Abstract
The intestine of marine teleosts must effectively absorb fluid from ingested seawater to avoid dehydration. This fluid transport has been almost exclusively characterized as driven by NaCl absorption. However, an additional feature of the osmoregulatory role of the intestine is substantial net HCO3− secretion. This is suggested to drive additional fluid absorption directly (via Cl−/HCO3− exchange) and indirectly by precipitating ingested Ca2+ as CaCO3, thus creating the osmotic gradient for additional fluid absorption. The present study tested this hypothesis by perfusing the intestine of the European flounder in vivo with varying [Ca2+]: 10 (control), 40, and 90 mM. Fractional fluid absorption increased from 47% (control) to 73% (90 mM Ca2+), where almost all secreted HCO3− was excreted as CaCO3. This additional fluid absorption could not be explained by NaCl cotransport. Instead, a significant positive relationship between Na+-independent fluid absorption and total HCO3− secretion was consistent with the predicted roles for anion exchange and CaCO3 precipitation. Further analysis suggested that Na+-independent fluid absorption could be accounted for by net Cl− and H+ absorption (from Cl−/HCO3− exchange and CO2 hydration, respectively). There was no evidence to suggest that CaCO3 alone was responsible for driving fluid absorption. However, by preventing the accumulation of luminal Ca2+ it played a vital role by dynamically maintaining a favorable osmotic gradient all along the intestine, which permits substantially higher rates of solute-linked fluid absorption. To overcome the resulting hyperosmotic and highly acidic absorbate, it is proposed that plasma HCO3− buffers the absorbed H+ (from HCO3− production), and consequently reduces the osmolarity of the absorbed fluid entering the body.
Keywords: osmoregulation, chloride/bicarbonate exchange, in vivo perfusion
for marine teleost fish the ability of the intestine to effectively absorb fluid is vital for coping with the challenge of living in seawater. Teleost fish are osmoregulators, tightly controlling the ionic composition of their body fluids and are distinctly hypoosmotic to seawater (∼320 mOsm/kg compared with ∼1,000 mOsm/kg). The osmotic gradient between these two compartments means that these fish are constantly faced with the loss of water and passive gain of ions. To avoid dehydration they must continuously drink the surrounding seawater, achieving ionic and osmotic homeostasis by integrating the functions of the gills, kidney, urinary bladder, and gastrointestinal tract (30). Overall, these processes result in a net retention of water, and excretion of the excess ions imbibed with seawater (monovalent ions Na+ and Cl− excreted via the gills; and divalent ions Ca2+, Mg2+, and SO42− via the intestine and kidney). The intestine therefore has a prominent role in osmoregulation, responsible for the absorption of water and elimination of the majority of ingested divalent ions that pass through unabsorbed.
Over 100 yr of investigation have failed to produce an overwhelming consensus on the routes and mechanism of fluid transport across vertebrate epithelia, but has generated a broad and often complex body of literature. The prevailing view is that fluid transport is driven by localized osmotic gradients generated by active solute transport, a process often referred to as solute-linked water transport. The general features of this solute-coupling model are that the movement of water is passive, secondary to net solute transport in the same direction, which can take place in the absence of, or even against, an osmotic gradient between bulk fluids with the resulting absorbed fluid being isoosmotic (35, 36, 40). The ideas behind this model, where solute absorption is typically dominated by Na+-coupled transport (i.e., apical NaCl cotransport and Na+-glucose cotransport), were largely the product of original observations on mammalian epithelia (8–10, 34, 44). By comparison, the mechanism of fluid transport is not considered to be exceptionally different in the marine teleost intestine receiving large amounts of Na+ and Cl− from drinking seawater (26, 28). Indeed, some of the earliest in vitro and in vivo experiments on this tissue revealed that fluid transport accommodated many features of the solute-linked water transport model (23, 24, 37, 38). However, drinking seawater presents the gastrointestinal tract with the challenge of not only dealing with high concentrations of Na+ and Cl−, but also coping with significant amounts of divalent ions (Ca2+, Mg2+, and SO42−).
Up to 85% of the imbibed seawater volume can be absorbed by the intestine (46), with the resulting excreted fluid being dominated by Mg2+ and SO42−. These divalent ions are poorly absorbed and therefore become progressively concentrated (frequently in excess of 100 mM) during transit along the gut as almost all of the Na+ and Cl− (typically >95%) are absorbed followed by a significant portion of water (30). The intestinal fluid is also characteristically alkaline (up to pH 9), which is the result of HCO3− secretion, with luminal concentrations typically ranging from 30 to over 100 mM (19). This unusual chemistry favors the precipitation of ingested Ca2+ to solid calcium carbonate (CaCO3) that is then excreted (47, 48). For almost all teleost species examined so far, this luminal alkalinization is mediated by apical Cl−/HCO3− exchange (2, 18–20, 22, 27, 48) where a significant portion (>50%) of secreted HCO3− is produced endogenously from the hydration of CO2 catalyzed by carbonic anhydrase within the intestinal epithelia itself (15–17, 22, 45, 46). When considered alongside protons (H+) yielded from the hydration of intracellular CO2, the exchange of HCO3− for Cl− will result in a net gain of osmolytes by the cell, therefore making a direct contribution to water absorption, alongside NaCl cotransport (22).
Despite observations of alkaline intestinal fluids and carbonate precipitates from as far back as the 1930s (39), insight into the functional aspects of HCO3− secretion and CaCO3 precipitation remains relatively limited. While there is recent evidence of a role in postprandial acid-base regulation (4, 5, 7, 41), it had previously been suggested that the primary function of CaCO3 precipitation is in osmoregulation by preventing the buildup of Ca2+ (and to a lesser extent, Mg2+) from imbibed seawater, which would otherwise accumulate following NaCl-linked water absorption, ultimately having an adverse effect on the osmotic gradient for fluid absorption (25, 42, 46). A simple theoretical treatment by Wilson et al. (48) showed that by secreting HCO3− and removing Ca2+ as CaCO3, the ionic content of the intestinal fluid could be reduced by up to 70 mM, thus offering a considerable osmotic advantage in terms of promoting fluid absorption. In addition to the direct role of Cl−/HCO3− exchange, it was hypothesized that the production of CaCO3 precipitates would also drive water absorption (48). This idea is unique in terms of our current understanding of epithelial fluid transport because it does not rely on net solute absorption followed by osmotically obliged water in the same direction. Rather, the formation of CaCO3 creates the osmotic gradient for water absorption by precipitating dissolved osmolytes and thus reducing the osmolality of the fluid within the intestinal lumen (48). On putting these observations to the test, Wilson et al. (48) presented the first evidence linking HCO3− secretion and CaCO3 precipitation with teleost osmoregulation after perfusing the intestine of the European flounder in vivo with saline containing 20 mM Ca2+, to deliberately stimulate HCO3− secretion and precipitation. Compared with a control perfusion with 5 mM Ca2+, almost all of the additional 15 mM Ca2+ could be accounted for by precipitation of CaCO3 and was associated with a significant reduction in the osmolality of both the gut fluid and blood plasma, as predicted. However, following 72 h of perfusion, there was only a very small 4% increase in water absorption, which was not statistically significant.
Given the fundamental importance of fluid absorption and existing controversies over the routes and mechanisms of fluid transport across vertebrate epithelia (35, 36, 40), the proposal of an additional, mechanism of epithelial water transport involving CaCO3 precipitation remains an intriguing possibility, but there is still only indirect evidence in support of this idea. By using the same perfusion technique as Wilson et al. (48), the present study set out to exaggerate the process of precipitation even further by perfusing the intestine with higher concentrations of Ca2+, 40 mM, and 90 mM, along with 10 mM Ca2+ as a control, in an attempt to try and resolve the influence of HCO3− secretion and CaCO3 production on fluid transport. These three Ca2+ concentrations would be equivalent to the amount being received by the intestine if the fish were living in normal, double, and triple-strength seawater in which the imbibed Ca2+ concentrations would be 10, 20, and 30 mM, respectively, but coupled with proportional increases in drinking rate (DR) at the elevated salinities (i.e., double DR × 20 ≡ 40 mM, and triple DR × 30 ≡ 90 mM).
MATERIALS AND METHODS
Experimental animals.
European flounder (Platichthys flesus) (mean body mass 465 ± 31 g; n = 23) were obtained from local fishermen in Flookburgh, Cumbria, UK and transported to the marine aquarium facilities at the School of Biosciences, University of Exeter. Fish were held in 150-liter tanks of flow-through, aerated, artificial seawater made with commercial marine salts (Tropic Marin, Tropical Marine Centre, Bristol, UK) as part of a recirculating seawater system maintained at a salinity of 33.8 ± 0.2 ppt and 12.5 ± 0.3°C under a 14:10-h light-dark photoperiod. Food was typically withheld for at least 72 h prior to experimentation; otherwise, the fish were maintained on a diet of fresh ragworm (Nereis virens) fed once per week. All experimental procedures were approved by the University of Exeter Ethical Committee and carried out under a UK Home Office license in accordance with the Animals (Scientific Procedures) Act 1986.
In vivo procedures.
Each fish, in preparation for surgery, was anesthetized in seawater containing 150 mg/l of tricaine methansulfonate (MS222; Pharmaq, Fordingbridge, UK) buffered with 300 mg/l NaHCO3 (followed by prolonged aeration to restore normal CO2 levels) before being placed on a custom-made wet surgery table. To maintain anesthesia during surgery, the gills were irrigated with aerated seawater containing 95 mg/l buffered MS-222. The surgery consisted of three consecutive procedures, cannulation of the caudal blood vessel, implantation of a stomach drain catheter (to allow imbibed fluid to freely exit the stomach) and an intestinal perfusion catheter, followed by fitting of the rectal catheter bag. Prior to attaching the rectal catheter, the intestine was flushed with 50 ml of the appropriate perfusion saline via the intestinal catheter to thoroughly rinse out the existing gut fluid and any precipitates. These procedures were carried out as described by Wilson et al. (48).
Saline composition and experimental design.
The perfusion salines were modified from Wilson et al. (48) and designed to test the influence of varying levels of Ca2+ on HCO3− secretion and precipitation rates in vivo. Fish were allocated to one of three nominal treatment groups (Table 1), one of which consisted of a control saline containing 10 mM Ca2+. To enhance CaCO3 precipitation, the second group was perfused with 40 mM Ca2+ and the third with 90 mM Ca2+. To preserve the Cl− concentration among salines, MgCl2 was exchanged for CaCl2. To prevent the spontaneous precipitation of CaSO4 in the presence of such high-Ca2+ concentrations, SO42− was held at 10 mM in all three treatments. Each saline was also deliberately HCO3− free and unbuffered, so the appearance of HCO3− and carbonate precipitates could only have originated from the intestine. Following the blood vessel cannulation (see above), a small blood sample was taken from each fish to determine plasma osmolality. The osmolality of the perfusion saline (Table 1) was then adjusted (if necessary) with deionized water to match that of the individual fish plasma.
Table 1.
The measured ion concentrations (mmol/l) and osmolalities (mOsm/kg) in each of the in vivo perfusion salines employed by the present study
| Perfusion Treatment |
|||
|---|---|---|---|
| Ion | Control, 10 mM Ca2+ | 40 mM Ca2+ | 90 mM Ca2+ |
| Na+ | 50.3 ± 1.6 | 47.5 ± 0.7 | 51.4 ± 1.6 |
| Cl− | 214.8 ± 4.9 | 206.7 ± 2.3 | 214.2 ± 3.0 |
| K+ | 4.9 ± 0.1 | 4.5 ± 0.1 | 5.2 ± 0.2 |
| Ca2+ | 9.3 ± 0.2 | 36.3 ± 0.3 | 82.7 ± 1.7 |
| Mg2+ | 85.0 ± 1.7 | 55.1 ± 0.7 | 10.0 ± 0.3 |
| SO42− | 8.8 ± 0.4 | 7.7 ± 0.1 | 9.0 ± 0.6 |
| Osmolality | 329 ± 6.8 | 318 ± 3.1 | 331 ± 5.1 |
Values are means ± SE, n = 8, 7, and 8 for the control, 40 mM, and 90 mM treatments, respectively.
Intestinal perfusion.
Once surgery was complete, the fish was placed in an individual chamber (11-liter capacity, filled to a volume of 8 liters) receiving a continuous supply of aerated seawater and was allowed to recover from anesthesia. During this recovery period the intestinal catheter was connected to a peristaltic pump (Minipuls 3; Gilson, Villiers-le-Bel, France), and perfusion commenced with one of the three salines listed in Table 1. The intestine of each fish was continuously perfused for 72 h at a mean perfusion rate of 5.04 ± 0.24 ml·kg−1·h−1 (n = 23). The volumes perfused were determined gravimetrically to the nearest 0.1 mg.
Sampling and analytical techniques.
Blood samples (∼800 μl) were taken at 24, 48, and 72 h using a gas-tight, 1-ml Hamilton syringe and processed immediately. Plasma was isolated from ∼500 μl of blood by centrifugation (11,600 g for 3 min; MSE Micro Centaur) and stored on ice. The remaining 300 μl was used to measure various blood acid-base parameters (see Ref. 6), with ∼250 μl being returned to the fish.
After 72 h, the experiment was terminated by stopping the intestinal perfusion pump and administering an overdose of anesthetic (250 mg/l buffered MS-222) into the chamber. The perfusion catheter was detached from the peristaltic pump, and a ligature was tied around the top of the rectal catheter before the fish was removed from the chamber. The abdominal cavity was opened, and another ligature was tied around the base of the rectum. The intestine was then carefully dissected out and its contents decanted into 50-ml centrifuge tubes. Similarly, the rectal catheter was removed, and the contents collected.
The contents of the intestine and rectal catheter were then centrifuged at 1,677 g for 4 min (MSE Mistral 3000), separating the precipitates from the fluid; the latter was decanted into preweighed tubes and subsequently weighed to the nearest 0.1 mg to determine the volume of fluid recovered. Measurements of osmolality (Wescor Vapro 5520), Cl− (Corning chloride analyzer model 925) and total CO2 (Mettler Toledo model 965D carbon dioxide analyzer) were made on all samples. The cations Na+, K+, Ca2+, and Mg2+ were also measured following appropriate dilution and addition of 0.1% LaCl3 (as 10% wt/vol solution) using a Pye Unicam model SP9 atomic absorption spectrophotometer. Levels of SO42− in the gut, rectal, and perfusion fluids were measured by ion chromatography (Dionex DX120 or ICS1000). The pH of the intestinal and rectal fluids was measured using an Accumet Microprobe combined pH electrode (Fisher Scientific, Loughborough, UK) connected to a Hanna model HI8314 membrane pH meter.
The precipitates collected from the intestine and rectal catheter of each fish were pooled and immediately rinsed three times in deionized water, recentrifuging each time, before they were finally sonicated (Vibra-Cell, sonics and materials) in 20 ml of Ultrapure water (Maxima Ultrapure water, ELGA). The content of bicarbonate equivalents (HCO3−+2CO32−) in the precipitates was determined by the double-titration method (48), by using an autotitration system (model TIM85; Radiometer) with autosampler (model SAC80; Radiometer). Following completion of titration, samples were manually acidified, and an aliquot was taken for subsequent analysis of Ca2+ and Mg2+ (by atomic absorption spectrophotometry; model SP9; Pye Unicam) and SO42− (by ion chromatography; Dionex) to determine the respective amounts of the divalent ions incorporated into the precipitates.
Calculations.
For the determination of bicarbonate equivalents in the intestinal and rectal fluids, the HCO3− concentration was calculated from a rearrangement of the Henderson-Hasselbach equation, but substituting pKapp for a second dissociation constant, pKII (9.46) that describes the HCO3−/CO32− reaction (22). This value is similar to the published pKII value of 9.52 for one-third strength seawater at the same temperature (43), but was obtained empirically by Wilson et al. (48) for intestinal fluids in a marine teleost. The overall net secretion rate of total bicarbonate equivalents (HCO3−+2CO32−) and net flux of each individual ion perfused into the intestine was calculated: JX = ([X]IN×VIN) − {([X]IF×VIF)+([X]RF×VRF)+XPPT}OUT/(M×t), where, [X]IN represents the concentration (μequiv/l) of ion X in the perfusion saline, and VIN is the total volume (ml) perfused into the intestine. [X]IF and [X]RF represent the concentration of ion X (μequiv/l) in the intestinal and rectal fluids, respectively, and VIF and VRF are the respective volumes (ml) of each of these fluids that were recovered. For divalent ions (Ca2+, Mg2+, and SO42−), as well as HCO3−, XPPT represents the total amount (μequiv) of each of these ion species incorporated into the precipitates, M is the mass of the fish (kg), t is the perfusion time period (h), and JX is the net flux expressed as μeq·kg−1·h−1; a positive value indicates net absorption and a negative value indicates net secretion.
Fluid absorption was calculated based on the difference between the total volume perfused and the volume recovered from the intestine and rectal catheter and expressed as a proportion: %Abs. = [VIN − (VIF+VRF)]/VIN×100, as well as a mass specific rate: Jv = [VIN − (VIF+VRF)]/(M×t), where, Jv is the net flux of fluid across the intestine (ml·kg−1·h−1).
Data presentation and statistical analysis.
The data are presented as means ± SE. Data expressed as a proportion were arcsine transformed prior to analysis, and significant differences between treatments were tested for by one-way ANOVA using a general linear modeling procedure. Post hoc, pairwise comparisons were made using Bonferroni simultaneous tests, where appropriate. For data failing to meet the assumptions of approximate normality and equality of variance, the nonparametric Kruskal-Wallis test was performed with post hoc comparisons made using Dunn's procedure. The results of all tests were accepted as significant at P < 0.05. Statistical analysis was carried out using Minitab version 13.1, and graphs were drawn using SigmaPlot version 9.0.
RESULTS
Bicarbonate production and excretion.
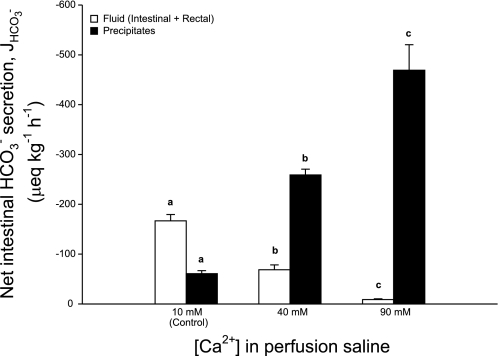
Increasing the concentration of Ca2+ perfusing the intestine produced a significant elevation in the overall rate of HCO3− equivalents excreted and dramatically altered its distribution between the fluid and precipitates. At 40 mM there was a significant 59% reduction in dissolved HCO3− in the intestinal and rectal fluids accompanied by a more than fourfold rise in the amount excreted as carbonate precipitates relative to the control. When Ca2+ was increased to 90 mM almost all (98%) of the HCO3− produced was recovered in the form of carbonate precipitates, approximately twice the rate of the 40 mM Ca2+ treatment and almost eight times higher than controls, with only a minor fraction remaining in the intestinal fluid (Fig. 1).
Fig. 1.
Net production and excretion of bicarbonate equivalents (μeq·kg−1·h−1) by the intestine of the European flounder perfused with salines containing varying concentrations of Ca2+ ([Ca2+]). The white bars represent the amount of bicarbonate equivalents recovered in the fluid (intestinal fluid+rectal fluid) and black bars show the amount incorporated into precipitates. J, flux. Data are means ± SE, and values labeled with different letters indicate a significant difference between treatments; n = 8, 7, and 8 for the control, 40-mM, and 90-mM treatments, respectively.
Predictably, the acid-base characteristics of the voided rectal fluid from flounder perfused with high Ca2+ were quite different compared with the control treatment. Rectal fluid pH under control conditions was alkaline (pH 8.57 ± 0.03) and rich in HCO3− equivalents (68.4 ± 5.6 μeq/l). Increasing the concentration of Ca2+ in the perfusion saline up to 40 mM reduced rectal fluid pH by 0.35 units and measured HCO3− equivalents by almost 50%. At 90 mM there was little alkalinization of the rectal fluid with a pH of 7.41, and only small amounts of dissolved HCO3− equivalents present (Table 2) due to almost all the secreted HCO3− being precipitated as CaCO3. Despite the increases in HCO3− secretion and precipitation rates across treatments, the resultant osmolality of rectal fluids collected from fish undergoing the 90-mM treatment was quite variable, and on average there were no significant differences between rectal fluid and perfusate osmolality within each treatment (assessed by paired t-tests), or rectal fluid and perfusate osmolality between treatments (Table 2).
Table 2.
Concentrations (mmol/l) of each ion recovered in the rectal fluid, including HCO3− equivalents (meq/l), pH, and osmolality (mOsm/kg), from the flounder following in vivo perfusion of the intestine with salines containing varying concentrations of Ca2+ ([Ca2+])
| Perfusion Treatment |
|||
|---|---|---|---|
| Ion | Control, 10 mM Ca2+ | 40 mM Ca2+ | 90 mM Ca2+ |
| Na+ | 16.9 ± 1.8a | 19.0 ± 3.2a | 62.8 ± 18.4b |
| Cl− | 162.4 ± 7.7 | 163.4 ± 6.2 | 178.1 ± 8.8 |
| K+ | 0.6 ± 0.2a | 0.5 ± 0.1a | 2.2 ± 0.4b |
| Ca2+ | 3.7 ± 1.0a | 4.3 ± 1.1a | 67.1 ± 6.5b |
| Mg2+ | 131.4 ± 3.9a | 115.9 ± 5.0a | 41.1 ± 7.8b |
| SO42- | 17.9 ± 1.3a | 18.2 ± 1.2a | 38.9 ± 9.3b |
| [HCO3−+2CO32− | 68.4 ± 5.6a | 37.3 ± 7.3b | 6.6 ± 1.3c |
| pH | 8.57 ± 0.03a | 8.22 ± 0.11b | 7.41 ± 0.11c |
| Osmolality | 320 ± 5 | 308 ± 4 | 327 ± 9 |
Values are means ± SE and means labeled with different letters indicate a significant difference between treatments, n = 8, 7, and 8 for the control, 40 mM, and 90 mM treatments, respectively.
Fluid transport.
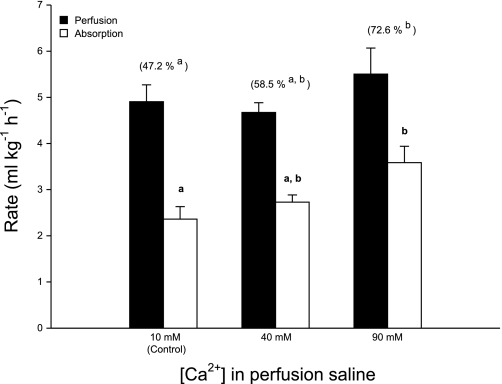
There was a significant, positive relationship between the luminal [Ca2+] and fractional fluid absorption (Fig. 2). On average, the proportion of fluid absorbed by the intestine was higher in the 40-mM treatment relative to the control (47.2 ± 3.5% and 58.5 ± 2.1%, respectively) but was not significantly different. At 90 mM, Ca2+ fluid absorption increased even further, reaching an average of 72.6 ± 4.8%, and although this was not significantly different from the 40-mM treatment, it represented a significant increase compared with the control treatment. A similar trend between treatments was revealed in terms of the absolute rate of fluid absorption (Fig. 2), with a significant increase up to 3.59 ± 0.36 ml·kg−1·h−1 in the 90-mM treatment, in relation to controls (2.36 ± 0.27 ml·kg−1·h−1).
Fig. 2.
Influence of luminal [Ca2+] on the rate (ml·kg−1·h−1) of intestinal fluid absorption (white bars) by the European flounder in relation to perfusion rate (black bars). Corresponding fractional absorption (%) is shown in parentheses. Data are means ± SE, and values labeled with different letters indicate a significant difference between treatments; n = 8, 7, and 8 for the control, 40-mM, and 90-mM treatments, respectively.
Blood chemistry.
By the final day of perfusion (day 3) the osmolality of the plasma for fish receiving the 40 mM and 90 mM Ca2+ were similarly and substantially reduced (318 ± 3 and 317 ± 5 mOsm/kg, respectively) compared with control fish whose plasma osmolality steadily rose over the 3-day perfusion period to an average of 335 ± 5 mOsm/kg. In terms of ion regulation there was no evidence of disruption, particularly in relation to plasma Ca2+, which was tightly regulated by fish from all three treatments (Table 3).
Table 3.
Summary of the various osmoregulatory parameters of plasma (osmolality, Na+, Cl−, K+, Ca2+, and Mg2+) as part of the daily blood sampling routine during in vivo perfusion of the flounder intestine with salines containing varying [Ca2+]
| Group | Osmolality, mOsm/kg | Na+, mM | Cl−, mM | K+, mM | Ca2+, mM | Mg2+, mM |
|---|---|---|---|---|---|---|
| Day 1 | ||||||
| Control | 326 ± 5.6 | 161.7 ± 3.6 | 147.1 ± 1.3 | 3.0 ± 0.1a,b | 2.2 ± 0.2a | 0.6 ± 0.1 |
| 40 mM | 330 ± 2.9 | 163.8 ± 1.1 | 145.9 ± 1.1 | 2.7 ± 0.1b | 2.8 ± 0.1b | 0.4 ± 0.1 |
| 90 mM | 323 ± 2.9 | 161.7 ± 2.6 | 148.0 ± 1.7 | 3.4 ± 0.2a | 2.0 ± 0.2a | 0.6 ± 0.2 |
| Day 2 | ||||||
| Control | 329 ± 5.9 | 159.2 ± 3.8 | 146.1 ± 1.6 | 3.0 ± 0.1a | 2.0 ± 0.2 | 0.7 ± 0.1 |
| 40 mM | 323 ± 2.8 | 156.7 ± 2.9 | 145.0 ± 1.3 | 2.6 ± 0.1b | 2.4 ± 0.1 | 0.4 ± 0.1 |
| 90 mM | 324 ± 6.8 | 158.6 ± 3.0 | 145.5 ± 1.6 | 3.1 ± 0.1a | 1.9 ± 0.1 | 0.6 ± 0.3 |
| Day 3 | ||||||
| Control | 335 ± 5.0a | 159.6 ± 4.2 | 148.4 ± 3.3 | 3.0 ± 0.1a,b | 2.0 ± 0.1 | 0.9 ± 0.2 |
| 40 mM | 318 ± 2.6b | 160.7 ± 2.4 | 144.3 ± 1.1 | 2.6 ± 0.1b | 2.4 ± 0.1 | 0.4 ± 0.1 |
| 90 mM | 317 ± 5.0b | 157.0 ± 3.4 | 146.8 ± 2.2 | 3.2 ± 0.1a | 1.9 ± 0.1 | 1.6 ± 0.9 |
Values are means ± SE. For each day, means labeled with different letters represent significant differences between treatments; n = 8, 7, and 8 for the control, 40 mM, and 90 mM treatments, respectively.
DISCUSSION
From a series of in vivo perfusion experiments, Wilson et al. (48) had presented the first indirect evidence for a unique mechanism of fluid transport by the teleost intestine, whereby increased CaCO3 precipitation was associated with a reduction in luminal osmotic pressure creating a favorable gradient for fluid absorption. However, Wilson et al. (48) were unable to detect a significant increase in fractional fluid absorption to support the theory. The present study employed the same intestinal perfusion technique but applied even higher concentrations of Ca2+ (40 mM and 90 mM). In terms of stimulating HCO3− production and precipitation, the results fit in well with and corroborate the prior observations made by Wilson et al. (48). Furthermore, the rate of CaCO3 precipitation increased with luminal [Ca2+] (Fig. 1) in conjunction with a greater capacity of the intestine to absorb fluid (Fig. 2). At 90 mM Ca2+, almost all of the HCO3− excreted in the rectal fluid was in the form of CaCO3, and the mean fractional fluid absorption had increased dramatically to over 70%.
Composition of fluid absorbed by the intestine.
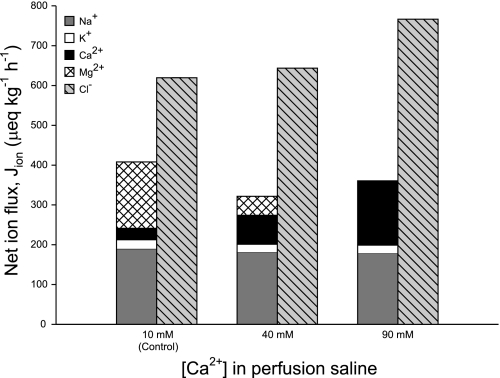
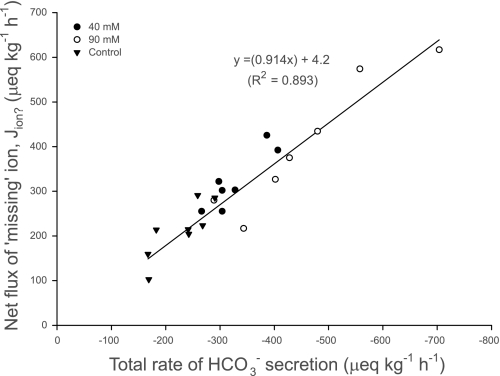
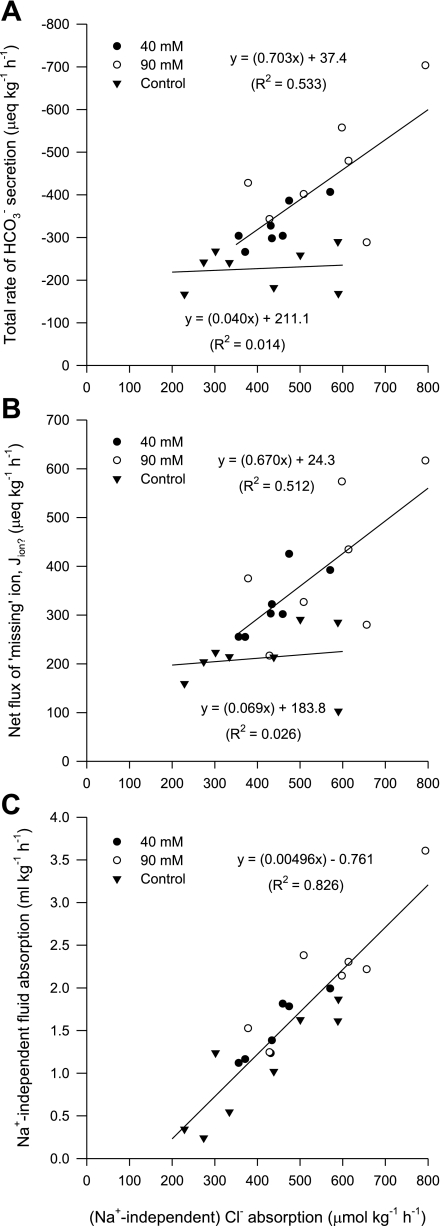
After accounting for the net fluxes of all ions, it is clear that the net absorption of anions (Cl− and SO42−) greatly exceeded cations (Na+, K+, Ca2+, and Mg2+) by an average of 50% across all treatments (Fig. 3), indicating a substantial shortfall in either absorbed cations or secreted anions. It has been proposed that this ion gap is related to HCO3− secretion, representing a net absorption of H+ incurred from CO2 hydration (13, 19, 22). The polarity of these acid-base transfers by the teleost intestine has subsequently been demonstrated in the Gulf toadfish Opsanus beta (15, 21). Previous characterization of HCO3− secretion by the intestine of the European flounder in vitro has revealed that the production of HCO3− was almost entirely dependent on endogenous CO2 hydration (45). It is therefore likely that this ion gap in the absorbed fluid also represents the movement of acid-base equivalents between the intestine and the blood. First, the net flux of this missing ion (from Fig. 3) is almost directly proportional to the corresponding rate of total HCO3− secretion (fluid+precipitates) across all treatments (Fig. 4). Second, intestinal HCO3− secretion represents a significant base efflux from the whole animal that needs to be balanced by an equivalent acid efflux (or base uptake) via other routes, primarily the gills (11, 44, 45). This is illustrated in the companion study by Cooper et al. (6) that revealed a significant positive relationship between the estimated net intestinal flux of this missing ion and the net (extraintestinal) flux of acid, excreted by the gills into the surrounding seawater.
Fig. 3.
Mean net fluxes of cations and anions (μeq·kg−1·h−1) by the intestine of the European flounder following perfusion with salines containing varying [Ca2+]; n = 8, 7, and 7 for the control, 40-mM, and 90-mM treatments, respectively. Values for SO42− are not shown since net fluxes were not significantly different from zero (0.7 ± 5.5, 0.0 ± 2.6, and −9.2 ± 12.8 μeq·kg−1·h−1, for each respective treatment).
Fig. 4.
Relationship between intestinal HCO3− secretion (μeq·kg−1·h−1) and the predicted net flux of the missing ion (Jion?; μeq·kg−1·h−1) in the fluid being absorbed by the intestine of the flounder perfused with salines containing varying [Ca2+]; n = 8, 7, and 7 for the control, 40-mM, and 90-mM treatments, respectively. (F1,20 = 167.24, P < 0.001).
NaCl cotransport as a driving force for intestinal fluid absorption.
The mechanism of fluid absorption by the teleost intestine (in terms of hypoosmoregulation) has previously been characterized in relation to the solute-coupling model, associated with the active transport of Na+ and Cl− across the epithelium, thus establishing the osmotic gradient along which water passively follows (26, 28). Examining the net intestinal fluxes of Na+ and Cl− across all treatments revealed that on average JNa did not change (Fig. 3), suggesting that the increases in intestinal fluid absorption observed in both of the high-Ca2+ treatments were independent of changes to rates of NaCl cotransport. This is further supported by the following calculations that have attempted to define the relative contribution of NaCl-driven, solute-linked water transport.
The transport of NaCl by the flounder intestine involves apical NaCl cotransport in parallel with Na+-K+−2Cl− cotransport (11, 29, 33); thus, the sum of the net absorptive fluxes of Na+ and K+ were considered to be associated with an equivalent flux of Cl−, according to the stoichiometry of their respective transporters. Taking the molarity of water as 55.56 M and assuming that the fluid being absorbed in association with NaCl would be isoosmotic (10, 40), the ratio between water and solutes in the absorbed fluid was therefore calculated as 170 mol water:1 mol solute. With the use of this information it was possible to calculate the theoretical rate of fluid absorbed (ml·kg−1·h−1) in association with Na+, K+, and Cl− cotransport (i.e., solute-linked water transport). By subtracting this value from the actual measured rate of fluid absorption (Fig. 2), it was possible to derive the rate of fluid absorption via Na+-independent pathways. This analysis revealed that only 58.5 ± 5.8% of fluid absorbed under control conditions (10 mM Ca2+) could be associated with NaCl cotransport. This is comparable to the 50% estimate previously calculated by Grosell et al. (14) for the lemon sole (Parophrys vetulus) in vivo. Interestingly, the relative contribution of NaCl cotransport to intestinal fluid absorption became smaller with increasing luminal [Ca2+], contributing 45.6 ± 2.3% and 36.2 ± 2.3% to overall fluid absorption during perfusion with 40 mM and 90 mM Ca2+, respectively.
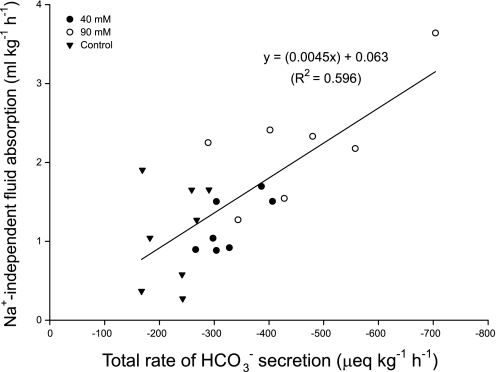
Having accounted for the contribution of NaCl cotransport to intestinal fluid absorption Fig. 5 subsequently reveals a significant relationship between the rate of Na+-independent fluid absorption and total HCO3− secretion, lending support to the hypothesis that intestinal HCO3− production, secretion, and CaCO3 precipitation are involved in driving a significant portion of intestinal fluid transport. This leads to consideration of how much of this Na+-independent fluid absorption remains solute linked, via the absorption of Cl− and H+ (from anion exchange and CO2 hydration, respectively), and how much is related to the precipitation of CaCO3.
Fig. 5.
Relationship between the calculated, Na+-independent fluid transport (ml·kg−1·h−1) and the total (fluid+precipitates) rate of HCO3− secretion (μeq·kg−1·h−1) by the intestine of the European flounder following perfusion with varying [Ca2+], n = 8, 7, and 7 for the control, 40-mM, and 90-mM treatments, respectively. (F1,20 = 29.44, P < 0.001).
Direct contribution of Cl−/HCO3− exchange to fluid absorption.
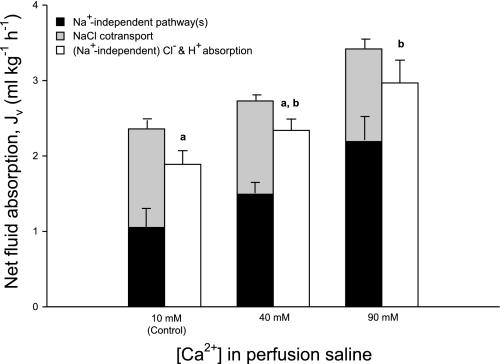
Intestinal fluid absorption linked to the absorption of Cl− and H+ (from apical anion exchange and CO2 hydration, respectively) has been highlighted by a number of recent studies (13, 21, 22), but as yet no attempt has been made to quantify its actual contribution in relation to NaCl cotransport and CaCO3 precipitation. To derive the fraction of Na+-independent fluid absorption linked to Cl−/HCO3− exchange, the Na+-independent Cl− flux has been calculated by subtracting the Cl− flux associated with NaCl cotransport (calculated above) from the total net Cl− flux. Combining this (Na+-independent) Cl− flux with the corresponding net absorption of H+ (from Fig. 4) provides an indication of the total solute flux associated with Cl−/HCO3− exchange and CO2 hydration. Subsequently, the predicted rate of fluid absorption associated with Cl− and H+ (assumed to be isoosmotic) is able to account for all of the fluid absorption taking place via Na+-independent pathways across all treatments (Fig. 6).
Fig. 6.
Predicted relative contributions of NaCl cotransport (gray bars) and Na+-independent pathway(s) (black bars) to overall measured fluid absorption (ml·kg−1·h−1) by the intestine of the European flounder following perfusion with varying [Ca2+]. Alongside is the predicted rate of fluid absorption (white bars) associated with (Na+-independent) Cl− absorption and the calculated H+ flux (thus providing an indication of solute absorption exclusively associated with Cl−/HCO3− exchange and CO2 hydration, respectively), assuming the associated water movement would be isoosmotic. Jv, net flux of fluid across the intestine. Data are means ± SE, and values labeled with different letters indicate a significant difference between treatments; n = 8, 7, and 7 for the control, 40-mM, and 90-mM treatments, respectively.
Role of CaCO3 precipitation in fluid absorption.
Although the data do not provide any evidence to suggest that CaCO3 precipitation can directly drive fluid absorption, it is considered to play a vital permissive role in fluid transport by helping to maintain a favorable osmotic gradient for the continuation of solute-linked fluid absorption. On the basis of the current hypothesis regarding the influence of CaCO3 precipitation on fluid transport, one might expect a corresponding reduction in the osmolality of the excreted rectal fluid at higher rates of precipitation (48). However, this may only hold true for static salt solutions, whereas the situation in the intestine is obviously dynamic, with CaCO3 precipitation (reducing osmolality) and fluid absorption occurring simultaneously. The overall result is no significant net change in rectal fluid osmolality due to the accumulation of unabsorbed solutes left behind (specifically Ca2+, Mg2+, and Cl−; Table 2). The lack of any corresponding reduction in rectal fluid osmolality in the high-Ca2+ treatments (Table 2) may therefore be a reflection of the dynamic nature of these simultaneous processes, where CaCO3 precipitation exactly cancels out the potential osmotic effect of Ca2+ accumulation that would otherwise follow on from solute-linked fluid absorption.
There can be no doubt that CaCO3 formation must have a major role in helping to maintain a favorable osmotic gradient allowing solute-linked water transport to continue. For example, based on the observed rates of fluid absorption in Fig. 2, if Ca2+ was neither precipitated nor absorbed in any treatment, but HCO3− production and secretion continued (with the associated absorption of Cl− and H+), the accumulated levels of Ca2+ and HCO3− would have increased the combined concentration of both of these ions in the rectal fluid by an average of 37, 221, and 877 mM for the control, 40-mM, and 90-mM treatments, respectively. Therefore, without the precipitation of CaCO3, ion absorption would not have been able to overcome such osmotic gradients, and consequently solute-linked fluid absorption would not have been able to continue in the high-Ca2+ treatments (Fig. 6). In reality, the process of precipitation actually allowed substantially greater rates of solute-linked fluid absorption in these high-Ca2+ treatments.
Generating the osmotic gradient for solute-linked fluid absorption.
The interpretation of the data so far suggest that HCO3− secretion and the subsequent absorption of Cl− and H+ (in association with CaCO3 precipitation removing luminal Ca2+), will help create the osmotic gradient to drive Na+-independent fluid absorption (Fig. 6). To help support this assertion, one would predict a positive relationship between the (Na+-independent) Cl− flux and total HCO3− secretion, as well as net H+ absorption across all treatments. Within each of the high-Ca2+ treatments, there were significant, proportional relationships between (Na+-independent) Cl− absorption and HCO3− secretion (Fig. 7A), as well as H+ absorption (Fig. 7B), which follows the assumption that (Na+-independent) Cl− absorption represents Cl−/HCO3− exchange supplied by CO2 hydration, respectively. However, within the control group this relationship was conspicuously absent, where for a number of individuals, elevated rates of (Na+-independent) Cl− absorption did not incur higher rates of HCO3− secretion or H+ absorption. Despite this, a strong correlation between (Na+-independent) Cl− absorption and Na+-independent fluid absorption was apparent across all three treatments (Fig. 7C). This leads to consideration of whether there is another process in the control treatment, which in addition to CaCO3 precipitation, may be involved in assisting Na+-independent fluid absorption.
Fig. 7.
Relationship between the rate of (Na+-independent) Cl− absorption (μeq·kg−1·h−1) and total (fluid+precipitates) HCO3− secretion (μeq·kg−1·h−1) (A), the net absorption of Jion? (μeq·kg−1·h−1) (B), and the rate of Na+-independent fluid absorption (ml·kg−1·h−1) (C) by the intestine of the flounder perfused with salines containing varying [Ca2+]; n = 8, 7, and 7 for the control, 40-mM, and 90-mM treatments, respectively.
To account for the trends in Fig. 7, it is proposed that in the control group, where CaCO3 was limited, there might have been a shift in polarity of H+ secretion from basolateral to apical to help support the osmotic gradient for fluid absorption. Previous studies on rainbow trout (16, 17, 46) and toadfish (20) have presented molecular in vitro and in vivo physiological evidence that there is scope for apical H+ secretion (via H+-V-ATPase or Na+/H+ exchange) by the intestinal epithelium. It has been predicted that the functional significance of this mechanism is to help maintain the osmotic gradient for fluid absorption when the availability of Ca2+ for CaCO3 formation becomes limited, with H+ titrating luminal HCO3− (to CO2 and H2O), catalyzed by extracellular, membrane-bound carbonic anhydrase (16, 17, 20).
The presence of a similar apical H+ secretion mechanism operating in the flounder intestine would explain why higher rates of (Na+-independent) Cl− absorption do not correlate with net HCO3− secretion or incur proportional increases in H+ absorption for fish undergoing the control treatment (Fig. 7, A and B), yet a close relationship with Na+-independent fluid absorption is maintained (Fig. 7C). Conversely, for the higher Ca2+ treatments, CaCO3 formation would have been less limiting (40 mM Ca2+) or not limiting at all (90 mM Ca2+). So, rather than utilizing apical H+ extrusion and subsequent titration of luminal HCO3− to maintain a favorable osmotic gradient, at higher luminal Ca2+ concentrations the intestine can upregulate Cl−/HCO3− exchange, absorbing both Cl− and H+ (Figs. 7, A and B, respectively) while benefitting from the removal of luminal Ca2+ and HCO3− (as CaCO3).
Implications of intestinal HCO3− secretion for whole animal water balance.
The final osmolality of the blood plasma was lower by almost 20 mOsm/kg in both 40- and 90- mM Ca2+ treatments, compared with controls (Table 3). This is consistent with the findings of Wilson et al. (48) who recorded a 7-mOsm/kg reduction in plasma osmolality with perfusate containing an extra 15 mM Ca2+ and supports their observation that intestinal fluid absorption can have a significant influence on overall fluid balance. Based on the net absorptive fluxes of all ions present (Na+, K+, Cl−, Ca2+, Mg2+, and SO42−), along with a net absorption of H+ (arising from intracellular CO2 hydration), and assuming an overall osmotic coefficient of 0.9, the theoretical osmolarity of the fluid absorbed from the intestine was distinctly hyperosmotic (450 ± 22, 406 ± 8, and 389 ± 14 mOsm/l for the control, 40-mM, and 90-mM treatments, respectively), relative to the plasma (Table 3). This calculation of a hyperosmotic absorbate agrees with previous in vivo and in vitro studies involving seawater-adapted teleosts (21, 24, 37, 38; for a review, see Ref. 13), which have all indicated absorption of a hyperosmotic fluid. This seems counterintuitive to the teleost strategy of hypoosmoregulation in seawater and therefore begs the question: how are marine teleosts able to absorb a hyperosmotic fluid from the intestine yet maintain overall fluid balance and, in the case of the present study (where HCO3− secretion has been hyperstimulated), actually reduce the osmotic pressure of their body fluids?
As pointed out by Grosell and Taylor (21), absorption of a hyperosmotic fluid is conceptual and will not necessarily represent actual conditions across the epithelia. For instance, given the rates of H+ absorption (Fig. 4), such an acidic fluid (theoretical pH <1) would need to be buffered. Therefore, one way to overcome the hyperosmotic, acidic absorbate would be the buffering of H+ by HCO3− within the interstitial fluid with the resulting CO2 transported in the blood and removed at the gills or even recycled back into the intestinal cells. This is similar, in principal, to how the mammalian duodenum neutralizes large amounts of gastric acid and absorbs the resulting CO2 into the blood (32). The low HCO3− content of teleost blood (3–10 mM; Ref. 31) relative to the concentration of acidic equivalents in the absorbate (up to 170 mM), suggests that the available HCO3− buffer capacity of the blood may be insufficient. However, this does not take into account gastrointestinal blood flow rate that ranges from 246 to 828 ml·kg−1·h−1 in resting, unfed fish (1, 3). Assuming plasma [HCO3−] of 6 mM, this equates to 1.5–5.0 mmol·kg−1·h−1 of HCO3− potentially available for buffering. By comparison, rates of H+ absorption were 0.1–0.6 mmol·kg−1·h−1 (Fig. 4), showing that HCO3− buffering capacity would be sufficient to resist major changes in blood pH. This process would also explain why fish undergoing the 40 mM and 90 mM Ca2+ treatments, and consequently absorbing more H+, exhibited a small respiratory acidosis (minor decrease in blood pH and increase in Pco2; see Ref. 6).
Given the negligible osmotic pressure exerted by CO2, a significant reduction in the resulting osmolarity of the absorbed fluid would be predicted. Assuming all of the H+ ions produced by the intestine were buffered, this reduces the osmolarity of the absorbed fluid by 87 ± 10, 106 ± 6, and 108 ± 12 mOsm/l for the control, 40 mM, and 90 mM Ca2+ treatments, respectively. For fish with significantly higher rates of HCO3− secretion (40 mM and 90 mM Ca2+ treatments) the predicted osmolarity of the absorbate therefore becomes distinctly hypoosmotic (300 ± 8 and 281 ± 15 mOsm/l, respectively), thus corroborating observations of a significant reduction in blood plasma osmolality in both of these treatments (Table 3).
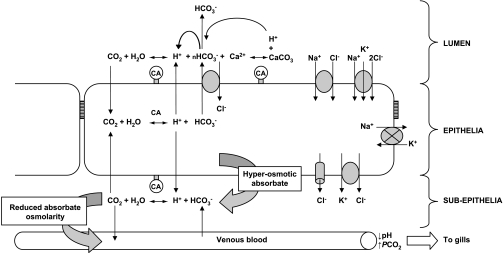
The driving forces for fluid transport across the marine teleost intestine in relation to HCO3− secretion and CaCO3 formation are summarized in Fig. 8. Alongside NaCl cotransport, Cl−/HCO3− exchange will incur the absorption of Cl− and H+; however, the relative importance of apical H+ secretion and CaCO3 formation to promoting a favorable osmotic gradient will likely depend on the availability of luminal Ca2+, as originally suggested by Grosell and colleagues (16, 17, 20). Where Ca2+ is limiting for CaCO3 formation, there may be an increased emphasis on apical H+ secretion and titration of luminal HCO3− to maintain fluid transport. At higher luminal Ca2+ concentrations, Cl−/HCO3− exchange is upregulated, CaCO3 production will consequently be higher, and combined with the absorption of Cl− and H+ provides the osmotic driving force for fluid transport. Given the electrochemical gradients for Cl− uptake and HCO3− secretion that exist across the apical membrane (20), and with the suggestion of a significant functional role for apical H+ secretion, it is interesting to consider whether apical Cl−/HCO3− exchange presented in Fig. 8 would be electrogenic (nHCO3−/Cl−) in nature, similar to the anion exchangers characterized recently from pufferfish (27) and toadfish (20). Finally, if the hyperosmotic and acidic absorbate entering the body is buffered by extracellular HCO3−, this will substantially reduce the osmolarity of the fluid entering the body. Furthermore, in the high-Ca2+ treatments where HCO3− secretion and CaCO3 formation have been upregulated together, incurring higher rates of net H+ absorption, consequent buffering in the interstitial fluid by HCO3− could be even more beneficial, producing a hypoosmotic absorbate relative to the body fluids.
Fig. 8.
Simple model illustrating the epithelial ion transport pathways and consequent reactions generating the driving forces for fluid absorption by the intestine of the European flounder as demonstrated and proposed by the present study (adapted from Ref. 20). Apical NaCl cotransporters (NaCl and NKCC) provide the primary driving force for fluid transport, fueled by the inward Na+ gradient created by basolateral Na+-K+-ATPase. Operating alongside is Cl−/HCO3− exchange, with the vast majority of HCO3− being supplied from intracellular CO2 hydration, catalyzed by carbonic anhydrase (CA), with preferential basolateral export of H+. However, the fate of secreted HCO3− will be determined by luminal chemistry. Under normocalcaemic control conditions (10 mM Ca2+), the availability of Ca2+ for precipitation will rapidly become limited; thus to maintain the osmotic gradient for fluid absorption apical H+ secretion becomes important, consuming luminal HCO3−, and yielding CO2, which can potentially recycle back into the cell. Higher luminal [Ca2+] will increase CaCO3 precipitation rates (as shown in 40 mM and 90 mM Ca2+ treatments), ultimately consuming 2HCO3− ions (as the resulting H+ will combine with HCO3− to produce CO2). The osmotic gradient generated by luminal CaCO3, in conjunction with elevated Cl− and H+ absorption, provides the driving force for Na+-independent fluid absorption. The overall absorbed fluid is distinctly hyperosmotic and acidic. In the subepithelial compartment, it is predicted that H+ in the absorbate will be buffered by extracellular HCO3−, producing CO2. This effectively both neutralizes and removes the osmotic influence of H+, consequently reducing the osmotic pressure of the fluid entering the blood. The CO2 generated enters the venous blood for excretion at the gills or alternatively could recycle back into the cell to support additional HCO3− production. In response to elevated luminal Ca2+, higher rates of intestinal HCO3− production and CaCO3 formation will incur additional H+, which not only leads to a reduction in blood pH and an increase in Pco2 (respiratory acidosis), but was predicted to produce a hypoosmotic absorbate, which significantly reduced the overall osmotic pressure of the body fluids.
Perspectives and Significance
The present study has focused on some intriguing aspects of epithelial transport and solute chemistry that together provide a major driving force for net water transport in the marine teleost intestine, beyond the well-studied role of NaCl cotransport. In conclusion, elevated HCO3− secretion and CaCO3 formation in response to elevated luminal Ca2+ significantly increased the capacity of the intestine to absorb fluid, independently of NaCl cotransport. CaCO3 formation appeared to play a vital but indirect role in water transport by removing nonabsorbed Ca2+ that would otherwise accumulate in the gut lumen and thus maintains a favorable osmotic gradient for solute-coupled fluid absorption to continue. This work provides an additional and valuable insight into the functional significance of intestinal HCO3− secretion, alongside CaCO3 precipitation and apical H+ secretion, for fluid transport and osmoregulation in vivo, yet it remains to be seen whether it is possible to fully characterize and directly quantify the relative contributions of these three components. In addition, the proposal of H+ exiting across the basolateral membrane and being buffered in the interstitial fluid and its associated benefit for overall fluid balance is an intriguing possibility offering an exciting area for further investigation. Fundamental studies of this nature seeking to understand the physiological mechanisms and roles of intestinal CaCO3 production now also have global environmental significance given the recent discovery that this piscine calcification process makes a previously unexpected but significant contribution to the marine inorganic carbon cycle (47).
GRANTS
This work was funded by the Biotechnology and Biological Sciences Research Council (BBSRC). J. M. Whittamore was supported by BBSRC studentship BBS/S/A/2004/11078 and BBSRC grant BB/F009364/1, and C. A. Cooper was supported by BBSRC grant BB/D005108/1.
Present address of C. Cooper: Integrative Biology, University of Guelph, New Science Complex, Guelph, ON, N1G 2W1, Canada.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGEMENTS
The authors thank Ian and Tony McClure, the local fishermen of Flookburgh, Cumbria, UK for collecting the fish used in this study, and Jan Shears for excellent fish husbandry assistance.
REFERENCES
- 1.Altimiras J, Claireaux G, Sandblom E, Farrell AP, McKenzie DJ, Axelsson M. Gastrointestinal blood flow and postprandial metabolism in swimming sea bassDicentrarchus labrax. Physiol Biochem Zool 81: 663–672, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Ando M, Subramanyam MVV. Bicarbonate transport systems in the intestine of the seawater eel. J Exp Biol 150: 381–394, 1990 [Google Scholar]
- 3.Axelsson M, Thorarensen H, Nilsson S, Farrell AP. Gastrointestinal blood flow in the red Irish lord, Hemilepidotus hemilepidotus: long-term effects of feeding and adrenergic control. J Comp Physiol B 170: 145–152, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Bucking C, Fitzpatrick JL, Nadella SR, Wood CM. Post-prandial metabolic alkalosis in the seawater-acclimated trout: the alkaline tide comes in. J Exp Biol 212: 2159–2166, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Bucking C, Wood CM. The alkaline tide and ammonia excretion after voluntary feeding in freshwater rainbow trout. J Exp Biol 211: 2533–2541, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Cooper CA, Whittamore JM, Wilson RW. Ca2+-driven intestinal HCO3− secretion and CaCO3 precipitation in the European flounder in vivo, and influences on acid-base regulation and blood gas transport. Am J Physiol Regul Integr Comp Physiol ( February 3, 2010). doi:10.1152/ajpregu.00513.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper CA, Wilson RW. Post-prandial alkaline tide in freshwater rainbow trout: effects of meal anticipation on recovery from acid-base and ion regulatory disturbances. J Exp Biol 211: 2542–2550, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Curran PF. Na, Cl and water transport by rat ileum in vitro. J Gen Physiol 43: 1137–1148, 1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curran PF, Solomon AK. Ion and water fluxes in the ileum of rats. J Gen Physiol 41: 143–168, 1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diamond JM. Mechanism of isotonic water transport. J Gen Physiol 48: 15–42, 1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Field M, Karnaky KJ, Smith PL, Bolton JE, Kinter WB. Ion transport across the isolated intestinal mucosa of the winter flounder, Pseudopleuronectes americanus . I. Functional and structural properties of cellular and paracellular pathways for Na and Cl. J Membr Biol 41: 265–293, 1978 [DOI] [PubMed] [Google Scholar]
- 12.Genz J, Taylor JR, Grosell M. Effects of salinity on intestinal bicarbonate secretion and compensatory regulation of acid-base balance in Opsanus beta. J Exp Biol 211: 2327–2335, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Grosell M. Intestinal anion exchange in marine fish osmoregulation. J Exp Biol 209: 2813–2827, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Grosell M, DeBoeck G, Johannsson O, Wood CM. The effects of silver on intestinal ion and acid-base regulation in the marine teleost fish, Parophrys vetulus. Comp Biochem Physiol C 124: 259–270, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Grosell M, Genz J. Ouabain-sensitive bicarbonate secretion and acid absorption by the marine teleost fish intestine play a role in osmoregulation. Am J Physiol Regul Integr Comp Physiol 291: R1145–R1156, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Grosell M, Genz J, Taylor JR, Perry SF, Gilmour KM. The involvement of H+-ATPase and carbonic anhydrase in intestinal HCO3− secretion in seawater-acclimated rainbow trout. J Exp Biol 212: 1940–1948, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Grosell M, Gilmour KM, Perry SF. Intestinal carbonic anhydrase, bicarbonate and proton carriers play a role in the acclimation of rainbow trout to seawater. Am J Physiol Regul Integr Comp Physiol 293: R2099–R2111, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Grosell M, Jensen FB. NO2− uptake and HCO3− secretion in the intestine of the European flounder (Platichthys flesus ). J Exp Biol 202: 2103–2110, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Grosell M, Laliberte CN, Wood S, Jensen FB, Wood CM. Intestinal HCO3− secretion in marine teleost fish: evidence for an apical rather than a basolateral Cl−/HCO3− exchanger. Fish Physiol Biochem 24: 81–95, 2001 [Google Scholar]
- 20.Grosell M, Mager EM, Williams C, Taylor JR. High rates of HCO3− secretion and Cl− absorption against adverse gradients in the marine teleost intestine: the involvement of an electrogenic anion exchanger and H+-pump metabolon? J Exp Biol 212: 1684–1696, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Grosell M, Taylor JR. Intestinal anion exchange in teleost water balance. Comp Biochem Physiol A 148: 14–22, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Grosell M, Wood CM, Wilson RW, Bury NR, Hogstrand C, Rankin C, Jensen FB. Bicarbonate secretion plays a role in chloride and water absorption of the European flounder intestine. Am J Physiol Regul Integr Comp Physiol 288: R936–R946, 2005 [DOI] [PubMed] [Google Scholar]
- 23.House CR, Green K. Sodium and water transport across isolated intestine of a marine teleost. Nature 199: 1293–1294, 1963 [DOI] [PubMed] [Google Scholar]
- 24.House CR, Green K. Ion and water transport in isolated intestine of marine teleost Cottus scorpius. J Exp Biol 42: 177–189, 1965 [DOI] [PubMed] [Google Scholar]
- 25.Humbert W, Kirsch R, Simonneaux V. Is mucus involved in biocrystallisation–study of the intestinal mucus of the sea-water eel Anguilla anguilla L. Cell Tissue Res 245: 599–604, 1986 [DOI] [PubMed] [Google Scholar]
- 26.Karnaky KJ. Osmotic and ionic regulation. In: The Physiology of Fishes (2nd ed.), edited by Evans DH.Boca Raton, FL: CRC, 1998 [Google Scholar]
- 27.Kurita Y, Nakada T, Kato A, Doi H, Mistry AC, Chang MH, Romero MF, Hirose S. Identification of intestinal bicarbonate transporters involved in formation of carbonate precipitates to stimulate water absorption in marine teleost fish. Am J Physiol Regul Integr Comp Physiol 294: R1402–R1412, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Loretz CA. Electrophysiology of ion transport in teleost intestinal cells. In: Cellular and Molecular Approaches to Fish Ionic Regulation, edited by Wood CM, Shuttleworth TJ. San Diego, CA: Academic, 1995 [Google Scholar]
- 29.MacKay WC, Lahlou B. Relationships between Na+ and Cl− fluxes in the intestine of the European flounder, Platichthys flesus. In: Epithelial Transport in the Lower Vertebrates, edited by Lahlou B. Cambridge, UK: Cambridge University Press, 1978 [Google Scholar]
- 30.Marshall WS, Grosell M. Ion transport and osmoregulation in fish. In: The Physiology of Fishes, (3rd ed.), edited by Evans DH, Claiborne JB., 3rd Boca Raton, FL: CRC, 2006 [Google Scholar]
- 31.McDonald DG, Milligan CL. Chemical properties of the blood. In: Fish Physiology: The Cardiovascular System , edited by Hoar WS, Randall DJ, Farrell AP. San Diego, CA: Academic, vol. XII, part B, 1992 [Google Scholar]
- 32.Mizumori M, Meyerowitz J, Takeuchi T, Lim S, Lee P, Supuran CT, Guth PH, Engel E, Kaunitz JD, Akiba Y. Epithelial carbonic anhydrases facilitate Pco2 and pH regulation in rat duodenal mucosa. J Physiol 573: 827–842, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Musch MW, Orellana SA, Kimberg LS, Field M, Halm DR, Krasny EJ, Frizzell RA. Na+-K+-Cl− co-transport in the intestine of a marine teleost. Nature 300: 351–353, 1982 [DOI] [PubMed] [Google Scholar]
- 34.Parsons DS, Wingate DL. The effect of osmotic gradients on fluid transfer across rat intestine in vitro. Biochim Biophys Acta 46: 170–183, 1961 [DOI] [PubMed] [Google Scholar]
- 35.Schultz SG. A century of (epithelial) transport physiology: from vitalism to molecular cloning. Am J Physiol Cell Physiol 274: C13–C23, 1998 [DOI] [PubMed] [Google Scholar]
- 36.Schultz SG. Epithelial water absorption: osmosis or cotransport? Proc Natl Acad Sci USA 98: 3628–3630, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Skadhauge E. Mechanism of salt and water absorption in intestine of eel (Anguilla anguilla ) adapted to waters of various salinities. J Physiol 204: 135–158, 1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Skadhauge E. Coupling of transmural flows of NaCl and water in intestine of eel (Anguilla anguilla ). J Exp Biol 60: 535–546, 1974 [DOI] [PubMed] [Google Scholar]
- 39.Smith HW. The absorption and excretion of water and salts by marine teleosts. Am J Physiol 93: 480–505, 1930 [Google Scholar]
- 40.Spring KR. Routes and mechanism of fluid transport by epithelia. Annu Rev Physiol 60: 105–119, 1998 [DOI] [PubMed] [Google Scholar]
- 41.Taylor JR, Grosell M. Feeding and osmoregulation: dual function of the marine teleost intestine. J Exp Biol 209: 2939–2951, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Walsh PJ, Blackwelder P, Gill KA, Danulat E, Mommsen TP. Carbonate deposits in marine fish intestines–a new source of biomineralisation. Limnol Oceanogr 38: 1227–1232, 1991 [Google Scholar]
- 43.Walton Smith FG. CRC Handbook of Marine Science Cleveland, OH: CRC, vol. I, 1974 [Google Scholar]
- 44.Whitlock RT, Wheeler HO. Coupled transport of solute and water across rabbit gallbladder epithelium. J Clin Invest 43: 2249–2265, 1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilson RW, Grosell M. Intestinal bicarbonate secretion in marine teleost fish–source of bicarbonate, pH sensitivity, and consequences for whole animal acid-base and calcium homeostasis. Biochim Biophys Acta 1618: 163–174, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Wilson RW, Gilmour KM, Henry RP, Wood CM. Intestinal base excretion in the seawater-adapted rainbow trout: a role in acid-base balance? J Exp Biol 199: 2331–2343, 1996 [DOI] [PubMed] [Google Scholar]
- 47.Wilson RW, Millero FJ, Taylor JR, Walsh PJ, Christensen V, Jennings S, Grosell M. Contribution of fish to the marine inorganic carbon cycle. Science 323: 359–362, 2009 [DOI] [PubMed] [Google Scholar]
- 48.Wilson RW, Wilson JM, Grosell M. Intestinal bicarbonate secretion by marine teleost fish–why and how? Biochim Biophys Acta 1566: 182–193, 2002. [DOI] [PubMed] [Google Scholar]