Abstract
Skeletal muscle, during periods of exertion, experiences several different fatigue-based changes in contractility, including reductions in force, velocity, power output, and energy usage. The fatigue-induced changes in contractility stem from many different factors, including alterations in the levels of metabolites, oxidative damage, and phosphorylation of the myosin regulatory light chain (RLC). Here, we measured the direct molecular effects of fatigue-like conditions on actomyosin's unloaded sliding velocity using the in vitro motility assay. We examined how changes in ATP, ADP, Pi, and pH affect the ability of the myosin to translocate actin and whether the effects of each individual molecular species are additive. We found that the primary causes of the reduction in unloaded sliding velocity are increased [ADP] and lowered pH and that the combined effects of the molecular species are nonadditive. Furthermore, since an increase in RLC phosphorylation is often associated with fatigue, we examined the differential effects of myosin RLC phosphorylation and fatigue on actin filament velocity. We found that phosphorylation of the RLC causes a 22% depression in sliding velocity. On the other hand, RLC phosphorylation ameliorates the slowing of velocity under fatigue-like conditions. We also found that phosphorylation of the myosin RLC increases actomyosin affinity for ADP, suggesting a kinetic role for RLC phosphorylation. Furthermore, we showed that ADP binding to skeletal muscle is load dependent, consistent with the existence of a load-dependent isomerization of the ADP bound state.
Keywords: motility assay, RLC, load-dependent kinetics, skeletal muscle
skeletal muscle exhibits several fatigue-based changes in contractility (for review, see Ref. 9). The physiological bases of fatigue-induced changes in contractility have been shown to be due to several different factors, including changes in excitation-contraction coupling, neuronal activity, and muscle contractility. The effects of fatigue on contractility are thought to result from several factors, including alterations in the levels of metabolites (ATP, ADP, Pi, pH, and others).
Fatigue-like changes in metabolites in skeletal muscle fibers have been shown to cause reductions in muscle fiber velocity, force production, and power output (for review, see Refs. 9 and 17). Myosin regulatory light-chain (RLC) phosphorylation, which occurs with repetitive twitch activations, may also play a role in the observed changes in muscle mechanics that occur during periods of exertion and fatigue (51). RLC phosphorylation appears to have a modulatory function in striated muscle fibers, in which both the magnitude and rate of tension development are enhanced, primarily at submaximal calcium levels (51). Furthermore, recent data suggest that phosphorylation also alters myosin kinetics under fatigue-like conditions, reducing myosin sliding velocity and increasing the affinity of myosin for ATP (18, 27, 50).
Taken together, these changes in muscle mechanics observed in muscle fiber studies suggest that fatigue-like changes in metabolites and phosphorylation affect one or more steps of the mechanochemical cycle of actomyosin. However, studying the direct molecular effects of fatigue and phosphorylation on the actomyosin contractile apparatus in muscle fibers can be extremely challenging because of several factors. Opposing effects have been observed in studies of living muscle fibers compared with skinned muscle fibers. For example, whereas in living muscle fibers, there is an increase in tension economy during fatigue, there is a decrease in tension economy in skinned muscle fibers (35). The effects of fatigue and phosphorylation are temperature dependent with major differences seen between studies of muscle fibers at 10–15°C and those conducted at more physiological temperatures [such as an enhancement of submaximal calcium activation in RLC-phosphorylated myosin, changes in lattice spacing, unloaded shortening velocity (for a review, see Ref. 9)]. With the exception of the jump plate technique implemented by Cooke and colleagues (40), studies in skinned muscle fibers, which allow for controlled examination of the effects of different biochemical conditions on actomyosin contractility, can suffer from disorganization of the myofilament proteins observed at physiological temperatures, which may ultimately lead to changes in muscle mechanics. In addition, in the study of the direct molecular effects of fatigue and phosphorylation on the actomyosin contractile apparatus, it is difficult to separate molecular-based changes in the actomyosin interaction from broader, cellular-based changes in the muscle fiber and in the organization of the myofilament lattice.
Here, we used the in vitro motility assay to study the direct molecular effects of fatigue-like metabolic molecular species (ATP, ADP, Pi, and pH) and myosin phosphorylation on the actomyosin contractile apparatus. The in vitro motility assay affords the ability to directly determine the molecular mechanism by which fatigue-like conditions affect the mechanochemistry of actomyosin at physiologically relevant temperatures without the complicating factors of the myofilament array. We observed that fatigue-like metabolites cause a reduction in unloaded sliding velocity in both phosphorylated and dephosphorylated myosins. Both lowering pH and increasing the concentration of ADP depresses sliding velocity independently, yet their combined effects are nonadditive because of a transition from unloaded to loaded motility. Furthermore, we showed that phosphorylated myosin has a higher affinity for ADP than dephosphorylated myosin, reinforcing the notion that phosphorylation of the myosin RLC plays a direct role in tuning actomyosin kinetics (18, 20). Lastly, we showed that the ADP affinity of actomyosin is load sensitive, suggesting that load sensitivity of ADP binding and release in skeletal muscle myosin is similar to the load sensitivity observed in nonmuscle and smooth muscle myosins (30, 58, 59). This load sensitivity is higher for phosphorylated than dephosphorylated myosin, which is consistent with a kinetic scheme, in which there is a load-sensitive isomerization of actomyosin in the ADP-bound state (49).
MATERIALS AND METHODS
Preparation of Proteins
Endogenously dephosphorylated and phosphorylated rabbit skeletal myosin.
All animal studies were conducted under an Institutional Animal Care and Use Committee-approved protocol in accordance with institutional guidelines (University of Miami Miller School of Medicine #A3711–01). Phosphorylated and dephosphorylated rabbit skeletal myosin was isolated from rabbit fast skeletal muscle taken from freshly killed New Zealand White rabbits, as described previously (20). Purity of myosin preparations was determined by SDS-PAGE analysis, and phosphorylation status was examined on 8% polyacrylamide gel in the presence of 8 M urea (55).
Actin purification and labeling.
Unlabeled actin was prepared from chicken pectoralis muscle acetone powder, as described previously (20). The actin was suspended in actin buffer (25 mM KCl, 1 mM EGTA, 10 mM DTT, 25 mM imidazole, 4 mM MgCl2). TRITC phalloidin-labeled actin was prepared by reacting a 1:1 molar ratio mixture of TRITC phalloidin and actin in actin buffer overnight at 4°C.
In Vitro Motility Assays
The in vitro motility assays were performed as previously described (20, 21, 54) with some subtle modifications. Approximately 200 μg of myosin in 50% glycerol was precipitated in 1 ml of 10 mM DTT for 1 h on ice and collected by centrifugation at 16,000 g for 30 min at 4°C. The pellet was resuspended in 200 μl of myosin buffer (300 mM KCl, 25 mM imidazole, 1 mM EGTA, 4 mM MgCl2, 10 mM DTT). The remainder of the protocol, including microscopy was identical to previous protocols published earlier (20, 21). All experiments were performed at 35°C with the temperature of the flow cell being regulated using a Bioptechs objective heater (Bioptechs, Butler, PA).
Motility Buffers
All buffers were designed using balanced ions as determined using the Bathe program as described by Greenberg et al. (20) and WinMaxC (http://www.stanford.edu/∼cpatton/maxc.html). All buffers had a total ionic strength of 150 mM, pCa 9, and contained 5 mM EGTA, 5 mM MgCl2, 25 mM imidazole, 25 mM MES, 0.5% methyl cellulose, 2 mM dextrose, 160 units glucose oxidase, and 2 μM catalase. Both imidazole and MES were used to ensure effective buffering over the full range of pH values (at 35°C, imidazole is effective from pH 6.29 to 7.29, while MES is effective from pH 5.49 to 6.49). A similar buffering scheme has been utilized in muscle fiber studies (27). In addition to the molecular species listed above, the standard buffer contained 5 mM ATP, 0.02 mM ADP, 2 mM Pi, at pH 7.0, and the fatigue-like buffer contained 3 mM ATP, 0.3 mM ADP, 30 mM Pi, at pH 6.2 (9). For the studies of the effects of individual molecular species on actin filament sliding velocity, standard buffer was prepared, and only one molecular species (i.e., ATP, ADP, Pi, or pH) at a time was altered.
Load-Dependent Kinetics Measurements
The procedure for measuring the load-dependent kinetics was identical to the standard motility assay, except the myosin was mixed with 1.25 μg/ml of α-actinin (Cytoskeleton, Denver, CO) before being incubated in the flow cell.
Data Fitting
All curve fitting was done in SigmaPlot (Systat Software, San Jose, CA) using a least-squares fitting algorithm. Measurements of velocity vs. ATP deviated from purely Michaelis-Menten kinetics since phosphate can act as a competitive inhibitor and affect velocity at low ATP (39). Thus, the measurement of sliding velocity vs. ATP was fit to a Hill curve:
| (1) |
where Vmax is the maximal sliding velocity, KM is the concentration of ATP at which half-maximal velocity is attained, and H is the Hill coefficient. Measurements of the effect of pH on velocity were fit to an analogous expression where KM is replaced with the pH50 and [ATP] is replaced with the pH.
For studies with ADP, the data were fit to a competitive inhibitor model:
| (2) |
For studies with Pi, the data were fit to a line.
Statistics
All experiments were repeated using at least two different myosin preparations. The filament velocity was determined by manual tracking using the freeware motility software, Retrac (http://mc11.mcri.ac.uk/Retrac). For each flow cell, the velocity and standard errors in the velocity were calculated for 15–30 sliding filaments over the course of 5–10 frames from at least two different areas of the flow cell (to ensure that there were no surface artifacts). Only moving filaments were counted in the average velocities. When the data were fit to a model, parameter values and the errors in the parameters were determined from the least squares fit. A two-tailed t-test was used to examine the significance of the differences between velocities. The P value was calculated from the Student's t-test distribution and corrected for multiple comparisons using the Holm t-test criteria when necessary.
RESULTS
Fatigue-Like Conditions Reduce Unloaded Shortening Velocity
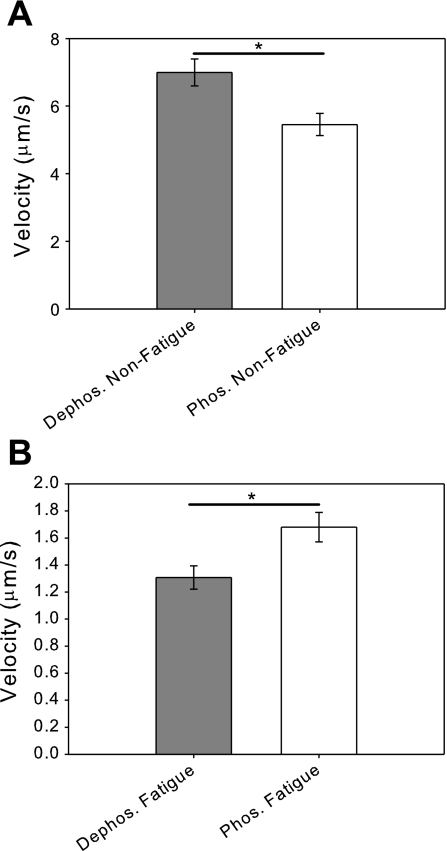
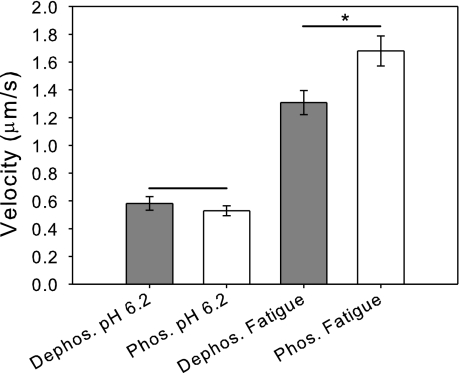
The unloaded sliding velocity of phosphorylated and dephosphorylated myosins were measured under both standard (5 mM ATP, 0.02 mM ADP, 2 mM Pi, pH 7.0) and fatigue-like conditions (3 mM ATP, 0.3 mM ADP, 30 mM Pi, pH 6.2) (Fig. 1). Under standard conditions, phosphorylation of the RLC causes a 22% percent depression in sliding velocity [V(dephos.) = 7.0 ± 0.4 μm/s, V(phos.) = 5.5 ± 0.3 μm/s; P < 0.005] (Fig. 1A). This is consistent with previous motility studies performed in the absence of exogenously added ADP and Pi (20). On the other hand, under fatigue-like conditions, phosphorylated myosin has a 28% higher sliding velocity than dephosphorylated myosin [V(dephos.) = 1.3 ± 0.1 μm/s, V(phos.) = 1.7 ± 0.1 μm/s; P < 0.02] (Fig. 1B). Thus, fatigue-like conditions cause an 81% depression in sliding velocity in dephosphorylated myosin yet only a 69% depression in sliding velocity in phosphorylated myosin. To examine the molecular basis for the effects of fatigue metabolites and myosin phosphorylation, we examined the individual effects of ATP, ADP, Pi, and pH on unloaded sliding velocity by adding ATP, ADP, Pi, or lowering pH in the standard motility buffer.
Fig. 1.
Fatigue-like conditions reduce actin filament sliding velocity. A: comparison of the unloaded sliding velocities of regulatory light-chain dephosphorylated and phosphorylated myosins under basal conditions. The velocity of phosphorylated myosin (V = 5.5 ± 0.3 μm/s) is significantly reduced compared with the dephosphorylated myosin (V = 7.0 ± 0.4 μm/s ; *P < 0.005). B: comparison of the unloaded sliding velocities of dephosphorylated and phosphorylated myosins under fatigue-like conditions shows that fatigue-like conditions cause a reduction of sliding velocity for both dephosphorylated and phosphorylated myosins. In contrast to the results under basal conditions, the velocity of phosphorylated myosin (V = 1.7 ± 0.1 μm/s) is significantly increased compared with dephosphorylated myosin (V = 1.3 ± 0.1 μm/s; *P < 0.02).
Fatigue-Like Reductions in the Concentration of ATP Has Little Effect on Sliding Velocity
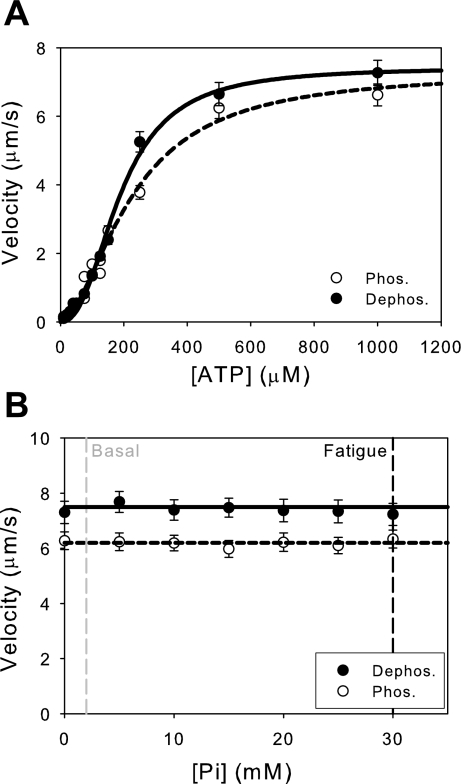
During fatigue, the available cellular pool of ATP decreases from 5 mM to 3 mM (9). The effect of ATP on unloaded sliding velocity was measured for both phosphorylated and dephosphorylated myosins over a range of ATP concentrations (Fig. 2A). As ATP is added, the unloaded sliding velocity increases steadily, reaching the maximum near 0.5 mM ATP. Because buffer solutions also contained 0.02 mM ADP and 2 mM Pi, the data deviated from standard Michaelis-Menten kinetics at low concentrations of ATP (39). Consequently, the data were fit to Hill plots, yielding the apparent KM for ATP determined at 50% of maximal velocity (V = ½ Vmax). There is no statistically significant difference between phosphorylated (KM = 225 ± 25 μM) and dephosphorylated (KM = 190 ± 10 μM) myosins (P = 0.21), consistent with the values measured in muscle fiber studies under nonfatigue conditions (18). Furthermore, fatigue-like reductions in the concentration of ATP from 5 mM to 3 mM under fatigue-like conditions has little effect on sliding velocity since these values are within the plateau region of the curve (data not shown), indicating that physiological fatigue-based changes in ATP alone have little effect on sliding velocity, consistent with fiber studies (2, 16).
Fig. 2.
Fatigue-like reductions in the concentration of ATP and phosphate have little effect on sliding velocity. A: effects of ATP concentration on unloaded sliding velocity. Reducing the available concentration of ATP from basal (5 mM) to fatigue-like conditions (3 mM) has no effect on sliding velocity (data not shown). Also, there is no significant difference in the KM between phosphorylated (KM = 225 ± 25 μM) and dephosphorylated myosins (KM = 190 ± 10 μM; P = 0.21). Data fit to the Hill equation (see text for details). B: effects of changing the concentration of Pi on unloaded sliding velocity. A line with zero slope was fit to the data for illustrative purposes. The addition of Pi has little effect on the unloaded sliding velocity of either phosphorylated or dephosphorylated myosins. Thus, the accumulation of Pi from basal levels (2 mM) to fatigue-like levels (30 mM) has little effect on unloaded sliding velocity.
Exogenously Added Phosphate Does Not Affect Unloaded Shortening Velocity
During fatigue, the concentration of Pi increases from 2 mM to 30 mM (9). The effect of Pi on unloaded sliding velocity was examined for both phosphorylated and dephosphorylated myosins (Fig. 2B). As can be seen from the data, Pi has no effect on unloaded sliding velocity for either phosphorylated or dephosphorylated myosins. The lack of effect of Pi on unloaded sliding velocity at saturating ATP is consistent with the data from skinned muscle fiber (10, 11, 13, 39) and in vitro studies (25).
Acidosis Decreases Unloaded Shortening Velocity
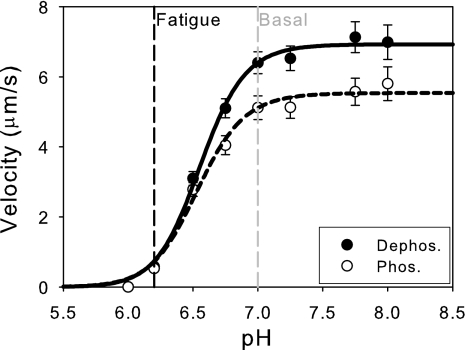
During periods of fatigue, the pH drops from 7.0 to 6.2 (46). The effects of alterations in pH on unloaded sliding velocity were measured for both dephosphorylated and phosphorylated myosins (Fig. 3). As can be seen from the data, both phosphorylated and dephosphorylated myosins showed significant reductions in sliding velocity when the pH was lowered from pH 7.0 (standard pH) to 6.2 (fatigue pH), consistent with skinned fiber studies (9, 14, 19, 36). These data were fit to Hill curves to obtain the pH50, the pH at which sliding velocity is reduced to 50% of the maximal sliding velocity. Both phosphorylated and dephosphorylated myosins show indistinguishable pH50 values [pH50(dephos.) = 6.55 ± 0.02, pH50(phos.) = 6.54 ± 0.03; P = 0.54]. Interestingly, at pH 7 phosphorylated myosin has a significantly lower sliding velocity than dephosphorylated myosin (P < 0.01), whereas at pH 6.2, there is no significant differences between the sliding velocities of phosphorylated and dephosphorylated myosins (P = 0.40).
Fig. 3.
Acidic conditions decrease actin filament velocity. Changing the pH from basal levels (7.0) to fatigue-like levels (6.2) causes a significant reduction in sliding velocity. The data were fit to the Hill equation, and it was shown that there is no difference in the pH50 for either phosphorylated (pH50 = 6.54 ± 0.03) or dephosphorylated myosins (pH50 = 6.55 ± 0.02; P = 0.54).
Exogenously Added ADP Decreases Velocity
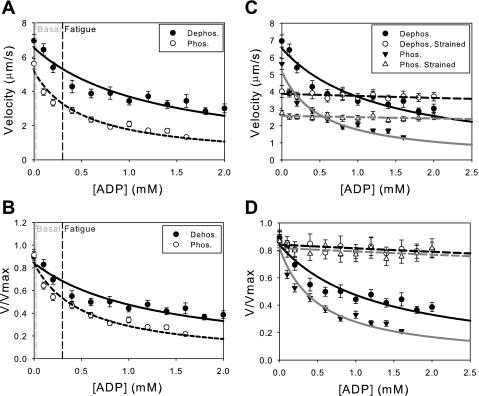
During fatigue, ADP accumulates, increasing in concentration from 0.02 mM to 0.3 mM (9). The effects of ADP accumulation on unloaded sliding velocity were examined for both phosphorylated and dephosphorylated myosins (Fig. 4). As can be seen from both the unnormalized (Fig. 4A) and normalized data (Fig. 4B), increasing [ADP] from 0.02 mM to 0.3 mM causes an 18% decrease in velocity for dephosphorylated myosin but a 34% decrease in sliding velocity for phosphorylated myosin. This is most likely due to the approximately twofold higher affinity for ADP observed for phosphorylated myosin compared with dephosphorylated myosin [KI(dephos.) = 200 ± 30 μM, KI(phos.) = 95 ± 10 μM; P < 0.01]. The KI measured for dephosphorylated myosin is consistent with values for skeletal muscle myosin measured in in vitro motility assays (1) and muscle fibers (8, 9, 28, 48).
Fig. 4.
Exogenous ADP decreases actin filament velocity. The unnormalized (A) and normalized (B) data are shown with fits to a competitive inhibitor model (Eq. 2). Changing the concentration of ADP from basal (0.02) to fatigue-like levels (0.3 mM) causes a significant depression in unloaded sliding velocity for both phosphorylated and dephosphorylated myosins. The inhibition constant for phosphorylated myosin (KI = 95 ± 10 μM) is significantly higher than dephosphorylated myosin (KI = 200 ± 30 μM; P < 0.01), demonstrating that phosphorylated myosin has an approximately 2-fold higher affinity for ADP than dephosphorylated myosin. C and D: load decreases the sensitivity of actin filament velocity to exogenously added ADP. A frictional load was introduced into the motility assay using the low-affinity actin binding protein, α-actinin. The unormalized (C) and normalized (D) data were fit to Eq. 2. Loading the myosin causes a significant reduction in the ability of ADP to depress actomyosin sliding. Loading both the phosphorylated (KI = 6 ± 4 mM) and dephosphorylated (KI = 5 ± 2 mM) myosins causes a lowering of the affinity for exogenously added ADP.
Load Decreases the Sensitivity of Myosin to Exogenous ADP
To examine the load sensitivity of ADP affinity for myosin, we introduced 1.25 μg/ml of α-actinin into the standard motility assay (see materials and methods) and examined the effect of exogenously added ADP (Fig. 4, C and D). α-Actinin is a low-affinity actin binding protein that transiently binds to translocating actin filaments in the in vitro motility assay, exerting a frictional load that opposes the driving force of myosin (4, 20). As can be seen from the unnormalized (Fig. 4C) and normalized data (Fig. 4D), introducing a resistive load causes an ∼10-fold decrease in the sensitivity of myosin to exogenously added ADP. For dephosphorylated myosin, the KI increases from 200 ± 30 μM in the absence of load to 5 ± 2 mM in the presence of load. For phosphorylated myosin, the KI increases from 95 ± 10 μM to 6 ± 4 mM in the presence of load.
DISCUSSION
In this study, we used the in vitro motility assay to examine the direct molecular effects of fatigue-like conditions and myosin regulatory light chain phosphorylation on the unloaded sliding velocity of the actomyosin contractile apparatus. One of the major findings of this study was that under fatigue-like conditions, there was a significant reduction in unloaded sliding velocity for both phosphorylated and dephosphorylated myosins. Although this result is consistent with fiber studies (for review, see Ref. 9), using the motility assay, we showed this effect can be ascribed directly to changes in the kinetics of the myosin motor, independent of fatigue condition-based changes in the myofilament lattice. To dissect the mechanism for these changes, we examined independent effects of ATP, ADP, Pi, pH, and phosphorylation on unloaded sliding velocity.
Physiological Fatigue-Like Changes in ATP and Inorganic Phosphate Do not Affect Unloaded Velocity
Fatigue-like changes in the levels of either ATP (5 to 3 mM) or Pi (2 to 30 mM) have no effect on the unloaded sliding velocity in the motility assay (Fig. 2). This result is consistent with fiber studies (2, 10, 11, 13, 39). Furthermore, the KM for ATP is unchanged when comparing phosphorylated and dephosphorylated myosins, consistent with the skinned fiber studies (18). It is important to note that although the accumulation of Pi has no effect on unloaded sliding velocity, it would be expected to play a role under loaded conditions, changing myosin force and power output (7, 10, 23, 39). While physiological reductions in ATP alone do not show any effect on sliding velocity, the 10-fold reduction in the affinity of ATP for myosin (12) and the inhibition of phosphocreatine resynthesis observed at low pH (16) could make changes in the cellular ATP levels more significant in determining unloaded sliding velocity in intact muscle fibers.
Acidosis Decreases Unloaded Sliding Velocity
During fatigue, there is a significant acidosis of the muscle primarily due to increased proton production from glycolysis (46). Here, we showed that reductions in pH cause a reduction in unloaded actin filament sliding in the in vitro motility assay, particularly below pH 6.7, consistent with skinned fiber (9, 14, 19, 36) and in vitro motility studies (12). The reduction in pH from pH 7.0 to 6.2 decreases sliding velocity by ∼91% and ∼90% for dephosphorylated and phosphorylated myosins, respectively, showing that both myosins are similarly affected by changes in pH alone. Interestingly, Pate et al. (38) observed less pronounced effects of velocity depression by pH measured at higher temperatures (30°C) in skinned muscle fibers. In contrast to this result, we and others (12) observe a significant reduction in velocity at 30–35°C with acidosis alone.
The effects of pH in muscle fibers have been proposed to result from changes in lattice spacing (57), constraints that are not present in the in vitro motility assay, suggesting that while changes in the lattice spacing play a role in affecting the mechanics of muscle fibers, there is also a direct molecular effect of acidosis on the actomyosin contractile apparatus. Furthermore, acidosis causes a reduction in the force per crossbridge (33). It is also possible that the observed differences in the effects of acidosis stem from the presence of regulated thin filaments (38) in the skinned fibers, whereas our in vitro studies employ unregulated thin filaments. It has been suggested that direct effects of the acidosis on the troponin complex (17, 34, 61) cause the observed shift in the intact fiber force-pCa relationship (15). Further in vitro motility studies utilizing regulated thin filaments could potentially help dissect the relative roles of acidosis on the thin filament regulatory apparatus and actomyosin.
ADP Inhibits Unloaded Sliding Velocity
Whereas decreasing pH affects both phosphorylated and dephosphorylated myosins similarly, increasing the ADP from 0.02 mM to 0.3 mM decreases sliding velocity 18% and 34% for dephosphorylated and phosphorylated myosins, respectively (Figs. 4, A and B). This is primarily due to the fact that phosphorylated myosin has a twofold higher affinity for ADP than dephosphorylated myosin. It has previously been proposed that the principal effect of skeletal muscle myosin phosphorylation is the release of the myosin heads from the thick-filament backbone, which would increase the rate of attachment of myosin to actin (32). The data obtained here were collected using the in vitro motility assay where there is no intact thick filament backbone, revealing a direct kinetic role for RLC phosphorylation in addition to the observed structural role, consistent with the suggestions of Franks-Skiba et al. (18) and Greenberg et al. (20).
Effects of the Individual Molecular Species Involved in Fatigue Are Nonadditive
Because both ADP and pH depressed velocity, one might expect that their combined effects would be additive. Surprisingly, this is not the case (Fig. 5), as the velocities of both phosphorylated and dephosphorylated myosins under fatigue-like conditions are significantly higher than their respective velocities at pH 6.2 in the absence of other fatigue-like factors. Whereas at pH 6.2, there is no difference in sliding velocity between phosphorylated and dephosphorylated myosins, phosphorylated myosin has a significantly higher velocity than dephosphorylated myosin under fatigue-like conditions. As will be discussed below, the increased velocity observed under fatigue-like conditions for phosphorylated myosin could be due to differences in ADP affinity, the only observed kinetic difference between phosphorylated and dephosphorylated myosins.
Fig. 5.
The combined effects of the individual molecular species involved in fatigue are nonadditive. Fatigue-like changes in pH (6.2) alone causes a significant depression in sliding velocity in the absence of other fatigue-like ionic molecular species. At pH 6.2, there is no significant difference in the unloaded sliding velocity between phosphorylated and dephosphorylated myosins (P = 0.40). On the other hand, the combined effect of all of the molecular species together (at pH 6.2) has an increased velocity compared with acidosis alone. This suggests that effects of the individual molecular species involved in fatigue are nonadditive. Furthermore, phosphorylated myosin has a greater recovery of velocity than dephosphorylated myosin (*P < 0.05) under fatigue-like conditions compared with the effects of acidosis alone.
The nonadditive effects of ADP and pH can be explained by the differences between loaded and unloaded kinetics. Under unloaded conditions, the velocity of actin in the motility assay is limited by myosin cross-bridge detachment (47). Thus, exogenously added ADP has a depressive effect on velocity, similar to what was observed in studies of the effect of ADP on velocity seen here (Fig. 4) and elsewhere (1, 8, 9, 28, 48). On the other hand, under loaded conditions, myosin sliding velocity is no longer limited by detachment of myosin from actin but rather the amount of force that can be generated by the ensemble of myosins working against the imposed load, which results from (strongly or weakly) attached, nonforce-producing myosin cross-bridges (12). In this case, the velocity can be described by
| 3 |
where Vmax is the maximal sliding velocity in the absence of a load, Fd(FL) is the average driving force of the bed of myosin [which is a function of load (24)], and FL is the imposed load.
It has been proposed that acidosis affects several biochemical transitions within the myosin biochemical cycle (12, 29), reducing the force per crossbridge (33), while increasing the population of weakly bound (29), dragging cross-bridges, introducing a load into the motility assay (60). Under loaded conditions, the detachment-limited model for motility no longer applies. Consistent with this notion, fatigue-like behavior in skinned fiber studies can be mimicked by agents that populate weak-binding states in the absence of acidosis (18, 27, 50).
Because under loaded conditions, ADP causes an increase in force (22, 35), the addition of ADP to the motility assay under acidotic conditions will increase the net myosin driving force and thus sliding velocity, according to Eq. 3. Thus, the competing effects of the load introduced into the motility assay with acidosis (12), along with the enhancement of force with ADP accumulation, explain the nonadditive effects of the individual molecular species involved with fatigue observed here. Consistent with this idea, Nosek et al. (36) showed that effects of the different molecular species involved with fatigue are additive over the range of pH 6.65–7 (19) but not below pH 6.65 (36). Similarly, as can be seen in Fig. 3, the effects of acidosis became appreciable once the pH dropped below 6.7. Because acidosis introduces a load into the motility assay and thus causes a transition from unloaded to loaded kinetics, it is not surprising that the effects of acidosis are additive over the range that the effects of acidosis on unloaded sliding velocity are not appreciable (Fig. 3). Moreover, the higher affinity of phosphorylated actomyosin for ADP should increase the driving force, explaining the greater recovery of velocity for phosphorylated myosin under fatigue-like conditions compared with the dephosphorylated myosin.
Phosphorylated Myosin Biochemistry Is More Sensitive to Load
Here (Fig. 1), and previously (20), we showed that phosphorylation causes a 20% reduction in unloaded velocity in the absence of exogenously added ADP and Pi at pH 7.4. This difference in sliding velocity is primarily due to an increase in the myosin duty cycle with phosphorylation (20). In the current work, we show that phosphorylated myosin has a twofold higher affinity for ADP, suggesting a biochemical basis for the reduced velocity and increased duty cycle of phosphorylated myosin under unloaded conditions. Consistent with this notion, Patel et al. (41) showed that phosphorylation slows the rate of transition from a strongly bound state to a weakly bound state (i.e., the detachment rate).
The myosin RLC binds the elongated α-helical neck region of the myosin molecule. In addition to its role as a force generator (43), the neck region has also been proposed to serve as a molecular load sensor, transmitting load directly to the active site, altering myosin kinetics (37). Previous motility data were consistent with a stiffening of the load-sensing myosin lever arm upon phosphorylation (20). Since ADP release is proposed to be the load-sensitive step of the myosin biochemical cycle (26, 59), one would expect greater load sensitivity to ADP release upon phosphorylation. We, therefore, introduced a load into our experiment by adding α-actinin to the motility assay surface and assayed the dependence of velocity on exogenously added ADP. We can define a load sensitivity parameter, Ψ, as
where a greater value of Ψ is indicative of a motor with a greater load sensitivity. Computing the load sensitivity parameter for data taken in the absence (Fig. 4, A and B) and the presence of a frictional load (Fig. 4, C and D), we saw that Ψ(dephos.) is ∼20 and Ψ(phos.) is ∼60, indicating that phosphorylated myosin is more load sensitive.
Studies of the load dependence of ADP affinity presented here show that ADP affinity is extremely load sensitive with an ∼10-fold lower affinity for exogenously added ADP under load. This fact requires that the ADP exchangeable state precedes the load-dependent transition. A lowering of affinity for exogenously added ADP under load is consistent with a kinetic scheme in which there is a load-dependent isomerization from a moderate-affinity ADP-bound state where ADP is exchangeable, to a state in which the affinity of myosin for ADP is low, and thus exogenously added ADP has little effect. It is possible that loading the myosin causes a closing of the active site, preventing ADP release and making the myosin less sensitive to exogenously added ADP (37).
Load sensitivity may be conveyed via prevention of an isomerization step that leads to the low-affinity state. Such an isomerization has been inferred from the crystal structures and EM reconstructions of several nonmuscle myosins and smooth muscle myosins (for review, see Ref. 37), all of which complete a working stroke in two steps (58, 59). Structural evidence for the isomerization has not been directly observed for skeletal muscle myosin (44); however, optical trapping, kinetic, and fluorescence data have all suggested the existence of multiple ADP-bound states (1, 6, 49, 56), consistent with our data. It is an intriguing possibility that this state is a common feature of all myosins and that structural studies have not observed this transition because of the large free energy change associated with ADP dissociation from skeletal muscle actomyosin (37).
Relation to Fiber Studies
Our data, showing a decrease in unloaded sliding velocity with phosphorylation (Fig. 1) in the absence of fatigue-like conditions and an increase in velocity with phosphorylation under fatigue conditions, contrast with fiber studies in which no change (5, 18, 42, 50, 52) or a decrease (18, 50) in velocity was observed. In a two-state model, with attachment rate, f, detachment rate, g, and duty cycle f/(f+g), it has been proposed that the increased mobility of the myosin heads upon phosphorylation is due to myosin heads detaching from the thick filament backbone, increasing f (32, 53). There is also evidence, however, that phosphorylation acts to additionally decrease g (41). Our data, which show a slowing of velocity and an increased duty cycle (20) with myosin phosphorylation, support the notion that phosphorylation of the RLC causes a decrease in g. Because the in vitro motility assays employed here used monomeric myosin lacking a thick filament backbone, any observed changes in actomyosin mechanics can be assigned to molecular-based changes in the actomyosin interaction, independent of the effects that have been proposed to be due to the thick-filament backbone (31, 32, 62). In muscle fibers in which release of the myosin heads from the thick-filament backbone with phosphorylation also increases f, there will be more complex effects of decreasing g and increasing f on the duty cycle. Because velocity is inversely proportional to the duty cycle, the competing effects on f and g can potentially explain the different effects on velocity observed in fiber studies (5, 18, 42, 50, 52). Future studies using thick filaments in motility assays will help to clarify the relative effects of phosphorylation on attachment and detachment of myosin crossbridges.
Our observation that ADP release from phosphorylated myosin is more load sensitive suggests that under fatigue-like (loaded) conditions, ADP release would be slowed, increasing the duty cycle. The resulting increase in the driving force (Eq. 3), provides a potential explanation for the greater recovery of velocity in the in vitro motility assay for phosphorylated myosin under fatigue-like conditions compared with the dephosphorylated myosin.
Perspectives and Significance
It has long been known that muscle fatigue is associated with the accumulation of metabolic end products and an increase in myosin RLC phosphorylation. In vitro motility assay experiments can provide insight into the complex molecular mechanism of muscle fatigue because they provide a means to quantify force and velocity in a simple, well-defined system. While motility assays provide important information about the molecularly based changes in the actomyosin interaction, the ultimate characteristics of muscle under fatigue conditions involves contributions from a host of factors, including changes in the myofilament array, changes in neuronal excitation, calcium handling, and cellular energetics (reviewed in Ref. 16).
Our studies have shown a direct effect of fatigue metabolites and RLC phosphorylation on the unloaded velocity of the actomyosin contractile apparatus, which should be considered when interpreting skinned and intact muscle fiber studies. Future in vitro motility studies that incorporate regulated thin filaments (3), filamentous myosin (45), and external loads (4) will help provide a more complete description of the underlying molecular basis for muscle fatigue.
GRANTS
This work was supported by National Institutes of Health-Heart, Lung, and Blood Institute Grants HL077280 (to J. Moore) and HL071778 (to D. Szczesna-Cordary), as well as American Heart Association Grants 0435434T (to J. Moore) and 0815704D (to M. Greeenberg).
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
The authors would like to thank Raymond Stephens for his helpful discussions and critical reading of the manuscript.
REFERENCES
- 1.Baker JE, Brosseau C, Joel PB, Warshaw DM. The biochemical kinetics underlying actin movement generated by one and many skeletal muscle myosin molecules. Biophys J 82: 2134–2147, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergstrom M, Hultman E. Energy cost and fatigue during intermittent electrical stimulation of human skeletal muscle. J Appl Physiol 65: 1500–1505, 1988 [DOI] [PubMed] [Google Scholar]
- 3.Bing W, Fraser ID, Marston SB. Troponin I and troponin T interact with troponin C to produce different Ca2+-dependent effects on actin-tropomyosin filament motility. Biochem J 327: 335–340, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bing W, Knott A, Marston SB. A simple method for measuring the relative force exerted by myosin on actin filaments in the in vitro motility assay: evidence that tropomyosin and troponin increase force in single thin filaments. Biochem J 350: 693–699, 2000 [PMC free article] [PubMed] [Google Scholar]
- 5.Butler TM, Siegman MJ, Mooers SU, Barsotti RJ. Myosin light chain phosphorylation does not modulate cross-bridge cycling rate in mouse skeletal muscle. Science 220: 1167–1169, 1983 [DOI] [PubMed] [Google Scholar]
- 6.Capitanio M, Canepari M, Cacciafesta P, Lombardi V, Cicchi R, Maffei M, Pavone FS, Bottinelli R. Two independent mechanical events in the interaction cycle of skeletal muscle myosin with actin. Proc Natl Acad Sci USA 103: 87–92, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caremani M, Dantzig J, Goldman YE, Lombardi V, Linari M. Effect of inorganic phosphate on the force and number of myosin cross-bridges during the isometric contraction of permeabilized muscle fibers from rabbit psoas. Biophys J 95: 5798–5808, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chase PB, Kushmerick MJ. Effect of physiological ADP concentrations on contraction of single skinned fibers from rabbit fast and slow muscles. Am J Physiol Cell Physiol 268: C480–C489, 1995 [DOI] [PubMed] [Google Scholar]
- 9.Cooke R. Modulation of the actomyosin interaction during fatigue of skeletal muscle. Muscle Nerve 36: 756–777, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Cooke R, Franks K, Luciani GB, Pate E. The inhibition of rabbit skeletal muscle contraction by hydrogen ions and phosphate. J Physiol 395: 77–97, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooke R, Pate E. The effects of ADP and phosphate on the contraction of muscle fibers. Biophys J 48: 789–798, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Debold EP, Beck SE, Warshaw DM. Effect of low pH on single skeletal muscle myosin mechanics and kinetics. Am J Physiol Cell Physiol 295: C173–C179, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Debold EP, Dave H, Fitts RH. Fiber type and temperature dependence of inorganic phosphate: implications for fatigue. Am J Physiol Cell Physiol 287: C673–C681, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Edman KA, Mattiazzi AR. Effects of fatigue and altered pH on isometric force and velocity of shortening at zero load in frog muscle fibres. J Muscle Res Cell Motil 2: 321–334, 1981 [DOI] [PubMed] [Google Scholar]
- 15.Fabiato A, Fabiato F. Effects of pH on the myofilaments and the sarcoplasmic reticulum of skinned cells from cardiace and skeletal muscles. J Physiol 276: 233–255, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fitts RH. Cellular mechanisms of muscle fatigue. Physiol Rev 74: 49–94, 1994 [DOI] [PubMed] [Google Scholar]
- 17.Fitts RH. The cross-bridge cycle and skeletal muscle fatigue. J Appl Physiol 104: 551–558, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Franks-Skiba K, Lardelli R, Goh G, Cooke R. Myosin light chain phosphorylation inhibits muscle fiber shortening velocity in the presence of vanadate. Am J Physiol Regul Integr Comp Physiol 292: R1603–R1612, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Godt RE, Nosek TM. Changes of intracellular milieu with fatigue or hypoxia depress contraction of skinned rabbit skeletal and cardiac muscle. J Physiol 412: 155–180, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greenberg MJ, Mealy TR, Watt JD, Jones M, Szczesna-Cordary D, Moore JR. The molecular effects of skeletal muscle myosin regulatory light chain phosphorylation. Am J Physiol Regul Integr Comp Physiol 297: R265–R274, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenberg MJ, Watt JD, Jones M, Kazmierczak K, Szczesna-Cordary D, Moore JR. Regulatory light chain mutations associated with cardiomyopathy affect myosin mechanics and kinetics. J Mol Cell Cardiol 46: 108–115, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo B, Guilford WH. Mechanics of actomyosin bonds in different nucleotide states are tuned to muscle contraction. Proc Natl Acad Sci USA 103: 9844–9849, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hibberd MG, Dantzig JA, Trentham DR, Goldman YE. Phosphate release and force generation in skeletal muscle fibers. Science 228: 1317–1319, 1985 [DOI] [PubMed] [Google Scholar]
- 24.Hill AV. The heat of shortening and the dynamic constants of muscle. Proc R Soc Lond B, 126: 136–195, 1938 [Google Scholar]
- 25.Hooft AM, Maki EJ, Cox KK, Baker JE. An accelerated state of myosin-based actin motility. Biochemistry 46: 3513–3520, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Kad NM, Patlak JB, Fagnant PM, Trybus KM, Warshaw DM. Mutation of a conserved glycine in the SH1-SH2 helix affects the load-dependent kinetics of myosin. Biophys J 92: 1623–1631, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karatzaferi C, Franks-Skiba K, Cooke R. Inhibition of shortening velocity of skinned skeletal muscle fibers in conditions that mimic fatigue. Am J Physiol Regul Integr Comp Physiol 294: R948–R955, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Karatzaferi C, Myburgh KH, Chinn MK, Franks-Skiba K, Cooke R. Effect of an ADP analog on isometric force and ATPase activity of active muscle fibers. Am J Physiol Cell Physiol 284: C816–C825, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Kentish JC. Combined inhibitory actions of acidosis and phosphate on maximum force production in rat skinned cardiac muscle. Pflügers Arch 419: 310–318, 1991 [DOI] [PubMed] [Google Scholar]
- 30.Laakso JM, Lewis JH, Shuman H, Ostap EM. Myosin I can act as a molecular force sensor. Science 321: 133–136, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levine RJ, Kensler RW, Yang Z, Stull JT, Sweeney HL. Myosin light chain phosphorylation affects the structure of rabbit skeletal muscle thick filaments. Biophys J 71: 898–907, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levine RJ, Yang Z, Epstein ND, Fananapazir L, Stull JT, Sweeney HL. Structural and functional responses of mammalian thick filaments to alterations in myosin regulatory light chains. J Struct Biol 122: 149–161, 1998 [DOI] [PubMed] [Google Scholar]
- 33.Metzger JM, Moss RL. Effects of tension and stiffness due to reduced pH in mammalian fast- and slow-twitch skinned skeletal muscle fibres. J Physiol 428: 737–750, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Metzger JM, Moss RL. Greater hydrogen ion-induced depression of tension and velocity in skinned single fibres of rat fast than slow muscles. J Physiol 393: 727–742, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Myburgh KH, Cooke R. Response of compressed skinned skeletal muscle fibers to conditions that simulate fatigue. J Appl Physiol 82: 1297–1304, 1997 [DOI] [PubMed] [Google Scholar]
- 36.Nosek TM, Fender KY, Godt RE. It is diprotonated inorganic phosphate that depresses force in skinned skeletal muscle fibers. Science 236: 191–193, 1987 [DOI] [PubMed] [Google Scholar]
- 37.Nyitrai M, Geeves MA. Adenosine diphosphate and strain sensitivity in myosin motors. Philos Trans R Soc Lond B Biol Sci 359: 1867–1877, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pate E, Bhimani M, Franks-Skiba K, Cooke R. Reduced effect of pH on skinned rabbit psoas muscle mechanics at high temperatures: implications for fatigue. J Physiol 486: 689–694, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pate E, Cooke R. A model of crossbridge action: the effects of ATP, ADP and Pi. J Muscle Res Cell Motil 10: 181–196, 1989 [DOI] [PubMed] [Google Scholar]
- 40.Pate E, Wilson GJ, Bhimani M, Cooke R. Temperature dependence of the inhibitory effects of orthovanadate on shortening velocity in fast skeletal muscle. Biophys J 66: 1554–1562, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patel JR, Diffee GM, Huang XP, Moss RL. Phosphorylation of myosin regulatory light chain eliminates force-dependent changes in relaxation rates in skeletal muscle. Biophys J 74: 360–368, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Persechini A, Stull JT, Cooke R. The effect of myosin phosphorylation on the contractile properties of skinned rabbit skeletal muscle fibers. J Biol Chem 260: 7951–7954, 1985 [PubMed] [Google Scholar]
- 43.Rayment I, Rypniewski WR, Schmidt-Base K, Smith R, Tomchick DR, Benning MM, Winkelmann DA, Wesenberg G, Holden HM. Three-dimensional structure of myosin subfragment-1: a molecular motor. Science 261: 50–58, 1993 [DOI] [PubMed] [Google Scholar]
- 44.Rosenfeld SS, Xing J, Whitaker M, Cheung HC, Brown F, Wells A, Milligan RA, Sweeney HL. Kinetic and spectroscopic evidence for three actomyosin:ADP states in smooth muscle. J Biol Chem 275: 25418–25426, 2000 [DOI] [PubMed] [Google Scholar]
- 45.Scholz T, Brenner B. Actin sliding on reconstituted myosin filaments containing only one myosin heavy chain isoform. J Muscle Res Cell Motil 24: 77–86, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Shulman RG. Glycogen turnover forms lactate during exercise. Exerc Sport Sci Rev 33: 157–162, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Siemankowski RF, Wiseman MO, White HD. ADP dissociation from actomyosin subfragment 1 is sufficiently slow to limit the unloaded shortening velocity in vertebrate muscle. Proc Natl Acad Sci USA 82: 658–662, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sleep J, Glyn H. Inhibition of myofibrillar and actomyosin subfragment 1 adenosinetriphosphatase by adenosine 5′-diphosphate, pyrophosphate, and adenyl-5′-yl imidodiphosphate. Biochemistry 25: 1149–1154, 1986 [DOI] [PubMed] [Google Scholar]
- 49.Sleep JA, Hutton RL. Exchange between inorganic phosphate and adenosine 5′-triphosphate in the medium by actomyosin subfragment 1. Biochemistry 19: 1276–1283, 1980 [DOI] [PubMed] [Google Scholar]
- 50.Stewart M, Franks-Skiba K, Cooke R. Myosin regulatory light chain phosphorylation inhibits shortening velocities of skeletal muscle fibers in the presence of the myosin inhibitor blebbistatin. J Muscle Res Cell Motil, 30: 17–27, 2009 [DOI] [PubMed] [Google Scholar]
- 51.Sweeney HL, Bowman BF, Stull JT. Myosin light chain phosphorylation in vertebrate striated muscle: regulation and function. Am J Physiol Cell Physiol 264: C1085–C1095, 1993 [DOI] [PubMed] [Google Scholar]
- 52.Sweeney HL, Kushmerick MJ. Myosin phosphorylation in permeabilized rabbit psoas fibers. Am J Physiol Cell Physiol 249: C362–C365, 1985 [DOI] [PubMed] [Google Scholar]
- 53.Sweeney HL, Stull JT. Alteration of cross-bridge kinetics by myosin light chain phosphorylation in rabbit skeletal muscle: implications for regulation of actin-myosin interaction. Proc Natl Acad Sci USA 87: 414–418, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Szczesna-Cordary D, Jones M, Moore JR, Watt J, Kerrick WG, Xu Y, Wang Y, Wagg C, Lopaschuk GD. Myosin regulatory light-chain E22K mutation results in decreased cardiac intracellular calcium and force transients. FASEB J, 21: 3974–3985, 2007 [DOI] [PubMed] [Google Scholar]
- 55.Szczesna D, Zhao J, Jones M, Zhi G, Stull J, Potter JD. Phosphorylation of the regulatory light chains of myosin affects Ca2+ sensitivity of skeletal muscle contraction. J Appl Physiol 92: 1661–1670, 2002 [DOI] [PubMed] [Google Scholar]
- 56.Trybus KM, Taylor EW. Transient kinetics of adenosine 5′-diphosphate and adenosine 5′-(β, γ-imidotriphosphate) binding to subfragment 1 and actosubfragment 1. Biochemistry 21: 1284–1294, 1982 [DOI] [PubMed] [Google Scholar]
- 57.Umazume Y, Onodera S, Higuchi H. Width and lattice spacing in radially compressed frog skinned muscle fibres at various pH values, magnesium ion concentrations and ionic strengths. J Muscle Res Cell Motil 7: 251–258, 1986 [DOI] [PubMed] [Google Scholar]
- 58.Veigel C, Coluccio LM, Jontes JD, Sparrow JC, Milligan RA, Molloy JE. The motor protein myosin-I produces its working stroke in two steps. Nature 398: 530–533, 1999 [DOI] [PubMed] [Google Scholar]
- 59.Veigel C, Molloy JE, Schmitz S, Kendrick-Jones J. Load-dependent kinetics of force production by smooth muscle myosin measured with optical tweezers. Nat Cell Biol 5: 980–986, 2003 [DOI] [PubMed] [Google Scholar]
- 60.Warshaw DM, Desrosiers JM, Work SS, Trybus KM. Smooth muscle myosin cross-bridge interactions modulate actin filament sliding velocity in vitro. J Cell Biol 111: 453–463, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wattanapermpool J, Reiser PJ, Solaro RJ. Troponin I isoforms and differential effects of acidic pH on soleus and cardiac myofilaments. Am J Physiol Cell Physiol 268: C323–C330, 1995 [DOI] [PubMed] [Google Scholar]
- 62.Yang Z, Stull JT, Levine RJ, Sweeney HL. Changes in interfilament spacing mimic the effects of myosin regulatory light chain phosphorylation in rabbit psoas fibers. J Struct Biol 122: 139–148, 1998 [DOI] [PubMed] [Google Scholar]