Abstract
Since omega–3 polyunsaturated fatty acids (n-3 PUFAs) can alter ventricular myocyte calcium handling, these fatty acids could adversely affect cardiac contractile function, particularly following myocardial infarction. Therefore, 4 wk after myocardial infarction, dogs were randomly assigned to either placebo (corn oil, 1 g/day, n = 16) or n-3 PUFAs supplement [docosahexaenoic acid (DHA) + eicosapentaenoic acid (EPA) ethyl esters; 1, 2, or 4 g/day; n = 7, 8, and 12, respectively] groups. In vivo, ventricular function was evaluated by echocardiography before and after 3 mo of treatment. At the end of the 3-mo period, hearts were removed and in vitro function was evaluated using right ventricular trabeculae and isolated left ventricular myocytes. The treatment elicited significant (P < 0.0001) dose-dependent increases (16.4-fold increase with 4 g/day) in left ventricular tissue and red blood cell n-3 PUFA levels (EPA + DHA, placebo, 0.42 ± 0.04; 1 g/day, 3.02 ± 0.23; 2 g/day, 3.63 ± 0.17; and 4 g/day, 6.97 ± 0.33%). Regardless of the dose, n-3 PUFA treatment did not alter ventricular function in the intact animal (e.g., 4 g/day, fractional shortening: pre, 42.9 ± 1.6 vs. post, 40.1 ± 1.7%; placebo: pre, 39.2 ± 1.3 vs. post, 38.4 ± 1.6%). The developed force per cross-sectional area, changes in length- and frequency-dependent behavior in contractile force, and the inotropic response to β-adrenoceptor activation were also similar for trabeculae obtained from placebo- or n-3 PUFA-treated dogs. Finally, calcium currents and calcium transients were the same in myocytes from n-3 PUFA- and placebo-treated dogs. Thus dietary n-3 PUFAs did not adversely alter either in vitro or in vivo ventricular contractile function in dogs with healed infarctions.
Keywords: contractility, Frank-Starling mechanism, force-frequency relationship, heart failure, sudden cardiac death, diet
the cardiovascular benefits of dietary omega–3 polyunsaturated fatty acids (n-3 PUFA) have been actively investigated for over 30 years. In the late 1970s, Dyerberg et al. (23) documented that age-adjusted mortality from myocardial infarction (MI) among the Greenland Inuit was ∼10 to 40% of that noted for Danes, despite similar consumption of dietary fat and cholesterol. Dietary analysis revealed that most of the fat and calories in the Inuit diet were from fish and marine mammals rich in long-chain n-3 PUFAs, eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA), whereas typical Western diets contain twice as much saturated fatty acids and more n-6 PUFAs (2). This initial observation has been confirmed by numerous epidemiological studies that provide further evidence for a strong inverse relationship between fatty fish consumption and cardiac mortality (18, 38, 41). In contrast to these observational studies, interventional studies using n-3 PUFAs for the secondary prevention of adverse cardiovascular events in patients recovering from MI have yielded conflicting results (14, 15, 43, 69). The Diet and Reinfarction Trial (DART) (14) and Gruppo Italiano per la Sperimentazione della Streptochinasi nell’Infarto Miocardico (GISSI)-Prevenzione trial (43) reported 10 to 20% reductions in all-cause mortality with up to a 45% reduction in sudden cardiac death (43). In marked contrast, the DART-2 study (15) reported that n-3 PUFAs increased, rather than decreased, all-cause mortality (26% increase over a 9-yr follow-up period, with a 54% increase sudden cardiac death), whereas the Japan Eicosapentaneoic Acid Lipid Intervention (JELIS) found that EPA supplements did not alter either sudden death or fatal MI despite decreasing nonfatal coronary events (69). Meta-analyses of trials in which subjects ingested either fish or fish oil supplements also yielded inconsistent results; some studies reported a reduced cardiovascular mortality (39, 70), whereas others did not find a reduced risk for sudden death (12, 35). An explanation for these conflicting results remains largely to be determined.
The acute application of n-3 PUFAs to cardiac myocytes has been shown to alter intracellular calcium handling by inhibiting both sarcolemmal calcium entry via the L-type calcium channels (24, 33, 67) and calcium release from the sarcoplasmic reticulum (34, 49, 52, 60). Dietary n-3 PUFAs have also been shown to alter intracellular calcium dynamics. Coronel and coworkers (64) found that myocytes from healthy pigs fed diets enriched with fish oil exhibited decreased L-type calcium (−15%) and sodium/calcium exchanger currents (−60%). Since calcium is critical for excitation-contraction coupling, these alterations in calcium homeostasis could provoke reductions in myocardial contractile performance that become particularly important in patients with compromised cardiac function. As such, it is possible that the consumption of n-3 PUFAs could reduce ventricular function and thereby increase the risk for adverse cardiac events in patients in whom cardiac function had been compromised by MI (13). Indeed, calcium channel antagonists have been shown to increase mortality in post-MI patients with left ventricular (LV) dysfunction (11, 28). The effects of n-3 PUFA supplements on ventricular function, particularly following MI, have not been extensively investigated.
It, therefore, was the purpose of this series of studies to investigate the effects of dietary n-3 PUFAs on ventricular function in animals with healed anterior wall MI. In particular, the hypothesis that n-3 PUFA treatment could dose-dependently alter ventricular contractile function was tested using a canine model of MI. Echocardiography was used to evaluate LV function in vivo, whereas in vitro studies were performed on isolated right ventricular trabeculae and LV myocytes.
METHODS
All the animal procedures were approved by the Ohio State University Institutional Animal Care and Use Committee and conformed to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publication No. 85-23, Revised 1996).
Surgical preparation.
Fifty-three heartworm-free mixed-breed dogs (2 to 3 yr old; male, n = 20; and female, n = 33) weighing 19.0 ± 0.4 kg (range, 13.6–24.1 kg) were used in this study. The animals were anesthetized and instrumented as previously described (3, 5–9, 36). Briefly, 24 h before surgery, a transdermal fentanyl patch that delivers 100 μg/h (Duragesic, Jansen Pharmaceutical, Titusville, NJ) was placed on the left side of the animal's neck and secured with tape. On the day of surgery, the dogs received 15 mg (1 ml im) morphine sulfate (Elkins-Sinn, Cherry Hill, NJ) and thiopental sodium (20 mg/kg iv; Baxter Healthcare, Glendale, CA) to induce anesthesia. The dogs were intubated and a surgical plane of anesthesia was maintained by the inhalation of isoflurane (1 to 1.5%, Baxter Healthcare). With the use of strict aseptic procedures, a left thoracotomy was made in the fourth intercostal space. The heart was exposed and supported by a pericardial cradle. The left anterior descending coronary artery was isolated, and a two-stage occlusion of this artery was then performed approximately one-third the distance from its origin. This vessel was partially occluded for 20 min and then tied off. The ligation of this vessel produced an anteriolateral MI [∼16% of LV mass (3)] from the region below the level of the papillary muscles to the apex. The infarction was confined to the mid- to the endocardial layers of the heart (i.e., was usually not transmural). A similar surgery was performed in five additional dogs (1 male, and 4 female; 19.8 ± 1.3 kg; range, 16.8–22.7 kg) with the exception that the left anterior descending was dissected from the surrounding tissue but was not ligated (sham operated, no MI control group).
In addition to the fentanyl patch described above, morphine sulfate (1.0 mg/kg sc) was given as needed to control any postoperative pain. The long-lasting local anesthetic 0.25% bupivacaine-HCl (Abbott, North Chicago, IL) was injected in each of three sites (0.5 ml) to block the intercostal nerves in the area of the incision to minimize discomfort to the animals. Each dog was placed on antibiotic therapy (amoxicillin, 500 mg po, Teva Pharmaceuticals, Sellersville, PA) twice daily for 7 days. The animals were placed in a quiet recovery area and were returned to their home kennel once the effects of the anesthesia had dissipated. To minimize the incidence of arrhythmias, the dogs received lidocaine-HCl (100 mg im; Elkin-Sinns) before surgery, which was supplemented (60 mg iv) before each of the two stages of the coronary occlusion. The dogs also received procainamide-HCl (500 mg im; Abbott) before the surgery.
Dietary n-3 polyunsaturated fatty acid protocol.
As noted in Surgical preparation, 53 dogs underwent surgery; 10 (18.9%) of these animals died within 72 h of the MI. Thus 43 dogs that survived MI were randomly assigned to one of the following groups: placebo (n = 16), 1 g/day n-3 PUFA (n = 7), 2 g/day n-3 PUFA (n = 8), or 4 g/day n-3 PUFA (n = 12). The five sham-operated (no MI) dogs received 4 g/day. All the n-3 PUFA-treated dogs were given supplements similar to those used in the GISSI-Prevenzione study (20, 26). The n-3 PUFA group received 465 mg ethyl eicosapentaenoate, EPA + ethyl docosahexaenoate, DHA, 375 mg per capsule (Lovaza, GlaxoSmithKline, Research Triangle Park, NC). Thus the 1 g/day group received one capsule per day, whereas the 2 g/day- and the 4 g/day-treated groups received two and four pills per day, respectively. The placebo was corn oil (1 g, 58% linoleic acid + 28% oleic acid). The capsules were given per os before the daily feeding (between 8:00 am and 10:00 am each day, 7 days/wk). Six dogs died suddenly during the 3-mo feeding study: placebo, n = 2; and n-3 PUFA, n = 4 (MI group: 1 dog with 1 g/day; 2 dogs with 4 g/day; and sham operated, 1 dog with 4 g/day). Thus the dogs included in the final evaluation were placebo, n = 14; 1 g/day, n = 5; 2 g/day, n = 8; 4 g/day, n = 10; and sham operated (no MI) 4 g/day, n = 4.
Red blood cell and cardiac tissue fatty acid analysis.
Fasting blood samples (5 ml) were drawn into EDTA tubes from a cephalic vein between 8:00 am and 9:00 am at the following times points: 1 day before treatment; 1, 2, 4, 8, and 12 wk during treatment; and when tissue was harvested at the end of the study (∼14 wk after the treatment began). Right atrial (RA), LV, and LV infarction tissues were obtained when the hearts were harvested. The infarction (scar) was visually identified and carefully dissected from the surrounding healthy tissue. A central portion of the scar that was free of any healthy muscle tissue (i.e., contained no red areas) was used for the lipid analysis. The tissue and red blood cells (RBCs) were flash frozen in liquid nitrogen and stored at −80°C for future analysis.
RBCs and phospholipids from cardiac tissue were analyzed for fatty acid composition using previously described techniques (10, 45). The samples were analyzed by gas chromatography using a GC2010-FID (Shimadzu, Columbia, MD) equipped with a 100-mm capillary column (SP-2560, Supelco, Bellefonte, PA). The fatty acids of interest were identified by a comparison with known standards and expressed as a percentage of total fatty acids. The coefficient of variation for the RBC EPA + DHA assays was <5%.
Echocardiography.
Echocardiography was performed 3 to 4 wk after surgically induced MI. Data were obtained the day before oil treatments began (pre) and at the end of the 3-mo treatment period (post). Echocardiograms were recorded in five of these dogs 1 wk before and 4 wk after the MI. Two-dimensional, M-mode (GE Vivid-7 Echocardiograph System, 3-MHz sector transducer, GE Vingmed Ultrasound, Horten, Norway) was performed on sedated (butorphanol 0.2 mg/kg iv, Mylan, Canonburgs, PA) dogs on a customized table. M-mode and two-dimensional images were used to assess LV systolic and diastolic function. All measurements were made in triplicate and averaged by a single investigator (Y. Nishijima).
Heart rate variability protocols.
Before the echocardiography studies, the animals were transported (between 8:00 am and 10:00 am) to the laboratory and placed in a harness that restricted movement. Body surface electrodes were placed in the standard configuration, and an electrocardiogram (lead II) was recorded. This procedure was repeated for 5 days. Data were obtained on the fifth day (when the animals had become habituated to the laboratory environment) for the heart rate variability analysis. At least 5 min of data were recorded during each session. Heart rate variability was obtained using a Delta-Biometrics Vagal Tone monitor (Urbana, Champaign, IL). This device employs the time-series signal processing techniques as developed by Porges (54) to estimate the amplitude of respiratory sinus arrhythmia. Details of this analysis have been described previously (5). Briefly, the ECG signal was digitized at 1 kHz, and sequential R-R intervals were timed to the nearest millisecond. The nonperiodic baseline fluctuations were removed using a moving third-order 21-point polynomial function. This procedure prevented leakage of trends and harmonics of nonsinusoidal periodic activity (i.e., transient changes) into the frequency component of interest (0.24 to 1.04 Hz). Once this “filtering” procedure had been performed, the output of the moving polynomial was processed with a digital band-pass filter to extract the variance in the 0.24- to 1.04-Hz frequency band. The variance measure was then transformed into its natural logarithm to “normalize” the distribution of the variance estimates to limit the impact of large differences (i.e., outlying values). Data were averaged over 30-s intervals, and the following indexes of heart rate variability were determined: vagal tone index, the high-frequency (0.24 to 1.04 Hz) component of R-R interval variability, and the standard deviation of the R-R intervals for the same 30-s time period. The studies were performed both before and at the end of the 3-mo treatment period.
Trabeculae studies.
At the end of the 3-mo treatment period, the hearts were excised and rapidly flushed with cardioplegic solution (59). Since myocytes were prepared from the LV, small (average width, ∼200 μm) nonbranching linear right ventricular trabeculae were dissected and used for the assessment of the effects of dietary n-3 PUFA on in vitro contractile function. Muscles were then mounted into the setup as previously described for rabbit muscle (63) and stimulated at 1 Hz (3-ms duration at 120% threshold) while being perfused with an oxygenated Krebs-Henseleit solution containing 2 mM calcium. Muscles were stretched until an increase in passive (diastolic) force was no longer accompanied by a substantial increase in developed force, representing optimal length, and were allowed time to equilibrate at 37°C (∼20 min). The Frank-Starling mechanism was then assessed by measuring developed force during steady state at 85, 90, 95, and 100% of optimal length. At optimal length, the muscles were next stimulated at 1, 2, 3, 4, and 5 Hz, and force was recorded when the muscle had equilibrated at each frequency. After this protocol, the muscle was returned to 1 Hz. The third protocol assessed the β-adrenoceptor responsiveness by the generation of an isoproterenol dose-response curve, obtained in semi-log steps between 1 nM and 1 μM.
Force development was normalized to the cross-sectional area of the trabeculae to allow for a comparison between muscles of different diameters. The average dimensions of the trabeculae were not different between groups. Twitches were recorded at each experimental condition upon stabilization of developed tension. Data from 14 placebo- and 20 n-3 PUFA (5 1 g/day, 5 2 g/day, and 10 4 g/day)-treated dogs were collected and analyzed using custom-designed software (LabView, National Instruments, Austin, TX).
Myocyte calcium currents and calcium transient studies.
Myocytes were isolated from LV midmyocardial wall from placebo- and only the 2 g/day n-3 PUFA-treated dogs by using techniques as previously described (59). Calcium currents were recorded using whole cell patch clamp with Axopatch 200B amplifier (Axon Instruments) (59). The external solution contained (in mM) 140 NaCl, 5.4 CsCl, 2.0 CaCl2, 0.5 MgCl2, 10 HEPES, and 5.6 glucose (pH 7.4). Patch pipettes were filled with a solution that contained (in mM) 123 CsCl, 20 tetraethylammonium chloride, 5 MgATP, 10 NaCl, 1 MgCl2, 0.1 Tris-GTP, 10 HEPES, and 0.1 Fluo-3 K-salt (Molecular Probes) (pH 7.2). Calcium current was evoked with 300-ms steps from −50 to indicated potentials every 5 s. The peak response was also determined in the presence of 100 nM isoproterenol, a β-adrenoceptor agonist. Cytosolic calcium transients were recorded using an Olympus Fluoview 1000 confocal microscope in line-scan mode. Fluo-3 was excited by the 488-nm line of an argon-ion laser, and the fluorescence was acquired at wavelengths > 510 nm. All studies were performed at room temperature.
Data analysis.
All the data are presented as means ± SE. The echocardiography, trabeculae, myocyte (i.e., calcium current and calcium transient amplitude), and RBC fatty acid data were analyzed using a two-factor mixed-design ANOVA with repeated measures on one factor. For example, the trabeculae data were evaluated using a two-factor [omega–3 dose (4 levels) × length (4 levels) or frequency (5 levels) or isoproterenol dose (7 levels)] mixed-design ANOVA with repeated measures on one factor (length or frequency or isoproterenol dose). Homogeneity of covariance (sphericity assumption, equal correlates between the treatments) was tested using Mauchley's test and, if appropriate, adjusted using Huynh-Feldt correction. Cardiac tissue lipid compositions were compared using a one-way ANOVA. Post hoc comparisons were made using Tukey-Kramer multiple comparison test. Linear regression was used to evaluate the relationship between RBCs and cardiac tissue n-3 PUFA levels. The data were analyzed using Number Crunching Statistical Software (NCSS) statistical software (Kaysville, UT).
RESULTS
Effect of n-3 PUFA on RBCs and cardiac fatty acid content.
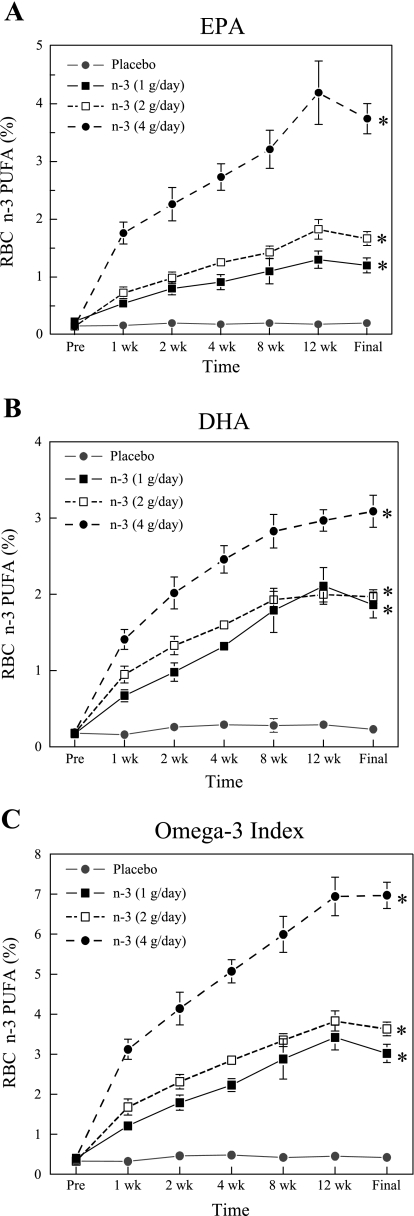
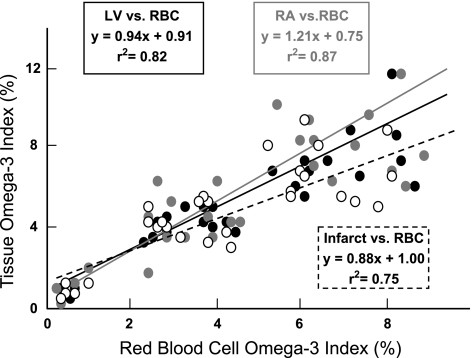
The RBC and the cardiac tissue n-3 PUFA content are displayed in Fig. 1 and Table 1, respectively. The n-3 PUFA supplements produced significant (dose effect, P < 0.0001) dose-dependent increases in the RBC membrane EPA (Fig. 1A) and DHA (Fig. 1B) content, as well as increasing the sum of EPA + DHA [the omega–3 index (29), Fig. 1C]. All three n-3 PUFA doses produced significant (time effect, P < 0.0001) increases by 7 days of treatment with peak values achieved between 8 and 12 wk of treatment. In contrast, n-3 PUFA content did not change over time in the placebo-treated animals. Interestingly, the 4 g/day dose of n-3 PUFA elicited an increase by 7 days of treatment that was higher than the peak values achieved by either the 1 or 2 g/day n-3 PUFA supplements (dose × time interaction, P < 0.0001) . Similarly, RA, LV, and infarction EPA levels; DHA levels; and the omega–3 index were all significantly (dose effect, P < 0.0001) higher in a n-3 PUFA dose-dependent manner (Table 1). No significant differences in the n-3 PUFA concentrations were noted between the RA, LV, or infarcted regions of the heart (e.g., LV vs. RA; P = 0.212). The RBC omega–3 index exhibited a strong positive correlation (LV, y = 0.94x + 0.91, r2 = 0.82, correlation coefficient = 0.91; RA, y = 1.21x + 0.75, r2 = 0.87, correlation coefficient = 0.93; and infarction, y = 0.88x + 1.00, r2 = 0.75, correlation coefficient = 0.87) with cardiac tissue n-3 PUFA content (Fig. 2). Each milligram per kilogram of DHA + EPA ingested produced a corresponding increase of roughly 0.03–0.05% in the n-3 PUFA content of RBCs and cardiac tissue.
Fig. 1.
Effect of increasing doses of omega–3 polyunsaturated fatty acid (n-3 PUFA) supplements on red blood cell (RBC) fatty acid content. A: effect on RBC eicosapentaenoic acid (EPA) content. B: effect on RBC docosahexaenoic acid (DHA) content. C: effect on the RBC omega–3 index (i.e., EPA + DHA). Note that the n-3 PUFA content increased significantly after 1 wk of supplementation, and peak values were reached between the 8th and 12th week of treatment. The final blood sample was collected when the cardiac tissue was harvest (∼14 wk after the treatment began). All values are reported as the percent n-3 PUFA in the total phospholipid content. Placebo, n = 6; 1 g/day, n = 7; 2 g/day, n = 8; and 4 g/day, n = 12. Every time point for each n-3 PUFA dose was significantly (*P < 0.01) different from the corresponding placebo time point, except at baseline (i.e., before treatment began).
Table 1.
Cardiac tissue n-3 PUFA levels
| Placebo | 1 g/day | 2 g/day | 4 g/day | |
|---|---|---|---|---|
| Right atrium | ||||
| EPA | 0.40 ± 0.11 | 1.20 ± 0.10* | 1.91 ± 0.18* | 3.27 ± 0.50* |
| DHA | 0.62 ± 0.15 | 3.25 ± 0.20* | 3.70 ± 0.26* | 4.26 ± 0.51* |
| OMX | 0.99 ± 0.21 | 4.46 ± 0.22* | 5.61 ± 0.43* | 7.54 ± 0.92* |
| Left ventricle | ||||
| EPA | 0.36 ± 0.04 | 1.22 ± 0.08* | 2.00 ± 0.19* | 3.68 ± 0.33* |
| DHA | 0.46 ± 0.10 | 2.92 ± 0.21* | 2.93 ± 0.17* | 3.54 ± 0.26* |
| OMX | 0.82 ± 0.11 | 4.14 ± 0.25* | 4.92 ± 0.33* | 7.22 ± 0.56* |
| Infarction | ||||
| EPA | 0.36 ± 0.09 | 1.20 ± 0.08* | 1.68 ± 0.15* | 3.44 ± 0.26* |
| DHA | 0.41 ± 0.07 | 2.66 ± 0.20* | 2.88 ± 0.17* | 3.15 ± 0.32* |
| OMX | 0.76 ± 0.13 | 3.86 ± 0.27* | 4.56 ± 0.32* | 6.59 ± 0.69* |
Values are means ± SE and are reported as the %omega–3 polyunsaturated fatty acids (n-3 PUFA) in the total phospholipid content. EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; OMX, omega–3 index. Placebo, n = 6; 1 g/day, n = 7; 2 g/day, n = 8; and 4 g/day, n = 12.
P < 0.01, placebo vs. a given dose of n-3 PUFA.
Fig. 2.
Correlation between RBC and left ventricular (LV) or right atrial (RA) or LV infarcted tissue n-3 PUFA content. The n-3 PUFA content is reported as the percentage in the total phospholipid content. Similar positive correlations were also noted between RBCs and LV or RA or LV infarcted tissue EPA or DHA composition (data not shown). For example, the correlation between RBC and LV and EPA was y = 1.38x + 1.82, r2 = 0.76, and correlation coefficient = 0.87, whereas the correlation RBC and DHA was y = 1.80x + 0.91, r2 = 0.75, and correlation coefficient = 0.87. The omega–3 index = EPA + DHA. RBC vs. LV is indicated by the solid black circles and solid black line, RBC vs. RV is indicated by the solid gray circles and gray line, and RBC vs. LV infraction is indicated by the open circles and dashed black line.
Effect of n-3 PUFA on LV function.
Previous studies demonstrated that the MI procedure used in the present study did not alter baseline ventricular function as assessed by either direct measurement of LV pressure (3, 8) or echocardiography (3, 9, 36, 59). In agreement with these previous studies, left anterior descending ligation did not induce heart failure in any dog in the present study. Indeed, LV function was not altered by MI in dogs (n = 5) in which echocardiographic data were obtained before and after MI (e.g., fractional shortening: pre-MI, 41.9 ± 2.7 vs. post-MI, 41.6 ± 1.7%; LV systolic diameter: pre-MI, 2.56 ± 0.12 vs. post-MI, 2.52 ± 0.13 mm; and LV diastolic diameter: pre-MI, 4.30 ± 0.17 vs. post-MI, 4.34 ± 0.29 mm). Furthermore, neither placebo nor n-3 PUFAs treatment significantly altered LV fractional shortening (treatment effect, P = 0.16; pre-post effect, P = 0.68; and treatment × pre-post interaction, P = 0.43), LV internal diastolic diameter (treatment effect, P = 0.83; pre-post effect, P = 0.97; and treatment × pre-post interaction, P = 0.42), or LV internal systolic diameter (treatment effect, P = 0.15; pre-post effect, P = 0.19; and treatment × pre-post interaction, P = 0.40) (Table 2) during the 3-mo time course of the study; i.e., no LV function variable changed over time. In contrast, all three n-3 PUFA treatments significantly (P < 0.05) reduced heart rate in a dose-independent manner, whereas heart rate did not change in the placebo group.
Table 2.
LV function as assessed by echocardiography
| Pre | Post | |
|---|---|---|
| Heart rate, beats/min | ||
| Placebo | 94.2 ± 5.1 | 86.9 ± 4.2 |
| 1 g/day | 116.3 ± 11.8 | 74.1 ± 4.1* |
| 2 g/day | 109.8 ± 5.4 | 79.3 ± 5.3* |
| 4 g/day | 99.5 ± 7.4 | 70.8 ± 7.0* |
| Sham (4 g/day) | 96.7 ± 6.3 | 64.1 ± 6.4* |
| Fractional shortening, % | ||
| Placebo | 39.2 ± 1.3 | 38.4 ± 1.6 |
| 1 g/day | 46.4 ± 2.8 | 44.5 ± 3.8 |
| 2 g/day | 42.2 ± 1.2 | 45.4 ± 2.2 |
| 4 g/day | 42.9 ± 1.6 | 40.1 ± 1.7 |
| Sham (4 g/day) | 39.1 ± 2.0 | 38.2 ± 2.7 |
| LV internal diastolic diameter, cm | ||
| Placebo | 4.15 ± 0.14 | 4.05 ± 0.14 |
| 1 g/day | 4.08 ± 0.16 | 3.84 ± 0.17 |
| 2 g/day | 4.11 ± 0.19 | 4.14 ± 0.17 |
| 4 g/day | 3.92 ± 0.12 | 4.21 ± 0.17 |
| Sham (4 g/day) | 4.00 ± 0.02 | 3.90 ± 0.18 |
| LV internal systolic diameter, cm | ||
| Placebo | 2.49 ± 0.12 | 2.48 ± 0.14 |
| 1 g/day | 2.20 ± 0.11 | 2.12 ± 0.16 |
| 2 g/day | 2.44 ± 0.11 | 2.25 ± 0.08 |
| 4 g/day | 2.30 ± 0.10 | 2.54 ± 0.13 |
| Sham (4 g/day) | 2.41 ± 0.09 | 2.40 ± 0.08 |
Values are means ± SE. LV, left ventricular; Pre, day before treatments began; Post, 3 mo after treatment began. Placebo, n = 13; 1 g/day, n = 5; 2 g/day, n = 8; 4 g/day, n = 10; and sham (4 g/day), n = 4.
P < 0.05, Pre vs. Post. Note that heart rate was not affected by the placebo treatment.
Effect of n-3 PUFA on heart rate variability.
The effects of the n-3 PUFA treatment on baseline heart rate and heart rate variability can be found in Table 3. The n-3 PUFA treatment significantly (pre-post effect, P < 0.001) reduced heart rate irrespective of the dose (i.e., there was no dose effect, P = 0.67, or dose × pre-post interaction, P = 0.15). In contrast, heart rate was not altered in the placebo-treated animals. In a similar manner, n-3 PUFA treatment elicited significant increases in heart rate variability. For example, the high-frequency component (0.24 to 1.04 Hz) of the R-R interval variability increased significantly (pre-post effect, P < 0.001) following all three doses of the n-3 PUFA, whereas this variable did not change in the placebo-treated animals. As with heart rate, the increase in heart rate variability occurred independently of the dose (dose effect, P = 0.74; dose × pre-post interaction, P = 0.13). Similar changes were noted in the sham-operated (no MI) dogs treated with 4 g/day n-3 PUFA (see Table 3).
Table 3.
Baseline heart rate and heart rate variability
| Pre | Post | |
|---|---|---|
| Heart rate, beats/min | ||
| Placebo | 120.8 ± 4.0 | 120.7 ± 4.4 |
| 1 g/day | 123.0 ± 7.6 | 104.7 ± 6.0* |
| 2 g/day | 123.0 ± 5.4 | 109.8 ± 5.8* |
| 4 g/day | 126.0 ± 5.4 | 117.3 ± 5.9* |
| Sham (4 g/day) | 131.3 ± 1.8 | 97.3 ± 3.5 |
| Vagal tone index, ln ms2 | ||
| Placebo | 7.37 ± 0.46 | 7.51 ± 0.34 |
| 1 g/day | 6.77 ± 0.38 | 8.15 ± 0.19* |
| 2 g/day | 6.97 ± 0.46 | 7.47 ± 0.22* |
| 4 g/day | 6.74 ± 0.40 | 7.66 ± 0.37* |
| Sham (4 g/day) | 4.87 ± 0.22 | 6.77 ± 0.73 |
| R-R interval standard deviation, ms | ||
| Placebo | 69.0 ± 9.1 | 72.8 ± 7.6 |
| 1 g/day | 53.9 ± 6.9 | 86.5 ± 12.1* |
| 2 g/day | 61.3 ± 9.1 | 77.5 ± 8.2* |
| 4 g/day | 48.6 ± 7.1 | 77.1 ± 8.1* |
| Sham (4 g/day) | 35.3 ± 11.3 | 58.1 ± 14.4 |
Values are means ± SE. Placebo, n = 13; 1 g/day, n = 5; 2 g/day, n = 8; 4 g/day, n = 10; and sham (4 g/day), n = 4.
P < 0.05, Pre vs. Post. Note that neither heart rate nor heart rate variability was affected by the placebo treatment.
Effect of n-3 PUFA on right ventricular trabeculae function.
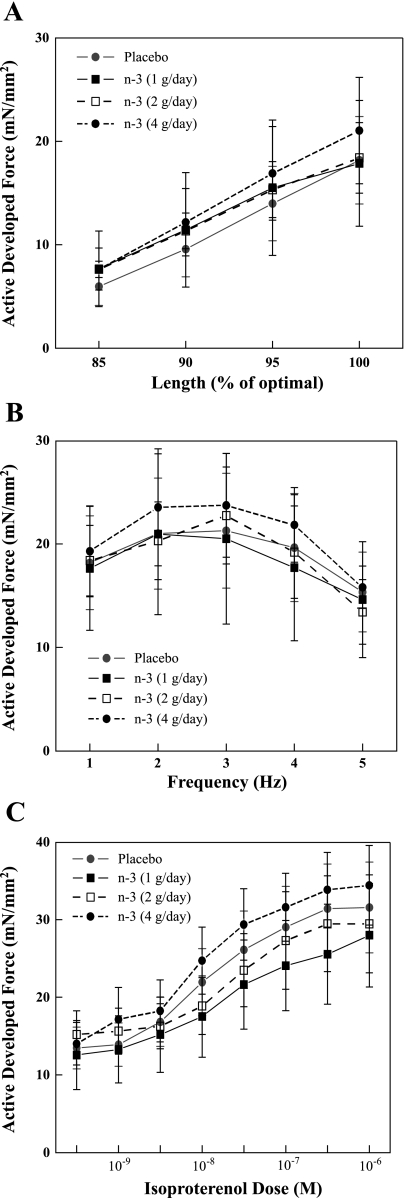
The effects of the n-3 PUFA on the length-tension (i.e., Frank-Starling mechanism) for isolated right ventricular trabeculae are displayed in Fig. 3A. As expected, stretching the muscle fibers elicited significant (length effect, P < 0.001) increases in the active force development. However, changes in active tension, induced by increasing muscle fiber length, were similar in all the treatment groups (i.e., no significant dose effect, P = 0.96; or dose × length interaction, P = 0.99).
Fig. 3.
Effect of increasing doses of dietary n-3 PUFA on the contractile response of isolated right ventricular trabeculae. A: effect on the length-tension (Frank-Starling) relationship. B: effect on the force-frequency relationship. C: effect on the positive inotropic response elicited by increasing dose of isoproterenol. Note that the contractile responses were similar in the n-3 PUFA- (regardless of dose) and the placebo-treated animals. Placebo, n = 14; 1 g/day, n = 5; 2 g/day, n = 5; and 4 g/day, n = 10; one muscle per animal.
The effects of the n-3 PUFA on the force-frequency relationship for isolated right ventricular trabeculae are displayed in Fig. 3B. As expected, increasing the simulation rate (frequency) of the muscle fibers elicited significant (frequency effect, P < 0.001) changes in the active force development. However, no differences in active tension, induced by increasing the stimulation rate of the muscle fibers, were noted between any of the treatment groups (i.e., no significant dose effect, P = 0.99; or dose × frequency interaction, P = 0.99).
Finally, the positive inotropic effects of isoproterenol are displayed in Fig. 3C. As expected, isoproterenol provoked significant (isoproterenol effect, P < 0.001) dose-dependent increases in active force development. However, no differences in the response to isoproterenol were noted between any of the treatment groups (i.e., no significant dose effect, P = 0.88; or dose × isoproterenol interaction, P = 0.99).
Effect of n-3 PUFA on myocyte calcium currents and calcium transients.
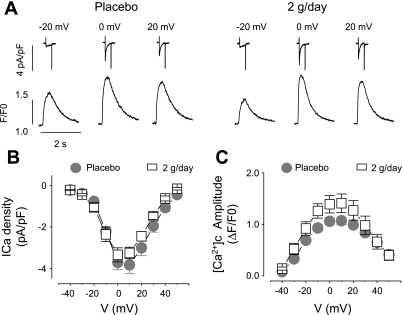
The effects of the n-3 PUFA treatments on calcium handling on acutely isolated voltage-clamped myocytes are displayed in Fig. 4. There were no differences in either peak calcium current density (treatment effect, P = 0.27; or treatment × voltage interaction, P = 0.25) or the corresponding calcium transient amplitudes (treatment effect, P = 0.24; or treatment × voltage interaction, P = 0.16) in the placebo and the n-3 PUFA groups. The peak calcium current density (at 0 mV) was −3.7 ± 0.4 in the placebo (n = 8) and −3.4 ± 0.3 pA/pF in the n-3 PUFA (n = 9) groups, whereas the corresponding calcium transient amplitudes were 1.1 ± 0.1 in placebo (n = 8) and 1.4 ± 0.2 ΔF/F0 in n-3 PUFA (2 g/day, n = 9) groups. Likewise, isoproterenol elicited similar increases in both the peak calcium density (P = 0.49) and the calcium transient amplitude (P = 0.16) in the placebo and in the n-3 PUFA (2 g/day) groups. The average peak calcium current density recorded at 1 Hz was −6.06 ± 0.45 (n = 5) in placebo and −5.51 ± 0.66 pA/pF in n-3 PUFA (n = 12) group, whereas the calcium transient amplitudes were 2.93 ± 0.25 in placebo (n = 14) and 2.41 ± 0.24 in the n-3 PUFA (n = 12) group. These data are consistent with the results obtained from the isoproterenol-treated trabeculae. Thus n-3 PUFA supplements did not alter either myocyte intracellular calcium handling or the inotropic response to β-adrenoceptor stimulation.
Fig. 4.
Effect of dietary n-3 PUFA on LV myocyte calcium current and calcium transients. A: representative calcium current traces recorded at the indicated membrane potentials, and corresponding temporal profiles of Fluo-3 fluorescence recorded in ventricular myocytes from placebo- and 2 g/day-treated groups. F/F0, calcium transient fluorescent signal normalized to background fluorescence. Voltage-dependence of the peak calcium current density (B) and amplitude of calcium transient ([Ca2+]c) (C) are recorded in ventricular myocytes from placebo- (n = 8) and 2 g/day (n = 9)-treated groups.
DISCUSSION
The major findings of this study are as follows. First, dietary EPA + DHA ethyl ester supplements elicited dose-dependent increases n-3 PUFA concentration in both RBCs and cardiac tissue, and furthermore, there was a near-perfect correlation between RBCs and LV, RA, or infarcted tissue n-3 PUFA levels. As such, RBC n-3 PUFA levels can serve as a reliable surrogate for cardiac tissue concentrations. Second, the n-3 PUFA supplements provoked significant reductions in heart rate that were accompanied by significant increases in heart rate variability that both occurred independently of the dose used. Third, despite large increases in cardiac tissue n-3 PUFA concentration (up to a 16.4-fold increase in the omega–3 index with 4 g/day n-3 PUFA), supplementation did not alter ventricular mechanical function either in vivo or in vitro in dogs with healed MIs. In vivo, fractional shortening, LV systolic internal diameter, and LV diastolic internal diameter did not change over time and were not altered by any dose of n-3 PUFAs. In vitro, the developed force per cross-sectional area, the length-tension (Frank-Starling mechanism) relationship, the force-frequency relationship, and the positive inotropic response to increasing doses of the nonselective β-adrenoceptor agonist isoproterenol were similar in right ventricular trabeculae from all treatment groups. Finally, both the L-type calcium current density and calcium transients (at baseline and during β-adrenoceptor activation) were the same in LV myocytes from n-3 PUFA- and placebo-treated animals. These in vivo and in vitro findings demonstrate that dietary n-3 PUFAs do not adversely affect ventricular function in damaged hearts.
RBC membrane and cardiac tissue n-3 PUFA levels.
Although the American Heart Association currently recommends about 1 g of EPA + DHA per day for patients with known coronary heart disease (37), the optimal n-3 PUFA dose has not been determined. The majority of the clinical or experimental studies that evaluated the cardiovascular benefits of dietary fish or fish oil supplements failed to report either blood or tissue n-3 PUFA levels. However, there are clinical studies in which blood/serum levels of n-3 PUFA have been reported (1, 55, 57). For example, 1 g/day of the same n-3 PUFA supplement that was used in the present study elicited a doubling of the n-3 PUFA portion of the plasma phospholipids (from 5.1 ± 0.5 to 10.4 ± 0.5%) after 12 wk of treatment in healthy volunteers (20). In a similar manner, epidemiological studies have reported a strong inverse relationship between the risk for sudden death and blood n-3 PUFA levels (1, 57). For example, Albert et al. (1) reported that subjects in the highest quartile for blood long-chain n-3 PUFA levels (mean, 6.9%; and range, 6.1–10.2%) had a 90% lower risk for sudden death compared with those in the lowest quartile (mean, 3.6%; and range, 2.1–4.3%). In agreement with the present study, n-3 PUFA supplements have also been shown to produce a dose-dependent enrichment of RBCs and cardiac tissue in healthy dogs (31) and human cardiac transplant patients (32).
Effect of n-3 PUFA on heart rate and heart rate variability.
In the present study, all three n-PUFA treatment doses elicited similar reductions in baseline heart rate that were accompanied by corresponding increases in heart rate variability. Interestingly, these heart rate and heart rate variability changes were similar to those induced by endurance exercise training in a postinfarction canine model of sudden cardiac death (3, 4, 6). In agreement with this finding, clinical and experimental studies report that n-3 PUFA ingestion (16, 17, 30, 46, 50) or acute intravenous administration (7) lowered heart rate and increased heart rate variability, suggestive of an increase in cardiac parasympathetic tone (3–6). However, there are also studies in which n-3 PUFA failed to alter either heart rate variability or other measures of autonomic function, such as baroreceptor reflex sensitivity (25, 27). Furthermore, the positive actions of n-3 PUFAs on heart rate or heart rate variability were modest in some studies (47, 48). For example, a meta-analysis of 30 trials (n = 1,678) found that fish oil supplements (∼3.5 g/day of EPA + DHA) reduced baseline heart rate by 2.5 beats/min (48), whereas Mozaffarian et al. (47) reported that individuals with the highest fish consumption (≥5 meals/wk) only exhibited 1.5 ms greater heart rate variability compared with those with the lowest fish consumption. Although this difference was statistically significant, the physiological relevance of such a small change remains an open question. Indeed, these investigators calculated that only a 1.1% reduction in the relative risk for sudden cardiac death could be associated with this increase in heart rate variability (47).
The mechanisms responsible for the n-3 PUFA-induced decreases in heart rate and increases in heart rate variability were not investigated in the present study. However, there are at least two possible explanations for these observations. First, the high-frequency component of the R-R interval variability increased following 3 mo of n-3 PUFA treatment. Although there are limitations with this index (53), it is now generally accepted that it can provide an indirect (qualitative) assessment of cardiac parasympathetic regulation (5, 62). As such, the reduction in heart rate that accompanies n-3 PUFA treatment could reflect an enhanced parasympathetic efferent (either of central or peripheral origin) regulation of the heart. Second, n-3 PUFA have been recently reported to reduce the pacemaker current (If) in sinoatrial node cells obtained from rabbits fed diets enriched with fish oil but not in cells isolated from the hearts of animals fed sunflower oil (65). It is interesting to note that in the present study, atrial tissue also tended to exhibit higher concentrations of n-3 PUFA than ventricular tissue. Thus the heart rate reduction induced by n-3 PUFA treatment could result from both direct (reductions in pacemaker current) and indirect (enhanced cardiac parasympathetic efferent activity) actions of these agents on the heart.
Effect of n-3 PUFA on ventricular contractile function.
As previously noted, clinical studies often produced conflicting results; some studies have reported significant reductions in all-cause mortality including a substantial reduction in sudden cardiac death (14, 43), whereas in other studies, n-3 PUFAs supplements or fish consumption either failed to alter mortality (69) or actually increased all-cause mortality (26% increase over 9 yr follow-up period) and sudden cardiac death (54% increase) (15), although the design of the latter study has been criticized (66). In a similar manner, EPA + DHA did not alter cardiac events or arrhythmias in patients with implantable cardioverter defibrillators (12). There have been at least three meta-analyses of cardiovascular outcome trials: one failed to find a relationship between fish or fish oil consumption and a reduction in cardiac mortality (35), a second analysis found a relationship between a reduced incidence of sudden death in MI patients and n-3 PUFA consumption but an increased risk for adverse cardiac events in patients with angina (70), and the third study reported a significant dose-independent reduction in cardiac mortality but not in sudden cardiac death or in all-cause mortality (39).
Since an acute administration of n-3 PUFAs to cellular preparations can reduce calcium currents and intracellular calcium, the negative inotropic actions of these lipids could adversely impact contractile function in patients with hearts compromised by MI, thereby accounting for the increased mortality reported in the DART-2 study (13, 15). Indeed, calcium channel antagonists have been shown to increase mortality in those patients with the greatest impairment of LV function following MI (11, 28).
To date, there have been relatively few studies that evaluated the effects of n-3 PUFAs on cardiac function and none that have examined these actions both in vivo and in vitro. Experimental studies demonstrated that dietary fish oil supplements attenuated cardiac hypertrophy and improved cardiac function in mice with genetic systemic carnitine deficiency (61) and prevented contractile dysfunction in a rat model of pressure overload-induced heart failure (21, 22). Dietary n-3 PUFAs also attenuated tachypacing-induced elevations in LV end-diastolic pressure in a canine model of heart failure (56). Similarly, isolated Langendorff-perfused hearts exhibited a better preservation of contractile function during reperfusion after global ischemia compared with hearts from rats that did not receive diets enriched with fish oil (67). In the nonhuman primate, tuna fish oil and sunflower oil enhanced ventricular filling and ejection fraction compared with diets enriched with saturated fat (44). In contrast, fish oil supplements reduced LV systolic pressure and maximum first derivative of left ventricular pressure by 20% in exercise-stressed rats compared with nonexercising animals (42). In humans, LV systolic function was not altered by 12 wk of n-3 PUFA supplementation in patients with MI (58), whereas fish oil supplements improved LV function in children with dilated cardiomyopathy (51). Collectively, these conflicting data suggest that dietary n-3 PUFAs have no major effects on contractile function. This conclusion is further supported by our findings.
Effect of n-3 PUFAs on myocyte calcium handling.
The initiation of contraction is critically dependent on calcium entry via L-type calcium channels during phase 2 of the cardiac action potential. This calcium entry triggers calcium release via action on ryanodine receptors (calcium release channels) from the sarcoplasmic reticulum, a process known as calcium-induced calcium release. Thus factors that alter calcium-induced calcium release could profoundly alter force development. The acute application of n-3 PUFAs to myocytes can inhibit L-type calcium currents (33, 67) with corresponding reductions in the amplitude of depolarization-induced calcium transients (49, 52, 60). Furthermore, n-3 PUFAs can also reduce the open-time probability of single ryanodine (i.e., the sarcoplasmic reticulum calcium release) channels incorporated into planar lipid bilayers (60) and to decrease spontaneous calcium waves and local calcium release events (calcium sparks) in cardiac myocytes (34, 52, 60). Recently, the acute administration of EPA (20 μM) to myocytes obtained from failing hearts lowered intracellular calcium (19). Thus the acute application of n-3 PUFAs to isolated myocytes could reduce intracellular calcium and, thereby, could decrease contractile responses.
While these studies evaluated the effects of acute applications of n-3 PUFAs, the chronic effects of n-3 PUFA supplementation remain largely to be determined. In the present study, neither L-type calcium currents nor calcium transients were altered in n-3 PUFA compared with placebo-treated dogs. These data are consistent with the neutral effects of EPA + DHA as described in the previous section. However, these results contrast with those described in the only other report in which the chronic effects of dietary fish oil on cardiac calcium currents have been examined. Coronel and coworkers (64) reported that myocytes from pigs fed EPA + DHA exhibited decreased L-type calcium current (−15%) and sodium/calcium exchanger current (−60%). The resulting decreased calcium entry would reduce systolic calcium levels (and thereby force development), whereas decreased sodium/calcium exchanger current activity would promote diastolic calcium accumulation (and thereby, delay relaxation). Interestingly, these investigators also found that the hearts isolated from the pigs given the fish oil supplement were more prone to ventricular arrhythmias during regional ischemia than hearts from pigs given the standard diet. Species differences likely account for the seemingly disparate results obtained in pigs and in dogs. For example, pigs possess a calcium-dependent transient-outward current, an important regulator of repolarization that is absent in both dogs and humans (40). Thus the route of n-3 PUFA administration can provoke different actions on calcium regulation and thereby contractile function. The acute application of n-3 PUFAs may reduce intracellular calcium levels, whereas dietary EPA + DHA may not alter either calcium entry or the resulting calcium transient.
Limitations of the study.
It must be acknowledged that there are a few limitations with the present study that could influence the interpretation of the results. First, the MI did not alter baseline contractile function as assessed by echocardiography. As such, the potential benefits of n-3 PUFA could be difficult to determine. It is possible that a larger response (either positive or negative) to the n-3 PUFA therapy might occur in models of heart failure or in animals in which the MI induced more overt mechanical dysfunction. Furthermore, it is important to emphasize that n-3 PUFA treatment reduced heart rate. A reduction in heart rate would reduce the metabolic stress placed upon the injured heart, a positive benefit that might help maintain contractile function by counterbalancing reductions that could occur over time (i.e., prevent decompensation). However, mechanical function was not altered in the placebo group, an observation that would tend to argue against a protection from a time-dependent worsening of ventricular pump function.
Second, one could argue that the lipid supplements given to the placebo group may have afforded some protection from a decline in mechanical function and as such would mask any putative benefits of the n-3 PUFA treatment. The present study did not include a nontreated group. However, in a previous study, we used echocardiography to evaluate ventricular function following MI in animals placed on either 10 wk of exercise training or an equivalent sedentary period (9). There were 10 sedentary dogs in this study from which data could be obtained. As with the placebo treatment, neither LV fractional shortening (pre, 40.8 ± 1.5% vs. post, 38.2 ± 2.0%) nor ventricular dimensions (diastolic: pre, 4.26 ± 0.16 vs. post, 4.15 ± 0.16; and systolic: pre, 2.51 ± 0.15 vs. post, 2.55 ± 0.15 mm) changed during the 10-wk sedentary period. It, therefore, seems unlikely that corn oil (placebo) treatment altered contractile function in the present study.
Third, for technical reasons (i.e., temperature-dependent compartmentalization of the calcium fluorescent dye, movement from the cytosol into the sacroplasmic reticulum and mitochondria at higher temperatures), the calcium current and calcium transient studies were performed at room temperature. As the fluidity of the membrane and lipid rafts in which the ion channels reside are affected by temperature, it possible that n-3 PUFA effects of calcium channel function would be more obvious at body temperature as opposed to room temperature. For the reasons stated above, caution must be used when extrapolating the findings of the present study to the clinical setting.
Summary and conclusions.
In the present study, EPA + DHA ethyl esters did not alter fractional shortening in intact animals, the contractile response of isolated right ventricular trabeculae, or L-type calcium current and calcium transients in isolated LV myocytes from dogs with healed MI, despite a nearly 17-fold increase in the LV n-3 PUFA concentrations. It, therefore, seems unlikely that n-3 PUFA-mediated reductions in cardiac performance contributed to the increased mortality reported in DART-2 (15). In fact, EPA + DHA ethyl ester supplements produced small but significant reductions in mortality (but not sudden death rates) in heart failure patients (26), data that also strongly suggest that n-3 PUFAs do not exert adverse actions on contractile function in individuals with advanced cardiac disease. One may, therefore, conclude that dietary n-3 PUFAs do not adversely affect contractile function even in patients in which the heart has been compromised by MI.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-086700 (to G. E. Billman), HL-074045 and HL-089836 (to C. A. Carnes), and HL-063043 (to S. Gyorke) and American Heart Association Grant EIA 0740040N (to P. M. L. Janssen).
DISCLOSURES
W. S. Harris is a scientific advisor to GlaxoSmithKline, Monsanto, and Unilever; a speaker for GlaxoSmithKline; and the owner of OmegaQuant Analytics, a company offering blood omega–3 testing.
ACKNOWLEDGMENTS
We thank Raven Morgan, Andrew Christianson, and Ingrid Bonilla for excellent technical assistance during the course of the studies. We also thank GlaxoSmithKline for generously providing the n-3 PUFA ethyl ester and placebo capsules for this study.
REFERENCES
- 1.Albert CM, Campos H, Stampfer MJ, Ridker PM, Mason JE, Willet WC, Ma J. Blood levels of long-chain n-3 fatty acids and the risk of sudden death. N Engl J Med 346: 1113–1118, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Bang HO, Dyerberg J, Sinclair HM. The composition of the Eskimo food in northwestern Greenland. Am J Clin Nutr 33: 2657–2661, 1980 [DOI] [PubMed] [Google Scholar]
- 3.Billman GE. A comprehensive review and analysis of 25 years of data from an in vivo canine model of sudden cardiac death: implications for future anti-arrhythmic drug development. Pharmacol Ther 111: 808–835, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Billman GE. Cardiac autonomic neural “remodeling” and susceptibility to sudden cardiac death: effect of endurance exercise training. Am J Physiol Heart Circ Physiol 297: H1171–H1193, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Billman GE, Dujardin J. Dynamic changes in cardiac vagal tone as measured by time-series analysis. Am J Physiol Heart Circ Physiol 258: H896–H902, 1990 [DOI] [PubMed] [Google Scholar]
- 6.Billman GE, Kukielka M. Effect of exercise training on heart rate variability and susceptibility to sudden cardiac death: protection is not due to enhanced cardiac vagal regulation. J Appl Physiol 100: 896–906, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Billman GE, Hallaq H, Leaf A. Prevention of ischemia-induced ventricular fibrillation by omega–3 fatty acids. Proc Natl Acad Sci USA 91: 4427–4430, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Billman GE, Schwartz PJ, Gagnol JP, Stone HL. The cardiac response to submaximal exercise in dogs susceptible to sudden cardiac death. J Appl Physiol 59: 890–897, 1985 [DOI] [PubMed] [Google Scholar]
- 9.Billman GE, Kukielka M, Kelley R, Mustafa-Bayoumi M, Altschuld RA. Endurance exercise training attenuates cardiac β2-adrenoceptor responsiveness and prevents ventricular fibrillation in animals susceptible to sudden death. Am J Physiol Heart Circ Physiol 290: H2590–H2599, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Bligh EG, Dyer WJ. A rapid method for total lipid extraction and purification. Can J Biochem Physiol 37: 911–917, 1959 [DOI] [PubMed] [Google Scholar]
- 11.Boden WE, Krone RJ, Kleiger RE, Oakes D, Greenberg H, Dwyer EJ, Jr, Miller JP, Abrams J, Coromilas J, Goldstein R. Electrocardiographic subset analysis of diltiazem on long-term outcome after acute myocardial infarction. The Multicenter Diltiazem Post-Infarction Trial Research Group. Am J Cardiol 63: 70–79, 1991 [DOI] [PubMed] [Google Scholar]
- 12.Brouwer IA, Riatt MH, Dullemeijer C, Kraemer DF, Zock PL, Morris C, Katan MB, Connor WE, Camm JA, Schouten EG, McAnulty J. Effect of fish oil on ventricular tachyarrhythmia in three studies in patients with implantable cardioverter defibrillators. Eur Heart J 30: 820–826, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burr ML, Dunstan FD, George CH. Is fish oil good or bad for heart disease? Two trials with apparently conflicting results. J Membr Biol 206: 155–163, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Burr ML, Fehily AM, Gilbert JF, Rogers S, Holliday RM, Sweetnam PM, Elwood PC, Deadman NM. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART). Lancet 2: 757–761, 1989 [DOI] [PubMed] [Google Scholar]
- 15.Burr ML, Ashfield-Watt PA, Dunstan FD, Fehily AM, Breay P, Ashton T, Zotos PC, Haboubi NA, Elwood PC. Lack of benefit of dietary advice to men with angina: results of controlled trial. Eur J Clin Nutr 57: 193–200, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Christensen JH, Gustenhoff P, Korup E, Aaroe J, Toft E, Moller J, Rasmussen K, Dyerberg J, Schmidt EB. Effect of fish oil on heart rate variability in survivors of myocardial infarction: a double blind randomized controlled study. Br Med J 312: 677–678, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dallongeville J, Yarnell J, Ducimetiere P, Arveiler D, Ferrieres J, Montaye M, Luc G, Evans A, Bingham A, Haas B, Ruidavets JB, Amouyel P. Fish consumption is associated with lower heart rates. Circulation 108: 820–825, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Daviglus ML, Stamler J, Orencia AJ, Dyer AR, Liu K, Greenland P, Walsh MK, Morris D, Shekelle RB. Fish consumption and the 30-year risk of fatal myocardial infarction. N Engl J Med 336: 1046–1053, 1997 [DOI] [PubMed] [Google Scholar]
- 19.Den Ruijter HM, Berecki G, Verkerk AO, Bakker D, Baartscheer A, Schumacher CA, Belterman CN, de Jonge N, Fiolet JW, Brouwer IA, Coroanel R. Acute administration of fish oil inhibits triggered activity in isolated myocytes from rabbits and patients with heart failure. Circulation 117: 536–544, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Di Stasi D, Bernasconi R, Marchioli R, Marfisi RM, Rossi G, Tognoni G, Tacconi MT. Early modifications of fatty acid composition in plasma phospholipids, platelets and mononucleates of healthy volunteers after low doses of n-3 polyunsaturated fatty acids. Eur J Clin Pharmacol 60: 183–190, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Duda MK, O'Shea KM, Lei B, Barrows BR, Azimzadeh AM, McElfresh TE, Hoit BD, Kop WJ, Stanley WC. Dietary supplementation with ω–3 PUFA increases adiponectin and attenuates ventricular remodeling and dysfunction with pressure overload. Cardiovasc Res 76: 303–310, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duda MK, O'Shea KM, Tintinu A, Xu W, Khairallah RJ, Barrows BR, Chess DJ, Azimzadeh AM, Harris WS, Sharon VG, Sabbah HN, Stanley WC. Fish oil, but not flaxseed oil, decreases inflammation and prevents pressure overload-induced cardiac dysfunction. Cardiovasc Res 81: 319–327, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dyerberg J, Bang HO, Stoffersen E, Moncada S, Vane JR. Eicosapentaenoic acid and prevention of thrombosis and atherosclerosis? Lancet 2: 117–119, 1978 [DOI] [PubMed] [Google Scholar]
- 24.Ferrier GR, Redundo I, Zhu J, Murphy MG. Differential effects of docosahexaenoic acid on contractions and L-type Ca2+ current in adult cardiac myocytes. Cardiovasc Res 54: 601–610, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Geelen A, Zock PL, Swenne CA, Brouwer IA, Schouten EG, Katan MB. Effect of n-3 fatty acids on heart rate variability and baroreflex sensitivity in middle-aged subjects. Am Heart J 146: E4, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Gissi-HF Investigators, Tavazzi L, Maggioni AP, Marchioli R, Barlera S, Franzosi MG, Latini R, Lucci D, Nicolosi GL, Porcu M, Tognoni G. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet 372: 1223–1230, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Hamaad A, Kaeng Lee W, Lip GY, MacFadyen RJ. Oral omega–3 PUFA therapy (Omacor) has no impact on indices of heart rate variability in stable post myocardial infarction patients. Cardiovasc Drug Ther 20: 359–364, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Hanson JF. Treatment with verapamil during and after acute myocardial infarction: a review based upon the Danish verapamil infarction trials I and II. J Cardiovasc Pharmacol 19, Suppl 6: S20–S25, 1991 [PubMed] [Google Scholar]
- 29.Harris WS, Von Schacky C. The Omega–3 Index: a new risk factor for death from coronary heart disease? Prev Med 39: 212–220, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Harris WS, Gonzles M, Laney N, Sastre A, Borkon AM. Effects of omega–3 fatty acids on heart rate in cardiac transplant recipients. Am J Cardiol 98: 1393–1395, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Harris WS, DiRienzo MA, Sands SA, George C, Jones PG, Eapen AK. Stearidonic acid increases red blood cell and heart EPA content in dogs. Lipids 42: 325–333, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Harris WS, Sands SA, Windsor SL, Ali HA, Stevens TL, Magalski A, Porter CB, Borkon AM. Omega–3 fatty acids in cardiac biopsies from heart transplantation patients: correlation with erythrocytes and response to supplementation. Circulation 110: 1645–1649, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Hazama H, Nakajima T, Asano M, Iwasawa K, Igarashi K, Nagata T, Horiuchi T, Suzuki J, Soma M, Okuda Y. Omega–3 polyunsaturated fatty acids—modulation of voltage-dependent L-type Ca2+ current in guinea-pig tracheal smooth muscle cells. Eur J Pharmacol 355: 257–266, 1998 [DOI] [PubMed] [Google Scholar]
- 34.Honen BN, Saint DA, Laver DR. Suppression of calcium sparks in rat ventricular myocytes and direct inhibition of sheep cardiac RyR channels by EPA, DHA and oleic acid. J Membr Biol 196: 95–103, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Hooper L, Thompson RL, Harrison RA, Summerbell CD, Moore H, Worthington HV, Durrington PN, Ness AR, Capps NE, Davey Smith G, Riemersma RA, Ebrahim SB. Omega–3 fatty acids for prevention and treatment of cardiovascular disease. Cochrane Database Syst Rev 4: CD003177, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Houle MS, Altschuld RA, Billman GE. Enhanced in vivo and in vitro responses with β2-adrenergic receptor stimulation in dogs susceptible to lethal arrhythmias. J Appl Physiol 91: 1627–1637, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Kris-Etherton PM, Harris WS, Appel LJ, AHA Nutrition Committee. American Heart Association Omega–3 fatty acids and cardiovascular disease: new recommendations from the American Heart Association. Arterioscler Thromb Vasc Biol 23: 151–152, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Kromhout D, Bosschieter EB, de Lezenne CC. The inverse relation between fish consumption and 20-year mortality from coronary heart disease. N Engl J Med 1985;312: 1205–1209, 1985 [DOI] [PubMed] [Google Scholar]
- 39.León H, Shibata MC, Sivakumaran S, Dorgan M, Chatterley T, Tsuyuki RT. Effect of fish oil on arrhythmias and mortality: systematic review. Br Med J 338: a2931, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li GR, Du XL, Siow YL, O K, Tse HF, Lau CP. Calcium-activated transient outward chloride current and phase 1 repolarization of swine ventricular action potential. Cardiovasc Res 58: 89–98, 2003 [DOI] [PubMed] [Google Scholar]
- 41.London B, Albert C, Anderson ME, Giles WR, Van Wagoner DR, Balk E, Billman GE, Chung M, Lands W, Leaf A, McAnulty J, Martens JR, Costello RB, Lathrop DA. Omega–3 fatty acids and cardiac arrhythmias: prior studies and recommendations for future research: a report from the National Heart, Lung, and Blood Institute and Office Of Dietary Supplements Omega-3 Fatty Acids and their Role in Cardiac Arrhythmogenesis Workshop. Circulation 116: e320–e335, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Lortet S, Verger P. Alteration of cardiovascular function in trained rats fed fish oil. Int J Sports Med 16: 519–521, 1995 [DOI] [PubMed] [Google Scholar]
- 43.Marchioli R, Barzi F, Bomba E, Chieffo C, Di Gregorio D, Di Mascio R, Franzosi MG, Geraci E, Levantesi G, Maggioni AP, Martini L, Marfisi RM, Mastrogiuseppe G, Mininni N, Nicolosi GL, Santini M, Schweiger C, Tavazzi L, Tognoni G, Tucci C, Valagussa F, GISSI-Prevenzione Investigators Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI)-Prevenzione. Circulation 105: 1897–1903, 2002 [DOI] [PubMed] [Google Scholar]
- 44.McLennan PL, Barnden LR, Bridle TM, Abeywardena MY, Charnock JS. Dietary fat modulation of left ventricular ejection fraction in the marmoset due to enhanced filling. Cardiovasc Res 26: 871–877, 1992 [DOI] [PubMed] [Google Scholar]
- 45.Morrison WR, Smith LM. Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride-methanol. J Lipid Res 5: 600–608, 1964 [PubMed] [Google Scholar]
- 46.Mozaffarian D, Prineas RJ, Stein PK, Siscovik DS. Dietary fish and n-3 fatty acid intake and cardiac electrocardiographic parameters in humans. J Am Coll Cardiol 48: 478–484, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Mozaffarian D, Stein PK, Prineas RJ, Siscovick DS. Dietary Fish ω–3 fatty acid consumption and heart rate variability in US adults. Circulation 117: 1130–1137, 2008 [DOI] [PubMed] [Google Scholar]
- 48.Mozaffarian D, Geelen A, Brouwer IA, Geleijinse JM, Zock PL, Katan MB. Effect of fish oil on heart rate in humans: meta-analysis of randomized controlled trials. Circulation 112: 1945–1952, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Negretti N, Perez MR, Walker D, O'Neill SC. Inhibition of sarcoplasmic reticulum by polyunsaturated fatty acids in intact, isolated myocytes from rat ventricular muscle. J Physiol 523: 367–375, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O'Keefe JH, Jr, Abuissa H, Sastre A, Steinhaus DM, Harris WS. Effects of omega–3 fatty acids on resting heart rate, heart rate recovery after exercise, and heart rate variability in men with healed myocardial infarctions and depressed ejection fraction. Am J Cardiol 97: 1127–1130, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Olgar S, Ertugrul T, Nisli K, Omeroglu RE, Dindar A, Aydogan U. Fish oil supplementation improves left ventricular function in children with idiopathic dilated cardiomyopathy. Congest Heart Fail 13: 308–312, 2007 [DOI] [PubMed] [Google Scholar]
- 52.O'Neill SC, Perez MR, Hammond KE, Sheader EA, Negretti N. Direct and indirect modulation of rat cardiac sarcoplasmic reticulum function by n-3 polyunsaturated fatty acids. J Physiol 538: 179–184, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parati G, di Rienzo M, Castiglioni P, Mancia G, Taylor JA, Studinger P. Point: Counterpoint: Cardiovascular variability is/is not an index of autonomic control of circulation. J Appl Physiol 101: 676–682, 2006 [DOI] [PubMed] [Google Scholar]
- 54.Porges SW. Respiratory sinus arrhythmia: physiological basis, quantitative methods and clinical implications. In: Cardiac, Respiratory, and Cardiosomatic Psychophysiology. New York: Plenum, 1986, p. 101–115 [Google Scholar]
- 55.Rissanen T, Voutilainen S, Nyyssonen K, Lakka TA, Salonen JT. Fish oil-derived fatty acids, docosahexaenoic acid and docosapentaenoic acid, and the risk of acute coronary events: the Kuopio ischaemic heart disease risk factor study. Circulation 102: 2677–2679, 2000 [DOI] [PubMed] [Google Scholar]
- 56.Sakabe M, Shiroshita-Takeshita A, Maguey A, Dumesnil C, Nigam A, Leung TK, Nattel S. Omega–3 polyunsaturated fatty acids prevent atrial fibrillation associated with heart failure but not atrial tachycardia remodeling. Circulation 116: 2101–2109, 2007 [DOI] [PubMed] [Google Scholar]
- 57.Siscovick DS, Raghunathan TE, King I, Weinmann S, Wicklund KG, Albright J, Bovbjerg V, Arbogast P, Smith H, Kushi LH, Cobb LA, Copass MK, Psaty BM, Lemaitre R, Retzlaff B, Childs M, Knopp R. Dietary intake and cell membrane levels of long-chain n-3 polyunsaturated fatty acids and the risk of primary cardiac arrest. JAMA 274: 1363–1367, 1995. [DOI] [PubMed] [Google Scholar]
- 58.Skou HA, Toft E, Christensen JH, Hansen JB, Dyerberg J, Schmidt EB. N-3 fatty acids and cardiac function after myocardial infarction in Denmark. Int J Circumpolar Health 60: 360–365, 2001 [PubMed] [Google Scholar]
- 59.Sridhar A, Nishijima Y, Terentyev D, Terentyeva R, Uelman R, Kukielka M, Bonilla IM, Robertson GA, Gyorke S, Billman GE, Carnes CA. Repolarization abnormalities and afterdepolarizations in a canine model of sudden cardiac death. Am J Physiol Regul Integr Comp Physiol 295: R1463–R1472, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Swan JS, Dibb K, Negretti N, Sitsapesan R. Effects of eicosapentaenoic acid on cardiac SR Ca2+-release and ryanodine receptor function. Cardiovasc Res 60: 337–346, 2003 [DOI] [PubMed] [Google Scholar]
- 61.Takahashi R, Okumura K, Asai T, Hirai T, Murakami H, Murakami R, Numaguchi Y, Matsui H, Ito M, Murohara T. Dietary fish oil attenuates cardiac hypertrophy in lipotoxic cardiomyopathy due to systemic carnitine deficiency. Cardiovasc Res 68: 213–223, 2005 [DOI] [PubMed] [Google Scholar]
- 62.Task Force of the European Society of Cardiology, and the North American Society of Pacing and Electrophysiology Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Circulation 93: 1043–1065, 1996 [PubMed] [Google Scholar]
- 63.Varian KD, Janssen PM. Frequency-dependent acceleration of relaxation involves decreased myofilament calcium sensitivity. Am J Physiol Heart Circ Physiol 292: H2212–H2219, 2007 [DOI] [PubMed] [Google Scholar]
- 64.Verkerk AO, van Ginneken AC, Berecki G, Den Ruijter HM, Schumacher CA, Veldkamp MW, Baartscheer A, Casini S, Opthof T, Hovenier R, Fiolet JW, Zock PL, Coronel R. Incorporated sarcolemmal fish oil fatty acids shorten pig ventricular action potentials in pigs. Cardiovasc Res 70: 509–520, 2006 [DOI] [PubMed] [Google Scholar]
- 65.Verkerk AO, den Ruijter HM, Bourier J, Boukens BJ, Brouwer IA, Wilders R, Coronel R. Dietary fish oil reduces pacemaker current and heart rate in rabbit. Heart Rhythm 6: 1485–1492, 2009 [DOI] [PubMed] [Google Scholar]
- 66.von Schacky C, Harris WS. Cardiovascular benefits of omega–3 fatty acids. Cardiovasc Res 73: 310–315, 2007 [DOI] [PubMed] [Google Scholar]
- 67.Xiao YF, Gomez AM, Morgan JP, Lederer WJ, Leaf A. Suppression of voltage-gated L-type Ca2+ currents by polyunsaturated fatty acids in adult and neonatal rat ventricular myocytes. Proc Natl Acad Sci USA 94: 4182–4187, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang BC, Saldeen TG, Bryant JL, Nichols WW, Mehta JL. Long-term dietary fish oil supplementation protects against ischemia-reperfusion-induced myocardial dysfunction in isolated rat hearts. Am Heart J 126: 1287–1292, 1993 [DOI] [PubMed] [Google Scholar]
- 69.Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K, Japan EPA lipid intervention study (JELIS) Investigators Effects of eicosapentaenoic acid on major coronary events in hypercholesterolemic (JELIS): a randomised open-label, blinded endpoint analysis. Lancet 369: 1090–1098, 2007 [DOI] [PubMed] [Google Scholar]
- 70.Zhao YT, Chen Q, Sun YX, Li XB, Zhang P, Xu Y, Guo JH. Prevention of sudden cardiac death with omega–3 fatty acids in patients with coronary heart disease: meta-analysis of randomized controlled trials. Ann Med 41: 301–310, 2009 [DOI] [PubMed] [Google Scholar]