Abstract
We aimed to identify which cytochrome P-450 (CYP) family/subfamily, as well as related transcription factor(s), is responsible for the estrogen-dependent synthesis of epoxyeicosatrienoic acids (EETs) to initiate shear stress-induced vasodilation. Microarray analysis indicated a significant upregulation of CYP2C29 and retinoid X receptor γ (RXRγ) in isolated mesenteric arteries/arterioles of female endothelial nitric oxide synthase-knockout mice, a result that was validated by real-time RT-PCR. The cannulated vessels were then perfused with 2 and 10 dyn/cm2 shear stress, followed by collection of the perfusate to determine EET concentrations and isoforms. Shear stress dose-dependently stimulated the release of EETs into the perfusate, associated with an EET-mediated vasodilation, in which predominantly 14,15-EET and 11,12-EET contributed to the responses (∼87.4% of total EETs). Transfection of vessels with CYP2C29 siRNA eliminated the release of EETs into the perfusate, which was evidenced by an abolished vasodilation, and confirmed by RT-PCR and Western blot analyses. Knockdown of RXRγ in these vessels significantly inhibited the production of EETs, parallel to a reduced vasodilation. RXRγ siRNA not only silenced the vascular RXRγ expression, but synchronously downregulated CYP2C29 expression, leading to a reduced EET synthesis. In conclusion, our data provide the first evidence for a specific signaling cascade, by which estrogen potentially activates the CYP2C29 gene in the absence of nitric oxide, to synthesize EETs in response to shear stress, via an RXRγ-related regulatory mechanism.
Keywords: estrogen, shear stress, endothelium cytochrome P-450, vasodilation
epoxyeicosatrienoic acids (EETs) are metabolized from arachidonic acid by cytochrome P-450 (CYP) epoxygenases. The activation of CYP/epoxygenases in vascular endothelial cells is an important step in the initiation of nitric oxide (NO)- and prostaglandin-independent vasodilation, and therefore, EETs have been characterized as endothelium-derived hyperpolarizing factors (EDHFs) (1, 4, 11). In addition to the regulation of vascular tone, the role of EETs in the modulation of cell-signaling cascades involving cell migration and proliferation, as well as angiogenesis, has received considerable attention (11, 20). More then 500 CYP genes have been identified and categorized into 78 families (16). Specifically, the epoxygenases of the CYP2C family, including CYP2C8, 2C9, 2C10 in humans, 2C34 in pigs, and 2C11 and 2C23 in rodents, generate a series of regiospecific and stereospecific EETs, such as 5,6-, 8,9-, 11,12-, and 14,15-EET, in the vascular endothelium. To date, increasing evidence highlights a physiologically significant CYP2C gene family in the mediation of EDHF-dependent responses initiated by physiological stimuli, such as shear stress (4, 12).
Published studies from our laboratory demonstrated that there is a sex difference in the mediation of shear stress-induced dilation, as a function of NO deficiency, which is characterized as a prostaglandin-mediated dilator response in vessels of male, but EET mediation of the response in female mice/rats (4, 17, 21). EETs are transferable mediators causing flow/shear stress-induced dilator responses, via directly hyperpolarizing vascular smooth muscle cells (4, 5). Also, the shear stress-induced release of EETs is estrogen dependent and occurs via an estrogen receptor (ER)-mediated activation of the PI3K/Akt signaling pathway, leading to transcriptional upregulation of CYP (6, 7). CYP2C is constitutively expressed in vascular endothelial cells (1, 2). It is believed to be inducible as evidence by the mRNA and protein levels in response to activation of several nuclear hormone receptors (NHRs), such as sex hormone receptors, pregnane X receptor (PXR), retinoid X receptor (RXR), and peroxisome proliferator-activated receptor (PPAR) (16).
Given the size of the CYP gene family, identification of specific gene(s) responsible for the estrogen-dependent upregulation of EET production in vessels was the first aim that guided our studies. Searching the literature so far, no evidence—on the basis of gene or protein expression—has been provided to answer the question of whether estrogen per se regulates the CYP2C expression, and if so, which NHRs are involved. Therefore, the present study aimed to address the issue of the signaling cascades involving the specific gene(s) of the CYP family/subfamily and related transcriptional factor(s) that are targeted/activated by estrogen to synthesize EETs, via performing microarray analysis of CYPs on single resistance arteries. On the basis of the gene profile detected, the specific target protein and its product(s) were characterized. The functional significance of the target gene(s)/protein/product(s) responsible for the estrogen-specific regulation of endothelial release of EETs to shear stress was confirmed by transfection of the vessels with specific short-interfering RNA (siRNA) oligonucleotides.
MATERIALS AND METHODS
Animals
Twelve- to fourteen-week-old endothelial nitric oxide synthase-knockout (eNOS-KO) and wild-type (WT) mice of both sexes were purchased from Jackson Laboratories (Bar Harbor, ME). All protocols were approved by the Institutional Animal Care and Use Committee of New York Medical College and conform to the guidelines of the National Institutes of Health and the American Physiological Society for the use and care of laboratory animals.
Microarray
Sample preparation.
Mice were killed by inhalation of 100% CO2. Four or five single mesenteric arteries/arterioles (∼7 mm in length), freshly isolated from eNOS-KO and WT mice, were pooled and pulverized in liquid nitrogen. Approximately ten micrograms of total RNA were isolated using a RNA isolation kit (TRIzol reagent, Sigma, St. Louis, MO, USA).
Isolation of RNA.
Total RNA was extracted using a commercial RNA isolation kit (TRIzol reagent; Sigma). Two quality control measures were carried out on a small aliquot of the isolated RNA samples: 1) a spectrophotometric analysis to confirm the concentration and to detect contaminating proteins and other molecules, and 2) a size fractionation procedure using the Agilent Bioanalyzer 2100 (Agilent Technologies, Colorado Springs, CO) to determine whether the RNA was intact.
Microarray labeling and hybridization.
These procedures were performed by SuperArray Bioscience (Frederick, MD). Oligo GEArray mouse toxicology and drug resistance microarray (SuperArray, Oligo GEArray OMM-401) were used. This microarray profiles the expression of 263 genes involved in the CYP family and related transcription factors, the list of which is available at www.superarray.com. Ten arrays from 2 WT and 3 eNOS-KO mice of each sex were performed.
The labeling and hybridization were carried out according to the instructions of the manufacturer (Superarray, Bethesda, MD). In brief, total RNA (2 μg) was annealed to 3-μl specific primers at 70°C for 3 min. Then, 10-μl master labeling mix, containing 4 μl special 5× GEA labeling buffer, 2 μl biotin-16-dUTP (1 mmol/l), 1 μl RNase inhibitor, and 1 μl reverse transcriptase (50 U/μl; Promega, Madison, WI) were added, and reverse transcription was performed for 2 h at 42°C. Oligo GEArray membranes were rinsed with DEPC-treated water and prehybridized with GEQ-hybridization solution containing sheared salmon sperm DNA (Sigma) for 2 h. After denaturation of labeled cDNA probes, membranes were hybridized at 60°C overnight. Then membranes were washed (twice in 2× SSC, 1% SDS for 10 min, then twice in 0.1× SSC, 0.5% SDS for 10 min), blocked in GEA-blocking solution Q (for 40 min), and incubated with alkaline phosphatase-conjugated streptavidine in 1:5,000 dilution for 30 min at room temperature. CDP-Star was used as chemiluminescent substrate. Membranes were exposed to X-ray film. Images were digitized (resolution: 300 dpi) using a charge-coupled device camera-based imaging system (Alpha Innotech, San Leandro, CA). Analysis of data was performed with an image analysis software by GEArrayAnalyzer software (SuperArray). Spots with anomalous pixel intensity-to-background ratios were filtered out. Background-corrected densities of each spot were normalized to the signals of the housekeeping gene GAPDH, and the mean value and standard deviation of normalized data from each array were computed. Pairwise comparisons were made between individual samples in each group, using Excel software. Genes that are upregulated or downregulated between sexes, as well as between WT and eNOS-KO mice, were listed and characterized. An average fold change, derived from all possible pairwise comparisons of 2.0 or greater, was used as the cut-off for significant differences in gene expression.
Quantitative real-time RT-PCR.
The microarray data were validated using a real-time quantitative PCR (LightCycler; Roche Diagnostics, Indianapolis, IN). Oligonucleotide primers for CYP2C29 and RXRγ primers were purchased from Qiagen (QIAGEN, nos. QT01076880 and QT00093590), and their expressions were normalized to the GAPDH (17). A relative quantitation method (ΔΔCt) was used to evaluate the relative expression of each gene between different groups of animals. All primer products were verified on a 1.5% agarose gel.
Perfused arteries and sample collection.
First-order mesenteric arteries were isolated from both sexes of eNOS-KO mice and cannulated in a vessel chamber filled with PSS (37°C) at 80 mmHg of intraluminal pressure. After equilibration, a known level of shear stress (2 and 10 dyn/cm2) was continually administered to the vessel that had been fully dilated with 10−4 M adenosine, for 10 min, and the perfusate was collected in the outflow tubing (5).
Quantitation and qualitation of EETs in perfusate.
Perfusate EETs were quantitated and qualitated by GC-MS and LC-MS, respectively. Two nanograms of 11,12-EET-d8 were added to the perfusate as an internal standard. After extraction, samples for GC-MS analysis were reconstituted in 80 μl acetonitrile and separated by HPLC to obtain total EET fractions. After derivatization, the samples were reconstituted in 50 μl iso-octane, and a 2-μl aliquot was injected into a GC-MS. Endogenous EETs were determined by comparison of GC retention time with authentic 11,12-EET-d8 (mass-to-charge ratio, m/z = 327.2) standard, quantified by calculating the ratio of abundance and further normalized by endothelial area of the vessels (mm2) (4–6). Extracted samples for LC-MS analysis were directly reconstituted in 18-μl acetonitrile, and a 2-μl aliquot was injected into a LC-MS. EET isoforms were characterized on the basis of their retention time with m/z (319.2) and expressed as the percentage of total EETs (8).
Flow/shear stress-induced dilation.
After 1-h equilibration, vessels developed spontaneous tone at 80 mmHg pressure. In the presence of indomethacin (10−5 M), initial shear stress of 2 and 10 dyn/cm2 was applied to the pressurized mesenteric arterioles of female eNOS-KO mice, and changes in diameter were recorded in control vessels and vessels transfected with specific siRNA.
Western blots.
Samples were loaded on a 10% SDS-PAGE gel and transferred to a PVDF membrane. Membranes were probed with primary antibodies of human CYP2C9 (Biodesign International, Saco, ME), or RXRγ (Santa Cruz Biotechnology, Santa Cruz, CA) and secondary antibodies. Specific bands were normalized to actin. Highly homologous and comparable product profiles between human CYP2C9 and mouse CYP2C29 have already been proven (15).
Experimental Protocols
Control study.
Perfusate samples from 2 and 10 dyn/cm2 shear stress-stimulated vessels were collected from control vessels; and the vessels that had been intraluminally and extraluminally administered 6-(2-proparglyoxyphenyl) hexanoic acid (PPOH; 5 × 10−5M) for 45 min to inhibit CYP/epoxygenase, for the measurement of EETs. In separate experiments, flow-induced dilation was also assessed. At the end of experiments, perfused vessels were snap frozen in liquid nitrogen to determine CYP2C29 or RXRγ mRNA and protein.
RNA Interference Study
Vessel culture perfusion system.
This has been described in detail previously (18). Briefly, all perfusion chambers, tubing, reservoirs, and connectors were autoclaved prior to use. The perfusion system was placed in a vertical cell-culture hood (EdgeCARD Hood, Baker Company, Sanford, ME) to maintain a sterile environment. The system consists of six 1-ml perfusion chambers that provide an identical experimental environment to six single vessels treated with different agents. The intravascular pressure of the vessels was maintained by six separate pressure reservoirs. The height of the reservoir was precisely controlled. Intraluminal flow was generated by a linear syringe pump coupled with an in-line pressure transducer to monitor the inflow pressure. The outflow pressure (the height of the reservoir) was adjusted accordingly to maintain intravascular pressure constant. The flow rate was adjusted within submicroliters per minute range. The diameter of vessels was measured by a microscope-television-image-shearing system and recorded in a computer.
RNA interference study.
The efficiency and specificity for siRNA transfection in isolated vessels have been proven in our previous study (18).
In each experiment, six first-order mesenteric arteries were cannulated in perfusion chambers. The vessels were superfused with DMEM with 1% antibiotic antimycotic solution without serum. After a 1-h equilibration, three vessels were transfected with CYP2C29 siRNA or RXRγ siRNA. The siRNA was mixed initially with 3 μl HiPerFect transfection reagent (Qiagen, Valencia, CA) per 100 μl DMEM at room temperature for 10 min. The mixture was further diluted 1:3 with DMEM to a final concentration of 25 nmol/l siRNA. The siRNA mixture was then administered intraluminally and extraluminally to the cannulated vessels at 37 C for 4–6 h without flow. The other three vessels were either untranfected as time-course controls, or transfected with nonsilencing siRNA. The vessels were then washed with DMEM and further incubated at 40 mmHg of intravascular pressure with 17β-estradiol (10−9 M) and 2 dyn/cm2 shear stress for 72 h. After that, shear stress-induced release of EETs and vasodilation were assessed by repeating the control experiments. The specificity for CYP2C29 and RXRγ siRNA transfection in the vessels was validated by real-time RT-PCR and Western blot at the end of experiments.
Chemicals
The RNA interference (RNAi) human/mouse Starter Kit and nonsilencing siRNA (ALLStars Negative Controls) as well as the primers, were purchased from Qiagen. All other chemicals were obtained from Sigma (St. Louis, MO).
Statistics
Data are expressed as means ± SE. N/n refers the number of mice/vessels. Statistical analysis was performed using repeated-measures ANOVA followed by the Tukey-Kramer post hoc test and the Student's t-test. Statistical significance was accepted at a level of P < 0.05.
RESULTS
Roles of Sex and NO Deficiency
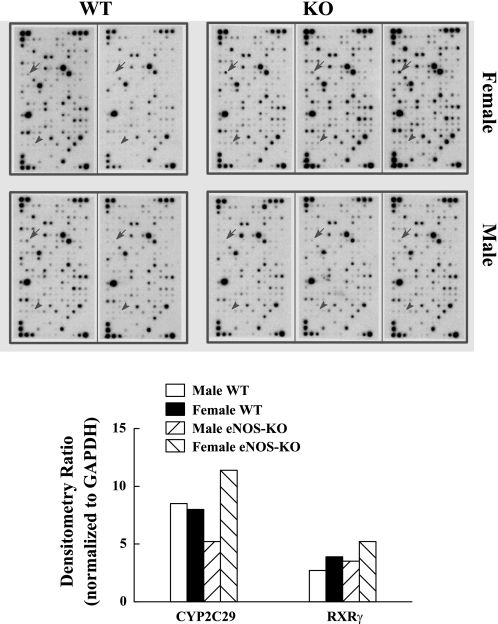
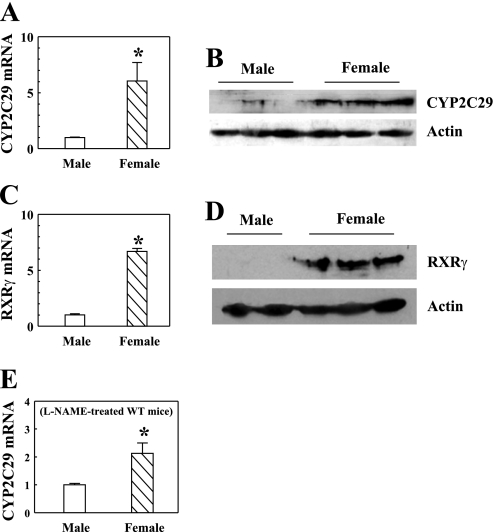
Microarray results focusing on the transcription factor and CYP global genes indicated a specific upregulation of CYP2C29 and RXRγ in female eNOS-KO vessels compared with male eNOS-KO, as well as to WT vessels of both sexes (Fig. 1). The difference was validated by real-time RT-PCR (Fig. 2, A and C) and Western blot analysis (Fig. 2, B and D). Importantly, there was no significant difference in the expression of CYP2C29 between the vessels of WT male and female mice, nor between the vessels of male WT and eNOS-KO mice, implying the necessity of both a female state and NO deficiency for the upregulation. To exclude the possibility that the upregulation of CYP2C29 might be a nonspecific result due to the knockout of certain gene(s), we also assessed CYP2C29 mRNA in mesenteric arteries isolated from WT male and female mice treated with nitro-l-arginine methyl ester (l-NAME) for 3 wk. Results indicated a significant increase in CYP2C29 mRNA in vessels of l-NAME-treated female WT compared with l-NAME-treated male WT mice (Fig. 2E), confirming the role of NO deficiency in the CYP2C29 expression in female vessels.
Fig. 1.
Top: microarray of isolated mesenteric arteries of wild-type (WT) male (n = 2) and female (n = 2), and endothelial nitric oxide synthase-knockout (eNOS-KO) male (n = 3) and female (n = 3) mice. A total of 288 (12 × 24) dots representing 263 genes in each array were analyzed. They were numbered in rows from the top-left to the bottom-right sequentially. Dots 1, 2, and 13 are the genes for GAPDH, used to normalize loading variations. Dots 86 (indicated by arrows) and 231 (indicated by arrowheads) are the genes for CYP2C29 and retinoid X receptor γ (RXRγ), respectively. Bottom: summarized densitometry ratio normalized to GAPDH (dot 2).
Fig. 2.
CYP2C29 mRNA (A) and protein expression (B); RXRγ mRNA (C) and protein expression (D) determined by real-time RT-PCR and Western blot analyses in mesenteric arteries of eNOS-KO male (n/n = 5/5) and female (n/n = 5/5) mice. E: CYP2C29 mRNA in isolated mesenteric arteries of WT male (n/n = 5/5) and female (n/n = 5/5) mice treated with nitro-l-arginine methyl ester (l-NAME; 50 mg/100 ml drinking water) for 3 wk. The mRNA data were normalized to GAPDH. *P < 0.05 compared with male mice.
CYP2C29 mRNA, Protein, Perfusate EETs, and Vasodilation in Shear Stress-Stimulated Vessels
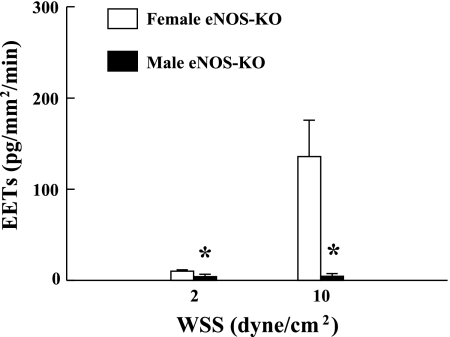
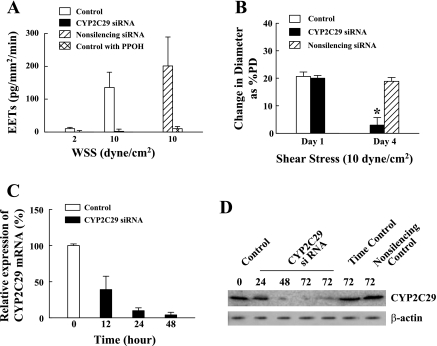
On the basis of the microarray studies, we hypothesized that an upregulation of CYP2C29 is responsible for the shear stress-stimulated release of EETs and flow-induced dilation in female NO-deficient vessels (3, 4, 6, 7, 21). Thus, we perfused isolated vessels of eNOS-KO mice with different levels of shear stress, followed by measurement of EET concentrations and determination of EET isoforms in the perfusate. Average diameter and length of perfused vessels were 226.8 ± 7.1 μm and 6.8 ± 0.2 mm, respectively, in female mice; and 245.7 ± 10.1 μm and 11.2 ± 1.1 mm, respectively, in male mice. In response to the stimulation with 2 and 10 dyn/cm2 shear stress, female eNOS-KO arteries demonstrated a dose-dependent release of EETs. Whereas, the release of EETs in male eNOS-KO vessels reached only the lowest detectable margin (Fig. 3), suggesting a negligible contribution of EETs to the mediation of flow/shear stress-induced dilation of male eNOS-KO arteries. Thus, the following studies were focused on female NO-deficient arteries that release EETs in response to shear stress. The efficiency and specificity for siRNA transfection in isolated vessels are shown in Fig. 4. Fig. 5A provides a link between CYP2C29 and release of EETs, as well as EET-mediated vasodilation (Fig. 5B), by transfection of vessels with CYP2C29 siRNA. In control conditions, shear stress stimulated the release of EETs from vascular endothelium, a response that was prevented by PPOH. After treatment of the vessels with CYP2C29 siRNA, shear stress-stimulated release of EETs in the perfusate was essentially undetectable but was maintained in the vessels that were transfected with nonsilencing siRNA (Fig. 5A), verifying a CYP2C29-dependent release of EETs. Consistently, EET-mediated shear stress-induced dilations were also eliminated after transfection of the vessels with CYP2C29 siRNA, whereas, vessels transfected with nonsilencing siRNA maintained dilator responses that were identical to those recorded before transfection (day 1) (Fig. 5B). Molecular evidence for the specific knockdown of CYP2C29 mRNA and protein expression in siRNA-transfected vessels was also provided by showing that after transfection for 24 h, arterial expression of CYP2C29 mRNA was reduced by 90% (Fig. 5C), whereas, most protein downregulation (CYP2C29 enzyme) occurred after transfection for 48 h (Fig. 5D), indicating the time necessary for target enzyme degradation. In both untransfected and nonsilencing siRNA transfected controls, CYP2C29 protein expression was maintained. Thus, results obtained from biochemical and molecular analyses, in combination with functional studies, strongly suggest that CYP2C29 is the EET synthase responsible for the shear stress-induced dilation in NO-deficient female vessels.
Fig. 3.
Quantitation of epoxyeicosatrienoic acids (EETs) in the perfusate collected from mesenteric arteries stimulated with shear stress (2 and 10 dyn/cm2) for 10 min, of male (n/n = 5/8) and female (n/n = 10/18) eNOS-KO mice. *P < 0.05 compared with female eNOS-KO mice.
Fig. 4.
Alexa Fluor 488-labeled nonsilencing siRNA (green; A) and propidium iodide-labeled nuclei (red; B) in the endothelial layer. C: merged image of A and B, showing an overlap of fluorescent staining from endothelial nuclei (red) and cytoplasm stained with fluorescently labeled siRNA (green).
Fig. 5.
A: quantitation of EETs in the perfusate collected from shear stress (2 and 10 dyn/cm2)-stimulated mesenteric arteries of female eNOS-KO mice in control conditions (n/n = 5/9), in the presence of 6-(2-proparglyoxyphenyl) hexanoic acid (PPOH; n/n = 5/7), and transfected with CYP2C29 siRNA (n/n = 5/12), or nonsilencing siRNA (n/n = 5/7) for 72 h, respectively. *P < 0.05 compared with control. †P < 0.05 compared to nonsilencing siRNA. B: shear stress (10 dyn/cm2)-induced vasodilation in control conditions (n/n = 5/10) (day 1) and after transfection with CYP2C29 siRNA (n/n = 5/10) or nonsilencing siRNA (n/n = 5/10) for 72 h (day 4). *P < 0.05 compared with nonsilencing siRNA. C and D: CYP2C29 mRNA (C) and protein (D) expressions in mesenteric arteries in control conditions (including untransfected for zero and 72 h), and transfected with CYP 2C29 siRNA, or nonsilencing siRNA, at different time points.
Role of RXRγ in the Mediation of CYP Signal Transduction
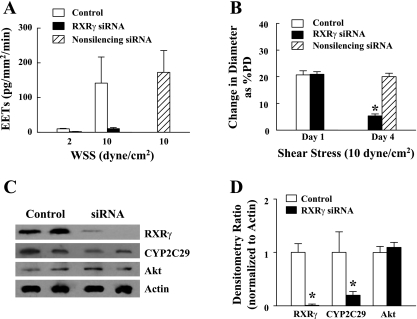
The finding of the upregulation of vascular RXRγ in an estrogen- and NO deficiency-dependent manner forms the basis of the following experiments presented in Fig. 6. Similar to the results shown in Fig. 5, shear stress dose-dependently stimulated the release of EETs in control vessels, a response that was eliminated by transfection of the vessels with specific RXRγ siRNA but was maintained in nonsilencing siRNA controls (Fig. 6A). Parallel to the abolished EET production in response to knockdown of RXRγ, EET-mediated shear stress-induced dilation was also significantly attenuated compared with the control, as well as nonsilencing siRNA control vessels (Fig. 6B), implying a role for RXRγ in the signaling pathway. Interestingly, knockdown of arterial RXRγ mRNA was also associated with a downregulation of CYP2C29 expression (Fig. 6, C and D), which accounts for the prevention of EET release (Fig. 6A) and the inhibition of shear stress-induced dilation (Fig. 6B) in these vessels, whereas, the expression of Akt was not affected by RXRγ siRNA. These results indicate that CYP2C29, as an EET synthase, is regulated by RXRγ.
Fig. 6.
A: quantitation of EETs in the perfusate collected from shear stress (2 and 10 dyn/cm2)-stimulated mesenteric arteries of female eNOS-KO mice in control conditions (n/n = 5/9) and transfected with RXRγ siRNA (n/n = 5/6) or nonsilencing siRNA (n/n = 5/5) for 72 h, respectively. *P < 0.05 compared with control. B: shear stress (10 dyn/cm2)-induced vasodilation in control conditions (n/n = 5/9) (day 1) and after transfection with RXRγ (n/n = 5/5) or nonsilencing siRNA (n/n = 4/5) for 72 h (day 4). *P < 0.05 compared with nonsilencing siRNA. C and D: original (C) and summarized data (D, 3 blots) for RXRγ, CYP2C29, and Akt protein expression of vessels in control conditions and transfected with RXRγ siRNA. *P < 0.05 compared with control.
EET Isoforms in the Perfusate
To clarify which specific EET(s) functions as downstream effector(s) of CYP2C29 in the estrogen-dependent signaling cascade, perfusate EETs were qualitatively analyzed by LC-MS. Results indicated that 14,15-EET and 11,12-EET are the major isoforms that account for 56.2% and 31.2% of total EETs released from shear stress-stimulated vessels. 8,9-EET and 5,6-EET provide lesser contributions (by ∼10.3% and 2.3%, respectively) to the responses.
DISCUSSION
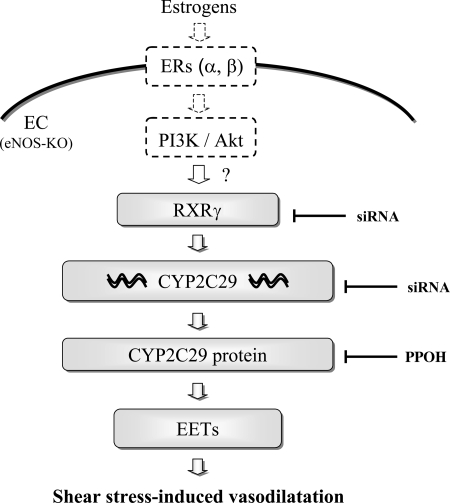
In combination with our previous findings, we drew conclusions depicted in Fig. 7, showing that when estrogens combine with/stimulate their membrane-bound estrogen receptors (ERs) on the endothelium, they activate tyrosine kinase (PI3K) phosphorylation, followed by activation of Akt (6, 7). The downstream transcription factor (RXRγ) is activated to initiate CYP2C29 gene expression. The target gene triggers the synthesis of CYP2C29 enzyme to produce EETs that are released from the endothelium (Figs. 5A and 6A) to initiate shear stress-induced vasodilation (Figs. 5B and 6B). To the best of our knowledge, this is the first study that profiles the specific cascade responsible for the estrogen-dependent upregulation of EET synthesis in NO-deficient resistance vessels, at the level of vascular gene expression, protein synthesis, product formation, and vascular functions. Additional salient findings of the present study are that we successfully screened CYP genes via microarray analysis in microvessels; and quantitated EET concentrations, as well as characterized EET isoforms in the perfusate passing through single mouse arteries stimulated with shear stress, neither technique of which was reported previously.
Fig. 7.
Schematic of signaling cascade of estrogen-dependent pathways in the absence of eNOS. When estrogen receptors (ERs) are activated, they, in turn, activate PI3 kinase (PI3K) to phosphorylate Akt, followed by activation of RXRγ to initiate CYP2C29 gene and protein expression, leading to the synthesis of EETs to cause vasodilation. Arrows indicate activation; flat arrows indicate inhibition. The upstream signaling effectors shown with a dotted line are based on our previous findings (6, 7). The question mark denotes an, as yet, unresolved mechanism.
Estrogen, NO, and CYP
We noted that CYP2C29 and RXRγ gene expression was increased only in female NO-deficient vessels (Figs. 1 and 2), revealing an essential role of estrogen and NO deficiency in the mediation of the response. This conclusion was further proven by the evidence of a significant release of EETs, in response to shear stress, in vessels of females vs. the lowest detectable level in those of males (Fig. 3). These results well explain and extend our previous findings that EET-mediated flow-induced dilation was only observed in female NO-deficient arteries/arterioles and was prevented by ovariectomy, as well as an ER antagonist but was restored by estrogen replacement (6, 7, 18, 21). ERs have been categorized as NHRs, transcriptional regulators responsible for endobiotically and xenobiotically mediated induction of CYP (13), by perhaps, cross-talk with other nuclear receptors, to control signaling pathways (14). In the present study, we suggested that the modulation of CYP2C29 by estrogen-dependent activation of other NHRs, such as RXRγ or PPARγ that was also upregulated in female eNOS-KO vessels (data not shown), initiates an increase in gene expression. Indeed, Liu et al. (9) described a role for PPARγ in the mediation of EET-related anti-inflammatory effect of shear stress, which, however, characterizes PPARγ to be a downstream effector rather than an upstream regulator of EET synthesis (9).
CYP enzyme activity and its contribution to the regulation of vascular function are dampened under physiological conditions and become discernible, in most instances, only in connection with an impaired endothelial NO bioavailability. Increasing evidence focused on the direct inhibitory effect of NO on CYP activity (3); our findings, however, were demonstrated to be genomically based, since this response was abolished by a transcriptional inhibitor (6). Additionally, we also noted that vascular EET concentrations were synchronously increased, along with the duration of chronic l-NAME treatment (unpublished data). These results support our hypothesis that estrogen and NO deficiency are essential factors for the upregulation of CYP2C29.
Perfusate EETs and Vasodilation in Response to Shear Stress
When EETs are synthesized in the endothelium, they are rapidly incorporated into membrane phospholipids, from which they are released in response to any of the stimuli that activate phospholipases (19), such as shear stress. Shear stress-induced dilation in vitro is an acute response that does not affect CYP gene/protein expression but stimulates endothelial cells to release presynthesized EETs. On the other hand, shear stress induces an increase in intracellular Ca2+, followed by the activation of phospholipase A2, which then liberates arachidonic acid from membrane phospholipids. The increases in the substrate activate CYP enzymes to synthesize EETs. We demonstrated that PPOH reduced perfusate EET levels by ∼90% (Fig. 5A), indicating that shear stress stimulates de novo EET synthesis. Thus, both mechanisms—the direct release of preformed EETs followed by de novo synthesized EETs—are believed to be responsible for the increased EET concentrations in the perfusate (Figs. 5A and 6A), which is associated with vasodilation (Figs. 5B and 6B). The functional significance of CYP2C29 gene(s)/protein and products (EETs) was further confirmed by an RNAi study, by which shear stress-stimulated release of EETs and the corresponding vasodilation were assessed before and after silencing the gene for CYP2C29. We demonstrated that CYP2C29 siRNA specifically knocked down the gene and protein expression (Fig. 5, C and D) in these vessels, resulting in an undetectable EET level in the perfusate, as well as an eliminated shear stress-induced dilation (Fig. 5, A and B), indicating a crucial role of CYP2C29 in the response. Shear stress-induced dilation in our preparation is exclusively an endothelium-dependent response that is initiated in the absence of endothelial NO and prostaglandins, revealing an EET/EDHF-mediated response. Despite the fact that much work on EET/EDHF has been performed, little is known about the CYP isoforms responsible for the production of EETs in mouse endothelial cells, nor is evidence provided for the relative contribution of specific EET(s) to the EDHF-mediated vasodilation. The present study provides answers addressing both issues, indicating first, that endothelial CYP2C29 is the enzyme responsible for the synthesis of EETs in mouse vessels, as evidenced by Fig. 5 showing that the elimination of EET release in the perfusate was paralleled with the inhibition of shear stress-induced dilation when the endothelial CYP2C29 gene was knocked down. Second, 14,15-EET and 11,12-EET are the major products of CYP2C29 in the vessels, as indicated by an approximate 56% and 30% contribution to the total EETs released to shear stress. This is in line with the report showing that in liver, kidney, and brain of mice, CYP2C29 preferentially metabolizes arachidonic acid to 14,15-EET (16). On the other hand, we cannot exclude the possibility that 11,12-EET in the perfusate could also be, at least in part, the product of other CYP subfamily, such as CYP2C38, the epoxygenase that mainly generates 11,12-EETs (10).
Role of RXRγ in the Signaling Cascade of CYP2C29
Microarray also provided a specific increase in RXRγ gene expression in line with the upregulation of CYP2C29 gene (Figs. 1 and 2). Retinoid X receptors are present in the nucleus as heterodimers bound to their responsive element in the promoter of target genes. When activated/phosphorylated, they undergo conformational change that leads to the recruitment of coactivators to initiate transcriptional regulation of target genes (13). We found in the present study that the signal transduction for the upregulation of CYP2C29 and synthesis of EETs was also dependent on RXRγ, as evidenced by the fact that RXRγ siRNA prevented the shear stress-stimulated release of EETs and vasodilation, via a downregulation of CYP2C29 expression to inhibit EET synthesis (Fig. 6). Interestingly, unlike its inhibitory effect on CYP2C29 expression, RXRγ siRNA did not affect Akt expression, indicating that RXRγ is upstream from the CYP2C29 gene but could be located downstream from Akt. At this moment, mechanisms responsible for the RXRγ-dependent regulation of CYP2C29 are unknown, nor is there evidence provided for the presence of an RXR response element in the CYP2C29 gene promoter, but given the fact that retinoic acid per se is a substrate of human CYP2C9 that is highly homologous to rodent CYP2C29 (11), it is reasonable to speculate that CYP2C29 is regulated by RXRγ, a receptor that is also activated by retinoic acid. Alternatively, an interaction between ERs and RXRγ may also be proposed, as some NHR family members, such as PXR, have been reported to bind to ERs (14); PXR, on the other hand, shares the response elements with RXR on the promoter of human CYP3A4 gene (13).
Perspectives and Significance
This is the first study demonstrating that CYP2C29 is an endothelial EET synthase, responsible for the estrogen-dependent production of EETs, as a back-up mechanism, to mediate shear stress-induced vasodilation of mice that are deficient in eNOS. Also, RXRγ regulates CYP2C29 via perhaps, upstream signaling factors, such as PI3K/Akt. Identification of the specific signal transduction pathway responsible for the estrogen-specific regulation of endothelial function brings to light the significance of the action of estrogen in the control of the synthesis of endothelial mediators and modulation of vascular tone, when NO synthesis is impaired. In the present study, we clarified the signaling cascade at the level of gene/protein expression and product formation, as well as mediation of changes in vascular function. We plan in future studies, to explore the interactions between CYP2C29 and other CYP subunits (such as 2C38/40), RXRγ, and PPARγ or estrogen and shear stress. We believe that these findings will deepen and broaden our understanding of the cardiovascular protective effects of estrogen, by which estrogen activates a CYP signaling pathway to compensate for NO deficiency, leading to a maintained shear stress-sensitive mechanism in the control of vascular tone. Moreover, these studies may lead to better strategies for the treatment of cardiovascular diseases, such as arteriosclerosis, heart failure, hypertension, and diabetes, pathological situations characterized by a reduction in the synthesis or bioactivity of NO.
GRANTS
This study was supported by Intramural Grants 49420 (to H. Jiang) and 49433 (to A. Huang), as well as National Institutes of Health Grants HL 070653 (to A. Huang) and HL 43023 (to G. Kaley).
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1.Campbell WB, Harder DR. Endothelium-derived hyperpolarizing factors and vascular cytochrome P450 metabolites of arachidonic acid in the regulation of tone. Circ Res 84: 484–488, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Fisslthaler B, Michaelis UR, Randriamboavonjy V, Busse R, Fleming I. Cytochrome P450 epoxygenases and vascular tone: novel role for HMG-CoA reductase inhibitors in the regulation of CYP 2C expression. Biochim Biophys Acta 1619: 332–339, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Huang A, Kaley G. Gender-specific regulation of cardiovascular function: estrogen as key player. Microcirculation 11: 9–38, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Huang A, Sun D, Carroll MA, Jiang H, Smith CJ, Connetta JA, Falck JR, Shesely EG, Koller A, Kaley G. EDHF mediates flow-induced dilation in skeletal muscle arterioles of female eNOS-KO mice. Am J Physiol Heart Circ Physiol 280: H2462–H2469, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Huang A, Sun D, Jacobson A, Carroll MA, Falck JR, Kaley G. Epoxyeicosatrienoic acids are released to mediate shear stress-dependent hyperpolarization of arteriolar smooth muscle. Circ Res 96: 376–383, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang A, Sun D, Wu Z, Yan C, Carroll MA, Jiang H, Falck JR, Kaley G. Estrogen elicits cytochrome P450–mediated flow-induced dilation of arterioles in NO deficiency: role of PI3K-Akt phosphorylation in genomic regulation. Circ Res 94: 245–252, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang A, Wu Y, Sun D, Koller A, Kaley G. Effect of estrogen on flow-induced dilation in NO deficiency: role of prostaglandins and EDHF. J Appl Physiol 91: 2561–2566, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Jiang H, Zhu AG, Mamczur M, Morisseau C, Hammock BD, Falck JR, McGiff JC. Hydrolysis of cis- and trans-epoxyeicosatrienoic acids by rat red blood cells. J Pharmacol Exp Ther 326: 330–337, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Y, Zhang Y, Schmelzer K, Lee TS, Fang X, Zhu Y, Spector AA, Gill S, Morisseau C, Hammock BD, Shyy JY. The antiinflammatory effect of laminar flow: the role of PPARγ, epoxyeicosatrienoic acids, and soluble epoxide hydrolase. Proc Natl Acad Sci USA 102: 16747–16752, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo G, Zeldin DC, Blaisdell JA, Hodgson E, Goldstein JA. Cloning and expression of murine CYP2Cs and their ability to metabolize arachidonic acid. Arch Biochem Biophys 357: 45–57, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Michaelis UR, Fleming I. From endothelium-derived hyperpolarizing factor (EDHF) to angiogenesis: Epoxyeicosatrienoic acids (EETs) and cell signaling. Pharmacol Ther 111: 584–595, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Miura H, Wachtel RE, Liu Y, Loberiza FR, Jr, Saito T, Miura M, Gutterman DD. Flow-induced dilation of human coronary arterioles: important role of Ca2+-activated K+ channels. Circulation 103: 1992–1998, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Pascussi JM, Gerbal-Chaloin S, Drocourt L, Maurel P, Vilarem MJ. The expression of CYP2B6, CYP2C9 and CYP3A4 genes: a tangle of networks of nuclear and steroid receptors. Biochim Biophys Acta 1619: 243–253, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Pascussi JM, Gerbal-Chaloin S, Duret C, Daujat-Chavanieu M, Vilarem MJ, Maurel P. The tangle of nuclear receptors that controls xenobiotic metabolism and transport: crosstalk and consequences. Annu Rev Pharmacol Toxicol 48: 1–32, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Pokreisz P, Fleming I, Kiss L, Barbosa-Sicard E, Fisslthaler B, Falck JR, Hammock BD, Kim IH, Szelid Z, Vermeersch P, Gillijns H, Pellens M, Grimminger F, van Zonneveld AJ, Collen D, Busse R, Janssens S. Cytochrome P450 epoxygenase gene function in hypoxic pulmonary vasoconstriction and pulmonary vascular remodeling. Hypertension 47: 762–770, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev 82: 131–185, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Sun D, Liu H, Yan C, Jacobson A, Ojaimi C, Huang A, Kaley G. COX-2 contributes to the maintenance of flow-induced dilation in arterioles of eNOS-knockout mice. Am J Physiol Heart Circ Physiol 291: H1429–H1435, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun D, Yan C, Jacobson A, Jiang H, Carroll MA, Huang A. Contribution of epoxyeicosatrienoic acids to flow-induced dilation in arteries of male ERα knockout mice: role of aromatase. Am J Physiol Regul Integr Comp Physiol 293: R1239–R1246, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.VanRollins M, Kaduce TL, Fang X, Knapp HR, Spector AA. Arachidonic acid diols produced by cytochrome P-450 monooxygenases are incorporated into phospholipids of vascular endothelial cells. J Biol Chem 271: 14001–14009, 1996 [DOI] [PubMed] [Google Scholar]
- 20.Webler AC, Michaelis UR, Popp R, Barbosa-Sicard E, Murugan A, Falck JR, Fisslthaler B, Fleming I. Epoxyeicosatrienoic acids are part of the VEGF-activated signaling cascade leading to angiogenesis. Am J Physiol Cell Physiol 295: C1292–C1301, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu Y, Huang A, Sun D, Falck JR, Koller A, Kaley G. Gender-specific compensation for the lack of NO in the mediation of flow-induced arteriolar dilation. Am J Physiol Heart Circ Physiol 280: H2456–H2461, 2001 [DOI] [PubMed] [Google Scholar]