Abstract
To determine the effect of irbesartan treatment on resting levels and arterial baroreflex control of cardiac sympathetic nerve activity (CSNA) in heart failure (HF), we studied conscious normal sheep and sheep with HF induced by rapid ventricular pacing for 8–10 wk (n = 7 per group). In HF, there is a large increase in CSNA that is detrimental to outcome. The causes of this increase in CSNA and the effect of angiotensin receptor blockers on CSNA in HF are unclear. CSNA, arterial blood pressure, heart rate (HR), and arterial baroreflex curves were recorded during a resting period and after 90 min of irbesartan infusion (12 mg·kg−1·h−1 iv). This dose of irbesartan abolished the pressor response to intravenous ANG II infusion but caused only a slight decrease in the pressor response to centrally administered ANG II. In HF, there was a large increase in CSNA (from 44 ± 3 to 87 ± 3 bursts/100 heartbeats). Irbesartan reduced arterial pressure in the normal and HF groups, but the usual baroreflex-mediated increases in CSNA and HR were prevented. This resulted from a significant leftward shift in the CSNA and HR baroreflex curves in both groups. Irbesartan also decreased the sensitivity of the arterial baroreflex control of CSNA. Short-term treatment with an angiotensin receptor blocker, at a dose that abolished the response to circulating, but not central, ANG II, prevented the reflex increase in CSNA in response to the drug-induced fall in arterial pressure.
Keywords: angiotensin receptor blockade, baroreflex
heart failure (HF) is associated with intense neurohumoral activation, in particular, increased sympathetic nerve activity (SNA) and activation of the renin-angiotensin system (RAS). Although activation of these systems is initially beneficial, in the long term these changes are detrimental (2, 24). Drugs that act on the RAS are a mainstay of the treatment of patients with HF (3), but it is unclear whether these treatments have direct actions to reduce the increased cardiac SNA (CSNA) that occurs in HF.
Several clinical trials have shown that blockade of the RAS, using angiotensin AT1 receptor blockers (ARBs) and angiotensin-converting enzyme (ACE) inhibitors, reduces mortality and morbidity in patients with HF (1, 11, 25). These drugs decrease the deleterious effects of increased circulating ANG II, which are thought to result from its actions to cause vasoconstriction, renal sodium retention, cardiac fibrosis, and increased SNA (3, 9). The exact mechanisms of the beneficial effects of ARBs in HF patients, however, remain unclear. Chronic treatment with ACE inhibitors and ARBs has been shown to reduce measures of SNA in HF patients (13, 17, 30), but it is uncertain whether this is a direct effect of RAS inhibition or secondary to the hemodynamic improvement that occurs with these drugs. In addition, there is evidence that ARBs can cross the blood-brain barrier and inhibit the effect of ANG II in the brain (12, 26), where it has been shown to act to increase CSNA (35).
To address these questions, we have examined the effect of intravenous infusion of irbesartan on directly recorded CSNA in sheep with HF. As with other ARBs, there is evidence that irbesartan can cross the blood-brain barrier, although its ability to do so is less than that of candesartan (12) and has been shown to be similar to or less than that of losartan (4, 26). Thus we examined the effect of short-term infusion of irbesartan to minimize any central effects due to its crossing of the blood-brain barrier. Furthermore, there is evidence that chronic administration of ARBs in HF patients improved baroreflex control of heart rate (HR) (33), and short-term angiotensin receptor blockade in an experimental animal model of HF sensitized the baroreflex control of HR (22). The effects of RAS inhibition on CSNA have been assessed using 123I-meta-iodobenzylguanidine scintigraphy or cardiac norepinephrine spillover (2, 17), but these indirect techniques do not allow beat-to-beat measurement of CSNA and construction of baroreflex curves. Therefore, we also examined the effects of irbesartan treatment on the baroreflex control of CSNA and HR in HF.
METHODS
Adult merino ewes (35–49 kg body wt) were housed in individual metabolism cages with other sheep. Experiments were started when sheep were accustomed to laboratory conditions and human contact. Sheep were fed a diet of oaten chaff (800 g/day) and had ad libitum access to water. Experimental procedures were approved by the Animal Experimental Ethics Committee of the Howard Florey Institute in accordance with the Prevention of Cruelty to Animals Act 1986, under the guidelines of the National Health and Medical Research Council of Australia's Code of Practice for the Care and Use of Animals for Experimental Purposes, which conforms with the Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996).
Surgical procedures.
Before the studies, sheep underwent two aseptic surgical procedures, each separated by ≥2 wk. Anesthesia was induced with thiopental sodium (15 mg/kg iv) and, after intubation, was maintained with 1.5–2.0% isoflurane-O2. The first surgical procedure involved preparation of a carotid arterial loop in all sheep and, in those to be paced into HF, percutaneous insertion of a pacemaking lead (Medtronic, Minneapolis, MN) via the right jugular vein into the right ventricle. HF was induced by rapid ventricular pacing for 8–10 wk. In a further operation, intrafascicular electrodes were implanted into the left cardiothoracic nerves (34, 35). In all operations, animals were treated with antibiotics (900 mg of procaine penicillin; Ilium Propen, Troy Laboratories, NSW, Australia) at the start of surgery and then for 2 days postoperatively. Postsurgical analgesia was maintained with flunixin meglumine (1 mg/kg im; Troy Laboratories) at the start of surgery and 4 and 16 h after surgery. Experiments were conducted on standing, conscious sheep; to minimize any effect of surgical stress, experiments were not started until 4 days after implantation of the electrodes. On the day before implantation of recording electrodes, cannulas were inserted into the carotid artery and jugular vein for measurement of arterial pressure and infusion, respectively (34, 35).
Experimental protocols.
The development of HF was assessed by measurement of ejection fraction using short-axis M-wave echocardiography on conscious sheep lying on their right side. After placement of ventricular pacing leads, a basal measurement was made before the start of ventricular pacing at 200–220 beats/min. Echocardiography was then performed weekly with the pacing switched off immediately before the measurement. Sheep were considered to be in HF when ejection fraction had fallen to <40%.
CSNA was recorded differentially between the pair of electrodes with the best signal-to-noise ratio. The signal was amplified (×100,000) and filtered (band pass 300–1,000 Hz), displayed on an oscilloscope, and passed through an audio amplifier and loudspeaker. SNA (5,000 Hz) and arterial blood pressure (100 Hz) were recorded on computer using a CED micro 1401 interface and Spike 2 software (Cambridge Electronic Design, Cambridge, UK).
Recordings of CSNA were started 4 days after surgical implantation of cardiac sympathetic nerve electrodes, and experiments were conducted between 10:00 and 15:00. The pacing was switched off 30 min before the start of experiments and restarted at the end of each day's experiment. A 5-min recording of resting CSNA and arterial pressure was made in conscious sheep from the normal and HF groups. Then baroreflex curves were generated by measurement of the CSNA and HR responses to increases and decreases in arterial pressure induced by intravenous administration of phenylephrine hydrochloride (33, 67, 133, and 330 mg/min; Neosynephrine, Abbot Australasia, Kurnell, NSW, Australia) and sodium nitroprusside (42, 83, 167, and 417 mg/min; David Bull Laboratories, Mulgrave, Vic, Australia) for 1–2 min at each dose, with treatments given in random order (27, 34). After construction of baroreflex curves, irbesartan (Bristol-Myers Squibb, Australia) was infused (12 mg·kg−1·h−1 iv). After 90 min of infusion, the baroreflex was retested during the irbesartan infusion. The dose of irbesartan infused (12 mg·kg−1·h−1 for 90 min) was demonstrated to abolish the pressor response (+19 ± 6 mmHg) to an intravenous bolus of ANG II (0.2 μg, n = 4).
The ability of systemically infused irbesartan to cross the blood-brain barrier and block AT1 receptors within the blood-brain barrier was assessed in a separate group of animals. In five normal sheep, guide tubes were implanted above the lateral ventricles, as previously described (35). The mean arterial pressure and HR responses to intracerebroventricular infusion of ANG II (10 nmol·ml−1·h−1 for 30 min) were recorded before and after infusion of irbesartan (12 mg·kg−1·h−1 iv for 90 min).
Data analysis.
All data were analyzed on a beat-to-beat basis using custom-written routines in the Spike 2 program (Cambridge Electronic Design) (34, 35). For each heartbeat, the program determined diastolic blood pressure, systolic blood pressure, mean arterial blood pressure, heart period, and the number of discriminated spikes above threshold between the following diastolic pressures as a measure of burst size. The background noise was taken as the spikes per second during the highest dose of phenylephrine, when CSNA was abolished, and this value was subtracted from the data collected on that day. We demonstrated previously that counting discriminated spikes per burst as a measure of sympathetic activity gives results similar to integration and rectification of the sympathetic signal (28). Burst frequency was calculated as the percentage of heartbeats that included spikes of activity above background. The accuracy of burst determination was checked by eye over the 5-min control data for each sheep.
Baroreceptor relations were constructed from data collected during infusion of phenylephrine and nitroprusside (34). CSNA was plotted against diastolic blood pressure, because it has a closer correlation to sympathetic activity than either systolic or mean arterial pressure (29). A four-parameter sigmoidal logistic equation was used to fit the data (SigmaPlot version 8.0, SPSS). Variables from the equations of all the graphs were grouped for the normal and HF animals, and average baroreflex relations were plotted.
Values are means ± SE. Data were analyzed using ANOVA (SigmaStat version 2.03, Access Softek). If significant, post hoc hypotheses were tested for significance, as planned beforehand, with the Dunn-Sidak method used for protection against multiple comparisons. P < 0.05 was considered statistically significant.
RESULTS
Comparison of normal and HF groups.
Left ventricular ejection fraction and fractional shortening, measured in conscious sheep by echocardiography, gradually decreased over the 8–10 wk of rapid ventricular pacing at 200–220 beats/min. In the HF animals, at 1–2 days before implantation of recording electrodes, ejection fraction (36 ± 3%) and fractional shortening (15 ± 2%) were significantly reduced compared with the prepacing values (82 ± 3% and 52 ± 2%, respectively, both P < 0.001). Pacing-induced left ventricular dilatation was demonstrated by the increases in end-systolic diameter (from 1.6 ± 0.2 to 3.5 ± 0.3 cm, P < 0.01) and end-diastolic diameter (from 3.2 ± 0.3 to 4.1 ± 0.4 cm, P < 0.05).
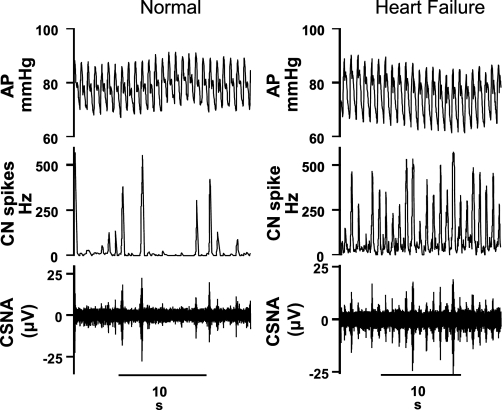
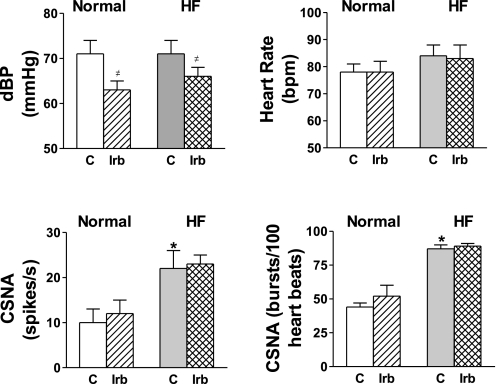
The resting level of CSNA was significantly higher in the HF group than in the normal group when assessed as burst incidence (87 ± 3 vs. 44 ± 3 bursts/100 heartbeats) or as total nerve activity (22 ± 4 vs. 10 ± 3 spikes/s, n = 7 per group; Figs. 1 and 2). There were no significant differences in the resting levels of systolic pressure, diastolic pressure, or HR between the normal and HF groups (Fig. 2). In HF, the baroreflex control of HR was impaired, with reductions in the maximum gain and top plateau compared with normal animals, but the baroreflex control of CSNA was not significantly altered (Fig. 3, Table 1).
Fig. 1.
Recordings of arterial pressure (AP), cardiac sympathetic nerve activity (CSNA), and cardiac nerve (CN) spikes in a conscious normal sheep and a sheep in heart failure (HF). Note much higher burst incidence of CSNA in the HF than in the normal sheep.
Fig. 2.
Resting levels of diastolic blood pressure (dBP), heart rate, CSNA, and CSNA burst incidence recorded in conscious normal and HF sheep during the control period (C) and after 90 min of irbesartan (Irb) infusion. Values are means ± SE (n = 7 in both groups). *P < 0.05 vs. normal. #P < 0.05 within groups (C vs. Irb).
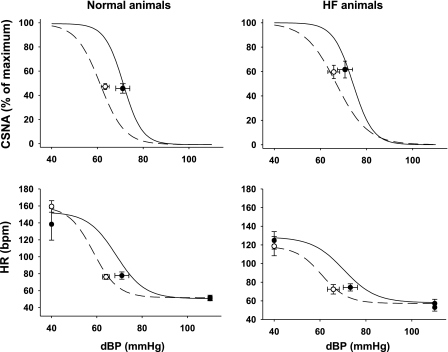
Fig. 3.
Arterial baroreflex curves for CSNA and heart rate (HR) during the control period (solid line, black circles) and after intravenous infusion of irbesartan (dashed line, white circles) in normal and HF sheep. CSNA is presented as percentage of maximum activity measured when arterial pressure was reduced with sodium nitroprusside. Resting points were taken from 5 min of recording during control periods. Values are means ± SE (n = 7 in both groups).
Table 1.
Baroreflex parameters for normal and HF groups before and after intravenous irbesartan
| Normal |
HF |
|||
|---|---|---|---|---|
| Control | After irbesartan | Control | After irbesartan | |
| dBP vs. HR | ||||
| Maximum slope | −4.2 ± 0.6 | −5.3 ± 0.8 | −2.78 ± 0.3* | −3.14 ± 0.6 |
| Top plateau | 153 ± 13 | 159 ± 7 | 125 ± 9* | 118 ± 10 |
| Bottom plateau | 51 ± 3 | 52 ± 3 | 53 ± 4 | 57 ± 4 |
| BP50 | 69 ± 3 | 58 ± 2† | 70 ± 4 | 61 ± 2† |
| Threshold | 84 ± 3 | 74 ± 3† | 91 ± 4 | 77 ± 4† |
| Saturation | 53 ± 5 | 43 ± 2† | 50 ± 6 | 44 ± 2 |
| dBP vs. CSNA | ||||
| Maximum slope | −9.9 ± 3.2 | −6.0 ± 1.3 | −6.9 ± 1.1 | −4.3 ± 0.6† |
| BP50 | 70 ± 2 | 61 ± 2† | 74 ± 4 | 67 ± 4† |
| Threshold | 83 ± 4 | 77 ± 3 | 87 ± 4 | 87 ± 7 |
| Saturation | 59 ± 4 | 46 ± 3† | 61 ± 5 | 47 ± 5† |
Values are means ± SE (n = 7). HF, heart failure; HR, heart rate; CSNA, cardiac sympathetic nerve activity; dBP, diastolic blood pressure; BP50, pressure at 50% of maximum CSNA.
P < 0.05 vs. normal.
P < 0.05 vs. control.
Effects of intravenous irbesartan in the normal group.
Irbesartan infusion significantly decreased mean arterial pressure (from 80 ± 3 to 75 ± 2 mmHg, P < 0.05), systolic pressure (from 100 ± 3 to 93 ± 2 mmHg, P < 0.05), and diastolic pressure (from 71 ± 3 to 63 ± 2 mmHg, P < 0.05) in the normal group, but this did not result in increases in CSNA or HR (Fig. 2). In the normal group, irbesartan caused a significant reduction in the pressure at 50% of maximum CSNA (BP50), indicating a leftward shift of the baroreflex curve (Table 1, Fig. 3), but the maximum gain of the CSNA baroreflex curve was unchanged. Irbesartan also caused a leftward shift in the HR baroreflex curve, as shown by the significant reduction in BP50, but had no effect on the maximum gain (Table 1, Fig. 3).
Effects of intravenous irbesartan in the HF group.
In the HF group, irbesartan significantly decreased mean arterial pressure (from 78 ± 4 to 73 ± 1 2 mmHg, P < 0.05) and diastolic pressure (from 71 ± 3 to 66 ± 2 mmHg, P < 0.05), but the fall in systolic pressure was not significant (from 93 ± 4 to 90 ± 2 mmHg). These changes were not accompanied by a reflex increase in HR or CSNA (Fig. 2). As in the normal group, in the HF group, irbesartan caused a leftward shift in the CSNA and HR baroreflex curves, indicated by the reductions in the BP50 for each curve (Table 1, Fig. 3). In contrast to the normal animals, in the HF group, irbesartan decreased the maximum gain of the CSNA baroreflex curve (Table 1, Fig. 3).
Response to central administration of ANG II during intravenous infusion of irbesartan.
During the control period, intracerebroventricular infusion of ANG II (10 nmol·ml−1·h−1) increased mean arterial pressure by 15 ± 1 mmHg. After intravenous infusion of irbesartan (12 mg·kg−1·h−1 for 90 min), the pressor response to intracerebroventricular ANG II was slightly, but significantly, reduced (12 ± 1 mmHg, P < 0.05).
DISCUSSION
This is the first study to examine the effects of systemic administration of an ARB on directly recorded CSNA in conscious animals in the normal healthy state and in HF. The major finding was that, in the normal state and in HF, short-term intravenous infusion of irbesartan caused a leftward shift in the CSNA and HR arterial baroreflex curves, so that the decreases in diastolic and mean arterial pressures caused by treatment did not induce reflex increases in CSNA or HR. In HF, irbesartan also decreased the maximum gain of the CSNA baroreflex curve but did not change the baroreflex control of HR. In addition, the results confirm our previous reports of a large increase in CSNA in HF, which occurred in the presence of normal arterial baroreflex control of CSNA but desensitized baroreflex control of HR (27, 34).
Effects of irbesartan on resting CSNA.
The finding that irbesartan decreased mean arterial pressure without inducing a reflex increase in CSNA in HF has important clinical implications in light of the extensive use of ARBs in HF and the well-established detrimental effect of increased sympathetic drive to the heart in HF (19). In the HF group, irbesartan reduced diastolic pressure by 5 ± 1 mmHg, but this baroreceptor unloading did not cause the expected reflex increase in CSNA. Calculated from the individual arterial baroreceptor curves from untreated HF animals, this decrease in diastolic pressure would normally increase CSNA by 22 ± 7% of the maximum level seen when arterial pressure was lowered by sodium nitroprusside (Fig. 3). Irbesartan had a similar effect in normal animals, which is consistent with the finding that, in normal subjects, intravenous enalapril reduced mean arterial pressure after 30–40 min but did not change cardiac norepinephrine spillover, although renal norepinephrine spillover increased (15). In two further studies of the acute effects of ACE inhibition, it was found that, in the presence of a decrease in blood pressure, muscle SNA was unchanged in normal subjects but reduced in HF patients (6, 23). In patients with HF, a number of studies used 123I-meta-iodobenzylguanidine scintigraphy as a measure of CSNA to examine the effect of 6–9 mo of treatment with ARBs or ACE inhibitors (16–18, 30, 32). In all cases, these studies demonstrated a reduction in this index of CSNA, but it is important to note that the treatments were associated with improved cardiac function, which would have acted independently to reduce CSNA.
The mechanisms by which systemically administered ARBs and ACE inhibitors reduce reflex increases in CSNA are not well defined. It is unlikely that the reduction in cardiac filling pressures that occurs with RAS inhibition (15) is responsible, since we have shown that, in the normal state, this acts to increase CSNA and, in HF, this reflex is abolished (28). Our finding that the dose of irbesartan had little effect on the pressor response to centrally administered angiotensin indicates that only a minor part of the response to irbesartan is likely to be due to its inhibition of angiotensin AT1 receptors within the blood-brain barrier. It is possible that the effect of irbesartan depends on inhibition of an action of ANG II on the circumventricular organs, areas outside the blood-brain barrier, including the lamina terminalis and area postrema. Indeed, there is extensive evidence that ANG II acts on the area postrema to reduce the sensitivity of the arterial baroreflex (20, 31). However, in the present study, angiotensin receptor blockade decreased the sensitivity of the baroreflex control of CSNA in HF sheep, although not in normal sheep.
Effects of irbesartan on the arterial baroreflex control of CSNA and HR.
The leftward shift in the CSNA and HR baroreflex curves caused by irbesartan in the normal and HF groups largely accounted for the lack of baroreflex-mediated increases in CSNA and HR in response to the decreased mean arterial pressure. Irbesartan also decreased the maximum baroreflex gain of CSNA in sheep with HF, whereas angiotensin receptor blockade in rabbits with HF increased the maximum gain of the baroreflex control of renal SNA (21). This difference may reflect the finding that HF is associated with impaired baroreflex control of renal SNA in rabbits (7, 36), whereas the baroreflex control of CSNA in sheep remains intact (34). The present finding that the arterial baroreceptor control of HR was desensitized in HF sheep is in accord with similar findings in HF patients (8, 10). The reported effects of long-term RAS blockade on the baroreflex control of HR in clinical studies are variable: both improvement and no effect have been reported (5, 14, 33). The enhancement in the baroreflex control of HR in HF patients may be related to inhibition of the direct actions of ANG II or treatment-induced improved cardiac function. In the present study, short-term angiotensin receptor blockade did not improve the sensitivity of the baroreflex control of HR, although in rabbits with HF it did partially restore the gain of the reflex (22). Long-term studies are required to determine whether chronic ARB treatment decreases resting levels of CSNA, which may result from an improvement in cardiac function and/or inhibition of central AT1 receptors due to increased amounts of drug crossing the blood-brain barrier with prolonged treatment.
Perspectives and Significance
It is well established that SNA is increased in HF, with the degree and timing of activation varying between different organs, indicating that multiple factors act at different times to stimulate activity in individual sympathetic nerves. Although the mechanisms are not well understood, previous studies have indicated that the activated RAS in HF plays an important role in determining the increased level of renal SNA. Our studies have focused on the factors leading to the increased CSNA in HF, which is particularly harmful, inasmuch as it predisposes to arrhythmias and sudden death. The present study indicates that, in HF, inhibition of angiotensin AT1 receptors with irbesartan reduced mean arterial pressure and shifted the CSNA baroreflex curve to the left, leading to a lack of a reflex-induced increase in CSNA. This finding may be clinically important because of the widespread use of ARBs in HF and the well-documented detrimental actions of cardiac sympathoexcitation in HF. Further experiments are required to establish the site at which irbesartan acts to modulate the baroreflex control of CSNA in HF.
GRANTS
This work was supported by National Health and Medical Research Council of Australia Grants 232313 and 509204 and National Heart, Lung, and Blood Institute Grant 5-R01 HL-074932. R. Ramchandra was the recipient of National Heart Foundation Postdoctoral Fellowship 07M 3293, and C. N. May was supported by National Health and Medical Research Council Research Fellowships 350328 and 566819.
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGEMENTS
The authors acknowledge the expert technical assistance of Alan McDonald and Tony Dornom.
Present address of A. M. D. Watson: Baker IDI Heart and Diabetes Institute, Melbourne, Victoria, Australia.
REFERENCES
- 1.Anonymous. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS) The CONSENSUS Trial Study Group. N Engl J Med 316: 1429–1435, 1987 [DOI] [PubMed] [Google Scholar]
- 2.Brunner-La Rocca HP, Esler MD, Jennings GL, Kaye DM. Effect of cardiac sympathetic nervous activity on mode of death in congestive heart failure. Eur Heart J 22: 1136–1143, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Brunner-La Rocca HP, Vaddadi G, Esler MD. Recent insight into therapy of congestive heart failure: focus on ACE inhibition and angiotensin-II antagonism. J Am Coll Cardiol 33: 1163–1173, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Culman J, von Heyer C, Piepenburg B, Rascher W, Unger T. Effects of systemic treatment with irbesartan and losartan on central responses to angiotensin II in conscious, normotensive rats. Eur J Pharmacol 367: 255–265, 1999 [DOI] [PubMed] [Google Scholar]
- 5.De Tommasi E, Iacoviello M, Romito R, Ceconi C, Guida P, Massari F, Francolini G, Bertocchi F, Ferrari R, Rizzon P, Pitzalis MV. Comparison of the effect of valsartan and lisinopril on autonomic nervous system activity in chronic heart failure. Am Heart J 146: E17, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Dibner-Dunlap ME, Smith ML, Kinugawa T, Thames MD. Enalaprilat augments arterial and cardiopulmonary baroreflex control of sympathetic nerve activity in patients with heart failure. J Am Coll Cardiol 27: 358–364, 1996 [DOI] [PubMed] [Google Scholar]
- 7.DiBona GF, Sawin LL. Reflex regulation of renal nerve activity in cardiac failure. Am J Physiol Regul Integr Comp Physiol 266: R27–R39, 1994 [DOI] [PubMed] [Google Scholar]
- 8.Eckberg DL, Drabinsky M, Braunwald E. Defective cardiac parasympathetic control in patients with heart disease. N Engl J Med 285: 877–883, 1971 [DOI] [PubMed] [Google Scholar]
- 9.Esler M. Differentiation in the effects of the angiotensin II receptor blocker class on autonomic function. J Hypertens Suppl 20: S13–S19, 2002 [PubMed] [Google Scholar]
- 10.Ferguson DW, Berg WJ, Roach PJ, Oren RM, Mark AL. Effects of heart failure on baroreflex control of sympathetic neural activity. Am J Cardiol 69: 523–531, 1992 [DOI] [PubMed] [Google Scholar]
- 11.Garg R, Yusuf S. Overview of randomized trials of angiotensin-converting enzyme inhibitors on mortality and morbidity in patients with heart failure. Collaborative Group on ACE Inhibitor Trials. JAMA 273: 1450–1456, 1995 [PubMed] [Google Scholar]
- 12.Gohlke P, Kox T, Jurgensen T, von Kugelgen S, Rascher W, Unger T, Culman J. Peripherally applied candesartan inhibits central responses to angiotensin II in conscious rats. Naunyn Schmiedebergs Arch Pharmacol 365: 477–483, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Grassi G, Cattaneo BM, Seravalle G, Lanfranchi A, Pozzi M, Morganti A, Carugo S, Mancia G. Effects of chronic ACE inhibition on sympathetic nerve traffic and baroreflex control of circulation in heart failure. Circulation 96: 1173–1179, 1997 [DOI] [PubMed] [Google Scholar]
- 14.Hikosaka M, Yuasa F, Yuyama R, Mimura J, Kawamura A, Motohiro M, Iwasaki M, Sugiura T, Iwasaka T. Candesartan and arterial baroreflex sensitivity and sympathetic nerve activity in patients with mild heart failure. J Cardiovasc Pharmacol 40: 875–880, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Johansson M, Rundqvist B, Petersson M, Lambert G, Friberg P. Regional norepinephrine spillover in response to angiotensin-converting enzyme inhibition in healthy subjects. J Hypertens 21: 1371–1375, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Kasama S, Toyama T, Hatori T, Sumino H, Kumakura H, Takayama Y, Ichikawa S, Suzuki T, Kurabayashi M. Comparative effects of valsartan and enalapril on cardiac sympathetic nerve activity and plasma brain natriuretic peptide in patients with congestive heart failure. Heart 92: 625–630, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kasama S, Toyama T, Kumakura H, Takayama Y, Ichikawa S, Suzuki T, Kurabayashi M. Effects of candesartan on cardiac sympathetic nerve activity in patients with congestive heart failure and preserved left ventricular ejection fraction. J Am Coll Cardiol 45: 661–667, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Kasama S, Toyama T, Kumakura H, Takayama Y, Ichikawa S, Suzuki T, Kurabayashi M. Effects of perindopril on cardiac sympathetic nerve activity in patients with congestive heart failure: comparison with enalapril. Eur J Nucl Med Mol Imag 32: 964–971, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Kaye DM, Lefkovits J, Jennings GL, Bergin P, Broughton A, Esler MD. Adverse consequences of high sympathetic nervous activity in the failing human heart. J Am Coll Cardiol 26: 1257–1263, 1995 [DOI] [PubMed] [Google Scholar]
- 20.Lumbers ER, McCloskey DI, Potter EK. Inhibition by angiotensin II of baroreceptor-evoked activity in cardiac vagal efferent nerves in the dog. J Physiol 294: 69–80, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murakami H, Liu JL, Zucker IH. Angiotensin II blockade [corrected] enhances baroreflex control of sympathetic outflow in heart failure. Hypertension 29: 564–569, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Murakami H, Liu JL, Zucker IH. Blockade of AT1 receptors enhances baroreflex control of heart rate in conscious rabbits with heart failure. Am J Physiol Regul Integr Comp Physiol 271: R303–R309, 1996 [DOI] [PubMed] [Google Scholar]
- 23.Noll G, Wenzel RR, de Marchi S, Shaw S, Luscher TF. Differential effects of captopril and nitrates on muscle sympathetic nerve activity in volunteers. Circulation 95: 2286–2292, 1997 [DOI] [PubMed] [Google Scholar]
- 24.Petersson M, Friberg P, Eisenhofer G, Lambert G, Rundqvist B. Long-term outcome in relation to renal sympathetic activity in patients with chronic heart failure. Eur Heart J 26: 906–913, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Pfeffer MA, Braunwald E, Moye LA, Basta L, Brown EJ, Jr, Cuddy TE, Davis BR, Geltman EM, Goldman S, Flaker GCet al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the Survival and Ventricular Enlargement Trial. The SAVE Investigators. N Engl J Med 327: 669–677, 1992 [DOI] [PubMed] [Google Scholar]
- 26.Polidori C, Ciccocioppo R, Nisato D, Cazaubon C, Massi M. Evaluation of the ability of irbesartan to cross the blood-brain barrier following acute intragastric treatment. Eur J Pharmacol 352: 15–21, 1998 [DOI] [PubMed] [Google Scholar]
- 27.Ramchandra R, Hood SG, Denton DA, Woods RL, McKinley MJ, McAllen RM, May CN. Basis for the preferential activation of cardiac sympathetic nerve activity in heart failure. Proc Natl Acad Sci USA 106: 924–928, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramchandra R, Hood SG, Watson AM, May CN. Responses of cardiac sympathetic nerve activity to changes in circulating volume differ in normal and heart failure sheep. Am J Physiol Regul Integr Comp Physiol 295: R719–R726, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sundlof G, Wallin BG. Human muscle nerve sympathetic activity at rest. Relationship to blood pressure and age. J Physiol 274: 621–637, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takeishi Y, Atsumi H, Fujiwara S, Takahashi K, Tomoike H. ACE inhibition reduces cardiac iodine-123-MIBG release in heart failure. J Nucl Med 38: 1085–1089, 1997 [PubMed] [Google Scholar]
- 31.Tan PS, Killinger S, Horiuchi J, Dampney RA. Baroreceptor reflex modulation by circulating angiotensin II is mediated by AT1 receptors in the nucleus tractus solitarius. Am J Physiol Regul Integr Comp Physiol 293: R2267–R2278, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Tsutamoto T, Tanaka T, Sakai H, Nishiyama K, Fujii M, Yamamoto T, Nakae I, Ohnishi M, Wada A, Horie M. Beneficial effect of perindopril on cardiac sympathetic nerve activity and brain natriuretic peptide in patients with chronic heart failure: comparison with enalapril. Circ J 72: 740–746, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Vaile JC, Chowdhary S, Osman F, Ross HF, Fletcher J, Littler WA, Coote JH, Townend JN. Effects of angiotensin II (AT1) receptor blockade on cardiac vagal control in heart failure. Clin Sci (Lond) 101: 559–566, 2001 [PubMed] [Google Scholar]
- 34.Watson AM, Hood SG, Ramchandra R, McAllen RM, May CN. Increased cardiac sympathetic nerve activity in heart failure is not due to desensitization of the arterial baroreflex. Am J Physiol Heart Circ Physiol 293: H798–H804, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Watson AM, Mogulkoc R, McAllen RM, May CN. Stimulation of cardiac sympathetic nerve activity by central angiotensinergic mechanisms in conscious sheep. Am J Physiol Regul Integr Comp Physiol 286: R1051–R1056, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Zucker IH, Wang W. Reflex control of renal sympathetic nervous activity in heart failure. Herz 16: 82–91, 1991. [PubMed] [Google Scholar]