Abstract
Escalating evidence indicates that disturbed flow patterns, characterized by the presence of retrograde and oscillatory shear stress, induce a proatherogenic endothelial cell phenotype; however, the mechanisms underlying oscillatory shear profiles in peripheral conduit arteries are not fully understood. We tested the hypothesis that acute elevations in muscle sympathetic nerve activity (MSNA) are accompanied by increases in conduit artery retrograde and oscillatory shear. Fourteen healthy men (25 ± 1 yr) performed three sympathoexcitatory maneuvers: graded lower body negative pressure (LBNP) from 0 to −40 Torr, cold pressor test (CPT), and 35% maximal voluntary contraction handgrip followed by postexercise ischemia (PEI). MSNA (microneurography; peroneal nerve), arterial blood pressure (finger photoplethysmography), and brachial artery velocity and diameter (duplex Doppler ultrasound) in the contralateral arm were recorded continuously. All maneuvers elicited significant increases in MSNA total activity from baseline (P < 0.05). Retrograde shear (−3.96 ± 1.2 baseline vs. −8.15 ± 1.8 s−1, −40 LBNP, P < 0.05) and oscillatory shear index (0.09 ± 0.02 baseline vs. 0.20 ± 0.02 arbitrary units, −40 LBNP, P < 0.05) were progressively augmented during graded LBNP. In contrast, during CPT and PEI, in which MSNA and blood pressure were concomitantly increased (P < 0.05), minimal or no changes in retrograde and oscillatory shear were noted. These data suggest that acute elevations in MSNA are associated with an increase in conduit artery retrograde and oscillatory shear, an effect that may be influenced by concurrent increases in arterial blood pressure. Future studies should examine the complex interaction between MSNA, arterial blood pressure, and other potential modulatory factors of shear rate patterns.
Keywords: sympathetic tone, blood pressure, blood flow, endothelium, atherosclerosis
atherosclerosis is a potentially life-threatening disease of large and medium-size arteries that is strongly associated with systemic risk factors such as physical inactivity, obesity, hypercholesterolemia, hypertension, smoking, and diabetes (30). However, the predictable distribution of atherosclerosis indicates that localized hemodynamic environments may also influence the atherosclerotic disease process (31). Indeed, postmortem (36) and in vivo imaging studies (10) demonstrate that atherosclerotic lesions are preferentially located in regions distinguished by oscillatory (bidirectional blood flow) and low mean shear stress, whereas areas exposed to unidirectional and moderate shear are protected. A causal link between disturbed flow patterns and a proatherogenic endothelial cell phenotype has been extensively confirmed by in vitro studies using cell culture and isolated perfused arteries (4a, 7, 9, 15, 17, 19, 23, 38). Furthermore, recent human data suggest that an acute increase in retrograde and oscillatory shear in the brachial artery blunts endothelium-dependent dilation (33).
The triphasic flow pattern in a peripheral conduit artery is primarily characterized by a large antegrade flow during systole, followed by a brief episode of retrograde flow in early diastole and a subsequent phase of antegrade flow in mid- and late diastole (4, 20). However, the shape of the flow wave varies markedly between individuals, physiological states, and arterial locations. In consideration of the recognized impact of oscillatory shear patterns on vascular phenotype, it appears fundamental to devote efforts toward elucidating factors that may influence flow profiles in peripheral conduit arteries. Previous (2) and more recent (33) observations that inflating a pressure cuff on the forearm results in greater retrograde flow at the brachial artery strongly suggests that changes in peripheral vascular resistance may explain differences in flow patterns. On this basis, it is tempting to speculate that factors altering vascular tone may be prime candidates participating in the regulation of upstream oscillatory shear patterns. In this regard, it has been recently proposed that increased sympathetic nerve activity may be associated with greater conduit artery retrograde and oscillatory flow (11); however, this hypothesis has not been tested. Pilot work in our laboratory provided anecdotal evidence that the abrupt and prominent increase in muscle sympathetic nerve activity (MSNA) induced by a voluntary apnea is followed by an enhanced retrograde flow at the brachial artery. These anecdotal observations prompted us to systematically evaluate the role of MSNA on modulating brachial artery shear rate profiles by applying a number of well-accepted sympathoexcitatory maneuvers. We hypothesized that an increase in MSNA would be accompanied by augmented retrograde and oscillatory shear rates.
METHODS
Fourteen healthy men with a mean age of 25 ± 1 yr, height of 177 ± 2 cm, and weight of 76 ± 3 kg (means ± SE) were recruited for voluntary participation in the study. All subjects were free of any recognized cardiovascular, pulmonary, metabolic, or neurological disease and were nonhypertensive (resting blood pressure < 140/80 mmHg), nonobese (body mass index < 30 kg/m2), and nonsmokers. Subjects were not taking prescribed or over-the-counter medications. Each subject received a verbal and written explanation of the study objectives, measurement techniques, and the risks and benefits associated with the investigation and provided written informed consent before participation. All procedures were approved by the University of Missouri-Columbia Health Sciences Institutional Review Board. On the experimental day, the subjects were instructed to report to the laboratory fasted for 4 h and abstained from caffeine and strenuous physical activity for 12 h.
Experimental Measurements
Subjects were positioned comfortably supine in a dark, climate-controlled quiet room (22 to 23°C) with both arms extended laterally. A handgrip dynamometer (model 78010; Lafayette Instrument, Lafayette, IN) was used to establish the maximal voluntary contraction (MVC) in the left arm. MVC was determined as the highest force produced during three to five maximal efforts, each separated by 1 min. Heart rate was monitored using a lead II electrocardiogram (ECG; Hewlett Packard 78353B, Philips Healthcare, Andover, MA). Beat-to-beat arterial blood pressure was monitored noninvasively by a servo-controlled finger photoplethysmograph (Finometer; Finapres Medical Systems, Amsterdam, The Netherlands) placed on the middle finger of the right hand. In addition, an automated sphygmomanometer (Welch Allyn, Skaneatles Falls, NY) was used to validate the Finometer measurements. Respiratory movements were monitored using a strain-gauge pneumograph placed in a stable position over the abdomen (Pneumotrace; UFI, Morro Bay, CA).
The right brachial artery was imaged longitudinally 2–5 cm above the antecubital fossa by a two-dimensional high-resolution ultrasound system (Terason T3000; Teratech, Burlington, MA), using a 10-MHz multifrequency linear-array transducer (25, 26). Continuous Doppler velocity was simultaneously obtained using the same ultrasound machine. Doppler velocity signals were corrected at an insonation angle of 60°, and measurements were performed with a large sample volume to encompass the vessel lumen without extending outside of it. Once a satisfactory image was acquired, the right arm was secured and the transducer was stabilized using a custom-designed clamp. The location of the transducer was marked on the skin to ensure an exact placement throughout the study. Ultrasound parameters were not changed during the study. Ultrasound images were recorded at 5 frames/s using Camtasia (TechSmith, Okemos, MI) and converted into an AVI file. Continuous measurements of brachial artery diameter and velocity were obtained at baseline, during each experimental maneuver and the following recovery period.
Multiunit postganglionic MSNA was recorded with standard microneurographic techniques, as described in detail previously (12, 24). Briefly, a unipolar tungsten microelectrode was inserted into muscle fascicles of the peroneal nerve near the fibular head of the right leg. Neural signals were amplified, filtered (bandwidth, 700–2,000 Hz), rectified, and integrated (time constant, 0.1 s) to obtain mean voltage neurograms. MSNA recordings were identified by their pulse synchronous burst pattern and increased burst frequency to an end-expiratory breath hold without any responses to arousal or skin stroking. Although MSNA was measured in the leg while blood flow was measured in the arm, it is well accepted from previous studies comparing simultaneous recordings made in the leg and arm that the MSNA response to sympathoexcitatory maneuvers is similar between the limbs (27, 28, 35). MSNA was not obtained in four subjects because an adequate recording site was not found in two subjects and an acceptable recording could not be maintained for the duration of the study in two others. MSNA, arterial blood pressure, heart rate, and respiratory movements were sampled at 1,000 Hz and stored for off-line analysis (PowerLab, AD Instruments, Bella Vista, NSW, Australia).
Experimental Protocols
To examine the impact of increased MSNA on brachial artery shear rate profiles, the subjects performed the following three sympathoexcitatory maneuvers: lower body negative pressure (LBNP), cold pressor test (CPT), and postexercise ischemia (PEI). These maneuvers were purposely selected to study the shear rate responses to varying increases in MSNA under conditions in which blood pressure was largely unchanged (LBNP), progressively increased (CPT), and steadily elevated (PEI). All three conditions were applied in a randomized order and separated by ∼10 min to ensure the reestablishment of baseline variables before commencing the subsequent protocol.
Protocol 1: LBNP.
The subject's lower body was enclosed in a negative pressure chamber to the level of the iliac crest. The pressure inside the chamber was measured continuously by a Statham transducer interfaced with PowerLab. Progressive LBNP at −10, −20, −30, and −40 Torr was conducted at 2 min per stage. The reduction of pressure between stages was applied gradually (∼0.5 Torr/s) to minimize the potential movement of the MSNA electrodes. LBNP was terminated in one individual at −40 Torr because of presyncopal symptoms, and therefore none of the LBNP data from this individual was included into the analysis. All variables were continuously recorded for 2 min at baseline, during all stages of LBNP, and for a 2-min recovery period upon the return of the chamber pressure to 0 Torr.
Protocol 2: CPT.
The left hand was submerged in ice water (∼4°C) for 2 min. All variables were continuously recorded for 2 min at baseline, during CPT, and for a 2-min recovery period that started 1 min after removing the hand from the ice water.
Protocol 3: PEI.
Subjects were instructed to perform left arm isometric handgrip exercise at 35% MVC for 2 min. Visual feedback of the force exerted by the subject, expressed as a percentage of maximum, was displayed on a computer screen positioned in front of the subject at eye level. Five seconds before the cessation of handgrip, an occlusion cuff placed over the upper arm was inflated to suprasystolic pressure (220 mmHg) and remained inflated for 2 min and 15 s. The additional 15 s was used to ensure 2 min of steady-state data for the PEI period because cessation of handgrip exercise causes a robust and transient decrease in blood pressure and MSNA. All variables were continuously recorded for 2 min at baseline, during the last 2 min of PEI, and for a 2-min recovery period that started 1 min after deflation of the arm cuff.
Data Analysis
Mean arterial pressure (MAP) was calculated as diastolic pressure plus one-third pulse pressure. MSNA bursts were identified from the mean voltage neurogram using a customized computer program employing fixed criteria, which accounted for the latency from the R wave of the ECG to the sympathetic burst (13) and incorporated a signal-to-noise ratio of at least 3:1 (18). Computer-identified bursts were subsequently evaluated and confirmed by an experienced investigator. The burst with the largest amplitude during the rest period was allocated a value of 1,000 (arbitrary units), and then all bursts within a trial were normalized with respect to this standard in each subject. MSNA burst frequency (in bursts/min), burst incidence (in bursts/100 heartbeats), and total activity (i.e., burst frequency × mean burst area) were calculated.
Off-line analyses of brachial artery diameters and velocities were performed using a custom-designed edge-detection and wall-tracking software (MatLab; MathWorks, Natick, MA). This software was validated against Brachial Analyzer (Medical Imaging Applications, Coralville, IA), a commercially available and commonly used program. In brief, the software allows the user to identify a region of interest on the portion of the image where the vessel walls are most clear. The arterial wall borders are then detected by determining the point at which the pixel density changes most rapidly. Each region of interest contains ∼100 diameter measures per frame, the average of which is calculated. Instantaneous mean velocity was measured throughout all cardiac cycles. Mean velocity was estimated from all of the Doppler shifts measured across the cross section of the vessel using a large sample volume. The software permits outputting time-average mean blood velocity, antegrade mean velocity, and retrograde mean velocity per cardiac cycle. Diameter and velocity measures obtained were synchronized and used to estimate brachial artery shear rate. Shear rate (in s−1) was defined as 4 × Vm/D, where Vm is mean blood velocity (in cm/s) and D is arterial diameter (in cm) (22, 26). For calculations of antegrade and retrograde shear rate, antegrade and retrograde mean blood velocities were used, respectively. Oscillatory shear index (OSI) is a dimensionless parameter that can be used as an indicator of the magnitude of oscillation and can be defined as follows: |retrograde shear|/(|antegrade shear| + |retrograde shear|) (22, 37). Note that the values for OSI range from 0 to 0.5, where a value of 0 corresponds to a unidirectional shear rate throughout the cardiac cycle, whereas a value of 0.5 represents pure oscillation with a time-average shear rate equal to zero. Forearm vascular resistance (in mmHg·ml−1·min−1) was calculated as the ratio between MAP and blood flow (Vm·Π·D2/4·60). All baseline and recovery data were averaged over the course of a 2-min period, LBNP data were averaged every 2 min according to the stages, and CPT and PEI data were averaged every minute.
Statistical Analysis
A one-way repeated-measures ANOVA was used to evaluate the time course of all variables of interest. When a significant time effect was found, Dunnet's post hoc procedure was used to identify what time points deviated from baseline. All data are presented as means ± SE. For all statistical tests, the α-level was set at 0.05. Statistical analyses were performed with SPSS v. 17.0. (SPSS, Chicago, IL).
RESULTS
Lower Body Negative Pressure
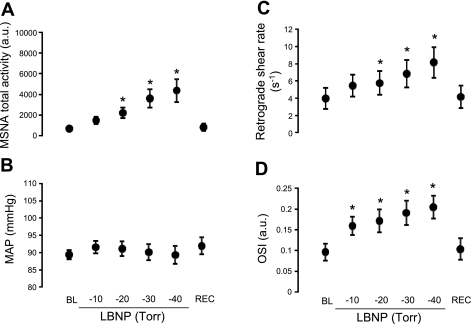
Figure 1A illustrates original records from one subject at baseline and during LBNP. Table 1 and Fig. 2 summarize the MSNA and hemodynamic responses to LBNP. Progressive increases in MSNA burst frequency, burst incidence, and total activity were evoked during graded LBNP (P < 0.0001, Table 1 and Fig. 2A). In addition, LBNP elicited decreases in mean and antegrade blood velocity (P < 0.0001, Table 1) and increases in retrograde shear rate and OSI (P < 0.0001, Fig. 2, C and D). MAP did not change throughout the protocol (P = 0.252, Fig. 2B).
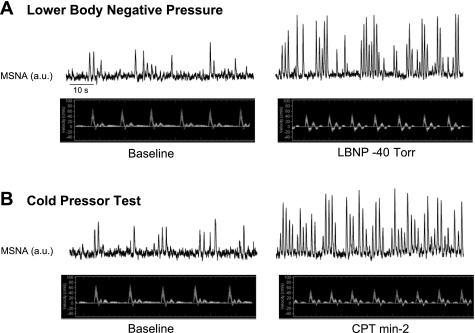
Fig. 1.
Original records from 1 representative subject illustrating the elevation in muscle sympathetic nerve activity (MSNA) and the accompanying changes in brachial artery blood velocity at baseline and during lower body negative pressure (LBNP, −40 Torr; A) and cold pressor test (CPT, minute 2; B). AU, arbitrary units.
Table 1.
MSNA and hemodynamic measurements at baseline and during LBNP and recovery
| LBNP, Torr |
|||||||
|---|---|---|---|---|---|---|---|
| Baseline | −10 | −20 | −30 | −40 | Recovery | ANOVA (F ratio; P value) | |
| MSNA burst frequency, bursts/min | 8.0 ± 2.1 | 13.2 ± 2.7 | 16.8 ± 2.7* | 22.0 ± 3.1* | 28.5 ± 4.0* | 7.6 ± 3.0 | 25.0;<0.0001 |
| MSNA bursts incidence, bursts/100 heartbeats | 14.8 ± 4.1 | 24.4 ± 5.2* | 30.2 ± 5.3* | 37.3 ± 6.1* | 42.3 ± 6.7* | 13.5 ± 5.1 | 16.4;<0.0001 |
| Heart rate, beats/min | 57 ± 3 | 55 ± 2 | 57 ± 2 | 61 ± 2* | 68 ± 3* | 57 ± 2 | 23.9;<0.0001 |
| SBP, mmHg | 125 ± 2 | 130 ± 2* | 128 ± 3 | 124 ± 3 | 121 ± 4 | 130 ± 3* | 5.6;<0.0001 |
| DBP, mmHg | 72 ± 1 | 72 ± 2 | 73 ± 2 | 73 ± 2 | 74 ± 2 | 73 ± 2 | 0.7;0.618 |
| BA diameter, mm | 4.03 ± 0.16 | 3.97 ± 0.14 | 3.97 ± 0.14 | 3.98 ± 0.15 | 3.97 ± 0.15 | 3.98 ± 0.15 | 1.5;0.190 |
| BA mean blood velocity, cm/s | 3.3 ± 0.3 | 2.0 ± 0.1* | 1.9 ± 0.1* | 1.9 ± 0.2* | 2.0 ± 0.1* | 3.1 ± 0.3 | 17.5;<0.0001 |
| BA antegrade velocity, cm/s | 3.7 ± 0.3 | 2.5 ± 0.1* | 2.5 ± 0.1* | 2.6 ± 0.1* | 2.8 ± 0.1* | 3.5 ± 0.2 | 15.0;<0.0001 |
| BA retrograde velocity, cm/s | 0.39 ± 0.12 | 0.52 ± 0.12* | 0.56 ± 0.12* | 0.67 ± 0.14* | 0.79 ± 0.16* | 0.40 ± 0.13 | 12.4;<0.0001 |
Values are means ± SE. MSNA, muscle sympathetic nerve activity; LBNP, lower body negative pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; BA, brachial artery.
Significantly different from baseline.
Fig. 2.
Summary data showing MSNA (A), mean arterial pressure (MAP; B), retrograde shear rate (C), and oscillatory shear index (OSI; D) at baseline (BL), during LBNP, and during recovery (Rec). *Significantly different from baseline; MSNA total activity (F = 11.45; P < 0.0001), MAP (F = 1.36; P = 0.252), retrograde shear rate (F = 10.8; P < 0.0001), and OSI (F = 14.0; P < 0.0001).
Cold Pressor Test
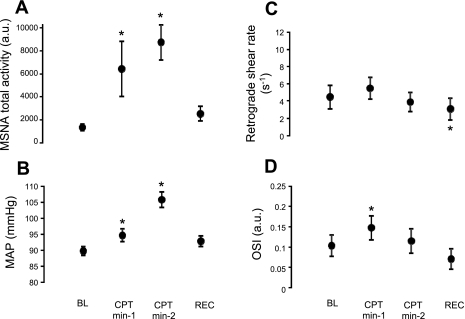
Figure 1B illustrates original records from one subject at baseline and during CPT. Table 2 and Fig. 3 summarize the MSNA and hemodynamic responses to CPT. CPT elicited increases in MSNA burst frequency, burst incidence, and total activity (P < 0.0001, Table 2 and Fig. 3A) and decreases in mean and antegrade blood velocity (P < 0.0001, Table 2). Retrograde shear was unaltered during CPT, and OSI was only increased (P = 0.001) at minute 1 (Fig. 3, C and D). MAP was modestly but significantly increased during minute 1 of CPT (Δ = 5 ± 1 mmHg), whereas during minute 2, MAP was markedly elevated (Δ = 16 ± 2 mmHg, P < 0.0001, Fig. 3B).
Table 2.
MSNA and hemodynamic measurements at baseline and during CPT and recovery
| Baseline | CPT, minute 1 | CPT, minute 2 | Recovery | ANOVA (F ratio; P value) | |
|---|---|---|---|---|---|
| MSNA burst frequency, bursts/min | 7.6 ± 1.6 | 20.5 ± 3.9* | 31.7 ± 4.7* | 11.8 ± 3.3 | 27.4;<0.0001 |
| MSNA bursts incidence, bursts/100 heartbeats | 13.4 ± 2.8 | 30.1 ± 5.8* | 48.1 ± 6.6* | 20.4 ± 5.5 | 20.1;<0.0001 |
| Heart rate, beats/min | 59 ± 2 | 70 ± 2* | 65 ± 2* | 58 ± 2 | 20.8;<0.0001 |
| SBP, mmHg | 125 ± 2 | 131 ± 3* | 145 ± 4* | 130 ± 2 | 23.7;<0.0001 |
| DBP, mmHg | 72 ± 1 | 77 ± 2* | 86 ± 2* | 74 ± 2 | 50.2;<0.0001 |
| BA diameter, mm | 3.93 ± 0.17 | 3.90 ± 0.17 | 3.81 ± 0.16* | 3.85 ± 0.16 | 2.9;0.049 |
| BA mean blood velocity, cm/s | 3.2 ± 0.3 | 2.5 ± 0.2* | 2.5 ± 0.2* | 3.5 ± 0.4 | 8.8;<0.0001 |
| BA antegrade velocity, cm/s | 3.6 ± 0.2 | 3.1 ± 0.2* | 2.8 ± 0.2* | 3.8 ± 0.3 | 8.5;<0.0001 |
| BA retrograde velocity, cm/s | 0.42 ± 0.13 | 0.53 ± 0.13 | 0.35 ± 0.10 | 0.28 ± 0.11* | 6.6;0.001 |
Values are means ± SE. CPT, cold pressor test.
Significantly different from baseline.
Fig. 3.
Summary data showing MSNA (A), MAP (B), retrograde shear rate (C), and OSI (D) at baseline, during CPT, and during recovery. *Significantly different from baseline; MSNA total activity (F = 9.2; P < 0.0001), MAP (F = 41.8; P < 0.0001), retrograde shear rate (F = 5.0; P = 0.005), and OSI (F = 7.0; P = 0.001).
Postexercise Ischemia
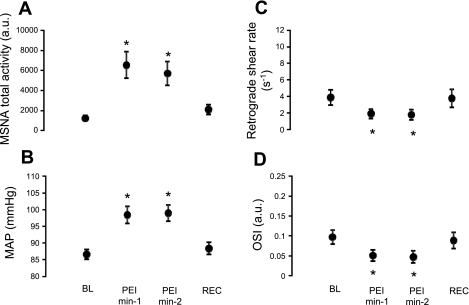
Table 3 and Fig. 4 summarize the MSNA and hemodynamic responses to PEI. PEI induced increases in MSNA burst frequency, burst incidence, and total activity (P < 0.0001, Table 3 and Fig. 4A) but had no significant effects on mean or antegrade blood velocity (Table 3). Retrograde shear and OSI were reduced during both minutes of exposure (P < 0.0001, Fig. 4, C and D). Similar to that of minute 2 of CPT, MAP was greatly and constantly elevated throughout PEI (Δ = 12 ± 2 mmHg, P < 0.0001, Fig. 4B).
Table 3.
MSNA and hemodynamic measurements at baseline and during PEI and recovery
| Baseline | PEI, minute 1 | PEI, minute 2 | Recovery | ANOVA (F ratio; P value) | |
|---|---|---|---|---|---|
| MSNA burst frequency, bursts/min | 7.3 ± 1.6 | 20.9 ± 2.2* | 20.5 ± 2.5* | 10.8 ± 2.7* | 37.4;<0.0001 |
| MSNA bursts incidence, bursts/100 heartbeats | 12.9 ± 2.8 | 34.8 ± 4.2* | 33.7 ± 4.1* | 20.2 ± 5.5* | 24.3;<0.0001 |
| Heart rate, beats/min | 58 ± 2 | 60 ± 2* | 61 ± 2* | 57 ± 2 | 3.8;0.018 |
| SBP, mmHg | 121 ± 2 | 138 ± 4* | 139 ± 4* | 126 ± 3 | 31.0;<0.0001 |
| DBP, mmHg | 70 ± 1 | 79 ± 2* | 79 ± 2* | 70 ± 2 | 35.7;<0.0001 |
| BA diameter, mm | 4.01 ± 0.15 | 3.96 ± 0.14 | 3.92 ± 0.14* | 3.95 ± 0.16* | 5.4;0.003 |
| BA mean blood velocity, cm/s | 3.1 ± 0.2 | 3.5 ± 0.2 | 3.5 ± 0.3 | 3.2 ± 0.2 | 2.1;0.111 |
| BA antegrade velocity, cm/s | 3.5 ± 0.2 | 3.7 ± 0.2 | 3.7 ± 0.2 | 3.6 ± 0.2 | 0.6;0.629 |
| BA retrograde velocity, cm/s | 0.37 ± 0.08 | 0.18 ± 0.05* | 0.17 ± 0.06* | 0.35 ± 0.10 | 10.2;<0.0001 |
Values are means ± SE. PEI, postexercise ischemia.
Significantly different from baseline.
Fig. 4.
Summary data showing MSNA (A), MAP (B), retrograde shear rate (C), and OSI (D) at baseline, during postexercise ischemia (PEI), and during recovery. *Significantly different from baseline; MSNA total activity (F = 13.6; P < 0.0001), MAP (F = 35.6; P < 0.0001), retrograde shear rate (F = 9.3; P < 0.0001), and OSI (F = 10.8; P < 0.0001).
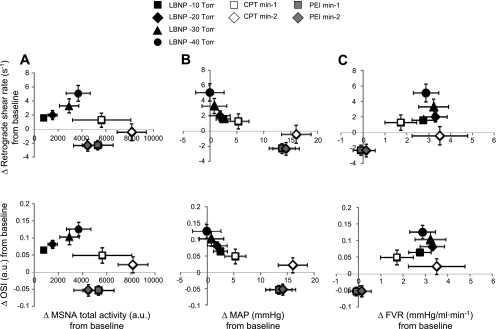
Figure 5 illustrates group changes in MSNA (Fig. 5A), MAP (Fig. 5B), and forearm vascular resistance (Fig. 5C) across all experimental maneuvers and their associations with alterations in retrograde shear rate (Fig. 5, top) and OSI (Fig. 5, bottom). As shown, when elevations in MSNA were accompanied by increases in MAP (i.e., CPT and PEI), MSNA-induced increases in retrograde shear and OSI were minimized, compared with when MSNA was elevated without concomitant changes in blood pressure (i.e., LBNP).
Fig. 5.
Group changes in MSNA (A), MAP (B), and forearm vascular resistance (FVR; C) across all experimental maneuvers and their associations with alterations in retrograde shear rate (top) and OSI (bottom) (n = 10).
DISCUSSION
The main novel finding of the present study is that acute increases in MSNA are associated with greater conduit artery retrograde and oscillatory shear. We also provide evidence that this effect of MSNA on shear patterns may be influenced by concomitant elevations in blood pressure. Indeed, in conditions in which increased MSNA was associated with significant elevations in blood pressure, a lesser augmentation in retrograde and oscillatory shear was observed.
It is becoming increasingly accepted that the vascular endothelium has the capacity to discern between different hemodynamic environments. From cell culture to in vivo human studies, extensive evidence indicates that disturbed flow patterns induce a proatherogenic endothelial cell phenotype (4a, 7, 9, 15, 17, 19, 23, 33, 38); however, an understanding of the factors contributing to such oscillatory shear profiles in peripheral conduit arteries remains incomplete. The rationale for considering elevated MSNA as a potential candidate stemmed from previous reports proposing that increased peripheral vascular resistance may influence flow profiles (2, 29). Indeed, the well-characterized observation that conduit artery blood flow is entirely antegrade during reactive hyperemia (induced by transient occlusion) suggests that retrograde flow can be abolished when selectively lowering downstream vascular resistance (29). Reciprocally, the inflation of a pressure cuff on the forearm results in greater retrograde flow at the brachial artery (2, 33), again indicating that changes in vascular resistance may contribute to differences in flow patterns. These observations led to the recent, but until now untested, hypothesis that sympathetic nerve activity may be a factor modulating conduit artery blood flow profiles (11). The present investigation provides the first evidence that elevated MSNA may indeed contribute to alterations in conduit artery shear rate patterns in that, during LBNP, graded increases in MSNA resulted in progressive increases in retrograde and oscillatory shear.
The finding that increased arterial blood pressure may counteract the effect of MSNA on shear patterns also deserves attention. During LBNP, when MAP was slightly elevated, lesser increments in retrograde and oscillatory shear were observed (Fig. 5B). Furthermore, during PEI, large increases in blood pressure were actually associated with a reduction of retrograde and oscillatory shear below baseline values. Together, the greatest augmentation in retrograde and oscillatory shear occurred when blood pressure was unchanged, and as arterial pressure increased, the changes in retrograde and oscillatory shear were gradually abolished. In this regard, the present study is not the first to observe and report a link between blood pressure and retrograde flow. Chilian and Marcus (8) experimentally altered coronary perfusion pressure in dogs and observed an attenuation in flow reversal with increasing aortic pressure.
The mechanism by which increased arterial blood pressure attenuates flow reversal may be related to changes in the pressure gradient within the artery. This concept that the pressure gradient can impact the nature of the oscillatory flow pattern in peripheral conduit arteries was eloquently described in the mid-1900s by McDonald (20). Using instrumented dogs, McDonald (20) measured the pressure gradient by simultaneously recording pressure at two points (a short distance apart) along the femoral artery. It was demonstrated that the presence of retrograde flow within each cardiac cycle could be predicted by the occurrence of a transient period of a negative pressure gradient between the upstream and downstream points. The current results may indeed be explained by this classic work. Our rationale was that increased MSNA would lead to increases in downstream vascular resistance, thus enhancing retrograde flow as previously suggested (2). While this was noted in situations in which arterial blood pressure remained unchanged, when blood pressure was concomitantly increased with MSNA, this may have altered the pressure gradient to one that favored decreases in retrograde flow. Although blood pressure appeared to be, at least circumstantially, associated with changes in shear patterns, we recognize that there may be other factors contributing to flow pattern changes. In this regard, although Fig. 5C suggests that part of the influence of MSNA on retrograde and oscillatory shear is dependent on changes in forearm vascular resistance, clearly other factors might play a role. Potential candidates include stroke volume, heart rate, compliance (capacitance) of the vessel, varying reflex pathways involved, and redistribution of regional blood flow.
Our finding that increased blood pressure neutralizes the effect of MSNA on shear patterns may also assist in the interpretation of current data in the literature. Physical exercise is a physiological scenario during which sympathetic outflow is increased in the inactive muscle beds (1). In this regard, the findings of the present study support the observations by Green et al. (16) and Thijssen et al. (32) that, during lower limb exercise, brachial artery retrograde and oscillatory shear in the resting arm are augmented. In a timely follow-up study (34), the same authors compared femoral retrograde shear during arm-crank exercise between spinal cord injury patients and matched controls. Based on the lack of a group difference, it was concluded that the sympathetic nervous system does not play a role in promoting retrograde shear in the inactive regions. While this was the logical interpretation of the results at that time, our finding that increased blood pressure offsets the impact of MSNA on retrograde shear may also offer new insights into the data of Thijssen et al. (34). Interestingly, the control subjects, but not the patients with spinal cord injury, exhibited an increase in blood pressure during the upper-body exercise bout. Thus it is possible that this increase in blood pressure masked the influence of MSNA on retrograde shear in the control group.
Furthermore, it should be noted that flow disturbance may not always represent a detrimental stimulus. While it is already well established that chronic exposure to oscillatory and retrograde shear is a deleterious signal to the vasculature (5, 6), it is possible that repeated episodes of disturbed flow, as it occurs in the inactive limb during exercise (16, 32), may provide favorable vascular adaptations over time; however, this interesting hypothesis has not been directly tested.
The differential effect of these sympathetic maneuvers on arterial diameter warrants consideration. CPT and PEI resulted in reductions in diameter, whereas LBNP did not. Because mean shear rate (data not included) was reduced during both CPT and LBNP maneuvers (but not PEI), it is unlikely that the reduction of shear-induced constriction was the main contributor to the reductions in diameter during CPT and PEI. Instead, it is possible that the increase in blood pressure, which occurred during CPT and PEI, but not LBNP, resulted in the reduction in arterial diameters through a myogenic response. Alternatively, it is also possible that MSNA had a direct constrictive effect on the conduit vessel and that this was only apparent in the maneuvers which elicited the greatest increases in MSNA total activity (CPT and PEI).
Potential limitations in the design and interpretation of the present investigation should be considered. First, our study is correlational, and therefore we cannot be conclusive that an increased MSNA caused greater retrograde and oscillatory shear. To definitively establish this link, future experiments should determine whether the relationship between MSNA and flow disturbance is abolished in the presence of adrenergic blockade. Second, because the Doppler ultrasound sampling rate was set at 5 frames/s, we are unable to explore to what extent changes in arterial diameter across the cardiac cycle could impact the estimates of antegrade and retrograde shear. Future studies using a higher sampling rate should address this issue.
Albeit these limitations, the clinical significance of the present findings is notable. Chronically elevated sympathetic nerve activity is a hallmark characteristic of several cardiovascular diseases (14). Elucidating the mechanisms responsible for the detrimental effect of sympathetic overactivity on the vasculature is a particular area of intense investigation. Importantly, there is evidence to suggest that sympathetic overactivity can have a deleterious effect on the vasculature that is independent of an increase in blood pressure (3). Because oscillatory flow patterns induce an endothelial perturbation (4a, 7, 9, 15, 17, 19, 23, 38), the vascular dysfunction in peripheral conduit arteries of patients with excessive sympathetic drive may be, at least in part, mediated by alterations in the shear profiles. Future studies examining whether individuals with persistently elevated sympathetic nerve activity exhibit enhanced oscillatory flow profiles are clearly warranted. In addition, since only young healthy men were included in the present study, further research is needed to determine the potential influence of age and sex on the MSNA-shear relationship.
In summary, acute increases in MSNA are associated with greater retrograde and oscillatory shear at the brachial artery of healthy adults; however, these effects may be influenced by simultaneous increases in blood pressure. This is the first study providing evidence of a relationship between elevated sympathetic outflow and a proatherogenic shear environment.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants RO1-HL-093167 (to P. J. Fadel) and PO1-HL-52490 (to M. H. Laughlin).
DISCLOSURES
None.
ACKNOWLEDGMENTS
We thank all the subjects for the time, effort, and willingness to participate in the study.
REFERENCES
- 1.American College of Sports Medicine ACSM's Advanced Exercise Physiology Baltimore: Lippincott Williams and Wilkins, 2006 [Google Scholar]
- 2.Baccelli G, Pignoli P, Corbellini E, Pizzolati PL, Bassini M, Longo T, Zanchetti A. Hemodynamic factors changing blood flow velocity waveform and profile in normal human brachial artery. Angiology 36: 1–8, 1985 [DOI] [PubMed] [Google Scholar]
- 3.Bevan RD. Trophic effects of peripheral adrenergic nerves on vascular structure. Hypertension 6: III19–III26, 1984 [DOI] [PubMed] [Google Scholar]
- 4.Blackshear WM, Phillips DJ, Strandness DE. Pulsed Doppler assessment of normal human femoral artery velocity patterns. J Surg Res 27: 73–83, 1979 [DOI] [PubMed] [Google Scholar]
- 4a.Chatzizisis YS, Coskun AU, Jonas M, Edelman ER, Feldman CL, Stone PH. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: molecular, cellular, and vascular behavior. J Am Coll Cardiol 49: 2379–2393, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Cheng C, Crom R, Haperen R, Helderman F, Gourabi BM, Damme LC, Kirschbaum SW, Slager CJ, Steen AF, Krams R. The role of shear stress in atherosclerosis. Cell Biochem Biophys 41: 279–294, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Cheng C, Tempel D, van Haperen R, van der Baan A, Grosveld F, Daemen MJ, Krams R, de Crom R. Atherosclerotic lesion size and vulnerability are determined by patterns of fluid shear stress. Circulation 113: 2744–2753, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Chien S. Effects of disturbed flow on endothelial cells. Ann Biomed Eng 36: 554–562, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chilian WM, Marcus ML. Effects of coronary and extravascular pressure on intramyocardial and epicardial blood velocity. Am J Physiol Heart Circ Physiol 248: H170–H178, 1985 [DOI] [PubMed] [Google Scholar]
- 9.Conway DE, Williams MR, Eskin SG, McIntire LV. Endothelial cell responses to atheroprone flow are driven by two separate flow components, low time-average shear stress and fluid flow reversal. Am J Physiol Heart Circ Physiol 298: H367–H374, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corti R, Fuster V, Badimon JJ, Hutter R, Fayad ZA. New understanding of atherosclerosis (clinically and experimentally) with evolving MRI technology in vivo. Ann NY Acad Sci 947: 181–195, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Credeur DP, Bobrosielski DA, Arce-Esquivel AA, Welsch MA. Brachial artery retrograde flow increases with age: relationship to physical function. Eur J Appl Physiol 107: 219–225, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fadel PJ, Ogoh S, Watenpaugh DE, Wasmund W, Olivencia-Yurvati AS, Smith ML, Raven PB. Carotid baroreflex regulation of sympathetic nerve activity during dynamic exercise in humans. Am J Physiol Heart Circ Physiol 280: H1383–H1390, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Fagius J, Wallin BG. Sympathetic reflex latencies and conduction velocities in normal man. J Neurol Sci 47: 433–448, 1980 [DOI] [PubMed] [Google Scholar]
- 14.Fisher JP, Young CN, Fadel PJ. Central sympathetic overactivity: maladies and mechanisms. Auton Neurosci 148: 5–15, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gambillara V, Chambaz C, Montorzi G, Roy S, Stergiopulos N, Silacci P. Plaque-prone hemodynamics impair endothelial function in pig carotid arteries. Am J Physiol Heart Circ Physiol 290: H2320–H2328, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Green DJ, Bilsborough W, Naylor LH, Reed C, Wright J, O'Driscoll JG, Walsh JH. Comparison of forearm blood flow responses to incremental handgrip and cycle ergometer exercise: relative contribution of nitric oxide. J Physiol 562: 617–628, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hahn C, Schwartz MA. Mechanotransduction in vascular physiology and atherogenesis. Nat Rev Mol Cell Biol 10: 53–62, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamner JW, Taylor JA. Automated quantification of sympathetic beat-by-beat activity, independent of signal quality. J Appl Physiol 91: 1199–1206, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Hastings NE, Simmers MB, McDonald OG, Wamhoff BR, Blackman BR. Atherosclerosis-prone hemodynamics differentially regulates endothelial and smooth muscle cell phenotypes and promotes pro-inflammatory priming. Am J Physiol Cell Physiol 293: C1824–C1833, 2007 [DOI] [PubMed] [Google Scholar]
- 20.McDonald DA. The relation of pulsatile pressure to flow in arteries. J Physiol 127: 533–552, 1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Newcomer SC, Sauder CL, Kuipers NT, Laughlin MH, Ray CA. Effects of posture on shear rates in human brachial and superficial femoral arteries. Am J Physiol Heart Circ Physiol 294: H1833–H1839, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Keeffe LM, Muir G, Piterina AV, McGloughlin T. Vascular cell adhesion molecule-1 expression in endothelial cells exposed to physiological coronary wall shear stresses. J Biomech Eng 131: 081003, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Ogoh S, Fisher JP, Raven PB, Fadel PJ. Arterial baroreflex control of muscle sympathetic nerve activity in the transition from rest to steady-state dynamic exercise in humans. Am J Physiol Heart Circ Physiol 293: H2202–H2209, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Padilla J, Johnson BD, Newcomer SC, Wilhite DP, Mickleborough TD, Fly AD, Mather KJ, Wallace JP. Adjusting flow-mediated dilation for shear stress stimulus allows demonstration of endothelial dysfunction in a population with moderate cardiovascular risk. J Vasc Res 46: 592–600, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Padilla J, Sheldon RD, Sitar DM, Newcomer SC. Impact of acute exposure to increased hydrostatic pressure and reduced shear rate on conduit artery endothelial function: a limb specific response. Am J Physiol Heart Circ Physiol 297: H1103–H1108, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Ray CA, Rea RF, Clary MP, Mark AL. Muscle sympathetic nerve responses to static leg exercise. J Appl Physiol 73: 1523–1529, 1992 [DOI] [PubMed] [Google Scholar]
- 28.Rea RF, Wallin BG. Sympathetic nerve activity in arm and leg muscles during lower body negative pressure in humans. J Appl Physiol 66: 2778–2781, 1989 [DOI] [PubMed] [Google Scholar]
- 29.Risoe C, Wille SO. Blood velocity in human arteries measured by a bidirectional ultrasonic Doppler flowmeter. Acta Physiol Scand 103: 370–378, 1978 [DOI] [PubMed] [Google Scholar]
- 30.Ross R. Atherosclerosis. An inflammatory disease. N Engl J Med 340: 115–128, 1999 [DOI] [PubMed] [Google Scholar]
- 31.Stone PH, Coskun AU, Yeghiazarians Y, Kinlay S, Popma JJ, Kuntz RE, Feldman CL. Prediction of sites of coronary atherosclerosis progression: in vivo profiling of endothelial shear stress, lumen, and outer vessel wall characteristics to predict vascular behavior. Curr Opin Cardiol 18: 458–470, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Thijssen DH, Dawson EA, Black MA, Hopman MT, Cable NT, Green DJ. Brachial artery blood flow responses to different modalities of lower limb exercise. Med Sci Sports Exerc 41: 1072–1079, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Thijssen DH, Dawson EA, Tinken TM, Cable NT, Green DJ. Retrograde flow and shear rate acutely impair endothelial function in humans. Hypertension 53: 986–992, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Thijssen DH, Green DJ, Steendijk S, Hopman MT. Sympathetic vasomotor control does not explain the change in femoral artery shear rate pattern during arm-crank exercise. Am J Physiol Heart Circ Physiol 296: H180–H185, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Wallin BG, Victor RG, Mark AL. Sympathetic outflow to resting muscles during static handgrip and postcontraction muscle ischemia. Am J Physiol Heart Circ Physiol 256: H105–H110, 1989 [DOI] [PubMed] [Google Scholar]
- 36.Wissler WR, Strong JP. Risk factors and progression of atherosclerosis in youth. PDAY Research Group. Pathological Determinants of Atherosclerosis in Youth. Am J Pathol 153: 1023–1033, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu SP, Ringgaard S, Oyre S, Hansen MS, Rasmus S, Pedersen EM. Wall shear rates differ between the normal carotid, femoral, and brachial arteries: an in vivo MRI study. J Magn Reson Imaging 19: 188–193, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Ziegler T, Bouzourene K, Harrison VJ, Brunner HR, Hayoz D. Influence of oscillatory and unidirectional flow environments on the expression of endothelin and nitric oxide synthase in cultured endothelial cells. Arterioscler Thromb Vasc Biol 18: 686–692, 1998 [DOI] [PubMed] [Google Scholar]