Abstract
Emotional and motivational dysfunction is fundamental to schizophrenia, and yet the nature and scope of associated deficits are not well understood. This study assessed the integrity of emotional responding from the perspective of its underlying motivational systems during different phases of schizophrenia. Evaluative, somatic, and autonomic responses were measured during viewing of pictures categorized by emotional content, including threat, mutilation, contamination, illness, pollution, mild erotica, families, food and nature. Participants were 13 patients at ultra high-risk or prodromal for psychosis, 40 first-episode schizophrenia patients, 37 chronic schizophrenia patients, and 74 healthy comparison subjects. Irrespective of phase of illness, schizophrenia patients showed a robust and normal pattern of response across multiple systems, with differential engagement of the defensive and appetitive systems as a function of the motivational significance assigned to specific emotional contexts. Although the integrity of core motivational states also appeared to be intact in prodromal patients, a less consistent pattern of response was observed. As continuing efforts are made to identify emotional and motivational abnormalities in schizophrenia, identified deficits will likely be independent of a fundamental dysfunction in basic emotion and motivation response systems and involve integration with higher order processes.
Keywords: schizophrenia, emotion, motivation, attention, startle reflex
Impaired emotional and motivational functioning is prominent in schizophrenia, including flat or inappropriate affect, a diminished ability to experience pleasure, deficits in processing facial expressions, and reductions in motivation. Research to date has targeted these and other components of emotion and motivation in an effort to delineate specific areas of dysfunction. There is a growing recognition, however, that these processes interact not only with each other but also with the prominent cognitive impairments characteristic of schizophrenia, and would benefit from being considered in tandem rather than in isolation. Determining the integrity of motivational processes in schizophrenia is of paramount importance in clarifying how motivation might influence or possibly underlie deficits in cognitive, social and occupational functioning (Barch, 2005, 2008). The present study adopts the perspective that motivation, attention, and emotion are inextricably linked (Bradley, 2009; Lang, Bradley, & Cuthbert, 1997) and examines the integrity of these processes in schizophrenia during engagement of defensive and appetitive motivational systems.
Phylogenetically, activation of the defensive system is engaged primarily in response to physical threat whereas the appetitive system is activated in situations that promote survival (Lang et al., 1997). The emotional content of a picture, therefore, can differentially activate underlying motivational systems and engage the range of behaviors necessary for adapting and responding to the demands of the environment. Pictures representing events that threaten life and physical safety, for example, are more motivationally salient than images of unpleasant but less devastating events (Bradley, Codispoti, Cuthbert, & Lang, 2001), and they activate well-established neural circuits that mediate the somatic and autonomic physiological systems involved in emotion and motivated behavior (Berridge & Robinson, 1998; Lang & Davis, 2006; Phelps & LeDoux, 2005). Similarly, pictures depicting sexual content, as compared with pleasant images of nature and families, evoke a robust motivational state so as to encourage survival through reproduction (Bradley et al., 2001).
When presented with evocative images, individuals with schizophrenia are similar to healthy individuals in their judgments of affective valence and arousal (Hempel et al., 2005; Rockstroh, Junghofer, Elbert, Buodo, & Miller, 2006; Taylor, Phan, Britton, & Liberzon, 2005; Volz, Hamm, Kirsch, & Rey, 2003) and reports of emotional experience (Heerey & Gold, 2007; Herbener, Rosen, Khine, & Sweeney, 2007; Herbener, Song, Khine, & Sweeney, 2008; Schlenker, Cohen, & Hopmann, 1995; Takahashi et al., 2004), although diminished responses have been reported in some patients (Curtis, Lebow, Lake, Katsanis, & Iacono, 1999; Paradiso et al., 2003; Quirk, Strauss, & Sloan, 1998; Seok et al., 2006).1 Irrespective of subjective appraisal, patients with schizophrenia exhibit a normal pattern of affective modulation of the startle eye blink reflex when viewing pictures (Curtis et al., 1999; Schlenker et al., 1995; Volz et al., 2003). Across other psychophysiological systems, including cardiovascular and electrodermal activity, schizophrenia patients and healthy individuals also demonstrate comparable patterns of responding to emotionally salient images (Hempel et al., 2005; Schlenker et al., 1995). In contrast, studies of brain activation have revealed reductions to emotionally potent pictures in schizophrenia patients (Paradiso et al., 2003; Rockstroh et al., 2006; Takahashi et al., 2004; Taylor et al., 2005).
One interpretation of these disparate findings is that the experience of emotion is intact in schizophrenia whereas neurocognitive activation for an emotion-eliciting situation may be inadequate to fully engage motivational systems. For instance, Heerey and Gold (2007) determined that although evocative images may be rated similarly on valence and arousal, schizophrenia patients and healthy participants diverge in subsequent behaviors especially when relying upon memory representations of their evaluative judgments. While these data support the role of cognitive influences on motivation, they leave open the possibility that the integrity of fundamental motivational processes also may be compromised in schizophrenia.
Another important consideration is that prior investigations of schizophrenia have relied exclusively on grouping images by hedonic valence (i.e., unpleasant, pleasant or neutral), possibly at the expense of considering reactions to specific content within each category. By disregarding variability in picture content rather than considering its specific motivational properties, differences between patients with schizophrenia and healthy individuals could be obscured. Patients, for instance, may not show strong discrimination between content categories and, instead, provide a less differentiated pattern of response as compared to healthy individuals. Alternatively, differences between groups could be confined to certain types of pictures with variations in the affective content of the images leading to differential engagement of motivational systems, depending upon clinical diagnosis. For example, affective modulation of the startle reflex is enhanced in some psychiatric conditions (Hamm, Cuthbert, Globisch, & Vaitl, 1997) but diminished in others depending upon picture content (Levenston, Patrick, Bradley, & Lang, 2000).
To assess the pattern of effects of picture content category on evaluative, somatic, and autonomic response systems in schizophrenia, we translated the startle eyeblink reflex paradigm developed by Bradley, Codispoti, Cuthbert, and Lang (2001) to evaluate primary motivational states across different phases of the illness. A distinct advantage of assessing motivation with the startle reflex during picture processing is that it provides a probe of emotional experience while avoiding demands on working memory which is compromised in schizophrenia (Lee & Park, 2005). Beyond observing that unpleasant images would enhance or potentiate the eyeblink reflex and pleasant images would inhibit or attenuate the response (Lang et al., 1997), we expected affective responding to be further modulated by the intensity of motivational activation, with more arousing picture contents eliciting heightened responses among healthy individuals. Hence, ordering of picture content by arousal ratings was expected to result in a strong linear association between the magnitude of the startle reflex and the intensity or motivational salience associated with the type of picture (Bradley et al., 2001).
While still assessing the appetitive system, the present study emphasized evaluating activation of the defensive system in an effort to better understand negative affect. In interpreting their findings, Heerey and Gold (2007) observed that relative to positive images, the capacity to assess and respond to the motivational salience of negative pictures may be particularly impaired in schizophrenia. Thus, we expected unpleasant and, to a lesser degree, pleasant pictures to be less motivationally significant for patients with schizophrenia, such that the overall pattern of affective modulation of the startle reflex would be diminished relative to the robust associations expected in healthy individuals in response to picture salience.
To evaluate whether other response systems are compromised in schizophrenia, subjective appraisal and cardiovascular and electrodermal activity also were examined to reflect the cascade of responses associated with the activation of defensive and appetitive motivational states (Lang et al., 1997). Consistent with reductions in motivational salience, we predicted that with increasing levels of motivational intensity patients would exhibit sustained cardiac deceleration and activation of a defensive response to unpleasant pictures, a triphasic (i.e., deceleratory, acceleratory, deceleratory) pattern of cardiovascular engagement to pleasant pictures and heightened electrodermal responding, and that the overall patterns would be dampened relative to those of healthy individuals.
In addition, we sought to determine whether differences exist in level of motivational and emotional responding across the course of illness. Accordingly, patients in the prodromal or ultra high-risk, first-episode, and chronic phases of schizophrenia were compared within a cross-sectional design. Prior investigations of the affective startle reflex have focused almost exclusively on individuals who were beyond the initial episode of schizophrenia, either as inpatients (Curtis et al., 1999; Schlenker et al., 1995) or outpatients (Volz et al., 2003). Although reductions in drive and motivation appear to be present as early as the prodrome (Yung & McGorry, 1996), there also is evidence that deficits in emotional functioning may increase with illness duration (Kucharska-Pietura, David, Masiak, & Phillips, 2005; Pinkham, Penn, Perkins, Graham, & Siegel, 2007). By determining which capacities are impaired or spared at different points in the illness, targets for intervention can be identified along with intact processes that may provide leverage in developing future treatment.
Methods
Participants
Participants were 13 patients in the prodromal phase of schizophrenia, 40 first-episode schizophrenia patients, 37 chronic schizophrenia patients, and demographically-matched comparison groups consisting of 13, 29 and 32 healthy individuals, respectively. All participants were part of the Center for Neurocognition and Emotion in Schizophrenia at UCLA. Prodromal patients were required to fall between the ages of 15 and 35 years and not meet DSM-IV criteria for schizophrenia, schizophreniform or schizoaffective disorder as assessed by the Structured Clinical Interview for the DSM-IV (SCID; First, 1995; Ventura, Liberman, Green, Shaner, & Mintz, 1998). They were instead required to meet criteria for one of three possible definitions of a prodromal state, as determined by the Structured Interview for Prodromal States (SIPS; Miller et al., 1999). Within the prodromal sample, 4 reported transient psychotic symptoms, and 9 met criteria for attenuated or subthreshold psychotic-like symptoms. First-episode patients were between 18 and 45 years of age, with an initial episode of psychotic illness occurring within the last 2 years. As determined by the SCID, first-episode patients met DSM-IV criteria for schizophrenia (n=23), schizophreniform (n=11) or schizoaffective (n=6) disorder. Patients were classified in the chronic phase if they were between 18 and 60 years of age, their first psychotic episode occurred at least 5 years earlier, and they met DSM-IV criteria for schizophrenia (n=34) or schizoaffective (n=3) disorder. The Scale for the Assessment of Positive Symptoms (SAPS; Andreasen, 1984b) and Scale for the Assessment of Negative Symptoms (SANS; Andreasen, 1984a) were administered to assess symptom levels. All patients were assessed in a clinically stable state. When clinically acceptable, antiparkinsonian medications were discontinued for at least 48 hours before the test session to reduce the possibility of anticholinergic effects.
Individuals were excluded as healthy comparison subjects if they reported a past or present Axis I psychotic disorder; any Axis II schizophrenia-spectrum disorder; any positive symptoms on the SIPS; a history of bipolar disorder, major depression, obsessive-compulsive disorder or post-traumatic stress disorder; alcohol or substance dependence; or presence of a psychotic disorder in a first-degree relative. Exclusion criteria for all participants in this study included evidence of a neurological disorder, major head trauma, significant drug or alcohol abuse, mental retardation or limited fluency in English.
Stimulus Presentation
Forty-four digital images were selected from the International Affective Picture System (IAPS; Lang, Bradley, & Cuthbert, 2005) for specific content and arousal characteristics, and divided into 11 categories based on ratings of hedonic valence and arousal obtained from the IAPS reference sample. The neutral category contained 4 images depicting common household objects and nature scenes rated as minimally arousing. The 7 unpleasant categories, organized from least to most arousing, consisted of images representing pollution and interpersonal loss, bodily illness, contamination, transportation accidents, mutilated human bodies, attacking animals, and attacking humans (i.e., criminal violence or threat). The 3 pleasant categories, ranging from least to most arousing, consisted of images depicting scenes of couples and families, food and nature, and mildly erotic couples or members of the opposite-sex.
Four blocks of images, each containing one exemplar from each of the 11 categories, were presented randomly, resulting in a total of 44 image presentations. Within each block, image order was randomized, resulting in a unique order of presentation for each participant. Each image was displayed for 6 s in 32 bit color at a resolution of 1024 × 768 pixels using a 19” CRT monitor set to a refresh rate of 75 Hz. After each image presentation, participants provided pleasure and arousal ratings on a 1–9 scale using the Self Assessment Manikin (SAM), a pictographic presentation that allows users to report subjective experience of stimuli (Bradley & Lang, 1994). Inter-picture intervals varied between 12 and 22 s, based on the individual’s speed of rating.
To elicit an auditory startle reflex, a burst of 104 dB SPL white noise was presented during half of the picture presentations through insert earphones. The acoustic probe was presented between 3 and 5 s after picture onset and lasted 50 ms after a near-instantaneous rise time. The pairing of pictures and the acoustic probe was counter-balanced between participants such that half of the sample received probes during one set of 22 images with 2 exemplars from each category, and the other half received probes during the remaining 22 images.
Physiological Recording and Data Reduction
In accordance with standard application (Blumenthal et al., 2005), the acoustic startle reflex was indexed through measurement of flexion in the orbicularis oculi muscle, using a pair of 4 mm sintered Ag-AgCl electrodes affixed under the left eye. Signal impedance of less than 5000 Ohms was achieved for all participants. Utilizing a Neuroscan SynAmps system, the raw electromyographic (EMG) signal was amplified with a gain of 250, and, post-recording, the signal was passed through an FIR digital bandpass filter set to simulate an analog filter. Frequencies outside the range of 28–500 Hz were removed. The signal was then rectified and smoothed using a low pass filter with a nominal time constant of 0.7 ms. Following inspection for electrical artifact, peak EMG activity after the acoustic probe was scored for absolute magnitude. These values were then converted to T-scores using the mean magnitude for each individual case to address variations between individuals in blink magnitude and facilitate between-case comparisons (Blumenthal et al., 2005).
The electrocardiogram was recorded with 8mm electrodes, placed on the right and left lower ribs, near the mid-axillary line. The signal was acquired with a bandpass set at 0.05 to 200 Hz and sampled at 2000 Hz. Offline, data from non-probed trials were down-sampled to 1000 Hz and reduced by identifying each R-wave and calculating inter-beat intervals to determine heart rate during each half-second for 1 s prior to, and 6 s during, picture viewing. Within each content category, the maximum deceleration during the initial 3 s of picture viewing and the maximum acceleration during the last 3 s of viewing were identified.
Electrodermal activity was measured using 8 mm electrodes placed on the volar surface of the distal phalanges of the second and third fingers of the non-dominant hand. After acquiring the signal with a Coulbourn V71-23 skin conductance coupler, conductance peaks of non-probed trials were scored by calculating the maximum change from baseline occurring between 1 and 4 s after picture onset and normalized with a log transformation (log [SCR + 1]).
Procedure
Upon obtaining written informed consent, electrodes were applied and each participant was seated in a dimly lit and acoustically-isolated room. A computer monitor was located 1 m in front of the participant and level with their field of vision. After being instructed in the use of the SAM and directed to attend to each image, stimulus presentation was initiated. Participants were monitored via an intercom and closed-circuit camera, and at the conclusion of the session they were fully debriefed and assessed for any adverse reactions.
Data Analysis
Mixed model analyses of variance (ANOVAs) were conducted with the Greenhouse-Geisser method to adjust degrees of freedom. Because age was not found to correlate with any of the dependent measures, yoking of patient groups to separate comparison groups was eliminated in favor of a single, healthy comparison group to maximize sample size for group contrasts. Significant interaction effects involving group were always followed initially by comparing each patient group to the healthy comparison sample. Post hoc analyses relied upon Newman-Keuls tests and univariate ANOVAs, along with the Bonferroni adjusted level of significance when appropriate. Analysis of covariance was used to determine the best-fitting model for averaged waveforms of heart rate change. As reflected in the degrees of freedom, SAM and autonomic activity data were not always available for all participants in a group due to technical difficulties, excessive movement, or with electrodermal activity, the presence of medications with anticholinergic effects.
Results
Demographic and Clinical Characteristics
As shown in Table 1, each patient group was demographically comparable to their healthy comparison sample in terms of age, gender, ethnicity, and parental education level, except that prodromal patients were significantly younger than their comparison sample by about one year on average, F(1,24)=6.75, p=.02. Attempts were not made to match the groups on education level given the potential influence of illness on educational attainment; prodromal and first-episode schizophrenia patients reported fewer years of education relative to their comparison groups, F(1,24)=2.47, p=.03 and F(1,67)=3.29, p=.01, respectively.
Table 1.
Demographic and Clinical Characteristics of Participants
| Healthy Comparison Subjects | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prodromal | First-Episode | Chronic | ||||||||||
| Prodromal | First-Episode | Chronic | Patients | Patients | Patients | |||||||
| (n=13) | (n=29) | (n=32) | (n=13) | (n=40) | (n=37) | |||||||
| Characteristic | M | SD | M | SD | M | SD | M | SD | M | SD | M | SD |
| Age (years) | 18.5 | 1.6 | 23.6 | 2.8 | 34.2 | 4.9 | 17.2 | 0.8 | 22.9 | 4.4 | 33.9 | 7.8 |
| Education (years) | 12.6 | 1.7 | 14.9 | 2.3 | 14.8 | 2.2 | 11.2 | 1.2 | 13.3 | 1.9 | 14.3 | 1.9 |
| Parental Education (years) | 15.1 | 4.5 | 14.2 | 3.1 | 15.2 | 1.9 | 16.3 | 2.3 | 14.3 | 3.9 | 15.3 | 2.7 |
| Duration of Schizophrenia (years) | - | - | - | - | - | - | - | - | 1.0 | 0.6 | 10.0 | 5.3 |
| Medication Dosage (mg/day, in Cpz Equiv)3 |
- | - | - | - | - | - | 90.61 | 81.2 | 217.1 | 95.3 | 426.02 | 317.7 |
| SAPS Total Score4 | - | - | - | - | - | - | 3.6 | 3.1 | 3.9 | 3.4 | 3.2 | 2.5 |
| SANS Total Score5 | - | - | - | - | - | - | 6.9 | 4.4 | 9.5 | 4.3 | 8.8 | 5.1 |
| n | n | n | n | n | n | |||||||
|
| ||||||||||||
| Gender (Female/Male) | 4/9 | 11/18 | 7/25 | 2/11 | 13/27 | 12/25 | ||||||
| Ethnicity | ||||||||||||
| African American | - | 4 | 5 | - | 15 | 5 | ||||||
| Asian American | 1 | 5 | 1 | 3 | 5 | 4 | ||||||
| Caucasian | 7 | 11 | 23 | 4 | 7 | 18 | ||||||
| Hispanic | 4 | 8 | 3 | 3 | 12 | 5 | ||||||
| Mixed | 1 | 1 | - | 3 | 1 | 5 | ||||||
n=4
n=34
Cpz Equiv, Chlorpromazine Equivalents
SAPS, Schedule for the Assessment of Positive Symptoms
SANS, Schedule for the Assessment of Negative Symptoms
As expected, first-episode patients had a significantly shorter duration of illness than chronic schizophrenia patients, F(1,70)=62.3, p=.001. Dosage of antipsychotics also was significantly higher in chronic patients as compared to prodromal and first-episode patients, F(2,75)=10.02, p=.001. The three patient groups, however, did not differ significantly in global symptom levels as assessed by the SAPS, F(2,85)=.65, p=.53, or the SANS, F(2,84)=1.61, p=.21. Negative symptoms were higher than positive symptoms across prodromal individuals, F(1,24)=4.70, p=.04, first-episode patients, F(1,75)=39.22, p=.001, and chronic patients, F(1,70)=34.70, p=.001.
Affective Modulation as a Function of Valence
To determine if prodromal, first-episode, and chronic schizophrenia patients exhibited an overall pattern of affective modulation consistent with previous studies (Curtis et al., 1999; Schlenker et al., 1995; Volz et al., 2003), responses to neutral images were compared to those elicited by unpleasant and pleasant pictures. To equate the number of content categories represented within each valence, a subset of unpleasant categories (i.e., contamination, mutilation, and animal attack) was selected for comparison with the pleasant categories (i.e., couples/families, food/nature, and mild erotica) to yield comparable levels of overall reported arousal (unpleasant: M=5.02; pleasant: M=5.08), as determined by standardized ratings of the IAPS (Lang, Bradley, & Cuthbert, 2005).
Regarding subjective evaluations of the images, significant main effects for valence, F(2,272)=231.50, p=.001, and arousal, F(2,272)=42.09, p=.001, indicated that unpleasant images were rated as low in experienced pleasure and high in experienced arousal while pleasant pictures were rated as high in experienced pleasure and arousal relative to neutral pictures (see Table 2). Although none of the clinical samples differed from the healthy comparison group, patients experiencing prodromal symptoms did rate pleasant pictures as less pleasurable than chronic schizophrenia patients and they found neutral images to be less pleasing than both groups of schizophrenia patients, as supported by a significant group-by-valence interaction effect, F(6,272)=3.56, p=.01. No significant main or interaction effects involving group were observed for ratings of arousal.
Table 2.
Evaluative and Physiological Responses to Pleasant, Neutral, and Unpleasant Pictures, Averaged Across Specific Picture Contents
| Dependent Measures |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pleasure Ratings (1–9) |
Arousal Ratings (1–9) |
Blink Magnitude (T score) |
Initial Heart Rate Deceleration (bpm) |
Peak Heart Rate Acceleration (bpm) |
Skin Conductance Δ (log µS+1) |
|||||||||||||
| M | SD | d | M | SD | d | M | SD | d | M | SD | d | M | SD | d | M | SD | d | |
| Healthy Subjects1 | ||||||||||||||||||
| Pleasant | 6.60 | 1.19 | 5.39 | 1.48 | 48.25 | 3.52 | −3.16 | 2.49 | 0.99 | 4.05 | 0.04g | 0.05 | ||||||
| Neutral | 5.03 | 1.00 | 3.36 | 1.64 | 48.81 | 6.37 | −2.43 | 2.88 | 2.41 | 4.82 | 0.04j | 0.07 | ||||||
| Unpleasant | 3.09 | 1.42 | 5.20 | 1.60 | 51.92 | 3.23 | −3.50 | 2.35 | 0.17 | 3.47 | 0.04m | 0.06 | ||||||
| Prodromal Pts.2 | ||||||||||||||||||
| Pleasant | 5.77a | 1.11 | .68 | 4.81 | 1.64 | .37 | 48.13 | 2.96 | .03 | −3.33 | 2.65 | .03 | 1.67d | 3.82 | .16 | 0.10g,h,i | 0.13 | 1.0 |
| Neutral | 4.54b,c | 1.15 | .40 | 3.69 | 1.54 | .21 | 49.13 | 5.12 | .07 | −4.65 | 5.46 | .33 | 2.38e | 10.12 | .01 | 0.09j,k,l | 0.11 | .83 |
| Unpleasant | 3.34 | 1.34 | .20 | 5.17 | 2.07 | .02 | 52.0 | 3.28 | .02 | −4.85 | 3.04 | .20 | 0.53f | 8.79 | .09 | 0.08m,n,o | 0.10 | .67 |
| First-Episode Pts.3 | ||||||||||||||||||
| Pleasant | 6.31 | 1.22 | .23 | 4.57 | 1.61 | .52 | 48.17 | 3.12 | .02 | −3.29 | 2.74 | .02 | 0.83 | 3.87 | .04 | 0.05h | 0.07 | .17 |
| Neutral | 5.42b | 1.40 | .32 | 3.39 | 1.87 | .02 | 50.39 | 7.06 | .34 | −3.20 | 3.44 | .11 | 1.56 | 4.50 | .20 | 0.03k | 0.05 | .17 |
| Unpleasant | 2.89 | 1.27 | .16 | 4.37 | 1.87 | .53 | 51.47 | 3.19 | .10 | −3.59 | 2.95 | .01 | −0.45 | 3.71 | .15 | 0.04n | 0.07 | .00 |
| Chronic Pts.4 | ||||||||||||||||||
| Pleasant | 6.83a | 1.36 | .19 | 5.04 | 1.44 | .22 | 47.49 | 3.05 | .16 | −3.01 | 2.27 | .02 | −0.63d | 2.15 | .39 | 0.02i | 0.04 | .33 |
| Neutral | 5.50c | 1.48 | .38 | 3.39 | 1.67 | .02 | 51.54 | 7.08 | .59 | −2.45 | 4.06 | .00 | −0.32e | 4.41 | .65 | 0.03l | 0.06 | .17 |
| Unpleasant | 2.54 | 1.17 | .45 | 5.21 | 1.52 | .01 | 51.65 | 2.44 | .06 | −3.32 | 3.29 | .03 | −1.37f | 3.91 | .37 | 0.05o | 0.08 | .17 |
Note. The effect sizes shown (Cohen’s d) compare each of the patient groups with the healthy comparison subjects. For each dependent measure, superscript letter sets are used to indicate the results of comparisons between the four groups within a valence category. Groups that share a letter significantly differ from each other.
Pleasure and arousal ratings (n=65). Blink magnitude (n=74). Initial and peak heart rate (n=66). Skin conductance (n=69).
Pleasure and arousal ratings (n=12). Blink magnitude (n=13). Initial and peak heart rate (n=12). Skin conductance (n=12).
Pleasure and arousal ratings (n=31). Blink magnitude (n=40). Initial and peak heart rate (n=37). Skin conductance (n=29).
Pleasure and arousal ratings (n=32). Blink magnitude (n=37). Initial and peak heart rate (n=36). Skin conductance (n=27).
As shown in Table 2, the prototypic valence effect on the eyeblink reflex was observed in the healthy comparison sample and in each of the patient groups, F(2,320)=13.71, p=.001. Unpleasant images were associated with significantly larger eyeblinks than pleasant images while eyeblinks to neutral images were intermediate. Neither the main effect for group, F(3,160)=1.40, p=.25, nor the group-by-valence interaction effect, F(6,320)=1.15, p=.33, was significant.
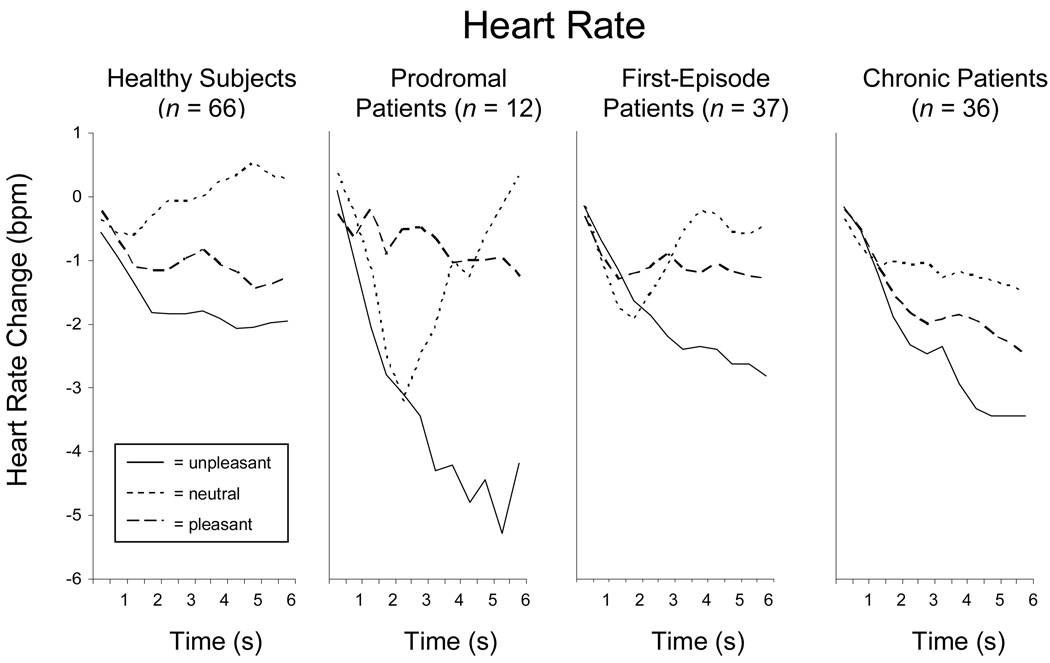
Heart rate response also was consistent with expected patterns: a sustained deceleration to unpleasant pictures and a triphasic response (deceleration-acceleration-deceleration) to pleasant images (see Figure 1). Thus, across the four groups, the best fitting function was quadratic when viewing unpleasant (r 2=.98) scenes and cubic when viewing pleasant (r 2=.91) or neutral (r 2=.92) images (Bradley et al., 2001). As shown in Table 2, the groups did not differ in the magnitude of their initial deceleration, F(3,147)=1.65, p=.18, and no group-by-valence interaction effect was observed, F(6,294)=.58, p=.74. Although pleasant and unpleasant pictures tended to prompt greater initial deceleration relative to neutral images, the effect did not achieve statistical significance, F(2,294)=1.80, p=.18. Later during picture viewing, a group effect did emerge with prodromal patients showing greater peak acceleration relative to chronic schizophrenia patients who persisted in a pattern of deceleration across valence categories during the last 3 s of picture viewing, F(3,147)=3.37, p=.02. A main effect for valence, F(2,294)=5.83, p=.01, replicated prior research with unpleasant scenes resulting in the least peak acceleration followed by pleasant and then neutral images.
Figure 1.
Averaged waveforms for heart rate change when viewing pleasant, neutral, and unpleasant images, with a sustained deceleratory pattern to unpleasant pictures and a triphasic (deceleratory, acceleratory, deceleratory) pattern to pleasant pictures, across healthy comparison subjects, prodromal patients, first-episode schizophrenia patients, and chronic schizophrenia patients.
For skin conductance responses (SCRs; see Table 2), the main effect of valence was not statistically significant, F(2,266)= 1.04, p=.36, probably owing to reliance on lower but more comparable levels of arousal for analyses of hedonic valence. The typical pattern would be increases in sympathetic activation with more arousing stimuli. Unexpectedly, SCRs were significantly higher in patients experiencing prodromal symptoms than in the other three groups, F(1,133)=2.92, p=.04, irrespective of valence.
Consideration of the average effect sizes between each patient group and the healthy comparison sample reveals a similar pattern of findings. With the exception of higher SCRs in prodromal patients, the effect sizes detected were generally quite modest with an occasional moderate effect but no consistent pattern of discrimination between patients and healthy individuals.
Evaluation of the Defensive Motivational System
Although all unpleasant images were rated as low in experienced pleasure (see Table 3), a significant main effect for picture content category, F(6,816)=31.40, p=.001, was modified by a significant group-by-content category interaction, F(18,816)=1.92, p=.02. Prodromal patients rated scenes of human attack as significantly less unpleasant than schizophrenia patients while pictures of accidents also were rated as less unpleasant when compared to judgments made by chronic patients. No significant differences were detected between healthy participants and the two schizophrenia patient groups.
Table 3.
| Table 3a. Pleasure Ratings to Specific Pleasant, Neutral, and Unpleasant Pictures Contents | ||||||||
|---|---|---|---|---|---|---|---|---|
| Healthy Comparison Subjects1 |
Prodromal Patients2 |
First-Episode Schizophrenia Patients3 |
Chronic Schizophrenia Patients4 |
|||||
| Pleasure Ratings (1–9) | ||||||||
| M | SD | M | SD | M | SD | M | SD | |
| Couples/Family | 6.71a | 1.47 | 5.14a,b | 2.16 | 6.54 | 1.45 | 7.07b | 1.60 |
| Food/Nature | 6.49c | 1.33 | 6.04c,d | 1.13 | 6.26 | 1.34 | 6.91d | 1.77 |
| Mild Erotica | 6.62e | 1.41 | 5.98e,f | 1.35 | 6.19 | 1.53 | 6.58f | 1.58 |
| Neutral | 5.03 | 1.00 | 4.54 | 1.15 | 5.42 | 1.40 | 5.50 | 1.48 |
| Loss/Pollution | 3.41 | 1.36 | 3.77 | 1.67 | 3.51 | 1.41 | 3.27 | 1.53 |
| Illness | 3.04 | 1.30 | 3.23 | 1.48 | 3.05 | 1.33 | 2.77 | 1.47 |
| Contamination | 2.94 | 1.45 | 2.73 | 1.61 | 3.01 | 1.46 | 2.37 | 1.29 |
| Accident | 3.05 | 1.37 | 3.60g | 1.51 | 2.91 | 1.42 | 2.34g | 1.32 |
| Mutilation | 2.47 | 1.62 | 2.62 | 1.39 | 2.19 | 1.52 | 1.87 | 1.23 |
| Animal Attack | 3.87 | 1.89 | 4.67 | 2.02 | 3.47 | 1.48 | 3.38 | 1.81 |
| Human Attack | 3.08 | 1.64 | 3.92h,i | 1.83 | 2.49h | 1.36 | 2.24i | 1.28 |
| Table 3b. Arousal Ratings to Specific Pleasant, Neutral, and Unpleasant Pictures Contents | ||||||||
|---|---|---|---|---|---|---|---|---|
| Healthy Comparison Subjects1 |
Prodromal Patients2 |
First-Episode Schizophrenia Patients3 |
Chronic Schizophrenia Patients4 |
|||||
| Arousal Ratings (1–9) | ||||||||
| M | SD | M | SD | M | SD | M | SD | |
| Couples/Family | 5.10 | 2.10 | 4.31 | 1.99 | 4.47 | 1.87 | 4.56 | 1.82 |
| Food/Nature | 5.18 | 1.64 | 4.65 | 1.75 | 4.33 | 1.89 | 4.83 | 1.83 |
| Mild Erotica | 5.81 | 1.71 | 5.35 | 2.07 | 4.89 | 1.90 | 5.61 | 1.66 |
| Neutral | 3.36 | 1.64 | 3.69 | 1.54 | 3.39 | 1.87 | 3.39 | 1.67 |
| Loss/Pollution | 4.61 | 1.51 | 3.92 | 1.29 | 3.87 | 1.74 | 4.34 | 1.48 |
| Illness | 4.55 | 1.46 | 3.98 | 1.96 | 3.79 | 1.71 | 4.31 | 1.65 |
| Contamination | 4.55 | 1.66 | 4.46 | 2.34 | 4.03 | 1.78 | 4.50 | 1.61 |
| Accident | 5.21 | 1.72 | 4.42 | 1.91 | 4.56 | 2.04 | 4.98 | 2.00 |
| Mutilation | 5.54 | 2.00 | 5.21 | 2.24 | 4.41 | 2.19 | 5.41 | 2.20 |
| Animal Attack | 5.52 | 1.80 | 5.83 | 2.07 | 4.66 | 2.29 | 5.71 | 1.77 |
| Human Attack | 5.92 | 1.84 | 5.75 | 2.03 | 5.04 | 2.24 | 6.14 | 1.92 |
| Table 3c. Initial Heart Rate Deceleration to Specific Pleasant, Neutral, and Unpleasant Pictures Contents | ||||||||
|---|---|---|---|---|---|---|---|---|
| Healthy Comparison Subjects1 |
Prodromal Patients2 |
First-Episode Schizophrenia Patients3 |
Chronic Schizophrenia Patients4 |
|||||
| Initial Heart Rate Deceleration (bpm) | ||||||||
| M | SD | M | SD | M | SD | M | SD | |
| Couples/Family | −3.04 | 3.76 | −4.84 | 5.17 | −3.81 | 6.50 | −2.68 | 2.14 |
| Food/Nature | −2.44 | 3.32 | −2.74 | 3.52 | −2.45 | 3.46 | −2.98 | 4.53 |
| Mild Erotica | −3.92 | 4.45 | −2.82 | 3.87 | −3.93 | 3.81 | −3.36 | 3.12 |
| Neutral | −2.43 | 2.88 | −4.65 | 5.46 | −3.20 | 3.44 | −2.45 | 4.06 |
| Loss/Pollution | −3.49 | 4.09 | −6.57a | 5.22 | −5.08 | 6.25 | −2.11a | 4.19 |
| Illness | −3.44 | 3.15 | −5.53 | 5.34 | −3.01 | 3.37 | −3.28 | 4.90 |
| Contamination | −3.32 | 4.04 | −3.98 | 5.56 | −3.41 | 5.32 | −3.57 | 4.00 |
| Accident | −3.06b | 3.43 | −5.29b,c,d | 5.11 | −1.75c | 1.76 | −2.30d | 2.34 |
| Mutilation | −3.78 | 4.05 | −3.18 | 6.22 | −4.25 | 3.63 | −3.06 | 2.88 |
| Animal Attack | −3.41e | 3.84 | −7.39e,f,g | 4.81 | −3.26f | 4.58 | −3.34g | 5.80 |
| Human Attack | −3.17h | 4.08 | −9.40h,i,j | 9.70 | −2.94i | 3.43 | −1.85j | 2.17 |
| Table 3d. Peak Heart Rate Acceleration to Specific Pleasant, Neutral, and Unpleasant Pictures Contents | ||||||||
|---|---|---|---|---|---|---|---|---|
| Healthy Comparison Subjects1 |
Prodromal Patients2 |
First-Episode Schizophrenia Patients3 |
Chronic Schizophrenia Patients4 |
|||||
| Peak Heart Rate Acceleration (bpm) | ||||||||
| M | SD | M | SD | M | SD | M | SD | |
| Couples/Family | 1.22 | 6.50 | 0.70 | 8.22 | −0.27 | 8.08 | −0.30 | 3.00 |
| Food/Nature | 2.00 | 6.12 | 3.12 | 5.26 | 1.60 | 4.15 | −0.26 | 3.02 |
| Mild Erotica | −0.25 | 6.17 | 1.71 | 5.75 | 1.16 | 6.38 | −1.32 | 4.34 |
| Neutral | 2.41 | 4.82 | 2.38 | 10.12 | 1.56 | 4.50 | −0.32 | 4.41 |
| Loss/Pollution | 0.67k | 4.70 | −4.75k,l,m | 6.55 | −1.16l | 6.10 | −0.10m | 4.78 |
| Illness | 0.39 | 4.96 | −0.98 | 5.92 | 0.33 | 4.75 | −1.13 | 3.33 |
| Contamination | 0.76 | 6.84 | 5.41u | 15.82 | 0.92 | 7.26 | −1.16u | 8.61 |
| Accident | 0.24 | 4.26 | −0.80 | 3.68 | 1.89 | 3.85 | −0.11 | 3.51 |
| Mutilation | −0.65 | 5.20 | 1.06 | 12.26 | −2.20 | 4.37 | −1.68 | 3.73 |
| Animal Attack | 0.42o | 4.89 | −4.89o,p,q | 5.34 | −0.01p | 5.93 | −1.27q | 4.03 |
| Human Attack | 1.27r | 6.06 | −4.22r,s,t | 11.63 | 1.74s | 6.97 | −0.16t | 3.12 |
For each dependent measure, superscript letter sets are used to indicate the results of group comparisons within a valence category. Groups that share a letter significantly differ from each other.
(n=65).
(n=12).
(n=31).
(n=32).
For each dependent measure, superscript letter sets are used to indicate the results of group comparisons within a valence category. Groups that share a letter significantly differ from each other.
(n=66).
(n=12).
(n=37).
(n=36).
As shown in Table 3, arousal ratings differed between specific categories, F(6,816)=36.17, p=.001, but followed the expected ordering across unpleasant contents as supported by a significant linear trend, F(1,136)=138.39, p=.001. Using the Bonferroni adjusted level of significance, scenes of human attack, animal attack, mutilations, and accidents received the highest ratings and differed significantly from pictures depicting contamination, loss/pollution, and illness, which did not differ statistically from each other. Human attack was rated significantly higher in subjective arousal than mutilations or accidents, while animal attack was viewed as more arousing than accidents. Analyses investigating group differences did not reveal a significant main effect, F(3,136)=1.91, p=.13, or interaction, F(18, 816)=1.09, p=.36.
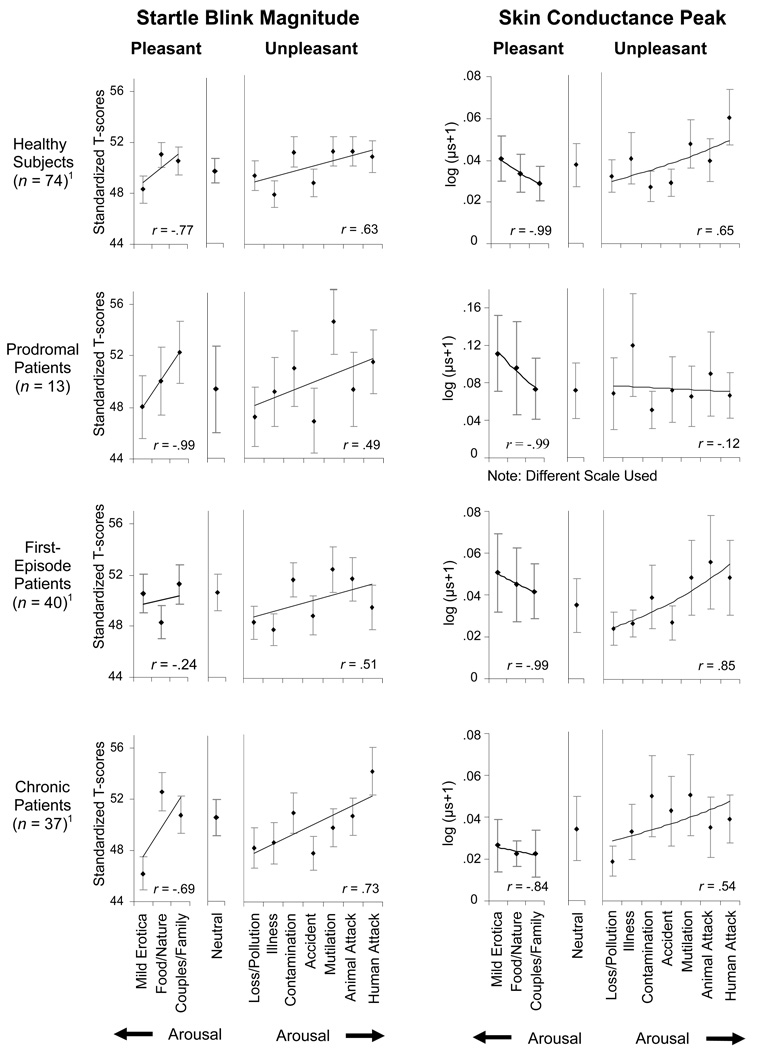
Further supporting the possibility that the defensive motivational system is not compromised by schizophrenia or its prodromal state, a significant main effect of unpleasant picture contents on startle reflex magnitude was obtained in healthy comparison subjects and across all three patient groups, F(6,960)=6.43, p=.001. The left side of Figure 2 illustrates the predicted linear association between increasing levels of self-reported arousal and potentiation of the startle eyeblink reflex in each group, as indicated by a significant effect for a linear trend, F(1,160)=17.45, p=.001. There were no significant effects involving group, F(3,160)=.31, p=.82, or the group-by-content category interaction, F(18,960)=1.04, p=.41. There also were no significant associations between total or subscale scores of the SAPS and SANS and magnitude of the startle reflex when picture contents were ordered by mean arousal ratings.
Figure 2.
Mean startle blink magnitude and skin conductance changes when viewing specific pleasant, neutral, and unpleasant picture contents, across healthy comparison subjects, prodromal patients, first-episode schizophrenia patients, and chronic schizophrenia patients. Picture contents are ordered by rated arousal within pleasant (right to left: low to high arousal) and unpleasant (left to right: low to high arousal) categories.
1For skin conductance data, healthy comparison subjects (n= 70), first-episode patients (n=30), chronic patients (n=29).
Analyses of heart rate response revealed significant effects for group, F(3,146)=6.83, p=.001, and group-by-content category, F(18,876)=2.05, p=.01, during initial deceleration, and for content category, F(6,876)=3.78, p=.01, and group-by-content category, F(18,876)=2.55, p=.01, during peak acceleration (see Table 3). Although rated as less unpleasant by prodromal patients, pictures of human attack, animal attack, and accidents resulted in significantly greater initial deceleration in the high-risk group than in healthy individuals or schizophrenia patients. Among prodromal patients, a larger initial deceleration also was associated with scenes of loss/pollution when compared with chronic patients. Images of human attack, animal attack, and loss/pollution continued to differentiate prodromal patients, who exhibited less acceleration towards the end of picture viewing than the other three groups. In contrast, peak acceleration to pictures of contamination was greater in prodromal individuals than in chronic patients.
The effect of unpleasant contents on SCRs differed by group, as indicated by a significant interaction effect, F(18,750)=2.13, p=.01, and significant group-by-content category trend effects involving linear, F(3,125)=3.43, p=.02, and quadratic, F(3,125)=3.12, p=.03, relationships (see right side of Figure 2). Following Bradley et al. (2001), a main effect of picture content was obtained in healthy comparison subjects, F(6,378)=3.47, p=.01, reflecting a predominately linear association, F(1,63)=14.11, p=.001, and a small quadratic contribution, F(1,63)=4.57, p=.04. Comparisons between each patient group and the healthy sample revealed that first-episode and chronic schizophrenia patients were no different from healthy individuals, showing larger SCRs as arousal levels increased with unpleasant contents. Prodromal patients failed to exhibit this relationship, however, and provided a relatively undifferentiated pattern of SCRs across unpleasant picture contents that tended to be larger overall than in the other three groups, F(3,125)=2.65, p=.06.
Evaluation of the Appetitive Motivational System
As shown in Table 3, all three pleasant content categories were rated as similarly high in experienced pleasure. A significant group effect, F(3,136)=2.80, p=.05, indicated that prodromal patients rated the images as less pleasant than healthy subjects and chronic schizophrenia patients. The expected linear effect of content category on subjective arousal ratings was obtained, F(1,136)=30.66, p=.001, such that pictures of mild erotica were rated as more arousing than images of food/nature and couples/families, F(2,272)=11.97, p=.001.
As further evidence that underlying motivational states showed no indication of abnormality in schizophrenia, startle eyeblink magnitude varied with pleasant picture contents, F(2,320)=6.91, p=.01, again reflecting an overall linear relationship, F(1,160)=11.39, p=.01. Mild erotica generally elicited greater inhibition or smaller eyeblinks than the other two pleasant categories (see left side of Figure 2), in the absence of a significant group effect, F(1,160)=.25, p=.86, or group-by-content category interaction, F(6,320)=1.88, p=.09. Neither total nor subscale scores of the SAPS and SANS corresponded with the effects of picture contents on the startle reflex.
When viewing different pleasant picture contents, the groups did not differ during initial heart rate deceleration, F(3,144)=.20, p=.90, or later peak acceleration, F(3,144)=2.07, p=.11, (see Table 3). The magnitude of these deceleratory and acceleratory responses were not significantly different between content categories, F(2,288)=1.79, p=.17 and F(2,288)=1.81, p=.17, respectively, suggesting comparable levels of cardiovascular activation across the pleasant images.
Consistent with arousal ratings and the eyeblink reflex, SCRs varied with pleasant picture contents, F(2,246)=4.35, p=.02, and as was best accounted for by a linear trend (see right side of Figure 2), F(1,123)=6.16, p=.02. Pictures of mild erotica prompted significantly larger SCRs than scenes of couples/families while food/nature images were associated with moderate responses. Prodromal patients exhibited overall larger SCRs to pleasant images as compared to the other groups, F(3,123)=5.06, p=.01, but the group-by-content category interaction was not significant, F(6,246)=1.20, p=.31.
Discussion
The principal finding from this investigation is that the integrity of the fundamental motivational systems underlying emotional responses in schizophrenia is intact. Notably, when cognitive demands are minimized, schizophrenia patients show a robust pattern of affective modulation across a wide range of picture contents, and a normal level of coherence between electromyographic, cardiovascular, and electrodermal response systems and subjective appraisal. Furthermore, any effects of stage of illness appear to be minimal and when present, confined to patients experiencing prodromal symptoms.
Extending beyond studies of the prototypic pattern of affective modulation when viewing images grouped as pleasant, unpleasant or neutral in valence (Curtis et al., 1999; Schlenker et al., 1995; Volz et al., 2003), the present data demonstrated that emotional reactions among schizophrenia patients are determined to a considerable degree by the intensity of activation of the underlying motivational systems, as are those of healthy individuals. Specifically, results of the present investigation confirm that emotional responses reflect differential engagement of the defensive and appetitive systems as a function of the motivational significance assigned to different emotional contexts (Bradley et al., 2001). During defensive activation, first-episode and chronic schizophrenia patients showed a normal pattern of response to threat when represented in visual images. Pictures of attacking humans or animals as well as mutilated bodies were judged to be the most arousing and prompted greater potentiation of the startle reflex and larger electrodermal responses than unpleasant pictures that are less likely to threaten survival (Lang et al., 1997). As observed in healthy individuals (Bradley et al., 2001), heart rate responses did not discriminate between unpleasant content categories. Instead, cardiac deceleration persisted during picture viewing, indicating sustained attention even at low levels of threat.
Among patients showing prodromal symptoms, the overall pattern of response to aversive picture contents was similar to that of healthy individuals and schizophrenia patients with a few exceptions. Prodromal patients found scenes of human attack as less unpleasant than other patients while also exhibiting pronounced cardiovascular responses to the more threatening images. Additionally, there was a suggestion of greater variance of responding in some instances and heightened electrodermal activity among patients with prodromal symptoms that may have contributed to the absence of a monotonic pattern of response with increasing defensive activation. The slightly different pattern of response among patients experiencing prodromal symptoms may be attributable to the smaller sample size along with substantial diagnostic heterogeneity reflected in this group, given the range of potential outcomes (e.g., schizophrenia, bipolar disorder, depression) as compared with the schizophrenia patient groups. Another possibility is that the greater variance in autonomic responding, the higher mean SCRs, and the lack of co-variation with less consistent self-evaluative reports may reflect a more agitated state as prodromal patients confront the initial emergence of significant clinical symptoms. Despite the lack of concordance between certain subjective ratings and autonomic activity, however, the data generally demonstrate that defensive motivation was strongly activated in prodromal patients. Nevertheless, these findings are in need of replication with a larger sample.
When presented with pleasant picture contents, all three patient groups exhibited patterns of response comparable to that of healthy comparison subjects. Images with erotic contents elicited higher ratings of arousal, greater inhibition of the startle reflex, and heightened electrodermal responses relative to other pleasant pictures that were judged to be less arousing and, presumably, less symbolically representative of promoting survival. Similar to the finding for unpleasant pictures, heart rate response did not vary with picture content.
The consistency of our findings across subjective judgments and measures of somatic and autonomic activity in response to varied picture-viewing contexts, both appetitive and defensive, suggests reliable and robust effects rather than a failure to detect group differences. One implication of these data is that the neural circuitry involved in emotional responses, from the perspective of their underlying motivational systems, is preserved in schizophrenia. This conclusion concurs with available evidence suggesting that individuals with schizophrenia generally report levels of emotional experience comparable to that of healthy individuals (Kring & Moran, 2008) and that deficits in emotional functioning more likely encompass the expression of emotion (Gur et al., 2006), recognition of facial expressions (Edwards, Jackson, & Pattison, 2002), and memory for emotionally evocative stimuli (Heerey & Gold, 2007; Herbener et al., 2007). Similarly, diminished motivation in schizophrenia does not appear to reflect deficits in determining the relative salience or significance of aversive and hedonic cues when represented symbolically in pictures but, instead, may be associated with impairments in attributing or predicting reward value to different situations and outcomes (Gold, Waltz, Prentice, Morris, & Heerey, 2008).
In the present study, we only assessed emotional responses to pictures and did not require participants to maintain a mental representation of emotional events or stimuli. To further specify the degree to which emotion and motivation are compromised in schizophrenia while placing few requirements on working memory, script-driven imagery might be used to generate emotional responses similar to those elicited by pictures. Patient participants in this study also were clinically stable and relatively low in symptoms. In addition, future studies are needed to examine the extent to which emotional and motivational deficits are reflected in other response domains and elicited by a broader array of pleasant stimuli. Research on regional brain activity, for instance, suggests that activation may be reduced to pleasant and unpleasant images in schizophrenia (Paradiso et al., 2003; Rockstroh et al., 2006; Takahashi et al., 2004; Taylor et al., 2005), although these studies have yet to consider the role of motivational systems or the impact of specific emotional contents. Nonetheless, as continuing efforts are made to disentangle neurocognitive, emotional, and motivational functioning in schizophrenia, it appears that identified deficits will likely be independent of a fundamental dysfunction in emotion and motivational response systems and, instead, involve integration with higher order processes.
Acknowledgments
This study was supported in part by grants R01 MH037705, T32 MH014584, and Center grant P50 MH066286 from the National Institute of Mental Health, Bethesda, MD. We gratefully acknowledge the important contributions of the patients and staff of the UCLA Center for Assessment and Prevention of Prodromal States and the UCLA Aftercare Research Program.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/abn.
Because emotional experience has been studied with a variety of stimuli, there is some divergence in findings that also likely reflects the different stimulus materials. This paper is confined primarily to considering prior research relying upon emotion-laden pictures.
References
- Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS) Iowa City, IA: University of Iowa; 1984a. [Google Scholar]
- Andreasen NC. Scale for the Assessment of Positive Symptoms (SAPS) Iowa City, IA: University of Iowa; 1984b. [Google Scholar]
- Barch DM. The relationships among cognition, motivation, and emotion in schizophrenia: how much and how little we know. Schizophr Bull. 2005;31(4):875–881. doi: 10.1093/schbul/sbi040. [DOI] [PubMed] [Google Scholar]
- Barch DM. Emotion, motivation, and reward processing in schizophrenia spectrum disorders: what we know and where we need to go. Schizophr Bull. 2008;34(5):816–818. doi: 10.1093/schbul/sbn092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28(3):309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Blumenthal TD, Cuthbert BN, Filion DL, Hackley S, Lipp OV, van Boxtel A. Committee report: Guidelines for human startle eyeblink electromyographic studies. Psychophysiology. 2005;42(1):1–15. doi: 10.1111/j.1469-8986.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- Bradley MM. Natural selective attention: Orienting and emotion. Psychophysiology. 2009;46(1):1–11. doi: 10.1111/j.1469-8986.2008.00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Cuthbert BN, Lang PJ. Emotion and motivation I: defensive and appetitive reactions in picture processing. Emotion. 2001;1(3):276–298. [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Measuring emotion: the Self-Assessment Manikin and the Semantic Differential. J Behav Ther Exp Psychiatry. 1994;25(1):49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Curtis CE, Lebow B, Lake DS, Katsanis J, Iacono WG. Acoustic startle reflex in schizophrenia patients and their first-degree relatives: evidence of normal emotional modulation. Psychophysiology. 1999;36(4):469–475. doi: 10.1017/s0048577299980757. [DOI] [PubMed] [Google Scholar]
- Edwards J, Jackson HJ, Pattison PE. Emotion recognition via facial expression and affective prosody in schizophrenia: a methodological review. Clin Psychol Rev. 2002;22(6):789–832. doi: 10.1016/s0272-7358(02)00130-7. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-P) New York: New York State Psychiatric Institute; 1995. [Google Scholar]
- Gold JM, Waltz JA, Prentice KJ, Morris SE, Heerey EA. Reward processing in schizophrenia: a deficit in the representation of value. Schizophr Bull. 2008;34(5):835–847. doi: 10.1093/schbul/sbn068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RE, Kohler CG, Ragland JD, Siegel SJ, Lesko K, Bilker WB, et al. Flat affect in schizophrenia: relation to emotion processing and neurocognitive measures. Schizophr Bull. 2006;32(2):279–287. doi: 10.1093/schbul/sbj041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm AO, Cuthbert BN, Globisch J, Vaitl D. Fear and the startle reflex: blink modulation and autonomic response patterns in animal and mutilation fearful subjects. Psychophysiology. 1997;34(1):97–107. doi: 10.1111/j.1469-8986.1997.tb02420.x. [DOI] [PubMed] [Google Scholar]
- Heerey EA, Gold JM. Patients with schizophrenia demonstrate dissociation between affective experience and motivated behavior. J Abnorm Psychol. 2007;116(2):268–278. doi: 10.1037/0021-843X.116.2.268. [DOI] [PubMed] [Google Scholar]
- Hempel RJ, Tulen JH, van Beveren NJ, van Steenis HG, Mulder PG, Hengeveld MW. Physiological responsivity to emotional pictures in schizophrenia. J Psychiatr Res. 2005;39(5):509–518. doi: 10.1016/j.jpsychires.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Herbener ES, Rosen C, Khine T, Sweeney JA. Failure of positive but not negative emotional valence to enhance memory in schizophrenia. J Abnorm Psychol. 2007;116(1):43–55. doi: 10.1037/0021-843X.116.1.43. [DOI] [PubMed] [Google Scholar]
- Herbener ES, Song W, Khine TT, Sweeney JA. What aspects of emotional functioning are impaired in schizophrenia? Schizophr Res. 2008;98(1–3):239–246. doi: 10.1016/j.schres.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kring AM, Moran EK. Emotional response deficits in schizophrenia: insights from affective science. Schizophr Bull. 2008;34(5):819–834. doi: 10.1093/schbul/sbn071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucharska-Pietura K, David AS, Masiak M, Phillips ML. Perception of facial and vocal affect by people with schizophrenia in early and late stages of illness. Br J Psychiatry. 2005;187:523–528. doi: 10.1192/bjp.187.6.523. [DOI] [PubMed] [Google Scholar]
- Lang P, Bradley M, Cuthbert B. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Gainesville, FL: University of Florida; 2005. Technical Report A-6. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Motivated attention: Affect, activation and action. In: L PJ, S RF, B MT, editors. Attention and orienting: Sensory and motivational processes. Hillsdale, N J: Erlbaum; 1997. pp. 97–135. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Gainesville, FL: University of Florida; 2005. Technical Report A-6. [Google Scholar]
- Lang PJ, Davis M. Emotion, motivation, and the brain: reflex foundations in animal and human research. Prog Brain Res. 2006;156:3–29. doi: 10.1016/S0079-6123(06)56001-7. [DOI] [PubMed] [Google Scholar]
- Lee J, Park S. Working memory impairments in schizophrenia: a meta-analysis. J Abnorm Psychol. 2005;114(4):599–611. doi: 10.1037/0021-843X.114.4.599. [DOI] [PubMed] [Google Scholar]
- Levenston GK, Patrick CJ, Bradley MM, Lang PJ. The psychopath as observer: emotion and attention in picture processing. J Abnorm Psychol. 2000;109(3):373–385. [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Woods SW, Stein K, Driesen N, Corcoran CM, et al. Symptom assessment in schizophrenic prodromal states. Psychiatr Q. 1999;70(4):273–287. doi: 10.1023/a:1022034115078. [DOI] [PubMed] [Google Scholar]
- Paradiso S, Andreasen NC, Crespo-Facorro B, O'Leary DS, Watkins GL, Boles Ponto LL, et al. Emotions in unmedicated patients with schizophrenia during evaluation with positron emission tomography. Am J Psychiatry. 2003;160(10):1775–1783. doi: 10.1176/appi.ajp.160.10.1775. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48(2):175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Pinkham AE, Penn DL, Perkins DO, Graham KA, Siegel M. Emotion perception and social skill over the course of psychosis: a comparison of individuals "at-risk" for psychosis and individuals with early and chronic schizophrenia spectrum illness. Cogn Neuropsychiatry. 2007;12(3):198–212. doi: 10.1080/13546800600985557. [DOI] [PubMed] [Google Scholar]
- Quirk SW, Strauss ME, Sloan DM. Emotional response as a function of symptoms in schizophrenia. Schizophr Res. 1998;32(1):31–39. doi: 10.1016/s0920-9964(98)00039-5. [DOI] [PubMed] [Google Scholar]
- Rockstroh B, Junghofer M, Elbert T, Buodo G, Miller GA. Electromagnetic brain activity evoked by affective stimuli in schizophrenia. Psychophysiology. 2006;43(5):431–439. doi: 10.1111/j.1469-8986.2006.00424.x. [DOI] [PubMed] [Google Scholar]
- Schlenker R, Cohen R, Hopmann G. Affective modulation of the startle reflex in schizophrenic patients. Eur Arch Psychiatry Clin Neurosci. 1995;245(6):309–318. doi: 10.1007/BF02191873. [DOI] [PubMed] [Google Scholar]
- Seok JH, An SK, Lee E, Lee HS, Lee YJ, Jeon JH, et al. Behavioural evidence of blunted and inappropriate affective responses in schizophrenia: lack of a "negativity bias.". Psychiatry Res. 2006;142(1):53–66. doi: 10.1016/j.psychres.2005.08.021. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Koeda M, Oda K, Matsuda T, Matsushima E, Matsuura M, et al. An fMRI study of differential neural response to affective pictures in schizophrenia. Neuroimage. 2004;22(3):1247–1254. doi: 10.1016/j.neuroimage.2004.03.028. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Phan KL, Britton JC, Liberzon I. Neural response to emotional salience in schizophrenia. Neuropsychopharmacology. 2005;30(5):984–995. doi: 10.1038/sj.npp.1300679. [DOI] [PubMed] [Google Scholar]
- Ventura J, Liberman RP, Green MF, Shaner A, Mintz J. Training and quality assurance with the Structured Clinical Interview for DSM-IV(SCID-I/P) Psychiatry Research. 1998;79:163–173. doi: 10.1016/s0165-1781(98)00038-9. [DOI] [PubMed] [Google Scholar]
- Volz M, Hamm AO, Kirsch P, Rey ER. Temporal course of emotional startle modulation in schizophrenia patients. Int J Psychophysiol. 2003;49(2):123–137. doi: 10.1016/s0167-8760(03)00100-4. [DOI] [PubMed] [Google Scholar]
- Yung AR, McGorry PD. The prodromal phase of first-episode psychosis: past and current conceptualizations. Schizophr Bull. 1996;22(2):353–370. doi: 10.1093/schbul/22.2.353. [DOI] [PubMed] [Google Scholar]