Abstract
Marine teleost fish continuously ingest seawater to prevent dehydration and their intestines absorb fluid by mechanisms linked to three separate driving forces: 1) cotransport of NaCl from the gut fluid; 2) bicarbonate (HCO3−) secretion and Cl− absorption via Cl−/HCO3− exchange fueled by metabolic CO2; and 3) alkaline precipitation of Ca2+ as insoluble CaCO3, which aids H2O absorption). The latter two processes involve high rates of epithelial HCO3− secretion stimulated by intestinal Ca2+ and can drive a major portion of water absorption. At higher salinities and ambient Ca2+ concentrations the osmoregulatory role of intestinal HCO3− secretion is amplified, but this has repercussions for other physiological processes, in particular, respiratory gas transport (as it is fueled by metabolic CO2) and acid-base regulation (as intestinal cells must export H+ into the blood to balance apical HCO3− secretion). The flounder intestine was perfused in vivo with salines containing 10, 40, or 90 mM Ca2+. Increasing the luminal Ca2+ concentration caused a large elevation in intestinal HCO3− production and excretion. Additionally, blood pH decreased (−0.13 pH units) and plasma partial pressure of CO2 (Pco2) levels were elevated (+1.16 mmHg) at the highest Ca perfusate level after 3 days of perfusion. Increasing the perfusate [Ca2+] also produced proportional increases in net acid excretion via the gills. When the net intestinal flux of all ions across the intestine was calculated, there was a greater absorption of anions than cations. This missing cation flux was assumed to be protons, which vary with an almost 1:1 relationship with net acid excretion via the gill. This study illustrates the intimate link between intestinal HCO3− production and osmoregulation with acid-base balance and respiratory gas exchange and the specific controlling role of ingested Ca2+ independent of any other ion or overall osmolality in marine teleost fish.
Keywords: carbon dioxide, oxygen, protons, gill, carbonic anhydrase
marine teleost fish drink the surrounding seawater and absorb the ingested fluid to replace water that has been lost to the hyperosmotic environment (10–12, 37–39, 44–46). It has long been established that fluid absorption in marine teleost fish is predominantly driven by active uptake of Na+ and Cl− ions along the gastrointestinal tract (25, 38). However, there is another mechanism that marine teleost fish utilize to facilitate fluid absorption and osmoregulation via the intestine, the production and secretion of bicarbonate (HCO3−). Seawater is rich in Ca2+ and Mg2+ (∼10 and ∼53 mM, respectively), and these divalent cations would accumulate in the intestine, if not otherwise dealt with, as water is preferentially absorbed. The build up of these divalent cations could potentially reach toxic levels but would also produce an osmotic gradient that would be detrimental for fluid absorption (42, 44–46). Intestinally secreted HCO3− circumvents this problem by reacting with Ca2+ and Mg2+ to form insoluble carbonate precipitates (CaCO3 and MgCO3). Once precipitated these elements no longer exert an osmotic pressure and are subsequently excreted from the intestine (20, 21, 42, 44–46). This process not only has physiological importance in facilitating water absorption by the gut, it also reduces Ca2+ absorption, which may secondarily protect the kidney from renal stone formation (44). Furthermore, intestinal bicarbonate secretion appears to be stimulated by conditions in which the amount of Ca2+, but not Mg2+, delivered to the intestine is enhanced, for example as salinity is increased (11, 37) or when intestinal Ca2+ is experimentally manipulated in vitro and in vivo (46).
Bicarbonate ions are produced via the hydration of endogenous CO2, a reaction that is catalyzed by intracellular carbonic anhydrase. The HCO3− is then transported out of the cell and into the intestine via apical Cl−/HCO3− exchange (1, 11, 13, 15–19, 39, 44, 46). In addition to endogenous CO2 being used to fuel intracellular HCO3− production, serosal HCO3− may be cotransported with Na+ (via sodium bicarbonate cotransporter) (24) and/or serosal CO2 may diffuse into the intestinal cell. Indeed, Grosell and Genz (14) demonstrated in isolated Gulf toadfish (Opsanus beta) intestinal preparations that approximately half of HCO3− secretion was supplied from endogenous CO2 with the remainder originating from extracellular sources of HCO3−/CO2. When apical HCO3− secretion was stimulated in the European flounder (Platichthys flesus) in vivo it was therefore not surprising to observe a significant decline in plasma total CO2 (44).
Changes in the rate of intestinal HCO3− secretion should also have proportional effects on overall acid-base balance as well as blood gas levels, in particular total CO2 concentration ([CO2]) because the apical export of base (HCO3−) must be balanced by the same basolateral export of acid equivalents for cellular pH homeostasis. A by-product of HCO3− production is the liberation of a proton (H+), and evidence from toadfish (O. beta) suggests that it is extruded out of the intestinal cell basolaterally in exchange for Na+ (14) and to a lesser extent apically, via vacuolar H+ ATPase (18, 19). Therefore, elevated rates of apical HCO3− secretion result in additional H+ loading into the blood, potentially causing an acidosis. In support of this idea, a significant decrease in blood pH of toadfish (O. beta) was observed following acclimation to 50 ppt compared with 35 ppt seawater (11). This blood acidosis was linked to increased intestinal HCO3− excretion in response to higher drinking rates and elevated [Ca2+] in the ingested fluid. A decrease in blood pH can also affect O2 transport in the blood via the Bohr/Root effect that decreases the hemoglobin-oxygen (Hb-O2) binding affinity, causing enhanced release of O2 and raised Po2 in the acidotic localities (3, 4, 30, 34, 35). This supports the link between osmoregulation, acid-base balance, and gas exchange in marine teleost fish. To compensate for such disturbances and therefore regulate blood pH, it has been demonstrated that fish transfer acid-base relevant ions across the gill epithelium via mitochondria-rich cells (7, 11). Although blood acidosis in fish has been commonly linked to a number of situations [e.g., during exhaustive exercise (28), hypercapnia, (27) or hypoxia (22, 32, 36)], there are very few examples in the literature about the extent and physiological effects of acid loading from the intestinal cells to the blood associated with osmoregulation.
The influence of ingested levels of Ca2+ on intestinal HCO3− production and carbonate excretion has yet to be fully characterized. Wilson et al. (46) showed that presenting the intestine of European flounder in vivo or in vitro with elevated concentrations of Ca2+ increased HCO3− secretion, and in vivo the majority of any additional Ca2+ ions were ultimately excreted from the intestine as precipitated calcium carbonate. The companion study of Whittamore et al. (43) has used a similar in vivo approach to determine the effects of intestinal HCO3− secretion on fluid transport and osmoregulation. The aim of the present study was to use this approach to examine the effects on whole animal acid-base balance and blood gas status.
To accomplish this, an in vivo technique was used (46) in which the intestine of the European flounder was perfused with salines containing increasing concentrations of Ca2+ (10, 40, or 90 mM). To negate the complicating influence of ion and osmotic exchanges at other sites (e.g., the gills), fish were maintained in normal seawater. By isolating the intestine in vivo and perfusing it with varying [Ca2+], it can be inferred that any changes in HCO3− excretion rates, and subsequently blood gas and acid-base status, can be directly attributed to the intestinal concentration of Ca2+ alone.
METHODS
Fish husbandry.
European flounder (P. flesus, 465 ± 31 g, n = 23) were obtained from local fishermen in Flookburgh, Cumbria, UK and transported to the University of Exeter. Fish were held in marine aquarium facilities in 150-liter tanks of flowing, aerated, artificial seawater (Tropic Marin) as part of a recirculating seawater system maintained at 33.8 ± 0.2 ppt and 12.5 ± 0.3°C, under a 14:10-h light-dark photoperiod. Fish were maintained on a diet of fresh ragworm (Nereis virens) fed once per week. Food was typically withheld for at least 72 h prior to experimentation. Animal care and experimental procedures were approved by and carried out within the guidelines of the University of Exeter Ethical Committee and under a UK Home Office license.
In vivo procedures.
Fish were anesthetized in seawater containing 150 mg/l of tricaine methanesulfonate (cat. no. MS-222; Pharmaq, Fordingbridge, UK) buffered with 300 mg/l NaHCO3 (followed by prolonged aeration to restore normal CO2 levels) before being placed on a custom-made wet surgery table. Anesthesia was maintained during surgery as the gills were constantly irrigated with aerated seawater containing 95 mg/l buffered MS-222. As described previously (46), the surgery consisted of three consecutive procedures: cannulation of a caudal vein, insertion of a stomach drain catheter (to allow imbibed fluid to freely exit the stomach), and an intestinal perfusion catheter, followed by a rectal catheter bag.
Following vessel cannulation, a small blood sample was taken from each fish and centrifuged (MSE Microcentaur) at 11,600 g for 3 min before plasma osmolality was measured on a vapor-pressure osmometer (Wescor Vapro 5520). The osmotic pressure of the perfusion saline was then adjusted (if necessary) with deionized water to match that of the fish plasma. In addition, prior to attachment of the rectal catheter, the intestine was flushed with 50 ml of the respective perfusion saline via the intestinal catheter to thoroughly rinse out the existing gut fluid and any precipitates.
To minimize the risk of infection, the incisions made for the blood and intestinal catheters were treated with a topical prophylactic antibiotic solution dissolved in Cortland saline (47) before closure of the wound.
Intestinal perfusion.
Once surgery was complete, the fish were recovered from anesthesia and placed in individual chambers (11-liter capacity, filled to a volume of 8 liters) where they received a continuous supply of aerated seawater. The intestinal catheters were connected to a peristaltic pump (model Minipuls 3; Gilson), and perfusion commenced with one of three salines with Ca2+ concentrations of 10, 40, and 90 mM (full details listed in Table 1 of Ref. 43). The intestine of each fish was continuously perfused for 72 h at a mean perfusion rate of 5.04 ± 0.24 ml·kg−1·h−1 (n = 23). The volumes perfused were determined gravimetrically to the nearest 0.1 mg. When the same perfusion rate was used, these salines delivered Ca2+ to the intestine at rates comparable with fish living in normal-, double-, and triple-strength seawater in which both drinking rates (DR) and ambient Ca2+ concentrations increased proportionally with salinity (i.e., double DR × 20 ≡ 40 mM, and triple DR × 30 ≡ 90 mM).
Table 1.
Changes in venous blood total O2 levels (TO2, mM), the partial pressure of O2 (PO2, mmHg), and hemoglobin O2 saturation (Hb-O2, %) in fish that had their intestines perfused with varying concentrations of Ca2+
| Blood TO2, mM | Blood Po2, mmHg | Hb-O2, saturation, % | |
|---|---|---|---|
| Day 1 | |||
| Control | 3.0 ± 0.4 | 41.0 ± 5.1 | 83.4 ± 5.7 |
| 40 mM | 2.4 ± 0.3 | 39.2 ± 3.7 | 81.6 ± 6.3 |
| 90 mM | 2.5 ± 0.2 | 39.9 ± 4.3 | 84.4 ± 5.7 |
| Day 2 | |||
| Control | 2.5 ± 0.4 | 39.9 ± 1.9 | 86.9 ± 8.6 |
| 40 mM | 2.0 ± 0.2 | 42.6 ± 8.3 | 79.9 ± 6.1 |
| 90 mM | 2.1 ± 0.2 | 50.5 ± 8.2 | 84.5 ± 3.9 |
| Day 3 | |||
| Control | 2.5 ± 0.3 | 26.4 ± 4.1 | 84.0 ± 6.8 |
| 40 mM | 1.9 ± 0.2 | 30.9 ± 2.0 | 85.0 ± 5.9 |
| 90 mM | 1.9 ± 0.4 | 37.0 ± 4.8 | 91.2 ± 7.9 |
Values are means ± SE; control, n = 8–7; 40 mM Ca2+, n = 8; and 90 mM Ca2+ n = 8–5.
Analysis of blood and plasma.
Blood samples (∼800 μl) were taken at 24, 48, and 72 h using a gas-tight 1-ml Hamilton syringe and processed immediately. Plasma was isolated from ∼500 μl of blood by centrifuging (11,600 g for 3 min; MSE Microcentaur) and was subsequently stored on ice. Various blood parameters were measured on the remaining 300 μl as previously described by Cooper and Wilson (8). Briefly, blood pH was measured on whole blood in a system thermostated to the experimental temperature (Cameron E301 glass and E351 electrodes connected to an Alpha 600 meter). Po2 was measured on whole blood in a system thermostated to the experimental temperature (Strathkelvin model 1302 electrode and model 781 meter). Total whole blood oxygen content (TO2) was measured using the method of Tucker (40) with a Cameron E101 electrode and BGS200 chamber at 38 ± 0.1°C (Grant GD120 thermostated water bath), connected to a Strathkelvin 781 meter. Blood Hb concentration ([Hb]) was measured spectrophotometrically (540 nm), with 10 μl of blood being added to 1 ml Drabkin's reagent (9), including Brij 35 (Sigma, St. Louis, MO). The dilution factor was accounted for and tetramer [Hb] (mM) was calculated by applying a millimolar extinction coefficient of 11.0 (6) and then multiplying by four. Combining the blood [Hb] with blood TO2 enabled the calculation of Hb-O2 saturation (i.e., blood O2 mM/blood [Hb tetramer] mM × 100 = % Hb-O2 saturation). Hematocrit was measured before the remaining blood (∼250 μl of total whole blood taken) was returned to the animal along with ∼550 μl Cortland's saline to replace the volume removed.
Plasma partial pressure of CO2 (Pco2) and HCO3− concentration ([HCO3−]) were calculated from plasma TCO2 (Mettler Toledo model 965 carbon dioxide analyzer) and venous blood pH measurements using a rearrangement of the Henderson-Hasselbalch equation. Values for solubility (αCO2 = 0.057 mM/mmHg) and pKapp [6.08–6.14 (temperature and pH dependent)] were based on Boutilier et al. (5).
Net acid-base fluxes.
Initial and final water samples were taken for each flux period for the measurement of net fluxes of acid-base relevant ions between the animal and its external medium. Fish were held in static water for up to 15 h in 8 liters, conditions in which average final water total ammonia concentrations did not exceed 200 μM. At the end of each flux period, chambers were flushed thoroughly with clean seawater. Total ammonia and titratable alkalinity were measured as described by Cooper and Wilson (8) and Wilson and Grosell (44). Total ammonia was measured on water samples by using the salicylate method (modified from Ref. 41), and the titratable alkalinity was measured using an autotitrator (model TIM845 titration manager and model SAC80 automated sample changer; Radiometer) performing single titrations with 0.02 N HCl. Under these experimental conditions, single titrations have been shown to yield the same results as double titrations (see Ref. 8).
The net fluxes of acid-base relevant ions between the fish and external water were subsequently calculated using the following equation:
, where V is the volume of water (liters) in the chamber (after the initial sample was taken), M is the mass of the fish (kg), t is the duration of the flux period (h), and [X]i and [X]f are the ion concentrations in the chamber water (μM or μeq) at the beginning and end of the flux period, respectively. Titratable alkalinity flux rates (JTAlk) were calculated from the above equation using titratable alkalinity measurements, but the initial and final values were reversed to achieve titratable acid instead of base fluxes, i.e., negative values represent net base uptake or branchial titratable acid excretion. For the remainder of the manuscript, branchial acid-base equivalent transfers will be referred to as gill acid excretion, rather than base uptake, as the former is more likely because of the gradients of HCO3− and H+ across the epithelium. The branchial net acid excretion was calculated by combining the flux of titratable alkalinity (JTAlk) and the flux of total ammonia (JTamm) to the external water (26). It should be noted that net acid excretion (JH+net) can result from the movement of any of the following: H+, NH4+, HCO3−, or OH−. While it is not possible to distinguish between these forms, H+ and NH4+ excretion and HCO3− and OH− uptake are all equivalent in terms of the acid-base status of the fish.
After 72 h, the intestinal perfusions were terminated by stopping the peristaltic pump and administering an overdose of anesthetic (250 mg/l buffered MS-222) into the flux chamber. Net fluxes of all ions were calculated, and in all instances there was a substantially greater absorption of anions than cations. Even though in the present study we could not measure the missing cation directly, it was assumed to be a proton (H+), and evidence supporting this hypothesis is covered in the discussion.
Statistical analysis.
All data are represented as means ± SE (SigmaPlot version 9.0). Normality was checked with the Kologorov-Smirnov test, and those data that were not normally distributed were log transformed. Where appropriate, an ANOVA followed by a Holm-Sidak post hoc test was used to test the normal and log normal data. Means were considered significantly different based on the adjusted P < 0.05 (SigmaStat 3.1 statistical program).
RESULTS
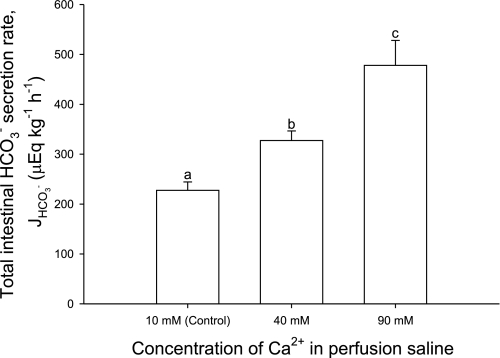
Increasing the perfusate Ca2+ concentrations from 10 mM (controls) to 40 and 90 mM significantly increased total intestinal HCO3− production rates, i.e., the sum of the fluid and precipitate HCO3− equivalent excretion rates by 44 and 100%, respectively (Fig. 1).
Fig. 1.
Net production and excretion of bicarbonate equivalents (HCO3− + 2CO32−; μeq·kg−1·h−1) by the intestine of the flounder perfused with salines containing varying concentrations of Ca2+. J, flux. Bars represent the total amount of bicarbonate equivalents excreted (via the intestinal fluid + rectal fluid + precipitates). a,b,cSignificant difference (P < 0.05; ANOVA followed by Holm-Sidak post hoc test). Values are means ± SE; n = 8, 7, and 8 for the control, 40 mM, and 90 mM Ca2+ treatments, respectively.
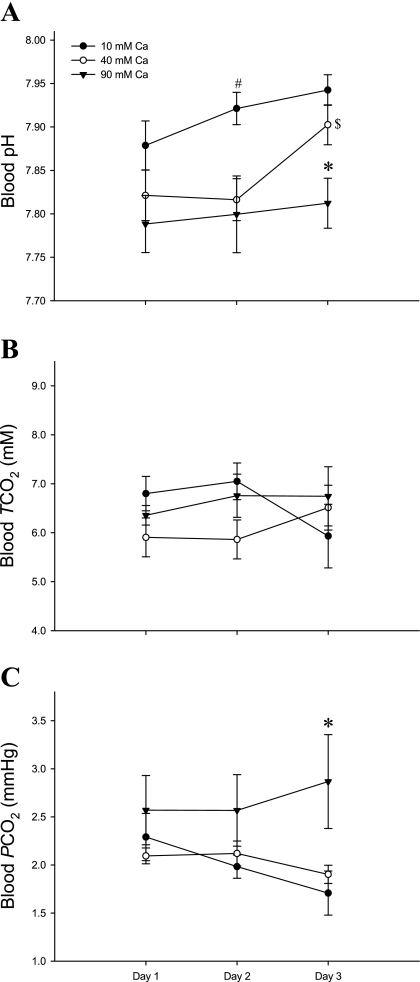
Fish with intestines perfused with 40 and 90 mM Ca2+ exhibited a significant blood acidosis at 2 days postsurgery. By day 3, blood pH had recovered in the 40 mM Ca2+ perfused fish but was still significantly acidotic by 0.13 pH units in the 90-mM perfusion group (Fig. 2A). In addition, although there were no significant differences in the blood total CO2 (Fig. 2B), Pco2 was significantly elevated after 3 days postsurgery in fish perfused with 90 mM Ca2+ compared with 10 and 40 mM Ca2+ (2.43 vs. 1.71 and 1.83 mmHg, respectively; Fig. 2C).
Fig. 2.
Changes in venous blood pH (A), total CO2 (TCO2; mM) (B), and the partial pressure of CO2 (Pco2; mmHg) (C) over 3 days when flounder intestines were perfused with varying concentrations of Ca2+. Black circles represent control (n = 8), white circles; 40 mM Ca2+ (n = 8); and black triangles, 90 mM Ca2+ (n = 8–7) treatments. A: *significant difference in blood pH on day 3 for fish perfused with 90 mM Ca2+ (P < 0.01; 90 vs. 10 and 40 mM Ca2+); #significant difference in blood pH on day 2 (P < 0.05, 10 vs. 90 and 40 mM Ca2+); $significant difference in blood pH for fish perfused with 40 mM Ca2+ between days 1 and 2 vs. day 3 (P < 0.05). C: *significant difference in blood Pco2 on day 3 for fish perfused with 90 mM Ca2+ (P < 0.01, 90 vs. 10 and 40 mM Ca2+). All statistical analyses were by ANOVA followed by Holm-Sidak post hoc test. Values are means ± SE.
In general, fish in the high [Ca2+] treatments had the lowest mean values for blood TO2 and Hb-O2 saturation, but the highest Po2 (Table 1). However, none of these differences were statistically significant.
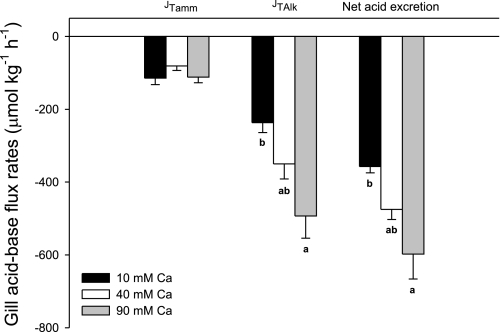
Figure 4 illustrates net acid-base flux movements between fish and the surrounding water (presumably via the gills as the gut was catheterized) in the three different intestinal perfusate [Ca2+] treatments. Increasing the [Ca2+] in the perfusion saline had no effect on ammonia excretion rates (JTamm; Fig. 3). However, branchial titratable acid excretion rates (negative JTAlk) rose by 113 and 143 μmol·kg−1·h−1 when the [Ca2+] was increased (to 40 mM and 90 mM, respectively), which resulted in significantly elevated net acid excretion rates (Fig. 3).
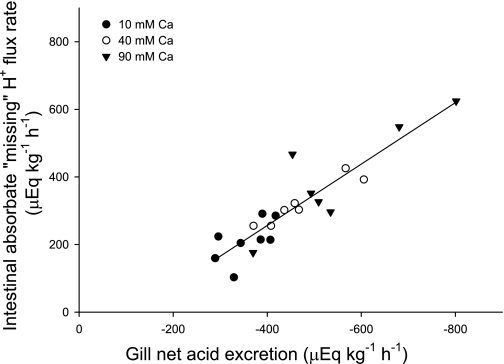
Fig. 4.
Relationship between the net acid excretion via the gills (μeq·kg−1·h−1) and the net flux of the missing cation, presumed to be H+ (μeq·kg−1·h−1), absorbed by the intestine of the flounder perfused with salines containing varying concentrations of Ca2+ (n = 8, 7, and 8 for control, 40 mM, and 90 mM Ca2+ treatments, respectively). Note that the linear regression crosses the x-axis at a value for gill net acid excretion of about 118 μeq·kg−1·h−1. This would represent the metabolic acid that is produced normally by metabolism in tissues other than the intestine and is typical of gill net acid excretion in many studies on fish.
Fig. 3.
Fluxes of titratable alkalinity (JTAlk, gray bars), total ammonia (JTamm, white bars), and net acidic equivalents (JH+net, black bars) between flounder and surrounding water. Negative values indicate fluxes that are equivalent to branchial acid excretion. Bars labeled with different letters indicate a significant difference (P < 0.05; ANOVA followed by Holm-Sidak post hoc test). Values are means ± SE; n = 8, 7, and 8 for control, 40 mM, and 90 mM Ca2+ treatments, respectively.
The rate of absorption (μeq·kg−1·h−1) of the missing cation (presumed to be H+, see discussion) within fluid absorbed by the intestine was plotted against net acid excretion via the gills, which revealed a significant positive correlation (P < 0.001; Fig. 4). High calculated rates of intestinal absorption of the missing H+ were matched by high net acid excretion rates via the gill, with a slope of 0.908, indicating this was close to a 1:1 ratio, and this relationship was consistent with the perfused [Ca2+]. For example, flounder perfused with 90 mM Ca2+ exhibited the highest rates of net acid excretion, which was matched by the highest calculated rates for apparent intestinal H+ absorption (Fig. 4).
DISCUSSION
This study provides an investigation into the effects of intestinal HCO3− secretion and subsequent CaCO3 formation driven specifically by Ca2+ on whole animal acid-base balance and respiratory gas transport in a euryhaline teleost fish, the European flounder. By isolating the flounder intestine in vivo it was possible to determine the specific effects of increased luminal Ca2+ concentrations on HCO3− production. The present data show that when teleost fish are faced with the same osmoregulatory challenge (i.e., breathing normal seawater and intestines perfused with salines that matched their blood osmolality) they are able to increase intestinal HCO3− production and excretion specifically in response to elevated Ca2+ concentrations in the intestine alone. In turn, this physiological response to intestinal Ca2+ affects both blood pH and plasma Pco2, further illustrating the intimate link between intestinal HCO3− production and whole animal acid-base balance and gas exchange in teleost fish. In previous studies using the same technique but lower levels of perfusate Ca2+, fish were able to maintain normal blood acid-base status (see Ref. 44 and discussion below). The present study is therefore the first to reveal a significant impact on blood acid-base status that can be specifically accounted for by Ca2+-driven processes occurring in the intestine alone and exclusively due to the upregulation of intestinal HCO3− secretion.
Effects of bicarbonate secretion on acid-base balance.
Using an in vitro Ussing chamber preparation with anterior intestinal sections from Gulf toadfish, Grosell and Genz (14) have shown that intestinal HCO3− secretion and subsequent basolateral H+ extrusion occur at equal rates, and partial inhibition of the latter by a reduction of serosal pH causes an equivalent reduction in luminal HCO3− secretion rates. Intestinal fluid absorption coupled with basolateral H+ extrusion into the extracellular fluid suggests that a highly acidic fluid is being absorbed via the intestine (12, 15). Whittamore et al. (43) calculated the charge balance of measured ions in the absorbed fluid (absorbate) in the same fish as used in the present study, and found that the absorption rate of measured anions greatly exceeded that of measured cations by a factor of 2- to 2.8-fold as perfusate Ca2+ was increased from 10 to 90 mM. The most likely candidate for this cation imbalance in the absorbate is H+, derived from intracellular CO2 hydration. However, we cannot distinguish this from transepithelial movement of HCO3− from the blood to the lumen, which would produce the exact same net effect (12, 15, 46) resulting from CO2 hydration in the blood, leaving behind an H+ ion for each translocated HCO3− ion. If the missing cation is presumed to be an H+, it will form part of the net solute flux, contributing to fluid transport and its composition (12, 15, 46). This explanation is corroborated by the finding that the net intestinal absorption of this missing cation is almost directly proportional to the corresponding rate of net acid excretion via the gills across all treatments (P < 0.001; Fig. 4). Such a highly acidic intestinal absorbate, which could theoretically be as low as pH ∼1.0 (12), would obviously have the potential to affect the blood acid-base status.
Consistent with this hypothesis, whole blood pH in flounder perfused with 90 mM Ca2+ (i.e., fish with the highest rates of intestinal HCO3− secretion; Fig. 1) remained low over the whole 3-day experiment. Perfusion with 40 mM Ca2+ resulted in a similarly acidotic blood pH after the first 2 days, which subsequently recovered to control pH levels by day 3 (Fig. 2A). As stated above, 10, 40, and 90 mM [Ca2+] in the perfusate provided intestinal Ca2+ loads equivalent to fish living in normal-, double-, and triple-strength seawater. However, the experimental design used here deliberately avoided any confounding influence of changes in ambient salinity (and the corresponding differences in multiple ions and osmolality) on both the gills and gut. Instead, this approach allowed us to focus solely on the whole animal physiological consequences of intestinal HCO3− secretion alone, which occurs exclusively in response to differential rates of Ca2+ delivery to this tissue.
The blood acidosis observed in fish intestinally perfused with high [Ca2+] resembles the reduced plasma pH observed in fish exposed to hypersaline ambient conditions, e.g., Gulf toadfish in 50 ppt (11). Reduced plasma pH was also observed in Gulf toadfish exposed to normal seawater containing 100 mM instead of 10 mM Ca2+ (Wilson RW and Grosell M, unpublished observations). Data from the present study therefore pinpoints the intestine rather than the gills, and specifically the upregulation of HCO3− secretion in response to Ca2+ alone, as the source of blood acidosis when fish encounter hypersaline conditions. It is worth noting that in the previous study of Wilson and Grosell (44), flounder intestinally perfused using identical methods but with saline containing 20 mM Ca2+, maintained a normal blood acid-base status relative to control fish (perfused with 5 mM Ca2+). It therefore appears that the blood's ability to buffer intestinally derived H+ ions, or the gill's ability to rapidly compensate via net acid excretion, are only exceeded at intestinal Ca2+ loads somewhere between two and four times that encountered by fish in oceanic salinities.
Effects of bicarbonate secretion on blood gases.
It is important to note that the caudal vein catheter used in the present study does not sample venous blood directly from the intestine; rather it draws from mixed venous blood largely derived from the tail musculature. Furthermore, the mixed arterial blood supplying this tail area would have passed through the liver (via the hepatic portal vein) and the gills after receiving the contribution from intestinal venous blood. It could therefore be argued that we are unlikely to see much in the way of blood chemistry changes caused by intestinal HCO3− secretion, given the capacity of the gills to regulate arterial blood gas levels (e.g., see Refs. 29 and 33). However, if this were the case, we should not have observed the significant blood pH differences between treatments. Therefore, for much of the remainder of this discussion, we have assumed that the caudal vein blood chemistry can reveal changes in venous blood resulting from intestinal processes, even though such modifications are likely to be substantially dampened by the time the blood reaches the caudal vein sampling site.
Interestingly, there were no changes in plasma total CO2 levels over the 3-day period, even when the intestine was being perfused with 90 mM Ca2+ (Fig. 2B). It is not possible from these present data to state whether endogenous CO2 or basolateral transport of HCO3− was the main source for apical HCO3− secretion from the enterocyte. Obviously, basolateral HCO3− transport would reduce plasma [HCO3−], but cellular CO2 hydration would produce the same outcome. The hydration of CO2 is dependent on H+ elimination from the enterocytes to the extracellular fluids, which would subsequently titrate and therefore reduce plasma [HCO3−]. However, venous blood Pco2 increased in fish perfused with 90 mM Ca2+ after 3 days (Fig. 2C). The most obvious explanation for this is that the basolaterally secreted H+ ions (from intracellular hydration of CO2) titrated some of the extracellular HCO3−, thus generating higher Pco2 values, while total CO2 remained the same. Although plasma [HCO3−] itself (∼6.4 mM for all fish in each treatment, data not shown) did not change relative to perfusion treatment, this may simply be because it is easier to detect a statistically significant change in Pco2 rather than [HCO3−]. Molecular CO2 is a much smaller component (few %) of the total inorganic carbon system in blood, so titration of a small amount of the dominant HCO3− form produces a relatively large change in Pco2 in plasma (e.g., a drop in [HCO3−] of just 0.04 mM would double Pco2, at a given TCO2).
It was originally hypothesized that a drop in blood pH would lead to a decrease in Hb-O2 saturation, releasing some oxygen from Hb and subsequently raising Po2, but not necessarily changing blood TO2. These pH-sensitive Hb-O2 affinity relationships are known as the Bohr/Root effect (3, 4, 34, 35). The general trends for the blood oxygen variables all followed the above prediction, i.e., fish in the high [Ca2+] treatments with greatest intestinal HCO3− secretion generally had the lowest mean TO2 and Hb-O2 saturation and the highest Po2 (Table 1). However, none of these differences were statistically significant. There are several potential reasons for this. First, the 0.13-unit drop in blood pH may have been insufficient to trigger a Bohr/Root effect. A separate study on flounder Hbs indicates this may be at least part of the explanation (Cooper CA, Regan MD, Brauner CJ, and Wilson RW, unpublished observation). Second, intracellular pH regulation by the erythrocytes may have been sufficient to compensate for the extracellular acidosis, thus protecting the Hb from acidosis. Third, as mentioned above, any effects of the intestine on blood acid-base status would presumably have been substantially attenuated by the time this blood reached the catheter sampling site (i.e., caudal vein). The pH in venous blood immediately downstream from the intestine has yet to be measured. The possibility of a Bohr/Root effect being utilized to supply O2 to the intestine during increased demands upon its osmoregulatory processes remains a plausible and unique prospect.
Perspectives and Significance
All marine teleost fish need to osmoregulate, a process that requires intestinal HCO3− production and secretion. In the past decade, a number of key studies have aided our understanding of this mechanism and subsequent physiological consequences for marine teleosts. However, this cellular process also has a global environmental significance. Recently, CaCO3 excretion by teleost fish has been shown to account for 3 to 15% of the total oceanic carbonate production and is therefore a significant and previously unrecognized component of the global inorganic carbon cycle (45). The present study adds to our growing understanding of the physiology behind the process of intestinal bicarbonate secretion and provides new data in support of the repercussions for other physiological systems, namely respiratory gas transport and acid-base regulation. High [Ca2+] perfused into the intestine increased HCO3− excretion rates, which appears to be accompanied by enhanced extrusion of acid (H+) out of the intestinal cell and into the extracellular fluid. Venous blood pH significantly decreased, but flounder were able to compensate by increasing net acid excretion via the gill. There is evidence for this H+ loading into the blood titrating some of the plasma HCO3− and causing an elevation in plasma Pco2. Other interesting areas for future work include trying to measure blood flow and blood pH in capillaries and veins in close proximity to the intestine. This would be a more reliable measure of the effects of the basolaterally extruded protons on blood pH regulation, buffering capacity, and red blood cell gas exchange. Additionally, a more detailed model of branchial acid-base regulation at the cellular level in response to intestinally derived acidosis would also be of interest. To conclude, the present study further demonstrates the complex and unique relationship between intestinal osmoregulation and whole animal blood acid-base regulation and gas transport in teleost fish.
GRANTS
Funding support from the Biotechnology and Biological Sciences Research Council contributed to this work (Research Grants BB/D005108/1 and BB/F009364/1 to R. W. Wilson and PhD Studentship BBS/S/A/2004/11078 to J. M. Whittamore).
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
We thank Jan Shears for excellent technical support and fish husbandry skills and local fishermen of Flookburgh, Cumbria (Ian and Tony McClure) for collecting the flukes (flounder) used in this study.
Present address of C. Cooper: Integrative Biology, University of Guelph, New Science Complex, Guelph, ON, N1G 2W1, Canada.
REFERENCES
- 1.Ando M, Subramanyam MVV. Bicarbonate transport systems in the intestine of the seawater eel. J Exp Biol 150: 381–394, 1990 [Google Scholar]
- 2.Berenbrink M. Historical reconstructions of evolving physiological complexity: O2 secretion in the eye and swimbladder of fishes. J Exp Biol 210: 1641–1652, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Berenbrink M, Koldkjaer P, Kepp O, Cossins AR. Evolution of oxygen secretion in fishes and the emergence of a complex physiological system. Science 307: 1752–1757, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Bohr C. The influence of section of the vagus nerve on the disengagement of gases in the air-bladder of fishes. J Physiol 15: 494–500, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boutilier RG, Heming TA, Iwama GK. Appendix–physicochemical parameters for use in fish respiratory physiology. Fish Physiol 10: 403–430, 1984 [Google Scholar]
- 6.Brauner CJ, Wang T, Jensen FB. Influence of hyperosmotic shrinkage and β-adrenergic stimulation on red blood cell volume regulation and oxygen binding properties in rainbow trout and carp. J Comp Physiol B 72: 251–262, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Claiborne JB, Edwards SL, Morrison-Shetlar AI. Acid-base regulation in fishes: cellular and molecular mechanisms. J Exp Zool 293: 302–319, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Cooper CA, Wilson RW. Post-prandial alkaline tide in freshwater rainbow trout: effects of meal anticipation on recovery from acid-base and ion regulatory disturbances. J Exp Biol 211: 2542–2550, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Drabkin DL, Austin JH., II Spectrophotometric studies. Preparations from washed blood cells; nitric oxide hemoglobin and sulfhemoglobin. J Biol Chem 112: 51–65, 1935 [Google Scholar]
- 10.Evans DH. Osmotic and ionic regulation. In: The Physiology of Fishes (1st ed.), edited by Evans DH. New York: CRC, 1993, p. 315–341 [Google Scholar]
- 11.Genz J, Taylor JR, Grosell M. Effects of salinity on intestinal bicarbonate secretion and compensatory regulation of acid-base balance in Opsanus beta. J Exp Biol 211: 2327–2335, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Grosell M. Intestinal anion exchange in marine fish osmoregulation. J Exp Biol 209: 2813–2827, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Grosell M, Jensen FB. NO2− uptake and HCO3− secretion in the intestine of the European flounder (Platichthys flesus). J Exp Biol 202: 2103–2110, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Grosell M, Genz J. Ouabain-sensitive bicarbonate secretion and acid absorption by the marine teleost fish intestine play a role in osmoregulation. Am J Physiol Regul Integr Comp Physiol 291: R1145–R1156, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Grosell M, Taylor JR. Intestinal anion exchange in teleost water balance. Comp Biochem Physiol A 148: 14–22, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Grosell M, Laliberte CN, Wood S, Jensen FB, Wood CM. Intestinal HCO3− secretion in marine teleost fish: evidence for an apical rather than a basolateral Cl−/HCO3− exchanger. Fish Physiol Biochem 24: 81–95, 2001 [Google Scholar]
- 17.Grosell M, Wood CM, Wilson RW, Bury NR, Hogstrand C, Rankin C, Jensen FB. Bicarbonate secretion plays a role in chloride and water absorption of the European flounder intestine. Am J Physiol Regul Integr Comp Physiol 288: R936–R946, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Grosell M, Genz J, Taylor JR, Perry SF, Gilmour KM. The involvement of H+-ATPase and carbonic anhydrase in intestinal HCO3− secretion in seawater-acclimated rainbow trout. J Exp Biol 212: 1940–1948, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Grosell M, Mager EM, Williams C, Taylor JR. High rates of HCO3− secretion and Cl− absorption against adverse gradients in the marine teleost intestine: the involvement of an electrogenic anion exchanger and H+-pump metabolon? J Exp Biol 212: 1684–1696, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Humbert W, Kirsch R, Simonneaux V. Is mucus involved in biocrystallisation? Study of the intestinal mucus of the sea-water eel Anguilla anguilla L. Cell Tissue Res 245: 599–604, 1986 [DOI] [PubMed] [Google Scholar]
- 21.Humbert W, Voegel JC, Kirsch R, Simonneaux V. Role of intestinal mucus in crystal biogenesis–an electron-microscopical, diffraction and X-ray microanalytical study. Cell Tissue Res 255: 575–583, 1989 [DOI] [PubMed] [Google Scholar]
- 22.Jackson DC. Acid-base balance during hypoxic hypometabolism: selected vertebrate strategies. Respir Physiol Neurobiol 141: 273–283, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Krogh A, Leitch I. The respiratory function of the blood in fishes. J Physiol 52: 288–300, 1919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurita Y, Nakada T, Kato A, Doi H, Mistry AC, Chang MH, Romero MF, Hirose S. Identification of intestinal bicarbonate transporters involved in formation of carbonate precipitates to stimulate water absorption in marine teleost fish. Am J Physiol Regul Integr Comp Physiol 294: R1402–R1412, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Marshall WS, Grosell M. Ion transport and osmoregulation in fish. In: The Physiology of Fishes, edited by Evans DH, Claiborne JB., 3rd Boca Raton, FL: CRC, 2006, p. 177–230 [Google Scholar]
- 26.McDonald DG, Wood CM. Branchial and renal acid and ion fluxes in the rainbow trout,Salmo gairdneri, at low environmental pH. J Exp Biol 93: 101–118, 1981 [DOI] [PubMed] [Google Scholar]
- 27.McKenzie DJ, Piccolella M, Dalla Valle AZ, Taylor EW, Bolis CL, Steffensen JF. Tolerance of chronic hypercapnia by the European eel Anguilla anguilla. J Exp Biol 206: 1717–1726, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Milligan CL, Wood CM. Tissue intracellular acid-base status and the fate of lactate after exhaustive exercise in the rainbow trout. J Exp Biol 123: 123–144, 1986 [DOI] [PubMed] [Google Scholar]
- 29.Nikinmaa M. Gas transport. In: The Physiology of Fishes, edited by Evans DH, Claiborne JB.(3rd ed.), 2006, p. 153–174 [Google Scholar]
- 30.Pelster B, Randall DJ. The physiology of the Root effect. In Fish Physiology: Fish Respiration, edited by Perry SF, Tufts BL. New York: Academic, 1998, vol. 17, p. 113–140 [Google Scholar]
- 31.Pelster B, Weber RE. The physiology of the Root effect. In: Advances in Comparative and Environmental Physiology, edited by Gilles R. Berlin: Springer, 1991, vol. 8, p. 51–77 [Google Scholar]
- 32.Perry SF. The regulation of hypercapnic acidosis in two Salmonids, the freshwater trout (Salmo gairdneri ) and the seawater salmon (Onchorynchus kisutch). Mar Fresh Behav Physiol 9: 73–79, 1982 [Google Scholar]
- 33.Randall DJ, Daxboeck C. Oxygen and carbon dioxide transfer across fish gills. In: Fish Physiology, Orlando, FL: Academic, 1984, vol. XA, p. 263–314 [Google Scholar]
- 34.Root RW. The respiratory function of the blood of marine fishes. Biol Bull 61: 427–456, 1931 [Google Scholar]
- 35.Scholander PF. Secretion of gases against high pressure in the swimbladder of deep sea fishes. II. The rete mirabile. Biol Bull 107: 260–277, 1954 [Google Scholar]
- 36.Scott GR, Wood CM, Sloman KA, Iftikar FI, De Boeck G, Almeida-Val VM, Val AL. Respiratory responses to progressive hypoxia in the Amazonian oscar, Astronotus ocellatus. Respir Physiol Neurobiol 162: 109–116, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Shehadeh ZH, Gordon MS. The role of the intestine in salinity adaptation of the rainbow trout, Salmo gairdneri. Comp Biochem Physiol 30: 397–418, 1969 [Google Scholar]
- 38.Smith HW. The absorption and excretion of water and salts by marine teleosts. Am J Physiol 93: 480–505, 1930 [Google Scholar]
- 39.Taylor JR, Grosell M. Evolutionary aspects of intestinal bicarbonate secretion in fish. Comp Biochem Physiol A 143: 523–529, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Tucker VA. Method for oxygen content and dissociation curves on microliter blood samples. J Appl Physiol 23: 410–414, 1967 [DOI] [PubMed] [Google Scholar]
- 41.Verdouw H, Van Echted CJA, Dekkers EMJ. Ammonia determination based on indophenol formation with sodium salicylate. Water Res 12: 399–402, 1978 [Google Scholar]
- 42.Walsh PJ, Blackwelder P, Gill KA, Danulat E, Mommsen TP. Carbonate deposits in marine fish intestines–a new source of biomineralisation. Limnol Oceanogr 38: 1227–1232, 1991 [Google Scholar]
- 43.Whittamore JM, Cooper CA, Wilson RW. HCO3− secretion and CaCO3 precipitation play major roles in intestinal water absorption in marine teleost fish in vivo. Am J Physiol Regul Integr Comp Physiol ( February3, 2010). doi:10.1152/ajpregu.00545.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilson RW, Grosell M. Intestinal bicarbonate secretion in marine teleost fish–source of bicarbonate, pH sensitivity, and consequences for whole animal acid-base and calcium homeostasis. Biochim Biophys Acta 1618: 163–174, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Wilson RW, Millero FJ, Taylor JR, Walsh PJ, Christensen V, Jennings S, Grosell M. Contribution of fish to the marine inorganic carbon cycle. Science 323: 359–362, 2009 [DOI] [PubMed] [Google Scholar]
- 46.Wilson RW, Wilson JM, Grosell M. Intestinal bicarbonate secretion by marine teleost fish–why and how? Biochim Biophys Acta 1566: 182–193, 2002 [DOI] [PubMed] [Google Scholar]
- 47.Wolf K. Physiological salines for freshwater teleosts. Prog Fish Cult 25: 135–140, 1963. [Google Scholar]