Abstract
To study changes in energy balance occurring during the initial phases of dieting, 18 adult ovariectomized female monkeys were placed on a low-fat diet, and available calories were reduced by 30% compared with baseline consumption for 1 mo. Surprisingly, there was not significant weight loss; however, daily activity level (measured by accelerometry) decreased soon after diet initiation and reached statistical significance by the 4th wk of dieting (18 ± 5.6% decrease, P = 0.02). During a 2nd mo of dieting, available calories were reduced by 60% compared with baseline consumption, leading to 6.4 ± 1.7% weight loss and further suppression of activity. Metabolic rate decreased by 68 ± 12 kcal/day, with decreased activity accounting for 41 ± 9 kcal/day, and the metabolic activity of the weight lost accounting for 21 ± 5 kcal/day. A second group of three monkeys was trained to run on a treadmill for 1 h/day, 5 days/wk, at 80% maximal capacity, leading to increased calorie expenditure of 69.6 ± 10.7 kcal/day (equivalent to 49 kcal/day for 7 days). We conclude that a diet-induced decrease in physical activity is the primary mechanism the body uses to defend against diet-induced weight loss, and undertaking a level of exercise that is recommended to counteract weight gain and promote weight loss is able to prevent the compensatory decrease in physical activity-associated energy expenditure that slows diet-induced weight loss.
Keywords: obesity, calorie reduction, activity
moderate weight loss in obese and overweight individuals is associated with major health benefits, including reductions in the risk of heart disease (37), stroke (37), type 2 diabetes (35, 42, 70), hypertension (18, 49), hyperlipidemia (21, 60), hypercholesterolemia (22, 60), cardiovascular disease (22, 60), osteoarthritis (60), and depression (22, 60). Thus it is not surprising that, at any time, two-thirds of obese North American adults are attempting to lose weight (58, 61). Currently, American consumers spend $33 billion annually on weight loss products and services (10). Despite these efforts, the prevalence of overweight and obese adults has escalated over the past several decades, such that 65% of adults in the United States have a body mass index above the healthy range (19). An important contributing factor to the increasing prevalence of obesity is that most of the individuals attempting to lose weight are unsuccessful (60, 63). Dieting is currently the most common weight loss strategy (14, 29, 31, 41, 81). However, the success rates for diet-induced weight loss are very low, ranging from 2 to 20% of individuals actually maintaining weight loss (30, 62, 72, 82). Weight loss in response to a diet is difficult to accomplish and maintain, as compensatory mechanisms act to prevent weight loss by decreasing energy expenditure (5, 11–13, 26, 43).
The majority of studies examining diet-induced weight loss in humans have not directly measured physical activity or food intake, but rather have relied upon self-report of these measures (28, 50, 71, 83), which has been shown to be unreliable (7, 28, 34, 44, 46, 69). There is clear evidence that dieting leads to a compensatory decrease in total metabolic rate in humans (5, 11–13, 26, 43) and nonhuman primates (38). It has been postulated that a decrease in physical activity contributes to the overall decrease in energy expenditure accompanying calorie reduction, and that this reduction in physical activity is at least partially responsible for the decreased effectiveness of dieting to promote weight loss. Evidence to support this hypothesis comes from several studies that suggest that people decrease physical activity level in response to reduced calorie consumption (13, 33, 43, 55). Also, a single report directly measured physical activity before and after a dietary weight loss intervention in women and found that physical activity decreased after the weight loss intervention (74). However, this study measured activity for short periods of time (5–6 days), and accelerometers were removed during periods of the day when individuals were sleeping or bathing. In contrast, in rodents, detailed measurements of physical activity indicate that activity increases in response to calorie reduction (8, 17, 45, 57). It is hypothesized that the increase in activity in rodent models reflects an increased drive to forage for food, as the elevated activity is decreased when food is made available (36). It is likely that the differential regulation of physical activity in response to calorie reduction in humans vs. rodents is due to species differences in stored energy.
To examine the compensatory decreases in energy expenditure that occur in response to diet-induced weight loss in detail, we have studied a primate species that shows metabolic regulation similar to that of humans (23, 73) (i.e., rhesus monkeys). A group of 18 ovariectomized adult female rhesus monkeys was put on a carefully controlled diet for 2 mo, and food intake, physical activity, total metabolic rate, and body composition were measured before and over the course of 2 mo of dieting: a 1st mo in which available calories were reduced by 30% compared with baseline consumption, and a 2nd mo in which available calories were reduced by 60% compared with baseline consumption.
MATERIALS AND METHODS
Animals
Twenty-one adult female rhesus monkeys (Macaca mulatta), 9–13 yr of age, were used in this study.
For experiment 1, 18 monkeys were housed in individual stainless steel cages (32 × 24 × 27 or 32 × 34 × 27 in.) in a temperature-controlled room (24 ± 2°C), with lights on between 0700 and 1900. Two and one-half years before the initiation of this study, these monkeys were ovariectomized and placed on a diet higher in fat than standard monkey chow (35% of calories from fat) to approximate the conditions experienced by many postmenopausal women in the Western world (77). This diet was formulated at the Oregon National Primate Research Center (ONPRC) following a modification of the recipe developed by Clarkson and colleagues to study diet-induced atherosclerosis (59, 77). The diet utilized in this study was modified to prevent loose stool by lowering the percent fat from 43 to 35%, increasing the amount of carbohydrate from 39 to 46%, and reducing the amount of calcium and phosphorus. The diet had a wheat flour base, and 35% of calories were derived from fat, 19% from protein, and 46% from carbohydrate. At the beginning of this study, during a 1-mo baseline period, monkeys continued to receive high-fat diet ad libitum. After the baseline period, monkeys were placed on monkey chow (5% fat), which involved switching their food back to the standard feeding regimen at ONPRC, in which they received high-protein monkey chow biscuits (no. 5047, jumbo biscuits, Ralston Purina, St. Louis, MO; ∼16.5 g each, 3.11 metabolizable kcal/g, 616 kcal/meal, 25% protein, 5% fat, 6.5% fiber, 6% ash, and 3% nutrients). During the 1st mo of the diet, the number of available calories was reduced by 30% compared with baseline consumption. During the 2nd mo of the diet, calories available were reduced by 60% compared with baseline calorie consumption. Throughout the study, two meals a day were provided at 0915 and 1515. All aspects of the study were reviewed and approved by the ONPRC Animal Care and Use Committee.
For experiment 2, three adult female monkeys, 13–14 yr of age, lived in social groups in pens measuring 14 × 11 × 10 ft, which had perches at various heights and various toys available. Skylights provided natural lighting supplemented with artificial lighting from ∼0730 to 1600 each day. Temperature was maintained at 24 ± 3°C. Monkeys were fed Purina high-protein monkey chow (no. 5045; Ralston Purina, St. Louis, MO), supplemented with seeds, fresh fruit, and vegetables. Monkeys were trained to enter transfer cages from their home pen so that they could be transported to a different room for running on treadmills during the experiment. All aspects of the study were reviewed and approved by the University of Pittsburgh Animal Care and Use Committee.
Experimental Design
Experiment 1.
The overall goal of this study was to examine the compensatory mechanisms that counteract diet-induced weight loss. During the baseline period (1 mo), initial measurements of body weight, body composition, food intake, activity, total energy expenditure, basal metabolic rate, thermic effect of food, and activity-associated energy expenditure were made. Monkeys were subsequently placed on a diet for 2 mo. In the 1st mo of the study, available calories were reduced by 30% compared with baseline consumption. In the 2nd mo of the study, calorie intake was reduced by 60% compared with baseline consumption. A second measurement of body composition, total energy expenditure, basal metabolic rate, thermic effect of food, and activity-associated energy expenditure was made at the end of the 2nd mo of dieting. Throughout the study, food intake was measured at every meal, body weight was measured weekly, and activity was measured continuously via accelerometry.
Experiment 2.
The overall goal of this study was to examine the change in daily activity that occurs when animals participate in an exercise program of 1 h of running a day, 5 days/wk, at levels recommended by the American College of Sports Medicine to prevent weight gain and promote weight loss (15). Monkeys wore collars with a small metal box attached, housing an omnidirectional acclerometer (Actical acclerometer, Respironics, Phoenix, AZ) throughout the entire study to measure daily level of physical activity. After a baseline period, monkeys were trained to run on motor-driven treadmills and ran for 1 h/day at 80% maximal capacity for 5 days/wk for 12 wk.
Experimental Measures
Food intake.
Total food consumption at each meal was recorded daily throughout the study, by counting the amount of food remaining before the next meal.
Body weight.
Body weight measurements were made weekly before consumption of the AM meal, at ∼0800.
Dual-energy X-ray absorptiometry measurements.
Percent body fat, percent central fat, percent peripheral fat, fat mass in grams, and lean tissue mass in grams were determined using dual-energy X-ray absorptiometry, as previously described (64, 66). Animals were sedated with Telazol (3 mg/kg im, Fort Dodge Animal Health, Fort Dodge, IA), supplemented with ketamine HCl (10–20 mg/kg im; Ketaset, Fort Dodge Animal Health), and were positioned supine on the bed of a Lunar DPX scanner (Lunar, Madison, WI). Total body scans were done in the “Pediatric Medium” scan mode with a voltage of 76 kV. Lunar software version 3.4 was used to calculate body composition. Two or three scans were performed for each monkey at the initiation of the baseline period and after 2 mo of dieting, and averages were calculated for each measure. To delineate central fat mass from peripheral fat mass, fat in the trunk (including both the subcutaneous and visceral compartments) and fat in the extremities were calculated using standard methodology (9, 68).
Metabolic rate.
Metabolic rate was measured by placing each monkey in a sealed Lexan metabolic chamber (Columbus Instruments, Columbus, OH) and measuring the amount of carbon dioxide produced and oxygen consumed over a 24-h period using a computer-controlled indirect open-circuit calorimeter (Oxymax System, Columbus Instruments) and previously published methods (66). The metabolic chamber was approximately the same size as the monkey's home cage (inside dimensions of 30 in. × 24 in. × 24 in.). To prevent social isolation stress during metabolic testing, two familiar monkeys (i.e., two monkeys routinely housed across the room from the experimental monkey) were placed in plain view of the subject. The metabolic rate of each monkey was assessed during the baseline period, when monkeys were consuming high-fat diet, and after 2 mo of dieting. To determine total daily energy expenditure, monkeys were placed in the metabolic chamber at 1000 and remained in the chamber until 0900 the next morning. Before placement in the chamber, monkeys were fed their standard meal at 0915. They were then fed a banana (114 ± 10 g, 108 calories) at 1515, while in the chamber. Water was available ad libitum throughout metabolic testing. BMR was calculated as the average number of kilocalories expended per hour from 2300 to 0300. This time period was selected because this is when monkeys typically sleep, and heart rate (J. Cameron, unpublished observations) and activity are lowest at this time of night (66).
Activity.
Activity was measured continuously throughout the experiment using omnidirectional Actical accelerometers (Respironics, Phoenix, AZ) and previously published methods (66). Each monkey was fitted with a loose-fitting metal collar (Primate Products, Immokalee, FL) that had an activity monitor, housed in a snug protective stainless steel box, mounted on it. The monitor was programmed to store the total number of activity counts per minute. During the study period, monkeys were sedated with ketamine hydrochloride (10–20 mg/kg im; Ketaset, Fort Dodge Animal Health), and the data from each activity monitor were downloaded at least every 45 days (the maximum number of days that the monitor can store data). After the data were downloaded and saved, the activity monitor was reprogrammed and replaced on the collar to resume collection of activity data. The monkeys in this study had been acclimated to wearing collars with activity monitors attached for over 6 mo before the collection of measurements for this study. Average activity during the light and dark cycle was calculated for the baseline period and each week of the diet. Activity-associated energy expenditure was calculated using a previously published calculation of the amount of energy expended (in kcal) per activity count (66). This was calculated by measuring total energy expenditure at times of day in which there would be little contribution of the thermic effect of food to the total metabolic rate (from 1400 to 1500 and 1800 to 1900), subtracting basal metabolic rate, and dividing the remaining energy expenditure by the number of activity counts occurring during this time period. The number of calories expended per activity count was multiplied by total daily activity counts to determine daily activity-associated energy expenditure.
Exercise training.
Monkeys were trained to run on a standard human-sized treadmill (model 910e, Precor, Bothell, WA), using previously published techniques (78–80). Each treadmill was covered by a Plexiglas box that had numerous air holes in the front and back panels for adequate ventilation. Initially, for several days, the monkeys were acclimated to the treadmill by sitting on it and being allowed to explore the treadmill belt and the Plexiglas box. Monkeys then learned to walk on the treadmill, and then speed and duration of each running session were slowly increased to 1.6 mph for 20 min/day. The treadmill adaptation period lasted 3–4 wk. Subsequently, each monkey underwent a “max test”, and their target exercise level was individually determined as 1 h of running, 5 days a wk, at 80% of their maximal capacity. Monkeys were trained by gradually increasing speed and duration of each running session until they reached their individual target speed. Each monkey's target amount of running was adjusted after a second max test, performed in week 7 of the study, so that they continued to train at 80% maximal capacity.
Maximal heart rate test procedures.
After monkeys were trained to walk on the treadmill, and again after 7 wk of running, each monkey had a maximal aerobic power test (max test) performed. Before the max test, monkeys were adapted to wearing nylon jackets that would protect ECG electrodes and leads for several days. For electrode placement, monkeys were sedated with 0.1 mg/kg ketamine hydrochloride (Ketaject, Phoenix Pharmaceuticals, St. Joseph, MO), and standard pediatric heart rate electrodes with self-adhesive pads were adhered to the monkey's chest. The distal ends of the electrodes were attached to a TM8 telemetry transmitter (Life Sensing Instruments, Tullahoma, TN) that was placed in an inside pocket of a jacket that the monkeys wore to prevent them from manipulating the heart rate electrodes and transmitter. The heart rate signal was received by a HST 220 telemetry receiver (Life Sensing Instruments) and recorded by a computer. Software for the heart rate data collection and storage (Samsedate Heart Rate Variability System) was developed by Autrec (Winston-Salem, NC).
For testing, heart rate was recorded while the monkey sat on the treadmill. Running was then initiated at a speed of 0.8 miles/h (mph; 1.28 km/h), and speed was increased by 0.2 mph every 2 min until the monkey was no longer able to keep pace with the treadmill. Heart rate was recorded for 6 s at the end of each speed interval. Once the monkey reached maximum speed, the treadmill was stopped briefly, and then running was reinitiated at 0.8 mph for a 5-min recovery period. Heart rate was recorded at 1, 3, and 5 min during the recovery period.
Statistical Analyses
For all analyses, normality and homoscedacity were initially tested. If these criteria were met, a repeated-measures ANOVA was utilized to look at differences in variables over time. The assumption of sphericity was examined with Mauchly's test. The Greenhouse-Geiser correction factor was used in cases where the assumption of sphericity was violated. least significant difference post hoc tests were used to determine time periods that were significantly different from each other. If the variables were measured twice, then a paired t-test was used to look for differences in the variable before and after dieting. Correlations were determined using a Pearson product-moment correlation. If data were not normally distributed and could not be normalized by transformation (using a square root or log transformation), then nonparametric tests were utilized. To look for differences in nonnormally distributed data over time, the Friedman test was used, followed by the Wilcoxon signed ranks test. If variables were measured twice, then a Wilcoxon signed ranks test was utilized. A Spearman's rho correlation was used to analyze relationships between parameters that were not normally distributed. Data are presented as means ± SE. α-Values are considered significant with P ≤ 0.05. All statistical analyses were conducted using the SPSS software package, version 13.0 (SPSS, Chicago, IL).
RESULTS
During the 1st mo of dieting, available calories were reduced by 30% compared with baseline consumption, and the percentage of calories from fat in the diet was reduced from 35 to 5%. During this 1-mo period, several monkeys ate fewer calories than provided (possibly due to differences in palatability and texture between the diets), so the actual percent decrease in calorie intake was 44 ± 2.6%. At the beginning of the 2nd mo of dieting, available calories were reduced by 60% compared with baseline consumption. A few monkeys continued to eat less food than provided, so that the actual average percent reduction of caloric intake was 68 ± 0.81%.
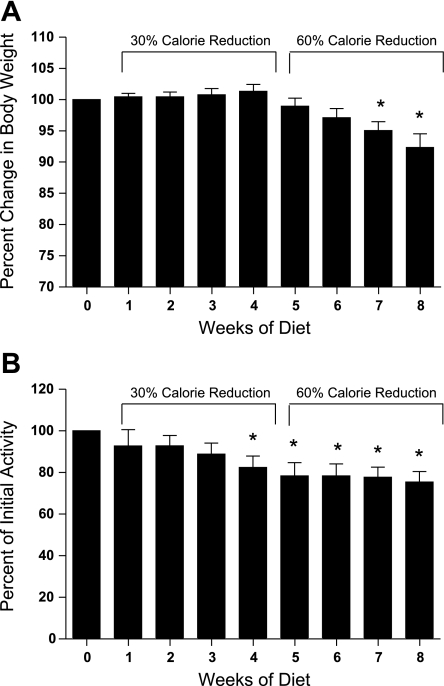
After 1 mo of dieting, there was no significant weight loss (P = 0.55). Body weight was significantly reduced by dieting (F1.85,31.5 = 19.41, P < 0.0001) during the 7th (P = 0.003) and 8th wk (P < 0.0001) of the dieting (Fig. 1A). The average percentage of weight loss over the 2-mo diet was 6.4 ± 1.7%.
Fig. 1.
A: percent change in body weight during 4 wk of 30% calorie restriction and 4 wk of 60% calorie restriction. Weight for each monkey was normalized to its body weight at the beginning of the study (week 0). B: percent change in activity measured by accelerometer during the periods of 30 and 60% calorie restriction, compared with week 0. *Significant change from baseline, P ≤ 0.05.
Body composition was measured by dual-energy X-ray absorptiometry scan at baseline and at the end of the 2-mo diet period. On average, fat mass decreased from 1,780 ± 365 to 1,568 ± 297 g [t = 2.3, degrees of freedom (df) = 15, P = 0.04; Table 1] and lean tissue mass from 5,483 ± 223 to 5,276 ± 225 g (t = 2.4, df = 15, P = 0.03; Table 1) during the 2-mo diet. However, dieting did not change percent body fat (P = 0.28; Table 1) or body fat distribution [as the percentage of fat distributed centrally (P = 0.36) and peripherally (P = 0.23) did not change; Table 1].
Table 1.
Body composition
| Baseline | After 2-mo Diet | |
|---|---|---|
| Fat mass, g | 1,780 ± 365 | 1,568 ± 297* |
| Lean tissue mass, g | 5,483 ± 223 | 5,276 ± 225* |
| Body fat, % | 20.6 ± 3.6 | 19.6 ± 3.1 |
| Central fat, % | 22.7 ± 3.9 | 22.3 ± 3.5 |
| Peripheral fat, % | 57.4 ± 9.8 | 53.2 ± 8.3 |
| Lean tissue, % | 79.4 ± 3.6 | 80.4 ± 3.1 |
Values are means ± SE.
Significant difference from baseline measures, P ≤ 0.05.
In contrast to weight loss, daily activity level began to decrease soon after placement on the diet (F3.4,47.4 = 5.13, P = 0.03; Fig. 1B) and was significantly decreased by the end of the 4th wk of dieting (18 ± 5.6% decrease in activity, P = 0.02). During the 2nd mo of dieting, physical activity was further suppressed (26 ± 7.6% decrease in activity, P < 0.0001).
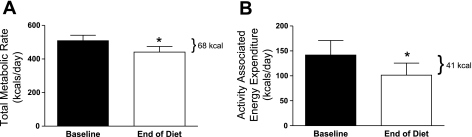
In response to the decrease in available calories, total daily energy expenditure decreased by 68 ± 12 kcal/day by the end of 2 mo of dieting (t = 5.3, df = 15, P < 0.0001; Fig. 2A). Activity-associated energy expenditure significantly decreased by 41 ± 9 kcal/day (t = −5.5, df = 16, P < 0.0001; Fig. 2B). The loss of metabolically active tissue due to dieting accounted for a decrease in energy expenditure of 21 ± 5 kcal/day. Dieting did not affect the thermic effect of an isocaloric meal (P = 0.81; data not shown) or respiratory quotient (P = 0.20; data not shown).
Fig. 2.
A: total metabolic rate, measured across a 24-h day in each monkey at baseline (solid bar) and at the end of the 8-wk diet period (open bar). The mean decrease in metabolic rate from the beginning to end of the diet period was 68 kcal. B: activity-associated energy expenditure measured across a 24-h day in each monkey at baseline (solid bar) and at the end of the 8-wk diet period (open bar). The mean decrease in activity-associated energy expenditure was 41 kcal. *Significant difference from baseline, P ≤ 0.05.
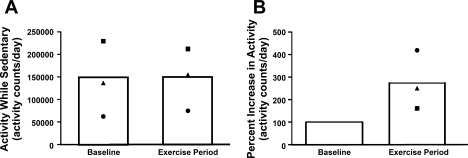
To determine whether participating in a regular exercise program that is generally recommended to prevent weight gain and promote weight loss (15) would be able to counteract the diet-induced decrease in physical activity, three additional adult female rhesus monkeys were trained to run on a treadmill at 80% maximal capacity for 1 h/day, 5 days/wk, for 3 mo. Participating in this exercise program was calculated to increase daily calorie expenditure by 69.6 ± 10.7 kcal/day (t = −5.82, df = 2, P = 0.03, equivalent to 49 kcal/day for a 7-day wk; Fig. 3A). The exercising monkeys experienced an average of a 6.1 ± 1.2% weight loss during the 3-mo exercise period. Exercising for 1 h a day did not significantly change total activity during the other 23 h of the day (t = −0.39 df = 2, P = 0.74; Fig. 3B).
Fig. 3.
A: mean activity measured across the 24-h day by accelerometer in 3 monkeys (symbols) at baseline, before monkeys started running on the treadmill 5 days/wk, and mean activity during the 23 h/day when monkeys were not running at the end of 12 wk of running (mean activity shown by bars). B: percent increase in total activity measured across the 24-h day at the end of 12 wk of running compared with the baseline period.
DISCUSSION
In this study, we characterized the compensatory decreases in activity and metabolic rate that accompany the initial stages of diet-induced weight loss in a nonhuman primate model of postmenopausal women. During the 1st mo of dieting, no significant weight loss occurred; however, daily physical activity level of the monkeys rapidly decreased after diet initiation and was significantly lower during the 4th wk of dieting (18 ± 5.6% decrease). During the 2nd mo of dieting, physical activity was further suppressed (26 ± 7.6% decrease), and, by the end of the 2nd mo of dieting, monkeys had lost a significant amount of weight (6.4 ± 1.7% of initial weight). However, it was minimal considering the substantial diet that they had been on for 2 mo. A compensatory decrease in total energy expenditure (13% decrease) occurred in response to the diet, similar in magnitude to what has been previously reported in humans during dieting (12, 13, 26, 43, 75). Two thirds of the decrease in energy expenditure resulted from the decrease in level of physical activity, and in a second study we showed a similar amount of calories is expended by undertaking a moderate exercise routine of running 5 h/wk, at a level recommended by the American College of Sports Medicine to prevent weight gain and promote weight loss (25). Thus we conclude that diet-induced decreases in the level of physical activity is the primary mechanism the body uses to defend against diet-induced weight loss, and undertaking an exercise program of 5 h of running per week is able to prevent the compensation in physical activity-induced calorie expenditure that slows diet-induced weight loss.
Activity-associated energy expenditure was reduced after 2 mo of dieting due to both the decrease in movement (i.e., amount and intensity of activity), and because it takes less energy to move a reduced body weight (1, 2). This finding supports previous reports in rhesus monkeys (32, 54) and humans (13, 33, 43, 74) that report a decrease in physical activity accompanying reductions in calorie intake. In contrast, rodents show an increase in activity in response to calorie reduction (8, 17, 45, 57). It is hypothesized that rodents increase their activity due to an increased drive to forage for food, as the elevated activity is decreased when food is made available (36). The differential regulation of physical activity in response to calorie reduction has been hypothesized to be dependent on whether an animal has a sufficient amount of stored energy to make it through a time of famine, metabolizing stored energy (slowing activity would protect their energy stores), or whether their stored energy is low, and thus survival would be dependent on finding food, and increasing activity would facilitate foraging (52, 53). A study in emperor penguins provides further support for this hypothesis, as the penguins decrease their activity in response to the first 3 mo of fasting, but, when their energy stores become depleted, their activity begins to increase (56).
A potential concern of using accelerometers mounted on collars to measure physical activity is the accuracy with which they detect whole body movement. To address this, we conducted a validation study in which we simultaneously measured physical activity by accelerometers mounted on collars and videotaped 16 monkeys (51). Frame-by-frame analysis was used to determine which behaviors generated activity counts, and we found that activity counts were strongly associated with whole body movement, but, not head, neck, or limb movement (51). More recently, we have used accelerometer-derived data to assess sleep, looked at the minute-by-minute data, and found the monkeys sit for longer periods of time than humans and that there are long stretches in the night and day in which zero activity counts are detected. Based on these studies, we believe that collar-worn accelerometers are an excellent way to accurately assess physical activity in nonhuman primates. It is also important to note that the monkeys used in experiment 1 were individually housed. Interestingly, we find that the activity level of an individual animal is similar in individual cages and group housing (65); thus we feel that the findings in this experiment are also applicable to individuals with access to a larger housing environment.
Two studies have reported that exercise can prevent a diet-induced decrease in energy expenditure (20, 47). In contrast, two other studies comparing weight loss in individuals that either dieted or dieted and exercised report similar weight loss (27), or greater weight loss in the group that only dieted (4). The studies showing an exercise-induced prevention of diet-induced energy expenditure documented participation in an exercise program, but did not directly measure daily level of physical activity, and so did not examine whether participating in purposeful exercise for a part of the day would change physical activity level during the remaining portion of the day. To address this directly, in the present study, a second group of three monkeys ran on a treadmill for 1 h/day, 5 days/wk. We found this led to a 6.1 ± 1.2% weight loss and a calculated increase in calorie expenditure of 69.6 ± 10.7 kcal/day (equivalent to 49 kcal/day for 7 days) and after a 3-mo period. Importantly, exercising did not change the amount of physical activity during the other 23 h of the day. This finding suggests that combining exercise with dieting will promote weight loss by compensating for the diet-induced decrease in energy expenditure. However, this conclusion is based on a calculation, and it would be worthwhile for future studies to directly test this hypothesis by measuring exercise-associated energy expenditure during dieting. In humans, running 1 h/day, five times a week expends between 700 (for the average woman) and 860 (for the average man) extra calories per day (2). As in monkeys, this expenditure would similarly compensate for the decrease in physical activity in response to dieting [167 kcal/day × 5 days/wk = 835 kcal (74)] and would thereby enhance diet-induced weight loss. In addition to participating in planned exercise regimens, recent studies indicate that it is possible to effectively increase daily physical activity by making small life-style changes, such as playing activity-promoting video games instead of traditional video games (39), and standing instead of sitting at desks while at school or work (40).
In this study, we found that dieting decreased both fat mass and lean tissue mass, but did not change overall percent body fat. Similar results have been reported in several human studies, which find that caloric restriction reduces both fat and lean tissue mass (48, 76). In contrast, other studies report that the majority of weight loss with a low-calorie diet is fat mass (75%) (3, 6). The loss in lean tissue with dieting is concerning, and dieting has also been reported to decrease muscle tissue mass, strength, and aerobic capacity (76). In contrast, weight loss occurring with exercise has been shown not to decrease lean tissue mass (67) and to actually improve strength and muscle mass (76). Thus combining exercise with dieting is important in maintaining physical fitness and preserving muscle mass, while still reducing body fat mass.
It is important to note that the monkeys in this study were ovariectomized females, an animal model of postmenopausal women. Several studies find that the body weight and fat responses to the initiation of exercise training and to energy deficit are sex dependent (16, 24). Thus the results of this study are most applicable to postmenopausal women, and caution should be used in extending the findings to premenopausal females and to males. Future studies are needed that objectively measure activity in premenopausal females and in males during diet-induced weight loss.
We conclude that decreased physical activity is the primary mechanism the body uses to defend against diet-induced weight loss, and an exercise program of 5 h of running per week is sufficient to prevent the diet-induced decrease in activity. As losing weight and then maintaining weight loss is quite difficult for most people, the findings of this study argue that increased emphasis should be placed on preventing weight gain over adulthood. Our previous studies indicate that the amount of physical activity that an individual undertakes is the best predictor of adult weight gain (66), suggesting that development of obesity in adulthood could be best prevented by maintaining elevated levels of physical activity across the adult years. Thus increased physical activity appears to be the most effective means of both preventing and treating adulthood obesity.
GRANTS
This work was supported by grants from the National Institutes of Health (DK55819, HD18185, MH067346, and RR00163).
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
The authors are grateful to the Division of Animal Resources at ONPRC and the University of Pittsburgh for expert care of the monkeys used in this study, and to Diana Takahashi, Lindsay Pranger, Randall Clark, Nicola Robertson, Kalen Abbott, Nathan Rockcastle, and Yasmin Aziz for technical assistance.
REFERENCES
- 1.Ainsworth BE, Haskell WL, Leon AS, Jacobs DR, Jr, Montoye HJ, Sallis JF, Paffenbarger RS., Jr Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc 25: 71–80, 1993 [DOI] [PubMed] [Google Scholar]
- 2.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, O'Brien WL, Bassett DR, Jr, Schmitz KH, Emplaincourt PO, Jacobs DR, Jr, Leon AS. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc 32: S498–S504, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Barrows K, Snook JT. Effect of a high-protein, very-low-calorie diet on body composition and anthropometric parameters of obese middle-aged women. Am J Clin Nutr 45: 381–390, 1987 [DOI] [PubMed] [Google Scholar]
- 4.Belko AZ, Van Loan M, Barbieri TF, Mayclin P. Diet, exercise, weight loss, and energy expenditure in moderately overweight women. Int J Obes 11: 93–104, 1987 [PubMed] [Google Scholar]
- 5.Bessard T, Schutz Y, Jequier E. Energy expenditure and postprandial thermogenesis in obese women before and after weight loss. Am J Clin Nutr 38: 680–693, 1983 [DOI] [PubMed] [Google Scholar]
- 6.Burgess NS. Effect of a very-low-calorie diet on body composition and resting metabolic rate in obese men and women. J Am Diet Assoc 91: 430–434, 1991 [PubMed] [Google Scholar]
- 7.Champagne CM, Baker NB, DeLany JP, Harsha DW, Bray GA. Assessment of energy intake underreporting by doubly labeled water and observations on reported nutrient intakes in children. J Am Diet Assoc 98: 426–433, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Chen D, Steele AD, Lindquist S, Guarente L. Increase in activity during calorie restriction requires Sirt1. Science 310: 1641, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Clark MK, Dillon JS, Sowers M, Nichols S. Weight, fat mass, and central distribution of fat increase when women use depot-medroxyprogesterone acetate for contraception. Int J Obes (Lond) 29: 1252–1258, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Cleland R, Graybill DC, Hubbard V, Khan LK, Stern JS, Wadden TA, Weinsier RL, Yanovski S. Commercial Weight Loss Products and Programs What Consumers Stand to Gain and Lose, edited by Gross WC, Daynard M. Washington, DC: Federal Trade Commission Building, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Dauncey MJ. Metabolic effects of altering the 24 h energy intake in man, using direct and indirect calorimetry. Br J Nutr 43: 257–269, 1980 [DOI] [PubMed] [Google Scholar]
- 12.de Boer JO, van Es AJ, Roovers LC, van Raaij JM, Hautvast JG. Adaptation of energy metabolism of overweight women to low-energy intake, studied with whole-body calorimeters. Am J Clin Nutr 44: 585–595, 1986 [DOI] [PubMed] [Google Scholar]
- 13.de Groot LC, van Es AJ, van Raaij JM, Vogt JE, Hautvast JG. Adaptation of energy metabolism of overweight women to alternating and continuous low energy intake. Am J Clin Nutr 50: 1314–1323, 1989 [DOI] [PubMed] [Google Scholar]
- 14.DiPietro L, Williamson DF, Caspersen CJ, Eaker E. The descriptive epidemiology of selected physical activities and body weight among adults trying to lose weight: the Behavioral Risk Factor Surveillance System survey, 1989. Int J Obes Relat Metab Disord 17: 69–76, 1993 [PubMed] [Google Scholar]
- 15.Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc 41: 459–471, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Donnelly JE, Smith BK. Is exercise effective for weight loss with ad libitum diet? Energy balance, compensation, and gender differences. Exerc Sport Sci Rev 33: 169–174, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Duffy PH, Feuers R, Nakamura KD, Leakey J, Hart RW. Effect of chronic caloric restriction on the synchronization of various physiological measures in old female Fischer 344 rats. Chronobiol Int 7: 113–124, 1990 [DOI] [PubMed] [Google Scholar]
- 18.Eliahou HE, Iaina A, Gaon T, Shochat J, Modan M. Body weight reduction necessary to attain normotension in the overweight hypertensive patient. Int J Obes 5, Suppl 1: 157–163, 1981 [PubMed] [Google Scholar]
- 19.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA 288: 1723–1727, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Frey-Hewitt B, Vranizan KM, Dreon DM, Wood PD. The effect of weight loss by dieting or exercise on resting metabolic rate in overweight men. Int J Obes 14: 327–334, 1990 [PubMed] [Google Scholar]
- 21.Giovannini C, Ciucci E, Clementi R, Cugini P, Facchinetti F, Negri M. Beta-endorphin, insulin, ACTH and cortisol plasma levels during oral glucose tolerance test in obesity after weight loss. Horm Metab Res 22: 96–100, 1990 [DOI] [PubMed] [Google Scholar]
- 22.Goldstein DJ. Beneficial health effects of modest weight loss. Int J Obes Relat Metab Disord 16: 397–415, 1992 [PubMed] [Google Scholar]
- 23.Grove KL, Grayson BE, Glavas MM, Xiao XQ, Smith MS. Development of metabolic systems. Physiol Behav 86: 646–660, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Hagobian TA, Sharoff CG, Stephens BR, Wade GN, Silva JE, Chipkin SR, Braun B. Effects of exercise on energy-regulating hormones and appetite in men and women. Am J Physiol Regul Integr Comp Physiol 296: R233–R242, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, Macera CA, Heath GW, Thompson PD, Bauman A. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation 116: 1081–1093, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Heilbronn LK, DE Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, Rood J, Nguyen T, Martin CK, Volaufova J, Most MM, Greenway FL, Smith SR, Deutsch WA, Williamson DA, Ravussin E. Effect of 6-mo calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA 295: 1539–1548, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heymsfield SB, Casper K, Hearn J, Guy D. Rate of weight loss during underfeeding: relation to level of physical activity. Metabolism 38: 215–223, 1989 [DOI] [PubMed] [Google Scholar]
- 28.Hoie LH, Bruusgaard D. Predictors of long-term weight reduction in obese patients after initial very-low-calorie diet. Adv Ther 16: 285–289, 1999 [PubMed] [Google Scholar]
- 29.Horm J, Anderson K. Who in America is trying to lose weight? Ann Intern Med 119: 672–676, 1993 [DOI] [PubMed] [Google Scholar]
- 30.Jeffery RW, Bjornson-Benson WM, Rosenthal BS, Lindquist RA, Kurth CL, Johnson SL. Correlates of weight loss and its maintenance over two years of follow-up among middle-aged men. Prev Med 13: 155–168, 1984 [DOI] [PubMed] [Google Scholar]
- 31.Keesey RE, Hirvonen MD. Body weight set-points: determination and adjustment. J Nutr 127: 1875S–1883S, 1997 [DOI] [PubMed] [Google Scholar]
- 32.Kemnitz JW, Weindruch R, Roecker EB, Crawford K, Kaufman PL, Ershler WB. Dietary restriction of adult male rhesus monkeys: design, methodology, and preliminary findings from the first year of study. J Gerontol 48: B17–26, 1993. [DOI] [PubMed] [Google Scholar]
- 33.Keys A, Brozek J, Henschel A, Mickelsen O, Taylor HL. The Biology of Human Starvation St. Paul, MN: University of Minnesota Press, 1950 [Google Scholar]
- 34.Klesges RC, Eck LH, Mellon MW, Fulliton W, Somes GW, Hanson CL. The accuracy of self-reports of physical activity. Med Sci Sports Exerc 22: 690–697, 1990 [DOI] [PubMed] [Google Scholar]
- 35.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346: 393–403, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koubi HE, Robin JP, Dewasmes G, Le Maho Y, Frutoso J, Minaire Y. Fasting-induced rise in locomotor activity in rats coincides with increased protein utilization. Physiol Behav 50: 337–343, 1991 [DOI] [PubMed] [Google Scholar]
- 37.Krauss RM, Eckel RH, Howard B, Appel LJ, Daniels SR, Deckelbaum RJ, Erdman JW, Jr, Kris-Etherton P, Goldberg IJ, Kotchen TA, Lichtenstein AH, Mitch WE, Mullis R, Robinson K, Wylie-Rosett J, St Jeor S, Suttie J, Tribble DL, Bazzarre TL. AHA Dietary Guidelines: revision 2000: a statement for healthcare professionals from the Nutrition Committee of the American Heart Association. Circulation 102: 2284–2299, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Lane MA, Baer DJ, Rumpler WV, Weindruch R, Ingram DK, Tilmont EM, Cutler RG, Roth GS. Calorie restriction lowers body temperature in rhesus monkeys, consistent with a postulated anti-aging mechanism in rodents. Proc Natl Acad Sci U S A 93: 4159–4164, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lanningham-Foster L, Foster RC, McCrady SK, Jensen TB, Mitre N, Levine JA. Activity-promoting video games and increased energy expenditure. J Pediatr 154: 819–823, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lanningham-Foster L, Foster RC, McCrady SK, Manohar CU, Jensen TB, Mitre NG, Hill JO, Levine JA. Changing the school environment to increase physical activity in children. Obesity (Silver Spring) 16: 1849–1853, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lappalainen R, Tuomisto MT, Giachetti I, D'Amicis A, Paquet S. Recent body-weight changes and weight loss practices in the European Union. Public Health Nutr 2: 135–141, 1999 [DOI] [PubMed] [Google Scholar]
- 42.Lean ME, Powrie JK, Anderson AS, Garthwaite PH. Obesity, weight loss and prognosis in type 2 diabetes. Diabet Med 7: 228–233, 1990 [DOI] [PubMed] [Google Scholar]
- 43.Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med 332: 621–628, 1995 [DOI] [PubMed] [Google Scholar]
- 44.Matthews CE, Freedson PS. Field trial of a three-dimensional activity monitor: comparison with self report. Med Sci Sports Exerc 27: 1071–1078, 1995 [DOI] [PubMed] [Google Scholar]
- 45.McCarter RJ, Shimokawa I, Ikeno Y, Higami Y, Hubbard GB, Yu BP, McMahan CA. Physical activity as a factor in the action of dietary restriction on aging: effects in Fischer 344 rats. Aging (Milano) 9: 73–79, 1997 [DOI] [PubMed] [Google Scholar]
- 46.Melanson EL, Jr, Freedson PS. Validity of the Computer Science and Applications, Inc (CSA) activity monitor. Med Sci Sports Exerc 27: 934–940, 1995 [PubMed] [Google Scholar]
- 47.Mole PA, Stern JS, Schultz CL, Bernauer EM, Holcomb BJ. Exercise reverses depressed metabolic rate produced by severe caloric restriction. Med Sci Sports Exerc 21: 29–33, 1989 [DOI] [PubMed] [Google Scholar]
- 48.Nicklas BJ, Wang X, You T, Lyles MF, Demons J, Easter L, Berry MJ, Lenchik L, Carr JJ. Effect of exercise intensity on abdominal fat loss during calorie restriction in overweight and obese postmenopausal women: a randomized, controlled trial. Am J Clin Nutr 89: 1043–1052, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oberman A, Wassertheil-Smoller S, Langford HG, Blaufox MD, Davis BR, Blaszkowski T, Zimbaldi N, Hawkins CM. Pharmacologic and nutritional treatment of mild hypertension: changes in cardiovascular risk status. Ann Intern Med 112: 89–95, 1990 [DOI] [PubMed] [Google Scholar]
- 50.Ogden J. The correlates of long-term weight loss: a group comparison study of obesity. Int J Obes Relat Metab Disord 24: 1018–1025, 2000 [DOI] [PubMed] [Google Scholar]
- 51.Papailiou A, Sullivan E, Cameron JL. Behaviors in rhesus monkeys (Macaca mulatta) associated with activity counts measured by accelerometer. Am J Primatol 70: 185–190, 2008 [DOI] [PubMed] [Google Scholar]
- 52.Perrigo G, Bronson FH. Behavioral and physiological responses of female house mice to foraging variation. Physiol Behav 34: 437–440, 1985 [DOI] [PubMed] [Google Scholar]
- 53.Perrigo G, Bronson FH. Foraging effort, food intake, fat deposition and puberty in female mice. Biol Reprod 29: 455–463, 1983 [DOI] [PubMed] [Google Scholar]
- 54.Rana SV, Mehta S. Effect of protein calorie malnutrition on in vitro incorporation of (U-C14)-glucose in brain of young rhesus monkeys. Indian J Exp Biol 29: 259–262, 1991. [PubMed] [Google Scholar]
- 55.Redman LM, Heilbronn LK, Martin CK, de Jonge L, Williamson DA, Delany JP, Ravussin E. Metabolic and behavioral compensations in response to caloric restriction: implications for the maintenance of weight loss. PLoS One 4: e4377, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Robin JP, Boucontet L, Chillet P, Groscolas R. Behavioral changes in fasting emperor penguins: evidence for a “refeeding signal” linked to a metabolic shift. Am J Physiol Regul Integr Comp Physiol 274: R746–R753, 1998 [DOI] [PubMed] [Google Scholar]
- 57.Russell JC, Epling WF, Pierce D, Amy RM, Boer DP. Induction of voluntary prolonged running by rats. J Appl Physiol 63: 2549–2553, 1987 [DOI] [PubMed] [Google Scholar]
- 58.Serdula MK, Mokdad AH, Williamson DF, Galuska DA, Mendlein JM, Heath GW. Prevalence of attempting weight loss and strategies for controlling weight. JAMA 282: 1353–1358, 1999 [DOI] [PubMed] [Google Scholar]
- 59.Shadoan MK, Anthony MS, Rankin SE, Clarkson TB, Wagner JD. Effects of tibolone and conjugated equine estrogens with or without medroxyprogesterone acetate on body composition and fasting carbohydrate measures in surgically postmenopausal monkeys. Metabolism 52: 1085–1091, 2003 [DOI] [PubMed] [Google Scholar]
- 60.Stern JS, Hirsch J, Blair SN, Foreyt JP, Frank A, Kumanyika SK, Madans JH, Marlatt GA, ST Jeor ST, Stunkard AJ. Weighing the options: criteria for evaluating weight-management programs. The Committee to Develop Criteria for Evaluating the Outcomes of Approaches to Prevent and Treat Obesity. Obes Res 3: 591–604, 1995 [PubMed] [Google Scholar]
- 61.Strychar I. Diet in the management of weight loss. CMAJ 174: 56–63, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stunkard A, McLaren-Hume M. The results of treatment for obesity: a review of the literature and report of a series. AMA Arch Intern Med 103: 79–85, 1959 [DOI] [PubMed] [Google Scholar]
- 63.Stunkard A, McLaren-Hume M. The results of treatment for obesity: a review of the literature and report of a series AMA. Arch Intern Med 103: 79–85, 1959 [DOI] [PubMed] [Google Scholar]
- 64.Sullivan EL, Daniels AJ, Koegler FH, Cameron JL. Evidence in female rhesus monkeys (Macaca mulatta) that nighttime caloric intake is not associated with weight gain. Obes Res 13: 2072–2080, 2005. [DOI] [PubMed] [Google Scholar]
- 65.Sullivan EL, Koegler FH, Cameron JL. Energy expenditure resulting from physical activity is similar in female rhesus monkeys (Macaca mulatta) housed in group pens vs. single cages. In: Proceedings of the 28th Annual Meeting of the American Society of Primatologists, Portland, OR, 2005 Seattle, WA: American Society of Primatologists, 2005 [Google Scholar]
- 66.Sullivan EL, Koegler FH, Cameron JL. Individual differences in physical activity are closely associated with changes in body weight in adult female rhesus monkeys (Macaca mulatta). Am J Physiol Regul Integr Comp Physiol 291: R633–R642, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Svendsen OL, Hassager C, Christiansen C. Effect of an energy-restrictive diet, with or without exercise, on lean tissue mass, resting metabolic rate, cardiovascular risk factors, and bone in overweight postmenopausal women. Am J Med 95: 131–140, 1993 [DOI] [PubMed] [Google Scholar]
- 68.Tanko LB, Christiansen C. Effects of 17beta-oestradiol plus different doses of drospirenone on adipose tissue, adiponectin and atherogenic metabolites in postmenopausal women. J Intern Med 258: 544–553, 2005 [DOI] [PubMed] [Google Scholar]
- 69.Treuth MS, Sherwood NE, Baranowski T, Butte NF, Jacobs DR, Jr, McClanahan B, Gao S, Rochon J, Zhou A, Robinson TN, Pruitt L, Haskell W, Obarzanek E. Physical activity self-report and accelerometry measures from the Girls Health Enrichment Multi-site Studies. Prev Med 38, Suppl: S43–S49, 2004 [DOI] [PubMed] [Google Scholar]
- 70.Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 344: 1343–1350, 2001 [DOI] [PubMed] [Google Scholar]
- 71.Vogels N, Diepvens K, Westerterp-Plantenga MS. Predictors of long-term weight maintenance. Obes Res 13: 2162–2168, 2005 [DOI] [PubMed] [Google Scholar]
- 72.Wadden TA, Sternberg JA, Letizia KA, Stunkard AJ, Foster GD. Treatment of obesity by very low calorie diet, behavior therapy, and their combination: a five-year perspective. Int J Obes 13, Suppl 2: 39–46, 1989 [PubMed] [Google Scholar]
- 73.Wagner JE, Kavanagh K, Ward GM, Auerbach BJ, Harwood HJ, JR, Kaplan JR. Old world nonhuman primate models of type 2 diabetes mellitus. ILAR J 47: 259–271, 2006 [DOI] [PubMed] [Google Scholar]
- 74.Wang X, Lyles MF, You T, Berry MJ, Rejeski WJ, Nicklas BJ. Weight regain is related to decreases in physical activity during weight loss. Med Sci Sports Exerc 40: 1781–1788, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Webb P, Abrams T. Loss of fat stores and reduction in sedentary energy expenditure from undereating. Hum Nutr Clin Nutr 37: 271–282, 1983 [PubMed] [Google Scholar]
- 76.Weiss EP, Racette SB, Villareal DT, Fontana L, Steger-May K, Schechtman KB, Klein S, Ehsani AA, Holloszy JO. Lower extremity muscle size and strength and aerobic capacity decrease with caloric restriction but not with exercise-induced weight loss. J Appl Physiol 102: 634–640, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Williams JK, Kaplan JR, Suparto IH, Fox JL, Manuck SB. Effects of exercise on cardiovascular outcomes in monkeys with risk factors for coronary heart disease. Arterioscler Thromb Vasc Biol 23: 864–871, 2003 [DOI] [PubMed] [Google Scholar]
- 78.Williams NI, Berga SL, Cameron JL. Synergism between psychosocial and metabolic stressors: impact on reproductive function in cynomolgus monkeys. Am J Physiol Endocrinol Metab 293: E270–E276, 2007 [DOI] [PubMed] [Google Scholar]
- 79.Williams NI, Caston-Balderrama AL, Helmreich DL, Parfitt DB, Nosbisch C, Cameron JL. Longitudinal changes in reproductive hormones and menstrual cyclicity in cynomolgus monkeys during strenuous exercise training: abrupt transition to exercise-induced amenorrhea. Endocrinology 142: 2381–2389, 2001 [DOI] [PubMed] [Google Scholar]
- 80.Williams NI, Helmreich DL, Parfitt DB, Caston-Balderrama A, Cameron JL. Evidence for a causal role of low energy availability in the induction of menstrual cycle disturbances during strenuous exercise training. J Clin Endocrinol Metab 86: 5184–5193, 2001 [DOI] [PubMed] [Google Scholar]
- 81.Williamson DF, Serdula MK, Anda RF, Levy A, Byers T. Weight loss attempts in adults: goals, duration, and rate of weight loss. Am J Public Health 82: 1251–1257, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wing RR, Hill JO. Successful weight loss maintenance. Annu Rev Nutr 21: 323–341, 2001 [DOI] [PubMed] [Google Scholar]
- 83.Wing RR, Phelan S. Long-term weight loss maintenance. Am J Clin Nutr 82: 222S–225S, 2005. [DOI] [PubMed] [Google Scholar]