Abstract
A filamentous non-N2-fixing cyanobacterium, Arthrospira (Spirulina) platensis, is an important organism for industrial applications and as a food supply. Almost the complete genome of A. platensis NIES-39 was determined in this study. The genome structure of A. platensis is estimated to be a single, circular chromosome of 6.8 Mb, based on optical mapping. Annotation of this 6.7 Mb sequence yielded 6630 protein-coding genes as well as two sets of rRNA genes and 40 tRNA genes. Of the protein-coding genes, 78% are similar to those of other organisms; the remaining 22% are currently unknown. A total 612 kb of the genome comprise group II introns, insertion sequences and some repetitive elements. Group I introns are located in a protein-coding region. Abundant restriction-modification systems were determined. Unique features in the gene composition were noted, particularly in a large number of genes for adenylate cyclase and haemolysin-like Ca2+-binding proteins and in chemotaxis proteins. Filament-specific genes were highlighted by comparative genomic analysis.
Keywords: cyanobacteria, Arthrospira, health supplement, genome, cAMP
1. Introduction
Cyanobacteria are prokaryotes that perform oxygenic photosynthesis and constitute a large taxonomic group within the domain of eubacteria. Cyanobacteria are divided morphologically (unicellular or filamentous) or functionally (N2-fixing and non-N2-fixing). Filamentous species are subdivided into those with and without a heterocyst which is a differentiation from vegetative cells for fixing nitrogen.1,2
Arthrospira is a representative filamentous non-N2-fixing cyanobacterium that lacks any differentiation such as for the heterocyst, akinete or hormogonium, which develops in some filamentous N2-fixing cyanobacteria. This cyanobacterium is also well known as ‘Spirulina’ because of its useful property as a food. The Aztecs consumed it regularly,3 and it is thought to be an important dietary element in tropical areas. Recently, Lake Chad has been famous for Arthrospira that is naturally cultivated as a food supply.4 However, current taxonomy claims that the name ‘Spirulina’ for strains used as food supplements is inappropriate, and there is agreement that Arthrospira is a distinct genus,5 consisting of over 30 different species including A. platensis and A. maxima. In the present study, we determined nearly the complete genome sequence of A. platensis NIES-39.6
Arthrospira platensis has physiologically particular diagnostic characteristics, and its biology is subject to a comprehensive book.7 Arthrospira platensis shows vigorous gliding motility of filamentous cells (trichomes) with rotation along their long axis. Gliding is a self-propulsion across a solid or semi-solid material without the aid of any visible flagellum8; however, the mechanism of gliding motility is not fully understood. This organism also possesses ecologically very valuable characteristics such as alkali and salt tolerance and algal mat production on the periphery of lakes. Arthrospira platensis is also able to grow under high salt concentrations of ∼1.5-fold higher than sea water.9 Accordingly, it often dominates in lakes with high carbonate/bicarbonate levels and high pH levels.10
Gene expression during adaptation to new environmental conditions like a high salt concentration has been determined in A. platensis, and the importance of the cAMP signalling cascade in its regulatory mechanism in response to changes in the external environment has been highlighted.11 Moreover, molecular analysis of adenylate cyclase genes has shown that A. platensis develops a number of diverse cAMP-dependent signal cascades to adapt to different severe environmental conditions.12–14
Arthrospira platensis has become an important industrial organic material as a health supplement, a source of beta-carotene and a natural colouring agent. It has been approved for treating symptoms of radiation sickness after the Chernobyl disaster in Russia.15 The presence of hydrogenase in its cells also makes this cyanobacterium a useful material for clean energy production.16
Despite its various useful applications, very little is known about the biology, physiology and genetic system of A. platensis. For production of useful products, gene manipulation through genetic engineering should be considered. However, genetic transformation of Arthrospira has had limited success to date,17 and thus commercial use of this organism has faced barriers due to difficulties in gene manipulation. To overcome these barriers, restriction-modification (RM) systems based on its genome sequence may prove useful.
In 1996, whole-genome sequencing was achieved for the first time in the unicellular cyanobacterium, Synechocystis sp. PCC 6803.18 Since then, whole genomes of 38 strains of cyanobacteria have been sequenced (Table 1). Of these strains, four are N2-fixing filamentous species, whereas the others are unicellular species, both N2-fixing and non-N2 fixing. Thus, this is the first study to sequence the genome of a filamentous non-N2-fixing species.
Table 1.
Strains used in the comparative genomic analysis
| CyanoClust grouping |
Species | Abbr. | Genes | Physiological indexes |
Number of Pfam domains |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Filament | N2-fixation | Heterocyst | Habitat | Motility | Total | HisKA | Respons_reg | PAS | GAF | |||
| I | − | − | − | Acaryochloris marina MBIC 11017 | amr | 8383 | Marine | − | 8273 | 75 | 161 | 54 | 74 |
| Gloeobacter violaceus PCC 7421 | gvi | 4430 | Rock | − | 5464 | 39 | 56 | 20 | 15 | ||||
| Microcystis aeruginosa NIES 843 | mar | 6312 | Freshwater | − | 5151 | 20 | 30 | 1 | 12 | ||||
| Prochlorococcus marinus SS120 | pma | 1883 | Marine | − | 1967 | 4 | 6 | 1 | 0 | ||||
| Prochlorococcus marinus AS9601 | pmb | 1921 | Marine | − | 1947 | 4 | 5 | 1 | 1 | ||||
| Prochlorococcus marinus MIT 9515 | pmc | 1906 | Marine | − | 1892 | 4 | 5 | 1 | 1 | ||||
| Prochlorococcus marinus NATL1A | pme | 2193 | Marine | − | 2074 | 5 | 6 | 1 | 1 | ||||
| Prochlorococcus marinus MIT 9303 | pmf | 2997 | Marine | − | 2908 | 7 | 8 | 1 | 0 | ||||
| Prochlorococcus marinus MIT 9301 | pmg | 1907 | Marine | − | 1928 | 4 | 6 | 1 | 1 | ||||
| Prochlorococcus marinus MIT 9215 | pmh | 1983 | Marine | − | 1941 | 3 | 5 | 1 | 1 | ||||
| Prochlorococcus marinus MIT 9312 | pmi | 1810 | Marine | − | 1928 | 5 | 6 | 1 | 1 | ||||
| Prochlorococcus marinus MIT 9211 | pmj | 1855 | Marine | − | 1963 | 4 | 6 | 1 | 0 | ||||
| Prochlorococcus marinus MED4 | pmm | 1717 | Marine | − | 1918 | 5 | 6 | 1 | 1 | ||||
| Prochlorococcus marinus NATL2A | pmn | 2163 | Marine | − | 2110 | 5 | 6 | 1 | 1 | ||||
| Prochlorococcus marinus MIT 9313 | pmt | 2269 | Marine | − | 2549 | 6 | 9 | 1 | 0 | ||||
| Synechococcus elongatus PCC 6301 | syc | 2527 | Freshwater | − | 3198 | 15 | 26 | 20 | 19 | ||||
| Synechococcus sp. CC9605 | syd | 2645 | Marine | − | 2571 | 5 | 9 | 1 | 2 | ||||
| Synechococcus sp. CC9902 | sye | 2307 | Freshwater | − | 2453 | 5 | 7 | 1 | 1 | ||||
| Synechococcus elongatus PCC 7942 | syf | 2662 | Freshwater | − | 3266 | 16 | 27 | 20 | 20 | ||||
| Synechococcus sp. CC9311 | syg | 2892 | Freshwater | − | 2696 | 12 | 15 | 1 | 1 | ||||
| Synechococcus sp. WH 5701 | syh | 3346 | Marine | − | nd | nd | nd | nd | nd | ||||
| Synechococcus sp. RCC307 | syr | 2535 | Marine | − | 2633 | 9 | 11 | 1 | 1 | ||||
| Synechococcus sp. WH 8102 | syw | 2519 | Marine | + | 2392 | 5 | 9 | 1 | 2 | ||||
| Synechococcus sp. WH 7803 | syx | 2533 | Marine | − | 2669 | 12 | 14 | 1 | 0 | ||||
| Synechocystis sp. PCC 6803 | syn | 3569 | Freshwater | + | 4269 | 41 | 67 | 29 | 33 | ||||
| Thermosynechococcus elongatus BP-1 | tel | 2476 | Hot spring | − | 2901 | 15 | 32 | 13 | 19 | ||||
| II | − | + | − | Crocosphaera watsonii WH 8501 | cro | 5958 | Marine | − | nd | nd | nd | nd | nd |
| Synechococcus sp. JA-3-3Ab | cya | 2760 | Hot spring | − | 3394 | 18 | 37 | 13 | 22 | ||||
| Synechococcus sp. JA-2-3B'a(2–13) | cyb | 2862 | Hot spring | − | 3469 | 20 | 36 | 15 | 18 | ||||
| Cyanothece sp. PCC 7424 | cyc | 5710 | Freshwater | − | 6407 | 62 | 120 | 67 | 47 | ||||
| Cyanothece sp. PCC 7425 | cyn | 5327 | Freshwater | − | 6255 | 70 | 145 | 122 | 68 | ||||
| Cyanothece sp. PCC 8801 | cyp | 4367 | Freshwater | − | 5182 | 38 | 81 | 32 | 39 | ||||
| Cyanothece sp. ATCC 51142 | cyt | 5304 | Marine | − | 5490 | 44 | 81 | 30 | 33 | ||||
| III | −/+ | − | − | Synechococcus sp. PCC 7002 | syp | 3186 | Marine | − | 3764 | 31 | 54 | 16 | 19 |
| IV | + | − | − | Arthrospira platensis NIES 39 | apl | 6631 | Freshwater | + | 6631 | 70 | 107 | 76 | 58 |
| V | + | + | − | Trichodesmium erythraeum IMS101 | ter | 4451 | Marine | − | 6159 | 26 | 47 | 10 | 24 |
| VI | + | + | + | Anabaena sp. PCC 7120 | ana | 6130 | Freshwater | − | 7207 | 103 | 153 | 72 | 80 |
| Anabaena variabilis ATCC 29413 | ava | 5661 | Freshwater | − | 7234 | 102 | 151 | 73 | 83 | ||||
| Nostoc punctiforme ATCC 29133 | npu | 6690 | Soil | + | 8751 | 125 | 194 | 92 | 121 | ||||
Abbr., abbreviation; nd, not determined. Pfam domains: HisKA (PF00512), Respons_reg (PF00072), PAS (PF00989) and GAF(PF01590).
This study determined the nearly complete genome sequence of A. platensis NIES-39 of ∼6.8 Mb. Some characteristic gene sets particularly in signal transduction and gene RM systems were determined, and additional properties in the genome structure were compared with those of other cyanobacteria.
2. Materials and methods
2.1. Bacterial strain and culture conditions
Arthrospira (Spirulina) platensis strain NIES-39 was obtained from the culture collection at the National Institute for Environmental Studies, which was originally isolated from Lake Chad and maintained in the Institute of Applied Microbiology, the University of Tokyo (strain IAM M-135).6 The cells were grown in the SOT medium19 at 30°C under continuous illumination at 30 µmol photon m−2 s−1 with aeration with 1% (v/v) CO2.
2.2. Sequencing and assembly
Genomic DNA was isolated with Genomic-tip 500/G (Qiagen, Valencia, CA, USA) as recommended by the supplier. A DNA shotgun library with 1.5- and 5-kb inserts in pUC118 vector (Takara, Otsu, Japan) was constructed as described previously.20 Plasmid clones were end-sequenced using dye terminator chemistry on an ABI Prism 3730 sequencer as described previously.20 Raw sequence data corresponding to 11-fold coverage were assembled using PHRED/PHRAP/CONSED software (http://www.phrap.org).21,22 For assembly validation, a fosmid library with 40-kb inserts in the pCC1FOS fosmid vector was constructed using the CopyControl Fosmid library production kit (Epicenter, Madison, WI, USA). Fosmid DNA was extracted from Escherichia coli transformants using a Montage BAC96 MiniPrep kit (Millipore, Billerica, MA, USA), and end sequencing was carried out using dye terminator chemistry on an ABI Prism 3730 as described previously.20 Fosmid end sequences were mapped onto the assembled sequence. Fosmid clones that link two contigs were selected and sequenced by primer walking to close any gaps. The sequencing of difficult templates was performed using a CUGA sequencing kit (Nippon Genetech, Tokyo, Japan). Contig sequences generated by Roche 454 FLX sequencer were also used to fill some gaps.
2.3. Optical mapping
The sequence assemblies were constructed by optical maps (OpGen Technologies, Madison, WI, USA) and PCR.23,24 Briefly, high molecular weight DNA was immobilized as individual molecules onto optical chips, digested with NcoI (New England Biolabs, Ipswich, MA, USA), fluorescently stained with YOYO-1 (Invitrogen, Carlsbad, CA, USA) and positioned onto an automated fluorescent microscope system for image capture and fragment size measurement to give high-resolution single-molecule restriction maps. Single-molecule maps were collected and then assembled to produce whole genome.
Optical maps and sequence contigs were compared as described previously.23 Sequence FASTA files were converted to in silico restriction maps via MapViewer software (OpGen Technologies) for direct comparison with the optical maps. Comparisons were accomplished by aligning the sequence with the optical maps according to their restriction fragment pattern. Alignments were generated with a dynamic programming algorithm that finds the optimal location or placement of a sequence contig by global alignment of the sequence contig against the optical map. Local alignment analysis was also performed to compare segments of the sequence contigs to the optical map.
2.4. Genome analysis and annotation
Putative non-translated genes were identified using the Rfam25 and tRNAscan-SE26 programs and rRNA genes using the RNAmmer27 and BLASTN28 programs, whereas protein-coding genes were identified using the GLIMMER program by obtaining potential open reading frames >150 bp.29,30 The genome sequence was translated into potential protein sequences in six frames and compared with sequences in the UniProt database31 using the BLASTP program28 for identification of additional genes not predicted by other methods and genes <150 bp, especially in the predicted intergenic regions. The start sites were manually inspected and altered based on predictions of GLIMMER and GeneHacker.32 For functional annotation, the non-redundant UniProt database and protein signature database, InterPro,33 were searched to assign the predicted protein sequences based on sequence similarities. Orthologous genes among cyanobacteria were manually curated using Gclust34 and CYORF.35 The KEGG database was used for pathway reconstruction.36 Signal peptides in proteins were predicted using SignalP,37 and transmembrane helices were predicted using TMHMM.38
2.5. Comparative genomic analysis
We first used the hmmer (v2.3.2) program and the Pfam_ls database (release 23) to analyse the cyanobacterial genomes (Table 1).
For comparative genomic analysis to determine genes related to trichome formation and N2-fixation, we applied the CyanoClust database.39 We classified all putative proteins from the 39 cyanobacterial genomes into nearly orthologous protein clusters; each cluster was statistically defined to minimize its size. This is particularly useful for analysing the many regulatory proteins in cyanobacteria which have multidomains. We divided A. platensis and the other strains of cyanobacteria into six groups (Table 1): unicellular non-N2-fixing cyanobacteria such as Synechocystis sp. PCC 6803 (group I), unicellular N2-fixing cyanobacteria such as Crocosphaera watsonii (group II), Synechococcus sp. PCC 7002 (a unicellular cyanobacterium which forms a short filament near optimal growth temperature; D. Bryant, personal communication) (group III), filamentous non-N2-fixing cyanobacteria (A. platensis, group IV), filamentous N2-fixing non-heterocyst-forming cyanobacteria (Trichodesmium erythraeum, group V) and filamentous N2-fixing heterocyst-forming cyanobacteria such as Anabaena sp. PCC 7120 (group VI). Gene clusters that were shared between groups were automatically extracted, and only those clusters present in all strains of the group were analysed.
3. Results and discussion
3.1. Sequencing, optical mapping and structural features of the A. platensis genome
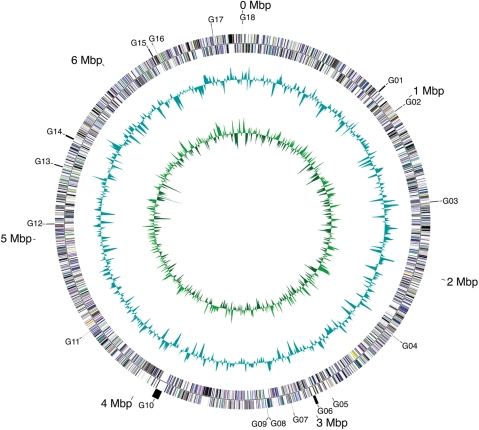
The nucleotide sequence of the whole genome of A. platensis NIES-39 was determined using the whole-genome shotgun method. Three different libraries with 1.5-, 5- and 40-kb inserts were prepared and each end sequence was determined. A total of 92 878 random sequences corresponding to 11 genome equivalents were assembled into 18 supercontigs. Final sequence assembly was carried out by visually editing the draft sequences and by optical mapping (data not shown) to determine the relative order/orientation of each supercontig. The 18 remaining gaps were estimated to be ∼95 kb. Most, if not all, gaps were flanked by repeated clusters of tandem sequences or the phage-like sequences. Gap closing including long PCR followed by primer walking and transposon-mediated sequencing was not successful due to unusually abundant repeats. The total sequence of the 18 supercontigs has a length of 6 692 865 bp with an average G + C content of 44.3%. Taken together, the A. platensis genome is composed of a single, circular chromosome of 6.8 Mb (Fig. 1); no plasmid DNA sequences were detected. On GC skew analysis to locate the probable origin and terminator of DNA replication, no apparent shift of skew was detected as in other cyanobacteria.
Figure 1.
Schematic representation of the circular chromosome of A. platensis. A scale indicates the coordinates in megabase pairs. From outside to inside: circle 1, the gaps in the genome; circles 2 and 3, predicted protein-coding genes on the forward and reverse strands; circle 4, G+C content; circle 5, GC skew. Eighteen contig gaps (G01-G18) are numbered in the clockwise direction starting from the end of the longest contig. Functional categories were colour-coded according to the standard colours used by COGs. The genome sequence and annotation of A. platensis NIES-39 are available at GenBank/EMBL/DDBJ under accession no. AP011615.
The estimated genome comprises 6630 potential protein-coding genes (average size, 835 bp), as well as 49 RNA genes consisting of 2 sets of rRNA genes, 40 tRNA genes representing 20 tRNA species, tmRNA, the B subunit of RNase P and signal recognition particle RNA. Translated amino acid sequences were compared with sequences in the UniProt database using the BLAST program. Of the 6630 potential protein-coding genes, 5157 (78%) were orthologous or had similarity to genes of known function or hypothetical genes (E-value of <10−3) and the remaining 1473 (22%) showed no significant similarity to any registered genes. On manual curation, 2539 (38%) genes could be assigned to biological roles. Genes for general metabolism in cyanobacteria were detected with no particular difference.
3.2. Comparative genomic analysis
3.2.1. Conserved domain analysis using Pfam
We obtained 2673 kinds of Pfam domains from 37 cyanobacterial genomes and 1537 kinds of Pfam domains from the A. platensis genome. Table 2 presents the top 50 Pfam domains in A. platensis, which contains highly repetitive motifs including TPR_1 (PF00515), TPR_2 (PF07719), WD40 (PF00400), HemolysinCabind (PF00353), Pentapeptide (PF00805), HNH (PF01844) and GIIM (PF08388).
Table 2.
Top 50 of Pfam domains of A. platensis
| domains | apl | amr | ana | ava | cya | cyb | cyc | cyn | cyp | cyt | gvi | mar | npu | pma | pmb | pmc | pme | pmf | pmg | pmh | pmi | pmj | pmm | pmn | pmt | syc | syd | sye | syf | syg | syn | syp | syr | syw | syx | tel | ter |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TPR_1 | 415 | 300 | 164 | 158 | 58 | 73 | 202 | 98 | 144 | 75 | 97 | 197 | 300 | 8 | 12 | 7 | 50 | 207 | 13 | 13 | 12 | 24 | 8 | 86 | 86 | 34 | 9 | 11 | 35 | 12 | 55 | 62 | 8 | 18 | 10 | 40 | 628 |
| TPR_2 | 398 | 308 | 166 | 163 | 76 | 90 | 199 | 115 | 147 | 72 | 123 | 202 | 298 | 7 | 16 | 7 | 49 | 210 | 13 | 13 | 10 | 20 | 9 | 87 | 76 | 42 | 11 | 16 | 43 | 12 | 53 | 63 | 13 | 21 | 17 | 48 | 593 |
| WD40 | 220 | 398 | 230 | 282 | 4 | 4 | 197 | 72 | 65 | 128 | 153 | 97 | 320 | 2 | 2 | 2 | 3 | 2 | 2 | 2 | 2 | 2 | 2 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 45 | 11 | 0 | 1 | 0 | 10 | 174 |
| HemolysinCabind | 191 | 172 | 72 | 60 | 0 | 0 | 53 | 3 | 10 | 27 | 10 | 52 | 123 | 0 | 6 | 0 | 3 | 17 | 0 | 0 | 0 | 0 | 0 | 1 | 3 | 7 | 0 | 5 | 7 | 10 | 14 | 0 | 27 | 11 | 5 | 0 | 156 |
| Pentapeptide | 160 | 252 | 98 | 89 | 40 | 44 | 111 | 106 | 101 | 111 | 68 | 50 | 123 | 8 | 6 | 7 | 8 | 13 | 6 | 6 | 6 | 8 | 7 | 8 | 15 | 20 | 11 | 11 | 20 | 12 | 48 | 62 | 13 | 13 | 13 | 41 | 107 |
| HNH* | 111 | 9 | 15 | 11 | 4 | 3 | 7 | 4 | 7 | 14 | 4 | 11 | 7 | 2 | 3 | 3 | 2 | 2 | 3 | 3 | 3 | 2 | 4 | 2 | 2 | 1 | 2 | 3 | 1 | 2 | 11 | 4 | 2 | 2 | 2 | 7 | 8 |
| Response_reg | 107 | 161 | 153 | 151 | 37 | 36 | 120 | 145 | 81 | 81 | 56 | 30 | 194 | 6 | 5 | 5 | 6 | 8 | 6 | 5 | 6 | 6 | 6 | 6 | 9 | 26 | 9 | 7 | 27 | 15 | 67 | 54 | 11 | 9 | 14 | 32 | 47 |
| GIIM* | 103 | 6 | 5 | 2 | 0 | 0 | 5 | 0 | 4 | 6 | 1 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 12 |
| HATPase_c | 82 | 95 | 139 | 135 | 26 | 27 | 76 | 102 | 47 | 52 | 49 | 23 | 168 | 6 | 5 | 5 | 6 | 9 | 6 | 5 | 6 | 6 | 6 | 6 | 8 | 17 | 7 | 7 | 18 | 13 | 48 | 36 | 10 | 7 | 11 | 21 | 38 |
| PAS_3 | 77 | 47 | 75 | 80 | 13 | 17 | 51 | 156 | 30 | 31 | 17 | 1 | 88 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 20 | 0 | 0 | 20 | 0 | 34 | 18 | 0 | 0 | 0 | 13 | 7 |
| PAS | 76 | 54 | 72 | 73 | 13 | 15 | 67 | 122 | 32 | 30 | 20 | 1 | 92 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 20 | 1 | 1 | 20 | 1 | 29 | 16 | 1 | 1 | 1 | 13 | 10 |
| PAS_4 | 76 | 54 | 73 | 81 | 19 | 24 | 57 | 128 | 28 | 32 | 30 | 3 | 85 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 19 | 1 | 1 | 19 | 1 | 24 | 12 | 1 | 1 | 1 | 14 | 5 |
| DUF820 | 70 | 26 | 56 | 48 | 15 | 21 | 67 | 53 | 85 | 54 | 93 | 89 | 63 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 2 | 0 | 35 | 26 | 0 | 0 | 0 | 1 | 7 |
| HisKA | 70 | 75 | 103 | 102 | 18 | 20 | 62 | 70 | 38 | 44 | 39 | 20 | 125 | 4 | 4 | 4 | 5 | 7 | 4 | 3 | 5 | 4 | 5 | 5 | 6 | 15 | 5 | 5 | 16 | 12 | 41 | 31 | 9 | 5 | 12 | 15 | 26 |
| ABC_tran | 65 | 79 | 106 | 105 | 55 | 63 | 78 | 88 | 65 | 73 | 68 | 66 | 106 | 31 | 32 | 28 | 34 | 43 | 30 | 31 | 32 | 29 | 31 | 34 | 44 | 50 | 40 | 38 | 51 | 38 | 60 | 60 | 36 | 47 | 42 | 49 | 56 |
| GAF | 58 | 74 | 80 | 83 | 22 | 18 | 47 | 68 | 39 | 33 | 15 | 12 | 121 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 19 | 2 | 1 | 20 | 1 | 33 | 19 | 1 | 2 | 0 | 19 | 24 |
| Pkinase | 46 | 58 | 49 | 56 | 9 | 10 | 28 | 30 | 16 | 20 | 15 | 17 | 56 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 5 | 0 | 1 | 5 | 1 | 7 | 8 | 1 | 0 | 1 | 11 | 42 |
| SBBP* | 46 | 0 | 0 | 0 | 0 | 1 | 0 | 7 | 0 | 0 | 9 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 13 |
| HEAT_PBS | 41 | 9 | 55 | 38 | 15 | 14 | 20 | 19 | 20 | 16 | 25 | 27 | 28 | 1 | 1 | 0 | 5 | 4 | 1 | 1 | 1 | 2 | 0 | 4 | 4 | 14 | 5 | 7 | 14 | 5 | 13 | 14 | 8 | 3 | 6 | 9 | 33 |
| PPC | 41 | 3 | 17 | 12 | 1 | 0 | 2 | 0 | 1 | 8 | 9 | 6 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 2 | 0 | 7 | 0 | 0 | 75 |
| Pkinase_Tyr | 40 | 54 | 46 | 52 | 8 | 9 | 26 | 23 | 16 | 20 | 12 | 16 | 51 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 5 | 0 | 6 | 8 | 0 | 0 | 0 | 10 | 38 |
| Transposase_35 | 40 | 2 | 25 | 13 | 47 | 65 | 46 | 4 | 30 | 7 | 2 | 92 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 2 | 0 | 0 | 0 | 32 | 4 |
| Transposase_14* | 39 | 3 | 0 | 0 | 16 | 0 | 5 | 1 | 1 | 0 | 3 | 20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 26 | 0 | 0 | 0 | 0 | 0 | 15 |
| Glycos_transf_1 | 38 | 48 | 52 | 45 | 7 | 8 | 34 | 56 | 33 | 36 | 29 | 28 | 57 | 10 | 8 | 12 | 12 | 12 | 6 | 8 | 9 | 7 | 8 | 9 | 10 | 13 | 12 | 19 | 13 | 11 | 26 | 19 | 15 | 14 | 17 | 12 | 30 |
| Glycos_transf_2 | 38 | 26 | 48 | 45 | 8 | 8 | 42 | 48 | 35 | 33 | 24 | 34 | 38 | 9 | 10 | 13 | 4 | 14 | 6 | 7 | 6 | 6 | 11 | 4 | 8 | 11 | 12 | 11 | 12 | 9 | 24 | 11 | 12 | 11 | 10 | 20 | 38 |
| Methyltransf_11 | 38 | 74 | 42 | 41 | 27 | 26 | 35 | 54 | 33 | 36 | 44 | 32 | 54 | 10 | 14 | 12 | 12 | 19 | 10 | 12 | 11 | 10 | 15 | 11 | 15 | 23 | 14 | 12 | 23 | 15 | 25 | 19 | 17 | 18 | 19 | 11 | 51 |
| RVT_1* | 37 | 9 | 8 | 3 | 0 | 0 | 6 | 0 | 4 | 7 | 1 | 5 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 5 | 11 |
| GGDEF | 33 | 54 | 14 | 14 | 3 | 4 | 34 | 19 | 23 | 31 | 1 | 2 | 21 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 17 | 0 | 0 | 17 | 4 | 23 | 13 | 0 | 0 | 0 | 8 | 4 |
| Methyltransf_12 | 33 | 66 | 34 | 32 | 23 | 22 | 28 | 46 | 28 | 34 | 39 | 28 | 46 | 8 | 11 | 10 | 12 | 19 | 9 | 9 | 7 | 9 | 12 | 10 | 13 | 20 | 14 | 13 | 20 | 14 | 24 | 17 | 15 | 16 | 17 | 10 | 44 |
| BPD_transp_1 | 27 | 24 | 34 | 39 | 28 | 34 | 25 | 30 | 23 | 23 | 19 | 19 | 31 | 8 | 5 | 8 | 8 | 14 | 6 | 5 | 7 | 9 | 6 | 8 | 13 | 22 | 13 | 12 | 23 | 11 | 21 | 22 | 13 | 13 | 15 | 16 | 19 |
| Epimerase | 27 | 54 | 37 | 42 | 16 | 14 | 30 | 44 | 31 | 30 | 35 | 27 | 56 | 16 | 18 | 20 | 18 | 22 | 16 | 17 | 14 | 12 | 18 | 16 | 17 | 16 | 18 | 20 | 16 | 19 | 25 | 17 | 21 | 19 | 20 | 13 | 27 |
| Transposase_2 | 27 | 2 | 24 | 12 | 42 | 62 | 42 | 4 | 26 | 5 | 2 | 84 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 2 | 0 | 0 | 0 | 32 | 6 |
| PIN | 26 | 10 | 17 | 9 | 1 | 2 | 10 | 18 | 10 | 14 | 22 | 31 | 14 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 4 | 1 | 1 | 5 | 3 | 13 | 22 | 1 | 5 | 2 | 1 | 1 |
| AAA | 25 | 34 | 34 | 37 | 23 | 18 | 28 | 25 | 19 | 19 | 25 | 23 | 37 | 13 | 13 | 13 | 15 | 16 | 13 | 13 | 12 | 12 | 14 | 15 | 15 | 16 | 18 | 14 | 16 | 16 | 18 | 17 | 14 | 16 | 15 | 14 | 37 |
| DAO | 25 | 32 | 29 | 37 | 20 | 22 | 31 | 28 | 22 | 25 | 28 | 20 | 34 | 16 | 16 | 13 | 15 | 23 | 12 | 11 | 14 | 14 | 15 | 15 | 21 | 23 | 25 | 21 | 24 | 27 | 25 | 22 | 21 | 22 | 21 | 13 | 24 |
| MMR_HSR1 | 25 | 35 | 32 | 28 | 20 | 21 | 24 | 30 | 24 | 28 | 18 | 24 | 31 | 16 | 16 | 16 | 16 | 17 | 17 | 17 | 17 | 16 | 16 | 16 | 17 | 21 | 18 | 18 | 21 | 19 | 23 | 19 | 17 | 15 | 17 | 24 | 27 |
| PG_binding_1 | 24 | 1 | 29 | 28 | 0 | 0 | 3 | 3 | 1 | 2 | 1 | 4 | 26 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 7 | 0 | 0 | 0 | 1 | 11 |
| adh_short | 23 | 47 | 38 | 41 | 14 | 12 | 28 | 39 | 23 | 28 | 43 | 25 | 79 | 10 | 9 | 8 | 9 | 16 | 7 | 9 | 11 | 10 | 7 | 8 | 16 | 12 | 13 | 16 | 12 | 20 | 17 | 12 | 15 | 17 | 16 | 11 | 16 |
| CBS | 23 | 24 | 21 | 21 | 8 | 6 | 18 | 16 | 11 | 13 | 9 | 10 | 19 | 3 | 3 | 3 | 3 | 5 | 4 | 4 | 3 | 4 | 3 | 3 | 5 | 11 | 6 | 6 | 11 | 5 | 8 | 14 | 5 | 6 | 5 | 7 | 13 |
| SLH | 23 | 24 | 29 | 32 | 10 | 9 | 9 | 11 | 11 | 13 | 11 | 8 | 40 | 2 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 1 | 0 | 0 | 4 | 7 | 6 | 4 | 7 | 6 | 9 | 11 | 2 | 4 | 4 | 8 | 10 |
| AAA_5 | 22 | 24 | 20 | 23 | 12 | 11 | 13 | 19 | 16 | 13 | 19 | 19 | 30 | 10 | 9 | 10 | 10 | 13 | 9 | 9 | 9 | 12 | 11 | 10 | 13 | 13 | 15 | 14 | 13 | 13 | 17 | 12 | 15 | 14 | 14 | 12 | 29 |
| Guanylate_cyc* | 22 | 7 | 6 | 5 | 1 | 2 | 2 | 5 | 2 | 3 | 0 | 1 | 8 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 1 | 0 | 2 | 1 | 3 | 1 | 0 | 0 | 1 | 2 | 13 |
| Radical_SAM | 22 | 24 | 25 | 26 | 13 | 15 | 31 | 31 | 23 | 20 | 24 | 21 | 25 | 11 | 10 | 10 | 8 | 14 | 12 | 11 | 10 | 12 | 10 | 8 | 13 | 17 | 16 | 13 | 17 | 14 | 22 | 19 | 13 | 15 | 15 | 16 | 20 |
| SMC_N | 20 | 13 | 20 | 22 | 11 | 11 | 20 | 24 | 18 | 16 | 18 | 18 | 22 | 10 | 9 | 7 | 12 | 8 | 7 | 6 | 7 | 8 | 6 | 11 | 11 | 8 | 11 | 8 | 9 | 6 | 12 | 6 | 8 | 10 | 9 | 9 | 13 |
| FHA | 19 | 38 | 13 | 13 | 10 | 9 | 15 | 23 | 13 | 15 | 6 | 7 | 13 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 2 | 1 | 11 | 9 | 1 | 0 | 1 | 20 | 14 |
| Abhydrolase_1 | 18 | 24 | 24 | 27 | 10 | 11 | 24 | 26 | 15 | 20 | 23 | 14 | 29 | 4 | 5 | 6 | 5 | 4 | 5 | 5 | 5 | 4 | 6 | 5 | 4 | 13 | 6 | 5 | 13 | 5 | 15 | 14 | 6 | 3 | 6 | 11 | 18 |
| GTP_EFTU | 18 | 18 | 19 | 19 | 15 | 14 | 15 | 14 | 18 | 17 | 12 | 13 | 18 | 9 | 10 | 11 | 11 | 12 | 10 | 9 | 10 | 10 | 10 | 11 | 12 | 12 | 13 | 11 | 12 | 11 | 17 | 16 | 11 | 11 | 11 | 14 | 17 |
| Hydrolase | 18 | 21 | 25 | 33 | 9 | 9 | 19 | 22 | 13 | 23 | 14 | 15 | 26 | 4 | 2 | 4 | 5 | 6 | 2 | 4 | 2 | 5 | 3 | 5 | 7 | 18 | 7 | 7 | 18 | 6 | 15 | 16 | 9 | 8 | 8 | 10 | 12 |
| NAD_binding_4 | 18 | 25 | 23 | 24 | 8 | 6 | 17 | 21 | 18 | 17 | 16 | 18 | 29 | 8 | 5 | 8 | 7 | 11 | 7 | 8 | 7 | 6 | 9 | 7 | 10 | 11 | 8 | 9 | 11 | 8 | 16 | 7 | 8 | 9 | 10 | 11 | 12 |
| 3Beta_HSD | 17 | 25 | 17 | 19 | 5 | 5 | 16 | 21 | 13 | 14 | 19 | 11 | 22 | 6 | 6 | 3 | 7 | 7 | 6 | 7 | 5 | 4 | 8 | 8 | 7 | 8 | 7 | 8 | 8 | 5 | 11 | 7 | 8 | 6 | 7 | 8 | 10 |
| Aminotran_1_2 | 17 | 12 | 17 | 20 | 12 | 13 | 14 | 13 | 15 | 15 | 17 | 15 | 22 | 7 | 8 | 5 | 7 | 10 | 8 | 6 | 5 | 6 | 5 | 8 | 9 | 11 | 9 | 7 | 13 | 9 | 13 | 11 | 12 | 7 | 7 | 11 | 16 |
| CbiA | 17 | 29 | 24 | 27 | 13 | 12 | 19 | 25 | 22 | 19 | 19 | 20 | 29 | 7 | 8 | 8 | 9 | 6 | 8 | 8 | 8 | 8 | 8 | 8 | 6 | 11 | 8 | 8 | 12 | 9 | 19 | 19 | 9 | 6 | 8 | 11 | 18 |
See Table 1 for abbreviations
*Highest domain numbers in A. platensis
Positive correlation was detected between the number of genes and number of Pfam domains of the 38 cyanobacterial species (r = 0.9X, P < 0.05), including A. platensis (Supplementary Fig. S1). Eighteen domains (DUF1064, BOF, Cytochrom_C_2, DUF1624, DUF1667, DUF2234, DUF2273, DUF268, DUF310, DUF579, Hp0062, JmjC, LAB_N, PhoU_div, PMSR, PPTA, SoxG and YibE_F) are unique to the A. platensis genome. The abundance of signalling domains, such as GAF (guanylate cyclase/adenylate cyclase/FhlA), PAS (Per/Arnt/Sim), HisKA and Respons_reg, depends on the habitat of the cyanobacteria; i.e. soil and freshwater cyanobacteria possess larger numbers of signalling domains than marine cyanobacteria (Supplementary Fig. S2). A. platensis, which lives in high salt lakes, was thus categorized as a soil/freshwater cyanobacterium.
3.2.2. Physiological profiling using CyanoClust
Arthrospira platensis is the first strain of filamentous non-N2-fixing cyanobacteria to have its genome sequenced. Whole-genome sequencing allows for comparative genomic analysis especially in regard to trichome formation and N2-fixation. Using the CyanoClust database, we extracted 694 gene clusters (938 genes in A. platensis) that were common to all six cyanobacteria groups (Table 3 and Supplementary Table S1). These clusters may be closely related to housekeeping, photosynthetic or cyanobacteria-specific genes. We also extracted 1066 gene clusters (2056 genes in A. platensis) that were specific to A. platensis (Table 3 and Supplementary Table S2); 71 (94 genes in A. platensis) common to only groups IV (A. platensis) and V (T. erythraeum) (Supplementary Table S3); and 223 common to the heterocyst-forming cyanobacteria. The latter clusters included known heterocyst-related genes such as patN and hetP (Table 3 and Supplementary Table S4). In addition, eight clusters were specifically conserved among N2-fixing cyanobacteria (groups II, V and VI) (Table 3 and Supplementary Table S5); most of these encode nif-related genes.
Table 3.
Summary of comparative genomic analysis using CyanoClust
| Physiological profiling |
|||||
|---|---|---|---|---|---|
| Common | A. platensis-specific | Heterocyst-specific | N2-fixing-specific | Filament-specific | |
| Group I | O | X | X | X | X |
| Group II | O | X | X | O | X |
| Group III | O | X | X | X | X/O |
| Group IV | O | O | X | X | O |
| Group V | O | X | X | O | O |
| Group VI | O | X | O | O | O |
| No. of clusters | 694 | 1066 | 223 | 8 | 29/7 |
| No. of genes in A. platensis | 938 | 2056 | 0 | 0 | 31/7 |
For cyanobacteria grouping, see Table 1.
Of the 29 gene clusters (31 genes in A. platensis, Table 4 and Supplementary Table S6) common to only filamentous cyanobacteria (groups IV, V and VI), fraC and fraG (sepJ) are already known to have an association with trichome formation.40,41 Notably, seven gene clusters (seven genes in A. platensis, Table 4 and Supplementary Table S7) were found to be common to the filamentous groups and Synechococcus sp. PCC 7002, which may be a primitive filamentous species (group III) and may also be related to trichome formation. Moreover, several genes (patU, hetR and hetF)42–44 that are required for heterocyst maturation and N2 fixation are conserved in A. platensis, although no nitrogenase genes were detected; this suggests that heterocyst formation could be developmentally coupled with trichome formation. Several contiguous genes (bolded in Table 4) are syntenically conserved among the five trichome-forming cyanobacteria. Particularly, NIES39_A00790 is located just downstream of fraC, implying that it is another candidate required for trichome development.
Table 4.
Gene clusters conserved only in filamentous cyanobacteria
| Cluster No. | Gene ID | Annotation |
|---|---|---|
| Specific for groups IV, V and VI | ||
| 4981 | NIES39_A00790 | Hypothetical protein |
| 5158 | NIES39_A00800 | Filament integrity protein (fraC) |
| 4909 | NIES39_A01310 | NUDIX hydrolase |
| 3571 | NIES39_A03140 | Hypothetical protein |
| 4548 | NIES39_A04850 | Hypothetical protein |
| 3571 | NIES39_C00130 | Hypothetical protein |
| 4852 | NIES39_C00870 | Hypothetical protein |
| 2616 | NIES39_D00070 | Hypothetical protein |
| 4736 | NIES39_D00920 | Hypothetical protein |
| 4710 | NIES39_E01660 | Nuclease (SNase-like) |
| 4588 | NIES39_E02590 | Serine/threonine protein kinase |
| 4667 | NIES39_F00600 | Probable glycosyl transferase |
| 4881 | NIES39_K02770 | Hypothetical protein |
| 4277 | NIES39_K02780 | Hypothetical protein |
| 4548 | NIES39_L02410 | Hypothetical protein |
| 4747 | NIES39_L03440 | Hypothetical protein |
| 2616 | NIES39_L06030 | Hypothetical protein |
| 5149 | NIES39_L06180 | Hypothetical protein |
| 5020 | NIES39_M00320 | Hypothetical protein |
| 4731 | NIES39_M02510 | DUF6 transmembrane protein (sepJ, fraG) |
| 5143 | NIES39_N00400 | Hypothetical protein (patU) |
| 2616 | NIES39_N00410 | Hypothetical protein |
| 5032 | NIES39_N00980 | Hypothetical protein |
| 4826 | NIES39_O03720 | Hypothetical protein |
| 5058 | NIES39_O03830 | hypothetical protein |
| 5102 | NIES39_O03850 | Hypothetical protein |
| 4991 | NIES39_O04240 | Hypothetical protein |
| 4590 | NIES39_O06790 | Alpha/beta hydrolase fold |
| 4996 | NIES39_Q00570 | Hypothetical protein |
| 5045 | NIES39_Q00580 | Hypothetical protein |
| 4607 | NIES39_R00660 | Hypothetical protein |
| Specific for groups III, IV, V and VI | ||
| 4298 | NIES39_C02810 | Hypothetical protein |
| 4333 | NIES39_C03480 | Heterocyst differentiation protein (hetR) |
| 4276 | NIES39_D04070 | Hypothetical protein |
| 4505 | NIES39_J00800 | Hypothetical protein |
| 3819 | NIES39_J02400 | Heterocyst differentiation protein (hetF) |
| 4311 | NIES39_O06320 | Hypothetical protein |
| 4255 | NIES39_Q02800 | Hypothetical protein |
Bolded genes are contiguous.
3.3. Mobile DNA elements
Group II introns include ribozymes and retroelements in the genomes of organelles, Eubacteria and Archaea. In bacteria, group II introns primarily act as retroelements consisting of six RNA structural domains (DI–DVI) and an intron-encoded protein, whereas in mitochondria and plastids, they frequently lack the intron-encoded protein and are mobile. At least 150 group II introns were identified in the A. platensis genome; 71 of these encode reverse transcriptase/maturase, whereas the other 79 do not. Additionally, 88 sequences were assigned to the group II catalytic intron (RF00029), DV and DVI. This number was much higher than that in previously reported abundant species (27 copies of group II introns in T. elongatus45 and 28 copies of RF00029 in T. erythraeum). These results suggested that group II introns have been self-propagating extensively in A. platensis cells for a long time.
Group I introns include ribozymes that catalyse their own RNA splicing to produce ligated exons (mature RNA) and the excised intron. We detected two group I introns in one gene comprising three ORFs (NIES39_L05970, NIES39_L05980 and NIES39_L05990) which encode class I ribonucleotide reductases (RNRs; Fig. 2). Notably, a stop codon was found just after the insertion position in each intron, indicative of translational regulation. It is extremely rare for a group I intron to be inserted in protein-coding genes in bacteria, although one report has detected this insertion in class II RNR of the cyanobacterium N. punctiforme.46 At least three different classes of RNRs that differ in primary sequence, substrate and cofactor requirements have been described in cyanobacteria. Since class I RNRs have no homology with class II RNRs, the essential DNA biosynthesis step from RNA could be an evolutionary hot spot for targeting these selfish genes. Cyanobacterial genome comparison revealed that classes I and II are distributed complementally in cyanobacteria, except for Gloeobacter violaceus in which no known RNR genes have been detected (Supplementary Table S8), whereas class III anaerobic RNRs are also present in some species. Many class I and II RNR genes are interrupted by introns and/or inteins which are spliced out for sequencing of RNA or protein. Inteins in A. platensis were also found in DnaB (NIES39_M02320), DnaX (NIES39_D01990) and thymidylate synthase (NIES39_Q01490) genes.
Figure 2.
Gene structure of the ribonucleotide reductase (RNR) gene with two group I intron insertions.
Novel phage-like sequences spanning from 12.5 to 25.5 kb were found in at least 18 loci in the A. platensis genome. Although there are a number of variations in deletion, insertion and rearrangement in each sequence, they consist of phage infection-related genes (e.g. NIES39_F00750) and small conserved putative genes, generating a direct repeat in some case. Such phage-like sequences contribute to at least 295 kb in the whole genome. As for transposases of the insertion sequence, 139 genes were found, which is comparable to other cyanobacteria.18 A total 612 kb of the genome are composed of group II introns, phage-like sequences, insertion sequences and some repetitive elements, which include clustered regulatory interspaced short palindromic repeat (CRISPR) sequences (see Section 3.5.2).
3.4. Signal transduction proteins
3.4.1. cAMP and c-di-GMP signal transduction
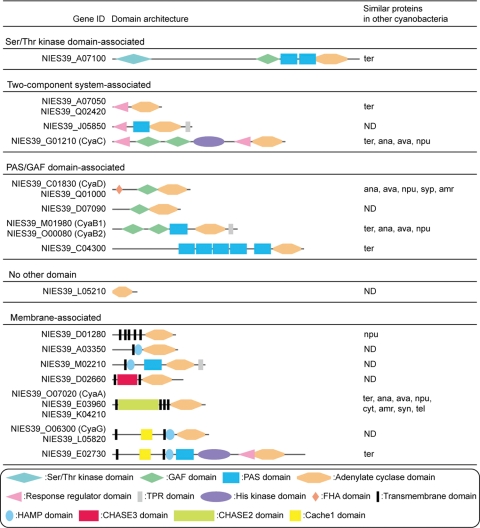
cAMP is an important signalling molecule in cyanobacteria.47 A. platensis genome encodes 22 adenylate cyclases that produce cAMP from ATP (Fig. 3). This number is quite prominent among cyanobacterial genomes, which usually encode less than 10 adenylate cyclases. Even T. erythraeum, the most closely related cyanobacterium to A. platensis, contains only 13 adenylate cyclase genes. In addition to the genes cyaC,13,14 cyaG,13,14 cyaA, cyaB1, cyaB2 and cyaD homologues (Fig. 3),48 many novel genes were detected. Another unique characteristic of A. platensis is its 10 membrane-associated adenylate cyclases, compared with most cyanobacteria which have only one or two. Thus, the cAMP signal transduction response to external stimuli may be highly developed in A. platensis. As for the domain structure of adenylate cyclase, NIES39_A07100 bears a unique domain architecture: a Ser/Thr protein kinase domain in the N-terminus and GAF, PAS and adenylate cyclase domains in the C-terminus (Fig. 3). This domain architecture is quite similar to that of the HstK family,49 a hybrid of Ser/Thr protein kinase and histidine kinase, except that the C-terminal histidine kinase domain is replaced with the adenylate cyclase domain.
Figure 3.
Domain architecture of adenylate cyclases in A. platensis. See Table 1 for abbreviations.
The A. platensis genome also contains 14 genes encoding cNMP-binding domains. Four of them [NIES39_C02730 (NtcA), NIES39_D00910 (CRP), NIES39_M01440 and NIES39_O00090] encode the helix-turn-helix DNA-binding motif of the CRP family, whereas the remaining encode unique domain architecture proteins. NIES39_A00950 bears a unique domain architecture, namely, an ATP:ADP antiporter-like domain in the N-terminus and a cNMP-binding domain in the C-terminus. The C-terminal cNMP-binding domain contains residues critical for binding cAMP. A previous report showed that A. platensis trichomes rapidly aggregate to form a mat in response to externally added cAMP.50 This aggregation is accompanied with an increase in the intracellular ATP level and a decrease in the intracellular ADP level,12 suggesting that NIES39_A00950 may contribute to this cAMP-dependent aggregation.
There are 33 GGDEF and 15 EAL domains, respectively, involved in the synthesis and hydrolysis of c-di-GMP,51 a widely distributed second messenger for biofilm formation in bacteria. One GGDEF (NIES39_C01090) and three GGDEF/EAL (NIES39_C03530, NIES39_L00190 and NIES39_Q02220) proteins contain putative flavin-binding PAS domains, suggestive of light- or redox-responsive c-di-GMP signalling for movement, aggregation or biofilm formation.
3.4.2. Two-component signal transduction systems
Two-component signal transduction systems canonically consist of two proteins, a histidine kinase and a response regulator.52 There are 84 putative genes for histidine kinases in the A. platensis genome. Among them, 33 encode hybrid histidine kinases containing not only a transmitter domain but also single or multiple receiver domain(s) or histidine phospho-transfer domain(s), which accept the phosphate group via intramolecular and/or intermolecular phospho-transfer. There are also 65 putative genes for response regulators.
Orthologous histidine kinases of Synechocystis, Hik2 (slr1147), Hik7 (SphS, sll0337), Hik8 (sasA, sll0750), Hik33 (sll0698) and Hik34 (slr1285), are conserved in almost all cyanobacterial genomes. The A. platensis genome contains orthologues of Hik2 (NIES39_J01640), Hik8 (NIES39_A03740), Hik33 (NIES39_M02760) and Hik34 (NIES39_J00420), but not SphS, a kinase that induces proteins to acquire inorganic phosphate (Pi) under Pi-deficient conditions. Although some marine cyanobacteria have been found to lack apparent orthologues of SphS, A. platensis is the first example among freshwater cyanobacteria. Accordingly, it does not contain a gene for SphU which regulates SphS.53 However, the response regulator SphR and its target genes phoA (alkaline phosphatase) and pts (high affinity Pi transporter) are present in the A. platensis genome, suggesting a different regulation system in this cyanobacterium.
Some histidine kinases in A. platensis contain multiple domains of PAS/PAC and/or GAF. These structures are also prevalent in other histidine kinases from filamentous cyanobacteria, such as Anabaena sp. PCC 712054 and N. punctiforme.55 Like in other cyanobacterial genomes, most genes for histidine kinases and response regulators in the A. platensis genome are not collocated to each other on the chromosomes, except for the 18 cases (Supplementary Table S9). Several genes encode fragments of histidine kinases in the A. platensis genome suggestive of pseudogenes (NIES39_A02200–NIES39_A02210, NIES39_D02110–NIES39_D02130–NIES39_D02160, NIES39_L02740–NIES39_L02750, NIES39_L05670–NIES39_L05680–NIES39_L05690).
3.4.3. Transcription factors
Cyanobacteria have multiple σ70-type sigma factors of RNA polymerase and no σ54-type sigma factor. In the A. platensis genome, there are seven σ70-type sigma factors. NIES39_L06110 encodes a group 1 sigma factor, which is also called the principal sigma factor and is essential for cell viability. In addition, three group 2 sigma factors (NIES39_A03770, NIES39_A08720 and NIES39_C01040), which show a high degree of sequence similarity with the principal sigma factor but are non-essential for cell growth, and three group 3 sigma factors (NIES39_C05220, NIES39_D05870 and NIES39_O06410), which are divergent from group 1 and 2 sigma factors in amino acid sequence, are identified. The B-, C- and D-type group 2 sigma factors and the F- and G-type group 3 sigma factors are conserved among non-marine cyanobacteria,56,57 all of which are present in A. platensis. An additional group 3 sigma factor which does not form a clade with previously analysed sigma factors was also determined.
In the A. platensis genome, there are 66 putative genes for transcriptional regulators. Compared with the genome size, this number is quite small for a non-marine cyanobacterium, being more comparable to marine cyanobacteria.58 For example, the freshwater cyanobacterium Anabaena sp. PCC 7120, having a comparable genome size to A. platensis, contains more than 100 transcriptional regulators.54 Some well-conserved transcriptional regulators among cyanobacteria were found in A. platensis, including genes for NtcA (NIES39_C02730),59 NtcB (NIES39_C02400),60 NdhR (NIES39_K02860),61 HrcA (NIES39_O04340)62 and CRP (NIES39_D00910).63 Two adjacent genes (NIES39_D06090 and NIES39_D06100) encode RbcR paralogues.64 Although A. platensis does not form a heterocyst, a gene for HetR (NIES39_C03480) has recently been found.65 Arthrospira platensis contains 20 response regulators that have a so-called receiver domain at the N-terminus and the helix-turn-helix type DNA-binding domain at the C-terminus. Of these regulators, 16 are classified in the OmpR family whereas the remaining belong to the LuxR family. Orthologous proteins of NrrA (NIES39_A06330),66 ManR (NIES39_G00800),67,68 RpaA (NIES39_H00910),69 SphR (NIES39_J04020),70 RpaB (NIES39_K03840)69 and NblR (NIES39_O05300)69 were also identified.
3.4.4. PAS/GAF domains
Most non-marine cyanobacteria contain a large number of PAS and GAF domains.54 The PAS and GAF domains are important signalling molecules that serve as specific sensors for light, redox, oxygen and many other signals or a structural element for dimerization.71,72 We detected 131 PAS domains using a custom HMM profile of Anabaena and Nostoc PAS domains.73 This number is almost comparable to those of other freshwater and soil cyanobacteria such as Anabaena and Nostoc. Six PAS domains encoded by NIES39_C01090, NIES39_C03530, NIES39_M00920, NIES39_L00190, NIES39_M02160 and NIES39_Q02220 are predicted to bind a flavin; the first three retain the conserved Cys residue that is essential for the photocycle of the LOV-type photoreceptors. One PAS domain encoded by NIES39_A03380 is predicted to bind a heme.
The A. platensis genome contains 58 GAF domains. Four GAF domains encoded by NIES39_M01980 (CyaB1) and NIES39_O00080 (CyaB2) are predicted to bind cAMP,74 whereas 18 are categorized into the phytochrome-type or cyanobacteriochrome-type GAF domain subfamilies that bind linear tetrapyrrole and exhibit reversible photoconversion.75 We found no Cph1 and AphA orthologues, which are cyanobacterial phytochromes, although bacteriophytochrome-type AphB (NIES39_A02210) and AphC (NIES39_C03450) orthologues are detected.76 In addition, there are two green/red reversible AnPixJ-type (NIES39_J03990_GAF2 and NIES39_C00690_GAF1)77 and six blue/green reversible TePixJ-type (NIES39_K04670_GAF1, NIES39_E01230_GAF1, NIES39_L03820_GAF1, NIES39_E04120_GAF3, NIES39_D04330_GAF1 and NIES39_M02490_GAF1) GAF domains.75,78
3.4.5. Chemotaxis regulators
In the A. platensis genome, there are eight methyl-accepting chemotaxis proteins (MCPs), which may serve as sensors for cell motility. In contrast with photoreceptor MCPs (e.g. PixJ) in many other cyanobacteria,79 any of Arthrospira MCPs does not harbour any photoreceptor domain. Three of these MCP genes are clustered with other chemotaxis-regulating che genes such as cheY, cheW and cheA (NIES39_A07840–NIES39_A07910, NIES39_E01010–NIES39_E01070 and NIES39_H00230– NIES39_H00290 gene clusters). Two che clusters (NIES39_A07840–NIES39_A07910 and NIES39_H00230–NIES39_H00290) lack the patA genes which are present in the che clusters of other cyanobacteria. These che clusters contain additional che genes (cheB, cheC and cheR in the NIES39_A07840–NIES39_A07910 gene cluster and cheB and cheR in the NIES39_H00230–NIES39_H00290 gene cluster), which have not been detected in che clusters of other cyanobacteria such as Synechocystis, whose motility and phototaxis regulation is well understood. CheR and CheB are known to methylate and demethylate the MCP, respectively, and thereby function as a kind of molecular memory for flagellar regulation in some proteobacteria such as E. coli.80 CheC is a phosphatase for the CheY response regulator.81 As MCPs of these clusters have no photosensory domains, these genes may function in chemotaxis. Molecular memory and a rapid turnover system of phosphorylation can facilitate highly sensitive chemotactic responses.
3.4.6. Ser/Thr protein kinases and protein phosphatases
There are 43 genes that encode Ser/Thr protein kinases in the A. platensis genome. As in other filamentous cyanobacteria such as Anabaena and Nostoc,54 HstK family (two), WD40-containing (nine) and TPR-containing (five) Ser/Thr kinases were detected.
Fourteen genes encoding phospho-Ser, -Thr and -Tyr phosphatases were also identified in the A. platensis genome. Among them, nine belong to the PP2C family including NIES39_O07130 and NIES39_R00570 that have response regulator domains. Other members of this family include NIES39_A03470, NIES39_C00770, NIES39_J01920, NIES39_J03440, NIES39_K03740, NIES39_M01820 and NIES39_M02650 (PphA) which dephosphorylates the nitrogen regulator PII protein.82 In addition, two phospho-Tyr (NIES39_B01000 and NIES39_N00600) and three PP1/2A/2B-type (NIES39_D01240, NIES39_G01060 and NIES39_G01070) phosphatases were identified.
3.5. Genome protection
3.5.1. RM system
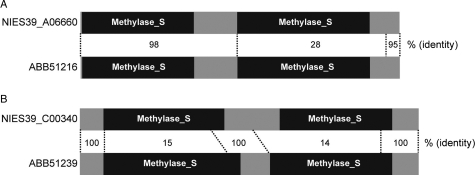
We detected three sets of type I RM systems (hsdMSR) in the A. platensis NIES-39 genome (Supplementary Table S10 and Supplementary Fig. S3) which are shared between a closely related strain reported by a Chinese group.83 Among them, two RM-specific genes (hsdS, NIES39_A06660 and NIES39_C00340) are mosaically conserved between the two strains (Fig. 4), whereas the full-length methylase and restriction genes hsdM and hsdR are highly conserved (>98%). HsdS protein is composed of two target recognition domains and the N-terminal, central and C-terminal regions. NIES39_A06660 is highly conserved with ABB51216 throughout the entire protein except for the second target recognition domain (Fig. 4A). NIES39_C00340 is highly conserved with ABB51239 at the N-terminal, central and C-terminal regions but not at the target recognition domains (Fig. 4B). These mosaically conserved HsdS proteins may be generated by one or two homologous recombinations through a mechanism similar to domain swapping of hsdS genes in other bacteria.84 This enables A. platensis to acquire the restriction system against a wider range of exogenous DNA elements, interfering with genetic transformation of A. platensis. On the other hand, another hsdS-like reading frame, which is interrupted by an inframe stop codon, is totally conserved between these two strains.
Figure 4.
Domain architecture of HsdS proteins. The target recognition domain roughly corresponds to Methylase_S (PF01420).
NIES-39 harbours eight clusters of the type II RM systems, four of which are identical to those of the strain of the Chinese group,83 although three are frame shifted. The other four clusters are novel in NIES-39. On the basis of this information of the RM systems, genetic transformation in A. platensis requires further improvement.
3.5.2. CRISPR system
CRISPRs are widespread in the genomes of many bacteria and almost all archaea, and they confer resistance to phages together with a group of CRISPR-associated (Cas) proteins.85–87 The A. platensis genome contains three regions of CRISPRs, which are located in close proximity to Cas proteins. CRISPR1 (at coordinates 2897603–2897927 and 2903170–2904079) is organized as 17 almost identical sequences of 35 nt interspaced by non-identical sequences of 34–43 nt. CRISPR2 (at coordinates 3587555–3589797) and CRISPR3 (at coordinates 5189874–5191453) are organized as 29 and 23 identical sequences of 36 and 35 nt interspaced by non-identical sequences of 37–49 and 32–41 nt, respectively. We also found 18 Cas genes. One of the three Cas1 proteins encoded by NIES39_F00150 is a fusion protein, having a C-terminal Cas1 domain and a reverse transcriptase domain similar to that of group II introns.
3.6. Membrane transporters
The A. platensis genome contains ∼180 genes encoding putative membrane transporters. NapA-type Na+/H+ antiporters (Nha3 in S. elongatus PCC 7942) are known to be involved in salt tolerance at alkaline pH in some cyanobacteria.88–90 Arthrospira platensis possesses seven putative Na+/H+ antiporters, one of which (NIES39_C00590) is an orthologue of NapA and Nha3.
Accumulation of bicarbonate in the cytoplasm is essential for photosynthesis under high pH conditions.91 Arthrospira platensis possesses two sets of genes for CO2-uptake modulation, NDH-1 (NdhF3-D3-CupA-CupS for high affinity and NdhF4-D4-CupB for low affinity), which converts CO2 to bicarbonate in the cytoplasm (Supplementary Table S11). Genes sbtA and bicA encode the sodium-dependent bicarbonate transporter in A. platensis. Two closely related copies of bicA (NIES39_B00700, NIES39_B00710), which are tandemly arranged on the genome, were also found. There is only one operon for the ABC-type transporter for either nitrate (NrtA-B-C-D) or bicarbonate (CmpA-B-C-D).
3.7. Photosynthesis-related genes
3.7.1. Photosystem components
Almost all photosynthesis genes were detected in A. platensis (Supplementary Table S11). For photosystem II, the reaction centre D1 protein is encoded by at least three identical copies and one divergent copy of psbA. Additional variant genes include cytochrome c550-like (NIES39_J05860) and psb28-2 (NIES39_A01290). For photosystem I, all known genes including psaX were detected. Genes for cytochrome b6/f, ATP synthase, and NDH were detected as in other cyanobacteria. We detected two copies of cytochrome c6, but no typical electron carrier proteins in the thylakoid lumen such as plastocyanin and cytochrome cM.
3.7.2. Genes for tetrapyrrole biosynthesis
One set of single-copy genes involved in tetrapyrrole biosynthesis was identified in the A. platensis genome. Almost all genes involved in porphyrin biosynthesis were assigned. For siroheme and heme biosynthesis, uroporphyrinogen-III C-methyltransferase (NIES39_H00180) and two types of ferrochelatase (HemH: NIES39_K00630 and NIES39_M01510) were also identified. The latter encodes the so-called ferrochelatase-like protein possessing characteristic poly-His sequence at the C-terminus, which are also found in some cyanobacteria. For heme metabolism and subsequent phycobilin biosynthesis, heme oxygenase (HO: NIES39_Q00930), heme o synthase (NIES39_O03120), protoheme IX farnesyltransferase (NIES39_E02030) and biliverdin reductase (BvdR: NIES39_B00110) were assigned. For chlorophyll a biosynthesis, all genes were identified as single-copy genes. As in other cyanobacteria, isoforms catalyzing the same reaction, i.e. aerobic (HemF: NIES39_D01090) and anaerobic (HemN: NIES39_E03410) coproporphyrinogen III oxidase and light-dependent (Por: NIES39_R00680) and dark-operative (ChlL, ChlN and ChlB: NIES39_L05610, NIES39_L05630 and NIES39_L01300, respectively) protochlorophyllide oxidoreductase, were identified, whereas only the aerobic form of Mg-protoporphyrin IX monomethylester cyclase (AcsF/CrdI/CHL27: NIES39_B00770) was found. These isoforms were assumed to function in the adaption to different oxygen environments.
3.7.3. Genes for biosynthesis of carotenoid, lipid and others
All known cyanobacterial genes involved in carotenoid biosynthesis were found except for beta-carotene ketolase (CrtW or CrtO) in the A. platensis genome. However, 3-hydroxyechinenone, which is produced by ketolase, is reported to bind to an orange carotenoid protein (NIES39_N00720) to regulate energy dissipation from phycobilisome to photosystem II.92,93 Therefore, a yet unknown type of ketolase may be present in the genome.
All known cyanobacterial genes involved in biosynthesis of glycerolipids, fatty acids, lipoic acid, vitamin E, lipopolysaccharides and polyhydroxyalkanoates were assigned. A gene for ω-3 fatty acid desaturase (desB) was not found, which is consistent with the biochemical analysis of A. platensis.94
The biosynthesis genes for cyanotoxins such as non-ribosomal peptide toxins (microcystins), ribosomal depsipeptide toxins (microviridins), alkaloid toxins (anatoxins and saxitoxins) and urea-derived toxins (cylindrospermopsin) were not present in the genome, which concurs with the long-time use of this organism as a food.
3.7.4. Carboxysome, reactive oxygen species protection and gas vesicles
A. platensis has eight genes for β-type carboxysome, which converts the accumulated bicarbonate ion to CO2 for ribulose 1,5-bisphosphate carboxylase. These genes are split into two operons (ccmK1-K2 and ccmK3-K4-L-M-N-O). Only one gene (ccmM) encodes carbonic anhydrase for conversion of bicarbonate to CO2.
We also detected some enzymes which protect against reactive oxygen species, except for catalase. However, the A. platensis genome harbours many genes for thioredoxin peroxidase, peroxiredoxin and other putative peroxidases including five genes for bacterioferritin co-migratory proteins (NIES39_A03490, NIES39_E01510, NIES39_E02230, NIES39_L02380, NIES39_O06760).
Genus Arthrospira possesses gas vesicles for buoyancy of its trichomes, which is in contrast with no gas vesicles in the morphologically similar genus Spirulina.95 Consequently, A. platensis has six gas vesicle genes (gvpA-C-N-J, gvpV and gvpW); GvpA is a structural protein to form the skeleton of the gas vesicle.
3.8. Cell surface proteins and motility
3.8.1. Cell surface and extracellular proteins
Concerning the cell surface structure and extracellular proteins, 42 genes were found to encode putative extracellular proteins that contain 191 haemolysin-like Ca2+-binding domains in the A. platensis genome (Table 2). This number is much higher than that in many other cyanobacteria. Fifteen of these genes consist of merely haemolysin-like Ca2+-binding domains, whereas 14 possess an additional SBBP-like domain, which is often found in S-layer-related proteins (i.e. NIES39_B00690, NIES39_C00400, NIES39_D06770, NIES39_D06910, NIES39_D06930, NIES39_D06980, NIES39_D07000, NIES39_D07020, NIES39_Q00770, NIES39_Q00840, NIES39_Q02140, NIES39_Q02160, NIES39_Q02170 and NIES39_Q02190).96 NIES39_D06770 also possesses the CalX-beta domain which is present in the cytoplasmic domains of CalX Na+/Ca2+ exchangers to expel calcium from cells,97 and NIES39_Q02140, NIES39_Q02160, NIES39_Q02170 and NIES39_Q02190 possess both the PPC domain found at the C-terminus of secreted bacterial peptidases and the CalX-beta domain. Three other genes (NIES39_C03760, NIES39_F00640 and NIES39_J05690) also possess the PPC domain. Other genes possess domains for cadherin (NIES39_C05180), SCP (NIES39_D00540, NIES39_D06960 and NIES39_L04630), Lipase-GDSL (NIES39_E00490), pro-isomerase (NIES39_L02390) and Chlam-PMP (NIES39_L00320 and NIES39_M01680); NIES39_L00320 also possesses the CalX-beta domain. Although pathogenic haemolysins are well known in Enterobacteria,98,99 it is interesting that non-toxic A. platensis has a large number of genes that contain the haemolysin-like Ca2+-binding domain.
The A. platensis genome has no genes coding siderophore to scavenge iron from the environment.
3.8.2. Gliding motility
The molecular mechanism for gliding motility has been poorly understood in cyanobacteria. The outer surface of gliding cyanobacteria in Oscillatoriaceae consists of a parallel, helically arranged protein array (S-layer).100,101 The S-layer protein, oscillin, required for gliding motility in Phormidium102 is conserved in A. platensis (NIES39_A01430, 42% identity). NIES39_A01430 consists of 19 haemolysin-like Ca2+-binding domains. This protein is also homologous to SwmA (27% identity) for swimming motility in unicellular Synechococcus sp. WH 8102.103,104
Gliding motility of cyanobacteria is reported to be driven by a secretion of mucilage from the junctional pore complex (JPC).105 The JPC consists of a channel through the outer membrane and the peptidoglycan layer. Although the subunits of JPC have not been identified, comparative genomics analysis may reveal their genes.
3.8.3. Type IV pilus-related proteins
Type IV pili are involved in twitching motility in many bacteria79,106–108 and have recently been reported to function in gliding motility in N. punctiforme109 and twitching motility in unicellular Synechocystis.110 A. platensis exhibits vigorous gliding motility and harbours type IV pilus-related genes (Supplementary Table S11), but shows no twitching motility, suggesting that this organism utilizes type IV pili as machinery for gliding motility like N. punctiforme.109 Typical pil genes for pili biogenesis are the pilMNOQ operon, pilB1T1C operon, pilD (prepilin leader peptidase) and three prepilin subunit genes (NIES39_C03030, NIES39_C00840 and NIES39_C00850). Additionally, A. platensis harbours the rather unusual pilT2-like (NIES39_A08210) and pilC-like (NIES39_A03250) genes. These copies may be involved in novel regulation of the type IV pili or type II secretion system, which has not yet been studied in cyanobacteria.
3.9. Microevolution
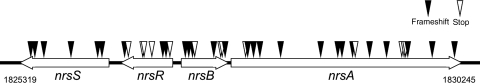
Arthrospira platensis NIES-39 harbours many pseudogenes, whose coding regions fragmentally correspond to known genes or ORFs (Supplementary Table S12). Many of them are not just accidental mutants resulting from single mutations but are generated by at least two events, frameshift and/or nonsense mutations. An extreme case is the operon of nrsS/R/B/A, which is inactivated by at least 27 frameshift mutations and 13 nonsense mutations (Fig. 5). NrsS/R involve the two-component signal transduction system for nickel sensing and transcriptional regulation, whereas NrsB/A form a cation-efflux pump to remove heavy metals.111 The extensive degradation of its operon strongly suggests that A. platensis NIES-39 is in the process of microevolution to adapt to its specific location in Lake Chad.
Figure 5.
Selective mutations in the nrsS/R/B/A region.
Rapid evolution is also noted in the hsdS gene which determines the RM specificity (see Section 3.5.1). Since many strains of A. platensis and A. maxima are the target of genome projects and economical applications, the microevolution of their genomes and diversity in phenotype are critical themes for post-genome studies.
Supplementary material
Supplementary Material is available at www.dnaresearch.oxfordjournals.org.
Supplementary Material
Acknowledgements
We thank Ms Miho Furumichi and Professor Minoru Kanehisa (KEGG) for kindly providing the CYORF data. We also thank Dr Fumie Kasai (NIES) for kindly providing Arthrospira platensis NIES-39.
Footnotes
Edited by Katsumi Isono
References
- 1.Desikachary T.V. In: Botanical Monographs. Carr N.G., Whitton B.A., editors. Oxford, London, Edinburgh, Melbourne: Blackwell Scientific Publications; 1973. pp. 473–81. [Google Scholar]
- 2.Doolittle W.F. In: The Biology of Cyanobacteria, Botanical Monographs. Carr N.G., Whitton B.A., editors. Berkeley and Los Angels: University of California Press; 1982. pp. 307–31. [Google Scholar]
- 3.Van Tuerenhout D. The Aztecs: New Perspectives. ABC-CLIO; 2005. [Google Scholar]
- 4.Belay A. In: Spirulina in Human Nutrition and Health. Gershwin M.E., Belay A., editors. CRC press: Florida; 2007. pp. 1–26. [Google Scholar]
- 5.Castenholz R.W., Rippka R., Herdman M., Wilmotte A. In: Bergey's Manual of Systematic Bacteriology. 2nd edition. Boone D.R., Castenholz R.W., Garrity G.M., editors. Springer: Berlin; 2007. pp. 542–3. [Google Scholar]
- 6.Kasai F., Kawachi M., Erata M., et al. NIES-collection list of strains 8th edition. Jpn. J. Phycol. 2009;57:162. [Google Scholar]
- 7.Vonshak A. Spirulina platensis (Arthrospira): Physiology, Cell-Biology, and Biotechnology. Taylor & Francis Group: London; 1997. [Google Scholar]
- 8.Hoiczyk E. Gliding motility in cyanobacteria: observations and possible explanations. Arch. Microbiol. 2000;174:11–7. doi: 10.1007/s002030000187. [DOI] [PubMed] [Google Scholar]
- 9.Zeng M.T., Vonshak A. Adaptation of Spirulina platensis to salinity stress. Comp. Biochem. Physiol. A. 1998;120:113–8. [Google Scholar]
- 10.Kebede E. Response of Spirulina platensis (Arthrospira fusiformis) from Chitu, Ethiopia, to salinity stress from sodium salts. J. Appl. Phycol. 1997;9:551–8. [Google Scholar]
- 11.Ohmori K., Ehira S., Kimura S., Ohmori M. Changes in the amount of cellular trehalose, the activity of maltooligosyl trehalose hydrolase, and the expression of its gene in response to salt stress in the cyanobacterium Spirulina platensis. Microb. Environ. 2009;24:52–6. doi: 10.1264/jsme2.me08537. [DOI] [PubMed] [Google Scholar]
- 12.Ohmori K., Ohmori M. cAMP stimulates Na+-dependent ATP formation in the alkalophylic cyanobacterium Spirulina platensis. Microb. Environ. 2002;17:144–7. [Google Scholar]
- 13.Yashiro K., Sakamoto T., Ohmori M. Molecular characterization of an adenylate cyclase gene of the cyanobacterium Spirulina platensis. Plant Mol. Biol. 1996;31:175–81. doi: 10.1007/BF00020618. [DOI] [PubMed] [Google Scholar]
- 14.Kasahara M., Unno T., Yashiro K., Ohmori M. CyaG, a novel cyanobacterial adenylyl cyclase and a possible ancestor of mammalian guanylyl cyclases. J. Biol. Chem. 2001;276:10564–9. doi: 10.1074/jbc.M008006200. [DOI] [PubMed] [Google Scholar]
- 15.Loseva L.P., Dardynskaya I.V. Czech Republic: 6th International Congress of Applied Algology; 1993. Spirulina natural sorbent of radionucleides. [Google Scholar]
- 16.Amao Y., Nakamura N. Biohydrogen production with the light-harvesting function of grana from Spirulina and colloidal platinum. Int. J. Hydro. Energy. 2006;29:39–42. [Google Scholar]
- 17.Kawata Y., Yano S., Kojima H., Toyomizu M. Transformation of Spirulina platensis strain C1 (Arthrospira sp. PCC 9438) with Tn5 transposase–transposon DNA-cation liposome complex. Mar. Biotechnol. 2004;6:355–63. doi: 10.1007/s10126-003-0037-1. [DOI] [PubMed] [Google Scholar]
- 18.Kaneko T., Sato S., Kotani H., et al. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res. 1996;3:109–36. doi: 10.1093/dnares/3.3.109. [DOI] [PubMed] [Google Scholar]
- 19.Ogawa T., Terui G. Studies on the growth of Spirulina platensis. J. Ferment. Technol. 1970;48:361–7. [Google Scholar]
- 20.Sekine M., Tanikawa S., Omata S., et al. Sequence analysis of three plasmids harboured in Rhodococcus erythropolis strain PR4. Environ. Microbiol. 2006;8:334–46. doi: 10.1111/j.1462-2920.2005.00899.x. [DOI] [PubMed] [Google Scholar]
- 21.Ewing B., Green P. Base-calling of automated sequencer traces using phred. II. Error probabilities. Genome Res. 1998;8:186–94. [PubMed] [Google Scholar]
- 22.Ewing B., Hillier L., Wendl M.C., Green P. Base-calling of automated sequencer traces using phred. I. Accuracy assessment. Genome Res. 1998;8:175–85. doi: 10.1101/gr.8.3.175. [DOI] [PubMed] [Google Scholar]
- 23.Chen Q., Savarino S., Venkatesan M. Subtractive hybridization and optical mapping of the enterotoxigenic Escherichia coli H10407 chromosome: isolation of unique sequences and demonstration of significant similarity to the chromosome of E. coli K-12. Microbiology. 2006;152:1041–54. doi: 10.1099/mic.0.28648-0. [DOI] [PubMed] [Google Scholar]
- 24.Reslewic S., Zhou S., Place M., et al. Whole-genome shotgun optical mapping of Rhodospirillum rubrum. Appl. Environ. Microbiol. 2005;71:5511–22. doi: 10.1128/AEM.71.9.5511-5522.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Griffiths-Jones S., Bateman A., Marshall M., Khanna A., Eddy S. Rfam: an RNA family database. Nucleic Acids Res. 2003;31:439–41. doi: 10.1093/nar/gkg006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lowe T.M., Eddy S.R. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1997;25:955–64. doi: 10.1093/nar/25.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lagesen K., Hallin P., Rodland E., Staerfeldt H.-H., Rognes T., Ussery D. RNAmmer: consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Res. 2007;35:3100–8. doi: 10.1093/nar/gkm160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Altschul S.F., Madden T.L., Schaffer A.A., et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Delcher A.L., Harmon D., Kasif S., White O., Salzberg S.L. Improved microbial gene identification with GLIMMER. Nucleic Acids Res. 1999;27:4636–41. doi: 10.1093/nar/27.23.4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salzberg S.L., Delcher A.L., Kasif S., White O. Microbial gene identification using interpolated Markov models. Nucleic Acids Res. 1998;26:544–8. doi: 10.1093/nar/26.2.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.The UniProt, C. The Universal Protein Resource (UniProt) 2009. Nucleic Acids Res. 2009;37:D169–74. doi: 10.1093/nar/gkn664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yada T., Hirosawa M. Detection of short protein coding regions within the cyanobacterium genome: application of the hidden Markov model. DNA Res. 1996;3:355–61. doi: 10.1093/dnares/3.6.355. [DOI] [PubMed] [Google Scholar]
- 33.Hunter S., Apweiler R., Attwood T.K., et al. InterPro: the integrative protein signature database. Nucleic Acids Res. 2009;37:D211–5. doi: 10.1093/nar/gkn785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sato N. Gclust: trans-kingdom classification of proteins using automatic individual threshold setting. Bioinformatics. 2009;25:599–605. doi: 10.1093/bioinformatics/btp047. [DOI] [PubMed] [Google Scholar]
- 35.Furumichi M., Sato Y., Omata T., Ikeuchi M., Kanehisa M. CYORF: community annotation of cyanobacteria genes. Genome Inform. 2002;13:402–3. [Google Scholar]
- 36.Kanehisa M., Goto S., Kawashima S., Okuno Y., Hattori M. The KEGG resource for deciphering the genome. Nucleic Acids Res. 2004;32:D277–80. doi: 10.1093/nar/gkh063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bendtsen J.D., Nielsen H., von Heijne G., Brunak S. Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 2004;340:783–95. doi: 10.1016/j.jmb.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 38.Krogh A., Larsson B.R., von Heijne G., Sonnhammer E. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J. Mol. Biol. 2001;305:567–80. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- 39.Sasaki V.N., Sato N. CyanoClust: comparative genome resources of cyanobacteria and plastids. Database. 2010 doi: 10.1093/database/bap025. doi:10.1093/database/BAP025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bauer C.C., Buikema W.J., Black K., Haselkorn R. A short-filament mutant of Anabaena sp. strain PCC 7120 that fragments in nitrogen-deficient medium. J. Bacteriol. 1995;177:1520–6. doi: 10.1128/jb.177.6.1520-1526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mullineaux C.W., Mariscal V., Nenninger A., et al. Mechanism of intercellular molecular exchange in heterocyst-forming cyanobacteria. EMBO J. 2008;27:1299–308. doi: 10.1038/emboj.2008.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wong F.C., Meeks J.C. The hetF gene product is essential to heterocyst differentiation and affects HetR function in the cyanobacterium Nostoc punctiforme. J. Bacteriol. 2001;183:2654–61. doi: 10.1128/JB.183.8.2654-2661.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buikema W.J., Haselkorn R. Characterization of a gene controlling heterocyst differentiation in the cyanobacterium Anabaena 7120. Genes Dev. 1991;5:321–30. doi: 10.1101/gad.5.2.321. [DOI] [PubMed] [Google Scholar]
- 44.Zhang W., Du Y., Khudyakov I., et al. A gene cluster that regulates both heterocyst differentiation and pattern formation in Anabaena sp. strain PCC 7120. Mol. Microbiol. 2007;66:1429–43. doi: 10.1111/j.1365-2958.2007.05997.x. [DOI] [PubMed] [Google Scholar]
- 45.Nakamura Y., Kaneko T., Sato S., et al. Complete genome structure of the thermophilic cyanobacterium Thermosynechococcus elongatus BP-1. DNA Res. 2002;9:123–30. doi: 10.1093/dnares/9.4.123. [DOI] [PubMed] [Google Scholar]
- 46.Meng Q., Zhang Y., Liu X.Q. Rare group I intron with insertion sequence element in a bacterial ribonucleotide reductase gene. J. Bacteriol. 2007;189:2150–4. doi: 10.1128/JB.01424-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ohmori M., Okamoto S. Photoresponsive cAMP signal transduction in cyanobacteria. Photochem. Photobiol. Sci. 2004;3:503–11. doi: 10.1039/b401623h. [DOI] [PubMed] [Google Scholar]
- 48.Katayama M., Ohmori M. Isolation and characterization of multiple adenylate cyclase genes from the cyanobacterium Anabaena sp. strain PCC 7120. J. Bacteriol. 1997;179:3588–93. doi: 10.1128/jb.179.11.3588-3593.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Phalip V., Li J.H., Zhang C.C. HstK, a cyanobacterial protein with both a serine/threonine kinase domain and a histidine kinase domain: implication for the mechanism of signal transduction. Biochem. J. 2001;360:639–44. doi: 10.1042/0264-6021:3600639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ohmori K., Hirose M., Ohmori M. An increase in the intracellular concentration of cAMP triggers formation of an algal mat by the cyanobacterium Spirulina platensis. Plant Cell Physiol. 1993;34:169–71. [Google Scholar]
- 51.Romling U., Simm R. Prevailing concepts of c-di-GMP signaling. Contrib. Microbiol. 2009;16:161–81. doi: 10.1159/000219379. [DOI] [PubMed] [Google Scholar]
- 52.Stock J.B., Ninfa A.J., Stock A.M. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol. Rev. 1989;53:450–90. doi: 10.1128/mr.53.4.450-490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Juntarajumnong W., Hirani T.A., Simpson J.M., Incharoensakdi A., Eaton-Rye J.J. Phosphate sensing in Synechocystis sp. PCC 6803: SphU and the SphS-SphR two-component regulatory system. Arch. Microbiol. 2007;188:389–402. doi: 10.1007/s00203-007-0259-0. [DOI] [PubMed] [Google Scholar]
- 54.Ohmori M., Ikeuchi M., Sato N., et al. Characterization of genes encoding multi-domain proteins in the genome of the filamentous nitrogen-fixing cyanobacterium Anabaena sp. strain PCC 7120. DNA Res. 2001;8:271–84. doi: 10.1093/dnares/8.6.271. [DOI] [PubMed] [Google Scholar]
- 55.Ashby M.K., Houmard J. Cyanobacterial two-component proteins: structure, diversity, distribution, and evolution. Microbiol. Mol. Biol. Rev. 2006;70:472–509. doi: 10.1128/MMBR.00046-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yoshimura H., Okamoto S., Tsumuraya Y., Ohmori M. Group 3 sigma factor gene, sigJ, a key regulator of desiccation tolerance, regulates the synthesis of extracellular polysaccharide in cyanobacterium Anabaena sp. strain PCC 7120. DNA Res. 2007;14:13–24. doi: 10.1093/dnares/dsm003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Osanai T., Ikeuchi M., Tanaka K. Group 2 sigma factors in cyanobacteria. Physiol. Plant. 2008;133:490–506. doi: 10.1111/j.1399-3054.2008.01078.x. [DOI] [PubMed] [Google Scholar]
- 58.Wu J., Zhao F., Wang S., et al. cTFbase: a database for comparative genomics of transcription factors in cyanobacteria. BMC Genomics. 2007;8:104. doi: 10.1186/1471-2164-8-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vega-Palas M.A., Madueno F., Herrero A., Flores E. Identification and cloning of a regulatory gene for nitrogen assimilation in the cyanobacterium Synechococcus sp. strain PCC 7942. J. Bacteriol. 1990;172:643–7. doi: 10.1128/jb.172.2.643-647.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aichi M., Takatani N., Omata T. Role of NtcB in activation of nitrate assimilation genes in the cyanobacterium Synechocystis sp. strain PCC 6803. J. Bacteriol. 2001;183:5840–7. doi: 10.1128/JB.183.20.5840-5847.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McGinn P., Price D., Maleszka R., Badger M. Inorganic carbon limitation and light control the expression of transcripts related to the CO2-concentrating mechanism in the cyanobacterium Synechocystis sp. strain PCC6803. Plant Physiol. 2003;132:218–29. doi: 10.1104/pp.019349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nakamoto H., Suzuki M., Kojima K. Targeted inactivation of the hrcA repressor gene in cyanobacteria. FEBS Lett. 2003;549:57–62. doi: 10.1016/s0014-5793(03)00768-3. [DOI] [PubMed] [Google Scholar]
- 63.Yoshimura H., Hisabori T., Yanagisawa S., Ohmori M. Identification and Characterization of a novel cAMP receptor protein in the cyanobacterium Synechocystis sp. PCC 6803. J. Biol. Chem. 2000;275:6241–5. doi: 10.1074/jbc.275.9.6241. [DOI] [PubMed] [Google Scholar]
- 64.Maier U.-G., Fraunholz M., Zauner S., Penny S., Douglas S. A nucleomorph-encoded CbbX and the phylogeny of RuBisCo regulators. Mol. Biol. Evol. 2000;17:576–83. doi: 10.1093/oxfordjournals.molbev.a026337. [DOI] [PubMed] [Google Scholar]
- 65.Zhang J.Y., Chen W.L., Zhang C.C. hetR and patS, two genes necessary for heterocyst pattern formation, are widespread in filamentous nonheterocyst-forming cyanobacteria. Microbiology. 2009;155:1418–26. doi: 10.1099/mic.0.027540-0. [DOI] [PubMed] [Google Scholar]
- 66.Ehira S., Ohmori M. NrrA, a nitrogen-responsive response regulator facilitates heterocyst development in the cyanobacterium Anabaena sp. strain PCC 7120. Mol. Microbiol. 2006;59:1692–703. doi: 10.1111/j.1365-2958.2006.05049.x. [DOI] [PubMed] [Google Scholar]
- 67.Ogawa T., Bao D.H., Katoh H., Shibata M., Pakrasi H.B., Bhattacharyya-Pakrasi M. A two-component signal transduction pathway regulates manganese homeostasis in Synechocystis 6803, a photosynthetic organism. J. Biol. Chem. 2002;277:28981–6. doi: 10.1074/jbc.M204175200. [DOI] [PubMed] [Google Scholar]
- 68.Yamaguchi K., Suzuki I., Yamamoto H., et al. A two-component Mn2+-sensing system negatively regulates expression of the mntCAB operon in Synechocystis. Plant Cell. 2002;14:2901–13. doi: 10.1105/tpc.006262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ashby M.K., Mullineaux C.W. Cyanobacterial ycf27 gene products regulate energy transfer from phycobilisomes to photosystems I and II. FEMS Microbiol. Lett. 1999;181:253–60. doi: 10.1111/j.1574-6968.1999.tb08852.x. [DOI] [PubMed] [Google Scholar]
- 70.Aiba H., Mizuno T. A novel gene whose expression is regulated by the response-regulator, SphR, in response to phosphate limitation in Synechococcus species PCC 7942. Mol. Microbiol. 1994;13:25–34. doi: 10.1111/j.1365-2958.1994.tb00399.x. [DOI] [PubMed] [Google Scholar]
- 71.Ponting C.P., Aravind L. PAS: a multifunctional domain family comes to light. Curr. Biol. 1997;7:R674–7. doi: 10.1016/s0960-9822(06)00352-6. [DOI] [PubMed] [Google Scholar]
- 72.Aravind L., Ponting C.P. The GAF domain: an evolutionary link between diverse phototransducing proteins. Trends Biochem. Sci. 1997;22:458–9. doi: 10.1016/s0968-0004(97)01148-1. [DOI] [PubMed] [Google Scholar]
- 73.Narikawa R., Okamoto S., Ikeuchi M., Ohmori M. Molecular evolution of PAS domain-containing proteins of filamentous cyanobacteria through domain shuffling and domain duplication. DNA Res. 2004;11:69–81. doi: 10.1093/dnares/11.2.69. [DOI] [PubMed] [Google Scholar]
- 74.Kanacher T., Schultz A., Linder J.U., Schultz J.E. A GAF-domain-regulated adenylyl cyclase from Anabaena is a self-activating cAMP switch. EMBO J. 2002;21:3672–80. doi: 10.1093/emboj/cdf375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ikeuchi M., Ishizuka T. Cyanobacteriochromes: a new superfamily of tetrapyrrole-binding photoreceptors in cyanobacteria. Photochem. Photobiol. Sci. 2008;7:1159–67. doi: 10.1039/b802660m. [DOI] [PubMed] [Google Scholar]
- 76.Okamoto S., Kasahara M., Kamiya A., Nakahira Y., Ohmori M. A phytochrome-like protein AphC triggers the cAMP signaling induced by far-red light in the cyanobacterium Anabaena sp. strain PCC7120. Photochem. Photobiol. 2004;80:429–33. doi: 10.1562/0031-8655(2004)080<0429:APPATT>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 77.Narikawa R., Fukushima Y., Ishizuka T., Itoh S., Ikeuchi M. A novel photoactive GAF domain of cyanobacteriochrome AnPixJ that shows reversible green/red photoconversion. J. Mol. Biol. 2008;380:844–55. doi: 10.1016/j.jmb.2008.05.035. [DOI] [PubMed] [Google Scholar]
- 78.Rockwell N.C., Njuguna S.L., Roberts L., et al. A second conserved GAF domain cysteine is required for the blue/green photoreversibility of cyanobacteriochrome Tlr0924 from Thermosynechococcus elongatus. Biochemistry. 2008;47:7304–16. doi: 10.1021/bi800088t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yoshihara S., Suzuki F., Fujita H., Geng X.X., Ikeuchi M. Novel putative photoreceptor and regulatory genes required for the positive phototactic movement of the unicellular motile cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol. 2000;41:1299–304. doi: 10.1093/pcp/pce010. [DOI] [PubMed] [Google Scholar]
- 80.Baker M.D., Wolanin P.M., Stock J.B. Signal transduction in bacterial chemotaxis. Bioessays. 2006;28:9–22. doi: 10.1002/bies.20343. [DOI] [PubMed] [Google Scholar]
- 81.Rao C.V., Glekas G.D., Ordal G.W. The three adaptation systems of Bacillus subtilis chemotaxis. Trends Microbiol. 2008;16:480–7. doi: 10.1016/j.tim.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Irmler A., Forchhammer K. A PP2C-type phosphatase dephosphorylates the PII signaling protein in the cyanobacterium Synechocystis PCC 6803. Proc. Natl Acad. Sci. USA. 2001;98:12978–83. doi: 10.1073/pnas.231254998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhao F., Zhang X., Liang C., Wu J., Bao Q., Qin S. Genome-wide analysis of restriction-modification system in unicellular and filamentous cyanobacteria. Physiol. Genomics. 2006;24:181–90. doi: 10.1152/physiolgenomics.00255.2005. [DOI] [PubMed] [Google Scholar]
- 84.O'Sullivan D., Twomey D.P., Coffey A., Hill C., Fitzgerald G.F., Ross R.P. Novel type I restriction specificities through domain shuffling of HsdS subunits in Lactococcus lactis. Mol. Microbiol. 2000;36:866–75. doi: 10.1046/j.1365-2958.2000.01901.x. [DOI] [PubMed] [Google Scholar]
- 85.Haft D.H., Selengut J., Mongodin E.F., Nelson K.E. A guild of 45 CRISPR-associated (Cas) protein families and multiple CRISPR/Cas subtypes exist in prokaryotic genomes. PLoS Comput. Biol. 2005;1:e60. doi: 10.1371/journal.pcbi.0010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Barrangou R., Fremaux C., Deveau H., et al. CRISPR provides acquired resistance against viruses in prokaryotes. Science. 2007;315:1709–12. doi: 10.1126/science.1138140. [DOI] [PubMed] [Google Scholar]
- 87.Sorek R., Kunin V., Hugenholtz P. CRISPR—a widespread system that provides acquired resistance against phages in bacteria and archaea. Nat. Rev. Microbiol. 2008;6:181–6. doi: 10.1038/nrmicro1793. [DOI] [PubMed] [Google Scholar]
- 88.Wang H.L., Postier B.L., Burnap R.L. Polymerase chain reaction-based mutageneses identify key transporters belonging to multigene families involved in Na+ and pH homeostasis of Synechocystis sp. PCC 6803. Mol. Microbiol. 2002;44:1493–506. doi: 10.1046/j.1365-2958.2002.02983.x. [DOI] [PubMed] [Google Scholar]
- 89.Laloknam S., Tanaka K., Buaboocha T., et al. Halotolerant cyanobacterium Aphanothece halophytica contains a betaine transporter active at alkaline pH and high salinity. Appl. Environ. Microbiol. 2006;72:6018–26. doi: 10.1128/AEM.00733-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Billini M., Stamatakis K., Sophianopoulou V. Two members of a network of putative Na+/H+ antiporters are involved in salt and pH tolerance of the freshwater cyanobacterium Synechococcus elongatus. J. Bacteriol. 2008;190:6318–29. doi: 10.1128/JB.00696-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kaplan A., Hagemann M., Bauwe H., Kahlon S., Ogawa T. In: The Cyanobacteria: Molecular Biology, Genomics and Evolution. Herrero A., Flores E., editors. UK: Caister; 2008. pp. 305–34. [Google Scholar]
- 92.Wilson A., Ajlani G., Verbavatz J.M., Vass I., Kerfeld C.A., Kirilovsky D. A soluble carotenoid protein involved in phycobilisome-related energy dissipation in cyanobacteria. Plant Cell. 2006;18:992–1007. doi: 10.1105/tpc.105.040121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kerfeld C.A., Sawaya M.R., Brahmandam V., et al. The crystal structure of a cyanobacterial water-soluble carotenoid binding protein. Structure. 2003;11:55–65. doi: 10.1016/s0969-2126(02)00936-x. [DOI] [PubMed] [Google Scholar]
- 94.Murata N., Wada H., Gombos Z. Modes of fatty-acid desaturation in cyanobacteria. Plant Cell Physiol. 1992;33:933–41. [Google Scholar]
- 95.Guglielmi G., Rippka R., Tandeau De Marsac N. Main properties that justify the different taxonomic position of Spirulina spp. and Arthrospira spp. among cyanobacteria. Bull. Inst. Oceanogr. 1993;12:13–23. [Google Scholar]
- 96.Smith D.R., Doucette-Stamm L.A., Deloughery C., et al. Complete genome sequence of Methanobacterium thermoautotrophicum deltaH: functional analysis and comparative genomics. J. Bacteriol. 1997;179:7135–55. doi: 10.1128/jb.179.22.7135-7155.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schwarz E.M., Benzer S. Calx, a Na-Ca exchanger gene of Drosophila melanogaster. Proc. Natl Acad. Sci. USA. 1997;94:10249–54. doi: 10.1073/pnas.94.19.10249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wiles T., Kulesus R., Mulvey M. Origins and virulence mechanisms of uropathogenic Escherichia coli. Exp. Mol. Pathol. 2008;85:11–9. doi: 10.1016/j.yexmp.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hahn H., Specht U. Secretory delivery of recombinant proteins in attenuated Salmonella strains: potential and limitations of Type I protein transporters. FEMS Immunol. Med. Microbiol. 2003;37:87–98. doi: 10.1016/S0928-8244(03)00092-0. [DOI] [PubMed] [Google Scholar]
- 100.Hoiczyk E., Baumeister W. Envelope structure of four gliding filamentous cyanobacteria. J. Bacteriol. 1995;177:2387–95. doi: 10.1128/jb.177.9.2387-2395.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Read N., Connell S., Adams D. Nanoscale visualization of a fibrillar array in the cell wall of filamentous cyanobacteria and its implications for gliding motility. J. Bacteriol. 2007;189:7361–6. doi: 10.1128/JB.00706-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hoiczyk E., Baumeister W. Oscillin, an extracellular, Ca2+-binding glycoprotein essential for the gliding motility of cyanobacteria. Mol. Microbiol. 1997;26:699–708. doi: 10.1046/j.1365-2958.1997.5971972.x. [DOI] [PubMed] [Google Scholar]
- 103.McCarren J., Brahamsha B. SwmB, a 1.12-megadalton protein that is required for nonflagellar swimming motility in Synechococcus. J. Bacteriol. 2007;189:1158–62. doi: 10.1128/JB.01500-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.McCarren J., Heuser J., Roth R., Yamada N., Martone M., Brahamsha B. Inactivation of swmA results in the loss of an outer cell layer in a swimming Synechococcus strain. J. Bacteriol. 2005;187:224–30. doi: 10.1128/JB.187.1.224-230.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hoiczyk E. Structural and biochemical analysis of the sheath of Phormidium uncinatum. J. Bacteriol. 1998;180:3923–32. doi: 10.1128/jb.180.15.3923-3932.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bhaya D., Bianco N.R., Bryant D., Grossman A. Type IV pilus biogenesis and motility in the cyanobacterium Synechocystis sp. PCC6803. Mol. Microbiol. 2000;37:941–51. doi: 10.1046/j.1365-2958.2000.02068.x. [DOI] [PubMed] [Google Scholar]
- 107.Terauchi K., Ohmori M. Blue light stimulates cyanobacterial motility via a cAMP signal transduction system. Mol. Microbiol. 2004;52:303–9. doi: 10.1111/j.1365-2958.2003.03980.x. [DOI] [PubMed] [Google Scholar]
- 108.Okamoto S., Ohmori M. The cyanobacterial PilT protein responsible for cell motility and transformation hydrolyzes ATP. Plant Cell Physiol. 2002;43:1127–36. doi: 10.1093/pcp/pcf128. [DOI] [PubMed] [Google Scholar]
- 109.Duggan P.S., Gottardello P., Adams D.G. Molecular analysis of genes in Nostoc punctiforme involved in pilus biogenesis and plant infection. J. Bacteriol. 2007;189:4547–51. doi: 10.1128/JB.01927-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yoshihara S., Ikeuchi M. Phototactic motility in the unicellular cyanobacterium Synechocystis sp. PCC 6803. Photochem. Photobiol. Sci. 2004;3:512–8. doi: 10.1039/b402320j. [DOI] [PubMed] [Google Scholar]
- 111.Lopez-Maury L., Garcia-Dominguez M., Florencio F.J., Reyes J.C. A two-component signal transduction system involved in nickel sensing in the cyanobacterium Synechocystis sp. PCC 6803. Mol. Microbiol. 2002;43:247–56. doi: 10.1046/j.1365-2958.2002.02741.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.