Abstract
In two-component signaling systems, phosphorylated response regulators (RRs) are often dephosphorylated by their partner kinases in order to control the in vivo concentration of phospho-RR (RR~P). This activity is easily demonstrated in vitro, but these experiments have typically used very high concentrations of the histidine kinase (HK) compared to the RR~P. Many two-component systems exhibit exquisite control over the ratio of HK to RR in vivo. The question thus arises as to whether the phosphatase activity of HKs is significant in vivo. This topic will be explored in the present review.
Keywords: phosphatase, fluorescence resonance energy transfer (FRET), fluorescence anisotropy, ATPase
Introduction
Two-component regulatory systems (TCS) are signal transduction systems that are composed of a histidine kinase (HK) that senses an environmental change and communicates it via phosphorylation to a response regulator (RR). The majority of RRs alter gene expression when activated. Most HKs are homodimeric transmembrane proteins. The transmembrane helix connects to a C-terminal portion that consists of two domains in the case of the Class I HKs (see Figure 1). One domain contains the histidine residue that is autophosphorylated and comprises a four-helix bundle (“DHp”). The second domain binds ATP and is catalytic (“CA”) [1,2]. The globular CA domains protrude on either side of the dimer helical stem [3]. Although autophosphorylation was initially proposed to occur in trans [4], the most recent evidence suggests that autophosphorylation is intramolecular [5••]. In the crystal structure of HK853 from Thermotoga maritima, the β–phosphorous of bound ADPβN (a non-hydrolytic ATP homologue) is much closer to the His260εN of the same subunit (~11A) than to the same atom of the other subunit (~24A).
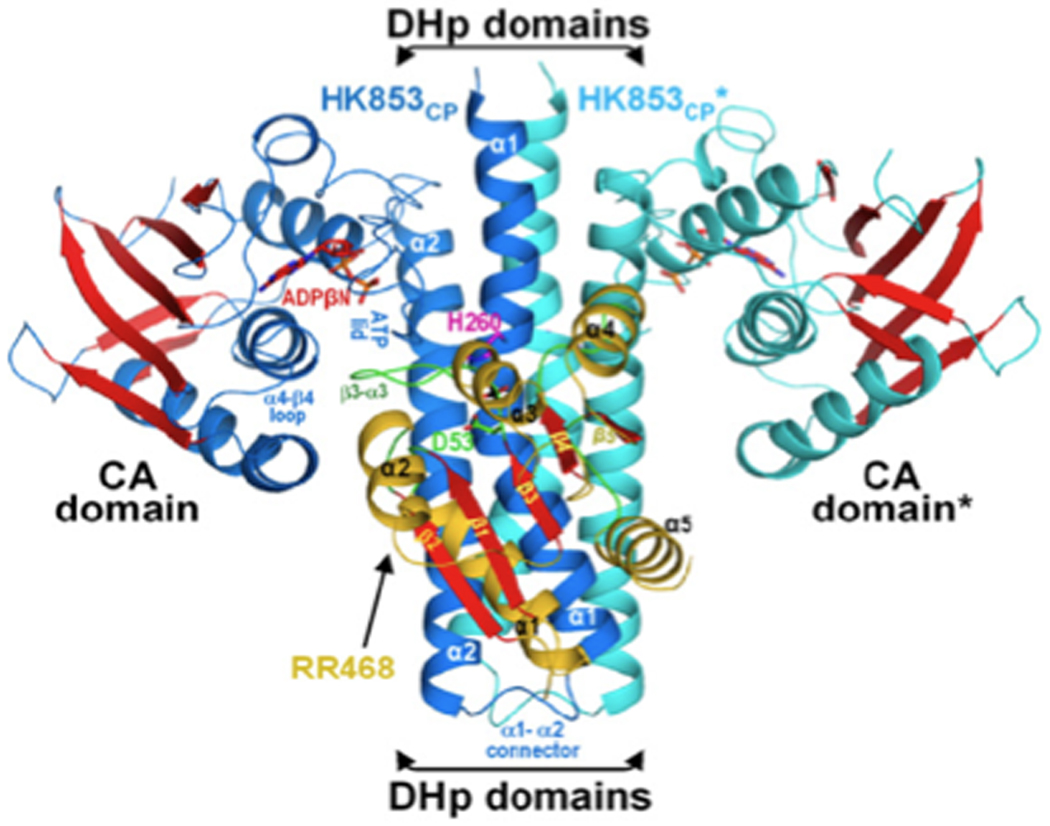
Figure 1.
Crystal structure of the HK853CP-RR468 Complex [5••], PDB accession number is 3DGE. Ribbon diagram of the complex viewed with the cell membrane at the top. The DHp and CA domains are indicated. Reprinted with permission from [5••].
In many two-component signaling systems, dephosphorylation of the RR~P via the HK (the so-called “phosphatase activity”) limits the level of the activated RR and resets the system. Although the PII-stimulated dephosphorylation of NtrC~P and CheY~P dephosphorylation by CheZ have been well-characterized, they will not be considered here, because in these examples, RR~P dephosphorylation occurs, or is stimulated by, accessory proteins [6–9]. The role of the HK in dephosphorylating the RR~P, with particular emphasis on the archetype EnvZ-OmpR system, is the subject of this review. Many other HKs have not been as extensively studied and much of what is known is derived from the EnvZ/OmpR system. As a cautionary note, it has become clear that although TCS are grouped into subfamilies based on structural similarity, this grouping does not necessarily reflect functional similarity. For example, the HK EnvZ is poised to increase autophosphorylation in response to signaling (i.e., OmpR~P levels are low in the absence of envZ) [10], whereas its close homologue HK CpxA is poised to regulate CpxR~P dephosphorylation (CpxR~P basal levels are high) [11]. Along the same line, although transmembrane signaling of Tar occurs via a piston movement, a uniform signaling mechanism for chemoreceptors and HKs is unlikely. A few of the interesting functional differences between HKs are highlighted in this review. Although some TCS have been studied extensively, many unanswered questions remain (see Box 1).
Box 1 Unanswered General Questions
How does the signaling state of the HK influence its activity?
What determines whether an HK will respond to environmental stimuli by altering autophosphorylation or RR~P dephosphorylation?
How does the behavior of single cells compare to the behavior of the population? Levels of EnvZ would be expected to fluctuate in individual cells and could dramatically affect signaling behavior.
Although many RR~Ps exhibit long half-lives in vitro, what is their half-life in vivo? Is it sufficiently brief that auto-dephosphorylation might play an important role?
Unanswered Specific Questions
How does MzrA influence EnvZ activity?
Does osmolality affect OmpR~P autodephosphorylation?
What is the Kd for binding of OmpR and OmpR~P to EnvZT247R, i.e. does a mutant with higher autophosphorylation and lower dephosphorylation exhibit altered affinity for OmpR and OmpR~P?
The two-component regulatory system that governs expression of the outer membrane porins OmpF and OmpC consists of the sensor kinase EnvZ and the response regulator OmpR. Activation of EnvZ, by an unknown signal, leads to phosphorylation of OmpR at aspartate 55 [12–14]. Phosphorylation of OmpR in its N-terminal receiver domain increases the affinity of its C-terminal output domain for the regulatory regions upstream of the ompF and ompC genes [15–18]. The porin genes are reciprocally regulated such that OmpF predominates at low osmolality and at high osmolality OmpC is the major porin in the outer membrane. The affinity of unphosphorylated OmpR for DNA was sufficiently high that it was postulated that OmpR was bound to DNA in vivo and became activated by EnvZ while bound [19]. DNA binding by OmpR would put it in a conformation more receptive to phosphorylation by EnvZ [19, 20]. A recent study that modeled OmpR bound to DNA, followed by substitution of relevant amino acids and mutation of the contacted bases, revealed that OmpR made surprisingly few specific DNA contacts and that these contacts could vary at different promoters [21•]. Because OmpR regulates many genes in addition to the porin genes [22] and has a high non-specific binding component [21•], the low level of OmpR~P produced during signaling (estimated at <10%) would be expected to be bound to DNA.
The EnvZ HK has the following enzymatic activities:
EnvZ +ATP ↔EnvZ~P +ADP (autophosphorylation)
EnvZ~P +OmpR ↔EnvZ +OmpR~P (phosphotransfer)
EnvZ +OmpR~P ↔EnvZ +OmpR+Pi (phosphatase)
By controlling the kinase, phosphotransfer and phosphatase activities, it is believed that EnvZ can modulate the level of OmpR~P in vivo. Because envZ deletion strains are effectively OmpF−OmpC−, unphosphorylated OmpR does not appear to play a role in porin gene expression [23]. A central role for the regulation of EnvZ phosphatase activity was proposed by Jin and Inouye (see below) [24]. Results from the EnvZ/OmpR system have motivated similar experiments with other TCS, often leading to the conclusion that the phosphatase activity of the sensor kinase is the step regulated by signal input [25–28]. Other studies are at odds with this view [29]. Qin et al. proposed that when OmpR~P bound to DNA, it was effectively made inaccessible to EnvZ to stimulate OmpR~P breakdown [20]. If EnvZ can’t recognize OmpR~P bound to DNA, it would be difficult to reconcile with the proposed role of the EnvZ phosphatase activity in breaking down OmpR~P and resetting OmpR~P levels in vivo [24]. In other words, how does EnvZ dephosphorylate OmpR~P bound to DNA if the OmpR~P/DNA complex is inaccessible? Furthermore, if the pool of unphosphorylated OmpR is already bound to DNA [19,20], how does DNA binding alter the interaction of OmpR with EnvZ? Our data suggest that the direct stimulation of OmpR~P breakdown by EnvZ probably does not play a role in osmotic signaling in vivo (see below). Recent kinetic studies proposed that the HK phosphatase activity functions to limit cross-talk between highly homologous TCS [31• •].
Two domains of sensor kinases
A commonly held view is that the kinase and phosphatase activities are regulated by a repositioning of the DHp and CA domains with respect to each other. ATP, ADP and non-hydrolyzable analogues of ATP bind to the nucleotide-binding site of the CA domain and stimulate phosphatase activity, presumably by re-positioning the CA and DHp domains into a conformation that allows higher activity. Earlier studies reported that the isolated DHp domain possesses phosphatase activity both in vivo and in vitro [32]. However, as in many studies on HK phosphatase activity, the ratio of HK to RR was high (2:1) and did not reflect in vivo ratios, which are reported to be 1:35–40; [33]; see also Figure 5, below). The phosphatase activity was barely apparent after 10 minutes in the presence of DHp, i.e., dephosphorylation was slow [32]. In any case, addition of the isolated DHp domain was substantially less effective at dephosphorylating OmpR~P than was the entire cytoplasmic domain of EnvZ containing both DHp and CA domains (EnvZc) [32]. As an aside, does the presence of isolated CA or DHp domains in vivo alter or disrupt OmpR phosphorylation by acetyl phosphate? Small changes in conformation can have dramatic effects on the active site of RRs, most often affecting phosphorylation by small molecules but not by HKs [34,35]. In a similar study with PhoR, the DHp domain appeared to slightly accelerate PhoB~P breakdown, although the low levels of PhoB~P present in the assay make it difficult to interpret [36]. This experiment was performed with a thioredoxin/six-His tag fusion protein at a ratio of 1.5 PhoB to 1 PhoR-DHp. Perhaps the low activity was caused by the fusion. Interestingly, a PhoR construct containing amino acids 83 to 431 of PhoR did not exhibit phosphatase activity [36], a result different from the one obtained with EnvZc. The assumption was that the CA domain inhibited the DHp domain of PhoR, further supporting the view that regulation repositions these two domains with respect to one another.
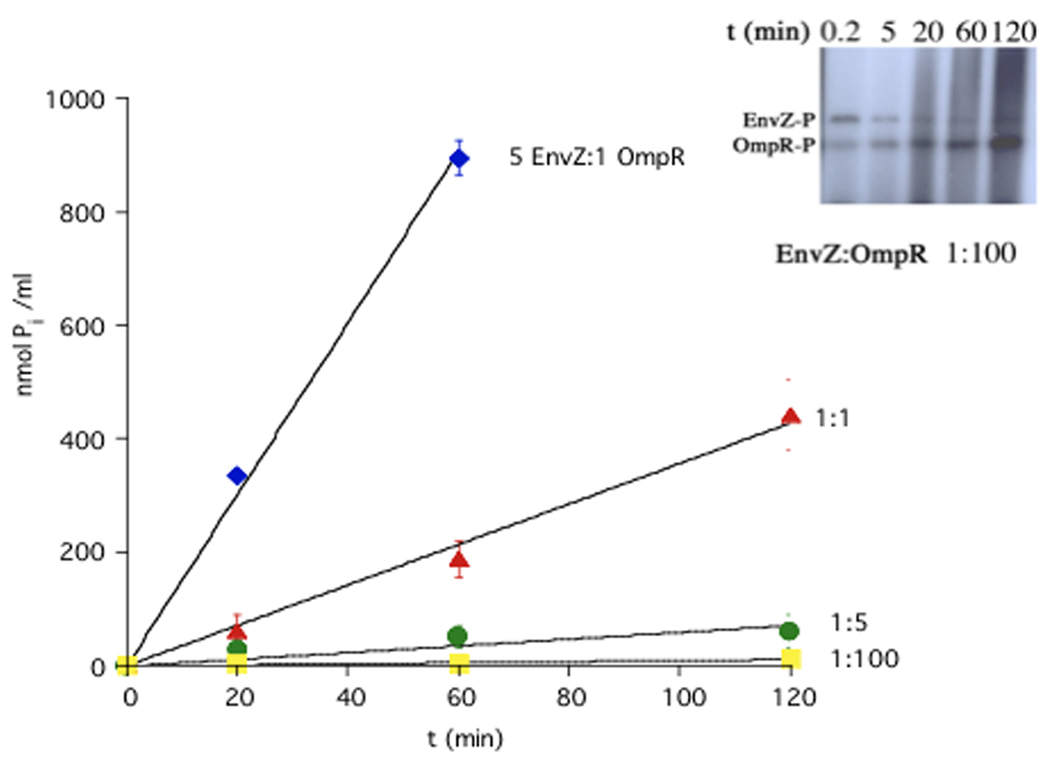
Figure 5.
Does a high kcat compensate for a high Kd? Measurements of OmpR-stimulated ATPase activity represent the sum of the phosphorylation/phosphotransfer reactions. The affinity of EnvZ for OmpR~P is >1.5 µM [50]. Can this low affinity be overcome by a high turnover rate that could rapidly reduce OmpR~P levels? ATPase assays were carried out in a 0.6-ml volume containing 125 mM NaCl, 4 mM MgCl2, 60 mM Tris-HCl (pH 7.5), 0.75 mM EDTA, and various concentrations of EnvZ (0.015–7.5 µM) and OmpR (1.5 µM). The apparent affinity of EnvZ for ATP is 200 µM [64]. Reactions were initiated by addition of 4 mM ATP and conducted as described in [29,64]. The Pi produced in the presence of EnvZ was subtracted from the total Pi produced in the presence of OmpR. The symbol represents the mean, and error bars indicate the standard deviation of three data points obtained at each time point. The data shown are a representative experiment. At ratios that approximate in vivo levels of EnvZ and OmpR (approximately 1:35) [33], there is almost no turnover of ATP. Inset: At low ratios of EnvZ to OmpR (1:100), the kinase is still active and OmpR~P levels actually accumulate over the incubation period. Phosphotransfer rates of EnvZ~P to OmpR are extremely fast [31••]. Figure 5 was part of Kirstin Mattison’s Ph.D thesis at Oregon Health and Sciences University.
The role of the autophosphorylated histidine in RR~P dephosphorylation
There was disagreement as to whether the phosphorylated histidine (H243 of EnvZ) was required for phosphatase activity [32,37,38]. In an in vivo experiment, the ability of plasmid-encoded EnvZ variants, in which different residues replaced H243, was examined in an envZ null strain [38]. In these cells, low-level phosphorylation of OmpR by endogenous acetyl phosphate supported a low level of OmpF production (< 10% of wildtype levels). EnvZ mutants that could support phosphatase activity would decrease ompF-lacZ. EnvZH243Y exhibited reduced β–galactosidase activity, consistent with the histidine not being required for dephosphorylation [38], although EnvZH243Y was over-expressed, i.e. EnvZ:OmpR ratios were skewed. In contrast, in an in vitro assay, DHpH243Y did not dephosphorylate OmpR~P [32]. Yet EnvZcH243Y, containing both DHp and CA domains showed significant OmpR~P phosphatase activity, although this study was also performed with high concentrations of EnvZc [39]. These differing results suggest that interactions between the DHp and CA domains contribute substantially to the ability of EnvZ to dephosphorylate OmpR~P. Thus, studies on isolated domains should be interpreted with caution. The conserved histidine residue seems to enhance, but is not required for, phosphatase activity, indicating that dephosphorylation of the RR~P does not involve a reverse phosphotransfer mechanism [39–42]. In studies with Thermotoga maritima HK853, replacing the histidine with alanine diminished its ability to dephosphorylate RR468~P [5••]. The histidine residue likely orients a water molecule for nucleophilic attack on the aspartyl phosphate of the RR and other residues with side chains that can form H-bonds might substitute for histidine. The active center of the crystal structure of the HK853 complexed with RR468 in which RR468 is in a phosphorylated conformation (the phosphate is replaced with sulfate), is consistent with this view [5••].
A classical “phosphatase-minus” EnvZ mutant also has elevated levels of autophosphorylation
The EnvZ11 mutant confers an OmpF− OmpC+ phenotype irrespective of the medium osmolality [43], which presumably results from elevated OmpR~P. OmpR~P levels could increase either from increasing phosphotransfer from EnvZ~P or from decreasing EnvZ-stimulated OmpR~P dephosphorylation, or both activities might be affected. The substitution replaced a highly conserved threonine with an arginine at residue 247 (T247R). This site is one helical turn away from the phosphorylated histidine. Purified EnvZT247R exhibited > 2-fold increase in autophosphorylation compared to wildtype EnvZ [44]. The EnvZ11 mutant also produced more OmpR~P and dephosphorylation of OmpR~P generated by phosphorylation from EnvZT247R was very slow. A more direct way to perform this experiment would be to prepare OmpR~P by phosphorylation from phosphoramidate and then monitor OmpR~P levels by HPLC under conditions in which the concentration of OmpR and OmpR~P can be readily compared [16]. The T247R substitution may alter the ability of OmpR~P to interact with EnvZ, thereby slowing OmpR~P turnover (see below and Box 1). A prediction is that the L16Q residue replacement in OmpR suppresses the envZ11 mutation by greatly decreasing the affinity of OmpR for EnvZ so that phosphotransfer is impaired [45].
When the substitution corresponding to T247R in EnvZ (resulting in enhanced autophosphorylation and reduced OmpR~P dephosphorylation) was made in the HK ResE (T378R), it did not increase autophosphorylation, but it did slow ResD~P dephosphorylation compared to wildtype ResE [27]. In the CpxA HK, the T253P replacement at the conserved threonine residue substantially reduced autophosphorylation [46]. This study employed MBP fusions to both CpxA and CpxR. Phosphotransfer to MBP-CpxR from MBP-CpxA~P was slow, and it was even slower with MBP-CpxAT253P. These results must be interpreted with caution, because we observed reduced phosphorylation of OmpR by an MBP-EnvZ fusion (L. Kenney, unpublished results). Substitutions at the critical threonine residue also inhibit autophosphorylation of the VicK HK homologue from Streptococcus pneumonia (M. Winkler, personal communication). In summary, similar substitutions in HKs do not all behave identically.
Which step in ompF/ompC regulation is sensitive to the osmotic signal?
The accepted view is that OmpR~P levels are low at low osmolality (Figure 2). This could result from a low activity of the EnvZ kinase, a low rate of phosphotransfer, or a high level of EnvZ phosphatase activity. As osmolality increases, it is presumed that the concentration of OmpR~P increases, either because of increased EnvZ kinase activity, increased phosphotransfer from EnvZ~P to OmpR, or decreased EnvZ phosphatase activity. Since OmpR~P levels are extremely low in the absence of EnvZ [10], it might be expected that the step regulated by osmolality would be EnvZ autophosphorylation. However, as mentioned above, it has been proposed that the regulated step in response to osmotic stress is inhibition of phosphatase activity [24]. This conclusion was based on a chimera called Taz1, in which the periplasmic, transmembrane and HAMP domains of the E. coli chemoreceptor Tar were fused to the cytoplasmic domain of EnvZ [47]. The authors found that addition of aspartate to cells expressing Taz1 induced ompC-lacZ expression. The level of Taz1 was estimated to be ~20-fold higher than normal EnvZ levels. Both Taz1 autophosphorylation and phosphotransfer to OmpR were not affected by aspartate, but extremely small aspartate-induced decreases in phosphatase activity were observed [48]. A concern about the physiological relevance of the aspartate response mediated by Taz1 is that, in contrast to Tar, Taz1 requires very high concentrations of aspartate to stimulate ompC-lacZ expression. Also, unlike E. coli Tar, Taz is insensitive to maltose.
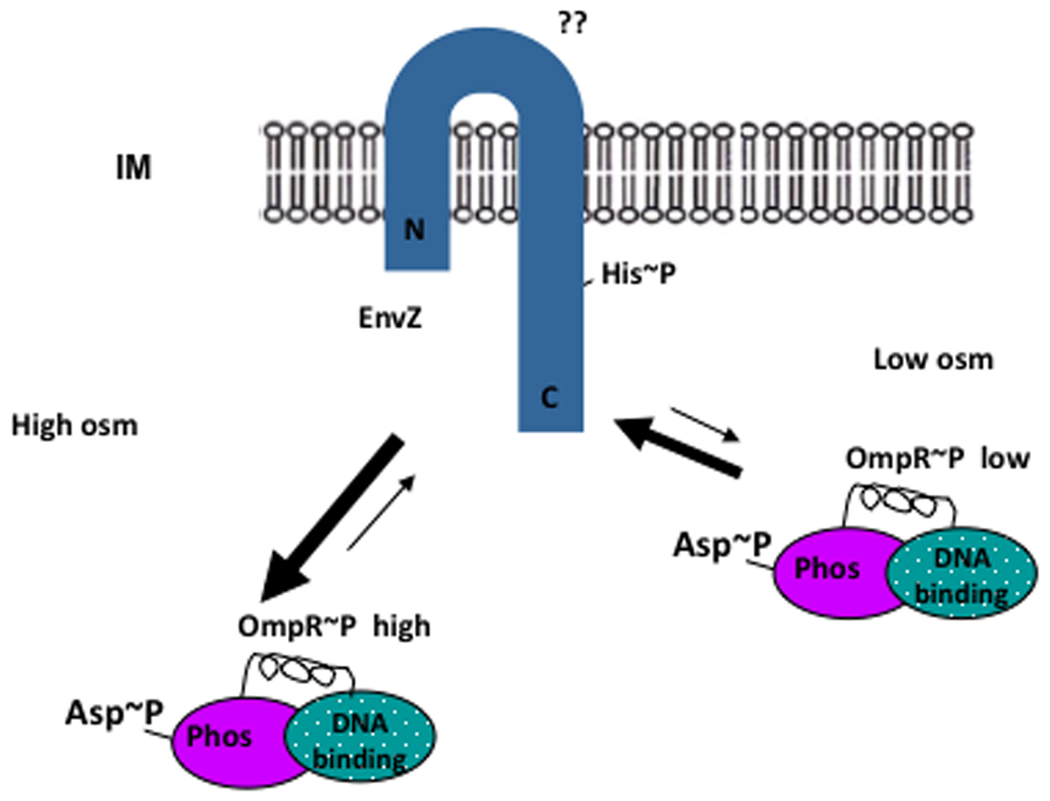
Figure 2.
Activities underlying OmpR/EnvZ signaling. It is presumed that, at low osmolality, the level of intracellular OmpR~P is low either because the kinase activity of EnvZ is low, or because EnvZ phosphatase activity is high. At high osmolality OmpR~P levels increase either because of an increase in the EnvZ kinase activity or a decrease in EnvZ phosphatase activity.
As mentioned earlier, it was reported that when OmpR~P was bound to DNA, it was sequestered from the phosphatase activity of EnvZ [20]. The implication is that OmpR~P/DNA and OmpR~P/EnvZ interactions are mutually exclusive, so that EnvZ could only dephosphorylate OmpR~P after it dissociates from the DNA. However, OmpR~P binds to the ompF and ompC promoters with a 25-fold higher affinity than unphosphorylated OmpR [16]. Therefore, it would seem unlikely that OmpR~P would be released from DNA to become a substrate for EnvZ. In support of this view, a recent kinetic model did not include OmpR~P sequestration from EnvZ [31••].
Formation of an EnvZ/OmpR/DNA ternary complex
If the interactions of OmpR~P with DNA and EnvZ are mutually exclusive [20], then a ternary complex should not form. We directly tested this hypothesis using fluorescence anisotropy to determine whether DNA binding altered the affinity of OmpR or OmpR~P for EnvZ [29]. OmpR was fluorescently labeled and after addition of various concentrations of EnvZc, equilibrium binding was measured in solution (Figure 3). The dissociation constant (Kd) for EnvZc binding to OmpR was 425 nM; for EnvZc binding to OmpR in the presence of ompC DNA (C1-C2-C3) the Kd was 385 nM. The presence of ompC DNA did not alter the binding of EnvZc to OmpR or to OmpR~P. Thus, the ternary complex forms, and we believe it is likely that this complex also occurs in vivo [19]. The surprising result was that phosphorylation of OmpR, and not DNA binding, reduced its affinity for EnvZ. We were able to measure this reduced affinity of OmpR~P to EnvZ using FRET (Figure 4 inset, below).
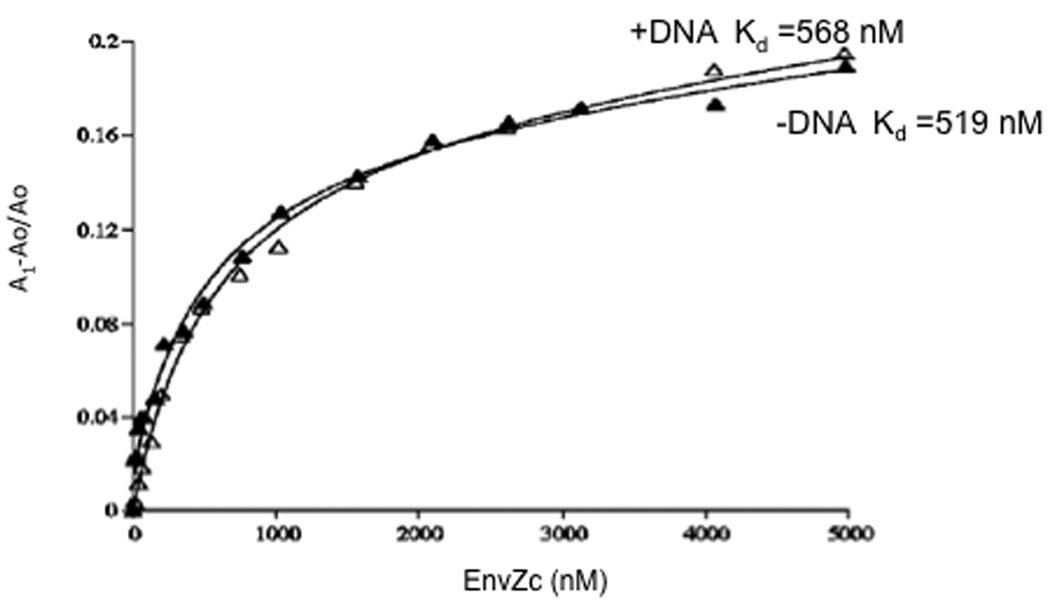
Figure 3.
EnvZc binding to OmpR using fluorescence anisotropy. The binding reactions contained 40 nM fluorescein-labeled OmpR. Closed triangles show OmpR binding to EnvZc with a Kd of 519 nM. The average Kd for EnvZc binding to OmpR from 10 independent experiments was 425 ± 127 nM. In the presence of specific DNA, EnvZc still binds OmpR, with a Kd of 568 nM (open triangles). The average Kd for EnvZc binding to OmpR C1-C2-C3 from six separate curves is 385 ± 162 nM. Reprinted with permission from [29].
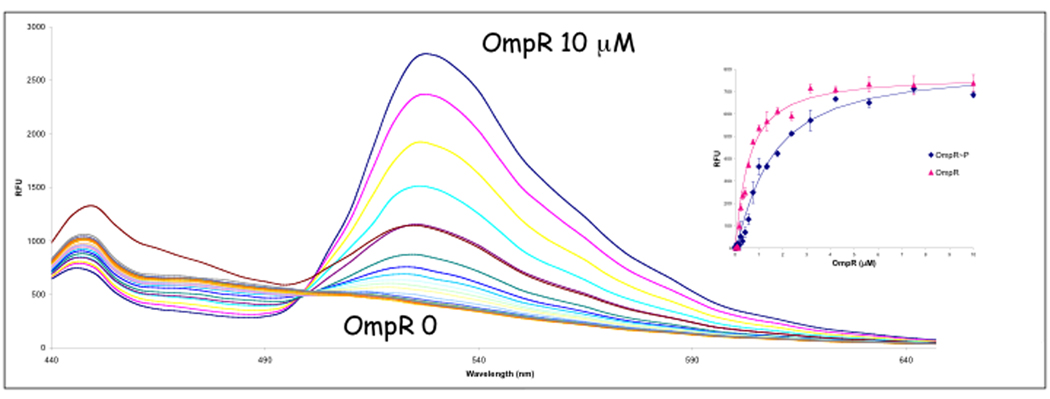
Figure 4.
Fluorescence resonance energy transfer (FRET) with EnvZ-GFP to fluorescent-OmpR. EnvZ-GFP was over-expressed, and spheroplasts were prepared and lysed in cold H2O according to Osborn et al. [63]. Fluorescent-OmpR concentrations ranged from 0 to 10 µM in the presence of EnvZ-GFP. A control experiment was performed with 0 to 1000 nM unconjugated fluorophore in the presence of 250 nM EnvZ-GFP to measure the amount of non-specific interaction of the donor (GFP) and acceptor fluorophores. A curve comparing FRET with OmpR and OmpR~P is shown in the inset. Kd OmpR = 0.5 µM; OmpR~P = 1.6 µM [50]. EnvZ-GFP was a kind gift from M. Goulian. Reprinted with permission from [50].
In contrast, a non-solution based approach using a His-OmpR pull-down assay followed by elution and SDS-PAGE separation reported a “Kd” of 1.25–1.42 µM for both OmpR and OmpR~P [33]. Additional analysis demonstrated that EnvZc dephosphorylated fluorescently-labeled OmpR~P, but that study employed high ratios (1:2) of EnvZc:fluorescent-OmpR~P [49].
Because the transmembrane or periplasmic domains could conceivably contribute to EnvZ/OmpR interactions, we performed an alternative experiment using an EnvZ-GFP chimera, lysing spheroplasts, and adding purified OmpR or OmpR~P fluorescently labeled at the lone native cysteine residue (labeling did not reduce activity). The Kd obtained by FRET was identical to that obtained by fluorescence anisotropy using EnvZc [50].
EnvZ exhibits higher affinity for OmpR than for OmpR~P
We reasoned that in order for the phosphatase activity of EnvZ to be important physiologically, EnvZ should have higher affinity for OmpR~P than for OmpR. That way, EnvZ could bind OmpR~P and stimulate its dephosphorylation. Then, its reduced affinity for OmpR would promote OmpR release, enabling EnvZ to bind another OmpR~P molecule. We used FRET to measure the Kd of EnvZ-GFP and purified OmpR (Figure 4) and OmpR~P (produced by phosphorylation by phosphoramidate) that was fluorescently labeled at Cys-67. OmpR~P had a >3-fold lower affinity for EnvZ than unphosphorylated OmpR (Figure 4, inset) [50]. Thus, the affinities are in the wrong ratio for the phosphatase of EnvZ to play a significant role in vivo. Perhaps osmotic signaling alters EnvZ/OmpR or EnvZ/OmpR~P affinities. The bacterial chemotaxis system exhibits similar behavior. CheY~P has a reduced affinity for its partner kinase CheA compared to unphosphorylated CheY. Measurements using isothermal titration calorimetry demonstrated that the affinity of CheA for CheY was 2 µM, and the affinity decreased 6-fold upon phosphorylation in the presence of magnesium [51]. This behavior would favor interaction of unphosphorylated CheY with CheA. CheY~P would then be more likely to dissociate from CheA, and the concomitant increase in affinity of CheY~P for FliM [52] would promote reversal of the flagellar motor from counterclockwise to clockwise rotation. In contrast to what we observed with EnvZ/OmpR, the phosphatase CheZ has higher affinity for CheY~P than for CheY [53].
OmpR~P turnover depends on high [EnvZ]
Although its low affinity for OmpR~P would make it difficult for EnvZ to play a significant role in dephosphorylating OmpR~P in vivo, a high kcat might compensate for a high Kd. We measured OmpR~P turnover by measuring inorganic phosphate production over time at different EnvZ ratios (see Figure 5). At high ratios of EnvZ to OmpR (blue diamonds), there is significant phosphatase activity and OmpR~P turnover is high. However, at the ratios of EnvZ to OmpR that prevail in vivo (between green circles and yellow squares), inorganic phosphate production is extremely low. At these low levels of EnvZ, the kinase is still active and capable of phosphorylating OmpR (see inset). In fact, OmpR~P actually accumulates during this assay, whereas at higher EnvZ levels, where there is substantial phosphatase activity, OmpR~P reaches a steady state and does not further increase. The results shown in Figure 5 suggest that the kcat is not sufficient to compensate for the high Kd. Thus, EnvZ would seem unlikely to play a significant role in OmpR~P turnover in vivo. This view is supported by a recent kinetic analysis of EnvZ/OmpR phosphorylation and dephosphorylation. The authors were required to use a cellular concentration of 2 µM for EnvZ in order to fit their data (close to the 1:1 plot in Figure 5), again suggesting that high HK levels are required for phosphatase activity to have a significant effect on OmpR~P levels [31••]. Many other studies have used high concentrations of soluble HK domains to study RR~P dephosphorylation. In vivo, however, the ratio of HK to RR is usually quite low, in part due to inefficient translational coupling of many TCS operons. For example, the stop codon for ompR overlaps the envZ start codon, leading to significantly higher levels of OmpR compared to EnvZ. Measurements of EnvZ and OmpR levels yield a ratio of 1:35–40 [33]. Lastly, the experiment shown in Figure 5 indicates that the EnvZ/OmpR system is not likely to be robust (i.e. insensitive to changes in protein concentration) and is probably highly sensitive to alterations of EnvZ levels in vivo [54–57]. Previous studies did not explore a wide enough range of protein concentrations for differences to become apparent [54,55].
Most measurements of OmpR~P half-life suggest that it is long-lived [31••]. Is autodephosphorylation of OmpR~P significant in vivo? Residues in the active site, as well as unknown factors, are known to modulate autodephosphorylation [58,59], but intracellular concentrations, and the in vivo stability of OmpR~P, have not been determined. Nor has the effect of osmolality on OmpR~P stability been examined.
Regulation by additional components
Some TCS possess additional components that may alter HK activity. For example, MzrA was recently identified as a modulator of EnvZ activity [60••]. MzrA appeared to reduce OmpR~P turnover, perhaps by decreasing the affinity of EnvZ/OmpR~P binding. The B1500 protein, like MzrA, localizes to the inner membrane and interacts with PhoQ [61]. How it activates the PhoQ system is not known. Inner-membrane proteins YycH and YycI interact with the HK YycG to decrease its activity, although the mechanism by which this occurs is not yet known [62].
Conclusions
Many in vitro experiments suggest a role for HKs in dephosphorylating RR~Ps, an activity that could reset the system or limit cross-talk. However, many of these studies used isolated domains or high concentrations of HK to RR that do not reflect in vivo levels. Thus, it remains to be determined whether HK phosphatase activity is significant in vivo. Results obtained with one HK-RR pair are not reliably extrapolated to other TCS, since so much diversity in behavior exists.
Acknowledgments
I am grateful to Yuhai Tu, IBM for helpful discussions and Malcolm Winkler and Alina Gutu, Indiana University, and Mike Manson, Texas A&M University for their comments on the manuscript. Supported by NIH GM-058746, VA 1I01BX000372 and RCE in Mechanobiology, National University of Singapore.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
- 1.Tanaka T, Saha SK, Tomomori C, Ishima R, Liu D, Tong KI, Park H, Dutta R, Qin L, Swindells MB, et al. NMR structure of the histidine kinase domain of the E. coli osmosensor EnvZ. Nature. 1998;396:88–92. doi: 10.1038/23968. [DOI] [PubMed] [Google Scholar]
- 2.Tomomori C, Tanaka T, Dutta R, Park H, Saha SK, Zhu Y, Ishima R, Liu D, Tong KI, Kurokawa H, et al. Solution structure of the homodimeric core domain of Escherichia coli histidine kinase EnvZ. Nat Struct Biol. 1999;6:729–734. doi: 10.1038/11495. [DOI] [PubMed] [Google Scholar]
- 3.Marina A, Waldburger CD, Hendrickson WA. Structure of the entire cytoplasmic portion of a sensor histidine-kinase protein. EMBO J. 2005;24:4247–4259. doi: 10.1038/sj.emboj.7600886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang Y, Inouye M. Intermolecular complementation between two defective mutant signal-transducing receptors of Escherichia coli. Proc Natl Acad Sci U S A. 1991;88:11057–11061. doi: 10.1073/pnas.88.24.11057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Casino P, Rubio V, Marina A. Structural insight into partner specificity and phosphoryl transfer in two-component signal transduction. Cell. 2009;139:325–336. doi: 10.1016/j.cell.2009.08.032. ••This is a co-crystal structure of an HK complexed with its RR from T. maritima. The authors make a strong case for HK phosphorylation occurring in cis rather than trans.
- 6.Boesch KC, Silversmith RE, Bourret RB. Isolation and characterization of nonchemotactic CheZ mutants of Escherichia coli. J Bacteriol. 2000;182:3544–3552. doi: 10.1128/jb.182.12.3544-3552.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pioszak AA, Ninfa AJ. Mechanism of the PII-activated phosphatase activity of Escherichia coli NRII (NtrB): how the different domains of NRII collaborate to act as a phosphatase. Biochemistry. 2003;42:8885–8899. doi: 10.1021/bi030065p. [DOI] [PubMed] [Google Scholar]
- 8.Pioszak AA, Ninfa AJ. Genetic and biochemical analysis of phosphatase activity of Escherichia coli NRII (NtrB) and its regulation by the PII signal transduction protein. J Bacteriol. 2003;185:1299–1315. doi: 10.1128/JB.185.4.1299-1315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao R, Collins EJ, Bourret RB, Silversmith RE. Structure and catalytic mechanism of the E. coli chemotaxis phosphatase CheZ. Nat Struct Biol. 2002;9:570–575. doi: 10.1038/nsb816. [DOI] [PubMed] [Google Scholar]
- 10.Slauch JM, Garrett S, Jackson DE, Silhavy TJ. EnvZ functions through OmpR to control porin gene expression in Escherichia coli K-12. J Bacteriol. 1988;170:439–441. doi: 10.1128/jb.170.1.439-441.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Wulf P, Lin EC. Cpx two-component signal transduction in Escherichia coli: excessive CpxR-P levels underlie CpxA* phenotypes. J Bacteriol. 2000;182:1423–1426. doi: 10.1128/jb.182.5.1423-1426.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delgado J, Forst S, Harlocker S, Inouye M. Identification of a phosphorylation site and functional analysis of conserved aspartic acid residues of OmpR, a transcriptional activator for ompF and ompC in Escherichia coli. Mol. Microbiol. 1993;10:1037–1047. doi: 10.1111/j.1365-2958.1993.tb00974.x. [DOI] [PubMed] [Google Scholar]
- 13.Igo MM, Ninfa AJ, Stock JB, Silhavy TJ. Phosphorylation and dephosphorylation of a bacterial transcriptional activator by a transmembrane receptor. Genes Develop. 1989;3:1725–1734. doi: 10.1101/gad.3.11.1725. [DOI] [PubMed] [Google Scholar]
- 14.Igo MM, Silhavy TJ. A Bacterial Environmental Sensor that Functions as a Protein Kinase and Stimulates Transcriptional Activation. Genes Develop. 1989;3:598–605. doi: 10.1101/gad.3.5.598. [DOI] [PubMed] [Google Scholar]
- 15.Aiba H, Nakasai F, Mizushima S, Mizuno T. Phosphorylation of a bacterial activator protein, OmpR, by a protein kinase, EnvZ, results in stimulation of its DNA-binding ability. J. Biochem. 1989;106:5–7. doi: 10.1093/oxfordjournals.jbchem.a122817. [DOI] [PubMed] [Google Scholar]
- 16.Head CG, Tardy A, Kenney LJ. Relative binding affinities of OmpR and OmpR-phosphate at the ompF and ompC regulatory sites. J. Mol. Biol. 1998;281:857–870. doi: 10.1006/jmbi.1998.1985. [DOI] [PubMed] [Google Scholar]
- 17.Huang KJ, Igo MM. Identification of the bases in the ompF regulatory region, which interact with the transcription factor OmpR. J. Mol. Biol. 1996;262:615–628. doi: 10.1006/jmbi.1996.0540. [DOI] [PubMed] [Google Scholar]
- 18.Rampersaud A, Harlocker SL, Inouye M. The OmpR protein of Escherichia coli binds to sites in the ompF promoter region in a hierarchical manner determined by its degree of phosphorylation. J. Biol. Chem. 1994;269:12559–12566. [PubMed] [Google Scholar]
- 19.Ames SK, Frankema J, Kenney LJ. C-terminal DNA binding stimulates N-terminal phosphorylation by the outer membrane protein regulator OmpR from Escherichia coli. Proc. Natl. Acad. Sci. USA. 1999;96:11792–11797. doi: 10.1073/pnas.96.21.11792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qin L, Yoshida T, Inouye M. The critical role of DNA in the equilibrium between OmpR and phosphorylated OmpR mediated by EnvZ in Escherichia coli. Proc. Natl. Acad. Sci. USA. 2001;98:908–913. doi: 10.1073/pnas.031383098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rhee JE, Sheng W, Morgan LK, Nolet R, Liao X, Kenney LJ. Amino acids important for DNA recognition by the response regulator OmpR. J Biol Chem. 2008;283:8664–8677. doi: 10.1074/jbc.M705550200. •This work examines chemical shift changes in OmpR by NMR as a result of DNA binding and substitutes amino acids and bases involved in DNA recognition.
- 22.Oshima T, Aiba H, Masuda Y, Kanaya S, Sugiura M, Wanner BL, Mori H, Mizuno T. Transcriptome analysis of all two-component regulatory system mutants of Escherichia coli K-12. Mol. Microbiol. 2002;46:281–291. doi: 10.1046/j.1365-2958.2002.03170.x. [DOI] [PubMed] [Google Scholar]
- 23.Slauch J, Silhavy TJ. Genetic analysis of the switch that controls porin gene expression in Escherichia coli K-12. J. Mol. Biol. 1989;210:291–292. doi: 10.1016/0022-2836(89)90330-6. [DOI] [PubMed] [Google Scholar]
- 24.Jin T, Inouye M. Transmembrane signaling. Mutational analysis of the cytoplasmic linker region of Taz1-1, a Tar-EnvZ chimeric receptor in Escherichia coli. J. Mol. Biol. 1994;244:477–481. doi: 10.1006/jmbi.1994.1746. [DOI] [PubMed] [Google Scholar]
- 25.Castelli ME, Garcia Vescovi E, Soncini FC. The phosphatase activity is the target for Mg2+ regulation of the sensor protein PhoQ in Salmonella. J Biol Chem. 2000;275:22948–22954. doi: 10.1074/jbc.M909335199. [DOI] [PubMed] [Google Scholar]
- 26.Montagne M, Martel A, Le Moual H. Characterization of the catalytic activities of the PhoQ histidine protein kinase of Salmonella enterica serovar Typhimurium. J Bacteriol. 2001;183:1787–1791. doi: 10.1128/JB.183.5.1787-1791.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakano MM, Zhu Y. Involvement of ResE phosphatase activity in down-regulation of ResD-controlled genes in Bacillus subtilis during aerobic growth. J Bacteriol. 2001;183:1938–1944. doi: 10.1128/JB.183.6.1938-1944.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wanner BL. Signal transduction in the control of phosphate-regulated genes of Escherichia coli. Kidney Int. 1996;49:964–967. doi: 10.1038/ki.1996.136. [DOI] [PubMed] [Google Scholar]
- 29.Mattison K, Kenney LJ. Phosphorylation alters the interaction of the response regulator OmpR with its sensor kinase EnvZ. J. Biol. Chem. 2002;277:11143–11148. doi: 10.1074/jbc.M111128200. [DOI] [PubMed] [Google Scholar]
- 30.Russo FD, Silhavy TJ. EnvZ controls the concentration of phosphorylated OmpR to mediate osmoregulation of the porin genes. J. Mol. Biol. 1991;222:567–580. doi: 10.1016/0022-2836(91)90497-t. [DOI] [PubMed] [Google Scholar]
- 31. Groban ES, Clarke EJ, Salis HM, Miller SM, Voigt CA. Kinetic buffering of cross talk between bacterial two-component sensors. J Mol Biol. 2009;390:380–393. doi: 10.1016/j.jmb.2009.05.007. • •This work measures EnvZ/OmpR and CpxA/R phosphorylation, phosphotransfer and phosphatase reactions of purified components.
- 32.Zhu Y, Qin L, Yoshida T, Inouye M. Phosphatase activity of histidine kinase EnvZ without kinase catalytic domain. Proc Natl Acad Sci U S A. 2000;97:7808–7813. doi: 10.1073/pnas.97.14.7808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cai SJ, Inouye M. EnvZ-OmpR interaction and osmoregulation in Escherichia coli. J. Biol. Chem. 2002;277:24155–24161. doi: 10.1074/jbc.M110715200. [DOI] [PubMed] [Google Scholar]
- 34.Mattison K, Oropeza R, Byers N, Kenney LJ. A phosphorylation site mutant of OmpR reveals different binding conformations at ompF and ompC. J. Mol. Biol. 2002;315:497–511. doi: 10.1006/jmbi.2001.5222. [DOI] [PubMed] [Google Scholar]
- 35.Tran VK, Oropeza R, Kenney LJ. A single amino acid substitution in the C-terminus of OmpR alters DNA recognition and phosphorylation. J. Mol. Biol. 2000;299:1257–1270. doi: 10.1006/jmbi.2000.3809. [DOI] [PubMed] [Google Scholar]
- 36.Carmany DO, Hollingsworth K, McCleary WR. Genetic and biochemical studies of phosphatase activity of PhoR. J Bacteriol. 2003;185:1112–1115. doi: 10.1128/JB.185.3.1112-1115.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hsing W, Russo FD, Bernd KK, Silhavy TJ. Mutations that alter the kinase and phosphatase activities of the two-component sensor EnvZ. J Bacteriol. 1998;180:4538–4546. doi: 10.1128/jb.180.17.4538-4546.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hsing W, Silhavy TJ. Function of conserved histidine-243 in phosphatase activity of EnvZ, the sensor for porin osmoregulation in Escherichia coli. J Bacteriol. 1997;179:3729–3735. doi: 10.1128/jb.179.11.3729-3735.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Skarphol K, Waukau J, Forst SA. Role of His243 in the phosphatase activity of EnvZ in Escherichia coli. J Bacteriol. 1997;179:1413–1416. doi: 10.1128/jb.179.4.1413-1416.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jagadeesan S, Mann P, Schink CW, Higgs PI. A novel "four-component" two-component signal transduction mechanism regulates developmental progression in Myxococcus xanthus. J Biol Chem. 2009;284:21435–21445. doi: 10.1074/jbc.M109.033415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kamberov ES, Atkinson MR, Chandran P, Ninfa AJ. Effect of mutations in Escherichia coli glnL (ntrB), encoding nitrogen regulator II (NRII or NtrB), on the phosphatase activity involved in bacterial nitrogen regulation. J Biol Chem. 1994;269:28294–28299. [PubMed] [Google Scholar]
- 42.Sheeler NL, MacMillan SV, Nodwell JR. Biochemical activities of the absA two-component system of Streptomyces coelicolor. J Bacteriol. 2005;187:687–696. doi: 10.1128/JB.187.2.687-696.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hall MN, Silhavy TJ. The ompB locus and the regulation of the major outer membrane porin proteins of Escherichia coli K12. J. Mol. Biol. 1981;146:23–43. doi: 10.1016/0022-2836(81)90364-8. [DOI] [PubMed] [Google Scholar]
- 44.Aiba H, Nakasai F, Mizushima S, Mizuno T. Evidence for the physiological importance of the phosphotransfer between the two regulatory components, EnvZ and OmpR, in osmoregulation in Escherichia coli. J. Biol. Chem. 1989;264:14090–14094. [PubMed] [Google Scholar]
- 45.Matsuyama S, Mizuno T, Mizushima S. Interaction between two regulatory proteins in osmoregulatory expression of ompF and ompC genes in Escherichia coli: a novel ompR mutation suppresses pleiotropic defects caused by an envZ mutation. J. Bacteriol. 1986;168:1309–1314. doi: 10.1128/jb.168.3.1309-1314.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raivio TL, Silhavy TJ. Transduction of envelope stress in Escherichia coli by the Cpx two-component system. J Bacteriol. 1997;179:7724–7733. doi: 10.1128/jb.179.24.7724-7733.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Utsumi R, Brissette RE, Rampersaud A, Forst SA, Oosawa K, Inouye M. Activation of bacterial porin gene expression by a chimeric signal transducer in response to aspartate. Science. 1989;245:1246–1249. doi: 10.1126/science.2476847. [DOI] [PubMed] [Google Scholar]
- 48.Jin T, Inouye M. Ligand binding to the receptor domain regulates the ratio of kinase to phosphatase activities of the signaling domain of the hybrid Escherichia coli transmembrane receptor, Taz1. J. Mol. Biol. 1993;232:484–492. doi: 10.1006/jmbi.1993.1404. [DOI] [PubMed] [Google Scholar]
- 49.Yoshida T, Qin L, Inouye M. Formation of the stoichiometric complex of EnvZ, a histidine kinase, with its response regulator, OmpR. Mol Microbiol. 2002;46:1273–1282. doi: 10.1046/j.1365-2958.2002.03239.x. [DOI] [PubMed] [Google Scholar]
- 50.King ST, Kenney LJ. Application of fluorescence resonance energy transfer to examine EnvZ/OmpR interactions. Methods Enzymol. 2007;422:352–360. doi: 10.1016/S0076-6879(06)22017-2. [DOI] [PubMed] [Google Scholar]
- 51.Li J, Swanson RV, Simon MI, Weis RM. The response regulators CheB and CheY exhibit competitive binding to the kinase CheA. Biochem. 1995;34:14626–14636. doi: 10.1021/bi00045a003. [DOI] [PubMed] [Google Scholar]
- 52.Welch M, Oosawa K, Aizawa S, Eisenbach M. Phosphorylation-dependent binding of a signal molecule to the flagellar switch of bacteria. Proc Natl Acad Sci U S A. 1993;90:8787–8791. doi: 10.1073/pnas.90.19.8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blat Y, Gillespie B, Bren A, Dahlquist FW, Eisenbach M. Regulation of phosphatase activity in bacterial chemotaxis. J Mol Biol. 1998;284:1191–1199. doi: 10.1006/jmbi.1998.2224. [DOI] [PubMed] [Google Scholar]
- 54.Batchelor E, Goulian M. Robustness and the cycle of phosphorylation and dephosphorylation in a two-component regulatory system. Proc Natl Acad Sci U S A. 2003;100:691–696. doi: 10.1073/pnas.0234782100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shinar G, Milo R, Martinez MR, Alon U. Input output robustness in simple bacterial signaling systems. Proc Natl Acad Sci U S A. 2007;104:19931–19935. doi: 10.1073/pnas.0706792104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alves R, Savageau MA. Comparative analysis of prototype two-component systems with either bifunctional or monofunctional sensors: differences in molecular structure and physiological function. Mol Microbiol. 2003;48:25–51. doi: 10.1046/j.1365-2958.2003.03344.x. [DOI] [PubMed] [Google Scholar]
- 57.Igoshin OA, Alves R, Savageau MA. Hysteretic and graded responses in bacterial two-component signal transduction. Mol Microbiol. 2008;68:1196–1215. doi: 10.1111/j.1365-2958.2008.06221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zapf J, Madhusudan M, Grimshaw CE, Hoch JA, Varughese KI, Whiteley JM. A source of response regulator autophosphatase activity: the critical role of a residue adjacent to the Spo0F autophosphorylation active site. Biochemistry. 1998;37:7725–7732. doi: 10.1021/bi9729615. [DOI] [PubMed] [Google Scholar]
- 59.Thomas SA, Brewster JA, Bourret RB. Two variable active site residues modulate response regulator phosphoryl group stability. Mol Microbiol. 2008;69:453–465. doi: 10.1111/j.1365-2958.2008.06296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gerken H, Charlson ES, Cicirelli EM, Kenney LJ, Misra R. MzrA: a novel modulator of the EnvZ/OmpR two-component regulon. Mol Microbiol. 2009;72:1408–1422. doi: 10.1111/j.1365-2958.2009.06728.x. ••This is one of a few recent examples of an extra component that modulates TCS signaling activity.
- 61.Eguchi Y, Itou J, Yamane M, Demizu R, Yamato F, Okada A, Mori H, Kato A, Utsumi R. B1500, a small membrane protein, connects the two-component systems EvgS/EvgA and PhoQ/PhoP in Escherichia coli. Proc Natl Acad Sci U S A. 2007;104:18712–18717. doi: 10.1073/pnas.0705768104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Szurmant H, Mohan MA, Imus PM, Hoch JA. YycH and YycI interact to regulate the essential YycFG two-component system in Bacillus subtilis. J Bacteriol. 2007;189:3280–3289. doi: 10.1128/JB.01936-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Osborn MJ, Gander JE, Parisi E, Carson J. Mechinism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972;247:3962–3972. [PubMed] [Google Scholar]
- 64.Kenney LJ. Kinase Activity of EnvZ, an osmoregulatory signal transducing protein of Escherichia coli. Arch. Biochem. Biophys. 1997;346:303–311. doi: 10.1006/abbi.1997.0315. [DOI] [PubMed] [Google Scholar]