Abstract
Male offspring of rats that were modestly protein restricted during pregnancy become hypertensive as adults, whereas their female littermates remain normotensive. The purpose of this study was to determine the role of testosterone in promoting this sexual dimorphism of prenatally programmed hypertension. Rats were fed either a normal (19% protein, NP) or modestly protein-restricted (8.5% protein, LP) diet throughout pregnancy. Male offspring either remained intact or were castrated (CAS) at 30 days of age. Female offspring remained intact. At ∼22 wk of age, the offspring were chronically instrumented for measurement of mean arterial pressure and renal function. Intact male LP offspring were hypertensive compared with male NP offspring (138 ± 2 vs. 130 ± 2 mmHg, P < 0.007), whereas female LP offspring were normotensive (123 ± 1 vs. 122 ± 2 mmHg in NP females). In CAS males, blood pressure in both diet groups was not different from that in intact males of the same group (138 ± 3 mmHg in LP CAS males, and 131 ± 2 mmHg in NP CAS males). Glomerular filtration rate and effective renal plasma flow were also not significantly affected by castration. However, castration significantly reduced protein excretion in LP males to levels not different from those in NP CAS and intact males. Renal histopathology scores showed a similar pattern. Thus removal of androgens by castration failed to provide any protective effect against the hypertension programmed by maternal protein restriction. Castration also failed to abolish the sex difference in blood pressure in both diet groups. These findings suggest that the lifelong presence of normal levels of testicular hormones does not play a major role either in maintaining baseline blood pressure higher in males than in females, or in promoting further elevations in blood pressure in males due to prenatal undernutrition. However, androgens such as testosterone may promote renal injury in LP males.
Keywords: dietary protein restriction, sexual dimorphism, glomerular filtration rate
epidemiological studies have shown an inverse relationship between early growth patterns and the risk for development of hypertension in adult life (3, 6, 7, 13, 23). In animal studies, prenatal undernutrition has been shown to program offspring for adult hypertension, although the mechanisms are still not completely understood. In the rat, we and others have provided evidence supporting a role for a suppressed renin-angiotensin system (RAS) in the fetal/newborn period, impaired renal development, and a reduced number of nephrons in causing the hypertension (26, 28–30). Alterations in the RAS in adult programmed animals have also been reported (14). Thus the RAS is thought to participate in both the development and the maintenance of prenatally programmed hypertension.
Our laboratory has shown previously that a sexual dimorphism exists in the hypertension programmed by maternal undernutrition, with female offspring being relatively protected. In the rat, maternal dietary protein restriction to 8.5% (from a control level of 19%) leads to hypertension in adult male offspring, but female littermates remain normotensive (26, 27). In contrast, a more severe maternal protein restriction to 5–6% of the diet programs both male and female offspring for hypertension (24, 30). Sexual dimorphism has also been reported in other models of prenatal programming (1, 11, 12, 18). However, the precise mechanisms responsible for the differences in responses between males and females are not fully known.
Testosterone is known to interact with the RAS (5, 9) and has been shown to participate in sexual dimorphism of blood pressure in other models (21). Indeed, in rats programmed for hypertension by placental insufficiency, testosterone appears to play an important role in maintaining the hypertension (17). The purpose of the present study was to determine the role of testosterone in the development and maintenance of hypertension programmed by maternal protein restriction.
METHODS
All procedures were approved by the Institutional Animal Care and Use Committee of Oregon Health & Science University. Female Sprague-Dawley rats (∼250–300 g) and proven breeder males were obtained from Harlan and housed in a room with a controlled temperature and a 12:12-h light-dark cycle. The basic diet (Purina basal diet 5755) contained normal levels of protein (19%, NP) and sodium (0.20%). Females were individually housed with males, and the day sperm were seen in a vaginal smear was considered day 1 of pregnancy. Seven dams were fed a low protein (8.5%, LP), normal sodium (0.20%) diet from day 1 of gestation until delivery; at this time, the diet was changed back to NP. Ten dams were kept on the normal diet throughout pregnancy. All dams were fed the NP diet throughout lactation; pups were weaned to the NP food at 22 days of age and remained on it until study. All pregnant animals were weighed daily. Litters were counted and weighed within 15 h of birth; pups were also counted and weighed at 1, 5, 10, 15, and 21 days. No more than two (randomly chosen) pups were used from a given litter for any group. Animals were housed overnight in metabolic cages before surgery for collection of 24-h urine samples.
Surgical preparation of the offspring.
One-half of the male offspring to be used (n = 8 NP, and n = 9 LP) were castrated at 30 days of age under anesthesia using sterile technique. The anesthetic cocktail contained 55% ketamine (100 mg/ml), 28% xylene (20 mg/ml), 11% acepromazine (10 mg/ml), and 6% sterile water and was administered at 1.0 ml/kg intraperitoneally. Intact and castrated (CAS) male and intact female offspring were chronically instrumented at ∼21 wk of age, as described previously (26, 30). Briefly, under ketamine-xylazine-acepromazine anesthesia, catheters were placed in the left femoral artery and vein and in the bladder. The animals were allowed to recover for at least 7 days, during which time they were trained in wire restrainers in the study room on at least three occasions. Vascular catheters were flushed every 2–3 days with 5% dextrose and filled with heparin.
Experimental protocol.
In conscious adult offspring at ∼22 wk of age, mean arterial pressure and renal function were measured, as described previously (26, 30). With the rat in a wire restrainer in the study room, urine was allowed to drain continuously through the bladder catheter into a test tube. Blood pressure was measured through the arterial catheter using a pressure transducer (Abbott Laboratories, North Chicago, IL) connected to a polygraph (Grass Instruments, Quincy, MA). Pressures were measured between 6:00 and 9:00 AM. A 15- to 30-min average was taken after allowing at least 30 min for the pressure to stabilize. After measurement of pressure, a blood sample was taken for measurement of microhematocrit and plasma protein (National Instrument, Baltimore, MD). Glomerular filtration rate (GFR) and effective renal plasma flow (ERPF) were measured using standard clearance techniques, as described previously (26, 30). A 0.6-ml blood sample was taken at the midpoint of each period. The red blood cells remaining after centrifugation and removal of the plasma were resuspended in an equal volume of saline and infused back into the animal. Samples were stored at −20°C until analysis. When the experiments were completed, the animals were killed with a commercial euthanasia solution, and the kidneys were removed and weighed.
Histopathological examination of kidneys.
Kidneys were coronally sectioned into three slices, fixed in 10% buffered formalin, paraffin embedded, and stained with periodic-acid Schiff. Stained sections were evaluated for glomerular and tubulointerstitial injury while blinded to experimental protocol. Tissues were scored as negative (0), mild focal tubuloglomerular disease (1), multifocal cortical disease (2), or severe diffuse cortical disease (3).
Analytical measurements.
Inulin and para-amino-hippuric acid in plasma and urine were measured by the methods of Waugh (25) and Brun (4), respectively. Urine protein was measured by precipitation with sulfosalicylic acid. Plasma renin activity (PRA) was measured by radioimmunoassay (Diasorin). Serum levels of testosterone were measured by the Endocrine Technology and Support Core Laboratory at the Oregon National Primate Research Center/Oregon Health & Science University (19). Briefly, serum samples (25 μl) were extracted with 6 ml redistilled diethyl ether, dried under an airstream, and re-dissolved in assay buffer (0.1% gel-PBS). Hormonal values were corrected for extraction losses determined by radioactive trace recovery at the same time with sample extraction. The sensitivity was <3.9 pg/tube. The overall interassay variation was <15%, and the intra-assay variations did not exceed 10%.
Statistical analysis.
Data are expressed as means ± SE. Data for the groups were compared using one-way or two-way ANOVA, followed by a post hoc test (Bonferroni). Offspring data represent eight offspring from eight litters (intact NP males), eight offspring from eight litters (CAS NP males), eight offspring from eight litters (NP females), nine offspring from seven litters (intact LP males), nine offspring from seven litters (CAS LP males), and eight offspring from six litters (LP females). Statistical significance was assumed with a value of P < 0.05. Histopathology scores were compared among groups using the nonparametric Kruskal-Wallis test, followed by Dunn's test.
RESULTS
There was no significant difference in the length of pregnancy between NP and LP litters (22.9 ± 0.1 vs. 22.7 ± 0.2 days), nor in the number of pups per litter (13 ± 1 vs. 12 ± 1). However, the birth weights in LP pups were significantly lower than those of NP pups (LP 5.62 ± 0.18 g vs. NP 6.21 ± 0.04 g, P < 0.004). By the time of weaning, body weights in LP offspring had caught up to those in NP offspring; there were no significant differences in weaning weights across the different groups (Table 1). At the time of study at 22 wk of age, intact males were significantly larger than CAS males and females, and CAS males were significantly larger than intact females. Although adult LP males were significantly smaller than adult NP males, there was no significant effect of maternal diet in CAS males or in females.
Table 1.
Physiological variables and body and organ weights in adult male, castrated male, and female offspring of rats fed either a normal or protein-restricted diet during pregnancy
| Normal Protein |
Low Protein |
|||||
|---|---|---|---|---|---|---|
| Males | CAS | Females | Males | CAS | Females | |
| n | 8 | 8 | 8 | 9 | 9 | 8 |
| Weaning weight, g | 57 ± 4 | 56 ± 3 | 50 ± 3 | 56 ± 1 | 56 ± 1 | 53 ± 1 |
| Body weight at study, g | 489 ± 15 | 412 ± 13* | 274 ± 10*† | 441 ± 10‡ | 398 ± 14* | 263 ± 5*† |
| Total kidney weight, g | 3.31 ± 0.15 | 2.35 ± 0.08* | 1.81 ± 0.04*† | 3.20 ± 0.08 | 2.32 ± 0.06* | 1.89 ± 0.04*† |
| Kidney/body wt, % | 0.684 ± 0.019 | 0.576 ± 0.013* | 0.677 ± 0.022† | 0.736 ± 0.012‡ | 0.581 ± 0.010* | 0.725 ± 0.011†‡ |
| GFR/KW, ml·min−1·g−1 | 0.97 ± 0.05 | 1.14 ± 0.03 | 1.35 ± 0.11* | 1.04 ± 0.06 | 1.23 ± 0.05 | 1.21 ± 0.07 |
| ERPF/KW, ml·min−1·g−1 | 3.29 ± 0.31 | 3.79 ± 0.13 | 4.41 ± 0.32* | 3.61 ± 0.21 | 4.08 ± 0.11 | 4.29 ± 0.26 |
| GFR/BW, ml·min−1·100 g−1 | 0.649 ± 0.022 | 0.653 ± 0.021 | 0.893 ± 0.069*† | 0.751 ± 0.049 | 0.718 ± 0.032 | 0.863 ± 0.042 |
| ERPF/BW, ml·min−1·100 g−1 | 2.20 ± 0.16 | 2.17 ± 0.10 | 2.89 ± 0.13*† | 2.61 ± 0.17‡ | 2.41 ± 0.10 | 3.07 ± 0.15† |
| Hematocrit | 0.48 ± 0.01 | 0.47 ± 0.01 | 0.43 ± 0.01* | 0.48 ± 0.01 | 0.45 ± 0.02 | 0.44 ± 0.01* |
| Plasma protein, g/dl | 6.9 ± 0.1 | 7.1 ± 0.1 | 6.6 ± 0.1 | 6.9 ± 0.1 | 7.1 ± 0.1 | 6.6 ± 0.2† |
| Urine protein excretion, mg/day | 21 ± 3 | 16 ± 3 | 3 ± 1*† | 27 ± 5 | 14 ± 3* | 4 ± 1* |
| Adrenal weight, g | 0.070 ± 0.004 | 0.074 ± 0.004 | 0.062 ± 0.004† | 0.067 ± 0.002 | 0.072 ± 0.003 | 0.061 ± 0.003 |
| Adrenal/body weight, % | 0.014 ± 0.001 | 0.018 ± 0.001* | 0.023 ± 0.001*† | 0.016 ± 0.001 | 0.018 ± 0.001 | 0.023 ± 0.001*† |
| Heart weight, g | 1.617 ± 0.066 | 1.365 ± 0.051* | 0.984 ± 0.027*† | 1.456 ± 0.055‡ | 1.317 ± 0.037 | 1.001 ± 0.043*† |
| Heart/body weight, % | 0.335 ± 0.009 | 0.335 ± 0.013 | 0.366 ± 0.008 | 0.334 ± 0.008 | 0.331 ± 0.011 | 0.384 ± 0.013*† |
| Plasma renin activity, ng AI·ml−1·h−1 | 4.29 ± 0.38 | 5.18 ± 0.65 | 6.15 ± 0.57 | 4.82 ± 0.79 | 4.96 ± 0.49 | 7.40 ± 0.91*† |
| Testosterone, ng/ml | 2.29 ± 0.42 | 0.35 ± 0.08* | 0.61 ± 0.07* | 1.80 ± 0.21 | 0.33 ± 0.09* | 0.61 ± 0.04* |
| RVR, mmHg·ml−1·min | 6.60 ± 0.51 | 7.97 ± 0.36 | 9.01 ± 0.60* | 6.35 ± 0.40 | 7.83 ± 0.29 | 8.74 ± 0.45* |
| RVR/KW, mmHg·ml−1·min·g−1 | 2.01 ± 0.17 | 3.43 ± 0.20* | 4.97 ± 0.33*† | 2.03 ± 0.16 | 3.39 ± 0.18* | 4.64 ± 0.25*† |
Values are means ± SE; n, no. of rats. CAS, castrated; GFR, glomerular filtration rate; KW, kidney weight; ERPF, effective renal plasma flow; BW, body weight; AI, angiotensin I; RVR, renal vascular resistance. P < 0.05 compared with
value for intact males,
value for CAS males,
normal protein animals of same sex group.
Effects of castration on physiological variables in the offspring.
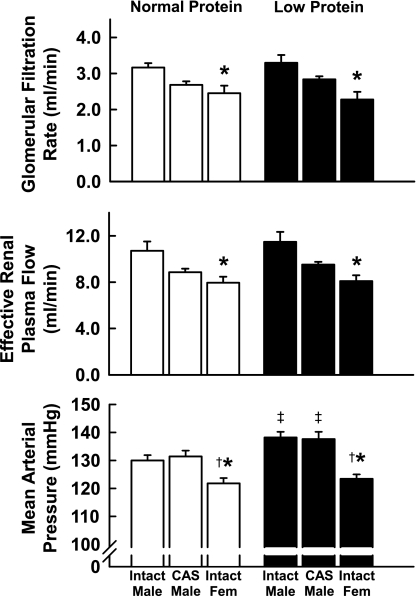
Physiological variables and organ weights in offspring exposed to NP and LP during gestation are shown in Fig. 1 and Table 1. In NP offspring, arterial pressures were significantly higher in males than in females, but castration of males during the juvenile period had no effect on later blood pressure. As we have previously reported (26, 27), maternal protein restriction at this level programmed for blood pressures that were higher in male LP than in male NP offspring, but had no effect on blood pressure in female offspring. Importantly, blood pressure was also elevated in CAS males that had been exposed to LP vs. NP prenatally. Thus castration failed to provide protection against the development of programmed hypertension. Absolute GFR was significantly greater in male than in female offspring; GFR in CAS males was intermediate between males and females. ERPF showed a similar pattern. Expressed per kidney weight, GFR and ERPF were significantly higher in NP females than in males. GFR and ERPF normalized to body weight remained elevated in females. In general, GFR and ERPF normalized to body weight tended to be higher in LP than in NP intact and CAS males, although this did not always reach statistical significance. Renal vascular resistance was significantly higher in females than in males in both diet groups. Values in CAS males were intermediate, but not significantly different than those for either males or females. When normalized to kidney weight, renal vascular resistance was significantly higher in females than in either intact or CAS males and was significantly higher in CAS than intact males. There was no effect of diet.
Fig. 1.
Arterial pressure and renal hemodynamics in adult intact male, castrated (CAS) male, and female offspring of rats fed either a normal or protein-restricted diet during pregnancy. Values are means ± SE. *P < 0.05 compared with value for intact males of same diet group. †P < 0.05 compared with value for CAS males. ‡P < 0.05 compared with normal protein animals of same sex group.
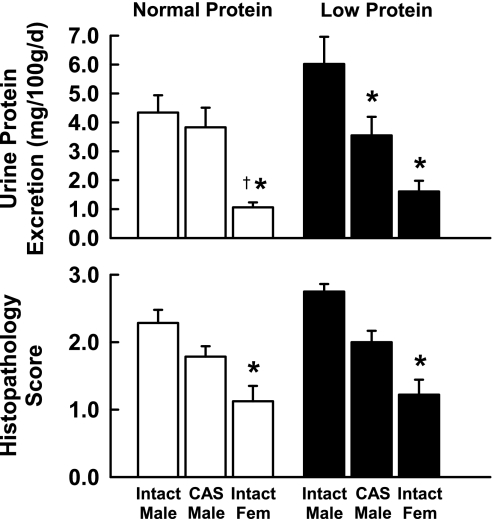
Hematocrit was significantly lower in females than in males in both diet groups, with CAS males showing an intermediate value. Urine protein excretion showed similar patterns in both diet groups, tending to be lower in females than in intact or CAS males, and lower in CAS than in intact males, although these differences did not always reach statistical significance. When normalized to body weight (Fig. 2), protein excretion was higher in LP than in NP intact males, although this did not quite reach statistical significance (P = 0.06). However, castration significantly reduced protein excretion in LP males to levels similar to those in NP CAS and intact males. In both diet groups, protein excretion was lower in females than in males. Absolute adrenal and heart weights were lower in females than in either intact or CAS males, but they tended to be higher than those in both groups of males when normalized to body weight. PRA tended to be higher in females than in males; this reached significance only in the LP group. Serum testosterone was significantly lower in females than in intact males, and castration of males reduced testosterone to levels not different from those in females. There were no significant differences in serum testosterone between LP and NP animals.
Fig. 2.
Urine protein excretion and histopathology scores in adult intact male, CAS male, and female offspring of rats fed either a normal or protein-restricted diet during pregnancy. Values are means ± SE. *P < 0.05 compared with value for intact males of same diet group. †P < 0.05 compared with value for CAS males.
Effects of castration on renal histopathology in the offspring.
Renal histopathology scores are also shown in Fig. 2. Although not reaching statistical significance, this index of renal damage showed a similar pattern to that of proteinuria; the mean score in intact LP males was higher than that in intact NP males, and castration reduced the scores in both diet groups to levels not different from each other. The histopathology score in both NP and LP females was significantly lower than that in intact males of the same diet group.
DISCUSSION
Maternal dietary protein restriction during rat pregnancy programs for hypertension in the adult male offspring (26). Our laboratory has shown previously that female offspring are relatively protected from this programming effect; modest maternal restriction (protein content reduced from 19 to 8.5% of the diet) fails to affect the arterial pressure of adult females, whereas a more severe maternal restriction (to 5% protein), either throughout or for the last one-half of pregnancy, programs hypertension in both males and females (27, 30). However, the mechanisms responsible for this sexual dimorphism in prenatal programming are not known. The major finding of the present study is that removal of the potential influence of testicular hormones, in particular testosterone, by castration at 30 days of age, failed to prevent the hypertension in male LP offspring. This suggests that the lifelong presence of normal levels of testosterone is not a major contributor to the sexual dimorphism of blood pressure in this model of programmed hypertension.
It is now well established that a variety of insults, when experienced in the prenatal period, can have long-term influences on the health of the individual. Factors such as maternal undernutrition and placental insufficiency that lead to impaired fetal growth are known to cause hypertension and other cardiovascular abnormalities in the offspring. In many animal models, the males and females are not equally affected; usually, but not always, males experience more profound effects (11). For example, in our laboratory, modest restriction of protein in the maternal diet has no effect on the adult blood pressure of the female offspring, but their male littermates become hypertensive (26, 27). Similar findings have been reported in a rat model of placental insufficiency (1). The sexual dimorphism of this prenatal programming suggests a role for the sex hormones in modulating the long-term changes in blood pressure in response to these prenatal insults. Two possible explanations are as follows: 1) the presence of testosterone and possibly other androgens in the male may promote the development of hypertension; or 2) the presence of estrogens in females may protect against the hypertensive effects of an adverse fetal environment.
In the present study, we found that castration during the juvenile period of the male offspring of protein-restricted mothers had no effect on the development of hypertension, suggesting that testosterone does not play an important role in promoting the sex-linked hypertension in this model. This finding is somewhat surprising, as it is in contrast to findings in other rat models. In one model of genetically programmed hypertension, the spontaneously hypertensive rat (SHR), Reckelhoff et al. (20) showed that castration at 3–4 wk of age reduced the blood pressure of male animals to the levels normally seen in females, indicating that testosterone is important in mediating the sex differences in blood pressure in this model. Castration also attenuated hypertension in the Dahl salt-sensitive rat model (31). In a model of placental insufficiency produced by clipping the uterine and ovarian arteries during pregnancy, male offspring exhibit sustained hypertension, whereas, in females, the elevated blood pressure is only a transient phenomenon (1). In this model of prenatal environmentally programmed hypertension, castration at 10 wk of age also reduced mean arterial pressure measured at 16 wk by telemetry in growth-retarded, but not in control, male offspring (17). Administration of a converting enzyme inhibitor for 2 wk reduced blood pressure more in intact than in CAS males, suggesting that the RAS also participates with testosterone in modulating the hypertension (17). The reasons for the apparent discrepancies between the above studies and the present study are not entirely clear. Both the SHR and the Dahl salt-sensitive rat are models of genetic hypertension and thus may involve different mechanisms than fetal programming models. On the other hand, one might expect mechanisms in the placental insufficiency model to be similar to those in the protein restriction model, as both are presumably initiated by fetal undernutrition. However, there are some differences between these models that could contribute to the differences in results. For example, maternal blood pressure is elevated in the placental-insufficiency model (2). Subtle methodological differences between studies might also play a role. We castrated the animals at 30 days of age, just before puberty, whereas, in the study of Ojeda et al (17)., castration was done in postpubertal animals, although one would expect that later castration would have less rather than more of an effect to lower adult blood pressure. In contrast to these previous rat studies, however, castration does not prevent programmed hypertension in male sheep, which is consistent with our findings (15). Furthermore, we have shown that nephron number is reduced in male LP rats, but not in females, and that a reduced number of nephrons from birth can cause hypertension (26, 27, 29). As nephrogenesis is complete by ∼10 days after birth in the rat, one would not expect castration in the juvenile period to have any effect on nephron number. Thus, to the extent that the reduced number of nephrons contributes to the programmed hypertension, it is not completely surprising that castration failed to prevent the increased blood pressure in our study. Finally, emerging evidence suggests that epigenetic factors are at play in fetal programming mechanisms, which also would fit with our findings. It is possible that the low levels of testosterone still present in CAS males play a permissive role, in combination with other more direct mechanisms, in allowing the hypertension to develop. However, the fact that testosterone levels in females are higher than those in CAS males, yet the females are normotensive, indicates that testosterone is not a direct mediator. At any rate, in our present model of programmed hypertension, castration at 30 days of age had no effect on long-term blood pressure, indicating that factors other than testosterone must account for the sexual dimorphism.
It is well known that the early postnatal period, around postnatal days 1–5, is the critical period in which testosterone produces its greatest effect in the developing rat brain, and sexual dimorphism of several structures and future reproductive behaviors are programmed at this time (32). Although the present studies clearly show that the lifelong presence of normal levels of testosterone is not required for the development of programmed hypertension in this model, we have not ruled out the possibility that testosterone might be involved in this programming during the neonatal period. Further studies, in which castration is performed immediately after birth, will be necessary to address this question.
Another group of factors that could play a role are the female sex steroids, estrogens. In the sheep, ovarian hormones are not required for programmed hypertension to be manifest (8). Although removal of the ovaries at 3–4 wk of age had no effect on blood pressure in female SHR (20), ovariectomy at 10 wk of age in the placental-insufficiency model led to hypertension in growth-restricted offspring that was reversible by replacement of estrogen for 2 wk (16). This hypertension was also abolished by RAS blockade (16). Thus it appears that both estrogen and testosterone contribute to the sexual dimorphism of blood pressure in the rat model of placental insufficiency, whereas only testosterone is important in maintaining the sex differences in blood pressure in the SHR. It seems likely that estrogens may play a central role in protecting the female from hypertension in our present model of prenatal programming by maternal diet, but further studies are needed to test this hypothesis.
As indicated above, the RAS has also been implicated in the sexual dimorphism of blood pressure in both genetically and environmentally programmed animal models. Although we did not test the effect of RAS blockade in the present study, PRAs were not significantly different between LP and NP intact males, suggesting that the RAS may not be a primary factor in causing the hypertension. Furthermore, PRA was actually higher in LP females than males, which also argues against a primary role for the RAS in promoting the hypertension in males. However, this finding would be consistent with a tendency for activation of the RAS in LP animals, a change that may be suppressed by the hypertension in males. In a model using a more severe maternal protein restriction in only the last part of gestation, PRAs were lower than controls at 1 and 2 mo of age, and higher than controls at 6 and 11 mo of age (14). It is possible that PRA has a similar pattern in our model, and that the crossover from lower to higher occurs near the 5-mo age at which we studied our animals, thus showing no significant difference.
Although testosterone does not appear to be involved in promoting the hypertension in male offspring of protein-restricted mothers, it may play a role in renal injury. We routinely find that urine protein excretion, on average, is higher in LP than in NP males. (When a comparison was done on 143 NP and 93 LP males of this age studied over the past 5 yr in our laboratory, urine protein excretion was 22 ± 1 mg/day NP and 32 ± 2 mg/day LP, P = 0.000001, unpublished results.) Differences in protein excretion do not always reach statistical significance when relatively small sample sizes are used, as in the present study (P = 0.06). However, urine protein excretion was reduced by castration in LP males, an effect that was statistically significant. This suggests a dissociation between blood pressure (which was not improved by castration) and renal damage (evidenced by proteinuria), which was improved by castration in LP males. Renal histopathology scores show a similar pattern. Thus testosterone may play a role in promoting renal damage in this model. Although this finding is consistent with reports in other models (31), in those models the effect of testosterone to promote renal injury is thought to be, at least in part, hemodynamically mediated, as blood pressure and indicators of renal damage change in parallel. In the present model, any renal damage related to testosterone must occur through mechanisms other than hemodynamic changes. The RAS has also been postulated to play a role in testosterone-mediated renal damage in other models (10). In the present model, plasma renin activities were not different among the four male groups, arguing against involvement of the RAS. However, our data do not rule out a possible role for changes in the intrarenal RAS in mediating testosterone-stimulated renal injury. Despite the suggestions of renal structural damage, we found no evidence of functional impairment: GFR and ERPF were not different between NP and LP males. Castration reduced the relative size of the kidneys (kidney-to-body weight ratio), but tended to result in increased GFR and ERPF per kidney weight, thus preserving renal function.
The results of the present study also suggest that testosterone does not play a major role in maintaining the normal differences in blood pressure between male and female rats. Although not always detected by other investigators in similar models, in our laboratory using chronic instrumentation in trained, conscious animals, we consistently find that mean arterial pressures of male Sprague-Dawley rats are 5–10 mmHg higher than those of their female littermates (26, 27). This is consistent with the findings of Sartori-Valinotti et al. using telemetry methods (22). In the present study, we found that the blood pressures of NP males were ∼8 mmHg higher than those of NP females. The fact that castration failed to reduce blood pressure in NP males indicates that testosterone does not play an important role in maintaining the normal sex differences in blood pressure in these animals.
Perspectives and Significance
Epidemiological studies in humans have established that factors in the prenatal environment, factors that affect fetal growth patterns, lead to increased adult risk for hypertension and other cardiovascular diseases. Indeed, developmental programming may account for a substantial portion of essential hypertension in humans. In some cases, the susceptibility to adverse effects of fetal programming is sex dependent, with males being more likely to be affected. The present study indicates that it is not the presence of testosterone in males that accounts for the sex difference in programming of hypertension, but does suggest that the accompanying renal damage may be at least partially dependent on testicular androgens.
GRANTS
These studies were supported by National Heart, Lung, and Blood Institute Grant R01HL070132, and a Grant-in-Aid from the American Heart Association. T. K. Morgan is a National Institutes of Health K12 BIRWCH scholar (2K12HD043488-06).
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Rachel Chin, Robert Osten, and Chava Sandoval for technical assistance, and Emily King for statistical assistance.
REFERENCES
- 1.Alexander BT. Placental insufficiency leads to development of hypertension in growth-restricted offspring. Hypertension 41: 457–462, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Alexander BT, Kassab SE, Miller MT, Abram SR, Reckelhoff JF, Bennett WA, Granger JP. Reduced uterine perfusion pressure during pregnancy in the rat is associated with increases in arterial pressure and changes in renal nitric oxide. Hypertension 37: 1191–1195, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Barker DJP, Bull AR, Osmond C, Simmonds SJ. Fetal and placental size and risk of hypertension in adult life. Br Med J 301: 259–262, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brun C. A rapid method for the determination of para-amino-hippuric acid in kidney function tests. J Lab Clin Med 37: 955–958, 1951 [PubMed] [Google Scholar]
- 5.Chen YF, Naftilan AJ, Oparil S. Androgen-dependent angiotensinogen, and renin messenger RNA expression in hypertensive rats. Hypertension 19: 456–463, 1992 [DOI] [PubMed] [Google Scholar]
- 6.Curhan GC, Chertow GM, Willett WC, Spiegelman D, Colditz GA, Manson JE, Speizer FE, Stampfer MJ. Birth weight and adult hypertension and obesity in women. Circulation 94: 1310–1315, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Curhan GC, Willett WC, Rimm EB, Spiegelman D, Ascherio AL, Stampfer MJ. Birth weight and adult hypertension, diabetes mellitus, and obesity in US men. Circulation 94: 3246–3250, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Dodic M, Samuel C, Moritz K, Wintour EM, Morgan J, Grigg L, Wong J. Impaired cardiac functional reserve and left ventricular hypertrophy in adult sheep after prenatal dexamethasone exposure. Circ Res 89: 623–629, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Ellison KE, Ingelfinger JR, Pivor M, Dzau VJ. Androgen regulation of rat renal angiotensinogen messenger RNA expression. J Clin Invest 83: 1941–1945, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fortepiani LA, Yanes L, Zhang H, Racusen LC, Reckelhoff JF. Role of androgens in mediating renal injury in aging SHR. Hypertension 42: 952–955, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Gilbert JS, Nijland MJ. Sex differences in the developmental origins of hypertension and cardiorenal disease. Am J Physiol Regul Integr Comp Physiol 295: R1941–R1952, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khan IY, Taylor PD, Dekou V, Seed PT, Lakasing L, Graham D, Dominiczak AF, Hanson MA, Poston L. Gender-linked hypertension in offspring of lard-fed pregnant rats. Hypertension 41: 168–175, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Law CM, de Swiet M, Osmond C, Fayers PM, Barker DJP, Cruddas AM, Fall CHD. Initiation of hypertension in utero and its amplification throughout life. BMJ 306: 24–27, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manning J, Vehaskari VM. Low birth weight-associated adult hypertension in rat. Pediatr Nephrol 16: 417–422, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Moritz KM, Dodic M, Jefferies AJ, Wintour EM, DeMatteo R, Singh RR, Evans RG. Haemodynamic characteristics of hypertension induced by prenatal cortisol exposure in sheep. Clin Exp Pharmacol Physiol 36: 981–987, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Ojeda NB, Grigore D, Robertson EB, Alexander BT. Estrogen protects against increased blood pressure in postpubertal female growth restricted offspring. Hypertension 50: 679–685, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ojeda NB, Grigore D, Yanes LL, Iliescu R, Robertson EB, Zhang H, Alexander BT. Testosterone contributes to marked elevations in mean arterial pressure in adult male intrauterine growth restricted offspring. Am J Physiol Regul Integr Comp Physiol 292: R758–R763, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Ozaki T, Nishina H, Hanson MA, Poston L. Dietary restriction in pregnant rats causes gender-related hypertension and vascular dysfunction in offspring. J Physiol 530: 141–152, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rasmussen LE, Buss IO, Hess DL, Schmidt MJ. Testosterone and dihydrotestosterone concentrations in elephant serum and temporal gland secretions. Biol Reprod 30: 352–362, 1984 [DOI] [PubMed] [Google Scholar]
- 20.Reckelhoff JF, Zhang H, Granger JP. Testosterone exacerbates hypertension and reduces pressure-natriuresis in male spontaneously hypertensive rats. Hypertension 31: 435–439, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Reckelhoff JF, Zhang H, Srivastava K. Gender differences in development of hypertension in spontaneously hypertensive rats: role of the renin-angiotensin system. Hypertension 35: 480–483, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Sartori-Valinotti JC, Iliescu R, Yanes LL, Dorsett-Martin W, Reckelhoff JF. Sex differences in the pressor response to angiotensin II when the endogenous renin-angiotensin system is blocked. Hypertension 51: 1170–1176, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Thame M, Osmond C, Wilks RJ, Bennett FI, McFarlane-Anderson N, Forrester TE. Blood pressure is related to placental volume and birth weight. Hypertension 35: 662–667, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Vehaskari VM, Aviles DH, Manning J. Prenatal programming of adult hypertension in the rat. Kidney Int 59: 238–245, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Waugh WH. Photometry of inulin and polyfructosan by use of a cysteine/tryptophan reaction. Clin Chem 23: 639–645, 1977 [PubMed] [Google Scholar]
- 26.Woods LL, Ingelfinger JR, Nyengaard JR, Rasch R. Maternal protein restriction suppresses the newborn renin-angiotensin system and programs adult hypertension in rats. Pediatr Res 49: 460–467, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Woods LL, Ingelfinger JR, Rasch R. Modest maternal protein restriction fails to program adult hypertension in female rats. Am J Physiol Regul Integr Comp Physiol 289: R1131–R1136, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Woods LL, Rasch R. Perinatal ANGII programs adult blood pressure, glomerular number, and renal function in rats. Am J Physiol Regul Integr Comp Physiol 275: R1593–R1599, 1998 [DOI] [PubMed] [Google Scholar]
- 29.Woods LL, Weeks DA, Rasch R. Hypertension after neonatal uninephrectomy in rats precedes glomerular damage. Hypertension 38: 337–342, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Woods LL, Weeks DA, Rasch R. Programming of adult blood pressure by maternal protein restriction: role of nephrogenesis. Kidney Int 65: 1339–1348, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Yanes LL, Sartori-Valinotti JC, Iliescu R, Romero DG, Racusen LC, Zhang H, Reckelhoff JF. Testosterone-dependent hypertension and upregulation of intrarenal angiotensinogen in Dahl salt-sensitive rats. Am J Physiol Renal Physiol 296: F771–F779, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Young WC, Goy RW, Phoenix CH. Hormones and sexual behavior. Science 143: 212–218, 1964. [DOI] [PubMed] [Google Scholar]