a linear regression between the two variables (r = 0.18, P = 0.07).
We also compared the catecholamine responses to the hemodynamic responses. The increase in epinephrine was correlated with the increase in heart rate (r = 0.55, P < 0.001), and weakly associated with the increase in systolic blood pressure (r = 0.27, P = 0.01) but not MAP or diastolic blood pressure. The increase in norepinephrine was also correlated to the increase in heart rate (r = 0.37, P < 0.001), but none of the blood pressure variables.
From the subset of individuals who completed the psychological distress survey, the mean cumulative score was 21.7 ± 0.6 and the mean perceived stress component was 7.7 ± 0.2. The perceived stress component was positively correlated with the increase in heart rate (r = 0.43, P = 0.02), and with the increase in FVC (r = 0.41, P = 0.03). When perceived stress was included in a multiple linear regression model with age, gender, BMI, resting blood pressure, hemodynamic and catecholamine changes, and the increase in heart rate as explanatory variables, heart rate was the only variable found to be significantly associated with the increase in FVC (P = 0.04).
Discussion
To our knowledge, this is the first report to demonstrate that the increase in FVC during mental stress is strongly correlated with the heart rate response. Generally accepted mechanisms evoking forearm vasodilation during mental stress include a combination of unchanged or decreased forearm muscle sympathetic nerve activity (MSNA), β2-adrenergic receptor mediated vasodilator effects from circulating epinephrine, and mechanical factors causing local release of nitric oxide [8]. The present findings suggest that the tachycardia response to mental stress has substantial influence on vascular conductance, an effect that is likely related to mechanical stimulation on the forearm vasculature. While the increases in blood pressure and arterial catecholamines were correlated with FVC by simple regression, the significance of these variables was nullified in a multivariate regression, leaving the increase in heart rate as the key independent variable. Moreover, this relationship remained significant when controlling for clinical risk factors for cardiovascular disease, including age, gender, BMI, and resting blood pressure. Finally, the degree of perceived stress in a subset of individuals was positively correlated with the heart rate and FVC responses, but the significance of perceived stress on FVC was also nullified in multivariate regression.
In addition to parasympathetic withdrawal, sympathetic activation during mental stress increases adrenomedullary release of epinephrine and total body spillover of norepinephrine into the circulation which determines the hemodynamic responses [4], including an increase in cardiac output which is largely dependent on heart rate [12]. Our correlations between the changes in arterial catecholamines and heart rate are consistent with these findings. Additionally, circulating epinephrine evokes beta adrenergic-receptor mediated forearm vasodilation that is thought to be responsible for up to 30% of the FBF response [10], and this effect is blunted by intra-arterial administration of beta blockers [6, 11]. The relationship between arterial epinephrine and FVC in this study was no longer significant after controlling for heart rate, consistent with a larger role for heart rate on FVC than regional beta-receptor stimulation.
With regard to neural control of FVC during mental stress, one study showed that MSNA in the arm decreases in relation to the increase in FVC, suggesting that individuals with the greatest sympathetic withdrawal also had the greatest vasodilation [6]. However, when regional sympathetic traffic is interrupted with local anesthetic, the forearm vasodilator response is preserved, and the hemodynamic and catecholamine responses are preserved [6] or even augmented [9]. Taken together, an interesting consideration would be whether high heart rate responders have a greater arm sympathetic withdrawal (and greater FVC) when sympathetic innervation is intact, or that high heart rate responders display greater FVC when sympathetic innervation is disrupted. The latter would suggest a greater role of hemodynamics, catecholamines, and local vasodilator responses. Importantly, another study measuring arm and leg MSNA simultaneously showed no change in MSNA in either limb, and MSNA was not correlated to flow or conductance in either limb [2]; however, the relationship between heart rate and the interindividual nerve traffic and FVC responses was not explored.
Local control of blood flow in the forearm vasculature remains a major focus of investigation. Green and colleagues demonstrated the importance of oscillatory antegrade plus retrograde flow patterns on shear-stress mediated release of nitric oxide from the endothelium that was associated with changes in heart rate and systolic blood pressure [5]. Regional nitric oxide synthase inhibition blunts the forearm vasodilator response to mental stress [3]. Therefore, our findings may be consistent with the general idea that the increase in cardiac output (via heart rate) increases perfusion pressure and pulsatile flow to the arm, which presumably increases shear stress and induces a dose-dependent vasodilation. Moreover, a component of both beta receptor-mediated vasodilation and cholinergic receptor-mediated dilation is through nitric oxide. As cholinergic vasodilator nerves are not thought to exist in humans, the interaction between local vasodilator mechanisms, pulsatile flow, and flow-mediated dilation during MS requires further elucidation.
Our findings may be limited by the wide age range of the cohort, which may have included individuals with and without cardiovascular risk factors that were not screened for, such as hypercholesterolemia. Although the increase in heart rate remained independently associated with FVC when adjusting for the available clinical data, we cannot fully exclude the possibility that all subjects were fully free of cardiovascular risk or endothelial dysfunction.
Conclusion
The forearm vasodilator response to mental stress is due to a constellation of hemodynamic, hormonal, neurogenic, and local vascular regulatory factors. There is a robust correlation between the increase in heart rate and the increase in FVC. This suggests a substantial role in the mechanical effects of the heart rate in response to mental stress, suggesting that flow-mediated dilation may be the major contributor to forearm conductance.
Acknowledgments
The authors thank Drs. Michael Joyner and Timothy Curry for medical assistance. We also thank Pamela Engrav and Cara Fernandez for recruitment and scheduling. Also, special thanks to all the participants. This study was supported by K23 RR17520, HL-089331, NS 32352, NS 90530056/5J4205, NIH/NCRR and NIH Roadmap for Medical Research 1 KL2 RR024151-01.
Footnotes
Disclosures: None
References
- 1.Barcroft H, Brod J, Hejl BZ, Hirsjarvi EA, Kitchin AH. The mechanism of the vasodilatation in the forearm muscle during stress (mental arithmetic) Clin Sci. 1960;19:577–586. [PubMed] [Google Scholar]
- 2.Carter JR, Kupiers NT, Ray CA. Neurovascular responses to mental stress. J Physiol. 2005;564:321–327. doi: 10.1113/jphysiol.2004.079665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dietz NM, Rivera JM, Eggener SE, Fix RT, Warner DO, Joyner MJ. Nitric oxide contributes to the rise in forearm blood flow during mental stress in humans. J Physiol. 1994;480:361–368. doi: 10.1113/jphysiol.1994.sp020366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldstein DS, Eisenhofer G, Sax FL, Keiser HR, Kopin IJ. Plasma norepinephrine pharmacokinetics during mental challenge. Psychosom Med. 1987;49:591–605. doi: 10.1097/00006842-198711000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Green DJ, Bilsborough W, Naylor LH, Reed C, Wright J, O’Driscoll G, Walsh JH. Comparison of forearm blood flow responses to incremental handgrip and cycle ergometer exercise: relative contribution of nitric oxide. J Physiol. 2005;562:617–628. doi: 10.1113/jphysiol.2004.075929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Halliwill JR, Lawler LA, Eickhoff TJ, Dietz NM, Nauss LA, Joyner MJ. Forearm sympathetic withdrawal and vasodilatation during mental stress in humans. J Physiol. 1997;504:211–220. doi: 10.1111/j.1469-7793.1997.211bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Houben H, Thien T, de Boo T, Lemmens W, Binkhorst R, van’t Laar A. Hemodynamic effects of isometric exercise and mental arithmetic in hypertension treated with selective and nonselective beta-blockade. Clin Pharmacol Ther. 1983;34:164–169. doi: 10.1038/clpt.1983.147. [DOI] [PubMed] [Google Scholar]
- 8.Joyner MJ, Halliwill JR. Sympathetic vasodilatation in human limbs. J Physiol. 2000;526:471–480. [PubMed] [Google Scholar]
- 9.Lindqvist M, Davidsson S, Hjemdahl P, Melcher A. Sustained forearm vasodilation in humans during mental stress is not neurogenically mediated. Acta Physiol Scand. 1996;158:7–14. doi: 10.1046/j.1365-201X.1996.529288000.x. [DOI] [PubMed] [Google Scholar]
- 10.Lindqvist M, Kahan T, Melcher A, Bie P, Hjemdahl P. Forearm vasodilator mechanisms during mental stress: possible roles for epinephrine and ANP. Am J Physiol. 1996;270:E393–399. doi: 10.1152/ajpendo.1996.270.3.E393. [DOI] [PubMed] [Google Scholar]
- 11.Lindqvist M, Melcher A, Hjemdahl P. Attenuation of forearm vasodilator responses to mental stress by regional beta-blockade, but not by atropine. Acta Physiol Scand. 1997;161:135–140. doi: 10.1046/j.1365-201X.1997.00192.x. [DOI] [PubMed] [Google Scholar]
- 12.Lindqvist M, Melcher A, Hjemdahl P. Hemodynamic and sympathoadrenal responses to mental stress during nitric oxide synthesis inhibition. Am J Physiol Heart Circ Physiol. 2004;287:H2309–2315. doi: 10.1152/ajpheart.01216.2003. [DOI] [PubMed] [Google Scholar]
- 13.Ramirez-Marrero FA, Charkoudian N, Liu Z, Hesse C, Eisenach JH. Balance between sympathetic response to head-up tilt and cardiac vagal factors in healthy humans. Clin Auton Res. 2007;17:227–230. doi: 10.1007/s10286-007-0427-y. [DOI] [PubMed] [Google Scholar]
- 14.Reims HM, Sevre K, Fossum E, Hoieggen A, Eide I, Kjeldsen SE. Plasma catecholamines, blood pressure responses and perceived stress during mental arithmetic stress in young men. Blood Press. 2004;13:287–294. doi: 10.1080/08037050410016474. [DOI] [PubMed] [Google Scholar]


