Abstract
Activation of naive T lymphocytes is regulated through a series of discrete checkpoints that maintain unresponsiveness to self. During this multistep process, costimulatory interactions act as inducible signals that allow APCs to selectively mobilize T cells against foreign Ags. In this study, we provide evidence that the anergy-associated E3 ubiquitin ligase GRAIL (gene related to anergy in lymphocytes) regulates expression of the costimulatory molecule CD40L on CD4 T cells. Using its luminal protease-associated domain, GRAIL binds to the luminal/extracellular portion of CD40L and facilitates transfer of ubiquitin molecules from the intracellular GRAIL RING (really interesting new gene) finger to the small cytosolic portion of CD40L. Down-regulation of CD40L occurred following ectopic expression of GRAIL in naive T cells from CD40−/− mice, and expression of GRAIL in bone marrow chimeric mice was associated with diminished lymphoid follicle formation. These data provide a model for intrinsic T cell regulation of costimulatory molecules and a molecular framework for the initiation of clonal T cell anergy.
The immune system uses an efficient dual-tiered system for the discrimination of Ags as nonself. Central tolerance selects developing lymphocytes that bind self-MHC but do not react strongly against host-derived Ags, whereas peripheral tolerance encompasses a wide array of processes in the secondary lymphoid organs that complement central tolerance and help prevent immune response to self. As a result, full activation of naive T cells requires coordinate signaling with APCs, where binding of TCR and MHC:peptide acts as an initial signal while CD28 interactions with the inducible costimulatory molecules CD80 and CD86 provide a second signal. However, engagement of TCR alone in the absence of costimulation can initiate T cell anergy, a state of induced unresponsiveness to proliferation and IL-2 production (1). Because T cells with reactivity to self can escape clonal deletion (2), anergy could serve as a component of peripheral tolerance that inhibits self-reactive T cells from initiating autoimmunity due to the absence of costimulatory signaling.
Whereas early molecular research into clonal T cell anergy suggested defects in signaling proteins and transcription factors, the role of protein stability controlled by the ubiquitin-protea-some pathway in anergy induction and peripheral tolerance has recently been investigated (3, 4). The gene related to anergy in lymphocytes (GRAIL4; Rnf128) is a type 1 transmembrane E3 ubiquitin ligase identified in a screen for transcripts differentially up-regulated in T cell clones following anergy induction by incubation with MHC:peptide alone (5). Naive CD4 T cells expressing GRAIL via bone marrow chimera production reduced their proliferation and IL-2 secretion in response to activation signals, suggesting that GRAIL acts as a potent, intrinsic anergy promoting factor (6). Endogenous GRAIL expression was subsequently found in multiple model systems of anergy induction, including anti-CD3 stimulation, ionomycin treatment, and in vivo adaptive tolerance (5–7).
Because costimulation abrogates anergy induction and GRAIL is differentially expressed 4–6 h following the initiation of anergy, we hypothesized that an inducible molecule downstream of TCR engagement and upstream of CD28 costimulatory signaling could serve as a target for GRAIL regulation. One potential candidate whose degradation could block T cell activation is CD40 ligand (CD40L; CD154), a type 2 transmembrane protein of the TNF superfamily (8). CD40L expression is low and intracellular on naive CD4 T cells, but following TCR stimulation cell surface CD40L increases rapidly by 6–8 h (9, 10). CD40L trimerization of CD40 on mature APCs further elevates cell surface CD80 and CD86, which in turn bind CD28 on T cells and provide reciprocal bidirectional costimulatory signals for full T cell activation (11). Therefore, modulating cell surface CD40L on the T cell could prevent APC-derived costimulatory signaling and promote anergy induction. Previous evidence lends support to this hypothesis, as anti-CD3 anergized T cells display a deficiency in CD40L expression, suggesting the presence of an anergy-specific factor that prevents CD40L up-regulation (12).
Materials and Methods
Reagents and Abs
Brij96, IPEGAL (Nonidet P-40), mouse anti-FLAG-HRP (clone M2), mouse anti-FLAG-conjugated agarose (M2), and rabbit anti-V5 were from Sigma Aldrich; anti-hemagglutinin (HA)-HRP (clone F7), anti-HA conjugated agarose (clone F7), from Santa Cruz Biotechnology; PE-conjugated anti-mouse CD40L (clone MR1) from BD Pharmingen; allophycocyanin-conjugatedanti-CD4 (GK1.5) from eBioscience; and PMA, ionomycin, and N-acetyl-leucinyl-leucinyl-norleucinal from Calbiochem.
Cell culture and transfection
293T Phoenix cells were grown in DMEM plus 5% heat-inactivated FBS supplemented with l-glutamine and penicillin/streptomycin. For transfection, TransIT-LT1 transfection reagent was used according to the manufacturer's standard protocol (Mirus Bio). Cells were lysedwith Brij96 lysis buffer (20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1% Brij96, and protease inhibitors) and protein concentration was measured by Bradford assay (Bio-Rad). For ubiquitination assays, cells were incubated with N-acetyl-leucinyl-leucinyl-norleucinal (10 μg/ml) for 1.5–2 h at 37°C before lysis. After cell lysis in 1% Brij buffer, lysates were denatured with 0.5% SDS at 100°C for 10 min to remove possible CD40L interacting proteins. Lysates were diluted to 0.1% SDS in Brij96 buffer before immunoprecipitation.
Ionomycin-induced anergy
Purified CD4 T cells from BALB/c mice were stimulated for 48 h with plate bound anti-CD3 (1 μg/ml) and soluble anti-CD28 (1 μg/ml). Cells were then washed and rested in fresh medium for 72 h in vitro. Cells were then incubated with vehicle, ionomycin, or PMA and ionomycin at the indicated concentrations for 18 h and then washed.
Real-time quantitative PCR (QPCR)
RNA was harvested and amplified as described previously (6) with actin as a normalizing gene. Primers used were as follows: GRAIL forward primer, 5′-AGAGAGAGGGGCTTCTGGAG-3′; GRAIL reverse primer, 5′-CGATGA CCATTGTGACTTGG-3′; β-actin forward primer, 5′-CAGGCATTGCTG ACAGGATGCA-3′; and β-actin reverse primer, 5′-GGCCAGGATGGAGCC ACCGATC-3′. All samples were analyzed in triplicate, and GRAIL mRNA arbitrary units are expressed as the mean of triplicate normalized values against β-actin.
Bone marrow chimeras and tissue sections
Generation of bone marrow chimeric mice was performed as described (6). Spleen and lymph nodes were removed and fixed in buffered formalin followed by paraffin embedding. Tissues sections were stained with H&E. Imaging was done using an inverted Nikon scope equipped with a Spot camera and software.
Results and Discussion
Up-regulation of endogenous GRAIL correlates with CD40L down-regulation
A recently described model system for clonal T cell anergy induction used unopposed calcium flux via ionomycin treatment to up-regulate a cohort of E3 ligases in CD4 T cells. These enzymes, including Cbl-b, GRAIL, and Itch, were implicated in controlling the expression of proteins involved in T cell activation via the ubiquitin-proteasome pathway (13). Because anergized T cell clones also exhibit reduced CD40L expression (12), we asked whether ionomycin treatment of recently activated naive T cells would correlate GRAIL expression with a deficiency in CD40L. Purified BALB/c CD4 T cells were stimulated with plate bound anti-CD3 and soluble anti-CD28 for 48 h, washed and rested in vitro for 72 h, and then treated with increasing concentrations of ionomycin overnight. GRAIL expression levels were then assayed by QPCR (Fig. 1A) or Western blotting (Fig. 1B). These data demonstrate that endogenous GRAIL expression is induced at the mRNA and protein level by ionomycin treatment and that GRAIL expression directly correlates with the ionomycin concentration added during anergy induction. In contrast, the addition of PMA with ionomycin prevented both anergy induction and up-regulation of GRAIL mRNA and protein (Fig. 1, A and B). To examine CD40L regulation in this context, we either incubated ionomycinanergized primary CD4 T cells in medium alone or exposed them to recall stimulation and then analyzed the cell surface expression of CD40L (Fig. 1C). CD40L up-regulation on ionomycin-anergized CD4 T cells was markedly reduced following recall activation. In addition, the absence of CD40L on anergized CD4 T cells without recall stimulation demonstrated that ionomycin treatment during anergy induction did not up-regulate CD40L itself or prevent its return to baseline (Fig. 1D). Overall, the percentage of CD40L seen on anergized CD4 T cells inversely correlated with GRAIL expression, as increasing amounts of ionomycin led to more GRAIL up-regulation and less CD40L expressed on the cell surface following recall stimulation (Fig. 1D). Finally, the strength of recall stimulation had no apparent effect on the relative reduction of cell surface CD40L expression seen (Fig. 1D). These data demonstrate a correlation between GRAIL expression and cell surface CD40L levels during anergy induction of primary T cells. The biological relevance of these findings was strengthened by demonstrating endogenous GRAIL up-regulation and CD40L down-regulation in DO11.10 CD4 T cells provided OVA peptide on APCs with costimulatory signals blocked by the addition of soluble CTLA4-Ig (data not shown).
FIGURE 1.

Ionomycin-up-regulated GRAIL expression is associated with reduced CD40L expression. A and B, MACS-purified CD4 T cells from BALB/c mice were anergized with a low (0.1 μM), medium (0.5 μM), or high (1.5 μM) ionomycin dose with or without PMA (200 ng/ml) overnight. Cells were then harvested for QPCR (A) or Western blot analysis (B) of GRAIL expression. N.S., nonspecific band used as a loading control; IB, immunoblot; Norm., normalized. C, MACS-purified BALB/c CD4 T cells were anergized with 0 μM (left) or 1.5 μM (right) ionomycin overnight. Live cells were then washed, stimulated, and stained for cell surface CD40L. D, Histograms representing the percentage of cell surface CD40L expressed on gated CD4 T cells with varying conditions of ionomycin anergy treatment and recall stimulation. Black histogram represents CD4 T cells treated with 0 μM ionomycin, dark gray histogram denotes CD4 T cells treated with 0.5 μM ionomycin, and light gray histogram represents CD4 T cells treated with 1.5 μM ionomycin.
GRAIL binds and ubiquitinates CD40L in vitro
GRAIL is a type 1 transmembrane protein that contains a luminal protease-associated (PA) domain and cytosolic RING (really interesting new gene) finger (Fig. 2A). The PA domain currently does not possess a described canonical function but is proposed to serve as a protein-protein interaction motif (14, 15). The RING finger confers E3 ubiquitin ligase activity by recruiting E2 transferase enzymes loaded with activated ubiquitin molecules for conjugation to substrate proteins (5, 16). CD40L is a type 2 transmembrane protein that contains a large extracellular domain of the TNF superfamily and a small cytoplasmic tail with unknown function (Fig. 2A). Although nearly every cellular protein is regulated by a component of the ubiquitin-proteasome pathway, it would appear difficult for cytosolic E3 ligases to simultaneously bind the 22-aa cytosolic tail of CD40L and retain space for conjugation of the 76-aa ubiquitin molecule. Thus, we hypothesized that the PA domain of GRAIL might provide an interaction interface for binding CD40L on the luminal/extracellular side of the membrane and facilitate ubiquitination of the intracellular tail of CD40L by the cytosolic RING domain of GRAIL.
FIGURE 2.

GRAIL binds and ubiquitinates CD40L. A, Schematic display of the domains and orientation of GRAIL and CD40L. B, 293T cells were transfected with 3× FLAG-tagged human CD40L (hCD40L) and the indicated V5-tagged GRAIL constructs. Eluted proteins from anti-FLAG conjugated agarose were separated by SDS-PAGE and blotted with the indicated Abs. IB, Immunoblot; IP, immunoprecipitation. C, 293T cells were transfected with 3× FLAG-tagged hCD40L, V5-tagged GRAIL constructs, and HA-tagged ubiquitin. Eluted proteins from anti-FLAG-conjugated agarose were separated by SDS-PAGE and blotted with the indicated Abs. D, 293T cells were transfected with 0.2 μg of 3× FLAG-tagged hCD40L and V5-tagged GRAIL at 0.4, 0.8, 1.2, and 1.6 μg (empty V5 vector was added for a total of 2 μg of total V5 plasmid for each transfection). Cell lysates were separated by SDS-PAGE and blotted with the indicated Abs.
To first test for an interaction between GRAIL and CD40L, 293T cells were transfected with N-terminal 3× FLAG-tagged CD40L and various C-terminal V5-tagged GRAIL constructs. Following immunoprecipitation of CD40L, both wild-type and an enzymatically inactive RING finger mutant of GRAIL (H2N2) were able to coimmunoprecipitate with CD40L (Fig. 2B). However, a GRAIL mutant lacking the PA domain (ΔPA) repeatedly failed to coimmunoprecipitate with CD40L, indicating that an intact GRAIL N-terminal PA domain was required for CD40L binding (Fig. 2B). Because the H2N2 mutant is more stable due to a marked reduction in autoubiquitination activity, the higher degree of association seen between CD40L and H2N2 results from the larger protein pool of H2N2 compared with GRAIL (Fig. 2B). To assess whether binding of the PA and TNF superfamily domains could then provide a favorable orientation for ubiquitination of the small cytosolic tail of CD40L by the intracellular GRAIL RING finger, we performed cellular ubiquitination assays by expressing epitope-tagged GRAIL, CD40L, and ubiquitin in 293T cells. A characteristic high m.w. ubiquitin ladder was seen on CD40L only in the presence of wild-type GRAIL and not with the PA or RING finger domain mutants (Fig. 2C). In addition, GRAIL did not ubiquitinate the related TNF superfamily member OX40L (OX40 ligand) in cellular ubiquitination assays, suggesting that the CD40L ubiquitination activity by GRAIL is specific (data not shown).
Next, we asked whether the consequence of GRAIL-mediated ubiquitination would lead to a lower steady state level of CD40L. Titrating the amount of GRAIL transfected into a fixed amount of transfected CD40L caused a reduction in the amount of CD40L present in whole cell lysates (Fig. 2D). Thus, the predominant consequence of GRAIL expression is the degradation of CD40L at the steady state level without any posttranslational modification required for substrate binding. Altogether, these biochemical data demonstrate that GRAIL uses a unique system for the capture and ubiquitination of substrates in which both functional elements are separated across a lipid bilayer. This model can be extrapolated to suggest how transmembrane substrates with small cytosolic domains that are not sufficiently large to support both E3 ligase binding and ubiquitin conjugation can be regulated at the protein level.
GRAIL down-regulates endogenous CD40L in T cells
We next wanted to confirm that GRAIL could regulate CD40L expression in primary CD4 T cells. To avoid the degradation of GRAIL seen following T cell stimulation without ionomycin anergy treatment (Fig. 1, A and B), which is required for CD40L up-regulation, we used the observation that naive CD4 T cells from CD40−/− mice express CD40L at levels well above those seen in naive CD4 T cells from wild-type mice (17). T cells from CD40−/− mice provide a full compartment of CD40L expression both intracellularly and at the cell surface as measured in fixed and live cells, respectively (Fig. 3A). Any decrease in CD40L levels in CD40−/− CD4 T cells transduced to express GRAIL should be indicative of GRAIL-mediated ubiquitination and degradation of endogenous CD40L. Following transduction of vector alone, wild-type GRAIL, or ΔPA constructs, each containing internal ribosome entry site (IRES)-enhanced GFP as a reporter, total CD40L on transduced T cells was reduced only in those cells expressing wild-type GRAIL (Fig. 3B). These data demonstrate that GRAIL down-regulates expression of the total cellular pool of endogenous CD40L in CD4 T cells, most likely CD40L molecules trafficking through the GRAIL-positive endosomal compartments. Targeting nascent CD40L molecules would be preferred for anergy induction instead of removing CD40L from the cell surface following CD40 binding and providing activation signals to the APC (18). Even though GRAIL does not appear to drastically reduce CD40L expression following retroviral transduction in T cells, the mechanism of action for diminished CD40L expression could be one of many elements that act in concert to modulate the activation signals being transmitted between the T cells and APC to create the anergic phenotype.
FIGURE 3.

GRAIL expression down-regulates endogenous CD40L expression. A, Freshly MACS-purified CD4 T cells from C57BL/6 and CD40−/− mice were stained for CD40L. Gray histogram represents unstimulated wild-type CD4 T cells, thick line denotes live CD40−/− CD4 T cells, and dotted line represents intracellular staining of CD40−/− CD4 T cells. B, MACS purified CD4 T cells from CD40−/− mice were stimulated overnight in vitro and then infected with retrovirus expressing the indicated GRAIL construct. After 36 h, cells were stained for total CD40L and gated GFP+ cells are shown. Thick line represents vector-transduced CD4 T cells, gray line denotes GRAIL transduced CD4 T cells, and dotted line represents ΔPA-transduced CD4 T cells.
GRAIL expression in vivo reduces lymphoid follicle formation
To ask whether GRAIL expression in naive CD4 T cells could result in a similar phenotype compared with the reduced lymphoid follicle formation seen in CD40L−/− mice, we generated bone marrow chimeric mice by infecting DO11.10 bone marrow cells with retrovirus expressing vector alone, wild-type GRAIL, H2N2, or a dominant negative mutant of Otubain-1, a negative regulator of GRAIL expression (19). Following transfer of GFP-sorted bone marrow into BALB/c mice, the spleen and lymph nodes were removed from reconstituted recipients for analysis. H&E staining showed defined primary follicles in vector control chimeras (Fig. 4A), whereas wild-type GRAIL-expressing chimeras exhibited smaller, diffuse primary follicles (Fig. 4B). Chimeric mice expressing the dominant negative H2N2 mutant contained extensive primary follicles that were larger than the vector control (Fig. 4C). In addition, bone marrow chimeric mice expressing a dominant negative mutant of Otubain 1, which stabilizes endogenous GRAIL levels, displayed very small and disorganized primary follicles (Fig. 4D), even smaller than those seen in wild-type GRAIL-expressing chimeric mice (Fig. 4B). These data demonstrate that expression of GRAIL as a transgene in vivo results in a phenotype consistent with reduced CD40L expression by peripheral CD4 T cells. Outcompeting endogenous GRAIL with the dominant negative H2N2 mutant lacking an E3 ubiquitin ligase function not only abrogated the effects seen with wild-type GRAIL, but also enlarged the primary follicles above that seen in vector control mice. In addition, stabilization of endogenous GRAIL by a dominant negative Otubain-1 mutant was sufficient to reduce primary follicle formation to that seen in chimeric mice expressing wild-type GRAIL. Together, these data also suggest that both ectopic and endogenous GRAIL can reduce primary follicle formation in vivo.
FIGURE 4.
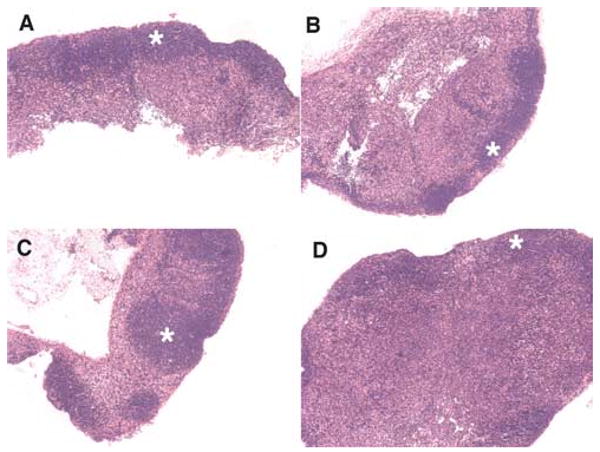
GRAIL expression in naive CD4 T cells results in diminished follicle formation in vivo. DO11.10 bone marrow chimeric mice were generated as described (6). Mice were sacrificed 28 days after injection of transduced hematopoietic cells and lymphoid tissue was processed for histological analysis. A, GFP vector control. B, Wild-type GRAIL. C, Dominant negative H2N2 GRAIL. D, Otubain-1 ARF-1 (epistatic protein stabilizer of GRAIL). Original magnification was × 10;✩ denotes follicular zone. One representative mouse is shown from two to three mice per group from three independent experiments.
Previous work suggested that diminished cell surface CD40L occurred following anergy induction, suggesting the possibility that an anergy-specific factor regulates CD40L expression (12). Because CD40L-CD40 interactions supply maturation signals for APCs to provide, in turn, costimulatory signals that prevent anergy induction, proteolytic regulation of CD40L during anergy induction provided a rational hypothesis for investigation. Furthermore, the small intracellular CD40L region suggested that cytosolic proteins would have difficulty mediating this effect, and a transmembrane E3 ubiquitin ligase associated with anergy induction would instead be an ideal candidate. In this study, we demonstrate that GRAIL expression in CD4 T cells following ionomycin-mediated anergy induction is associated with decreased expression of CD40L. Biochemical evidence demonstrates that GRAIL uses a novel split substrate capture ubiquitination model for regulation of CD40L and possibly other transmembrane proteins with very small intracellular regions. Retroviral transduction of GRAIL into T cells resulted in diminished expression of total CD40L, and a GRAIL mutant lacking a substrate capture domain blocked this effect. GRAIL expression in vivo in bone marrow chimeras resulted in diminished follicle formation. Although GRAIL appears to be selective for CD40L among other TNF superfamily members assayed, E3 ligases target multiple substrates. Therefore, it is possible that modulation of other GRAIL targets also play a significant role in anergy induction in addition to the effects seen with CD40L expression. These data suggest a molecular model for CD4 T cell anergy induction in which TCR signaling in the absence of costimulation leads to GRAIL expression, reduced CD40L up-regulation, and inhibition of the bidirectional costimulatory signaling cascade required for full CD4 T cell activation.
Acknowledgments
We are indebted to Cariel Taylor for excellent technical assistance. We gratefully acknowledge Shoshana Levy for the hCD40L cDNA and Ron Kopito for HA-tagged ubiquitin. Special thanks for Robyn Rajkovich for administrative support.
Footnotes
This work was supported by National Institutes of Health Grants CA 65237-17, T32-AI07290-21, and U19-AI070352 and the Tom and Susan Ford Stanford Graduate Fellowship (to N.L.).
Abbreviations used in this paper: GRAIL, gene related to anergy in lymphocytes; CD40L, CD40 ligand; HA, hemagglutinin; PA, protease-associated domain; QPCR, real-time quantitative PCR; RING, really interesting new gene.
Disclosures: The authors have no financial conflict of interest.
References
- 1.Schwartz RH. Tcell anergy. Annu Rev Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 2.Bouneaud C, Kourilsky P, Bousso P. Impact of negative selection on the T cell repertoire reactive to a self-peptide: a large fraction of T cell clones escapes clonal deletion. Immunity. 2000;13:829–840. doi: 10.1016/s1074-7613(00)00080-7. [DOI] [PubMed] [Google Scholar]
- 3.Fathman CG, Lineberry NB. Molecular mechanisms of CD4+ T-cell anergy. Nat Rev Immunol. 2007;7:599–609. doi: 10.1038/nri2131. [DOI] [PubMed] [Google Scholar]
- 4.Mueller DL. E3 ubiquitin ligases as T cell anergy factors. Nat Immunol. 2004;5:883–890. doi: 10.1038/ni1106. [DOI] [PubMed] [Google Scholar]
- 5.Anandasabapathy N, Ford GS, Bloom D, Holness C, Paragas V, Seroogy C, Skrenta H, Hollenhorst M, Fathman CG, Soares L. GRAIL: an E3 ubiquitin ligase that inhibits cytokine gene transcription is expressed in anergic CD4+ T cells. Immunity. 2003;18:535–547. doi: 10.1016/s1074-7613(03)00084-0. [DOI] [PubMed] [Google Scholar]
- 6.Seroogy CM, Soares L, Ranheim EA, Su L, Holness C, Bloom D, Fathman CG. The gene related to anergy in lymphocytes, an E3 ubiquitin ligase, is necessary for anergy induction in CD4 T cells. J Immunol. 2004;173:79–85. doi: 10.4049/jimmunol.173.1.79. [DOI] [PubMed] [Google Scholar]
- 7.Safford M, Collins S, Lutz MA, Allen A, Huang CT, Kowalski J, Blackford A, Horton MR, Drake C, Schwartz RH, Powell JD. Egr-2 and Egr-3 are negative regulators of T cell activation. Nat Immunol. 2005;6:472–480. doi: 10.1038/ni1193. [DOI] [PubMed] [Google Scholar]
- 8.Foy TM, Aruffo A, Bajorath J, Buhlmann JE, Noelle RJ. Immune regulation by CD40 and its ligand GP39. Annu Rev Immunol. 1996;14:591–617. doi: 10.1146/annurev.immunol.14.1.591. [DOI] [PubMed] [Google Scholar]
- 9.Castle BE, Kishimoto K, Stearns C, Brown ML, Kehry MR. Regulation of expression of the ligand for CD40 on T helper lymphocytes. J Immunol. 1993;151:1777–1788. [PubMed] [Google Scholar]
- 10.Roy M, Waldschmidt T, Aruffo A, Ledbetter JA, Noelle RJ. The regulation of the expression of gp39, the CD40 ligand, on normal and cloned CD4+ T cells. J Immunol. 1993;151:2497–2510. [PubMed] [Google Scholar]
- 11.Grewal IS, Flavell RA. CD40 and CD154 in cell-mediated immunity. Annu Rev Immunol. 1998;16:111–135. doi: 10.1146/annurev.immunol.16.1.111. [DOI] [PubMed] [Google Scholar]
- 12.Bowen F, Haluskey J, Quill H. Altered CD40 ligand induction in tolerant T lymphocytes. Eur J Immunol. 1995;25:2830–2834. doi: 10.1002/eji.1830251018. [DOI] [PubMed] [Google Scholar]
- 13.Heissmeyer V, Macian F, Im SH, Varma R, Feske S, Venuprasad K, Gu H, Liu YC, Dustin ML, Rao A. Calcineurin imposes T cell unresponsiveness through targeted proteolysis of signaling proteins. Nat Immunol. 2004;5:255–265. doi: 10.1038/ni1047. [DOI] [PubMed] [Google Scholar]
- 14.Luo X, Hofmann K. The protease-associated domain: a homology domain associated with multiple classes of proteases. Trends Biochem Sci. 2001;26:147–148. doi: 10.1016/s0968-0004(00)01768-0. [DOI] [PubMed] [Google Scholar]
- 15.Mahon P, Bateman A. The PA domain: a protease-associated domain. Protein Sci. 2000;9:1930–1934. doi: 10.1110/ps.9.10.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joazeiro CA, Weissman AM. RING finger proteins: mediators of ubiquitin ligase activity. Cell. 2000;102:549–552. doi: 10.1016/s0092-8674(00)00077-5. [DOI] [PubMed] [Google Scholar]
- 17.Lesley R, Kelly LM, Xu Y, Cyster JG. Naive CD4 T cells constitutively express CD40L and augment autoreactive B cell survival. Proc Natl Acad Sci USA. 2006;103:10717–10722. doi: 10.1073/pnas.0601539103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yellin MJ, Sippel K, Inghirami G, Covey LR, Lee JJ, Sinning J, Clark EA, Chess L, Lederman S. CD40 molecules induce down-modulation and endocytosis of T cell surface T cell-B cell activating molecule/CD40-L. Potential role in regulating helper effector function. J Immunol. 1994;152:598–608. [PubMed] [Google Scholar]
- 19.Soares L, Seroogy C, Skrenta H, Anandasabapathy N, Lovelace P, Chung CD, Engleman E, Fathman CG. Two isoforms of otubain 1 regulate T cell anergy via GRAIL. Nat Immunol. 2004;5:45–54. doi: 10.1038/ni1017. [DOI] [PubMed] [Google Scholar]


