Abstract
Using a systematic and comprehensive gene expression library (the ASKA library), we have carried out a genome-wide screening of the genes whose increased plasmid-directed expression affected glycogen metabolism in Escherichia coli. Of the 4123 clones of the collection, 28 displayed a glycogen-excess phenotype, whereas 58 displayed a glycogen-deficient phenotype. The genes whose enhanced expression affected glycogen accumulation were classified into various functional categories including carbon sensing, transport and metabolism, general stress and stringent responses, factors determining intercellular communication, aggregative and social behaviour, nitrogen metabolism and energy status. Noteworthy, one-third of them were genes about which little or nothing is known. We propose an integrated metabolic model wherein E. coli glycogen metabolism is highly interconnected with a wide variety of cellular processes and is tightly adjusted to the nutritional and energetic status of the cell. Furthermore, we provide clues about possible biological roles of genes of still unknown functions.
Keywords: ASKA library, carbohydrate metabolism, functional genomics
1. Introduction
Glycogen is a major intracellular reserve polymer consisting of α-1,4-linked glucose subunits with α-1,6-linked glucose at the branching points, which accumulates in Escherichia coli and other bacteria under conditions of limiting growth when an excess of carbon source is available and other nutrients are deficient.1–3 The exact role of this polyglucan in bacteria is still not well-defined, but several works have linked glycogen metabolism to environmental survival, symbiotic performance and colonization and virulence.4–12
Bacterial glycogen is produced by the concerted action of glycogen synthase (GlgA) and branching enzyme (GlgB) using ADP-glucose (ADPG) as the sugar donor nucleotide.1 Since the initial demonstration that ADPG serves as the precursor molecule for bacterial glycogen biosynthesis,13 it has been considered that ADPG pyrophosphorylase (GlgC) is the sole enzyme catalyzing the production of ADPG in these organisms.14 However, recent reports have provided evidence about the occurrence of other important sources of ADPG linked to glycogen biosynthesis in bacterial species such as E. coli, Salmonella, Streptomyces coelicolor and Mycobacterium tuberculosis.11,15–17 Genes involved in glycogen metabolism in enterobacterial species, such as E. coli and Salmonella enterica, are clustered in two apparently independent transcriptional units designated as glgBX (encoding GlgB and debranching GlgX enzymes) and glgCAP [comprising genes coding for the glycogen anabolic enzymes GlgC and GlgA, and the catabolic glycogen phosphorylase (GlgP)].1
Regulation of E. coli glycogen metabolism involves a complex assemblage of factors that are adjusted to the physiological and energetic status of the cell,2,3,18,19 and cell-to-cell communication.20 At the level of enzyme activity, glycogen metabolism is subjected to the allosteric regulation of GlgC by different glycolitic intermediates.14 Also, E. coli GlgP activity is regulated by the phosphorylation status of the carbohydrate phosphotransferase system (PTS) protein Hpr.21 At the level of gene expression, several factors have been described to control E. coli glycogen accumulation. This includes negative regulation by the carbon storage regulator CsrA and by the still unidentified glgQ regulatory locus,22–24 and positive regulation by guanosine 5′-triphosphate 3′-diphosphate and/or guanosine 5′-diphosphate 3′-diphosphate [(p)ppGpp] stringent response regulators3,25–28 and by the PhoP–PhoQ regulatory system at low environmental Mg2+ concentration.3 Different experimental evidences also indicate positive regulation of glgCAP expression by the cyclic AMP/cyclic AMP receptor protein complex29–31 (however, for an opposite view, see Montero et al.3 and Hengge-Aronis and Fischer32). The general stress regulator RpoS does not regulate glgCAP expression, but positively controls the expression of glgS, a gene whose product exerts a positive effect on glycogen accumulation.32
We have recently initiated a series of studies aimed to uncover mechanisms regulating bacterial glycogen metabolism and its connection with other biological processes. Using a systematic and comprehensive gene-disrupted mutant collection of E. coli (the Keio collection33), we carried out genome-wide screenings of genes affecting glycogen metabolism in this bacterial species.2,3 Our studies revealed that bacterial glycogen metabolism is highly interconnected with a wide variety of cellular processes and proposed an integrated metabolic model wherein glycogen metabolism is influenced by the stringent and general stress responses, end-turnover of tRNA, intracellular AMP levels, nutrient transport and metabolism, low extra-cellular Mg2+ availability and energy production.3 To further investigate the mechanisms regulating bacterial glycogen metabolism and its connection with other biological processes, in this work, we have carried out a genome-wide analysis of glycogen content using the ASKA library, a set of 4123 clones expressing all predicted ORFs of an E. coli K-12 derivative.34 The overall data presented in this work reinforce the idea that glycogen metabolism is highly interconnected with a wide variety of cellular processes and is adjusted to the bacterial energy and nutritional status. Furthermore, we provide evidence showing that glycogen metabolism is also affected by factors determining intercellular communication, aggregative and social behaviour modes.
2. Materials and methods
2.1. Bacterial strains and culture conditions
We used the ASKA library, a set of 4123 different clones of the AG1 E. coli K-12 strain (recA1 endA1 gyrA96 thi-1 hsdR17(rK_mKþ) supE44 relA1), each expressing one of all predicted E. coli K-12 ORFs.34 For quantitative measurement of glycogen content, cells were grown at 37°C with rapid gyratory shaking in liquid Kornberg medium (1.1% K2HPO4, 0.85% KH2PO4, 0.6% yeast extract from Duchefa, Haarlem, the Netherlands) supplemented with 50 mM glucose and 1 mM MgCl2 after inoculation with 1 vol. of an overnight culture per 50 volumes of fresh medium. The culture medium was not supplemented with IPTG. Cultures entering the stationary phase were centrifuged at 4400g for 15 min, and the collected cells were rinsed with fresh Kornberg medium, resuspended in 40 mM Tris–HCl (pH 7.5) and disrupted by sonication as described previously.2 Solid culture medium was prepared by adding 1.8% bacteriological agar to liquid Kornberg medium before autoclaving.
2.2. Screening of ASKA clones with altered glycogen content
A first screening of glycogen in the different bacterial clones of the ASKA collection after growth on solid glucose Kornberg medium was carried out employing the iodine staining method. In the presence of iodine vapours, ‘glycogen-excess’ clones stained darker than its brownish parent cells, whereas ‘glycogen-deficient’ clones stained yellow.16 Clones identified using this procedure were subsequently cultured in liquid glucose Kornberg medium and subjected to the quantitative measurement of glycogen content at the onset of the stationary phase using an amyloglucosidase/hexokinase/glucose-6P dehydrogenase-based test kit from Sigma. Intracellular glycogen content was referred to protein, which was measured using a Bio-Rad (USA) prepared reagent. The function of each gene whose enhanced expression affects glycogen accumulation was assigned by referring to the EchoBASE (http://ecoli-york.org/)35 and EcoCyc (http://www.ecocyc.org/)36 databases.
2.3. Morphotype evaluation
To monitor the expression of curli and cellulose biosynthesis, 10 µl of a bacterial overnight culture suspended in water to an absorbance at 600 nm of five were spotted onto TY agar plates (1% Bacto Tryptone, 0.5% yeast extract, 1.5% bacteriological agar) supplemented with 40 µg ml−1 Congo red and 20 µg ml−1 Coomassie brilliant blue.37 Plates were incubated at 28°C for 5 days, and dye binding was evaluated by red colour intensity. The multicellular rdar morphotype is characterized by a red, dry and rough aspect on Congo red agar plates, which is determined by the expression of extracellular matrix components such as cellulose and adhesive curli fimbriae.38 The appearance of a pink colony (pdar morphotype) is indicative of cellulose biosynthesis.39 Capacity for cellulose production was also qualitatively analyzed by assessing the level of calcofluor white (Fluorescent brightener 28; Sigma) binding of colonies grown on TY agar plates supplemented with 50 µg ml−1 of this dye. Fluorescence of the cells was observed under a 366 nm UV light source and compared with the wild-type (WT) strain.
2.4. General molecular techniques
Routine DNA manipulations were performed following standard procedures.40 Plasmids were extracted by Quantum Prep plasmid mini-prep kit (Bio-Rad). ΔrelA cells of the Keio collection33 expressing relA in trans were obtained by incorporation of relA- expression vector of the ASKA library. DNA sequencing was carried out in Secugen (Madrid). Sequence homologies to genes in the GenBank database were determined by using the BLAST algorithm of the National Center for Biotechnology Information at the National Library of Medicine.
2.5. Analytical procedures
Bacterial growth was followed spectrophotometrically by measuring the absorbance of cultures at 600 nm. Protein contents in bacterial extracts were measured by the Coomassie G dye-binding method using a Bio-Rad prepared reagent.
3. Results and discussion
3.1. Screening, identification and classification of genes whose enhanced expression affects glycogen accumulation
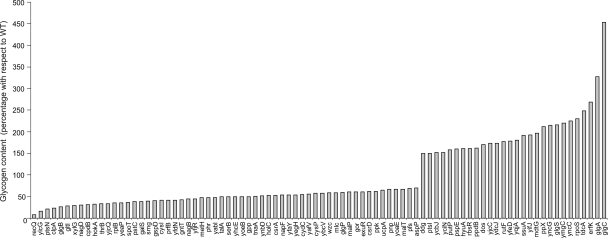
Clones of ASKA collection were first screened for altered glycogen content in solid glucose Kornberg medium. In the presence of iodine vapours, ‘glycogen-excess’ clones stained darker than their brownish parent cells, whereas ‘glycogen-deficient’ clones stained yellow. On inspecting the ASKA library, 28 clones (0.7% of the library) displayed ‘glycogen-excess’ phenotypes, whereas 58 clones (1.4% of the library) displayed yellow, ‘glycogen-deficient’ phenotypes. Subsequent quantitative glycogen measurement analyses on cells entering the stationary phase confirmed that the 86 selected clones accumulate altered levels of glycogen (Fig. 1).
Figure 1.
Glycogen content (referred as percentage of glycogen accumulated by WT cells) of glycogen-excess and glycogen-deficient clones of the ASKA library. Averaged glycogen content in WT cells was 45 nmol glucose mg protein−1. Cells were grown at 37°C with rapid gyratory shaking in liquid Kornberg medium (1.1% K2HPO4, 0.85% KH2PO4, 0.6% yeast extract) supplemented with 50 mM glucose. Because some yeast extracts are deficient in Mg2+,3 and because Mg2+ is a major determinant of cell metabolic and energetic status and of expression of genes affecting glycogen metabolism,91,92 the Kornberg medium was also supplemented with 1 mM MgCl2.
The 86 genes whose enhanced expression showed modified glycogen accumulation were classified into clusters of orthologous groups (COGs).41 Tables 1 and 2 show the genes whose enhanced expression leads to glycogen-excess and glycogen-deficient phenotypes, respectively, whereas Supplementary Table S1 shows the function of each gene product. In some cases, the families are clearly meaningful, with the presence of multiple genes of related function, reinforcing the validity of their identification in the survey. Yet, a large group of 28 clones, representing one-third of the clones identified, express genes about which little or nothing is known such as glgS, gspD, mdtG, ppdB, rutF, smg, ucpA, yabI, yafV, ybcV, ycbJ, yciN, ydcJ, yegH, yfaY, yfdN, yfeD, yfjR, yhcE, yjcC, yjcQ, ylcG, ymgC, ynbD, yncC, yncG, yoaE and yqjA.
Table 1.
Escherichia coli genes whose enhanced expression caused a ‘glycogen-excess’ phenotype in cells of the ASKA library entering the stationary phase
| Metabolism | |
| E | Amino acid transport and metabolism (1/432): putP |
| F | Nucleotide transport and metabolism (1/94): hyuA |
| G | Carbohydrate transport and metabolism (3/395): glgA, glgC, ptsI |
| P | Inorganic ion transport and metabolism (3/273): ppx, pspE, ssuA |
| Cellular processes | |
| M | Cell wall/membrane/envelope biogenesis (1/227): ddg |
| O | Posttranslational modification, protein turnover, chaperones (1/144): yncG |
| U | Intracellular trafficking (1/116): ppdB |
| Information, storage and processing | |
| K | Transcription (5/321): rbsR, rpoS, tdcA, yfeD, yncC |
| T | Signal Transduction (2/186): dos, yjcC |
| Poorly characterized | |
| R | General function prediction only (3/510): mdtG, rutF, yifJ |
| S | Function unknown (3/315): erfK, ydcJ, yqjA |
| No COG assignment (4/590): glgS, ycbJ, yciN, ymgC | |
Table 2.
Escherichia coli genes whose enhanced expression caused a ‘glycogen-deficient’ phenotype
| Metabolism | |
| C | Energy production and conversion (2/301): gor, napF |
| E | Amino acid transport and metabolism (5/432): gltI, metH, serB, thrB, tnaA |
| F | Nucleotide transport and metabolism (3/94): cpdB, gpp, pfs |
| G | Carbohydrate transport and metabolism (10/395): glgB, glgP, gntT, malP, nagB, nagD, ptsN, rpiB, talA, xylG |
| I | Lipid transport and metabolism (1/104): ynbD |
| P | Inorganic ion transport and metabolism (4/273): cysI, cysP, ppK, pstC |
| Cellular processes | |
| M | Cell wall/membrane/envelope biogenesis (1/227): wzc |
| O | Posttranslational modification, protein turnover, chaperones (2/144): clpA, cydC |
| T | Signal transduction mechanisms (3/186): csrA, csrD, yeaP |
| U | Intracellular trafficking, secretion, and vesicular transport (1/116): gspD |
| Information, storage and processing | |
| J | Translation, ribosomal structure and biogenesis (2/188): pnp, prfB |
| K | Transcription (6/321): exuR, galS, malT, mlc, spoT, yfjR |
| L | DNA replication, recombination and repair (3/224):holC, phr, recQ |
| Poorly characterized | |
| R | General function prediction only (7/510): ucpA, yafV, ybcV, yegH, yfaY, yoaE, aspP |
| S | Function unknown (4/315): smg, yabI, yjcQ, yoeB |
| No COG assignment (4/590): hokA, yfdN, yhcE, ylcG | |
The general trend observed after this analysis indicates that glycogen metabolism of E. coli cells cultured in glucose Kornberg medium is affected by genes whose products can be embodied in the following groups:
Carbon sensing, transport and metabolism;
general stress response;
stringent response;
factors determining intercellular communication, aggregative and social behaviour;
nitrogen metabolism;
energy status.
3.1.1. Carbon sensing, transport and metabolism
As expected from the glycogen synthetic roles of GlgA and GlgC, glgA and glgC over-expressing bacteria of the ASKA library displayed glycogen-excess phenotypes (Fig. 1). In fact, these bacteria presented the highest levels of glycogen accumulation of the whole collection. In agreement also with the assigned function of GlgP and AspP in E. coli glycogen breakdown,42,43 AG1 cells over-expressing glgP and aspP showed reduced glycogen accumulation (Fig. 1). Noteworthy, although GlgB is a glycogen anabolic enzyme, glgB over-expressing cells of the ASKA library displayed a glycogen-deficient phenotype (Fig. 1). This could be ascribed to the fact that glycogen granule architecture is the result of the highly orchestrated actions of GlgB and other glycogen enzymes, which may collapse under GlgB overproduction conditions.44
The global regulator of carbon metabolism CsrA is an RNA-binding protein, which is thought to prevent glycogen biosynthesis by both promoting glgCAP decay and translation.24,45 Consistently, csrA over-expressing bacteria of the ASKA library displayed a glycogen-deficient phenotype (Fig. 1). CsrA activity is antagonized by the two CsrB and CsrC non-coding RNAs,46–48 which in turn are targeted by CsrD for RNase E degradation.49 Thus and consistent with the assigned role of CsrD as relieving CsrA function from CsrB and CsrC, csrD over-expressing cells of the ASKA library displayed a glycogen-deficient phenotype (Fig. 1).
malP and malT over-expressing bacteria of the ASKA collection displayed glycogen-deficient phenotypes (Fig. 1). MalT is a transcriptional regulator of genes involved in maltose/maltodextrin transport and metabolism.50,51 MalP, over which MalT exerts a positive control, catalyzes the phosphorolytic breakdown of maltodextrins. However, MalP poorly recognizes large and highly branched polyglucans such as glycogen,42 suggesting an indirect rather than a direct effect of malP over-expression on glycogen accumulation. In this respect, previous studies have indicated tight, albeit still not well characterized, links between glycogen and maltodextrin metabolisms.52,53
PTS is a major determinant of transport and phosphorylation of a large number of carbohydrates including glucose.21,54,55 PTS mutants impaired in sensing and transport of glucose accumulate low glycogen content.3 It is therefore conceivable that over-expression of some PTS components would result in enhanced glycogen content in cells cultured in glucose Kornberg medium, whereas cells over-expressing the Mlc transcriptional repressor of PTS genes56 would display a glycogen-deficient phenotype. Confirming this presumptions ptsI over-expressing bacteria of the ASKA library displayed a glycogen-excess phenotype (Fig. 1), whereas cells ectopically expressing the Mlc transcriptional repressor of PTS genes displayed a glycogen-deficient phenotype (Fig. 1).
3.1.2. General stress response
Different genetic studies indicate a requirement of the general stress regulator RpoS as a positive modulator of glycogen biosynthesis.2,3,57 In agreement, rpoS over-expressing cells of the ASKA library displayed a glycogen-excess phenotype (Fig. 1). It has been shown that RpoS up-regulates the expression of glgS, a gene whose product exerts a positive effect on glycogen accumulation.2,32 Consistently, glgS over-expressing cells of the ASKA library displayed a glycogen-excess phenotype (Fig. 1).
3.1.3. Stringent response
During nutrient starvation, E. coli elicits the so-called ‘stringent response’ that switches the cell from a growth-related mode to a maintenance/survival mode.58,59 The hallmark of this pleiotropic physiological response is the accumulation of the alarmones pppGpp and ppGpp.58–60 Although ppGpp is more abundant than pppGpp, the relative effects of these two regulatory nucleotides have not been thoroughly examined, their levels depending on the synthesis of pppGpp by RelA and SpoT, the hydrolysis of pppGpp to ppGpp by Gpp, and the breakdown of ppGpp by the bifunctional enzyme SpoT.58–62 (p)ppGpp binds bacterial RNA polymerase to increase transcription of amino acid biosynthesis genes during amino acid starvation and to down-regulate the transcription of ‘stable’ RNAs (rRNAs and tRNAs) genes.58,59 As transcription of genes coding for components of the translation apparatus account for a large percentage of transcription in exponentially growing cells, the liberation of RNA polymerase from these genes is thought to passively allow up-regulation of diverse promoters activated at the onset of stationary phase.63
Different in vivo and in vitro experimental evidences have linked the E. coli stringent response and (p)ppGpp accumulation with increased glycogen contents and enhanced expression of glg genes at the onset of the stationary phase.25–28 Consistent with the involvement of (p)ppGpp in regulatory aspects of glycogen metabolism, and also consistent with the assigned functions of SpoT and Gpp in (p)ppGpp degradation,64,65 both spoT and gpp over-expressing cells of the ASKA collection displayed glycogen-deficient phenotypes (Fig. 1).
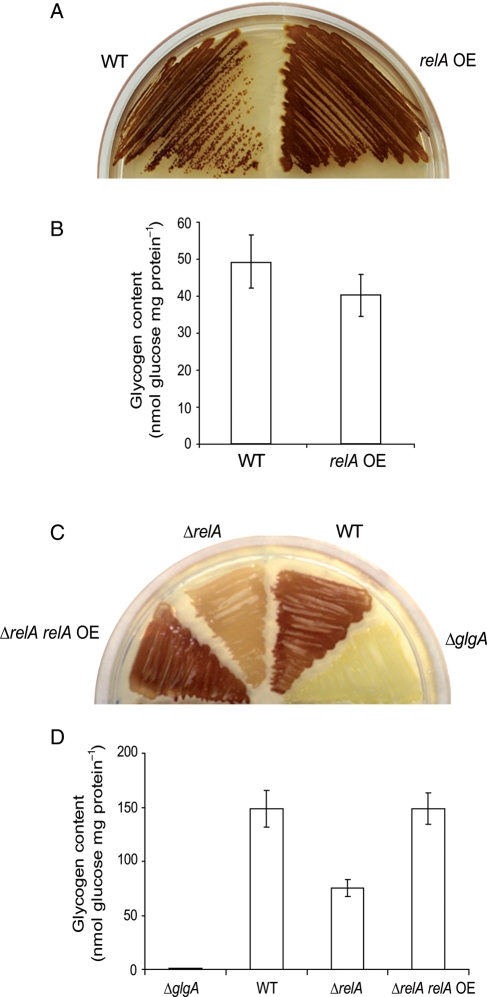
We recently found that ΔrelA cells of the E. coli Keio collection33 display reduced glycogen contents and restricted expression of glgC::lacZ transcriptional fusions3 (see also Fig. 2), which further fortifies the view that (p)ppGpp plays an important role in glycogen accumulation in E. coli. AG1 strain used in the ASKA library as plasmids recipient has been annotated as a K-12 derivative relA1 mutant.34 relA1 mutants possess little or residual pppGpp synthase activity, which is due to an IS2 insertion between the 85th and 86th codons of the WT relA structural gene.66 It is thus conceivable that relA over-expressing cells of the ASKA collection would display a glycogen-excess phenotype. Surprisingly, however, these cells displayed glycogen levels similar to those of control AG1 cells (Fig. 2A and B). To understand why the relA over-expressing cells of the ASKA library accumulate glycogen levels comparable to those of control cells, we sequenced the relA gene of AG1 cells. This analysis revealed that relA of AG1 does not contain any mutation (not shown). To explore whether this phenomenon could be ascribed to the possible occurrence of secondary mutations in AG1 cells or to defects of the ASKA library relA expression vector, we analyzed the glycogen contents in ΔrelA cells of the Keio mutant collection transformed with the ASKA library relA expression vector. These cells were constructed on a K-12 derivative BW25113 strain, which is normal for the relA function. As shown in Fig. 2C and D, ectopic expression of relA complemented the glycogen-deficient phenotype of ΔrelA cells, the overall data thus showing that (i) AG1 cells are not relA1 mutants, (ii) the relA expression vector of the ASKA library codes for an active RelA form and (iii) ectopic expression of relA does not lead to enhancement of glycogen accumulation.
Figure 2.
AG1 is not a relA1 mutant. In (A) and (B), relA over-expressing cells of the ASKA library accumulate WT glycogen content. In (C) and (D), relA expression vector of the ASKA library complements the glycogen-deficient phenotype of ΔrelA cells of the Keio collection. ΔglgA cells of the Keio collection are used as negative control for glycogen accumulation.
3.1.4. Factors determining intercellular communication, aggregative and social behaviour modes
We have recently shown that glycogen metabolism may also be subjected to regulation by cell-to-cell communication.20 In E. coli, swimming, swarming and adherence of cells to surfaces or to one another by biofilm formation are fundamental modes to communicate and to coordinately regulate metabolic processes. Communication, aggregative and social behaviour modes are highly determined by environmental cues and act as major determinants of the nutritional status of the cell, which as discussed above is a major determinant of glycogen accumulation. The following data provided evidence that factors determining intercellular communication, aggregative and social behaviour (Supplementary Fig. S1) are important determinants of glycogen content in E. coli, although further studies are required to get a clear picture of the link between the different factors involved.
First, enhanced expression of the poorly characterized yncC, yncG and ymgC genes (all down-regulated in the mqsR biofilm deficient mutants67) resulted in increased glycogen content (Fig. 1). yncC encodes a transcription factor that positively affects biofilm formation by repressing production of the biofilm matrix component colanic acid,68 whereas ymgC is an orphan gene belonging to the ymgABC operon whose transcription is repressed in young and mature biofilms, but is induced in the intermediate, developed biofilms.69 Although the function of ymgC is still unknown, ymgA and ymgB strongly promote the synthesis of colanic acid, and virtually eliminate the expression of adhesive curli fimbriae genes.
Second, gaining-of-function of GGDEF and EAL domain enzymes controlling the intracellular levels of cyclic diguanylate (a secondary messenger that regulates the transition from the motile, planktonic state to sessile, community-based behaviours in different bacteria39,70,71) resulted in changes in the intracellular glycogen content. For instance, up-regulation of YeaP (a diguanylate cyclase that positively regulates the expression of csg genes involved in curli and cellulose production72) exerted a negative effect on glycogen accumulation (Fig. 1). In contrast, up-regulation of YjcC (a predicted cyclic diguanylate phosphodiesterase that down-regulates the expression of the CsgD central regulator of extra-cellular matrix components37,38,73) and Dos (a cyclic diguanylate phosphodiesterase74) resulted in enhanced glycogen content (Fig. 1).
Third, some ASKA clones ectopically expressing functions that participate in the synthesis of biofilm components and/or precursors displayed glycogen deficient phenotypes. Thus, ectopic expression of Wzc (an autophosphorylating protein-tyrosine kinase that prevents the production of colanic acid75,76), GalS (a repressor of metabolism of galactose linked to the synthesis of colanic acid and other exopolysaccharide components77,78), and NagB (a d-glucosamine 6-P isomerase that prevents the synthesis of major components of the cell envelope such as peptidoglycans and lipopolysaccharides79) resulted in a glycogen-deficient phenotype (Fig. 1).
3.1.5. Nitrogen metabolism
It is known that carbon metabolism is subject to regulation by nitrogen availability, although the mechanisms involved are still obscure. PtsN is a member of the nitrogen-related PTS, which has been associated with balancing of nitrogen and carbon metabolism,80 and regulation of the RpoE-dependent cell envelope stress response and potassium uptake.81,82 Most recently, evidence has been provided suggesting the occurrence of cross-talk between sugar-PTS and nitrogen-related PTS.83 Consistent with this view, the ptsN overproducing cells of the ASKA library displayed a marked glycogen-deficient phenotype when cultured in glucose Kornberg medium (Fig. 1).
Yeast extract (the amino acid source of the Kornberg medium employed in this work) is deficient in various amino acids.2,84 Mutants impaired in cysteine biosynthesis entering the stationary phase display a glycogen-excess phenotype when they are cultured in the Kornberg medium, which is ascribed to stringent response-mediated up-regulation of glg genes occurring when the culture medium is deficient in cysteine precursors.2 Consistent with the view that the stringent response favourably affects glycogen accumulation in E. coli, serB, cysI and cysP over-expressing cells of the ASKA library (all ectopically expressing genes involved in cysteine biosynthesis) accumulated lower levels of glycogen than WT cells at the onset of the stationary phase (Fig. 1). tdcA positively regulates the expression of tdcB and tdcC genes, which code for biodegradative proteins involved in threonine and serine metabolism.85,86 Also consistent with the view that the lack of internal amino acid provision positively affects glycogen accumulation, tdcA over-expressing cells of the ASKA library displayed a glycogen-excess phenotype (Fig. 1).
3.1.6. Energy status
Because intracellular ATP level is a major determinant of glycogen accumulation,3,18 it is conceivable that any factor affecting ATP availability will also affect glycogen accumulation. In fact, deletion mutants lacking components required for the proper functioning of the aerobic electron transport chain and ATP generation displayed a glycogen-deficient phenotype.2,3 Consistent with this view, bacteria with enhanced expression of cytosolic enzymes likely competing with GlgC for the same ATP pool, such as RecQ (an ATP-dependent DNA helicase87), NagD (a promiscuous ribo and deoxyribonucleoside tri-, di- and monophosphatase88), and Ppk (an ATP requiring enzyme that catalyzes the production of polyphosphate89) displayed glycogen-deficient phenotypes (Fig. 1).
Glutathione is a major determinant of cell redox status, playing a prime role in maintaining the correct assembly of electron transport chain components.90 It is therefore conceivable that factors altering the intracellular glutathione levels will also affect ATP and glycogen formation. In agreement with this presumption, bacteria with enhanced expression of GorA (a glutathione reductase) and CydC (a protein involved in the transport of glutathione from the cytosol to the periplasm90) displayed glycogen deficient phenotypes (Fig. 1).
3.2. Proposal of an integrated model for the regulation of glycogen metabolism in E. coli
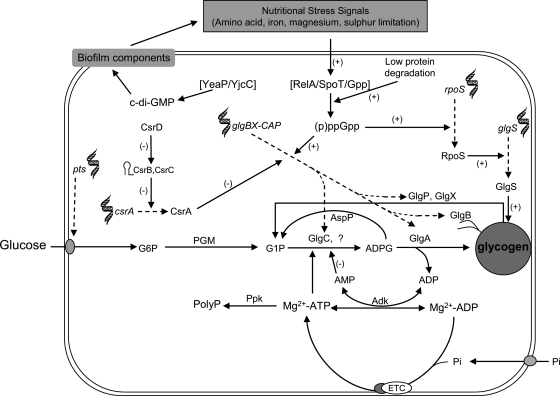
Results presented in this work further strengthen the view that glycogen metabolism is highly interconnected with a wide variety of cellular processes.2,3 Figure 3 illustrates a suggested model of glycogen metabolism in E. coli wherein major determinants of glycogen accumulation include intracellular concentration and availability of ATP for ADPG synthesis, levels of ppGpp (which accumulates in a RelA- SpoT- and/or Gpp-dependent manner under conditions of limited provision of nutrients such as amino acids, sulphur, Mg2+, iron, etc.), factors determining intercellular communication, aggregative and social behaviour modes (which in turn determine the nutritional status of the cell), expression levels of the general stress regulator RpoS and of the global regulator CsrA, availability of a carbon source and less well-defined systems sensing the cell energy status through the activity of the electron transport chain.
Figure 3.
Suggested model of glycogen metabolism in E. coli wherein major determinants of glycogen accumulation include availability of ATP for ADPG synthesis, levels of (p)ppGpp (which accumulates in a RelA- SpoT- and/or Gpp-dependent manner under conditions of limited provision of nutrients such as amino acids, sulphur, Mg2+, iron, etc.), factors determining intercellular communication, aggregative and social behaviour modes (which in turn determine the nutritional status of the cell), expression levels of the general stress regulator RpoS and of the global regulator CsrA, availability of a carbon source, redox status of the cell and less well-defined systems sensing the cell energy status through the activity of the electron transport chain. CsrB and CsrC small non-coding RNAs, represented by stem-loops, are likely involved in the regulation of functions strongly affecting glycogen accumulation through interaction with CsrA (Adk, adenylate kinase; c-di-GMP, cyclic diguanylate; ETC, electron transport chain).
Supplementary data
Supplementary data are available at www.dnaresearch.oxfordjournals.org.
Funding
This research was partially supported by the grant BIO2007-63915 from the Comisión Interministerial de Ciencia y Tecnología and Fondo Europeo de Desarrollo Regional (Spain) and by Iden Biotechnology S.L.
Supplementary Material
Acknowledgements
G.E. and G.A. acknowledge their pre-doctoral fellowships from the Public University of Nafarroa. M.M. acknowledges a post-doctoral contract from I3P program of Consejo Superior de Investigaciones Científicas. A.M.V. expresses his gratitude to the Ministerio de Educación y Cultura, the Consejo Superior de Investigaciones Científicas and the Public University of Nafarroa for their support. M.R. acknowledges a JAE pre-doctoral fellowship from CSIC. We thank María Angeles Barado, Maite Hidalgo and Jessica Díaz de Cerio for technical assistance and support. We also thank Dr Cristina Solano (Institute of Agrobiotechnology) for highly stimulating and constructive discussions. We thank the National Institute of Genetics (Shizuoka, Japan) for providing us the ASKA library. We also thank Dr H. Mori (Nara Institute of Science and Technology, Japan) and Dr M. Tomita (Keio University, Japan) to provide us the Keio collection.
Footnotes
Edited by Katsumi Isono
References
- 1.Preiss J., Romeo T. Molecular biology and regulatory aspects of glycogen biosynthesis in bacteria. Prog. Nucleic Acid Res. Mol. Biol. 1994;47:299–329. doi: 10.1016/s0079-6603(08)60255-x. [DOI] [PubMed] [Google Scholar]
- 2.Eydallin G., Viale A.M., Morán-Zorzano M.T., et al. Genome-wide screening of genes affecting glycogen metabolism in Escherichia coli K-12. FEBS Lett. 2007;581:2947–53. doi: 10.1016/j.febslet.2007.05.044. [DOI] [PubMed] [Google Scholar]
- 3.Montero M., Eydallin G., Almagro G., et al. Escherichia coli glycogen metabolism is controlled by the PhoP–PhoQ regulatory system at submillimolar environmental Mg2+ concentrations, and is highly interconnected with a wide variety of cellular processes. Biochem. J. 2009;424:129–41. doi: 10.1042/BJ20090980. [DOI] [PubMed] [Google Scholar]
- 4.Strange R.E. Bacterial ‘glycogen’ and survival. Nature. 1968;220:606–7. doi: 10.1038/220606a0. [DOI] [PubMed] [Google Scholar]
- 5.Van Houte J., Jansen H.M. Role of glycogen in survival of Streptococcus mitis. J. Bacteriol. 1970;101:1083–5. doi: 10.1128/jb.101.3.1083-1085.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonafonte M.A., Solano C., Sesma B., et al. The relationship between glycogen synthesis, biofilm formation and virulence in Salmonella enteritidis. FEMS Microbiol. Lett. 2000;191:321–36. doi: 10.1111/j.1574-6968.2000.tb09315.x. [DOI] [PubMed] [Google Scholar]
- 7.Marroquí S., Zorreguieta A., Santamaría C., et al. Enhanced symbiotic performance by Rhizobium tropici glycogen synthase mutants. J. Bacteriol. 2001;183:854–64. doi: 10.1128/JB.183.3.854-864.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lepek V.C., D′Antuono A.L., Tomatis P.E., Ugalde J.E., Giambiagi S., Ugalde R.A. Analysis of Mesorhizobium loti glycogen operon: effect of phosphoglucomutase (pgm) and glycogen synthase (glgA) null mutants on nodulation of Lotus tenuis. Mol. Plant Microbe Interact. 2002;15:368–75. doi: 10.1094/MPMI.2002.15.4.368. [DOI] [PubMed] [Google Scholar]
- 9.Chang D.E., Smalley D.J., Tucker D.L., et al. Carbon nutrition of Escherichia coli in the mouse intestine. Proc. Natl Acad. Sci. USA. 2004;101:7427–32. doi: 10.1073/pnas.0307888101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones S.A., Jorgensen M., Chowdhury F.Z., et al. Glycogen and maltose utilization by Escherichia coli O157:H7 in the mouse intestine. Infect. Immun. 2008;76:2531–40. doi: 10.1128/IAI.00096-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sambou T., Dinadayala P., Stadthagen G., et al. Capsular glucan and intracellular glycogen of Mycobacterium tuberculosis: biosynthesis and impact on the persistence in mice. Mol. Microbiol. 2008;70:762–74. doi: 10.1111/j.1365-2958.2008.06445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bourassa L., Camilli A. Glycogen contributes to the environmental persistence and transmission of Vibrio cholerae. Mol. Microbiol. 2009;72:124–38. doi: 10.1111/j.1365-2958.2009.06629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Preiss J., Shen L., Greenberg E., Gentner N. Biosynthesis of bacterial glycogen. IV. Activation and inhibition of the adenosine diphosphate glucose pyrophosphorylase of Escherichia coli. Biochemistry. 1966;5:1833–45. doi: 10.1021/bi00870a008. [DOI] [PubMed] [Google Scholar]
- 14.Ballicora M.A., Iglesias A.A., Preiss J. ADP-glucose pyrophosphorylase, a regulatory enzyme for bacterial glycogen synthesis. Microbiol. Mol. Biol. Rev. 2003;67:213–25. doi: 10.1128/MMBR.67.2.213-225.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin M.C., Scheneider D., Bruton C.J., Chater K.F., Hardisson C. A glgC gene essential only for the first two spatially distinct phases of glycogen synthesis in Streptomyces coelicolor. J. Bacteriol. 1997;179:7784–9. doi: 10.1128/jb.179.24.7784-7789.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morán-Zorzano M.T., Alonso-Casajús N., Muñoz F.J., et al. Occurrence of more than one important source of ADP-glucose linked to glycogen biosynthesis in Escherichia coli and Salmonella enterica. FEBS Lett. 2007;581:4423–9. doi: 10.1016/j.febslet.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 17.Eydallin G., Morán-Zorzano M.T., Muñoz F.J., et al. An Escherichia coli mutant producing a truncated inactive form of GlgC synthesizes glycogen: further evidences for the occurrence of various important sources of ADP-glucose in enterobacteria. FEBS Lett. 2007;581:4417–22. doi: 10.1016/j.febslet.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 18.Shen L.C., Atkinson D.E. Regulation of adenosine diphosphate glucose synthase from Escherichia coli. Interactions of adenylate energy charge and modifier concentrations. J. Biol. Chem. 1970;245:3996–4000. [PubMed] [Google Scholar]
- 19.Dietzler D.N., Lais C.J., Leckie M.P. Simultaneous increases of the adenylate energy charge and the rate of glycogen synthesis in nitrogen-starved Escherichia coli W4957 (K) Arch. Biochem. Biophys. 1974;160:14–25. doi: 10.1016/s0003-9861(74)80003-2. [DOI] [PubMed] [Google Scholar]
- 20.Morán-Zorzano M.T., Montero M., Muñoz F.J., et al. Cytoplasmic Escherichia coli ADP sugar pyrophosphatase binds to cell membranes in response to extracellular signals as the cell population density increases. FEMS Microbiol. Lett. 2008;288:25–32. doi: 10.1111/j.1574-6968.2008.01319.x. [DOI] [PubMed] [Google Scholar]
- 21.Deutscher J., Francke C., Postma P.W. How phosphotransferase system-related protein phosphorylation regulates carbohydrate metabolism in bacteria. Microbiol. Mol. Biol. Rev. 2006;70:939–1031. doi: 10.1128/MMBR.00024-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Romeo T., Gong M., Liu M.Y., Brun-Zinkernagel M. Identification and molecular characterization of csrA, a pleiotropic gene from Escherichia coli that affects glycogen biosynthesis, gluconeogenesis, cell size, and surface properties. J. Bacteriol. 1993;175:4744–55. doi: 10.1128/jb.175.15.4744-4755.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang H., Liu M.Y., Romeo T. Coordinate genetic regulation of glycogen catabolism and biosynthesis in Escherichia coli via the CsrA gene product. J. Bacteriol. 1996;178:1012–7. doi: 10.1128/jb.178.4.1012-1017.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baker C.S., Morozov I., Suzuki K., Romeo T., Babitzke P. CsrA regulates glycogen biosynthesis by preventing translation of glgC in Escherichia coli. Mol. Microbiol. 2002;44:1599–610. doi: 10.1046/j.1365-2958.2002.02982.x. [DOI] [PubMed] [Google Scholar]
- 25.Bridger A.A., Paranchych W. relA gene control of bacterial glycogen synthesis. Can. J. Biochem. 1978;56:403–6. doi: 10.1139/o78-063. [DOI] [PubMed] [Google Scholar]
- 26.Taguchi M., Izui K., Katsuki H. Augmentation of glycogen synthesis under stringent control in Escherichia coli. J. Biochem. 1980;88:379–87. doi: 10.1093/oxfordjournals.jbchem.a132983. [DOI] [PubMed] [Google Scholar]
- 27.Romeo T., Black J., Preiss J. Genetic regulation of glycogen biosynthesis in Escherichia coli: in vivo effects of the catabolite repression and stringent response systems in glg gene expression. Curr. Microbiol. 1990;21:131–7. [Google Scholar]
- 28.Traxler M.F., Summers S.M., Nguyen H-T., et al. The global ppGpp-mediated stringent response to amino acid starvation in Escherichia coli. Mol. Microbiol. 2008;68:1128–48. doi: 10.1111/j.1365-2958.2008.06229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dietzler D.N., Leckie M.P., Magnani J.L., Sughrue M.J., Bergstein P.E., Sternheim W.L. Contribution of cyclic adenosine 3′:5′-monophosphate to the regulation of bacterial glycogen synthesis in vivo. J. Biol. Chem. 1979;254:8308–17. [PubMed] [Google Scholar]
- 30.Urbanowski J., Leung P., Weissbach H., Preiss J. The in vitro expression of the gene for Escherichia coli ADP glucose pyrophosphorylase is stimulated by cyclic AMP and cyclic AMP receptor protein. J. Biol. Chem. 1983;258:2782–4. [PubMed] [Google Scholar]
- 31.Romeo T., Preiss J. Genetic regulation of glycogen biosynthesis in Escherichia coli: in vitro effects of cyclic AMP and guanosine 5′-diphosphate 3′-diphosphate and analysis of in vivo transcripts. J. Bacteriol. 1989;171:2773–82. doi: 10.1128/jb.171.5.2773-2782.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hengge-Aronis R., Fischer D. Identification and molecular analysis of glgS, a novel growth-phase-regulated and rpoS-dependent gene involved in glycogen synthesis in Escherichia coli. Mol. Microbiol. 1992;6:1877–86. doi: 10.1111/j.1365-2958.1992.tb01360.x. [DOI] [PubMed] [Google Scholar]
- 33.Baba T., Ara T., Hasegawa M., et al. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2006 doi: 10.1038/msb4100050. doi:10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kitagawa M., Ara T., Arifuzzaman M., et al. Complete set of ORF clones of Escherichia coli ASKA library (a complete set of E. coli K-12 ORF archive): unique resources for biological research. DNA Res. 2005;12:291–9. doi: 10.1093/dnares/dsi012. [DOI] [PubMed] [Google Scholar]
- 35.Misra R.V., Horler R.S.P., Reindl W., Goryanin I.I., Thomas G.H. EchoBASE: an integrated post-genomic database for Escherichia coli. Nucleic Acids Res. 2005;33(Database issue):D329–33. doi: 10.1093/nar/gki028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keseler I.M., Collado-Vides J., Gama-Castro S., et al. EcoCyc: a comprehensive database resource for Escherichia coli. Nucleic Acids Res. 2005;33(Database issue):D334–7. doi: 10.1093/nar/gki108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simm R., Lusch A., Kader A., Andersson M., Römling U. Role of EAL-containing proteins in multicellular behavior of Salmonella enterica serovar Typhimurium. J. Bacteriol. 2007;189:3613–23. doi: 10.1128/JB.01719-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Römling U. Characterization of the rdar morphotype, a multicellular behaviour in Enterobacteriaceae. Cell. Mol. Life Sci. 2005;62:1234–46. doi: 10.1007/s00018-005-4557-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simm R., Morr M., Kader A., Nimtz M., Romling U. GGDEF and EAL domains inversely regulate cyclic di-GMP levels and transition from sessility to motility. Mol. Microbiol. 2004;53:1123–34. doi: 10.1111/j.1365-2958.2004.04206.x. [DOI] [PubMed] [Google Scholar]
- 40.Ausubel F.M., Brent R., Kingston R.E., et al. Current Protocols in Molecular Biology 2. New York: Wiley Intersciencie; 2001. [Google Scholar]
- 41.Tatusov R.L., Fedorova N.D., Jackson J.D., et al. The COG database: an updated version includes eukaryotes. BMC Bioinformatics. 2003;4:41. doi: 10.1186/1471-2105-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alonso-Casajús N., Dauvillée D., Viale A.M., et al. Glycogen phosphorylase, the product of the glgP gene, catalyzes glycogen breakdown by removing glucose units from the non-reducing ends in Escherichia coli. J. Bacteriol. 2006;188:5266–72. doi: 10.1128/JB.01566-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moreno-Bruna B., Baroja-Fernández E., Muñoz F.J., et al. Adenosine diphosphate sugar pyrophosphatase prevents glycogen biosynthesis in Escherichia coli. Proc. Natl Acad. Sci. USA. 2001;98:8128–32. doi: 10.1073/pnas.131214098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilson W.A., Hughes W.E., Tomamichel W., Roach P.J. Increased glycogen storage in yeast results in less branched glycogen. Biochem. Biophys. Res. Commun. 2004;320:416–23. doi: 10.1016/j.bbrc.2004.05.180. [DOI] [PubMed] [Google Scholar]
- 45.Liu M.Y., Yang H., Romeo T. The product of the pleiotropic Escherichia coli csrA modulates glycogen biosynthesis via effects on mRNA stability. J. Bacteriol. 1995;177:2663–72. doi: 10.1128/jb.177.10.2663-2672.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu M.Y., Gui G., Wei B., et al. The RNA molecule CsrB binds to the global regulatory protein CsrA and antagonizes its activity in Escherichia coli. J. Biol. Chem. 1997;272:17502–10. doi: 10.1074/jbc.272.28.17502. [DOI] [PubMed] [Google Scholar]
- 47.Weilbacher T., Suzuki K., Dubey A.K., et al. A novel sRNA component of the carbon storage regulatory system of Escherichia coli. Mol. Microbiol. 2003;48:657–70. doi: 10.1046/j.1365-2958.2003.03459.x. [DOI] [PubMed] [Google Scholar]
- 48.Dubey A.K., Baker C., Romeo T., Babitzke P. RNA sequence and secondary structure participate in high-affinity CsrA–RNA interaction. RNA. 2005;11:1579–87. doi: 10.1261/rna.2990205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suzuki K., Babitzke P., Kushner S.R., Romeo T. Identification of a novel regulatory protein (CsrD) that targets the global regulatory RNAs CsrB and CsrC for degradation by RNase E. Genes Dev. 2006;20:2605–17. doi: 10.1101/gad.1461606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cole S.T., Raibaud O. The nucleotide sequence of the malT gene encoding the positive regulator of the Escherichia coli maltose regulon. Gene. 1986;42:201–8. doi: 10.1016/0378-1119(86)90297-0. [DOI] [PubMed] [Google Scholar]
- 51.Boos W., Bohm A. Learning new tricks from an old dog: MalT of the Escherichia coli maltose system is part of a complex regulatory network. Trends. Genet. 2000;16:404–9. doi: 10.1016/s0168-9525(00)02086-2. [DOI] [PubMed] [Google Scholar]
- 52.Boos W., Shuman H. Maltose/maltodextrin system of Escherichia coli: transport, metabolism, and regulation. Microbiol. Mol. Biol. Rev. 1998;62:204–29. doi: 10.1128/mmbr.62.1.204-229.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dippel R., Bergmiller T., Böhm A., Boos W. The maltodextrin system of Escherichia coli: glycogen-derived endogenous induction and osmoregulation. J. Bacteriol. 2005;187:8332–9. doi: 10.1128/JB.187.24.8332-8339.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kotrba P., Inui M., Yukawa H. Bacterial phosphotransferase system (PTS) in carbohydrate uptake and control of metabolism. J. Biosci. Bioeng. 2001;92:502–17. doi: 10.1263/jbb.92.502. [DOI] [PubMed] [Google Scholar]
- 55.Nishio Y., Usuda Y., Matsui K., Kurata H. Compute-aided rational design of the phosphotransferase system for enhanced glucose uptake in Escherichia coli. Mol. Syst. Biol. 2008;4:160. doi: 10.1038/msb4100201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Plumbridge J. Regulation of gene expression in the PTS in Escherichia coli: the role and interactions of Mlc. Curr. Opin. Microbiol. 2002;5:187–93. doi: 10.1016/s1369-5274(02)00296-5. [DOI] [PubMed] [Google Scholar]
- 57.Lange R., Hengge-Aronis R. Identification of a central regulator of stationary-phase gene expression in Escherichia coli. Mol. Microbiol. 1991;5:49–59. doi: 10.1111/j.1365-2958.1991.tb01825.x. [DOI] [PubMed] [Google Scholar]
- 58.Dennis P.P., Ehrenberg M., Bremer H. Control of rRNA synthesis in Escherichia coli: a systems biology approach. Microbiol. Mol. Biol. Rev. 2004;68:639–68. doi: 10.1128/MMBR.68.4.639-668.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Potrykus K., Cashel M. (p)ppGpp: still magical? Annu. Rev. Microbiol. 2008;62:35–51. doi: 10.1146/annurev.micro.62.081307.162903. [DOI] [PubMed] [Google Scholar]
- 60.Hara A., Sy J. Guanosine 5′-triphosphate,3′-diphosphate 5′-phosphohydrolase. Purification and substrate specificity. J. Biol. Chem. 1983;258:1678–83. [PubMed] [Google Scholar]
- 61.Xiao H., Kalman M., Ikehara K., Zemel S., Glaser G., Cashel M. Residual guanosine 3′,5′-bispyrophosphate synthetic activity of relA null mutants can be eliminated by spoT null mutations. J. Biol. Chem. 1991;266:5980–90. [PubMed] [Google Scholar]
- 62.Kuroda A., Murphy H., Cashel M., Kornberg A. Guanosine tetra- and pentaphosphate promote accumulation of inorganic polyphosphate in Escherichia coli. J. Biol. Chem. 1997;272:21240–3. doi: 10.1074/jbc.272.34.21240. [DOI] [PubMed] [Google Scholar]
- 63.Barker M.M., Gaal T., Gourse R.L. Mechanism of regulation of transcription initiation by ppGpp. II. Models for positive control based on properties of RNAP mutants and competition for RNAP. J. Mol. Biol. 2001;305:689–702. doi: 10.1006/jmbi.2000.4328. [DOI] [PubMed] [Google Scholar]
- 64.Belitskii B.R., Shakulov R.S. Cloning of Escherichia coli gpp gene and insertion of its mutant allele into chromosome of recBC, sbcB cells. Genetika. 1987;24:1333–42. [PubMed] [Google Scholar]
- 65.Cashel M., Gentry D.R., Hernandez V.J., Vinella D. The stringent response. In: Neidhardt F.C., Curtiss R. III, Ingraham L., Lin E.C.C., et al., editors. Escherichia coli and Salmonella: Cellular and Molecular Biology. 2nd edition. Washington, DC: ASM Press; 1996. pp. 1458–96. [Google Scholar]
- 66.Metzger S., Schreiber G., Aizenman E., Cashel M., Glaser G. Characterization of the relA1 mutation and a comparison of relA1 with new relA null alleles in Escherichia coli. J. Biol. Chem. 1989;264:21146–52. [PubMed] [Google Scholar]
- 67.González-Barrios A.F., Zuo R., Hashimoto Y., Yang L., Bentley W.E., Wood T.K. Autoinducer 2 controls biofilm formation in Escherichia coli through a novel motility quorum-sensing regulator (MqsR, B3022) J. Bacteriol. 2006;188:305–16. doi: 10.1128/JB.188.1.305-316.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang X.S., García-Contreras R., Wood T.K. Escherichia coli transcription factor YncC (McbR) regulates colanic acid and biofilm formation by repressing expression of periplasmic protein YbiM (McbA) ISME J. 2008;2:615–31. doi: 10.1038/ismej.2008.24. [DOI] [PubMed] [Google Scholar]
- 69.Domka J., Lee J., Wood T.K. YliH (BssR) and YceP (BssS) regulate Escherichia coli K-12 biofilm formation by influencing cell signalling. Appl. Environ. Microbiol. 2006;72:2449–59. doi: 10.1128/AEM.72.4.2449-2459.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wolfe A.J., Visick K.L. Get the message out: cyclic-Di-GMP regulates multiple levels of flagellum-based motility. J. Bacteriol. 2008;190:463–75. doi: 10.1128/JB.01418-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hengge R. Principles of c-di-GMP signalling in bacteria. Nat. Rev. Microbiol. 2009;7:263–73. doi: 10.1038/nrmicro2109. [DOI] [PubMed] [Google Scholar]
- 72.Sommerfeldt N., Possling A., Becker G., Pesavento C., Tschowri N., Hengge R. Gene expression patterns and differential input into curli fimbriae regulation of all GGDEF/EAL domain proteins in Escherichia coli. Microbiology. 2009;155:1318–31. doi: 10.1099/mic.0.024257-0. [DOI] [PubMed] [Google Scholar]
- 73.Christen M., Christen B., Folcher M., Schauerte A., Jenal U. Identification and characterization of a cyclic di-GMP-specific phosphodiesterase and its allosteric control by GTP. J. Biol. Chem. 2005;280:30829–37. doi: 10.1074/jbc.M504429200. [DOI] [PubMed] [Google Scholar]
- 74.Schmidt A.J., Ryjenkov D.A., Gomelsky M. The ubiquitous protein domain EAL is a cyclic diguanylate-specific phosphodiesterase: enzymatically active and inactive EAL domains. J. Bacteriol. 2005;187:4774–81. doi: 10.1128/JB.187.14.4774-4781.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vincent C., Duclos B., Grangeasse C., et al. Relationship between exopolysaccharide production and protein-tyrosine phosphorylation in gram-negative bacteria. J. Mol. Biol. 2000;304:311–21. doi: 10.1006/jmbi.2000.4217. [DOI] [PubMed] [Google Scholar]
- 76.Grangeasse C., Obadia B., Mijakovic I., Deutscher J., Cozzone A.J., Doublet P. Autophosphorylation of the Escherichia coli protein kinase Wzc regulates tyrosine phosphorylation of Ugd, a UDP-glucose dehydrogenase. J. Biol. Chem. 2003;278:39323–9. doi: 10.1074/jbc.M305134200. [DOI] [PubMed] [Google Scholar]
- 77.Weickert M.J., Adhya S. The galactose regulon of Escherichia coli. Mol. Microbiol. 1993;10:245–51. doi: 10.1111/j.1365-2958.1993.tb01950.x. [DOI] [PubMed] [Google Scholar]
- 78.Semsey S., Krishna S., Sneppen K., Adhya S. Signal integration in the galactose network of Escherichia coli. Mol. Microbiol. 2007;65:465–76. doi: 10.1111/j.1365-2958.2007.05798.x. [DOI] [PubMed] [Google Scholar]
- 79.Vogler A.P., Trentmann S., Lengeler J.W. Alternative route for biosynthesis of amino sugars in Escherichia coli K-12 mutants by means of a catabolic isomerase. J. Bacteriol. 1989;171:6586–92. doi: 10.1128/jb.171.12.6586-6592.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Reizer J., Reizer A., Saier M.H., Jacobson G.R. A proposed link between nitrogen and carbon metabolism involving protein phosphorylation in bacteria. Protein Sci. 1992;1:722–6. doi: 10.1002/pro.5560010604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee C-R., Cho S-H., Yoon M-J., Peterkofsky A., Seok J. Escherichia coli enzyme IIANtr regulates the K+ transporter TrkA. Proc. Natl. Acad. Sci. USA. 2007;104:4124–9. doi: 10.1073/pnas.0609897104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hayden J.D., Ades S.E. The extracytoplasmic stress factor, σE, is required to maintain cell envelope integrity in Escherichia coli. PLoS One. 2008;6:e1573. doi: 10.1371/journal.pone.0001573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zimmer B., Hillman A., Görke B. Requirements for the phosphorylation of the Escherichia coli EIIANtr protein in vivo. FEMS Microbiol. Lett. 2008;286:96–102. doi: 10.1111/j.1574-6968.2008.01262.x. [DOI] [PubMed] [Google Scholar]
- 84.Reitzer L.J. Ammonia assimilation and the biosynthesis of glutamine, glutamate, aspartate, asparagines, L-alanine, and D-alanine. In: Neidhart F.C., Curtis R. III, Ingraham J.L., et al., editors. Escherichia coli and Salmonella: Cellular and Molecular Biology. 2nd edition. Washington, DC: ASM Press; 1996. pp. 391–407. [Google Scholar]
- 85.Ganduri Y.L., Sadda S.R., Datta M.W., Jambukeswaran R.K., Datta P. TdcA, a transcriptional activator of the tdcABC operon of Escherichia coli, is a member of the LysR family of proteins. Mol. Gen. Genet. 1993;240:395–402. doi: 10.1007/BF00280391. [DOI] [PubMed] [Google Scholar]
- 86.Hagewood B.T., Ganduri Y.L., Datta P. Functional analysis of the tdcABC promoter of Escherichia coli: roles of TdcA and TdcR. J. Bacteriol. 1994;176:6214–20. doi: 10.1128/jb.176.20.6214-6220.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Umezu K., Nakayama K., Nakayama H. Escherichia coli RecQ protein is a DNA helicase. Proc. Natl Acad. Sci. USA. 1990;87:5363–7. doi: 10.1073/pnas.87.14.5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kuznetsova E., Proudfoot M., Gonzalez C.F., et al. Genome-wide analysis of substrate specificities of the Escherichia coli haloacid dehalogenase-like phosphatase family. J. Biol. Chem. 2006;281:36149–61. doi: 10.1074/jbc.M605449200. [DOI] [PubMed] [Google Scholar]
- 89.Wood H.G. Biological aspects of inorganic polyphosphates. Annu. Rev. Biochem. 1988;57:235–60. doi: 10.1146/annurev.bi.57.070188.001315. [DOI] [PubMed] [Google Scholar]
- 90.Pittman M.S., Robinson H.C., Poole R.K. A bacterial glutathione transporter (Escherichia coli cydDC) export reductant to the periplasm. J. Biol. Chem. 2005;280:32254–61. doi: 10.1074/jbc.M503075200. [DOI] [PubMed] [Google Scholar]
- 91.Garcia-Vescovi E., Soncini F.C., Groisman E.A. Mg2+ as an extracellular signal: environmental regulation of Salmonella virulence. Cell. 1996;84:165–74. doi: 10.1016/s0092-8674(00)81003-x. [DOI] [PubMed] [Google Scholar]
- 92.Spinelli S.V., Pontel L.B., García Véscovi E., Soncini F.C. Regulation of magnesium homeostasis in Salmonella: Mg2+ targets the mgtA transcript for degradation by RNase E. FEMS Microbiol. Lett. 2008;280:226–34. doi: 10.1111/j.1574-6968.2008.01065.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.