Abstract
The importance of gonadal hormones in the regulation of vascular function has been documented. An alternate and essential contribution of the sex chromosomes to sex differences in vascular function is poorly understood. We reported previously sex differences in microvessel permeability (Ps) responses to adenosine that were mediated by the cAMP signaling pathway (Wang J, PhD thesis, 2005; Wang J and Huxley V, Proceedings of the VIII World Congress of Microcirculation, 2007; Wang J and Huxley VH, Am J Physiol Heart Circ Physiol 291: H3094–H3105, 2006). The two cyclic nucleotides, cAMP and cGMP, central to the regulation of vascular barrier integrity, are hydrolyzed by phosphodiesterases (PDE). We hypothesized that microvascular endothelial cells (EC) would retain intrinsic and inheritable sexually dimorphic genes with respect to the PDEs modulating EC barrier function. Primary cultured microvascular EC from skeletal muscles isolated from male and female rats, respectively, were used. SRY (a sex-determining region Y gene) mRNA expression was observed exclusively in male, not female, cells. The predominant isoform among PDE1–5, present in both XY and XX EC, was PDE4. Expression mRNA levels of PDE1A (male > female) and PDE3B (male < female) were sex dependent; PDE2A, PDE4D, and PDE5A were sex independent. Barrier function, Ps, was determined from measures of albumin flux across confluent primary cultured microvessel XY and XX EC monolayers. Consistent with intact in situ microvessels, basal monolayer Ps did not differ between XY (1.7 ± 0.2 × 10−6 cm/s; n = 8) and XX (1.8 ± 0.1 × 10−6 cm/s; n = 10) EC. Cilostazol, a PDE3 inhibitor, reduced (11%, P < 0.05) Ps in XX, not XY, cells. These findings demonstrate the presence and maintenance of intrinsic sex-related differences in gene expression and cellular phenotype by microvascular EC in a gonadal-hormone-free environment. Furthermore, intrinsic cell-sex likely contributes significantly to sexual dimorphism in cardiovascular function.
Keywords: gender, microvessel, permeability, cyclic nucleotide, skeletal muscle
sex has been identified as an important independent factor contributing to cardiovascular disease morbidity and mortality. Lower incidence of cardiovascular disease in premenopausal women, relative to age-matched men, implicates a significant role for female gonadal hormones in the beneficial effects on cardiovascular function. A large body of clinical and basic research has also documented sex-specific differences in offspring with high blood pressure and endothelial dysfunction, due to a variety of stresses experienced by pregnant mothers (15, 22, 36). This phenomenon, fetal programming of hypertension/endothelial dysfunction, is obviously not mediated by the gonadal hormones and implicates that the sex genome can contribute to the development of vascular sex-related differences in the mother's uterus. Relatively, the contribution of sex chromosomes to cardiovascular health and disease remains to be elucidated.
Vascular endothelial cells (ECs) at the blood/tissue interface play a vital role in the regulation of vascular homeostasis by controlling coagulation, vascular tone, wound healing, angiogenesis, and microvascular exchange. Sex-related differences in endothelium-dependent vasomotion have been documented (16, 21, 41, 44). To date, studies of sex-related differences have focused on gonadal hormones, or their receptors, in reproductively mature experimental animal models. Interestingly, our laboratory's previous studies showed the profound influence of sex on vascular barrier behavior initially in coronary microvessels from reproductively mature pigs (17, 18) and again in skeletal muscle microvessels from both reproductively mature and immature rats (45). Furthermore, we found that the nature of sex differences observed in the immature animals changed with onset of reproductive maturity (45). As gonadal hormone levels in juvenile animals are relatively low and comparable between males and females, our findings in the juveniles lead us to hypothesize that the observed sexually dimorphic vascular barrier responses result, at least partially, from genes encoded on the sex chromosomes and/or fetal gonadal hormone-induced permanent or organizational effects on the cardiovascular system. Therefore, this study used primary cultured microvascular ECs derived from male and female rats, respectively, independent of the gonadal hormone environment, to elucidate signaling mechanisms by which vascular barrier function differs with intrinsic sex chromosome genes.
Phosphodiesterases (PDE), enzymes key to determining the degradation rate of cyclic nucleotides, modulate vascular function through altering intracellular cAMP and cGMP levels. Extensive research on the role of PDE in vascular functions led to the development of PDE inhibitors as therapeutic agents, reducing platelet aggregation and inflammation and facilitating vasodilation (34). Eleven PDE superfamilies have been cloned; of those, five have been identified in vascular endothelium (34). Although important roles for PDE in endothelium-dependent blood flow regulation, microvascular barrier function, and microvascular inflammatory responses have been demonstrated in substantially pharmacological studies (7, 27), relatively little is known of the influence of sex on microvascular functions in the context of PDE-mediated signaling pathways.
Importantly, we found that permeability (Ps) responses to adenosine differed with sex in microvessels from rat skeletal muscle of adults and reproductively immature juveniles, and adenosine action was mediated via A2A receptor-cAMP signaling pathway (46). We further found that the differences could not be accounted for by sex differences in adenosine receptor subtypes, per se. Since this phenomenon was observed in juvenile rats, whose circulating gonadal hormone levels are not only low and steady, but similar between males and females, a role for sexual genes encoded on XY or XX chromosomes was suggested to contribute independently to the sex-related differences in regulation of barrier function (45). We hypothesized that expression and function of the PDEs, focusing on isoforms 1–5 in EC, differ with intrinsically genetic (XX vs. XY), not gonadal hormone, sex, per se.
We found sex-based differences in messenger RNA of isoforms of PDE1 and PDE3, but not PDE2, PDE4, or PDE5, in microvascular EC derived from XY vs. XX cells. Barrier function was assessed using EC monolayer derived from the skeletal muscle microvessels. While basal Ps to albumin did not differ with sex, Ps responses to the PDE3 inhibitor, cilostazol, were present in cells from females, not males, further demonstrating functional consequences of intrinsic XX-XY differences. These results in culture mirrored the in situ results from sexually immature female and male rats (45).
MATERIALS AND METHODS
Experimental animals and microvessel preparation.
All animal care and experimental protocols on male (200–300 g) and female (150–250 g) Sprague-Dawley rats (Hilltop Lab Animals, Scottsdale, PA) were conducted in accordance with the National Institutes of Health “Guide for the Care and Human Use of Laboratory Animals” under the supervision of the Office of Laboratory Medicine at the University of Missouri-Columbia. All experimental protocols were approved by the Institutional Animal Care and Use Committee.
Isolation and culture of rat skeletal muscle microvascular EC.
Unless otherwise noted, reagents were purchased from Sigma (St. Louis, MO). On the day of EC isolation, the animals were anesthetized with an intraperitoneal injection of 130 mg/kg thiobutabarbital (Inactin). Following removal of fur and skin from the anterior abdomen, the abdominal wall muscle was excised carefully using sterile procedures.
The EC isolation procedure was modified from the method of Lidington et al. (23). In brief, the excised abdominal skeletal muscles were cut into ∼0.5 mm2 pieces; digested in 25 ml of enzyme solution, consisting of dispase (0.12 mg/ml; Worthington, Lakewood, NJ), trypsin (0.12 mg/ml; Invitrogen, Carlsbad, CA), collagenase type II (0.84 mg/ml), and bovine serum albumin (BSA; 1.62 mg/ml) in Medium 199 (M199; Invitrogen, Carlsbad, CA) at 37°C until the solution became cloudy (∼40–50 min). The cellular suspension was separated from tissue by filtration through sterile gauze and then a cell strainer (100 μm in pore size; BD Falcon, San Jose, CA). EC were isolated using Dynabeads (Dynal, Brown Deer, WI) coated with Griffonia Simplicifolia lectin for 10–15 min at room temperature, collected using a magnet (Dynal), and cultured with M199 containing 20% fetal bovine serum (FBS), EC growth supplement (50 μg/ml), heparin (5 U/ml), antimycotic-antibiotic solution (10 μl/ml), and l-glutamine (0.1 mg/ml). Subcultures (passage number ≥ 2) were grown in culture medium containing 10%, instead of 20%, FBS (vol/vol). For all experiments, EC were incubated with the culture medium without FBS (starving medium) 8–10 h before experiments to minimize the potential influence of gonadal hormones present in FBS. The isolated cells were identified as vascular EC by identification of EC markers, including PECAM-1 and von Willebrand factor, and EC physiological features, including uptake of 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate-labeled acetylated low-density lipoprotein and capillary-like tube formation. The methods and results for the validation are presented in supplemental materials (Supplemental Figs. 1–3; the online version of this paper contains supplemental data).
RT-PCR assay for SRY gene expression.
EC sex-typing (XY vs. XX) was determined by a gene of SRY (sex-determining region Y) on the Y chromosome using RT-PCR assay described previously (47). Primers for specific SRY (Gene Bank, NM_012772) were as follows: forward 5′-62GCAAGTTGGCTCAACAGA79-3′; reverse 5′-442GTTTCTGCTGTAGTGGGTA424-3′. PCR for this gene was conducted with Mastercycler gradient (Eppendorf, Westbury, NY) for 30 cycles at 94°C for 45 s (denature), 55°C for 1 min (anneal), and 72°C for 1 min (extension) following an initial denaturation step of 95°C for 15 min. A final extension of 72°C for 10 min was added after the 30th amplification cycle. PCR products were separated by electrophoresis through 2% (wt/vol) agarose gel and visualized under UV light with ethidium bromide.
Assessment of PDE mRNA expression by real-time RT-PCR analysis.
Primary cultured EC (passages 3–8) derived from abdominal skeletal muscle microvessels of male and female rats, respectively, was employed to assess mRNA expression of PDEs. Total RNA was extracted from EC derived from male and female rats with RNeasy kit (QIAGEN, Valencia, CA), followed by DNA digestion with RNase-Free DNase (QIAGEN, Valencia, CA), in accordance with the manufacturer's instruction. The total RNA was quantified by NanoDrop 1000 (Thermo Scientidic, Wilmington, DE). First-strand cDNA was synthesized from 2 μg of RNA using reverse transcriptase SuperScript III (Invitrogen, Carlsbad, CA) in a total volume of 20 μl. Taqman gene expression assay (Applied Biosystem) was used for PDE real-time PCR analysis: PDE1A, Rn00578422_m1; PDE1B, Rn00575591_m1; PDE1C, Rn00579334_m1; PDE2A, Rn00579346_m1; PDE3A, Rn00569192_m1; PDE3B, Rn00568191_m1; PDE4A, Rn00565354_m1; PDE4B, Rn00566785_A1; PDE4D, Rn00566798_m1; and PDE5A, Rn00592185_m1. β-Actin (Rn00667869_m1) served as a housekeeping gene, because no significant difference in the expression of its gene of EC derived from abdominal skeletal muscle microvessels of male and female rats was observed. Rat brain was used as a positive control for the expression of PDE when we optimized conditions for the assays (data not shown). To exclude external contamination in the reaction components, no cDNA template in the reaction (cDNA template replaced by H2O) served as a negative control. Amplification of cDNA was performed on a real-time quantitative PCR BioRad IQ5 (Bio-Rad, Hercules, CA) for 40 cycles at 95°C for 15 s and 60°C for 1 min, following manufacturer's instruction.
Cell lysate preparation and immunoblot analysis.
Protein extraction from primary cultured EC was performed on ice. Culture media was removed from cells; the cells were then washed with ice-cold PBS. Cultured EC were incubated with 10 μl/cm2 (plate area) of cold lysis buffer containing 30 mM Tris·HCl (pH = 7.5), 0.075% SDS (vol/vol), 5.6% glycerol (vol/vol), and Halt protease inhibitor cocktail (1:100 dilution; Pierce, Rockford, IL). Cells were scraped, transferred to 1.5-ml microfuge tubes, and homogenized (ULTRA-TURRAX T8) at speed 6 for 1–2 min. Cell lysates were obtained from the supernatant after centrifugation at 14,000 rpm for 15 min at 4°C. The protein was quantified with Micro BCA protein assay kit (Pierce, Rockford, IL). The lysate proteins (20–40 μg) were separated by electrophoresis on 4–12% Nupage gel (Invitrogen, Carlsbad, CA) and then transferred to polyvinylidene difluoride membrane, in accordance with the manufacturer's instructions. The polyvinylidene difluoride membrane was incubated overnight at 4°C with primary polyclonal antibody against PDE2A (1:250 dilution in blocking solution; FabGennix International, Frisco, TX) or PDE4D (1:500, FabGennix International). The blot was then incubated with secondary goat anti-rabbit antibody (1:1,500 dilution) for 1 h at room temperature. The immunoblot was detected using chemiluminescence substrate SuperSignal West Dura (Pierce, Rockford, IL) and exposed to X-ray film. The PDE2A and PDE4D antibodies have been successfully used for immunoblot and fluorescent immunohistochemistry assays in rat tissue or cells (12, 14, 31).
Immunofluorescence assays for the distribution of PDE in skeletal muscle microvessels.
Localization of PDE in skeletal muscle microvessels was assessed by immunofluorescence and confocal laser-scanning microscopy, described previously (47). In brief, abdominal skeletal muscles were embedded in OCT compound (Sakura Finetek, Torrance, CA) and then sectioned using cryostat. The tissue sections were fixed with methanol-acetone (1:1 vol/vol) at −20°C for 10 min and then blocked with 5% normal goat sera (Calbiochem, San Diego, CA) and 5% IgG-free BSA (Jackson Immunoresearch, West Grove, PA) for 1 h at room temperature. The following procedure was performed the same as described in Immunofluorescence Assays for Identification of Cultured EC in the supplemental data. PDE2A (1:50 dilution) and PDE4D (1:100 dilution) (FabGennix International) were incubated with sections overnight at 4°C. Secondary antibodies used were Alexa-488 goat anti-rabbit IgG (1:300 dilution) and Alexa-568 goat anti-mouse IgG (1:300 dilution; Molecular Probes). Immunofluorescence-negative controls performed via incubation of cross sections with omission of primary antibodies, as described above (image not shown), have been validated previously (47). Images were taken on a confocal laser (Krypton-Argon)-scanning microscope (Radiance 2000 Confocal Microscopy System, Zeiss, Thornwood, NY) in the Cytology and Molecular Core of University of Missouri-Columbia.
Preparation of BSA labeled with Alexa 488.
Albumin flux across the cultured EC monolayer barrier was detected using BSA conjugated with the Alexa Fluor 488 (Molecular Probes, Eugene, OR), which was prepared having modified the manufacturer's protocol. Briefly, Alexa 488 and BSA (3:1 molar/molar) were reacted for 30 min at room temperature. The free fluorescent dye was removed by centrifugation using Vivaspin 20, 30-kDa nominal molecular weight limit (VivaScience, Hanover, Germany) tube. Finally, buffer exchange and further removal of free dye were performed using a chromatography-desalting column (Sephadex G-25, Fine, Amersham Bioscience). The protein concentration of the conjugate was determined by micro BCA assay (Thermo Fisher Scientific, Rockford, IL). The final concentration of BSA was adjusted to ∼25 mg/ml.
Measurement of EC monolayer Ps.
EC (passages 3–8) were seeded in Costar Transwell 24-well plate (Corning, Acton, MA) and grown to confluence as above (see Determination of Cultured EC Monolayer Confluence in the supplemental data). Flux measurements for determination of permeability were made using M199 without phenol red; otherwise, the conditions were those described for the starving medium in Isolation and culture of rat skeletal muscle microvascular EC. Accordingly, cells were washed with PBS, following the removal of growth medium from both transwell plate (lower) and insert (upper) chambers. Two hundred microliters of BSA-Alexa 488 (2 mg/ml in growth medium) were applied to cell monolayer, and 500 μl of growth medium were added into the plate chamber. After 20-min incubation at 37°C, medium samples from the plate chamber were withdrawn to determine fluorescence intensity using a fluorescence microplate reader (SpectraMax M2, Molecular Devices, Sunnyvale, CA). Albumin concentration in the samples was calculated from the standard curves of BSA-Alexa 488 concentration-fluorescence intensity. The EC monolayer permeability coefficient to albumin, Ps (cm/s), was determined from the equation:
where V (ml) is the volume of medium in the plate; [C]p and [C]i (mg/ml) are the concentration of BSA-Alexa 488 in the transwell plate (p) and insert (i), respectively; Δt (s) is the period of time during observation of BSA-Alexa 488 crossing EC barrier; and S (cm2) is the surface area of the insert.
Experimental protocol.
To determine the role of PDE3B in the regulation of EC monolayer permeability, EC were incubated with PDE3 inhibitor, cilostazol, for 10 min before measurement of Ps. Albumin flux was evaluated as described in Measurement of EC monolayer Ps in the presence of tested reagents in BSA-Alexa 488 solution applied to the transwell insert.
Statistical analysis.
All values are expressed as means ± SE. One-sample t-test was performed by using GraphPad Prism version 4.0 (Graph Pad Software, San Diego, CA) to determine the differences in PDE mRNA levels among isoforms 1–5 within XY or XX EC, and between XY and XX EC. PDE2A, comparably and moderately expressed between XY and XX EC, was arbitrarily chosen as a reference for comparison of PDE1–5 levels in XY or XX cells. Therefore, the level of each PDE mRNA expression for each sample is presented as the change in expression of specific PDE compared with PDE2A (determining the difference within the same origin of cells) or PDE1–5 in XX cells (determining the difference between sexes), following normalization to β-actin (housekeeping gene) mRNA levels obtained from the same sample in the same PCR reaction. The relative quantification of gene expression analysis (2−ΔΔCt method) was employed. Thus the null hypothesis, ΔΔCt = 0, was tested (24, 38, 53). The difference was assessed by one-sample t-test. EC permeability responses to cilostazol, a PDE inhibitor, are presented as the ratio of Ps in the presence of cilostazol to basal Ps (Pstest/Pscon). The ratio Pstest/Pscon was analyzed by one-sample t-test to compare with hypothetical value 1. An unpaired t-test was used to determine the differences in Ps responses between XX and XY cells. Statistical significance in all cases was defined as P < 0.05.
RESULTS
Maintenance of sex-specific gene expression in microvascular EC.
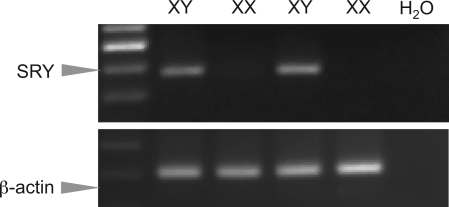
The messenger RNA of the sex-determining gene on the Y chromosome, SRY, was detected only in EC derived from male rats and absent from EC from female rats (Fig. 1). Positive bands demonstrate the presence of SRY mRNA. Water was used as a negative control. β-Actin served as the loading control of transcript, indicating relatively equal amount of cDNA being used for RT-PCR assay.
Fig. 1.
A representative RT-PCR assay shows expression of SRY, a sex-determining region Y gene, in cultured endothelial cells (EC) derived from male (XY), but not in EC cells from female (XX), rats.
Sex-based differences in microvascular EC PDE mRNA expression.
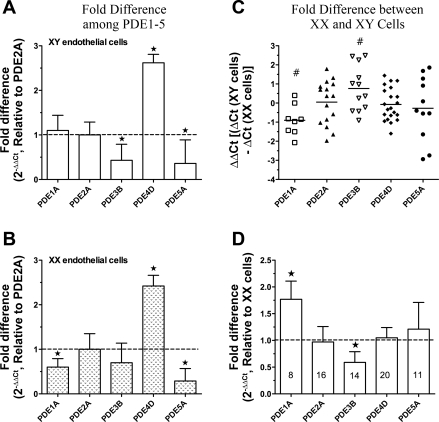
The PDE mRNA levels in microvascular EC derived from abdominal skeletal muscles of males and females were analyzed by real-time RT-PCR assay. PDE1A, -2A, -3B, -4D, and -5A mRNA were expressed in primary microvascular EC; PDE1B, -1C, -3A, -4A, and -4B were barely detectable. To identify which PDE isoform predominates in EC of the same origin, mRNA levels of PDE1–5 were normalized to PDE2A. The results, shown in Fig. 2, A (XY cells) and B (XX cells), indicate that PDE4D for specific cAMP hydrolysis is the major PDE isoform in EC from both sexes. The relative levels are as follows: PDE4D > PDE2A = PDE1A > PDE3B = PDE5A in XY (male) EC, and PDE4D > PDE2A = PDE3B > PDE1A = PDE5A in XX (female) EC.
Fig. 2.
Expression of phosphodiesterase (PDE) mRNA in microvessel EC derived from rat skeletal muscles of males and females via real-time RT-PCR analysis (n = 8–11). A and B: fold changes in PDE expression normalized to PDE2A are given on the ordinate for cultured XY (A) or XX (B) endothelium, providing a comparison among the 5 PDE isoforms within EC of the same origin (XX or XY). If the value (A and B) is larger than 1, the expression level is greater than that for PDE2A mRNA, and vice versa. C and D: ΔΔCt values (C) defined as ΔCtXY cells subtracting ΔCtXX cells, and the fold differences (D) of PDE1–5 expression in XY cells normalized to XX EC. If the value is <0 in C or >1 in D, it indicates that this gene mRNA is expressed higher in XY EC relative to XX EC, and vice versa. P < 0.05 vs. #0 (C) and ★1 (A, B, and D). Numbers in bars (D) represent experimental replicates.
Interestingly, the sex-related differences in PDE1A and PDE3B mRNA levels were observed as higher PDE1A (P < 0.05) and lower PDE3B (P < 0.05) in XY EC compared with XX EC shown in Fig. 2, C and D. The mRNA levels of PDE2A, PDE4D, and PDE5A in EC were comparable between XY and XX cells (Fig. 2, C and D).
Microvessel EC PDE2A and PDE4D protein expression.
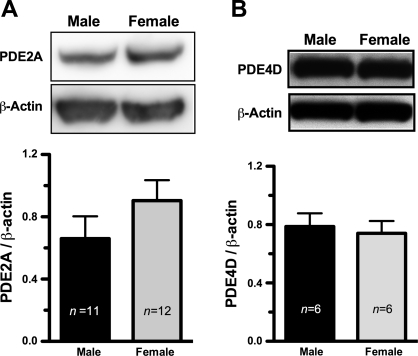
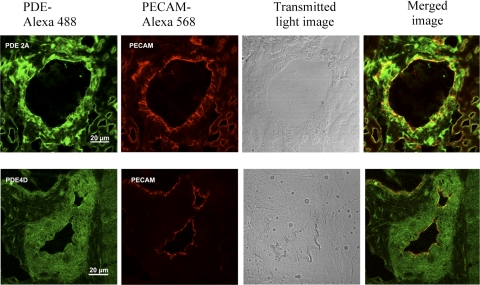
A representative immunoblot assay for the PDE (n = 4) is shown in Fig. 3; similar protein levels of PDE2A (3A) and PDE4D (3B) were expressed by XY and XX EC. Unfortunately, we have been unable to conduct the immunoblot assay in rat tissue for PDE3B because of our inability to locate a suitable antibody. Immunofluorescence and confocal laser scanning microscopy analysis, shown in Fig. 4, revealed the distribution of PDE2A and PDE4D in microvessels of rat abdominal skeletal muscles in situ. The green color in the images represents the presence of PDE2A and PDE4D protein. Endothelium was identified by specific antibody against PECAM, an EC marker, followed by detection with secondary antibody conjugated with Alexa Fluoro 568 (red color). Yellow denotes the superimposed images from the red and the green images, indicating the presence of PDE2A or PDE4D in the microvascular endothelium in the tissue sections.
Fig. 3.
Comparable protein levels of PDE2A (A) and PDE4D (B) were expressed in EC from male (XY) and female (XX) EC. The representative immunoblot results for PDE2A and PDE4D are displayed. n represents the experimental numbers. β-actin serves as the controls for the amount of protein loaded for this assay.
Fig. 4.
The distribution of PDE2A and PDE4D in abdominal skeletal muscle microvessels was assessed by immunofluorescence staining and confocal laser scanning microscopy. PDE2A and PDE4D were detected by primary antibody, followed by secondary antibody conjugated with AlexaFluro 488 (green). Endothelium was identified with antibody against an EC marker, PECAM, and subsequently detected by secondary antibody conjugated with Alexa Fluro 568 (red). The yellow color is the superimposed image of the green and red images of PDE2A and PDE4D in microvascular endothelium. The bright-field images are from the tissue sections.
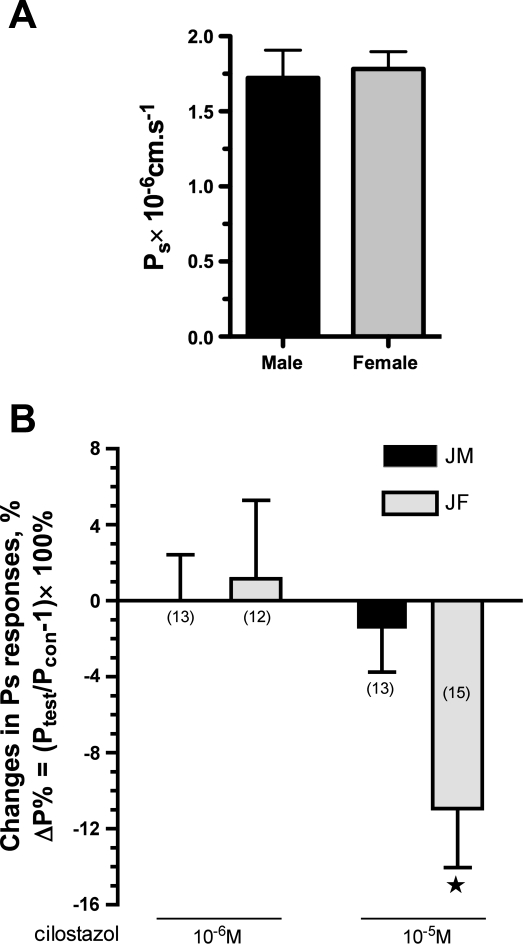
Primary EC Ps in response to cilostazol, PDE3B inhibitor.
Basal Ps did not differ between monolayers of XY (1.72 ± 0.18 × 10−6 cm/s; n =8) and XX (1.78 ± 0.12 × 10−6 cm/s; n =10) cells. Addition of the PDE3 inhibitor, cilostazol at 10−6 M, in the superfusate failed to change either XY (n = 13) or XX (n = 12) monolayer Ps from basal levels. At a high dose of cilostazol (10−5 M), sex differences in Ps responses were observed. In this case, cilostazol at 10−5 M induced no change in Ps for XY cells (Pstest/Pscon = 0.99 ± 0.02, n = 13), while inducing a reduction in Ps for XX cells (Pstest/Pscon = 0.89 ± 0.03, n = 15, P < 0.05). The percentage changes in Ps induced by cilostazol were defined as ΔP% = (Pstest/Pscon − 1)× 100% shown in Fig. 5.
Fig. 5.
EC permeability to albumin in primary cultured male and female cells. Basal permeability Ps to albumin of EC derived from skeletal muscle microvessels of male and female rats did not differ with sex (A), while sexually dimorphic EC Ps responses to cilostazol, a PDE3 inhibitor, were observed (B). Relative EC Ps responses (normalized to its basal Ps) were expressed as a percentage of the changes (Ptest/Pcon − 1) × 100% (see materials and methods for explanation of equation terms). The numbers in the parentheses represent the experimental numbers. ★P < 0.05 compared with 0 (B).
DISCUSSION
We demonstrate in this study, for the first time, that microvascular ECs in primary culture retain sex-dependent differences with respect to both signaling molecule expression and exchange barrier function. We show that SRY, a sex-determining region Y gene, was expressed exclusively in EC derived from male (XY) animals. Furthermore, the primary cultured microvessel EC manifest sex-related differences in mRNA levels of PDE isoforms, PDE1A and PDE3B. The sex-related difference in EC barrier function was observed when PDE3 activity was blocked by the PDE3 inhibitor, cilostazol.
Sex-related differences in microvascular EC signaling molecule expression and cellular response.
The basis of sexual differences with respect to health and disease has been explored to a lesser extent in the cardiovascular than the central nervous system. Knowledge gained from studies of the central nervous system indicates that three components contribute to sexual dimorphism: 1) genes encoded on the sex chromosomes; 2) increased gonadal hormone levels during embryonic gonadal organ differentiation, leading to permanent/organizational effects; and 3) circulating and fluctuating gonadal hormone levels following reproductive maturity. Extensive research on brain demonstrates the important role of intrinsic sex-related differences in gene expression and neuronal cell differentiation in XY and XX cultured brain cells harvested from embryonic brain (3, 4, 52). In heart and blood vessels, the role of X and Y genes in sex-related differences in cardiovascular structures and functions is very poorly understood. To address this issue, it becomes important to identify the sexually dimorphic phenotypes associated with intrinsic sex genes using appropriate models. The present study focused on vascular EC, the prominent and constitutive structure and function of microvasculature, to identify intrinsic sex dimorphism in EC cellular gene expression and responses. We used primary cultures of microvascular EC derived from skeletal muscle of males and females using the same microvessels on which our earlier studies of exchange were performed. Our finding that SRY gene is expressed exclusively in XY EC, but not in XX EC, provides evidence that cells in culture possess and retain sex-related differences in gene expression. Therefore, cells have sex and might be suitable for study of sex-related differences of phenotypes, resulting from intrinsic sex genes between males and females.
Although gonadal hormones, particular female reproductive hormones, have been studied in the context of sex-related differences in endothelium-dependent vascular tone (21, 25, 44), a paucity of literature explores the relationship of sex chromosomes to vascular function. While we found that the mRNA levels of PDE2A, PDE4D, and PDE5A did not differ with sex, the levels of PDE1A and PDE3B mRNA expression differed in primary cultured EC derived from skeletal muscle microvessels of male compared with female rats. The data revealed that microvascular EC carry/retain sex-related variant signaling potential in an environment essentially free of gonadal hormones. The sexually dimorphic gene expression in primary cultured EC provides a mechanism whereby EC-mediated vascular functions, including exchange, can vary between males and females of the same species and age. Consistent with our findings that sexual dimorphisms are not all the consequences of the actions of circulating gonadal hormones, intrinsic sex chromosome could directly affect somatic cell responses evidenced by in vitro studies with cultured neuronal cells (11, 54), splenocytes (11), muscle-derived stem cells (10), mesenchymal stem cells (9), and human umbilical vein ECs (HUVECs) (6) under the gonadal hormone-free condition. Importantly, most recent evidence provided by Kunert and coworkers (22) using consomic rat strains derived from a cross between Dahl salt-sensitive and Brown Norway normotensive rats supports strongly the important role of sex chromosomes in the differential regulation of aortic tone between males and females. Furthermore, Turner et al. (43) demonstrated the SRY loci as the hypertensive component of the SHR Y chromosome. These findings provide strong evidence to support that genes encoded on sex chromosomes contribute to vascular diseases.
As for signaling molecules, PDEs have been shown to mediate sex-related differences in vasodilatation induced by C-type natriuretic peptide in porcine coronary arteries (5). PDEs are autosomal genes. For instance, rat PDE3B is located on the chromosome 1q34. The mechanism of interaction between PDE genes and sex chromosome-linked genes remains to be elucidated. However, differential dosages of genes encoded on sex chromosomes between XY and XX cells, referred to as intrinsic cellular sex-specific differences, have been found to contribute to sexually dimorphic phenotypes (3, 4). The disparate doses of genes encoded on sex chromosome between XY and XX cells result from the presence or absence of Y-linked genes, incomplete inactivation, and paternal imprint of X-linked genes in XX cells (3). The present findings will facilitate additional studies for elucidating the linkage between intrinsic cellular sex-related differences and variations in vascular behaviors in health and disease.
The cellular distribution and functions of PDE1–5 in EC.
Among the 11 members of the PDE family, the present study focused on PDE1–5 in primary cultured EC and whether the sex of the animal of origin influenced their presence, distribution, and function in the regulation of EC barrier integrity.
PDE1 is Ca2+/calmodulin dependent and most likely involved in the regulation of cell growth, survival, and migration (34, 40). That we identify the presence of PDE1A mRNA in primary cultured EC derived from skeletal muscle microvessels is novel. Previously, PDE1 had been reported to exist in cultured aortic EC with high passage number (20). In contrast, PDE1 was undetectable in cultured bovine or human aortic EC, HUVEC, or human dermal microvessel EC in the presence of Ca2+/calmodulin (32). In fact, we found higher PDE1A mRNA levels in XY relative to XX skeletal microvascular EC. The physiological significance of this difference in EC proliferation and migration remains to be elucidated.
A large body of evidence demonstrates that the cAMP-dependent signaling pathway is of major importance for the protective regulation of microvascular barrier integrity (28, 30). PDE2, PDE3, and PDE4 have been shown to be essential regulators of intracellular cAMP activity, which, in turn, modulates the cAMP-PKA and/or the cAMP-exchange protein activated by cAMP pathways in microvascular EC (33). Consistent with findings in cultured bovine and human aortic EC, HUVEC, and human dermal microvessel EC, we found PDE2, PDE3, and PDE4 mRNA to be present in primary cultured EC derived from rat skeletal muscle microvessels of both sexes. The amount of PDE4 mRNA was higher relative to PDE2 and PDE3, suggesting that PDE4 is the major PDE isoform for specific cAMP hydrolysis in microvessel EC. The findings from this study provided molecular evidence to support pharmacological data that PDE4 inhibitor, rolipram, induced a decrease in another index of exchange function, hydraulic conductivity, in rat mesentery venules (2).
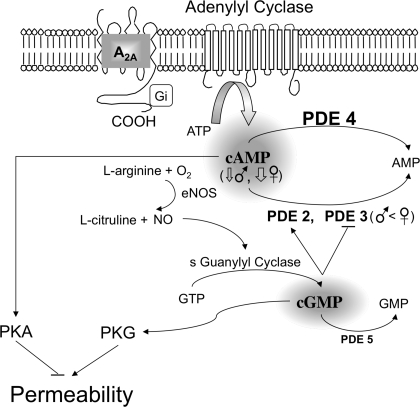
Importantly, the levels of PDE3B mRNA were found to differ in EC derived from males compared with females. In most cases, gene transcript levels correspond to levels of expressed protein as both real-time PCR and immunoblot assays demonstrated the presence of comparable PDE2A and PDE4D between XY and XX EC. Unfortunately, at this time, it was not possible to verify the relationships between the PDE3B protein level and distribution in EC and skeletal muscle vasculature due to the lack of a suitable PDE3B antibody for use in rat. Therefore, we used available pharmacological tools to identify the role of differential PDE3B mRNA expression in the regulation of EC barrier function. Cilostazol, a putative selective PDE3 inhibitor, at 10−6 and 10−5 M was selected based on previous studies on the effect of PDE3 on human and rat ECs or endothelium-dependent vascular function (26, 39). We found that inhibition of PDE3 caused different Ps responses in XY cells vs. XX cells. Only XX cells, having higher PDE3B gene expression relative to XY cells, showed a change in exchange function on exposure to cilostazol with a reduction in EC Ps. Given that cilostazol elevates greater cAMP concentration in XX cells due to the inhibition of higher cellular PDE3B levels relative to XY cells, the greater reduction in Ps of XX EC induced by cilostazol provides functional evidence to support that sexually dimorphic signaling molecules could attribute to the distinct EC barrier behavior between sexes (Fig. 6). To completely verify this conclusion, the activities of PDE and cAMP need to be determined.
Fig. 6.
Schematic of the mechanisms whereby PDEs mediate endothelial barrier integrity. PDE isoforms 2–5 are expressed constitutively in primary EC derived from rat skeletal muscle microvessels. PDE4 and -5 hydrolyze intracellular cAMP and cGMP, respectively. Both PDE2 and PDE3, hydrolyzing cAMP, are the critical molecules bridging between cGMP and cAMP. Their activity creates the cross talk, resulting in loosening and tightening the endothelial barrier via PKG and PKA pathways, respectively. PDE2 is activated, while PDE3 is inhibited by cGMP. Given that XX EC express higher levels of PDE3 and comparable levels of PDE2, -4, and -5 relative to XY EC, the intracellular levels of cAMP are expected to be elevated greatly in XX EC compared with XY EC. This appears to be the case only when the system is activated (e.g., when cilostazol is given to block PDE3-mediated cAMP degradation). Consequently, while basal Ps did not differ in monolayers of XX and XY EC, the decrease in Ps in response to cilostazol was greater in XX than XY EC. eNOS, endothelial nitric oxide synthase.
PDE5, a cGMP-specific hydrolysis enzyme, has been documented mostly in vascular smooth muscle cells. Substantial evidence supports that inhibition of PDE5 results in vasodilatation via increases in the cellular level of cGMP (37). We found evidence for the presence of PDE5A, at levels significantly lower relative to PDE1–4, in both primary cultured XX and XY EC derived from rat abdominal skeletal muscle microvessels. This novel evidence suggests direct involvement of PDE5 in the regulation of cGMP in microvessel EC. It should be noted that our findings are not universal, as Zhu and coworkers (55) found PDE5 expressed in pulmonary conduit arterial EC, but failed to detect it in pulmonary microvessel EC. While the physiological significance of PDE5 in the regulation of microvascular barrier integrity remains to be elucidated, we and others have shown a role for increases in cGMP, whether in response to activation of the particulate guanylyl cyclase by ANP, or in response to soluble guanylyl cyclase via NO donors, with increases in microvessel hydraulic conductivity and permeability in in situ microvessels (29).
Endothelial barrier permeability.
Assessment of barrier properties from measures of albumin flux through in vitro EC monolayer has been widely used for the research on understanding regulatory mechanisms mediating vascular barrier integrity. The present study is the first to employ primary cultured EC derived from skeletal muscle microvessels used for studies of the regulation of microvascular exchange in the intact microvasculature. Before the primary cultured EC monolayers were used for the permeability studies, EC monolayers were characterized using at least one of the assays described in the Supplemental Materials and Results. Of all the primary cells placed into conditions of culture, >95% possessed EC characteristics, consistent with findings from Tyml's group (23, 49). A prerequisite for the in vitro EC culture model used to evaluate EC barrier integrity and to elucidate the mechanisms regulating vascular permeability is that the monolayer be confluent. We also validated the confluence of primary cultured EC by assessment of cell-cell adhesion using labeled lectin, WGA-Alexa 488 binding to cell surface glycoproteins (Supplemental Fig. 2A).
In this study, the basal Ps of primary cultured EC derived from rat skeletal muscle microvessels was 1.72 ± 0.18 and 1.78 ± 0.12 × 10−6 cm/s for XY and XX EC, respectively. This finding of no difference in basal microvessel permeability with sex is consistent with what we found for rat skeletal muscle microvessels (45) and porcine coronary arterioles (48). To date, to the best of our knowledge, no absolute basal value of skeletal muscle microvessel endothelial monolayer permeability has been reported to make a comparison with our findings. Only one recent publication on the study of endothelial barrier function using EC derived from mouse skeletal muscle microvessels was noticed in which no basal Ps values were reported (50). For bovine pulmonary artery EC grown on gelatin-coated filters, Ps was 4.8 × 10−6 cm/s (13); for HUVEC monolayer, Ps was 19.4 ± 0.8 × 10−6 cm/s (51). The apparent Ps value in our in vitro model EC monolayer was 1.5–2 times higher than basal Ps measured from corresponding intact rat abdominal skeletal muscle microvessels (arteriolar Ps = 0.67 ± 0.16 × 10−6 cm/s, venular Ps = 0.91 ± 0.33 × 10−6 cm/s in juvenile males; and arteriolar Ps = 0.99 ± 0.04 × 10−6 cm/s, venular Ps = 1.2 ± 0.06 × 10−6 cm/s in juvenile females) (45).
Likely reasons for in vitro and ex vivo differences in Ps are 1) the contribution of components of extracellular matrix present in vivo (including collagen, movement across fibronectin, elastin), providing resistance to solute movement relative to the resistance offered by the gelatin substrata for EC monolayer; 2) absence of the resistance offered by accessory cells from pericytes in capillaries to vascular smooth muscle cells in arterioles and venules in vivo relative to culture; 3) absence or differences in EC glycocalyx structure and density (35) shown to contribute to Ps measured in vivo (1, 19); and 4) hemodynamic environment (lack of blood pressure, hydraulic flow across the monolayer, and surface shear in vitro relative to their presence in vivo). These differential structures and dynamic environments have been shown to influence cell-cell junctional formation, EC morphology, as well as cellular signaling and protein activities (8, 42).
Of prime importance, independent of being grown in culture, the EC derived from males and females retain the sex-related genetic features with respect to only expression of sexual determinant gene on Y chromosome, SRY, in XY cells. Furthermore, as shown previously in vivo, Ps responses to vasoactive substances demonstrated sex-related differences in the in vitro culture model.
Taking together, the findings in this study are the first to demonstrate that at least one component of the in vivo sex-sensitive differences in exchange function reside with the ECs themselves. It suggests that cellular intrinsic sex-related differences, at least partially, contribute to differences observed between males and females under physiological and pathological conditions. In vitro cultured cells have been used widely for the cell and molecular biology research and have made significant contributions to better understanding mechanisms of a variety of diseases and development of therapeutic interventions. Similarly, not all studies using the cultured cell model have consistent results, nor is the sex of the animals from which the cultures were derived noted. In fact, sex could be a confounding variable in interpretation of data derived from widely used sources of ECs. For example, HUVEC are likely derived equally from males and females, whereas bovine materials have a higher likelihood of being derived from males, as males are culled for slaughter more frequently than females (retained for milking and reproduction). In light of the present findings, it is necessary to claim the origin (male vs. female) of cells used for research in the future.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant RO1HL078816 and National Aeronautics and Space Administration Grant NNJ05HF37G.
DISCLOSURES
No conflicts of interest are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank Steven W. Sieveking and Qi Feng for expert technical assistance. We are grateful for Dr. Karel Tyml's advice on isolation and culture of microvascular ECs from skeletal muscles.
REFERENCES
- 1.Adamson RH. Permeability of frog mesenteric capillaries after partial pronase digestion of the endothelial glycocalyx. J Physiol 428: 1–13, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adamson RH, Liu B, Fry GN, Rubin LL, Curry FE. Microvascular permeability and number of tight junctions are modulated by cAMP. Am J Physiol Heart Circ Physiol 274: H1885–H1894, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Arnold AP, Burgoyne PS. Are XX and XY brain cells intrinsically different? Trends Endocrinol Metab 15: 6–11, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Arnold AP, Rissman EF, De Vries GJ. Two perspectives on the origin of sex differences in the brain. Ann N Y Acad Sci 1007: 176–188, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Barber DA, Burnett JC, Jr, Fitzpatrick LA, Sieck GC, Miller VM. Gender and relaxation to C-type natriuretic peptide in porcine coronary arteries. J Cardiovasc Pharmacol 32: 5–11, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Batres RO, Dupont J. Gender differences in prostacyclin and prostaglandin E2 synthesis by human endothelial cells. Prostaglandins Leukot Med 22: 159–171, 1986 [DOI] [PubMed] [Google Scholar]
- 7.Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev 58: 488–520, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Chien S. Mechanotransduction and endothelial cell homeostasis: the wisdom of the cell. Am J Physiol Heart Circ Physiol 292: H1209–H1224, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Crisostomo PR, Wang M, Herring CM, Markel TA, Meldrum KK, Lillemoe KD, Meldrum DR. Gender differences in injury induced mesenchymal stem cell apoptosis and VEGF, TNF, IL-6 expression: role of the 55 kDa TNF receptor (TNFR1). J Mol Cell Cardiol 42: 142–149, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deasy BM, Lu A, Tebbets JC, Feduska JM, Schugar RC, Pollett JB, Sun B, Urish KL, Gharaibeh BM, Cao B, Rubin RT, Huard J. A role for cell sex in stem cell-mediated skeletal muscle regeneration: female cells have higher muscle regeneration efficiency. J Cell Biol 177: 73–86, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Du L, Bayir H, Lai Y, Zhang X, Kochanek PM, Watkins SC, Graham SH, Clark RS. Innate gender-based proclivity in response to cytotoxicity and programmed cell death pathway. J Biol Chem 279: 38563–38570, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Fujita M, Imaizumi M, D'Sa C, Zoghbi SS, Crescenzo MS, Hong J, Musachio JL, Gee AD, Seidel J, Green MV, Pike VW, Duman RS, Innis RB. In vivo and in vitro measurement of brain phosphodiesterase 4 in rats after antidepressant administration. Synapse 61: 78–86, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Garcia JG, Siflinger-Birnboim A, Bizios R, Del Vecchio PJ, Fenton JW, 2nd, Malik AB. Thrombin-induced increase in albumin permeability across the endothelium. J Cell Physiol 128: 96–104, 1986 [DOI] [PubMed] [Google Scholar]
- 14.Giorgi M, Modica A, Pompili A, Pacitti C, Gasbarri A. The induction of cyclic nucleotide phosphodiesterase 4 gene (PDE4D) impairs memory in a water maze task. Behav Brain Res 154: 99–106, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Grigore D, Ojeda NB, Alexander BT. Sex differences in the fetal programming of hypertension. Gend Med 5, Suppl A: S121–S132, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang A, Kaley G. Gender-specific regulation of cardiovascular function: estrogen as key player. Microcirculation 11: 9–38, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Huxley VH, Wang J, Whitt SP. Sexual dimorphism in the permeability response of coronary microvessels to adenosine. Am J Physiol Heart Circ Physiol 288: H2006–H2013, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huxley VH, Wang JJ, Sarelius IH. Adaptation of coronary microvascular exchange in arterioles and venules to exercise training and a role for sex in determining permeability responses. Am J Physiol Heart Circ Physiol 293: H1196–H1205, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huxley VH, Williams DA. Role of a glycocalyx on coronary arteriole permeability to proteins: evidence from enzyme treatments. Am J Physiol Heart Circ Physiol 278: H1177–H1185, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Keravis T, Komas N, Lugnier C. Cyclic nucleotide hydrolysis in bovine aortic endothelial cells in culture: differential regulation in cobblestone and spindle phenotypes. J Vasc Res 37: 235–249, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Kublickiene K, Luksha L. Gender and the endothelium. Pharmacol Rep 60: 49–60, 2008 [PubMed] [Google Scholar]
- 22.Kunert MP, Dwinell MR, Drenjancevic Peric I, Lombard JH. Sex-specific differences in chromosome-dependent regulation of vascular reactivity in female consomic rat strains from a SSxBN cross. Am J Physiol Regul Integr Comp Physiol 295: R516–R527, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lidington D, Ouellette Y, Tyml K. Endotoxin increases intercellular resistance in microvascular endothelial cells by a tyrosine kinase pathway. J Cell Physiol 185: 117–125, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2[-delta delta C(T)] method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Luksha L, Agewall S, Kublickiene K. Endothelium-derived hyperpolarizing factor in vascular physiology and cardiovascular disease. Atherosclerosis 202: 330–344, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Matsumoto T, Noguchi E, Ishida K, Nakayama N, Kobayashi T, Kamata K. Cilostazol improves endothelial dysfunction by increasing endothelium-derived hyperpolarizing factor response in mesenteric arteries from Type 2 diabetic rats. Eur J Pharmacol 599: 102–109, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Maurice DH, Palmer D, Tilley DG, Dunkerley HA, Netherton SJ, Raymond DR, Elbatarny HS, Jimmo SL. Cyclic nucleotide phosphodiesterase activity, expression, and targeting in cells of the cardiovascular system. Mol Pharmacol 64: 533–546, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev 86: 279–367, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Meyer DJ, Jr, Huxley VH. Capillary hydraulic conductivity is elevated by cGMP-dependent vasodilators. Circ Res 70: 382–391, 1992 [DOI] [PubMed] [Google Scholar]
- 30.Michel CC, Curry FE. Microvascular permeability. Physiol Rev 79: 703–761, 1999 [DOI] [PubMed] [Google Scholar]
- 31.Mongillo M, Tocchetti CG, Terrin A, Lissandron V, Cheung YF, Dostmann WR, Pozzan T, Kass DA, Paolocci N, Houslay MD, Zaccolo M. Compartmentalized phosphodiesterase-2 activity blunts beta-adrenergic cardiac inotropy via an NO/cGMP-dependent pathway. Circ Res 98: 226–234, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Netherton SJ, Maurice DH. Vascular endothelial cell cyclic nucleotide phosphodiesterases and regulated cell migration: implications in angiogenesis. Mol Pharmacol 67: 263–272, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Netherton SJ, Sutton JA, Wilson LS, Carter RL, Maurice DH. Both protein kinase A and exchange protein activated by cAMP coordinate adhesion of human vascular endothelial cells. Circ Res 101: 768–776, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Omori K, Kotera J. Overview of PDEs and their regulation. Circ Res 100: 309–327, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Potter DR, Damiano ER. The hydrodynamically relevant endothelial cell glycocalyx observed in vivo is absent in vitro. Circ Res 102: 770–776, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Rodford JL, Torrens C, Siow RC, Mann GE, Hanson MA, Clough GF. Endothelial dysfunction and reduced antioxidant protection in an animal model of the developmental origins of cardiovascular disease. J Physiol 586: 4709–4720, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rybalkin SD, Yan C, Bornfeldt KE, Beavo JA. Cyclic GMP phosphodiesterases and regulation of smooth muscle function. Circ Res 93: 280–291, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3: 1101–1108, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Schror K. The pharmacology of cilostazol. Diabetes Obes Metab 4, Suppl 2: S14–S19, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Sharma RK, Das SB, Lakshmikuttyamma A, Selvakumar P, Shrivastav A. Regulation of calmodulin-stimulated cyclic nucleotide phosphodiesterase (PDE1): review. Int J Mol Med 18: 95–105, 2006 [PubMed] [Google Scholar]
- 41.Sumanasekera WK, Sumanasekera GU, Mattingly KA, Dougherty SM, Keynton RS, Klinge CM. Estradiol and dihydrotestosterone regulate endothelial cell barrier function after hypergravity-induced alterations in MAPK activity. Am J Physiol Cell Physiol 293: C566–C573, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Tarbell JM, Weinbaum S, Kamm RD. Cellular fluid mechanics and mechanotransduction. Ann Biomed Eng 33: 1719–1723, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Turner ME, Farkas J, Dunmire J, Ely D, Milsted A. Which Sry locus is the hypertensive Y chromosome locus? Hypertension 53: 430–435, 2009 [DOI] [PubMed] [Google Scholar]
- 44.Villar IC, Hobbs AJ, Ahluwalia A. Sex differences in vascular function: implication of endothelium-derived hyperpolarizing factor. J Endocrinol 197: 447–462, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Wang J. Modulation of Coronary and Skeletal Muscle Exchange by Adenosine: Role of Adenosine Receptors (PhD thesis). Columbia, Missouri: University of Missouri-Columbia, 2005 [Google Scholar]
- 46.Wang J, Huxley V. Sexual Dimorphism in the Regulation of Skeletal Muscle Arteriole Macromolecule Permeability. In: Proceedings of the VIII World Congress of Microcirculation. Microcirculation 14: 439–569, 2007 [Google Scholar]
- 47.Wang J, Huxley VH. Adenosine A2A receptor modulation of juvenile female rat skeletal muscle microvessel permeability. Am J Physiol Heart Circ Physiol 291: H3094–H3105, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang J, Whitt SP, Rubin LJ, Huxley VH. Differential coronary microvascular exchange responses to adenosine: roles of receptor and microvessel subtypes. Microcirculation 12: 313–326, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilson JX, Dixon SJ, Yu J, Nees S, Tyml K. Ascorbate uptake by microvascular endothelial cells of rat skeletal muscle. Microcirculation 3: 211–221, 1996 [DOI] [PubMed] [Google Scholar]
- 50.Wu F, Tyml K, Wilson JX. iNOS expression requires NADPH oxidase-dependent redox signaling in microvascular endothelial cells. J Cell Physiol 217: 207–214, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu MH, Guo M, Yuan SY, Granger HJ. Focal adhesion kinase mediates porcine venular hyperpermeability elicited by vascular endothelial growth factor. J Physiol 552: 691–699, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu J, Taya S, Kaibuchi K, Arnold AP. Sexually dimorphic expression of Usp9x is related to sex chromosome complement in adult mouse brain. Eur J Neurosci 21: 3017–3022, 2005 [DOI] [PubMed] [Google Scholar]
- 53.Yuan JS, Reed A, Chen F, Stewart CN., Jr Statistical analysis of real-time PCR data. BMC Bioinformatics 7: 85, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang L, Li PP, Feng X, Barker JL, Smith SV, Rubinow DR. Sex-related differences in neuronal cell survival and signaling in rats. Neurosci Lett 337: 65–68, 2003 [DOI] [PubMed] [Google Scholar]
- 55.Zhu B, Strada S, Stevens T. Cyclic GMP-specific phosphodiesterase 5 regulates growth and apoptosis in pulmonary endothelial cells. Am J Physiol Lung Cell Mol Physiol 289: L196–L206, 2005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.