Abstract
Objective
Compare the efficacy and adverse effects of CDB-2914, a new progesterone receptor modulator, to levonorgestrel for emergency contraception.
Methods
We performed a randomized, double-blinded noninferiority trial, enrolling healthy women seeking emergency contraception within 72 hours of unprotected intercourse. Participants were randomly assigned to receive a single dose of 50 mg of CDB-2914, plus a placebo 12 hours later or two doses of 0.75 mg of levonorgestrel taken 12 hours apart. Follow-up was scheduled 5 to 7 days after the expected onset of the next menstrual period. Posttreatment pregnancy was established by a positive urine test at follow-up and confirmed by quantitative serum β-hCG. Daily diaries were used from the time of emergency contraception use until next menses to record adverse effects and sexual activity.
Results
Product efficacy was evaluable in 775 of CDB-2914 users and 774 of levonorgestrel users. Pregnancies occurred in 7 (0.9%, 95% confidence interval 0.2–1.6%) and 13 (1.7%, 95% confidence interval 0.8–2.6%) women, respectively. Based on the estimated cycle day of unprotected intercourse, 85% and 69% of anticipated pregnancies, respectively, were averted. Nausea was reported by a somewhat greater percentage of CDB-2914 than levonorgestrel users (29% compared with 24%, P=.03), but the distribution of other adverse effects was similar in both groups. Women in both groups experienced considerable variation in menstrual cycle length as compared with their reported individual normal cycle lengths.
Conclusion
CDB-2914 is at least as effective as levonorgestrel in preventing pregnancies after unprotected intercourse and has a similar side effect profile.
Emergency contraception, by using a drug or device after unprotected intercourse, is an important means of preventing unwanted pregnancy.1,2 In the United States, where emergency contraception use is relatively low, approximately one half of the pregnancies are unintended and one half of these unintended pregnancies result in abortion.3 The Netherlands, in contrast, has a higher awareness and use of emergency contraception, which has been cited as one of the factors resulting in the very low rates of abortion and teenage pregnancy.4
Several approaches to emergency contraception have been described, including high-dose estrogens, danazol, intrauterine devices, oral contraceptives with estrogen and progestin (Yuzpe Method), a progestin alone (levonorgestrel), and an antiprogestin (mifepristone).1,5 The first dedicated emergency contraceptive product available in the U.S. was an estrogen-progestin combination (Preven, Barr Laboratories, Pomona, NY). More recently, the estrogen-progestin product has been phased out in favor of a more effective and better-tolerated progestin-only product containing levonorgestrel (Plan B, DuraMed Pharmaceuticals, Pomona, NY). Both products were indicated for use within 72 hours of unprotected intercourse with instructions to take an initial dose followed by a second dose 12 hours later. The progestin-only product has recently been shown to be equally as effective when both doses are taken simultaneously (single dose) and to maintain some efficacy through 120 hours.6,7
Progesterone receptor modulators offer another option for emergency contraception that also maintain effectiveness longer than 72 hours in a single-dose regimen.7–9 The potential for widespread availability of mifepristone as an emergency contraceptive is often limited for social or political reasons. However, CDB-2914 (17-acetoxy-11-(4-N, N-dimethylamino phenyl)-19-norpregna-4, 9-diene-3,20 dione) is a second-generation progesterone receptor modulator that has lower antiglucocorticoid activity than mifepristone, a first generation progesterone receptor modulator.10,11 This trial was developed with the hope that CDB-2914 could represent the next evolutionary step in emergency contraception treatment. This report summarizes our evaluation of the efficacy and adverse effects of CDB-2914 compared with levonorgestrel in a randomized, double-blinded noninferiority trial involving 1,672 healthy women seeking emergency contraception within 72 hours of unprotected intercourse. This study was initiated before data were available demonstrating the efficacy of the progestin-only method for emergency contraception both as a single-dose regimen and at intervals exceeding 72 hours from the act of unprotected intercourse.
Materials and Methods
This study was conducted in accordance with International Committee on Harmonization, Good Clinical Practices, Declaration of Helsinki, and U.S. Code of Federal Regulations and was approved by institutional review boards of each of the six participating institutions. The study centers included a consortium of family planning clinics in the Los Angeles, California area and five university-based clinical research centers. A signed informed consent was obtained from each potential participant after counseling and before enrollment.
Study participants were healthy women aged at least 18 years not using any hormonal contraception who requested emergency contraception within 72 hours after unprotected intercourse as a result of using no contraception, condom breakage or slippage, or failure of another barrier method. To be eligible for enrollment, they were required to have had a recent history of regular menstrual cycles (mean length of 24–42 days with intraindividual variation of ±5 days). At least one normal menstrual cycle (two menses) was required after delivery, abortion, or discontinuation of hormonal contraceptive (including depomedroxyprogesterone acetate; Depo-Provera, Pfizer, Morris Plains, NJ.).
Women were excluded who were pregnant at screening or enrollment (assessed by a high-sensitivity urine pregnancy test), pregnant or breastfeeding within the 2 months before screening, using an intrauterine device or female or male sterilization as a contraceptive method, uncertain about the date of the last menstrual period (±3 days), nauseated or vomited within the 2 weeks before screening, using oral glucocorticoid replacement therapy in the year before screening, or currently enrolled in any other investigational trial.
The study drug was supplied in sequentially numbered sealed packages containing two opaque capsules. The packages either contained a single opaque capsule with 50 mg of CDB-2914 plus an identical placebo capsule, or two opaque capsules, each with a tablet of 0.75 mg of levonorgestrel. The identification of the contents of the capsules was unknown to the investigators and the subjects. A portion of the label on each package of study drug was affixed to the case report form. This portion of the label had a removable opaque panel to allow for emergency unblinding. Once removed, these labels could not be replaced. Randomization was performed in blocks of eight such that, within each block of study drug, the chance of getting each treatment was equal. Study drug was supplied to each study site in these blocks of eight. The study site gave out study drug packages in numerical order.
At the screening visit, a high-sensitivity urine pregnancy test (level of detection, 25 milli-International Unit/mL) was performed and a blood sample was drawn before ingestion of the drug. The blood sample was centrifuged and the serum decanted and frozen at −70°C. If the woman's urine pregnancy test was negative, the next sequentially numbered envelope was then opened and the first dose of study drug taken in the office. The first capsule was to be used within 72 hours of unprotected intercourse followed by the second capsule 12 hours later. Participants recorded adverse effects daily from the time of use until next menses on a diary. Enrolled subjects agreed to abstain from further acts of unprotected intercourse until follow-up was complete. However, subjects were asked to record any such activity, if it did occur, and the contraceptive used on the diary. No hormonal or intrauterine contraception was allowed to be used until after follow-up was completed.
Each woman returned for a follow-up visit 5 to 7 days after the expected onset of her next menstrual period, based on her reported prior menstrual period and her typical menstrual cycle length. At this time, a high-sensitivity urine pregnancy test was performed. If the subject reported having a normal menstrual period since using the study medication and the urine pregnancy test was negative, she was considered not pregnant and her participation in the study was complete. If she had not had a normal menses and the urine pregnancy test was negative, she returned a week later for another urine pregnancy test. This procedure was repeated until the subject had a menstrual period and a negative urine pregnancy test or was diagnosed clinically as amenorrheic. If a urine pregnancy test was positive then a blood sample was drawn; the serums from this sample and the sample obtained at enrollment were assayed for quantitative β-hCG. A woman was considered pregnant if either a urine test or a serum test was positive for β-hCG. If the serum sample from the enrollment visit was positive, the pregnancy was classified as a pretreatment pregnancy. Confirmation of an intrauterine pregnancy and gestational age was determined by ultrasonography when possible.
The study protocol was designed as a noninferiority trial to test the hypothesis that CDB-2914 has a pregnancy rate no worse than that of levonorgestrel with a noninferiority margin (δ) of 2%. Using Black-welder's12 approach for equivalence testing, the null hypothesis is HO: πc ≥ πL + δ, where πc and πL represent the proportions of subjects who become pregnant during the menstrual cycle in which they received CDB-2914 or levonorgestrel, respectively. Utilizing πL=2%, δ=2%, a 2.5% level of significance (one-tailed), and 80% statistical power to reject the null hypothesis, the study goal was to enroll 770 subjects in each treatment group. We increased these numbers by 5%, to 811 per treatment arm, to allow for anticipated loss of participants to follow-up.
We planned the study with analyses of efficacy evaluable and modified intent-to-treat populations. The efficacy evaluable population included the women who were not pregnant at enrollment, took the study drug without additional emergency contraception during the treatment cycle, and met one of the following posttreatment criteria: had menses and a negative urine pregnancy test, verified amenorrheic with a negative pregnancy test, or verified pregnancy. The modified intent-to-treat population included all women who were randomly assigned and for whom a pregnancy outcome was known.
The primary outcome of pregnancy was evaluated by calculating the upper bound of the 97.5% one-tailed confidence interval (CI) for the difference in pregnancy probabilities between the two treatment groups. To assess noninferiority, we evaluated whether the upper bound of the one-tailed CI was less than our predetermined noninferiority margin of 2%. If the upper bound was less than 2%, then the null hypothesis was rejected, indicating that CDB-2914 was noninferior to levonorgestrel. Descriptive results, including frequency and proportion of subjects with a pregnancy by site, were similarly evaluated.
The probabilities of conception on each day of the cycle before ovulation, on the day of ovulation and after the day of ovulation have been estimated by Wilcox et al.13,14 A method's efficacy in preventing pregnancy based on a specific act of unprotected intercourse can be approximated using those probability estimates in conjunction with an estimate of the day of ovulation of a subject based on menstrual history. Calculations were performed for the number of observed and expected pregnancies in each treatment group using the efficacy evaluable population. These calculations were also stratified by 24-hour intervals since exposure (unprotected intercourse). The expected number of pregnancies was determined by using the estimated date of ovulation and the single-day pregnancy probabilities using the pooled recognized pregnancies.13 The effectiveness rate was calculated by 100 (expected–observed)/expected.
Descriptive statistics were used to summarize the number of pregnancies according to the proximity to the estimated day of ovulation. Pregnancy outcome was too infrequent to allow for exploration of covariates that potentially affect this outcome. We evaluated adverse effects by descriptive statistics. Logistic regression was used in separate models to assess nausea and vomiting; the final model retained site and body mass index as covariates.
Menstrual cycle length changes were calculated using a subject-reported average cycle length in the 6 months before being enrolled in the study and the observed cycle length while in the study. Differences in menstrual cycle length changes between the treatments were analyzed using an analysis of variance.
Results
From September 1999 to June 2001, 1,672 women were enrolled and randomly assigned, 832 to CDB-2914 and 840 to levonorgestrel (Fig. 1). The modified intent-to-treat population included 1,578 women: 792 in the CDB-2914 group and 786 in the levonorgestrel group. This population did not include the 94 women (40 CDB-2914, 54 levonorgestrel) who were lost to follow-up or had incomplete data. The efficacy evaluable population included 1,549 women: 775 in the CDB-2914 treatment group and 774 in the levonorgestrel treatment group. In addition to women who were designated lost to follow-up, subjects excluded from the efficacy evaluable population included 5 women (4 CDB-2914, 1 levonorgestrel) who had a pretreatment pregnancy and 24 women (13 CDB-2914, 11 levonorgestrel) who used additional emergency contraception during the treatment cycle.
Fig. 1.
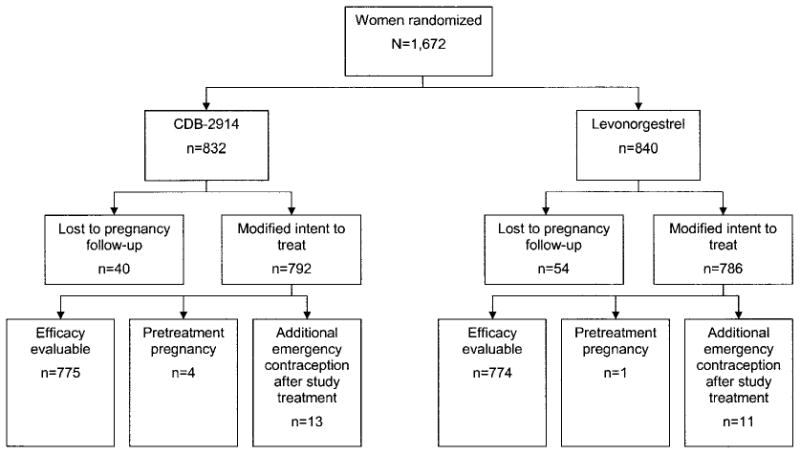
Trial profile. Women for whom a pregnancy outcome could not be evaluated (lost to follow-up) were excluded from the modified intent-to-treat population. Women who were pregnant before treatment (based on serum hCG levels) or who used additional emergency contraception during the treatment cycle were excluded from the efficacy evaluable population.
Creinin. CDB-2914 Emergency Contraception. Obstet Gynecol 2006.
Study participants were primarily young (mean age 24.3 years), white (73%), single (88%), not living with her partner (84%), and well educated (86% with some college or more). There were no notable differences between the characteristics of the treatment groups (Table 1).
Table 1.
Participant Characteristics
| CDB-2914 Users (n = 775) |
Levonorgestrel Users (n = 774) |
|
|---|---|---|
| Age (years at randomization) | ||
| 18–19 | 19 | 21 |
| 20–24 | 42 | 42 |
| 25–29 | 22 | 20 |
| 30–34 | 10 | 10 |
| 35 or more | 7 | 7 |
| Race | ||
| White | 74 | 73 |
| Black | 16 | 14 |
| Asian | 6 | 7 |
| Other | 4 | 6 |
| Marital status | ||
| Single | 89 | 87 |
| Married | 5 | 5 |
| Separated | 3 | 3 |
| Divorced or widowed | 4 | 5 |
| Living arrangement | ||
| Not living with partner | 83 | 84 |
| Education | ||
| Less than 12 years | 4 | 5 |
| High school completed | 9 | 12 |
| Some college | 54 | 50 |
| Completed college or beyond | 33 | 33 |
| Smoking | ||
| Current smoker | 31 | 35 |
| Less than 1 pack/d | 28 | 31 |
| 1 pack/d or more | 3 | 5 |
| Former smoker | 16 | 14 |
| Never smoked | 53 | 50 |
| Alcohol use frequency | ||
| Never | 14 | 13 |
| Less than once/mo | 15 | 16 |
| At least once/mo | 30 | 26 |
| At least once/wk | 42 | 45 |
| Intercourse (number of acts) without birth control in previous cycle | ||
| 0 | 80 | 79 |
| 1–2 | 17 | 16 |
| 3 or more | 3 | 5 |
| Birth control currently used | ||
| Male condom | 85 | 83 |
| Abstinence | 19 | 20 |
| Withdrawal | 11 | 13 |
| Spermicides | 3 | 4 |
| Female barrier (cap, diaphragm, female condom) | 2 | 1 |
| Number of pregnancies | ||
| 0 | 55 | 58 |
| 1 | 25 | 20 |
| 2 | 10 | 12 |
| 3 or more | 10 | 10 |
| Number of deliveries | ||
| 0 | 82 | 82 |
| 1 | 10 | 10 |
| 2 or more | 8 | 8 |
| Number of induced abortions | ||
| 0 | 68 | 70 |
| 1 | 23 | 20 |
| 2 or more | 9 | 10 |
| Number of unplanned pregnancies | ||
| 0 | 60 | 61 |
| 1 | 25 | 32 |
| 2 | 8 | 11 |
| 3 or more | 7 | 5 |
Data are %.
In the modified intent-to-treat population, 26 pregnancies were observed. Twelve pregnancies occurred in the CDB-2914 users (1.5%, 95% CI 0.7–2.4%) and 14 occurred in the levonorgestrel users (1.8%, 95% CI 0.9–2.7%). This difference between treatments of −0.3%, with an upper limit of the 97.5% one-tailed CI of 1.42%, was statistically noninferior (P=.003). Four pregnancies from the CDB-2914 users and one from the levonorgestrel users were found to be pretrial pregnancies based on enrollment serum β-hCG levels and ultrasound measurements. One additional pregnancy in the CDB-2914 group occurred in an individual who had further acts of unprotected intercourse and also used prescription Plan B in the treatment cycle. The estimated conception date for this pregnancy occurred in the cycle after the treatment cycle but before an exit visit. Additional analysis of effectiveness at pregnancy prevention was performed only on the efficacy evaluable population.
In the efficacy evaluable population, a total of 20 pregnancies occurred in subjects after treatment, 7 in subjects who received CDB-2914 and 13 in levonorgestrel users. Pregnancy rates in the two groups were 0.9% (95% CI 0.2–1.6%) and 1.7% (95% CI 0.8–2.6%), respectively. The difference between treatments of −0.8%, with an upper limit of the 97.5% one-tailed CI of 0.77%, was statistically noninferior (P<.001). Using expected pregnancies based on the day of exposure relative to estimated day of ovulation, CDB-2914 users experienced an 85% reduction in the number of pregnancies compared with what would be expected with no treatment, whereas levonorgestrel users had a reduction of 69% (Table 2).
Table 2.
Effectiveness of Drug Based on the Interval From Exposure to Treatment
| Total | 0-24 h | More Than 24-48 h | More Than 48-72 h | |||||
|---|---|---|---|---|---|---|---|---|
| CDB | Levo | CDB | Levo | CDB | Levo | CDB | Levo | |
| Exposed (n) | 775 | 774 | 273 | 263 | 268 | 298 | 234 | 213 |
| Expected pregnancies (n)* | 47 | 42 | 19 | 14 | 14 | 16 | 14 | 12 |
| Observed pregnancies (n) | 7 | 13 | 0 | 4 | 6 | 3 | 1 | 6 |
| Effectiveness (%, 95% CI) | 85, 68–93 | 69, 46–82 | 100, N/E | 71, 28–89 | 57, 6–81 | 81, 42–94 | 93, 52–99 | 50, 0–77 |
We examined whether the time elapsed from exposure (unprotected intercourse) to treatment had an effect on the effectiveness of the two drugs. The number of individuals in each 24-hour period was similar between the two user groups and was fairly evenly distributed across each interval (Table 2). The women who used levonorgestrel had lower estimated rates of effectiveness at periods after 48 hours, suggesting a possible trend toward lower effectiveness with increasing time from exposure to treatment. In contrast, the women who used CDB-2914 did not seem to exhibit similar lower effectiveness rates after 48 hours. When evaluating the lower calculated efficacy with CDB-2914 in the more than 24 – 48 hour range, we observed a cluster of failures during the 37- to 48-hour interval.
Adverse effects were generally similar in both treatment groups. More CDB-2914 users than levonorgestrel users experienced nausea (29% compared with 24%, P=.03), whereas subjects in both groups had similar incidence of bleeding and spotting (Table 3).
Table 3.
Adverse Effects and Cycle Length After Treatment
| CDB-2914 Users (n = 775) |
Levonorgestrel Users (n = 774) |
P | |
|---|---|---|---|
| Cycle length (d)* | |||
| Before treatment | 29.13 | 29.04 | .36 |
| After treatment | 31.77 | 26.94 | <.001 |
| Change | +2.64 | −2.10 | <.001 |
| Symptoms experienced after treatment (%) | |||
| None | 30 | 30 | .96 |
| Nausea | 29 | 24 | .03 |
| Vomiting | 2 | 2 | .60 |
| Headache | 29 | 29 | .77 |
| Dizziness | 20 | 18 | .36 |
| Fatigue | 37 | 37 | .98 |
| Breast tenderness | 16 | 15 | .81 |
| Lower abdominal pain | 31 | 27 | .08 |
| Diarrhea | 12 | 11 | .84 |
| Spotting† | 4 | 6 | .08 |
| Bleeding† | 1 | 1 | 1.00 |
Analysis based on one-way analysis of variance, with treatment as the factor; remainder based on χ2 test.
Occurred in more than 25% of follow-up days.
Women in both groups experienced a wide variation in length of the cycle during which the drugs were taken as compared with what they each reported as their individual average cycle length. A reduction of 4 to 19 days in the menstrual interval during the treatment cycle occurred in 35% of levonorgestrel users and 17% of CDB-2914 users (P<.001). Similarly, an increase in the interval by 4 to 19 days occurred in 16% and 25%, respectively (P<.001). On average, the onset of menses after emergency contraception use was 2.1 days earlier than anticipated in levonorgestrel users and 2.6 days later in CDB-2914 users (Table 2).
When the change in cycle length was assessed according to the estimated cycle day of drug dosing, the levonorgestrel users exhibited, on average, shortening of cycle length if the drug was taken in the proliferative phase, less shortening if the drug was taken at ovulation or in the early secretory phase and lengthening of the cycle when the levonorgestrel was taken in the mid to late secretory phase. In contrast, no such trend was seen between the different cycle phases for women who took CDB-2914 (Fig. 2).
Fig. 2.
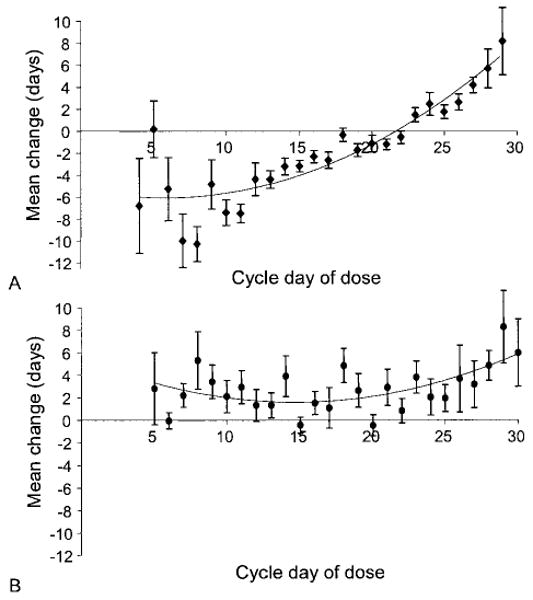
Mean change in menstrual cycle length based on the cycle day of treatment. A. Levonorgestrel users. B. CDB-2914 users.
Creinin. CDB-2914 Emergency Contraception. Obstet Gynecol 2006.
Using menstrual cycle history to predict the estimated day of ovulation during the treatment cycle, individuals were grouped according to proximity of unprotected intercourse to the predicted day of ovulation (Table 4). The distribution of the number of subjects on each day was similar between the two user groups (P=.96). Based on this grouping, all of the treatment failures in the CDB-2914 users occurred in women who had unprotected intercourse before or within one day of the estimated day of ovulation. Approximately one half of the treatment failures in the levonorgestrel group occurred in women who had exposures after their estimated day of ovulation. These pregnancies were confirmed by ultrasound dating to correlate with conception on or around the estimated day of unprotected intercourse and not the “predicted” day of ovulation based on menstrual history.
Table 4.
Exposure and Pregnancies Relative to Estimated Day of Ovulation Based on Menstrual History
| CDB-2914 Users | Levonorgestrel Users | |||
|---|---|---|---|---|
| Days Relative to Ovulation | Subjects | Pregnancies | Subjects | Pregnancies |
| No. days before | ||||
| 7 or more | 124 | 1 | 138 | 0 |
| 6 | 32 | 0 | 28 | 0 |
| 5 | 36 | 0 | 41 | 0 |
| 4 | 29 | 1 | 33 | 0 |
| 3 | 35 | 1 | 31 | 0 |
| 2 | 48 | 0 | 42 | 1 |
| 1 | 47 | 2 | 40 | 5 |
| Day of ovulation | 52 | 1 | 44 | 0 |
| No. days after | ||||
| 1 | 44 | 1 | 43 | 2 |
| 2 | 38 | 0 | 43 | 0 |
| 3 | 32 | 0 | 38 | 0 |
| 4 or more | 258 | 0 | 253 | 5 |
| Total | 775 | 7 | 774 | 13 |
Data are n.
Discussion
Both CDB-2914 and levonorgestrel are effective in preventing pregnancies after unprotected intercourse; pregnancy rates were less than 2% in both groups. The statistical comparison of CDB-2914 with levonorgestrel in this clinical trial was designed as a noninferiority study, and the efficacy difference of −0.3%, with an upper CI bound of less than 2%, implies that CDB-2914 is at least as effective as levonorgestrel. When effectiveness was determined by an analysis that considers pregnancy probabilities based on when intercourse occurred relative to the estimated date of ovulation, CDB-2914 users averted 85% of pregnancies, whereas levonorgestrel users averted 69%, an effectiveness rate ratio of 1.2. This difference, however, was not statistically significant.
Strengths of the current study include its large sample size, confirmation of each pregnancy by quantitative serum β-hCG analysis, and estimation of the date of conception of each pregnancy by cycle history and, in most cases, by ultrasound evaluation of gestational age. As compared with past studies that either did not include ultrasound confirmation or used less sensitive hormonal evaluation, our approach allowed a more precise estimation of the date of conception.7,15,16
Some imprecision is inherent in calculating efficacy because the actual fertile window of each cycle is not known and likely varies from cycle to cycle in normally cycling women.17–20 Most studies use cycle day to calculate an efficacy value based on predictions of the number of pregnancies that result from a single act of intercourse in women who measure their fertile window by some biologic marker such as urinary luteinizing hormone, basal body temperature, or cervical mucus. Some of the pregnancies that occurred in our study corresponded to an ovulatory event that was either before or after the fertile window based on menstrual cycle data. The random assignment of the subjects in the study assured that approximately equal numbers of subjects received either treatment for each cycle day (Table 4). For example, 156 and 166 women were treated with either CDB-2914 or levonorgestrel, respectively, for unprotected intercourse that occurred six or more days before the date of expected ovulation, during which time the likelihood of pregnancy is considered to be negligible in the standard calculation methods.20 Of these individuals, none of the women who took levonorgestrel and only one woman who took CDB-2914 became pregnant. At a later point in the menstrual cycle, there were 258 and 253 women who took CDB-2914 or levonorgestrel, respectively, after an exposure that occurred 4 or more days after the expected day of ovulation. Had ovulation occurred at the expected time, these women would have virtually no risk of pregnancy from intercourse that took place 4 or more days after ovulation, yet five women who took levonorgestrel under these conditions became pregnant. No pregnancies were observed in the corresponding CDB-2914 group. The standard calculations assign very low values to these cycle days.17–20 However, our data indicate that the risk of pregnancy at that time of the cycle is not negligible, most likely because of the lack of reliability of “normal” or “average” cycle lengths to accurately predict the fertile window in each future cycle.21 Accordingly, women who experience unprotected intercourse late in the cycle would be advised to seek treatment rather than assume that they are in a safe window of the cycle.
When the efficacy data are expressed according to the amount of time between exposure and treatment, CDB-2914 does not seem to exhibit loss of effectiveness at more than 48–72 hours after exposure (Fig. 2). The decrease in calculated efficacy in the more-than-24–48-hour range in this group is most likely a statistical aberration given the high efficacy in the more-than-24–48-hours group. This lack of a consistent trend is different from the results of previous studies with levonorgestrel and combined ethinylestradiol–norgestrel regimens, which reported a trend toward decreased effectiveness with increased time between exposure and treatment.7,15,16 Our data with levonorgestrel are consistent with previously reported studies in which the effectiveness of the drug after 48 hours from exposure seems to be decreasing. The fact that studies of levonorgestrel conclude that the effectiveness of the drug is greatest if taken within the first 12 hours of exposure and effectiveness decreases subsequently is an important point, because it may be difficult for women to obtain a prescription for the medication from a clinician especially over a weekend. The effectiveness of CDB-2914 was similar during the more-than-48–72-hour period to that observed during the initial 24 hour period, and no consistent trend was apparent. This lack of a clear trend, however, implies that more studies are needed to understand whether this high efficacy is truly maintained over time. Both levonorgestrel and another progesterone receptor modulator, mifepristone, have been shown to be efficacious beyond 72 hours. The efficacy of these interventions is lower in the more-than-72–120-hour window as compared with 72 hours or less.7,8 We have no comparable data for CDB-2914.
Adverse effects are an important determinant of how a drug is used and accepted by patients and providers. Although a statistically significantly greater proportion of CDB-2914 users reported nausea than those who used levonorgestrel, we believe that the incidence of nausea observed in the CDB-2914 group may be anomalous and not clinically significant. Other studies evaluating higher doses of CDB-2914 (up to 200 mg) in smaller numbers of women reported no evidence of nausea.22,23 The value of 29% found in this study may reflect a variable background incidence rather than an increase attributable to the drug itself. This is plausible, because no mechanism has been established by which nausea would be expected to be associated with a progesterone receptor blocker. Additionally, although the rates of the subjective complaint of nausea differed, the rates of the objective variable, vomiting, were identical. No differences in any other adverse effects were observed between the two groups (Table 3).
The statistically significant delay in resumption of menses in women who used CDB-2914, especially when compared with levonorgestrel, is consistent with evaluations of mifepristone that indicate delays of similar magnitude.7 Whether a delay of 2 to 3 days is clinically relevant to potential users is unknown. Importantly, the concept that a delay in resumption of menses may occur should be explained to women using CDB-2914 within the context that such a delay is entirely expected and normal and does not imply method failure.
The treatments to avoid pregnancy after unprotected intercourse have evolved from estrogen only to combined estrogen–progestin to levonorgestrel alone. With each advance, efficacy and adverse effects were reduced, and with the introduction of levonorgestrel regimens, efficacy significantly improved. Mifepristone, a first-generation progesterone receptor modulator, has overall efficacy and adverse effect rates comparable to levonorgestrel.7 However, mifepristone is also used as an abortifacient and its potential use for emergency contraception in the United States is unlikely in the near future. This study was initiated at a time when single-dose levonorgestrel was not yet studied nor used in clinical practice, with the expectation that CDB-2914, which is a single-dose treatment, could represent the next evolutionary step in emergency contraception. We are encouraged that CDB-2914 was not less effective than levonorgestrel and that the results suggest the possibility that CDB-2914 may be more efficacious for women who cannot obtain emergency contraception within 48 hours of exposure. Given the high efficacy of both CDB-2914 and levonorgestrel for use in emergency contraception, much larger studies would be necessary to address whether either treatment will provide better protection from unwanted pregnancy.
Acknowledgments
Supported by Federal funds from the National Institute of Child Health and Human Development, National Institutes of Health, Clinical Contraceptive Trials Network under Contract Numbers N01-HD-6–3261 and N01-HD-9–3297 through 3303 and, in part, by National Institutes of Health General Clinic Research Center Grants MO1RR000056 at the University of Pittsburgh and M01RR00096 at New York University.
The authors thank our colleagues, Dr. Susan Ballagh, Dr. Ann Davis, Dr. Michelle Fox, Dr. Bryna Harwood, Dr. George Kovalevsky, Dr. Amitasrigowri Murthy, Dr. Anita Nelson, Dr. Courtney Schreiber, and Terri Walsh for their assistance in design and performance of the study.
Footnotes
CLINICAL TRIAL REGISTRATION: ClinicalTrials.gov, www. clinicaltrials.gov, NCT00271583
References
- 1.Cheng L, Gulmezoglu AM, Oel CJ, Piaggio G, Ezcurra E, Look PF. Interventions for emergency contraception. Cochrane Database Syst Rev. 2004;(3):CD001324. doi: 10.1002/14651858.CD001324.pub2. [DOI] [PubMed] [Google Scholar]
- 2.The Kaiser Family Foundation. Women's health care providers' experiences with emergency contraception. Available at: http://www.kff.org/womenshealth/3343-index.cfm. Retrieved June 23, 2003.
- 3.Trussell J, Stewart F, Guest F, Hatcher RA. Emergency contraceptive pills: a simple proposal to reduce unintended pregnancies. Fam Plann Perspect. 1992;24:269–73. [PubMed] [Google Scholar]
- 4.Haspels AA. Emergency contraception: a review. Contraception. 1994;50:101–8. doi: 10.1016/0010-7824(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 5.Trussell J, Ellertson C, Stewart F, Raymond EG, Shochet T. The role of emergency contraception. Am J Obstet Gynecol. 2004;190:S30–8. doi: 10.1016/j.ajog.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 6.Arowojolu AO, Okewole IA, Adekunle AO. Comparative evaluation of the effectiveness and safety of two regimens of levonorgestrel for emergency contraception in Nigerians [published erratum appears in Contraception. 2003;67:165] Contraception. 2002;66:269–73. doi: 10.1016/s0010-7824(02)00337-2. [DOI] [PubMed] [Google Scholar]
- 7.von Hertzen H, Piaggio G, Ding J, Chen J, Song S, Bartfai G, et al. Low dose mifepristone and two regimens of levonorgestrel for emergency contraception: a WHO multicentre randomised trial. Lancet. 2002;360:1803–10. doi: 10.1016/S0140-6736(02)11767-3. [DOI] [PubMed] [Google Scholar]
- 8.Xiao BL, Von Hertzen H, Zhao H, Piaggio G. A randomized double-blind comparison of two single doses of mifepristone for emergency contraception. Hum Reprod. 2002;17:3084–9. doi: 10.1093/humrep/17.12.3084. [DOI] [PubMed] [Google Scholar]
- 9.Comparison of three single doses of mifepristone as emergency contraception: a randomized trial. Task Force on Postovulatory Methods of Fertility Regulation. Lancet. 1999;353:697–702. [PubMed] [Google Scholar]
- 10.Attardi BJ, Burgenson J, Hild SA, Reel JR, Blye RP. CDB-4124 and its putative monodemethylated metabolite, CDB-4453, are potent antiprogestins with reduced antiglucocorticoid activity: in vitro comparison to mifepristone and CDB-2914. Molec Cell Endocrinol. 2002;188:111–23. doi: 10.1016/s0303-7207(01)00743-2. [DOI] [PubMed] [Google Scholar]
- 11.Reel JR, Hild-Petito S, Blye RP. Antiovulatory and postcoital antifertility activity of the antiprogestin CDB-2914 when administered as single, multiple, or continuous doses to rats. Contraception. 1998;58:129–36. doi: 10.1016/s0010-7824(98)00067-5. [DOI] [PubMed] [Google Scholar]
- 12.Blackwelder W. “Proving the null hypothesis” in clinical trials. Controlled Clin Trials. 1982;3:345–53. doi: 10.1016/0197-2456(82)90024-1. [DOI] [PubMed] [Google Scholar]
- 13.Wilcox AJ, Weinberg CR, Baird DD. Timing of sexual intercourse in relation to ovulation: effects on the probability of conception, survival of the pregnancy, and sex of the baby. N Engl J Med. 1995;333:1517–21. doi: 10.1056/NEJM199512073332301. [DOI] [PubMed] [Google Scholar]
- 14.Wilcox AJ, Weinberg CR, Baird DD. Post-ovulatory ageing of the human oocyte and embryo failure. Hum Reprod. 1998;13:394–7. doi: 10.1093/humrep/13.2.394. [DOI] [PubMed] [Google Scholar]
- 15.Randomised controlled trial of levonorgestrel versus the Yuzpe regimen of combined oral contraceptives for emergency contraception. Task Force on Postovulatory Methods of Fertility Regulation. Lancet. 1998;352:428–33. [PubMed] [Google Scholar]
- 16.Ho PC, Kwan MS. A prospective randomized comparison of levonorgestrel with the Yuzpe regimen in post-coital contraception. Hum Reprod. 1993;8:389–92. doi: 10.1093/oxfordjournals.humrep.a138057. [DOI] [PubMed] [Google Scholar]
- 17.Wilcox AJ, Dunson DB, Weinberg CR, Trussell J, Baird DD. Likelihood of conception with a single act of intercourse: providing benchmark rates for assessment of post-coital contraceptives. Contraception. 2001;63:211–5. doi: 10.1016/s0010-7824(01)00191-3. [DOI] [PubMed] [Google Scholar]
- 18.Dixon GW, Schlesselman JJ, Ory HW, Blye RP. Ethinyl estradiol and conjugated estrogens as postcoital contraceptives. JAMA. 1980;244:1336–9. [PubMed] [Google Scholar]
- 19.Trussell J, Rodriguez G, Ellertson C. New estimates of the effectiveness of the Yuzpe regimen of emergency contraception. Contraception. 1998;57:363–9. doi: 10.1016/s0010-7824(98)00042-0. [DOI] [PubMed] [Google Scholar]
- 20.Mikolajczyk RT, Stanford JB. A new method for estimating the effectiveness of emergency contraception that accounts for variation in timing of ovulation and previous cycle length. Fertil Steril. 2005;83:1764–70. doi: 10.1016/j.fertnstert.2005.01.097. [DOI] [PubMed] [Google Scholar]
- 21.Creinin MD, Keverline S, Meyn LA. How regular is regular? An analysis of menstrual cycle regularity. Contraception. 2004;70:289–92. doi: 10.1016/j.contraception.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 22.Stratton P, Hartog B, Hajizadeh N, Piquion J, Sutherland D, Merino M, et al. A single mid-follicular dose of CDB-2914, a new antiprogestin, inhibits folliculogenesis and endometrial differentiation in normally cycling women. Hum Reprod. 2000;15:1092–9. doi: 10.1093/humrep/15.5.1092. [DOI] [PubMed] [Google Scholar]
- 23.Blithe DL, Nieman LK, Blye RP, Stratton P, Passaro M. Development of the selective progesterone receptor modulator CDB-2914 for clinical indications. Steroids. 2003;68:1013–7. doi: 10.1016/s0039-128x(03)00118-1. [DOI] [PubMed] [Google Scholar]


