Abstract
Extracellular fluid volume is highly regulated, at least in part, by peripheral resistance and renal function. Nitric oxide (NO) produced by NO synthase type 3 (NOS 3) in the nonrenal vasculature may promote fluid retention by reducing systemic vascular resistance and arterial pressure. In contrast, NO produced by renal NOS 3 promotes water excretion by reducing renal vascular resistance, increasing glomerular filtration, and inhibiting reabsorption along the nephron. Thus, the net effect of NO from NOS 3 on urinary volume (UV) is unclear. We hypothesized that NO produced by NOS 3 promotes water excretion primarily due to renal tubular effects. We gave conscious wild-type and NOS 3 −/− mice an acute volume load and measured UV, blood pressure, plasma renin concentration (PRC), Na+, vasopressin, and urinary Na+ and creatinine concentrations. To give the acute volume load, we trained mice to drink a large volume of water while in metabolic cages. On the day of the experiment, water was replaced with 1% sucrose, and mice had access to it for 1 h. Volume intake was similar in both groups. Over 3 h, wild-type mice excreted 62 ± 10% of the volume load, but NOS 3 −/− excreted only 42 ± 5% (P < 0.05). Blood pressure in NOS 3 −/− was 118 ± 3 compared with 110 ± 2 mmHg in wild-type mice (P < 0.05), but it did not change following volume load in either strain. PRC, vasopressin, and glomerular filtration rate were similar between groups. Urinary Na+ excretion was 49.3 ± 7.0 in wild-type vs. 37.8 ± 6.4 μmol/3 h in NOS 3 −/− mice (P < 0.05). Bumetanide administration eliminated the difference in volume excretion between wild-type and NOS 3 −/− mice. We conclude that 1) NO produced by NOS 3 promotes water and Na+ excretion and 2) the renal epithelial actions of NO produced by NOS 3 supersede the systemic and renal vascular actions.
Keywords: nitric oxide, nitric oxide synthase 3, diuresis, transport
extracellular volume is regulated by a number of parameters, including water intake, total peripheral resistance (and thus perfusion pressure), and volume excretion by the kidneys (12). Urinary volume (UV) is determined by glomerular filtration, water absorption along the nephron, renal perfusion pressure (and therefore blood pressure) (29–31, 41, 47), and vasopressin status (18, 19). Nitric oxide (NO) is one of the main factors that regulates total peripheral resistance and renal volume excretion (24, 32).
NO is synthesized from the amino acid l-arginine by the enzyme NO synthase (NOS). Three different NOS isoforms have been identified: neuronal (NOS 1 or nNOS), inducible (NOS 2 or iNOS), and endothelial (NOS 3 or eNOS) (15). Regulation of total peripheral resistance and renal function by NOS 1 and NOS 2 has been extensively investigated through the use of selective inhibitors of these enzymes (1, 3, 17, 28, 39). NOS 1 is expressed in vascular smooth muscle cells (4, 45) but appears to play little or no role in acute regulation of peripheral resistance under physiological circumstances. NOS 1 is also expressed in several cell types within the kidney. In the macula densa, it inhibits tubuloglomerular feedback, and consequently enhances glomerular filtration rate (22). In the collecting duct, it mediates reductions in transport caused by endothelin (28). NOS 2 is also expressed in the kidney, primarily along the nephron (26, 27). However, NO produced by this NOS isoform appears to play little role in regulation of UV or urinary Na+ excretion (UNaV) (3). Similarly, there is no evidence that, under normal conditions, NO from NOS 2 regulates peripheral resistance.
NOS 3 is widely expressed in endothelial cells of both the systemic (35, 42) and renal (2, 46) vasculature and along the nephron. NOS 3 is expressed in proximal tubules, thick ascending limbs, and collecting ducts (2). NO from NOS 3 in the systemic vasculature causes vasodilation (37, 48), which would tend to reduce renal perfusion pressure and UV, thus promoting increases in extracellular fluid volume. In contrast, NO from NOS 3 in the renal vasculature contributes to a basal dilated vascular tone, increasing renal blood flow and glomerular filtration (21). Along the nephron, NO from NOS 3 inhibits fluid reabsorption in the proximal tubule (7) and collecting duct (9, 10). It also inhibits Na+ reabsorption by the medullary thick ascending limb (33, 34). The rate of fluid reabsorption by the collecting duct depends on the osmotic gradient generated by Na+ transport by the thick ascending limb and, vasopressin-stimulated collecting duct water permeability. Given that 1) glomerular filtration rate is the first factor in determining UV (47), 2) the proximal tubule reabsorbs 70% of the filtered volume, and 3) the thick ascending limb generates the osmotic gradient necessary for fluid absorption in the collecting duct (14), one would expect that NO from renal NOS 3 would promote reductions in extracellular fluid volume. Thus, the systemic actions of NO from NOS 3 would appear to oppose its renal actions. The net effect of NO produced by NOS 3 in the kidney and systemic vasculature on the regulation of extracellular fluid volume is unclear because there are no selective inhibitors of NOS 3.
We hypothesized that NO produced by NOS 3 promotes excretion of water primarily due to renal tubular effects. To test this, we examined the ability of conscious wild-type mice (C57BL/6J) and NOS 3 knockout mice (−/−) to excrete a mild volume load.
MATERIALS AND METHODS
Animals.
We used C57BL/6J wild-type and B6129P2-NOS 3tm1Unc homozygous knockout mice (NOS 3 −/−, Jackson Laboratories, Bar Harbor, ME) for this study. C57BL/6J is the appropriate control strain for the NOS 3 −/− mice (44). This study was approved by the Henry Ford Health System Institutional Animal Care and Use Committee and was conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. The mice were fed standard mouse chow, and water was available ad libitum for at least 5 days before the start of training.
In vivo studies.
Mice were placed in metabolic cages for 7 days. They were trained to drink a large amount of water in a short period of time by giving them access to water only 3 h/day from 4 PM to 7 PM on days 1–6 (training period). On day 7, the water was changed to a 1% sucrose solution and only given for 1 h at 4 PM. We have added sucrose to the water on the day of the experiment only to accelerate intake. Our empirical evidence shows this was successful. The amount of water that the mice drank during the training period is not different from the day of the experiment, it just occurred faster. This procedure was developed to give conscious mice a modest rapid volume load without anesthesia or handling. Once mice were given the sucrose solution, we collected urine every 30 min for 3 h. At the end of the experiment, mice were killed, and kidneys and plasma were harvested for analysis. We measured water intake, UV, urinary osmolality, Na+, and creatinine. UV was corrected by water intake, and the values were reported as percent of intake. UNaV was calculated. We also measured plasma Na+, creatinine, renin, and vasopressin concentrations (see below). Glomerular filtration rate was calculated as clearance of creatinine: {[urinary creatinine] × urinary flow rate}/[plasma creatinine].
For experiments in which bumetanide was used, it was given to the mice prior to the sucrose solution. Bumetanide tablets were pulverized and mixed with chocolate frosting. The final mixture contained 1.66 mg bumetanide/g. Mice had access to the 0.4-g mixture for 75 min. Consumption was monitored by weight. Bumetanide intake was 24.0 ± 1.4 mg/kg for wild-type and 21.5 ± 1.6 mg/kg for NOS 3 −/− mice [NS, (not significant)].
Blood pressure by telemetry.
Wild-type and NOS 3 −/− mice (8-wk-old males) were implanted with telemetry devices (model no. TA11PA-C10, Data Sciences International, St. Paul, MN) to measure blood pressure and heart rate. Under ketamine/xylazine anesthesia (100 and 20 mg/kg ip, respectively), the telemeter catheter tip was inserted into the aortic arch via the left carotid artery, with the telemeter body positioned subcutaneously on the right flank. The recording room was maintained at 21–22°C and had a radio turned on at a low volume to reduce the impact of environmental noise. Mice were housed in individual cages. The telemeter signal was processed using a RLA1020 receiver, a 20-channel data exchange matrix, a APR-1 ambient pressure monitor and a DataQuest ART Gold 3.01 acquisition system (Data Sciences International). The system was set to sample the mean, systolic, and diastolic blood pressure and heart rate at 500 Hz for 15 s at 15-min intervals, and record their average values.
Plasma renin concentration.
To measure plasma renin concentration, 15-μl blood samples were obtained from the orbital venous plexus of conscious mice using capillary tubes containing disodium ethylene diamine tetraacetic acid (EDTA; 10 mM) and stored at −80°C. Samples were thawed and incubated with an excess of exogenous partially purified rat angiotensinogen at 30°C for 30 min. The supernatant was analyzed by radioimmunoassay for generation of ANG I using a Gamma Coat kit (DiaSorin, Stillwater, MN). Values were expressed as ng ANG I·ml−1·h−1.
Plasma vasopressin concentration.
At the end of the experiments, mice were anesthetized (ketamine: 100 mg/kg body wt and xylazine: 20 mg/kg body wt ip), and blood samples were collected from the vena cava in tubes containing EDTA (10 mM), aprotonin (10.6 TIU/ml), and protease inhibitor cocktail containing: antipain (5 μg/ml), aprotonin (10 μg/ml), leupeptin (5 μg/ml), benzamidine (4 mM), chymostatin (5 μg/ml), pepstatin A (5 μg/ml), 4-(2-aminoethyl)benzenesulfonyl fluoride (25 μg/ml). The plasma was separated by centrifugation at 1,000 g at 4°C for 15 min. Plasma was then purified using Bond Elut C18 columns (Varian, Palo Alto, CA). The columns were activated with 2 ml of methanol (HPLC grade) and equilibrated with 2 ml of deionized water. Plasma (0.3 ml) was acidified with 30 μl of 1 mol/l HCl and loaded onto the column. The columns were then washed with 3 ml acetic acid (0.67 mol/l). Elution was carried out with 0.5 ml of trifluoroacetic acid/methanol (1% trifluoroacetic acid in 1 liter methanol). Samples were dried (Savant speed vac system, Ramsey, MN) and resuspended for the assay. A vasopressin enzyme immunoassay kit (Assay Designs, Ann Arbor, MI) was used to analyze samples.
Plasma and urinary Na+ and creatinine concentrations.
Plasma and urinary Na+ concentrations were measured with a Nova autoanalyzer (Nova Biomedical, Waltham, MA). UNaV was calculated from UV and urinary Na+ concentration.
Creatinine in plasma and urine was measured using a colorimetric assay (Pointe Scientific, Canton, MI). Creatinine excretion was calculated from UV and urinary creatinine concentration.
Statistics.
Data are reported as means ± SE. Cumulative UV and UNaV were analyzed by ANOVA for repeated measures followed by an interaction test. The other parameters were evaluated with Student's t-test. The criterion for statistical significance was P ≤ 0.05 in all experiments. Statistical analysis was performed by the Biostatistics Department at Henry Ford Hospital.
RESULTS
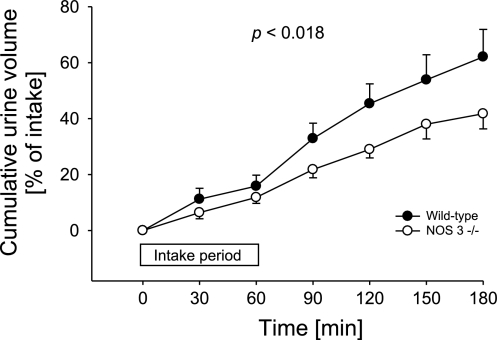
To test our hypothesis, we first evaluated the ability of wild-type and NOS 3 −/− mice to eliminate a volume load (Fig. 1). From the first 30-min collection period, NOS 3 −/− mice had lower UV compared with the wild-type-mice group. This difference increased during the course of the experiment. The volume of sucrose solution consumed by both groups was the same (wild-type mice: 1.1 ± 0.1 ml; NOS 3 −/− mice: 1.2 ± 0.1 ml). As a result, after 3 h of urine collection, the wild-type mice excreted 62 ± 10% of the volume load, whereas NOS 3 −/− mice excreted only 42 ± 5% (n = 21 per group; P < 0.018). These data indicate that selective genetic ablation of NOS 3 decreases the rate at which mice eliminate a volume load.
Fig. 1.
Cumulative urinary volume excretion over 3 h after volume load in wild-type (●) and NOS 3 −/− mice (○); n = 21 for each group. P < 0.018 indicates that excretion over time is different between groups.
We next measured basal 24-h water intake and urine output in a different group of wild-type and NOS 3 −/− mice. Over the 24-h period, wild-type mice drank 1.79 ± 0.36 ml water and NOS 3 −/− mice 1.63 ± 0.16 ml (n = 5 per group, NS). Over the same 24-h period, urine output was 2.03 ± 0.24 ml for wild-type and 2.2 ± 0.3 ml for NOS 3 −/− mice (n = 5 per group, n.s.) (Table 1). Body weight was 28.2 ± 0.3 and 28.2 ± 0.5 g for the wild-type and NOS 3 −/− mice groups, respectively (n = 5 per group, NS). These data indicate that genetic deletion of NOS 3 does not alter basal water intake or urine output.
Table 1.
Characteristics of experimental animals (NOS 3 −/− mice and their wild-type controls)
| Parameter | Wild-Type | NOS 3−/− |
|---|---|---|
| 24 h water intake, ml | 1.8 ± 0.4 | 1.6 ± 0.2 |
| 24 h urine excretion, ml | 2.1 ± 0.2 | 2.2 ± 0.3 |
| Body weight, g | 24.3 ± 0.7 | 23.8 ± 0.8 |
| 1% sucrose intake, ml/h | 1.1 ± 0.1 | 1.2 ± 0.1 |
| MAP before load, mmHg | 108 ± 4 | 121 ± 3* |
| MAP after load, mmHg | 110 ± 2 | 118 ± 3* |
| Heart rate after load, beats/min | 543 ± 10 | 562 ± 8 |
| Plasma Na+, mEq/l, after load | 156 ± 1 | 155 ± 1 |
| Plasma vasopressin after load, pg/ml | 5 ± 1 | 7 ± 3 |
| PRC after load, ng ANG I•ml−1•h−1 | 364 ± 51 | 377 ± 41 |
| Plasma creatinine after load, μg/ml | 2.0 ± 0.2 | 2.4 ± 0.2 |
| GFR during first 30 min after load, ml/min | 2.0 ± 0.4 | 1.6 ± 0.4 |
Values are presented as means ± SE. MAP, mean arterial pressure; NOS 3, nitric oxide synthase 3; PRC, plasma renin concentration; GFR, glomerular filtration rate.
P ≤ 0.05 vs. wild-type mice.
We measured blood pressure in wild-type and NOS 3 −/− mice by telemetry before and after the water load. We found that NOS 3 −/− mice had significantly higher systolic and diastolic blood pressures than wild-type mice under basal conditions. Blood pressure did not change after volume loading with respect to basal conditions in either group (Table 1).
We next investigated whether plasma vasopressin concentrations differed between wild-type and NOS 3 −/−. Plasma vasopressin levels were 5 ± 1 pg/ml in wild-type mice and 7 ± 3 pg/ml in NOS 3 −/− mice after water loading, not significantly different (n = 14 per group; Table 1).
We then studied whether plasma renin concentrations differed between wild-type and NOS 3 −/− mice. Plasma renin concentrations in wild-type and NOS 3 −/− mice were not significantly different [364 ± 51 and 377 ± 41 ng ANG I·ml−1·h−1 for wild-type and NOS 3 −/− mice (n = 8 per group; Table 1)] after volume loading.
To investigate whether differences in glomerular filtration rate could account for the differences in the rate at which mice eliminate a water load, we measured urinary and plasma creatinine and calculated clearance of creatinine as an indicator of glomerular filtration rate. Because of the nature of the experimental protocol, blood samples for determination of plasma creatinine could only be collected at the end of the experiment. During the first 30-min collection period after the water load, glomerular filtration rate was 2.0 ± 0.4 ml/min in wild-type and 1.6 ± 0.4 ml/min in NOS 3 −/− mice (NS; n = 6 per group; Table 1).
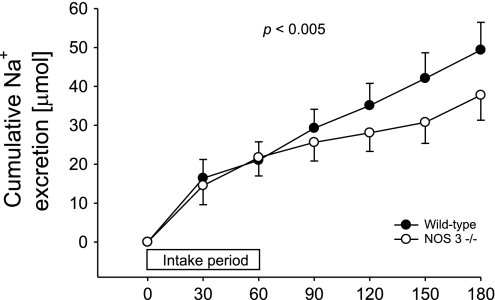
Next, we calculated UNaV for each strain. Cumulative UNaV over the 3-h study period was 49.3 ± 7.0 μmol in wild-type mice, but only 37.8 ± 6.4 μmol in NOS 3 −/− mice, 23% less (n = 21 per group; P < 0.005) (Fig. 2). Plasma Na+ concentration was not significantly different between groups (Table 1).
Fig. 2.
Cumulative urinary Na excretion over 3 h after volume load in wild-type (●) and NOS 3 −/− mice (○); n = 21 for each group. P < 0.005 indicates that excretion over time is different between groups.
We also investigated whether differences in the ability to excrete a volume load between NOS 3 −/− and wild-type mice were due to different effects of the training regimen or hydration status. To do this, we measured body weight in both experimental groups before and after the training period. Before training, both groups had similar body weights (24.3 ± 0.7 g for wild-type and 23.8 ± 0.8 g for NOS 3 −/−). After the training period, both groups had lost the same amount of weight (2.3 ± 0.5 and 2.4 ± 0.5 g for wild-type and NOS 3 −/− mice). To study whether this weight loss was due to a change in fluid intake, we measured water intake throughout the experiment in both groups of mice. On the first day, mice drank about 1 ml of water. However, on subsequent days, both groups drank about 3.5 ml in 3 h. These data suggest that weight loss due to dehydration is not likely the explanation for the difference in urinary volume, because both wild-type and NOS 3 −/− mice lost the same amount of weight.
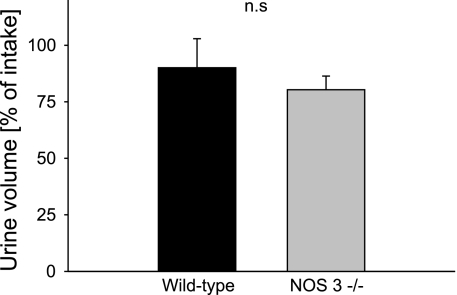
Finally, to investigate whether the reduced ability to eliminate a water load in NOS 3 −/− mice was due to a tubular effect, we performed the experiments in mice treated with the loop diuretic bumetanide. The volume of sucrose solution consumed by both groups was the same (wild-type mice: 1.9 ± 0.1 ml; NOS 3 −/− mice: 1.8 ± 0.1 ml). As a result, after 3 h of urine collection, the wild-type mice excreted 90 ± 13% of the volume load, and the NOS 3 −/− mice excreted 80 ± 6% (n = 5–7 per group; NS) (Fig. 3).
Fig. 3.
Cumulative urinary volume excretion over 3 h after volume load in wild-type and NOS 3 −/− mice treated with the loop diuretic, bumetanide; n = 5–7 for each group.
DISCUSSION
NO produced by NOS 3 in the kidney promotes natriuresis and diuresis by dilating the renal vasculature and inhibiting transport along the nephron (23, 25, 34). In contrast, NO produced by NOS 3 in endothelial cells of the systemic vasculature favors Na+ and fluid retention by dilating blood vessels and reducing renal perfusion pressure. The net effect of NOS 3-derived NO on UV and UNaV in vivo is unknown. Our hypothesis was that genetic deletion of NOS 3 would reduce the rate at which mice eliminate a volume load due to a predominant renal tubular effect.
To test our hypothesis, we used a method to induce a water load in conscious mice. This so-called volume load is not truly a volume load. It is just 24 h worth of water consumed over a 1-h period. The animals were trained to drink water during only 3 h/day. Therefore, they stopped urine production during at least the last 12 h of the “water deprivation” period. Once they are allowed to drink, it takes some time (about 20 min) for them to start producing urine again, as indicated from our data (Fig. 1). As expected, the urine that they start producing is concentrated. The Na+ excreted in the urine during this time comes from the food they ate during the previous 24 h, because the animals have no access to food during the water-load period.
We found that after an acute water load, NOS 3 −/− mice excreted urine more slowly than wild-type mice. This difference is not likely due to alterations in intestinal fluid reabsorption since 24-h water intake and urine excretion were not statistically different in both groups of mice. The different ability to eliminate a water load between wild-type and NOS 3 −/− mice was not due to differences in water intake because both groups drank the same amount of solution during the “water load” period. This difference was also not due to differences in hydration status or the effects of training insofar as we could measure. The reduction in UV was accompanied by a 23% reduction in UNaV. It is important to note that we have only measured excretion for 180 min. It is expected that eventually, urine volume and Na+ excretion over time will be equal since 1) intake must equal excretion for both, water and Na+; 2) volume of intake is not different; and 3) food intake is the same as reflected by body weight. The lack of NOS 3 only changes the rate at which volume and Na+ are excreted. It should not change the total amount. Given that eliminating NO from the systemic vasculature would promote fluid and Na+ excretion, while eliminating it from the kidney would favor fluid and Na+ retention, our data indicate that the renal effects of genetically deleting NOS 3 outweigh the systemic vascular effects.
A difference in perfusion pressure is a potential explanation for the reduced ability of NOS 3 −/− mice to eliminate a volume load. We found that the blood pressure of NOS 3 −/− was higher than that of wild type by about 10 mmHg before a water load. This difference in blood pressure is similar to values reported by others (16, 20). However, the blood pressure of neither strain changed after water loading. Thus, the reduced ability to eliminate a water load observed in the NOS 3 −/− mice does not seem to be related to differences in the change of renal perfusion pressure after the load or renal vascular resistance.
Vasopressin is the primary regulator of urine volume. It directly stimulates water reabsorption by the collecting duct via increasing the amount of aquaporin-2 at the apical membrane (18, 19). It also stimulates Na+ transport by the thick ascending limb (19). Thus, vasopressin enhances water retention by augmenting the osmotic gradient necessary for water reabsorption by the collecting ducts and also by direct stimulation of collecting duct water flux. NO has been reported to inhibit vasopressin release in the brain during acute water load in rats (38). Thus, the reduced rate at which NOS 3 −/− mice eliminate a water load could be explained by differences in circulating vasopressin concentration. To investigate whether this was the case, we measured plasma vasopressin. We found that plasma vasopressin levels were not different between strains. Thus, differences in vasopressin are not likely to account for the altered ability of NOS 3 −/− mice to eliminate water load. However, we recognize that the large variability of the vasopressin measurements may mask a physiological significance of the 30% increase in the mean value observed. Thus, differences in vasopressin could potentially account, but only in part, for the reduced rate at which NOS 3 −/− mice eliminate a water load.
The renin-angiotensin-aldosterone system (RAAS) is also an important regulator of Na+ and water homeostasis (13). Activation of RAAS increases Na+ retention, which leads to reduced water loss into the urine (5). Renin is the limiting enzyme in this system, and NO has been shown to regulate renin release by the juxtaglomerular cells (43). Therefore, one possible explanation of the reduced ability of NOS 3 −/− mice to eliminate water load is inappropriate suppression of renin release. To investigate whether this was the case, we measured plasma renin concentration (PRC) after water load. PRC was similar in both groups 3 h after the water load was given. These results suggest that alterations of the RAAS system are not responsible for the reduced ability to eliminate a water load in NOS 3 −/− mice. These data are consistent with Shesely et al. (44), who showed that renin release in vivo was unaltered in NOS 3 −/− compared with wild-type mice. Because of the nature of the experiments, both vasopressin and renin were measured at the end of the experimental period (180 min after water load). We found no differences in any of these peptides at the end of the experiment, where the major difference in cumulative volume excretion was observed. However, we recognize that differences at early time points cannot be completely ruled out.
Glomerular filtration rate (GFR) directly determines urinary volume (12). Thus, to investigate whether the reduced ability to eliminate a water load was due to reduced GFR in NOS 3 −/− mice, we measured urinary and plasma creatinine and calculated GFR during the first 30-min collection period. We found no differences between strains, suggesting that GFR did not differ between strains immediately after water load. These data suggest that the reduced rate at which NOS 3 −/− mice eliminate a water load is not likely due to a reduction in GFR.
Given that PRC, vasopressin levels, and GFRs were not different between NOS 3 −/− and wild-type mice, it is likely that the reduced ability of NOS 3 −/− to excrete a volume load is due to eliminating the direct actions of NOS 3-derived NO along the nephron.
Water reabsorption by the cortical collecting duct is an important determinant of UV. Vasopressin-stimulated water reabsorption of the collecting duct depends on the generation of an osmotic gradient across the epithelium generated by Na+ reabsorption in the thick ascending limb. The thick ascending limb and distal convoluted tubules are water impermeable and therefore dilute the urine. The difference in osmolality between the diluted urine and the interstitium allows absorption of water in the collecting duct. We found that the rate of UNaV excretion was reduced in NOS 3 −/− mice compared with wild-type mice. Given that GFR did not differ between strains, these data indicate that the lack of NO derived from NOS 3 in NOS 3 −/− mice results in an enhanced rate of Na+ absorption along the nephron. Thus, increased osmotic gradient resulting from enhanced Na+ reabsorption by the loop of Henle is likely to be part of the mechanism, whereby NOS 3 −/− mice excrete a water load at a slower rate.
To directly test whether the reduced ability to eliminate a water load in NOS 3 −/− mice was due to enhanced Na+ reabsorption by the thick ascending limb, we repeated the experiments in the presence of the loop diuretic, bumetanide. Our results showed that in the presence of bumetanide, urinary volume was similar between wild-type and NOS 3 −/− mice over the 180-min period after the water load. These results indicate that the reduced ability to eliminate a water load in NOS 3 −/− mice is due, at least in part, to the lack of NO-dependent effects at the level of the thick ascending limb. The possibility that other nephron segments are involved in the NO-dependent effect seems unlikely. In the proximal tubule, ANG II is the main regulator of Na+ and fluid reabsorption (11, 36). We found that renin did not differ between strains after the water load. Similarly, vasopressin is the main stimulator of water permeability in the collecting duct (18). We found that vasopressin levels are similar between groups after the water load. In addition, inhibition of Na+ reabsorption by the thick ascending limb would increase distal Na+ delivery, which, in turn, stimulates Na+ reabsorption by the collecting duct (6). This, in fact, would enhance the differences between wild-type and NOS 3 −/− mice if the effect of NO occurs in the distal nephron.
Perspectives and Significance
In summary, our hypothesis was that NO produced by NOS 3 in the nephron supersede the actions of it in the systemic and renal vasculature. Because there are no selective inhibitors of NOS 3, we used NOS 3 −/− mice. To test our hypothesis, we have developed an in vivo, noninvasive method to administer an acute water load in animals. This has allowed us to test our hypothesis in conscious animals reducing stress that often occurs when the water load is given intraperitoneally. We found that conscious NOS 3 −/− mice have a reduced ability to eliminate a volume load. These data indicate that the effects of NOS 3-derived NO in the kidney supersede those in the systemic vasculature. Furthermore, the loop diuretic bumetanide, eliminated the difference between wild-type and NOS 3 −/− mice. Thus, reduced ability to excrete a volume load after genetic deletion of NOS 3 is unlikely to be caused by differences in circulating hormones of GFR, but rather the direct effects of NOS 3-derived NO along the distal nephron.
Our in vivo findings are supported by data showing that NO inhibits Na+ absorption in the proximal tubule, thick ascending limb, and collecting duct (32). The thick ascending limb is of particular interest because we have shown that NO from NOS 3 acts as an autacoid in this segment to reduce NaCl absorption (34). The thick ascending limb is the primary segment where the osmotic gradient for water absorption by the collecting duct is created. Previous studies have shown that Na+ reabsorption by the thick ascending limb is enhanced in salt-sensitive hypertensinve rats (40). Furthermore, the inhibitory actions of NO on Na+ reabsorption are diminished in thick ascending limbs from salt-sensitive rats (8). Thus, reduced NO bioavailability at the thick ascending limb, as well as defects in the signaling cascade beyond NO, are likely to play an important role in the pathogenesis of salt-sensitive hypertension.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute grants HL-28982, HL-70985, and HL-090550 to J. L. Garvin.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
This work was presented, in part, at the Experimental Biology Meeting, April 5–9, 2008, San Diego, California, USA.
REFERENCES
- 1.Ait ST, Hoekstra D. Sphingolipid trafficking and protein sorting in epithelial cells. FEBS Lett 529: 54–59, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Bachmann S, Bosse HM, Mundel P. Topography of nitric oxide synthesis by localizing constitutive NO synthases in mammalian kidney. Am J Physiol Renal Fluid Electrolyte Physiol 268: F885–F898, 1995 [DOI] [PubMed] [Google Scholar]
- 3.Bloch J, Qiu C, Erdely A, Baylis C. Inhibition of inducible nitric oxide synthase during high dietary salt intake. Am J Hypertens 15: 230–235, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brophy CM, Knoepp L, Xin J, Pollock JS. Functional expression of NOS 1 in vascular smooth muscle. Am J Physiol Heart Circ Physiol 278: H991–H997, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Burns KD, Li N. The role of angiotensin II-stimulated renal tubular transport in hypertension. Curr Hypertens Rep 5: 165–171, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Cupples WA, Sonnenberg H. Load dependency of sodium chloride reabsorption by medullary collecting duct in rat. Am J Physiol Renal Fluid Electrolyte Physiol 253: F642–F648, 1987 [DOI] [PubMed] [Google Scholar]
- 7.Eitle E, Hiranyachattada S, Wang H, Harris PJ. Inhibition of proximal tubular fluid absorption by nitric oxide and atrial natriuretic peptide in rat kidney. Am J Physiol Cell Physiol 274: C1075–C1080, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Garcia NH, Plato CF, Stoos BA, Garvin JL. Nitric oxide-induced inhibition of transport by thick ascending limbs from Dahl salt-sensitive rats. Hypertension 34: 508–513, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Garcia NH, Pomposiello SI, Garvin JL. Nitric oxide inhibits ADH-stimulated osmotic water permeability in cortical collecting ducts. Am J Physiol Renal Fluid Electrolyte Physiol 270: F206–F210, 1996 [DOI] [PubMed] [Google Scholar]
- 10.Garcia NH, Stoos BA, Carretero OA, Garvin JL. Mechanism of the nitric oxide-induced blockade of collecting duct water permeability. Hypertension 27: 679–683, 1996 [DOI] [PubMed] [Google Scholar]
- 11.Garvin JL. Angiotensin stimulates glucose and fluid absorption by rat proximal straight tubules. J Am Soc Nephrol 1: 272–277, 1990 [DOI] [PubMed] [Google Scholar]
- 12.Guyton AC, Hall JE, Lohmeier TE, Jackson TE, Kastner PR. Blood pressure regulation: basic concepts. Fed Proc 40: 2252–2256, 1981 [PubMed] [Google Scholar]
- 13.Hall JE, Guyton AC, Jackson TE, Coleman TG, Lohmeier TE, Trippodo NC. Control of glomerular filtration rate by renin-angiotensin system. Am J Physiol Renal Fluid Electrolyte Physiol 233: F366–F372, 1977 [DOI] [PubMed] [Google Scholar]
- 14.Hebert SC, Andreoli TE. Control of NaCl transport in the thick ascending limb. Am J Physiol Renal Fluid Electrolyte Physiol 246: F745–F756, 1984 [DOI] [PubMed] [Google Scholar]
- 15.Herrera M, Silva-Laciar GB, Ortiz PA, Garvin JL. Nitric oxide. In: Comprehensive Hypertension, edited by Lip G. Y. H., Hall J. E., New York: Elsevier, 2007, p. 325–336 [Google Scholar]
- 16.Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, Fishman MC. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature 377: 239–242, 1995 [DOI] [PubMed] [Google Scholar]
- 17.Kakoki M, Mattson DL. Effects of isoform-specific nitric oxide synthase inhibitors on renal blood flow (Abstract). FASEB J 14: A135 3–15- 2000 [Google Scholar]
- 18.Knepper MA. Molecular physiology of urinary concentrating mechanism: regulation of aquaporin water channels by vasopressin. Am J Physiol Renal Physiol 272: F3–F12, 1997 [DOI] [PubMed] [Google Scholar]
- 19.Knepper MA, Kim GH, Fernandez-Llama P, Ecelbarger CA. Regulation of thick ascending limb transport by vasopressin. J Am Soc Nephrol 10: 628–634, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Kuenzli KA, Bradley ME, Buxton IL. Cyclic GMP-independent effects of nitric oxide on guinea-pig uterine contractility. Br J Pharmacol 119: 737–743, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lahera V, Salom MG, Miranda-Guardiola F, Moncada S, Romero JC. Effects of NG-nitro-l-arginine methyl ester on renal function and blood pressure. Am J Physiol Renal Fluid Electrolyte Physiol 261: F1033–F1037, 1991 [DOI] [PubMed] [Google Scholar]
- 22.Liu R, Carretero OA, Ren Y, Garvin JL. Increased intracellular pH at the macula densa activates nNOS during tubuloglomerular feedback. Kidney Int 67: 1837–1843, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Majid DS, Navar LG. Nitric oxide in the mediation of pressure natriuresis. Clin Exp Pharmacol Physiol 24: 595–599, 1997 [DOI] [PubMed] [Google Scholar]
- 24.Majid DS, Navar LG. Nitric oxide in the control of renal hemodynamics and excretory function. Am J Hypertens 14: 74S–82S, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Mattson DL, Roman RJ, Cowley AW., Jr Role of nitric oxide in renal papillary blood flow and sodium excretion. Hypertension 19: 766–769, 1992 [DOI] [PubMed] [Google Scholar]
- 26.Mohaupt MG, Elzie JL, Ahn KY, Clapp WL, Wilcox CS, Kone BC. Differential expression and induction of mRNAs encoding two inducible nitric oxide synthases in rat kidney. Kidney Int 46: 653–665, 1994 [DOI] [PubMed] [Google Scholar]
- 27.Morrissey JJ, McCracken R, Kaneto H, Vehaskari M, Montani D, Klahr S. Location of an inducible nitric oxide synthase mRNA in the normal kidney. Kidney Int 45: 998–1005, 1994 [DOI] [PubMed] [Google Scholar]
- 28.Nakano D, Pollock JS, Pollock DM. Renal medullary ETB receptors produce diuresis and natriuresis via NOS1. Am J Physiol Renal Physiol 294: F1205–F1211, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Navar LG. Renal autoregulation: perspectives from whole kidney and single nephron studies. Am J Physiol Renal Fluid Electrolyte Physiol 234: F357–F370, 1978 [DOI] [PubMed] [Google Scholar]
- 30.Navar LG, Bell PD, Burke TJ. Autoregulatory responses of superficial nephrons and their association with sodium excretion during arterial pressure alterations in the dog. Circ Res 41: 487–496, 1977 [DOI] [PubMed] [Google Scholar]
- 31.Orlov SN, Mongin AA. Salt-sensing mechanisms in blood pressure regulation and hypertension. Am J Physiol Heart Circ Physiol 293: H2039–H2053, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Ortiz PA, Garvin JL. Role of nitric oxide in the regulation of nephron transport. Am J Physiol Renal Physiol 282: F777–F784, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Ortiz PA, Hong NJ, Wang D, Garvin JL. Gene transfer of eNOS to the thick ascending limb of eNOS-KO mice restores the effects of l-arginine on NaCl absorption. Hypertension 42: 674–679, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Plato CF, Shesely EG, Garvin JL. eNOS mediates l-arginine-induced inhibition of thick ascending limb chloride flux. Hypertension 35: 319–323, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Pollock JS, Forstermann U, Mitchell JA, Warner TD, Schmidt HH, Nakane M, Murad F. Purification and characterization of particulate endothelium-derived relaxing factor synthase from cultured and native bovine aortic endothelial cells. Proc Natl Acad Sci USA 88: 10480–10484, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Quan A, Baum M. Endogenous production of angiotensin II modulates rat proximal tubule transport. J Clin Invest 97: 2878–2882, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rapoport RM, Draznin MB, Murad F. Endothelium-dependent relaxation in rat aorta may be mediated through cyclic GMP-dependent protein phosphorylation. Nature 306: 174–176, 1983 [DOI] [PubMed] [Google Scholar]
- 38.Reis WL, Giusti-Paiva A, Ventura RR, Margatho LO, Gomes DA, Elias LL, Antunes-Rodrigues J. Central nitric oxide blocks vasopressin, oxytocin and atrial natriuretic peptide release and antidiuretic and natriuretic responses induced by central angiotensin II in conscious rats. Exp Physiol 92: 903–911, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Ren YL, Garvin JL, Ito S, Carretero OA. Role of neuronal nitric oxide synthase in the macula densa. Kidney Int 60: 1676–1683, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Roman RJ, Kaldunski ML. Enhanced chloride reabsorption in the loop of Henle in Dahl salt-sensitive rats. Hypertension 17: 1018–1024, 1991 [DOI] [PubMed] [Google Scholar]
- 41.Romero JC, Knox FG. Mechanisms underlying pressure-related natriuresis: the role of the renin-angiotensin and prostaglandin systems. State of the art lecture. Hypertension 11: 724–738, 1988 [DOI] [PubMed] [Google Scholar]
- 42.Sasser JM, Sullivan JC, Elmarakby AA, Kemp BE, Pollock DM, Pollock JS. Reduced NOS3 phosphorylation mediates reduced NO/cGMP signaling in mesenteric arteries of deoxycorticosterone acetate-salt hypertensive rats. Hypertension 43: 1080–1085, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Sayago CM, Beierwaltes WH. Nitric oxide synthase and cGMP-mediated stimulation of renin secretion. Am J Physiol Regul Integr Comp Physiol 281: R1146–R1151, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Shesely EG, Maeda N, Kim HS, Desai KM, Krege JH, Laubach VE, Sherman PA, Sessa WC, Smithies O. Elevated blood pressures in mice lacking endothelial nitric oxide synthase. Proc Natl Acad Sci USA 93: 13176–13181, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sullivan JC, Giulumian AD, Pollock DM, Fuchs LC, Pollock JS. Functional NOS 1 in the rat mesenteric arterial bed. Am J Physiol Heart Circ Physiol 283: H658–H663, 2002 [DOI] [PubMed] [Google Scholar]
- 46.Terada Y, Tomita K, Nonoguchi H, Marumo F. Polymerase chain reaction localization of constitutive nitric oxide synthase and soluble guanylate cyclase messenger RNAs in microdissected rat nephron segments. J Clin Invest 90: 659–665, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thomson SC, Blantz RC. Glomerulotubular balance, tubuloglomerular feedback, and salt homeostasis. J Am Soc Nephrol 19: 2272–2275, 2008 [DOI] [PubMed] [Google Scholar]
- 48.Vallance P, Collier J, Moncada S. Effects of endothelium-derived nitric oxide on peripheral arteriolar tone in man. Lancet 2: 997–1000, 1989 [DOI] [PubMed] [Google Scholar]