Abstract
One proposed explanation for the V̇o2 slow component is that lower-threshold motor units may fatigue and develop little or no tension but continue to use O2, thereby resulting in a dissociation of cellular respiration from force generation. The present study used intact isolated single myocytes with differing fatigue resistance profiles to investigate the relationship between fatigue, tension development, and aerobic metabolism. Single Xenopus skeletal muscle myofibers were allocated to a fast-fatiguing (FF) or a slow-fatiguing (SF) group, based on the contraction frequency required to elicit a fall in tension to 60% of peak. Phosphorescence quenching of a porphyrin compound was used to determine Δ intracellular Po2 (PiO2; a proxy for V̇o2), and developed isometric tension was monitored to allow calculation of the time-integrated tension (TxT). Although peak ΔPiO2 was not different between groups (P = 0.36), peak tension was lower (P < 0.05) in SF vs. FF (1.97 ± 0. 17 V vs. 2. 73 ± 0.30 V, respectively) and time to 60% of peak tension was significantly longer in SF vs. FF (242 ± 10 s vs. 203 ± 10 s, respectively). Before fatigue, both ΔPiO2 and TxT rose proportionally with contraction frequency in SF and FF, resulting in ΔPiO2/TxT being identical between groups. At fatigue, TxT fell dramatically in both groups, but ΔPiO2 decreased proportionately only in the FF group, resulting in an increase in ΔPiO2/TxT in the SF group relative to the prefatigue condition. These data show that more fatigue-resistant fibers maintain aerobic metabolism as they fatigue, resulting in an increased O2 cost of contractions that could contribute to the V̇o2 slow component seen in whole body exercise.
Keywords: oxidative phosphorylation, skeletal muscle, fiber type, oxygen uptake
the kinetics of the rise in whole body oxygen uptake during exercise (V̇o2 kinetics) vary with exercise intensity (3, 7). During low-to-moderate intensity exercise, the V̇o2 response, whether measured at the muscle or at the mouth, is characterized by a short time delay followed by a monoexponential increase to a steady state (7, 29, 44). During more intense exercise, V̇o2 does not reach a steady state but instead a second, slower, rise appears and remains until exercise is terminated (the so-called V̇o2 slow component) (4, 7, 20, 30). The V̇o2 slow component has been attributed to a variety of factors and continues to be an area of considerable debate (6, 25, 46). Among the many ideas that have been postulated to account for this phenomenon are changes in muscle recruitment patterns (5, 34, 36, 38) and changes in contractile efficiency (32, 44, 46). The idea of muscle recruitment patterns and/or muscle efficiency relates to the concept that the V̇o2 slow component could arise because of progressive recruitment of less-efficient fast-twitch fibers or because of persistent aerobic metabolism in fatigued muscle fibers, resulting in a reduced economy of contraction (36, 38, 46). There are at least two reasons that this may occur: 1) during fatiguing contractions, some active muscle fibers may fatigue to the point where they are producing negligible tension, but still consuming oxygen, resulting in a greater oxygen cost of contractions, or 2) higher-threshold motor units are recruited during fatiguing contractions, resulting in a greater oxygen cost of contractions due to the higher oxygen cost of force production of fast-twitch fibers (9). Although a developing inefficiency of contractions remains a popular view in accounting for the V̇o2 slow component, the extent to which this involves recruitment of less efficient fibers at higher-exercise intensity vs. persistent respiration in fatiguing fibers, remains unclear. For example, it was recently shown that neuromuscular blockade of slow-twitch muscle fibers in humans increases the oxygen cost of single leg knee extensor exercise (25), which supports the notion that the V̇o2 slow component may be due to recruitment of less efficient fast-twitch fibers. On the other hand, it was also observed recently that a slow component was present during electrically stimulated contractions in canine gastrocnemius muscle (46, 54), and since electrical stimulation recruits all fibers simultaneously, these results suggest that recruitment of less efficient fibers is not a prerequisite for the development of a V̇o2 slow component. One difficulty in determining the cause(s) of the slow component in whole body exercise or in situ whole muscle preparations is that it is very difficult to account for the physiological responses of different individual muscle fibers or of motor units comprising distinct fiber types. For instance, does a fatigued fiber contribute significantly to the V̇o2 even when producing negligible tension, or is its lack of tension simply replaced by less efficient fibers, or do both of these events occur in concert and contribute to the V̇o2 slow-component phenomenon? To help address this issue, it would be valuable to know whether there was a change in efficiency in single fibers of different fiber types as muscle fatigue progressed.
Therefore, to address this problem more directly, we studied fast-fatiguing and slow-fatiguing Xenopus single isolated myocytes using a measure of intracellular Po2 [as a surrogate for cellular V̇o2, as done previously (14, 16, 18, 39)], in conjunction with calculations of time-integrated tension during a contractile protocol eliciting fatigue, to facilitate examination of the relationship between aerobic metabolism and contractile demand at the single-cell level. On the basis of the view that the V̇o2 slow component may be due, in part, to a dissociation of aerobic metabolism and contractile demand during fatigue [i.e., less-efficient tension generation; (33, 35, 38, 46)], we hypothesized that slow-fatiguing fibers would exhibit less attenuation of aerobic metabolism during fatigue and that this would be manifested in an elevation of the estimated O2 cost of tension development.
METHODS
Animal care.
All procedures were approved by the University of California, San Diego Institutional Animal Care and Use Committee and conform to American Physiological Society and National Institutes of Health guidelines. Female adult Xenopus laevis were doubly pithed and decapitated, and the lumbrical muscles (II-IV) were dissected free from the hind feet.
Fiber preparation.
All dissections and experiments were conducted at 20°C. Single living muscle fibers (n = 18) were microdissected from the lumbrical muscle strips with intact tendons in a chamber filled with Ringer solution (112 mM NaCl, 1.87 mM KCl, 0.82 mM CaCl2, 2.38 mM NaHCO3, 0.07 mM NaH2PO4, 0.1 mM EGTA; pH 7.0). Intact cells were then microinjected with a solution of 0.5 mM palladium-meso-tetra (4-carboxyphenyl) porphine bound to BSA (containing 10 mM fura-2 for visual monitoring) by micropipette pressure injection (World Precision Instruments PV830 pneumatic picopump, Sarasota FL). Subsequent experiments were only performed on fibers with visually intact plasma membranes that exhibited normal contractile properties and that did not display any difficulties in propagating action potentials in response to electrical stimulation. To attempt to have equal numbers of slow-fatiguing (SF) and fast-fatiguing (FF) fibers in the two groups, muscle fibers were discriminated for fiber type by visual inspection during dissection using size, opacity, and twitch characteristics. Subsequent separation into fatigue response groups was accomplished as below.
Measurement systems.
Phosphorescence signals were recorded using a Nikon 40× Fluor objective (0.70 numerical aperture) used dry. The phosphorescence quenching of the Pd-porphyrin oxygen probe within each cell was measured through a system consisting of a flash lamp (Oxygen Enterprises, Philadelphia, PA), a 425-nm bandpass excitation filter, a 630-nm cut-on emission filter, and a photomultiplier tube for collection of the phosphorescence signal. To calculate phosphorescence lifetimes from the intracellular O2 probe, the phosphorescent decay curves from a series of 10–15 flashes (15 Hz) were averaged, and a monoexponential function was fit to the subsequent best-fit decay curve (analysis software from Medical Systems, Greenvale, NY). Phosphorescent decay curves were recorded every 4 s from each cell throughout the experimental period. Previously determined values for the measured phosphorescence lifetime decay in a zero oxygen environment and the phosphorescence quenching constant for the intracellular oxygen probe were used to calculate PiO2 (15). This technique has been previously validated for the measurement of PiO2 within single skeletal muscle cells injected with the porphyrin compound (15). In addition, this technique has been used in several studies examining single muscle cell respiration under varied conditions (22, 23, 39), and we have specifically demonstrated that the fall in PiO2 during contractions is a reliable surrogate for the change in cell V̇o2 that occurs during different contractile intensities (17). Furthermore, in our previous study examining pH changes during fatiguing contractions in our model preparation (40), pH fell from ∼7.1 to 6.6 (M. C. Hogan, unpublished observations), which is close to the range that Dunphy et al. (11) have shown that the sensitivity of the phosphorescent probe, which we employed, is not adversely impacted by changes in pH.
Experimental protocol.
Platinum clips were attached to the tendons of the cells, and they were mounted in a chamber filled with Ringer solution. One end of the fiber was fixed, and the other free end was attached to an adjustable force transducer (Aurora Scientific, Model 400A, Aurora, Ontario), allowing the muscle to be set at a length (Lo) that produced maximal tetanic peak tension (Po). The analog signal from the force transducer was sampled at 200 Hz and converted to a digital signal via an MP100WSW A-D converter and analyzed with AcqKnowledgeIII 3.2.6 analysis software (Biopac Systems, Santa Barbara, CA). To reduce the possible occurrence of unstirred layers surrounding the cell, the chamber was perfused throughout the experimental protocol with Ringer solution equilibrated with a gas mixture to produce a Po2 of ∼60 Torr.
Isometric tetanic contractions were elicited using direct (8–10 V) stimulation of the myocytes (Grass model S48; Grass Instruments, Warwick, RI). Stimulation consisted of 200-ms trains of 70-Hz pulses of 1-ms duration. Baseline measurements (no electrical stimulation) were collected for ∼25 s prior to stimulation. The stimulation regime was targeted to elicit fatigue to below 60% of the initial peak tension development in all fibers. Specifically, fibers were stimulated for 60 s at each of the following stimulation frequencies in sequential order: 0.167, 0.25, 0.33, and 0.5 Hz. If at this point, tension had fallen below 60% of initial tension, then the fibers were stimulated for a further 30 s at 0.5 Hz (i.e., for a total of 90 s at 0.5 Hz), and this became the end point of the experiment for these fibers. If by the end of 60 s at 0.5 Hz, a fiber continued to produce tension >60% of the initial level, contraction frequency was increased to 1 Hz for 90 s, and this was the end point of the experiment for these fibers. At completion of the experiment, the rate of fatigue development during the contractions was used to distinguish muscle fiber type, with FF fibers classified as those that reached 60% of initial tension at a frequency of 0.5 Hz and SF fibers classified as those that reached 60% of initial tension at a frequency of 1 Hz. We have previously demonstrated (41) that these fatigue characteristics correlate directly with mitochondrial density, as would be expected in fast- vs. slow-fatiguing fibers.
Estimation of time-integrated tension and O2 cost of contractions.
The time-integrated tension was estimated in each muscle fiber at two points within each contraction frequency by summing the tensions over the five consecutive contractions preceding the time interval, multiplying by the contraction duration (fixed at 200 ms), and dividing by the time interval required for five contractions. To estimate the O2 cost of contractions, we took the quotient of ΔPiO2 (initial PiO2-PiO2 observed for a given time period) and the time-integrated tension produced at each time interval.
Statistics.
Values are expressed as means ± SE. Differences between groups of fibers were assessed by Student's t-test. Differences between the estimated O2 cost of contractions as a function of time within a group were assessed by a one-way ANOVA with repeated measures, and a Holm-Sidak post hoc multiple-comparison test. The α was set at 0.05 for t-tests and ANOVAs.
RESULTS
General characteristics of fiber preparations.
Table 1 shows some of the defining characteristics of the fibers designated as FF or SF. Whereas peak tension was lower in SF fibers, the time at which developed tension fell to 60% of peak was significantly lower in the FF fibers (i.e., they exhibited more rapid fatigue). There was no difference in the PiO2 under resting (noncontracting) conditions, nor was there a difference in the peak ΔPiO2 (i.e., greatest fall in PiO2 from rest to contractions) between FF and SF fibers.
Table 1.
Skeletal muscle fiber type descriptive data
| Peak Tension, V | Time to 60% of Peak Tension, s | PiO2 Rest, Torr | ΔPiO2 Peak, Torr | |
|---|---|---|---|---|
| FF (n = 9) | 2.73 ± 0.30 | 203 ± 10 | 59 ± 2 | 30 ± 3 |
| SF (n = 9) | 1.97 ± 0.17* | 242 ± 10* | 56 ± 2 | 37 ± 6 |
Values are expressed as means ± SE;
P < 0.05 vs. FF. FF, fast-fatiguing fibers (final contraction frequency =0.5 Hz); SF, slow-fatiguing fibers (final contraction frequency =1.0 Hz).
Contractile responses.
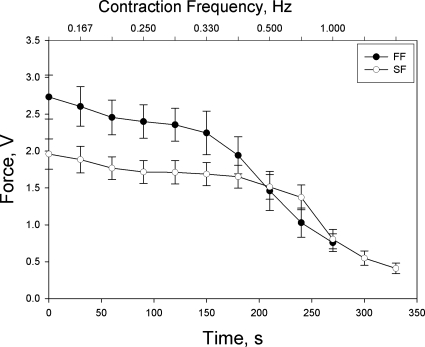
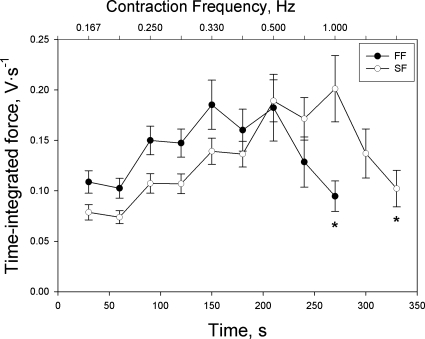
The tension profile and time-integrated tension profile for each group of fibers are depicted in Figs. 1 and 2, respectively. In both groups of fibers, tension development showed little decline until the highest frequency of contractions but exhibited a precipitous drop thereafter. Time-integrated tension generally increased with increases in contraction frequency until reaching a peak at the first time interval of the highest contraction frequency in both groups of fibers. However, time-integrated tension also exhibited a precipitous fall after reaching this peak, as fatigue became severe.
Fig. 1.
Tension development as a function of time and contraction intensity in fast-fatiguing (FF; final contraction intensity = 0.5 Hz) and slow-fatiguing (SF; final contraction intensity = 1.0 Hz) myocytes. Values are expressed as means ± SE.
Fig. 2.
Time-integrated tension as a function of contraction intensity in FF (final contraction intensity = 0.5 Hz) and SF (final contraction intensity = 1.0 Hz) myocytes. Values are expressed as means ± SE; *P < 0.05 vs. first sampling point of final contraction intensity.
Intracellular Po2 responses.
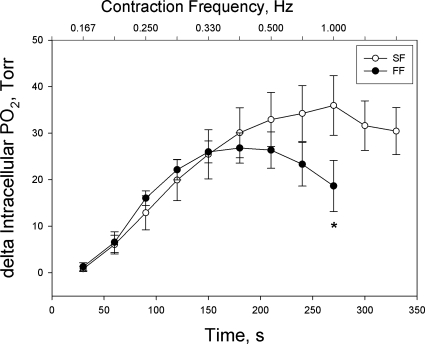
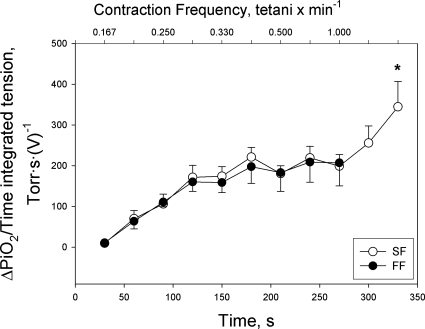
The ΔPiO2, used as a proxy measure for V̇o2, exhibited a gradual increase with increases in time-integrated tension development until it reached a peak in both groups of fibers, as reflected by a fall in intracellular Po2 (Fig. 3). Beyond this point, however, the responses of the two groups of fibers diverged, with the FF fibers exhibiting a significant reduction in ΔPiO2, as fatigue became severe, but the SF fibers exhibiting no significant decline despite a similar degree of fatigue as seen in FF fibers. Thus, the plot of the quotient of ΔPiO2 and time-integrated tension (an estimate of the O2 cost of tension development) vs. contraction intensity shows (Fig. 4) that after an initial increase (a response that was complete ∼midway through the 0.25-Hz contraction intensity), the quotient of ΔPiO2 and time-integrated tension leveled off and was identical in both groups of fibers until fatigue became severe during the highest contraction intensity within each group. At this point, because of the divergent ΔPiO2 responses during fatigue in the two groups of fibers, the quotient of ΔPiO2 and time-integrated tension rose dramatically in the SF fibers (but not FF fibers), such that the final measurement point (330 s) was significantly greater than every point prior to 300 s, with the exception of 180 s, where the critical P was 0.001 and the actual P was 0.002 (Holm-Sidak post hoc test). This response is consistent with an increase in O2 cost of tension development when fatigue became severe in the SF fibers.
Fig. 3.
Delta intracellular Po2 (as a proxy for V̇o2) as a function of contraction intensity in FF (final contraction intensity = 0.5 Hz) and SF (final contraction intensity = 1.0 Hz) myocytes. Values are expressed as means ± SE; *P < 0.05 vs. first sampling point of final contraction intensity.
Fig. 4.
The quotient of delta intracellular Po2 (ΔPiO2) and time-integrated tension in FF (final contraction intensity = 0.5 Hz) and SF (final contraction intensity = 1.0 Hz) myocytes. Values are expressed as means ± SE; *P < 0.05 vs. first sampling point at final contraction intensity.
DISCUSSION
The physiological basis of the V̇o2 slow component, a phenomenon that occurs during whole body exercise at intensities exceeding the lactate threshold, remains unclear. The two best supported hypotheses involve 1) the recruitment of less efficient fast-twitch muscle fibers at higher exercise intensities; and/or 2) persistent aerobic metabolism in fibers that are producing less tension consequent to fatigue in conjunction with recruitment of additional fibers to make up the required tension. In the present experiment, fibers were distinguished by their fatigability profile, either fast or slow. After the first 2 min of stimulation, both groups of fibers maintained a very tight coupling of oxidative metabolism and time-integrated tension until the point of rapid fatigue. At the point of rapid fatigue, those fibers that were more fatigue resistant (SF) maintained their respiratory rate, despite the decreasing force production, whereas the more fatigable fibers (FF) exhibited a parallel decrease in respiratory rate as tension fell. The implication of our results is that fatigue-resistant fibers show a greater O2 cost of contractions during fatigue, whereas FF fibers maintain the O2 cost of contractions regardless of fatigue. As such, our results show that one factor that could contribute to the V̇o2 slow component in vivo is persistent aerobic metabolism in relatively fatigue-resistant muscle fibers as they fatigue, independent of any recruitment of less efficient fast-twitch motor units.
Factors contributing to increased O2 cost of time-integrated tension during fatigue of single myocytes.
In general, there is a linear relationship between increasing work output and muscle oxygen uptake. Across the range from rest to sublactate threshold exercise intensities, this relationship generally holds whether measured during whole body exercise (2), the level of the whole muscle (21, 24, 42) or even at the single myocyte level (18). Indeed, data from the current study show that after the initial 2 min, when the kinetics of the V̇o2 response are likely contributing to the gradual increase seen, there was a remarkably constant quotient of ΔPiO2 and time-integrated tension from 0.25 Hz to 0.5 Hz (a doubling in contraction frequency), showing that respiration was well matched to contractile demand at the single-cell level during this interval. However, once exercise intensity exceeds the lactate threshold during prolonged [>15-min duration (10)] whole body exercise, there is a disproportionately greater increase in V̇o2 for a given increment in work output, manifested as the V̇o2 slow component during whole body exercise (3, 4, 30). Similar to the whole body exercise response, our current data also show that there is a developing disproportionality between aerobic metabolism and tension production at the single fiber level. Interestingly, this characteristic was unique to SF fibers. As such, these observations suggest that one of the contributing factors to the rise in V̇o2 during the slow component may be persistent aerobic metabolism in a subpopulation of fibers that are undergoing fatigue.
Previously, we reported a proportional fall in V̇o2 and tension development during high-intensity fatiguing muscle contractions in electrically stimulated rat distal hindlimb muscles pump-perfused in situ (13). Because this response occurred after the attainment of V̇o2max regardless of whether the contraction intensity was high from the beginning of stimulation (a protocol that induced more rapid fatigue) or whether contraction intensity was incrementally increased (a protocol that delayed fatigue development), we argued that V̇o2 fell in these experiments, consequent to the contractile demand having fallen below that necessitating maximal activation of aerobic metabolism (13). Given the high fast-twitch fiber proportion in the rat distal hindlimb muscles (1), the current results in FF fibers are consistent with these previous observations and further support the idea that respiration falls during fatigue in fast-twitch fibers because of a decreased contractile ATP demand.
It has long been recognized that the direct coupling of respiration and tension development is transduced and modulated through factors such as ADP, Pi, and H+. Increased ADP and Pi, which are produced during ATP hydrolysis at muscle cross-bridges and other cellular processes like Ca2+ pumping, are potent activators of mitochondrial respiration (19, 37). In the current study, as isometric tension and ATP hydrolysis decline with reduced cross-bridge turnover and reduced Ca2+ pumping during fatigue, this could explain the fall in respiration in FF fibers. On the other hand, the current results in SF fibers show that respiration and tension development become dissociated during fatigue of these fibers. As recent evidence shows that Pi stimulates respiration in both fast- and slow-twitch muscle bundles and isolated mitochondria from fast- and slow-twitch fibers (37), the most likely explanation for our results is that the factors driving respiration and tension production can be decoupled in some circumstances and that this is a property of SF fibers in particular. There are likely several important differences between FF and SF fibers that bear consideration in this context.
First, on the basis of our previous observation that fatigability correlates with mitochondrial volume density in the single fibers that we typically study (41), one difference between FF and SF fibers is a higher mitochondrial content in SF fibers. Although a greater mitochondrial content increases sensitivity to drivers of respiration, our results seem paradoxical to the expected superior coupling of aerobic metabolism to tension development in SF fibers, suggesting that some aspect of fatigue per se is responsible for the differential responses between FF and SF fibers during fatigue.
In this respect, a second difference between SF and FF fibers that we have observed is that the dynamics of pH changes during fatiguing contractions are different between fiber types (43). Since acidosis can inhibit respiration (12) and since pH changes in slow-twitch muscle are less severe (and perhaps opposing) to those observed in fast-twitch muscle (26, 27), this may allow aerobic metabolism to persist at or near prefatigue levels in SF fibers.
Third, another difference between FF and SF fibers may be in the cost of the relative energy requirements for Ca2+ handling. Current estimates suggest that up to 80% of the overall ATP demand in mouse fast-twitch muscle fibers may be accounted for by Ca2+ handling (45). Because the SF fibers develop less tension than the FF fibers, and the cross-bridges turn over more slowly, it follows that the energetic requirements for Ca2+ handling are significantly less in SF fibers. Thus, during fatigue, when the amount of Ca2+ released by the sarcoplasmic reticulum (SR) is reduced, tension generation falls and the energetic requirement for pumping Ca2+ back into the SR is thereby diminished. This large requirement for ATP for Ca2+ pumping in FF fibers, and the relatively larger fall in this energetic process in the FF fibers compared with the SF fibers during fatigue development, may contribute to the differences observed in coupling between tension development and the O2 cost of contractions in the FF fibers vs. the SF fibers. Further experiments are warranted to test this possibility.
Perspectives on the Causes of the V̇o2 Slow Component
The notion of a V̇o2 slow component was first raised by Linnarson in 1974 (28) and referred to by the term “slow component” by Casaburi and colleagues in 1989 (7). Many previous studies have attempted to illuminate the causes of the V̇o2 slow component observed during higher-intensity exercise and/or muscle contractions. Although there is general consensus that this event occurs when exercise/contraction intensities exceed the lactate threshold (7, 20), an increase in lactate per se is not the cause (31), and many contradictions remain regarding the underlying mechanisms of this event. For example, several studies have shown that the upward rise in V̇o2 coincides with an increase in muscle EMG, an observation taken to suggest that recruitment of additional (faster and less efficient) motor units might contribute to this phenomenon (5, 34, 36). On the other hand, other studies reject the notion of recruitment of less efficient muscle fibers (8, 38), suggesting instead that there is some developing inefficiency in the fibers that have already been recruited. Consistent with this latter viewpoint, a V̇o2 slow component was recently reported in electrically stimulated canine gastrocnemius muscle (46), a situation in which there is no additional recruitment of muscle fibers. Muscle fatigue appears to play an important role in the V̇o2 slow component response, as Rossiter et al. (33) previously showed that infusion of the pyruvate dehydrogenase activator, dichloroacetate, not only slowed the acidification of muscle and reduced PCr hydrolysis (both of which would attenuate fatigue), it also reduced the amplitude of the V̇o2 slow component. Thus, our current results, showing that in SF fibers, fatigue is associated with a developing inefficiency of tension production, are consistent with the notion that fatigue of fibers already recruited, and subsequent dissociation of aerobic metabolism and tension output in some of these fibers, may contribute to the V̇o2 slow-component phenomenon. In this paradigm, we speculate that during whole body exercise above the lactate threshold, fatigue of some fibers requires the recruitment of additional fibers to maintain tension output, and it is a disproportionate maintenance of aerobic metabolism relative to declining tension production in some of these fatiguing fibers that produces a gradual upward drift of V̇o2 with continued exercise. Although our study does not prove this previous point conclusively, it provides the first cellular-based evidence of developing aerobic inefficiency in fatiguing fibers, which is a valuable observation in seeking a cellular-based explanation to the origins of the V̇o2 slow component.
GRANTS
This study was supported by operating grants from the National Institutes of Health (AR40155 to M. C. Hogan) and the Natural Sciences and Engineering Research Council (RPGIN 238805 to R. T. Hepple). Dr. Hepple was supported by a Canadian Institute of Health Research New Investigator Award.
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1.Armstrong RB, Phelps RO. Muscle fiber type composition of the rat hindlimb. Am J Anat 171: 259–272, 1984 [DOI] [PubMed] [Google Scholar]
- 2.Astrand PO. Textbook of Work Physiology: Physiological Bases of Exercise New York: McGraw-Hill, 1986, p. 230–300 [Google Scholar]
- 3.Barstow TJ, Casaburi R, Wasserman K. O2 uptake kinetics and the O2 deficit as related to exercise intensity and blood lactate. J Appl Physiol 75: 755–762, 1993 [DOI] [PubMed] [Google Scholar]
- 4.Barstow TJ, Mole PA. Linear and nonlinear characteristics of oxygen uptake kinetics during heavy exercise. J Appl Physiol 71: 2099–2106, 1991 [DOI] [PubMed] [Google Scholar]
- 5.Borrani F, Candau R, Millet GY, Perrey S, Fuchslocher J, Rouillon JD. Is the V̇o2 slow component dependent on progressive recruitment of fast-twitch fibers in trained runners? J Appl Physiol 90: 2212–2220, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Cannon DT, Kolkhorst FW, Cipriani DJ. Electromyographic data do not support a progressive recruitment of muscle fibers during exercise exhibiting a V̇o2 slow component. J Physiol Anthropol 26: 541–546, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Casaburi R, Barstow TJ, Robinson T, Wasserman K. Influence of work rate on ventilatory and gas exchange kinetics. J Appl Physiol 67: 547–555, 1989 [DOI] [PubMed] [Google Scholar]
- 8.Cleuziou C, Perrey S, Borrani F, Lecoq AM, Courteix D, Germain P, Obert P. V̇o2 and EMG activity kinetics during moderate and severe constant work rate exercise in trained cyclists. Can J Appl Physiol 29: 758–772, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Crow MT, Kushmerick MJ. Chemical energetics of slow- and fast-twitch muscles of the mouse. J Gen Physiol 79: 147–166, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis JA, Whipp BJ, Lamarra N, Huntsman DJ, Frank MH, Wasserman K. Effect of ramp slope on determination of aerobic parameters from the ramp exercise test. Med Sci Sports Exerc 14: 339–343, 1982 [PubMed] [Google Scholar]
- 11.Dunphy I, Vinogradov SA, Wilson DF. Oxyphor R2 and G2: phosphors for measuring oxygen by oxygen-dependent quenching of phosphorescence. Anal Biochem 310: 191–198, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Harkema SJ, Meyer RA. Effect of acidosis on control of respiration in skeletal muscle. Am J Physiol Cell Physiol 272: C491–C500, 1997 [DOI] [PubMed] [Google Scholar]
- 13.Hepple RT, Krause DJ, Hagen JL, Jackson CC. V̇o2max is unaffected by altering the temporal pattern of stimulation frequency in rat hindlimb in situ. J Appl Physiol 95: 705–711, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Hogan MC. Fall in intracellular Po2 at the onset of contractions in Xenopus single skeletal muscle fibers. J Appl Physiol 90: 1871–1876, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Hogan MC. Phosphorescence quenching method for measurement of intracellular Po2 in isolated skeletal muscle fibers. J Appl Physiol 86: 720–724, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Howlett RA, Hogan MC. Dichloroacetate accelerates the fall in intracellular Po2 at onset of contractions in Xenopus single muscle fibers. Am J Physiol Regul Integr Comp Physiol 284: R481–R485, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Howlett RA, Hogan MC. Intracellular Po2 decreases with increasing stimulation frequency in contracting single Xenopus muscle fibers. J Appl Physiol 91: 632–636, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Howlett RA, Kindig CA, Hogan MC. Intracellular Po2 kinetics at different contraction frequencies in Xenopus single skeletal muscle fibers. J Appl Physiol 102: 1456–1461, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Jeneson JAL, Westerhoff HV, Kushmerick MJ. A metabolic control analysis of kinetic controls in ATP free energy metabolism in contracting skeletal muscle. Am J Physiol Cell Physiol 279: C813–C832, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Jones AM, Carter H, Doust JH. A disproportionate increase in V̇o2 coincident with lactate threshold during treadmill exercise. Med Sci Sports Exerc 31: 1299–1306, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Jorfeldt L, Wahren J. Leg blood flow during exercise in man. Clin Sci 41: 459–473, 1971 [DOI] [PubMed] [Google Scholar]
- 22.Kindig CA, Howlett RA, Hogan MC. Effect of extracellular Po2 on the fall in intracellular Po2 in contracting single myocytes. J Appl Physiol 94: 1964–1970, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Kindig CA, Kelley KM, Howlett RA, Stary CM, Hogan MC. Assessment of O2 uptake dynamics in isolated single skeletal myocytes. J Appl Physiol 94: 353–357, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Knight DR, Poole DC, Schaffartzik W, Guy HJ, Prediletto R, Hogan MC, Wagner PD. Relationship between body and leg V̇o2 during maximal cycle ergometry. J Appl Physiol 73: 1114–1121, 1992 [DOI] [PubMed] [Google Scholar]
- 25.Krustrup P, Secher NH, Relu MU, Hellsten Y, Soderlund K, Bangsbo J. Neuromuscular blockade of slow twitch muscle fibres elevates muscle oxygen uptake and energy turnover during submaximal exercise in humans. J Physiol 586: 6037–6048, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kushmerick MJ. Energetics studies of muscles of different types. Basic Res Cardiol 82 Suppl 2: 17–30, 1987 [DOI] [PubMed] [Google Scholar]
- 27.Kushmerick MJ. Patterns in mammalian muscle energetics. J Exp Biol 115: 165–177, 1985 [DOI] [PubMed] [Google Scholar]
- 28.Linnarsson D. Dynamics of pulmonary gas exchange and heart rate changes at start and end of exercise. Acta Physiol Scand Suppl 415: 1–68, 1974 [PubMed] [Google Scholar]
- 29.Ozyener F, Rossiter HB, Ward SA, Whipp BJ. Influence of exercise intensity on the on- and off-transient kinetics of pulmonary oxygen uptake in humans. J Physiol 533: 891–902, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poole DC, Barstow TJ, Gaesser GA, Willis WT, Whipp BJ. V̇o2 slow component: physiological and functional significance. Med Sci Sports Exerc 26: 1354–1358, 1994 [PubMed] [Google Scholar]
- 31.Poole DC, Gladden LB, Kurdak S, Hogan MC. l-(+)-lactate infusion into working dog gastrocnemius: no evidence lactate per se mediates V̇o2 slow component. J Appl Physiol 76: 787–792, 1994 [DOI] [PubMed] [Google Scholar]
- 32.Pringle JS, Doust JH, Carter H, Tolfrey K, Campbell IT, Jones AM. Oxygen uptake kinetics during moderate, heavy and severe intensity ‘submaximal’ exercise in humans: the influence of muscle fibre type and capillarisation. Eur J Appl Physiol 89: 289–300, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Rossiter HB, Ward SA, Howe FA, Wood DM, Kowalchuk JM, Griffiths JR, Whipp BJ. Effects of dichloroacetate on V̇o2 and intramuscular 31P metabolite kinetics during high-intensity exercise in humans. J Appl Physiol 95: 1105–1115, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Sabapathy S, Schneider DA, Morris NR. The V̇o2 slow component: relationship between plasma ammonia and EMG activity. Med Sci Sports Exerc 37: 1502–1509, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Sahlin K, Sorensen JB, Gladden LB, Rossiter HB, Pedersen PK. Prior heavy exercise eliminates V̇o2 slow component and reduces efficiency during submaximal exercise in humans. J Physiol 564: 765–773, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saunders MJ, Evans EM, Arngrimsson SA, Allison JD, Warren GL, Cureton KJ. Muscle activation and the slow component rise in oxygen uptake during cycling. Med Sci Sports Exerc 32: 2040–2045, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Scheibye-Knudsen M, Quistorff B. Regulation of mitochondrial respiration by inorganic phosphate; comparing permeabilized muscle fibers and isolated mitochondria prepared from type-1 and type-2 rat skeletal muscle. Eur J Appl Physiol 105: 279–287, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Scheuermann BW, Hoelting BD, Noble ML, Barstow TJ. The slow component of O2 uptake is not accompanied by changes in muscle EMG during repeated bouts of heavy exercise in humans. J Physiol 531: 245–256, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stary CM, Hogan MC. Effect of varied extracellular Po2 on muscle performance in Xenopus single skeletal muscle fibers. J Appl Physiol 86: 1812–1816, 1999 [DOI] [PubMed] [Google Scholar]
- 40.Stary CM, Hogan MC. Intracellular pH during sequential, fatiguing contractile periods in isolated single Xenopus skeletal muscle fibers. J Appl Physiol 99: 308–312, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Stary CM, Mathieu-Costello O, Hogan MC. Resistance to fatigue of individual Xenopus single skeletal muscle fibres is correlated with mitochondrial volume density. Exp Physiol 89: 617–621, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Sullivan MJ, Binkley PK, Unverferth DV, Leier CV. Hemodynamic and metabolic responses of the exercising lower limb of humans. J Lab Clin Med 110: 145–152, 1987 [PubMed] [Google Scholar]
- 43.Walsh B, Stary CM, Howlett RA, Kelley KM, Hogan MC. Glycolytic activation at the onset of contractions in isolated Xenopus laevis single myofibres. Exp Physiol 93: 1076–1084, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Whipp BJ, Ward SA, Rossiter HB. Pulmonary O2 uptake during exercise: conflating muscular and cardiovascular responses. Med Sci Sports Exerc 37: 1574–1585, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Zhang SJ, Andersson DC, Sandstrom ME, Westerblad H, Katz A. Cross bridges account for only 20% of total ATP consumption during submaximal isometric contraction in mouse fast-twitch skeletal muscle. Am J Physiol Cell Physiol 291: C147–C154, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Zoladz JA, Gladden LB, Hogan MC, Nieckarz Z, Grassi B. Progressive recruitment of muscle fibers is not necessary for the slow component of V̇o2 kinetics. J Appl Physiol 105: 575–580, 2008. [DOI] [PubMed] [Google Scholar]