Abstract
This study examined mechanisms by which immune cells participate in the development of hypertension and renal disease in Dahl salt-sensitive (SS) rats. Increasing dietary salt from 0.4% to 4.0% NaCl significantly increased renal infiltration of T lymphocytes from 8.8 ± 1.2 × 105 to 14.4 ± 2.0 × 105 cells/2 kidneys, increased arterial blood pressure from 131 ± 2 to 165 ± 6 mmHg, increased albumin excretion rate from 17 ± 3 to 129 ± 20 mg/day, and resulted in renal glomerular and tubular damage. Furthermore, renal tissue ANG II was not suppressed in the kidneys of SS rats fed 4.0% NaCl. Administration of the immunosuppressive agent mycophenolate mofetil (MMF; 20 mg·kg−1·day−1) prevented the infiltration of T lymphocytes and attenuated Dahl SS hypertension and renal disease. In contrast to vehicle-treated rats, Dahl SS rats administered MMF demonstrated a suppression of renal tissue ANG II from 163 ± 26 to 88 ± 9 pg/g of tissue when fed high salt. Finally, it was demonstrated that the T lymphocytes isolated from the kidney possess renin and angiotensin-converting enzyme activity. These data indicate that infiltrating T cells are capable of participating in the production of ANG II and are associated with increased intrarenal ANG II, hypertension, and renal disease. The suppression of T-cell infiltration decreased intrarenal ANG II and prevented Dahl SS hypertension and kidney damage. As such, infiltrating cells are capable of participating in the established phase of Dahl SS hypertension.
Keywords: sodium-dependent hypertension, rats, blood pressure
a number of experimental studies in the past several decades have indicated that the immune system may play a role in hypertension (7, 9, 15, 16, 18, 40). Studies in which animals received a thymectomy (17, 46), antithymocyte serum (3), immunosuppressant therapy (2), or genetic manipulation of T and B lymphocytes (11), all indicated that the immune system was a permissive or causative factor in the development of hypertension. Moreover, it has been recognized that the infiltration or activation of immune cells in the kidney may participate in the pathogenesis of salt-sensitive hypertension and/or kidney disease (15, 16, 33, 39, 45).
The localization and characterization of different immune cell types infiltrating the renal interstitial space in nonimmune models of hypertension and kidney disease have been documented by a number of different groups (24, 28, 33, 39, 42, 45). Both lymphocytes and macrophages have been localized in the renal interstitium of different rat models, including hypertension induced by genetic or experimental elevation of ANG II (23, 28, 33), two-kidney, one-clip hypertension (24), l-NAME-induced hypertension (36), and genetically hypertensive rats (42). Moreover, treatment with immunosuppressive drugs has proven beneficial to reduce arterial blood pressure and the severity of kidney damage in a number of different experimental and/or genetic models of rodent hypertension (1, 3, 4, 17, 28, 36, 39, 41, 42).
Immunohistochemical and immunoblotting studies have characterized the infiltration of macrophages and lymphocytes (28, 33, 39, 45), as well as cells staining positive for superoxide (8, 42, 39) and angiotensin (8, 36, 41, 39) in the kidneys of hypertensive rats. Though the different cell types have been characterized, the mechanisms by which these immune cells lead to elevated levels of arterial blood pressure and renal end-organ damage is not clear. It has been proposed that the infiltration of immune cells leads to oxidative stress, and/or increased release of ANG II (8, 39, 40). This hypothesis is consistent with results of other studies that have indicated that 1) intrarenal ANG II is increased in hypertension by a mechanism that is not modulated by standard physiological stimuli (8, 20, 31), 2) angiotensin-converting enzyme (ACE) inhibition or angiotensin II type 1 (AT1) receptor blockade attenuates renal damage (12, 21, 32) and/or the elevation of arterial blood pressure (13, 22) in Dahl salt-sensitive (SS) hypertensive rats fed a high-salt diet, and 3) clinical observations that have demonstrated the beneficial effects of AT1 receptor blockers or ACE inhibitors for the treatment of renal disease and hypertension in humans (5, 34, 47).
Recent experiments have demonstrated that treatment of Dahl SS rats with immunosuppressive agents leads to the attenuation of sodium-dependent hypertension and kidney damage. Associated with the immunosuppressive therapy was a decrease in markers related to T lymphocytes in the kidney tissue (25). The present studies were performed to determine mechanisms by which infiltrating T lymphocytes may mediate hypertension and associated kidney damage in Dahl SS rats fed a high-salt diet. Experiments in this manuscript tested the hypothesis that renal infiltration of immune cells following an increase in salt intake mediates the further development of hypertension and renal damage by increasing intrarenal levels of ANG II.
METHODS
Experimental animals.
Experiments were performed on male Dahl SS rats (SS/JrHSDMcwi) obtained from a colony maintained at the Medical College of Wisconsin. One group of Dahl SS rats, used to measure blood pressure in protocol 1, were purchased from Charles River Laboratories (SS/JrHsdMcwiCrl). The breeders and weanlings were fed purified AIN-76A rodent diet (Dyets, Bethlehem, PA) containing 0.4% NaCl. At 9 wk of age, the salt content of the chow was increased to 4.0% NaCl for 3 wk in some of the rats; other rats were maintained on the 0.4% NaCl chow throughout the study. The Medical College of Wisconsin Institutional Animal Care and Use Committee approved all experimental protocols.
Surgical preparation.
In survival surgical procedures, the rats were deeply anesthetized with a mixture of ketamine (50 mg/kg im), acepromazine (5 mg/kg im), and xylazine (5 mg/kg im). Supplemental anesthesia was administered as needed. Using an aseptic technique, we implanted a telemetry transmitter (Data Sciences International, St. Paul, MN) for measuring arterial blood pressure with the catheter in the femoral artery, and the body of the transmitter was implanted subcutaneously on the animal's flank. The rats were kept warm during and following surgery on a specially designed warming table. Analgesics and antibiotics were administered postoperatively to control pain and infection. The rats were allowed to recover for 5–7 days before the experimental protocol.
T-cell isolation.
Isolation of infiltrating cells was performed using a modification of methods that we have previously described (27). Briefly, rats were anesthetized with pentobarbital sodium (50 mg/kg ip), the abdominal aorta was isolated and cannulated, and the kidneys were perfused with a solution containing 154 mM NaCl and 100 Units/ml heparin. The kidneys were removed and cut into 1- to 2-mm-thick sections; the sections were incubated at 37 C for 60 min in dissection solution containing (in mM) 135 NaCl, 3 KCl, 2 KH2PO4, 5.5 glucose, 20 HEPES (pH 7.2), and 0.85 mg/ml collagenase (573 Units/mg; Sigma, St. Louis, MO). During incubation, the samples were gently shaken and bubbled with 95% O2-5% CO2. The digested solution was then filtered through 100- and 70-μm filters and centrifuged at 300 g for 10 min. The pellet was resuspended in PBS containing DNase 1 (20 units/ml). The isolated cells were layered over 5 ml of Histopaque (Sigma), centrifuged at 400 g for 30 min at room temperature, and the mononuclear cell layer was collected. The separated mononuclear cells were resuspended and washed in PBS, counted, and incubated with a rat Pan T-cell antibody coupled to magnetic microbeads (MACS Rat Pan T Cell Microbeads, Miltenyi Biotec, Gladbach, Germany) for 15 min at 4–8°C. The cells were then washed, resuspended in labeling buffer, and the cell suspension was applied to a magnetic column (MACS Separation Columns; Miltenyi Biotec) to isolate T lymphocytes.
Histological analysis and immunohistochemistry of kidney tissues.
Rats were deeply anesthetized with pentobarbital sodium (50 mg/kg ip); the kidneys were removed, bisected, and placed in 10% formaldehyde in phosphate buffer. The tissue was paraffin embedded (Microm HMP 300), cut in 3-μm sections (Microm HM355S), mounted on silanized/charged slides, and stained with Gomori's One-Step Trichrome. Slides were photographed using a Nikon E-400 fitted with a Spot Insight camera; digital micrographs were obtained at different magnifications. Individual glomeruli (30–40 per rat) were evaluated using a modification of the semiquantitative index method of Raij et al. (37); glomeruli were scored from 0 (best) to 4 (worst) on the basis of glomerulosclerosis and mesangial expansion (25, 26). The percentage of the outer medullary tissue containing dilated tubules was quantified by determining the proportion of red-stained structures in this region using Metamorph Image Analysis software (ver. 4.6, Universal Imaging Systems) (25, 26).
Immunohistochemistry was performed on tissue sections to localize T lymphocytes in the kidney. The sections were prepared as described above, deparaffinized with xylene and ethanol, and incubated with Proteinase K for antigen retrieval. Endogenous biotin and peroxidase activity was blocked by incubation with avidin and biotin, and hydrogen peroxide, respectively. The primary monoclonal antibodies used to detect T cells were anti-CD43 (Abcam, Cambridge, MA) or anti-CD3 (BD Pharmingen, San Diego, CA). A biotinylated horse anti-mouse secondary antibody was used for development with avidin-biotinylated horseradish peroxidase complex (Vectastain ABC kits; Vector Laboratory, Burlingame, CA) and 0.02% H2O2 + 0.1% diaminobenzidine tetrahydrochloride (DAB). The slides were lightly counterstained with aniline blue dye and photographed under the microscope.
Renin, ANG II, and ACE measurements.
All assays were performed as previously described (6). Plasma renin activity was measured by RIA using a modification of the method of Sealey and Laragh (44) with nephrectomized rat plasma used as the substrate for plasma renin concentration measurements. Plasma ANG II was measured by RIA following HPLC separation of ANG I, ANG II, and the primary angiotensin metabolites due to the strong antibody cross reactivity with the other metabolites. The comparison of the total concentration of ANG II-like fragments [including ANG II-(2–8), ANG II-(3–8), and ANG II-(4–8)] indicates that there is as much as a five-fold greater concentration in plasma of these fragments compared with ANG II-(1–8). As previously reported for this assay, the recovery of [3H] ANG II from plasma processed through the entire extraction and HPLC separation procedure averaged 83% (38). Renal tissue ANG II was measured in samples obtained from pentobarbital sodium-anesthetized rats (50 mg/kg ip) that were snap-frozen on dry ice and stored at −80°C. The tissue samples were homogenized in a large volume (∼10 ml/25 mg) of ice-cold methanol. The homogenate obtained from the tissue samples was centrifuged, and the supernatant was collected and dried (Speedvac; Savant Instruments, Nassau-Suffolk, NY). ANG II was measured by RIA following HPLC separation of ANG I, ANG II, and the primary angiotensin metabolites as described above. Tissue ACE activity was measured by assessing the conversion of Hip-His-Leu to His-Leu using an adaptation of the protocol developed by Santos (43).
For protocol 1, the influence of elevated sodium intake on blood pressure and renal damage was assessed in Dahl SS rats fed 0.4% or 4.0% NaCl chow for 3 wk. Further studies examined the renal histological changes and the infiltration of T lymphocytes into the kidney of Dahl SS rats fed 0.4% or 4.0% NaCl.
For protocol 2, the influence of treatment with the immunosuppressive drug mycophenolate mofetil (MMF) or dextrose vehicle during the 3-wk period of elevated sodium intake on blood pressure, and renal damage was assessed in Dahl SS rats. Additional experiments assessed the renal infiltration of T lymphocytes and the renal tissue level of ANG II in vehicle and MMF-treated Dahl SS rats fed 0.4% or 4.0% NaCl chow. A final set of experiments assessed renin activity in T lymphocytes isolated from the kidneys of Dahl SS rats fed 0.4% or 4.0% NaCl chow for 3 wk.
RESULTS
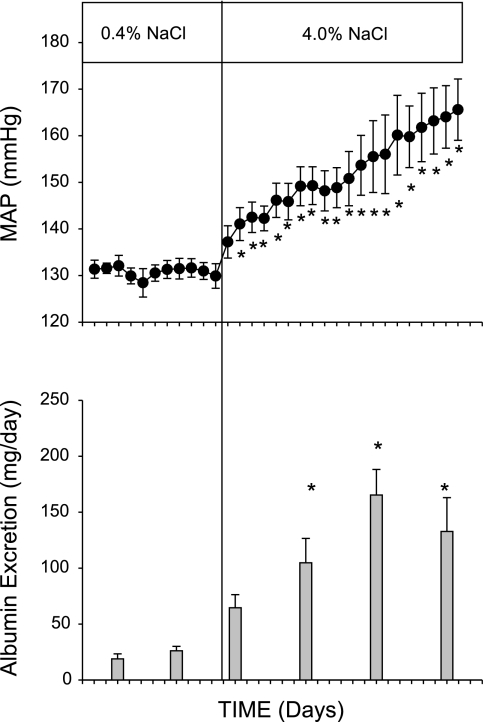
Figure 1 illustrates the changes in arterial blood pressure, assessed by 24 h/day telemetry measurements, and renal injury, assessed by albumin excretion rate, when the salt content of the chow was increased from 0.4% to 4.0% NaCl in Dahl SS rats (n = 4 or 5). Blood pressure averaged ∼131 mmHg during the 10-day period of 0.4% NaCl intake. The rats rapidly developed elevated arterial pressure following 2–3 days on the high-salt diet. The arterial pressure plateaued for several days and then slowly and progressively increased to levels ∼30 mmHg higher than the 0.4% NaCl level following 3 wk of the high sodium intake. The development of hypertension was accompanied by increased albumin excretion following the elevated salt intake. The albumin excretion rate also increased with time and averaged 129 ± 20 mg/day after 3 wk of the 4.0% NaCl diet.
Fig. 1.
Changes in mean arterial pressure (top) and albumin excretion rate (bottom) in Dahl salt-sensitive (SS) rats fed AIN-76A diet containing 0.4% NaCl followed by 20 days of 4.0% NaCl chow. *P < 0.05 vs. values obtained on 0.4% NaCl chow.
Figure 2 illustrates the histological changes associated with 3 wk of 4.0% NaCl intake in these rats. Compared with rats maintained on the 0.4% NaCl chow, the high-sodium diet led to both glomerular damage (blue fibrotic tissue and collapsed capillary structure) and tubular damage (red protein deposition casts indicating blocked tubules in the outer medulla). Visibly less glomerular and tubular injury was observed in the kidneys of the SS rats maintained on the 0.4% NaCl chow. Quantitative grading of the histological damage demonstrated that kidneys from the rats fed 4.0% NaCl had an increased degree of glomerular damage (2.8 ± 0.1 vs. 2.1 ± 0.2 on a scale of 0-best to 4-worst), as well as a greater number of blocked tubules in the outer medulla (15.8 ± 1.7%) compared with the kidneys from SS rats maintained on the low-salt diet (5.1 ± 1.6%) (n = 4/group).
Fig. 2.
Light microscopy of Trichrome-stained sections of renal cortex (A and C, ×40 original magnification) and renal outer medulla (B and D, ×10 magnification) of Dahl SS rats fed 0.4% NaCl (A and B) or 4.0% NaCl chow for 3 wk (C and D). The lower panels depict the calculated glomerular injury score (E), and the percentage of renal outer medulla consisting of protein casts (F) in kidneys of rats fed the 0.4% or 4.0% NaCl diets. *P < 0.05 vs. 0.4% NaCl.
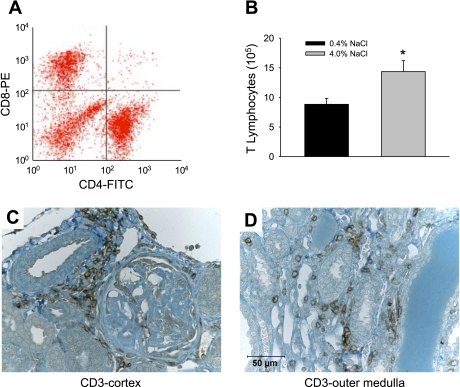
A representative two-dimensional plot of a flow cytometry analysis of mononuclear cells isolated from the kidneys of rats fed 4% NaCl for 3 wk is presented in Fig. 3A. Approximately equal numbers of helper (CD4+) and cytotoxic (CD8+) T cells infiltrated the kidneys. A minor fraction of the mononuclear cells were positive for both markers (upper right), while other mononuclear cell types were negative for both markers (lower left), indicating that there were also other subtypes of mononuclear cells infiltrating the kidneys. Further experiments to isolate total T cells in the kidney demonstrated that the number of T lymphocytes in the kidneys of SS rats fed 4.0% NaCl was ∼62% greater than the number of cells in kidneys of rats fed 0.4% NaCl (Fig. 3B, n = 11/group). The number of circulating T lymphocytes was not different between the groups, averaging 0.79 ± 0.1 × 106 cells/ml in the blood of rats fed the low-salt diet. Immunohistochemistry demonstrated that infiltration of T lymphocytes in the kidneys of rats fed 4.0% NaCl was localized around glomeruli and preglomerular vessels in the renal cortex and in the areas surrounding damaged tubules and vasa recta bundles in the renal outer medulla (Fig. 3, C and D).
Fig. 3.
Identification of cytotoxic (CD8+) and helper (CD4+) T lymphocytes in mononuclear cells infiltrating the kidneys of Dahl SS rats (A), absolute number of T-lymphocytes isolated from the kidneys of Dahl SS rats maintained on the 0.4% or 4.0% NaCl chow (B), and immunohistochemical localization of T cells in the kidney of Dahl SS rats fed 4.0% NaCl chow (C, D, ×40 original magnification). T cells were localized in the renal tissue with antibodies directed against the cell surface markers CD3. *P < 0.05 vs. 0.4% NaCl.
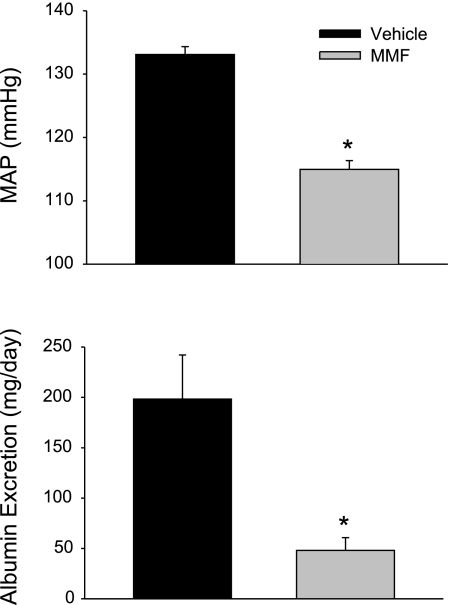
Figure 4 illustrates the MAP and albumin excretion rate in rats treated with vehicle or the immunosuppressive agent MMF following 3 wk of the 4.0% NaCl diet (n = 5). As we previously reported (25), both the development of hypertension and albumin excretion were significantly attenuated in Dahl SS rats treated with MMF. Figure 5, top, demonstrates the changes in infiltrating T cells in vehicle-treated and MMF-treated rats when maintained on 0.4% NaCl chow and following 3 wk of 4.0% NaCl (n = 5–8). As observed earlier, there was a significant increase in infiltrating T lymphocytes in the kidney of vehicle-treated Dahl SS rats when salt intake was increased (from 5.06 ± 1.56 × 105 cells/2 kidneys to 8.93 ± 1.15 × 105 cells/2 kidneys). Treatment with the immunosuppressive agent MMF prevented the increase in T-cell infiltration when NaCl intake was increased (5.19 ± 2.00 × 105 cells/2 kidneys on the 0.4% NaCl diet vs. 5.40 ± 0.99 × 105 cells/2 kidneys on the 4.0% NaCl diet). The bottom panel of Fig. 5 illustrates the changes in intrarenal ANG II that occurred in vehicle or MMF-treated Dahl SS rats when dietary salt intake was altered (n = 6). Intrarenal ANG II was not different in vehicle-treated rats fed 4.0% NaCl compared with values obtained from rats fed 0.4% NaCl (134 ± 26 pg/g of tissue). This failure to suppress intrarenal ANG II occurred despite a significant decrease in plasma renin concentration from 7.4 ± 0.9 ng ANG I·ml−1·h−1 to 2.1 ± 0.4 ng ANG I·ml−1·h−1 and a significant reduction in plasma ANG II from 14 ± 2 pg/ml to 7 ± 1 pg/ml when dietary salt was increased from 0.4% to 4.0% NaCl (data not shown). In contrast to the response in vehicle-treated Dahl SS rats, MMF-treated rats, in which infiltrating T cells were decreased, demonstrated a significant decrease in intrarenal ANG II when NaCl intake was elevated (0.4% NaCl: 163 ± 26 pg/g of tissue vs. 4.0% NaCl: 88 ± 9 pg/g tissue; Fig. 5).
Fig. 4.
Mean arterial pressure (top) and urinary albumin excretion rate (bottom) in Dahl SS rats maintained on a 4.0% NaCl diet and administered vehicle or mycophenolate mofetil (MMF; 20 mg·kg−1·day−1 ip) for 3 wk prior to these measurements (n = 5/group). *P < 0.05 vs. vehicle-treated rats on the same diet.
Fig. 5.
Number of infiltrating T lymphocytes in the kidney (top) and renal concentration of ANG II (bottom) in vehicle-treated and MMF-treated Dahl SS rats fed 0.4% or 4.0% NaCl. *P < 0.05 vs. vehicle-treated rats on the same diet.
Additional experiments were then performed to determine whether T lymphocytes isolated from kidneys of Dahl SS rats are capable of participating in the intrarenal formation of ANG II. As illustrated in Fig. 6, both renin and angiotensin-converting enzyme activity were detected in T cells isolated from the kidneys of Dahl SS rats. Renin activity from T lymphocytes tended to increase in Dahl SS rats fed 4.0% NaCl (1.7 ± 0.4 ng ANG I/min, n = 14) compared with rats fed the 0.4% NaCl chow (1.4 ± 0.3 ng ANG I/min, n = 11) but was not different. Similarly, angiotensin-converting enzyme activity in T cells was not different between rats fed 4.0% NaCl (31.3 ± 7 pmol His-Leu/min, n = 9) and rats fed the 0.4% NaCl chow (34.8 ± 25 pmol His-Leu/min, n = 10).
Fig. 6.
Calculated total renin tissue activity and angiotensin-converting enzyme activity contributed by infiltrating T cells in kidneys of rats fed 0.4% and 4.0% NaCl.
DISCUSSION
The present set of studies demonstrates a role for infiltrating T lymphocytes and ANG II in the development of salt-sensitive hypertension and renal injury in the Dahl SS rat. Experiments indicated that infiltration of T cells into the kidney following elevated sodium intake is associated with enhanced formation of intrarenal ANG II and the development of hypertension and renal injury in the SS rat. Chronic administration of the immunosuppressive agent MMF decreased the number of T lymphocytes in the kidney, reduced intrarenal ANG II, and attenuated salt-sensitive hypertension and albuminuria. In vitro experiments demonstrated significant renin enzymatic activity in T lymphocytes isolated from the kidney of rats on a high-salt diet, demonstrating that these cells may participate in the intrarenal production of ANG II. These data, therefore, indicate that infiltrating T cells can participate in the development and/or maintenance of salt-sensitive hypertension by producing elevated ANG II.
Similar to some human forms of hypertension, Dahl SS rats exhibit salt-sensitive hypertension that is associated with a progressive decline in renal function. Interestingly, sodium-sensitive hypertension and renal disease in the Dahl SS rat and a number of other rodent models is ameliorated by pharmacological suppression or genetic deletion of components of the immune system (7, 9, 15, 16, 18, 40). The mechanisms of hypertension mediated by infiltrating immune cells in the Dahl SS rat have not been elucidated. It has been speculated from data in other animal models that the infiltrating cells could produce free radicals that lead to kidney damage. Alternatively, other studies have indicated that the infiltrating cells may release ANG II, which could potentiate renal disease (8). These studies demonstrate that ANG II may mediate a portion of the prohypertensive effects of infiltrating immune cells.
The present data, indicating that infiltrating cells participate in the development or maintenance of hypertension by increasing intrarenal ANG II, are in agreement with results from a number of different laboratories. It has previously been demonstrated that intrarenal ANG II levels are elevated in hypertensive animals (8, 30). Immunohistochemical and immunoblotting techniques have shown that infiltrating cells stain positive for angiotensin in the kidneys of hypertensive rats (36, 39, 41). The present data indicate that T lymphocytes have renin and angiotensin-converting enzyme activity. Moreover, macrophages, monocytes, and leukocytes have been demonstrated to contain ANG II or angiotensinogen, or are capable of de novo production of ANG II (10, 14, 19, 29, 35, 48); any or all of these infiltrating immune cells may provide an important source of ANG II, which may exaggerate renal damage and hypertension. Moreover, angiotensin-converting enzyme inhibition or AT1 receptor blockade attenuates renal damage and/or the elevation of arterial blood pressure (13, 22) in Dahl SS hypertensive rats fed a high-salt diet. Despite the presence of renin and angiotensin-converting enzyme activity in T cells and the positive association between infiltrating T lymphocytes in the kidney and intrarenal ANG II, it is not clear to what extent T cells participate in ANG II formation in vivo. The apparent localization of the infiltrating T cells around blood vessels, however, supports the concept that vasoactive factors released by these cells can have a marked influence on renal hemodynamics.
A number of different types of infiltrating immune cells, including macrophages, T-lymphocytes, and B-lymphocytes, have been identified in the diseased kidney of hypertensive rats. It is, therefore, possible that the beneficial effects of MMF, the pharmacological agent used in the present study, were mediated by a reduction in multiple cell types. Further T-cell-specific approaches will need to be performed before a definitive conclusion can be made regarding the role of this cell type in Dahl SS hypertension. In support of the concept that T cells are mediating the present effects, recent studies in RAG1−/− mice have indicated that T lymphocytes mediate a significant portion of ANG II-mediated hypertension (11). The mechanisms leading to the infiltration of these cells into the kidney in hypertension remain to be determined. We speculate that infiltration of immune cells in the kidney of hypertensive rats is a secondary effect resulting from kidney damage mediated by a primary increase in arterial pressure. We base this hypothesis on reports from a number of laboratories, indicating that treatment with immunosuppressive drugs has proven beneficial to reduce arterial blood pressure and the severity of kidney damage in a number of different experimental and/or genetic models of rodent hypertension (1, 3, 4, 17, 28, 36, 39, 41, 42).
The present studies focused upon T lymphocytes and their potential role in Dahl SS hypertension; it is also likely that other infiltrating cells participate in the development of hypertension and renal disease in these rats. The effects of other infiltrating cell types and/or the interactions between infiltrating cells and native renal cells remain to be elucidated. The present experiments focused upon the kidney because of this organ's role in sodium-sensitive hypertension. It is possible that immune cells infiltrate other organs and tissues throughout the body; nonrenal effects of infiltrating cells could, therefore, influence the hypertensive disease phenotype. The infiltration of immune cells in other organs and tissues was not examined in this study. Results of this study demonstrate that ANG II may be released from T cells and participate in the development of hypertension and renal disease in the Dahl SS rat. It is possible, however, that other factors released from T cells or other immune cells (i.e., free radicals, cytokines, and others) are also involved in this response and that ANG II is one of many players in this response.
In conclusion, the present data indicate that infiltrating T lymphocytes are increased in the kidney of Dahl SS rats when placed on a 4.0% NaCl diet. The T cells are capable of participating in the production of ANG II and are associated with increased intrarenal ANG II and the development of SS hypertension and kidney damage. The suppression of T-cell infiltration decreased intrarenal ANG II and attenuated Dahl SS hypertension and kidney damage. As such, infiltrating T lymphocytes are capable of participating in the established phase of Dahl SS hypertension.
GRANTS
This work was partially supported by National Institutes of Health Grants HL-29587 and DK-62803.
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1.Alvarez V, Quiroz Y, Nava M, Pons H, Rodríguez-Iturbe B. Overload proteinuria is followed by salt-sensitive hypertension caused by renal infiltration of immune cells. Am J Physiol Renal Physiol 283: F1132–F1141, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Bataillard A, Vincent M, Sassard J, Tourain JL. Antihypertensive effect of cyclophosphamide in genetically hypertensive rats. Int J Immunopharmacol 11: 377–384, 1989 [DOI] [PubMed] [Google Scholar]
- 3.Bendich A, Belisle EH, Strausser HR. Immune system modulation and its effect on the blood pressure of the spontaneously hypertensive male and female rat. Biochem Biophys Res Commun 99: 600–607, 1981 [DOI] [PubMed] [Google Scholar]
- 4.Bravo Y, Quiroz Y, Ferrebuz A, Vaziri ND, Rodriguez-Iturbe B. Mycophenolate mofetil administration reduces renal inflammation, oxidative stress, and arterial pressure in rats with lead-induced hypertension. Am J Physiol Renal Physiol 293: F616–F623, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar Sthe RENAAL Study Investigators Effects of losartan on renal and cardiovascular outcomes in patients with Type 2 diabetes and nephropathy. N Engl J Med 345: 861–869, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Cholewa BC, Meister CJ, Mattson DL. Importance of the renin-angiotensin system in the regulation of arterial blood pressure in conscious mice and rats. Acta Physiol Scand 183: 309–320, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Dzielak DJ. The immune system and hypertension. Hypertension 19: 36–44, 1992 [DOI] [PubMed] [Google Scholar]
- 8.Franco M, Martinez F, Quiroz Y, Galicia O, Bautista R, Johnson RJ, Rodriguez-Iturbe B. Renal angiotensin II concentration and interstitial infiltration of immune cells are correlated with blood pressure levels in salt-sensitive hypertension. Am J Physiol Regul Integr Comp Physiol 293: R251–R256, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Fu MLX. Do immune system changes have a role in hypertension? J Hypertens 13: 1259–1265, 1995 [DOI] [PubMed] [Google Scholar]
- 10.Fukuda N, Nakayama M, Jian T, Satoh C, Nakayama T, Soma M, Izumi Y, Kanmatsuse K. Leukocyte angiotensin II levels in patients with essential hypertension: relation to insulin resistance. Am J Hypertens 16: 129–134, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II-induced hypertension and vascular dysfunction. J Exp Med 204: 2449–2460, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayakawa H, Coffee K, Raij L. Endothelial dysfunction and cardiorenal injury in experimental salt-sensitive hypertension: effects of antihypertensive therapy. Circulation 96: 2407–2413, 1997 [DOI] [PubMed] [Google Scholar]
- 13.Huang BS, Leenen FHH. Both brain angiotensin II and “ouabain” contribute to sympathoexcitation and hypertension in Dahl S rats on high salt intake. Hypertension 32: 1028–1033, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Jankowski V, Vanholder R, van der Giet M, Henning L, Tolle M, Schonfelder G, Krakow A, Karadogan S, Gustavsson N, Gobom J, Webb J, Lehrach H, Giebing G, Schluter H, Hilgers KF, Zidek W, Jankowski J. Detection of angiotensin II in supernatants of stimulated mononuclear leukocytes by matrix-assisted laser desorption ionization time-of-flight/time-of-flight mass analysis. Hypertension 46: 591–597, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Johnson RJ, Rodriguez-Iturbe B, Nakagawa T, Kang DH, Feig DI, Herrera-Acosta J. Subtle renal injury is a likely common mechanism for salt-sensitive essential hypertension. Hypertension 45: 326, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Johnson RJ, Herrera-Acosta J, Schreiner GF, Rodriguez-Iturbe B. Subtle acquired renal injury as a mechanism of salt-sensitive hypertension. N Engl J Med 346: 913–923, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Khraibi AA, Smith TL, Hutchins PM, Lynch CD, Dusseau JW. Thymectomy delays the development of hypertension in Okamoto spontaneously hypertensive rats. J Hypertens 5: 537–541, 1987 [DOI] [PubMed] [Google Scholar]
- 18.Khraibi AA. Association between disturbances in the immune system and hypertension. Am J Hypertens 4: 635–635, 1991 [DOI] [PubMed] [Google Scholar]
- 19.Kitazano T, Padgett RC, Armstrong ML, Tompkins PK, Heistad DD. Evidence that angiotensin II is present in human monocytes. Circulation 91: 1129–1134, 1995 [DOI] [PubMed] [Google Scholar]
- 20.Kobori H, Ozawa Y, Suzaki Y, Prieto-Carrasquero MC, Nishiyama A, Shoji T, Cohen EP, Navar LG. Young scholars award lecture: Intratubular angiotensinogen in hypertension and kidney diseases. Am J Hypertens 19: 541–550, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kodama K, Adachi H, Sonoda J. Beneficial effects of long-term enalapril treatment and low-salt intake on survival rate of Dahl salt-sensitive rats with established hypertension. J Pharmacol Exp Ther 283: 625–629, 1997 [PubMed] [Google Scholar]
- 22.Leenen FHH, Yuan B. Prevention of hypertension by irbesartan in Dahl S rats relates to central angiotensin II type 1 receptor blockade. Hypertension 37: 981–984, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Lombardi D, Gordon K, Polinksy P, Suga S, Schwartz S, Johnson R. Salt sensitive hypertension develops after short-term exposure to angiotensin II. Hypertension 33: 1013–1019, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Mai M, Geiger H, Hilgens KF, Veelken R, Mann JFE, Daemmrich J, Luft FC. Early changes in hypertension induced renal injury. Hypertension 22: 754–765, 1993 [DOI] [PubMed] [Google Scholar]
- 25.Mattson DL, James L, Berdan EA, Meister CJ. Immune suppression attenuates hypertension and renal disease in the Dahl salt-sensitive rat. Hypertension 48: 149–156, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Mattson DL, Kunert MP, Kaldunski ML, Greene AS, Roman RJ, Jacob HJ, Cowley AW., Jr Influence of diet and genetics on hypertension and renal disease in Dahl salt-sensitive rats. Physiol Genomics 16: 194–203, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Mattson DL, Wu F. Nitric oxide synthase activity and isoforms in the rat renal vasculature. Hypertension 35: 337–341, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Muller DN, Shagdarsuren E, Park JK, Dechend R, Mervaala E, Hampich F, Fiebeler A, Ju X, Finckenberg P, Theuer J, Viedt C, Kreuzer J, Heidecke H, Haller H, Zenke M, Luft FC. Immunosuppressive treatment protects against angiotensin II-induced renal damage. Am J Pathol 161: 1679–1693, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nahmod KA, Vermeulen ME, Radien S, Salamone G, Gamberale R, Fernandez-Calotti P, Alvarez A, Nahmod V, Giordana M, Geffner JR. Control of dendritic cell differentiation by angiotensin II. FASEB J 17: 491–493, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Navar LG, Harrison-Bernard LM, Nishiyama A, Kobori H. Regulation of intrarenal angiotensin II in hypertension. Hypertension 39: 316–322, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishiyama A, Seth DM, Navar LG. Renal interstitial fluid concentrations of angiotensins I and II in anesthetized rats. Hypertension 39: 129–134, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Otsuka F, Yamauchi T, Kataoka H, Mimura Y, Ogura T, Makino H. Effects of chronic inhibition of ACE and AT1 receptors on glomerular injury in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 274: R1797–R1806, 1998 [DOI] [PubMed] [Google Scholar]
- 33.Ozawa Y, Kobori H, Suzaki Y, Navar LG. Sustained renal interstitial macrophage infiltration following chronic Angiotensin II infusions. Am J Physiol Renal Physiol 292: F330–F339, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parving HH, Lehnert H, Brochner-Mortensen J, Gomis R, Andersen S, Arner Pthe Irbesartan in Patients with Type 2 Diabetes and Microalbuminuria Study Group The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med 345: 870–878, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Potter DD, Sobey CG, Tompkins PK, Rossen JD, Heistad DD. Evidence that macrophages in atherosclerotic lesions contain angiotensin II. Circulation 98: 800–807, 1998 [DOI] [PubMed] [Google Scholar]
- 36.Quiroz Y, Pons H, Gordon KL, Rincón J, Chávez M, Parra G, Herrera-Acosta J, Gómez-Garre D, Larfo R, Egido J, Johnson RJ, Rodríguez-Iturbe B. Mycophenolate mofetil prevents salt-sensitive hypertension resulting from nitric oxide synthase inhibition. Am J Physiol Renal Physiol 281: F38–F47, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Raij L, Azar S, Keane W. Mesangial immune injury, hypertension, and progressive glomerular damage in Dahl rats. Kidney Int 26: 137–143, 1984 [DOI] [PubMed] [Google Scholar]
- 38.Rieder MJ, Roman RJ, Greene AS. Reversal of microvascular rarefaction and reduced renal mass hypertension. Hypertension 30: 120–127, 1997 [DOI] [PubMed] [Google Scholar]
- 39.Rodriguez-Iturbe B, Vaziri ND, Herrera Acosta J, Johnson RJ. Oxidative stress, renal infiltration of immune cells, and salt-sensitive hypertension: all for one and one for all. Am J Physiol Renal Physiol 286: F606–F616, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Rodriguez-Iturbe B, Johnson RJ. The role of inflammatory cells in the kidney in the induction and maintenance of hypertension. Nephrol Dial Transplant 21: 260–263, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Rodríguez-Iturbe B, Pons H, Quiroz Y, Gordon K, Rincón J, Chávez M, Parra G, Herrera-Acosta J, Gomez-Garre D, Largo R, Egido J, Johnson RJ. Mycophenolate mofetil prevents salt-sensitive hypertension resulting from angiotensin II exposure. Kidney Int 59: 2222–2232, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Rodríguez-Iturbe B, Quiroz Y, Nava M, Bonet L, Chávez M, Herrera-Acosta J, Johnson RJ, Pons HA. Reduction of renal immune cell infiltration results in blood pressure control in genetically hypertensive rats. Am J Physiol Renal Physiol 282: F191–F201, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Santos RA, Krieger EM, Greene LJ. An improved fluorometric assay of rat serum and plasma converting enzyme. Hypertension 7: 244–252, 1985 [DOI] [PubMed] [Google Scholar]
- 44.Sealey JE, Laragh JH. How to do a plasma renin assay. Cardiovasc Med 2: 1079–1092, 1977 [Google Scholar]
- 45.Stewart T, Jung FF, Manning J, Vehaskari VM. Kidney immune cell infiltration and oxidative stress contribute to prenatally programmed hypertension. Kidney Int 68: 2180–2188, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Svendsen UG. Evidence for an initial, thymus independent, and a chronic, thymus-dependent phase of DOCA and salt hypertension in mice. Path Microbiol Scand Acta 84: 523–528, 1976 [DOI] [PubMed] [Google Scholar]
- 47.Wolf G, Ritz E. Combination therapy with ACE inhibitors and angiotensin II receptor blockers to halt progression of chronic renal disease: pathophysiology and indications. Kidney Int 67: 799–812, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Yanagitani Y, Rakugi H, Okamura A, Moriguchi K, Takiuchi S, Ohishi M, Suzuki K, Higaki J, Ogihara T. Angiotensin II type 1 receptor-mediated peroxide production in human macrophages. Hypertension 33: 335–339, 1999 [DOI] [PubMed] [Google Scholar]