Abstract
Although bradykinin (BK) is known to exert effects on the myocardium, its intracellular signaling pathways remain poorly understood. Experiments in other cell types indicated that p21-activated kinase-1 (Pak1), a Ser/Thr kinase downstream of small monomeric G proteins, is activated by BK. We previously reported that the expression of active Pak1 in adult cardiac myocytes induced activation of protein phosphatase 2A and dephosphorylation of myofilament proteins (Ke et al. Circ Res 94: 194–200, 2004). In experiments reported here, we tested the hypothesis that BK signals altered protein phosphorylation in adult rat cardiac myocytes through the activation and translocation of Pak1. Treatment of myocytes with BK resulted in the activation of Pak1 as demonstrated by increased autophosphorylation at Thr423 and a diminished striated localization, which is present in the basal state. BK induced dephosphorylation of both cardiac troponin I and phospholamban. Treatment of isolated myocytes with BK also blunted the effect of isoproterenol to enhance peak Ca2+ and relaxation of Ca2+ transients. Protein phosphatase 2A was demonstrated to associate with both Pak 1 and phospholamban. Our studies indicate a novel signaling mechanism for BK in adult rat cardiac myocytes and support our hypothesis that Pak 1 is a significant regulator of phosphatase activity in the heart.
Keywords: cardiac troponin I, phospholamban, dephosphorylation
bradykinin (BK) plays an important role in pre- and postconditioning and in cardiac protection during ischemia-reperfusion (3, 10, 14). BK has been demonstrated in the heart or cardiomyocytes to inhibit apoptosis (49), to activate ATP-sensitive K+ channels, and to induce the generation of nitric oxide and reactive oxygen species (8, 11). The key link between BK and changes in these cardioprotective effectors through signaling molecules and protein phosphorylation and the subsequent functional changes is poorly understood. One potential pathway to mediate BK signaling is through the p21-activated kinase-1 (Pak1). Pak1 is a member of a highly conserved family of Ser/Thr protein kinases that are downstream effectors of the small G proteins, Cdc42, and Rac1 (32). Constitutively active Pak1 induces cytoskeletal reorganization including the loss of stress fibers and the dissolution of focal adhesion complexes (31). In cardiac myocytes, we reported that constitutively active Pak1 induced the dephosphorylation of cardiac troponin I (cTnI) through the activation of protein phosphatase 2A (PP2A) (22). In myocytes expressing constitutively active Pak1, the Ca2+ sensitivity of myofilament tension increased as expected with the dephosphorylation of cTnI (22). Pak1 expression also blunted the sarcoplasmic reticulum (SR) Ca2+ release in the presence of isoproterenol (Iso) with no coincident loss of caffeine-releasable Ca2+, indicating that the action was on the release mechanism (42). Pak1 inhibited L-type Ca2+ channels and delayed rectifier K+ channel activities in the presence of Iso in sinoatrial nodal cells (20). Pak1 may also mediate a cardioprotective effect by FTY720 in isolated rat heart subjected to ischemia and reperfusion (12).
Although there is evidence that Pak1 might be a downstream effector of BK (25) in nonmuscle cells, there is no direct evidence that Pak1 is activated by BK in cardiac myocytes. BK is a peptide, which acts in a paracrine and autocrine manner as the major signaling factor in the kalikrein kinin system (3). BK acts in various systems including alterations in function of heart (9), kidney (40), and blood vessels (33, 36), where it binds to B2 kinase receptor, a G protein-coupled receptor (35). In patients with essential hypertension, treatment with angiotensin-converting enzyme inhibitors increased the plasma BK level associated with reduced blood pressure (24). Thus BK is significant clinically, though the intracellular mechanisms of its function in cardiac myocytes remain unclear. In experiments reported here, we show evidence that the treatment of cardiac myocytes with BK induces an activation of Pak1 that is associated with the dephosphorylation of cTnI, phospholamban (PLB), and slowed the relaxation of Ca2+ transients in cardiac myocytes stimulated with Iso.
MATERIALS AND METHODS
Bradykinin.
All experiments employed BK1–9 (B-3259, Arg-Pro-Pro-Gly-Phe-Ser-Pro-Phe-Arg), which was purchased from Sigma (St. Louis, MO).
Isolation and culture of Ca2+-tolerant cardiac ventricular myocytes.
Cardiac ventricular myocytes were isolated from the hearts of adult male rats, 2 to 3 mo old, using a collagenase perfusion method as previously described (14). All investigations conformed to the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health (NIH Publication No. 85-23, Revised 1996) and were approved by the Institutional Animal Care and Use Committee of the University of Illinois at Chicago. Two hours before plating the myocytes, the culture dishes were coated with laminin at 10 μg/ml. The cells were first cultured for 2 h in DMEM (D6421, Sigma) plus 10% FBS for cell attachment. The medium was then changed to serum-free DMEM, and the cells were cultured overnight. The cultured ventricular myocytes were treated with vehicle or BK at 0, 0.1, 1, and 10 μM for 10 min before the preparation of the samples.
AdPak1 construction, viral amplification, and plaque assays.
The preparation of recombinant adenovirus that expresses constitutively active Pak1 (AdPak1) was described previously (22). The viral titer (in plaque forming units/ml) and the multiplicity of infection were determined by standard techniques (2).
Metabolic labeling of cardiac myocytes with [32P].
The levels of protein phosphorylation in myocyte preparations were determined using a protocol modified from that described by Fentzke et al. (13). The freshly isolated adult rat cardiac myocytes were allowed to settle onto 35-mm tissue culture plates coated with laminin as described in Isolation and culture of Ca2+-tolerant cardiac ventricular myocytes and were cultured for 2 h in DMEM with 10% FBS. Viral infection (multiplicity of infection of 100) was carried out when the media was exchanged for serum free. The cells were infected with AdPak1 or AdLacZ for 8 h, in which time the level of transgene expression was substantial, and DMEM was replaced with Na-HEPES phosphate-free buffer containing (in mM) 1.0 CaCl2, 4.8 KCl, 1.2 MgSO4, 132 NaCl, 10 HEPES, 2.5 Na-pyruvate, and 10 glucose (pH 7.4) with 0.1 mCi [32P]orthophosphate for 30 min at room temperature. At 30 min of incubation in buffer containing [32P]orthophosphate, Iso or BK was added and was incubated for 2 min more. The cells were then washed twice with the Na-HEPES solution with 1 mM CaCl2. Two minutes after adding vehicle, Iso, or Iso + BK, we added an equal volume of SDS-stop solution containing (in mM) 1 DTT, 30 Tris·HCl, and 3 EDTA with 6% SDS, 15% glycerol, and a trace of bromophenol blue. An aliquot of cells containing 50 μg protein, as determined using the Lowry method, was analyzed by SDS-PAGE. The samples were first boiled for 10 min, and PAGE was performed using either 12% or a linear 5–20% polyacrylamide gradient gel as previously described (13).
Western blot analysis and immunoprecipitation.
To prepare cell lysates for Western blot analysis, cultured adult cardiac myocytes were incubated with a modified radioimmunoprecipitation assay buffer containing 20 mM Tris·HCl (pH 7.4), 0.5% deoxycholate, 0.1% SDS, and 0.1% Triton X-100. Cellular and nuclear debris were removed by centrifugation at 1,200 g for 15 min, and the pellet was resuspended in 50 μl of lysis buffer and 50 μl of sample buffer containing 37% deionized water, 13% 0.5 M Tris·HCl (pH 6.8), 26% glycerol, 21–10% sodium dodecylsulfate, and 2–0.5% (wt/vol) bromophenol blue. For immunoprecipitation, the same procedures were used as described previously (22).
Primary antibodies used included the following: Pak1 (Cell Signaling Technology, Hitchin, UK; and SC-881, Santa Cruz), PLB (No. 05-205, Upstate) and phospho-PLB (No. 07-052, Upstate), and polyclonal antibody against residues surrounding Thr423 of endogenous PAK1 protein raised in rabbit (Cell Signaling Technology).
Labeling of proteins for immunofluorescence.
For immunolabeling, coverslips containing myocytes were washed twice with PBS, and the cells were fixed with 2% paraformaldehyde. The cells were washed twice (5 min) with PBS containing 0.25% NH4Cl, 0.01% saponin, and 0.02% NaN3. The cells were then coated with PBS containing 0.5% BSA, 0.01% saponin, and 0.02% NaN3. The primary antibody was added in coating reagent and incubated for 30 min. The cells were washed three times in PBS containing 0.05% saponin and 0.02% NaN3 and incubated in secondary antibody for 15 min in PBS containing 0.5% BSA, 0.01% saponin, and 0.02% NaN3 and then washed three times (5 min) with PBS containing 0.01% saponin and 0.02% NaN3. Anti-Pak1 (α-Pak) polyclonal antibody of rabbit origin was from Santa Cruz Biotechnology (Cat No. SC-881). For immunofluorescence studies, we used a 1:50 dilution, and for Western blot analysis, a 1:200 dilution. The secondary antibody (1:200 dilution) was fluorescein isothiocyanate-conjugated anti-rabbit IgG of goat origin (Sigma, Cat No. F-9887). Rhodamine-conjugated phalloidin was purchased from Sigma (Cat No. 1951). Images were acquired using a Bio-Rad laser scanning confocal microscope Radiance 2000 equipped with a ×60 water immersion objective. A 488- and a 568-nm beam from an argon-krypton laser was used for excitation. The detection of the emissions from green and red fluorescence was through HQ515/30 and HQ590/70 filters, respectively. The collected images were processed using LaserPix version 4.0 (Bio-Rad).
Intracellular Ca2+ concentration imaging.
Adult rat ventricular myocytes were loaded with the Ca2+-indicator fluo-4 by exposure for 20 min at room temperature to 1 ml standard Tyrode solution containing 10 μM fluo-4 acetoxymethyl ester (Molecular Probes, Eugene, OR) made from a DMSO stock solution. A minimum of 15 min was allowed for de-esterification of the indicator. Coverslips containing single myocytes were mounted on the stage of an inverted Nikon microscope and continuously perfused with a solution containing (in mM) 137 NaCl, 5 KCl, 10 HEPES, 1 MgCl2, and 1 CaCl2 (pH 7.4). In some experiments, 100 nM Iso or 1 μM propranolol were added to the perfusate. The indicator was excited with the 488-nm line of an argon ion laser attached to the microscope via a confocal laser-scanning unit (Radiance 2100, Bio-Rad) equipped with a ×60 water immersion objective. Emitted fluo-4 fluorescence was measured at wavelengths > 515 nm. Linescan images were recorded from a central focal plane. Intracellular Ca2+ concentration ([Ca2+]i) images were calculated according to the following formula (4): [Ca2+]i = KdR/(Kd/[Ca2+]rest − R + 1), where R is the fluorescence (F) normalized to resting (F0) fluorescence (R = F/F0) and Kd is the dissociation constant for the Ca2+ dye complex. The value of Kd for fluo-4 was taken to be 1.1 μM (43). [Ca2+]rest was taken as 100 nM (19, 29). In experiments where diastolic Ca2+ increased, F0 from the control condition was used for the calculation of R in subsequent images for each cell. The time constant of the [Ca2+]i-transient decay (τdecay) was evaluated by a monoexponential fit to the declining phase of the transient. All experiments were performed at room temperature (26–28°C).
Statistical analysis.
All data are reported as means ± SE. Repeated-measures one-way ANOVA and two-tailed t-test were used with a P value <0.05 taken as a measure of significance.
RESULTS
Altered intracellular localization and increased autophosphorylation of Pak1 at Thr423 by BK treatment of cardiac myocytes.
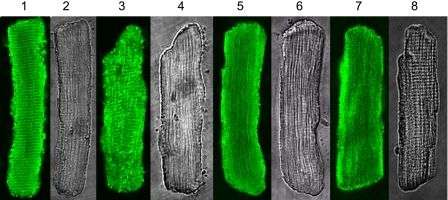
Figure 1 demonstrates the intracellular distribution of Pak1 as a function of the concentration levels of BK using immunofluorescence in fixed myocytes and confocal microscopy. Also shown is a phase-contrast image of the myocytes. In the control myocytes with no BK treatment (Fig. 1, lanes 1 and 2), endogenous Pak1 demonstrated defined intracellular localizations. As we previously reported (22), endogenous Pak1 localizes to the Z-line, intercalated disc, and cell and nuclear membranes. When the cardiac myocytes were treated with BK at low (0.1 μM; Fig. 1, lanes 3 and 4) to high concentrations (1.0 and 10 μM; Fig. 1, lanes 5 and 6 and lanes 6 and 7), the endogenous Pak1 changed its intracellular distribution. BK treatment abolished the membrane localization and striated pattern of the endogenous Pak1 with the translocation of Pak1 occurring in a concentration-dependent manner. Thus the confocal images indicate that the striated localization of Pak1 became blurred when myocytes were treated with BK at 0.1 μM and disappeared completely when myocytes were treated with BK at 1 and 10 μM. The phase-contrast images demonstrate that this was not due to an altered sarcomeric structure. We identified the same changes in Pak1 localization induced by BK in more than 20 myocytes for each treatment.
Fig. 1.
Intracellular localization of endogenous p21-activated kinase-1 (Pak1) regulated by bradykinin (BK) in rat ventricle myocytes. Lanes 1, 3, 5, and 7: confocal images with immunofluorescence signals for Pak1. Lanes 2, 4, 6, and 8: transmitted light images of the myocytes. Lanes 1 and 2: control myocytes without BK treatment. Lanes 3 and 4: myocytes treated with 0.1 μM BK. Lanes 5 and 6: myocytes treated with 1 μM BK. Lanes 7 and 8: myocytes treated with 10 μM BK. For immunofluorescence, the primary antibody was from Santa Cruz (sc-881). The secondary antibody was FITC-conjugated anti-rabbit IgG from Sigma (F-9887).
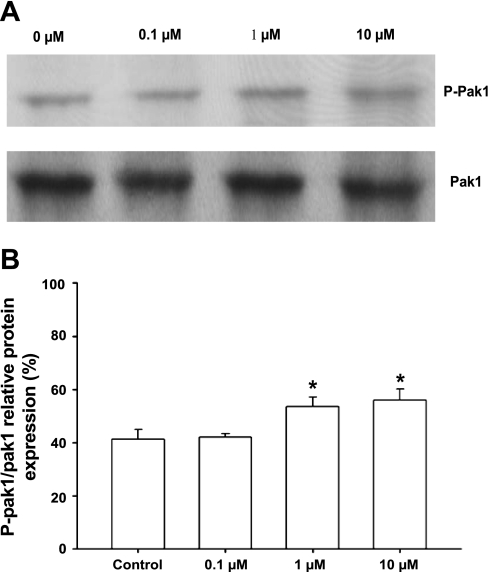
To further determine whether BK acts as an upstream agonist for Pak1 in adult rat cardiac myocytes, we measured autophosphorylated Pak1 (Fig. 2) using Western blot analysis with an antiphosphopeptide antibody specific for Thr423 (41). Thr423 is localized in the activation loop of the catalytic domain, which is the most important phosphorylation site for Pak1 activation. When the myocytes were treated with BK from 0.1 to 10 μM, Pak1 autophosphorylation increased gradually, with significant increases at 1 and 10 μM of BK treatment (Fig. 2, A and B).
Fig. 2.
A: Western blot analysis of autophosphorylation of endogenous Pak1 in myocytes treated with BK at different concentrations. A, lanes 1–4: myocytes treated with BK at 0, 0.1, 1, and 10 μM, respectively, for 10 min. Pak1 autophosphorylation was detected by phospho (p)-specific (Pak1 Thr423) antibody. Total Pak1 served as an internal control. B: histogram showing increase of Pak1 Thr423 autophosphorylation normalized to total Pak1. Results shown are representative of 3 independent experiments. p-Pak1 Thr423 levels are expressed as percentage of total Pak1. *P < 0.05 compared with control without BK exposure.
BK induced dephosphorylation of troponin I and PLB.
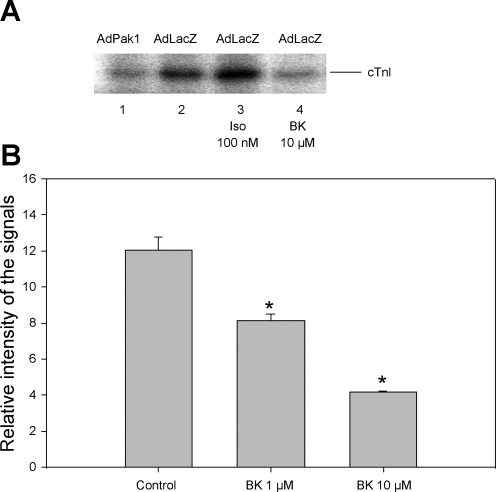
Our previous studies indicated that an adenoviral transfer of active Pak1 into adult rat cardiac myocytes induced the dephosphorylation of cTnI and myosin-binding protein C with no effect on myosin light chain phosphorylation (22). Here we tested whether an agonist-induced increase in Pak1 activity is also able to dephosphorylate cTnI. To test whether both BK and constitutively active Pak1 induce changes in the phosphorylation of cTnI, we cultured freshly isolated cardiac myocytes in DMEM plus 10% FBS for 2 h. The cells were then infected with AdPak1 or AdLacZ as control and were incubated in serum-free media overnight. The phosphorylation of cTnI was detected by metabolic labeling with [32P]orthophosphate. BK or Iso were added to the [32P] incorporation buffer to test their effect on the phosphorylation of cTnI. Myocytes infected with AdPak1 (Fig. 3A, lane 1) demonstrated significantly reduced cTnI phosphorylation compared with those infected with control virus AdLacZ (Fig. 3A, lane 2) or AdLacZ with 100 nM Iso (Fig. 3A, lane 3). In myocytes infected with AdLacZ and treated with BK at 10 μM, the phosphorylation at cTnI was also significantly reduced (Fig. 3A, lane 4). Data from a series of experiments are summarized in Fig. 3B.
Fig. 3.
BK-induced activation of Pak1 associated with dephosphorylation of cardiac troponin I (cTnI). A: inhibition of phosphorylation of cTnI by constitutively active Pak1 and by BK, as detected by [32P] incorporation: myocytes infected with adenovirus Pak1 (AdPak1; lane 1), myocytes infected with AdLacZ (control; lane 2), myocytes infected with AdLacZ and treated with 100 nM isoproterenol (Iso; lane 3), and myocytes infected with AdLacZ and treated with BK at 10 μM (lane 4). B: histogram showing dephosphorylation of cTnI induced by BK at 1 and 10 μM (n = 3). Signal intensity is given in arbitrary units. Results shown are measurements from 4 independent experiments. *P < 0.05 compared with control without exposure to BK.
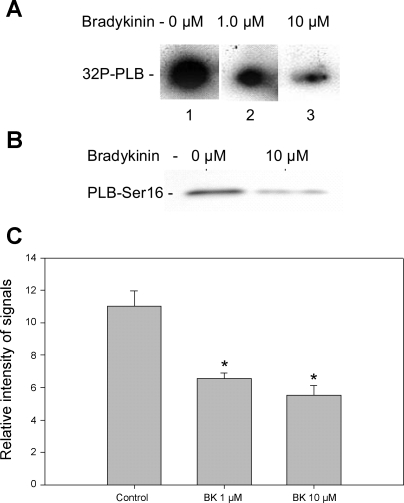
Results of experiments shown in Fig. 4A, in which we employed metabolic labeling of ATP with [32P], demonstrated that the treatment of myocytes with either 1 or 10 μM BK induced a dephosphorylation of PLB, compared with the myocytes without BK treatment. As illustrated in Fig. 4B, BK also induced a decrease in the phosphorylation of PLB, also detected by an antiphosphopeptide antibody specific for Ser16. The data summarized in Fig. 4C summarize the results of a series of experiments and indicate that BK is an effective agent in inducing the dephosphorylation of PLB.
Fig. 4.
Bradykinin induced a reduction in phosphorylation of phospholamban (PLB) in cardiac myocytes. A: [32P] incorporation into PLB from control myocytes (lane 1) and from myocytes treated with 1 μM (lane 2) and 10 μM (lane 3) BK, respectively. Equal amounts of cardiac myocytes were plated in 35-mm culture dish. [32P] incorporation was carried out at room temperature for 30 min. At 30 min of incubation in buffer containing [32P]orthophosphate, BK was added and was incubated for 2 min more. Equal amounts of total protein were loaded in each sample lane as demonstrated by Coommassie blue staining. B: Western blot detection of PLB-Ser16 phosphorylation in control myocytes treated with 100 nM Iso (lane 1) and Western blot of proteins from myocytes treated with 100 nM Iso + 10 μM BK for 2 min (lane 2). C: histogram summarizing the effect of BK effect on PLB phosphorylation corresponding to lanes 1, 2, and 3 of [32P] incorporation shown in A. Results shown are from 3 independent experiments. *P < 0.05 compared with control without exposure to BK.
Pak1 and PLB associate with PP2A.
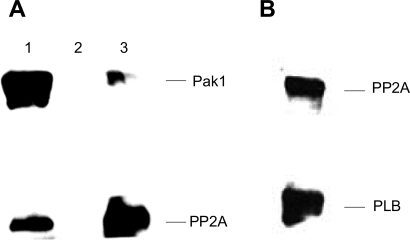
We have previously reported evidence that Pak1 associates with PP2A (22). In the present experiments, coimmunoprecipitation experiments demonstrated a complex between Pak1-PP2A and PLB (Fig. 5). The catalytic subunit of PP2A was detected in an immunoprecipitation product using the Pak1 antibody. Pak1 was also detected as an immunoprecipitation product pulled down by a PP2A antibody (Fig. 5A). The catalytic subunit of PP2A was also detected in the immunoprecipitation product with an antibody against PLB (Fig. 5B).
Fig. 5.
Demonstration of protein phosphate 2A (PP2A) association with both Pak1 and PLB by immunoprecipitation (IP). A: PP2A association with Pak1 when Pak1 antibody was used in IP (lane 1), negative control with the same experimental conditions except without the primary antibody (lane 2), and Pak1 association with PP2A when PP2Ac antibody was used in IP. B: Western blotting showing the presence of both PP2Ac and PLB in IP products using a PLB antibody. A control with no PLB or PP2A antibody showed no staining.
Treatment with BK slows the relaxation of [Ca2+]i transients in cardiac myocytes stimulated with Iso.
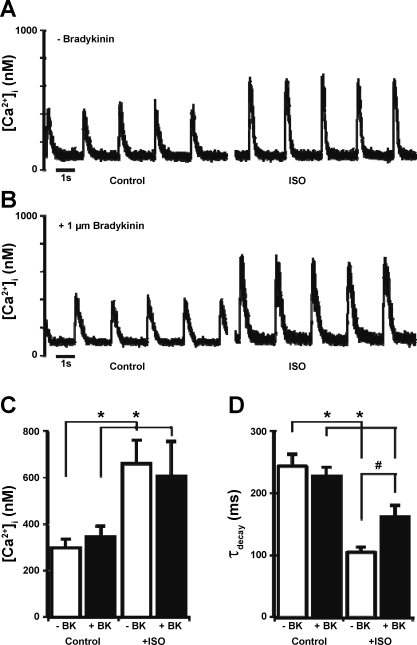
The effects of BK on the contractile function of cardiac myocytes have not been previously defined; however, one potential role of BK is in the modification of cellular Ca2+ fluxes. To test this hypothesis, we used fluo-4 fluorescence and laser-scanning confocal microscopy to measure changes in the [Ca2+]i transient during field stimulation with and without a preincubation with 1 μM BK. Figure 6A illustrates the response of an untreated adult rat ventricular myocyte to the application of 100 nM Iso in the perfusion with a significant increase in peak Ca2+ and faster τdecay. A myocyte pretreated with 1 μM BK (Fig. 6B) showed an increase in the amplitude of the [Ca2+]i transient but a blunted response of τdecay to the Iso application. Figure 6C shows that the average peak [Ca2+]i transient in untreated cells under control conditions was 297 ± 39 nM and following Iso was 660 ± 101 nM (n = 7 cells from 3 hearts). In cells that were pretreated with 1 μM BK, the average [Ca2+]i transient peak was 345 ± 45 nM under control conditions and, after Iso treatment, increased to 606 ± 147 nM (n = 9 cells from 3 hearts). Figure 6D shows that the average value of τdecay measured from the same records was similar between treatment groups under control conditions (−BK, 243 ± 19 ms; and +BK, 227 ± 15); however, the cells that had been pretreated with BK had a significantly slower τdecay following the application of Iso (−BK, 105 ± 9 ms; and +BK, 162 ± 19 ms, P < 0.05).
Fig. 6.
Intracellular Ca2+ concentration ([Ca2+]i) transients recorded from representative field-stimulated single adult rat ventricular myocytes in control conditions and after application of 100 nM Iso in an untreated cell (A) and a cell pretreated for 20 min with 1 μM BK (B) are shown. Summaries of peak [Ca2+]i transients (C) and the [Ca2+]i-transient time constant of decay (τdecay; D) from the same set of records are shown. Data are presented as means ± SE. *P < 0.05 compared with control; #P < 0.05 compared with −BK.
DISCUSSION
Our results provide the first evidence that Pak1 is a functionally significant downstream signaling molecule in a cascade initiated by the receptor binding of BK in adult cardiac myocytes. We demonstrated that Pak1 activity and its intracellular localization are regulated by BK in these myocytes. Moreover, both active Pak1 and BK promoted the dephosphorylation of PLB and cTnI and slowed the fall in Ca2+ during myocyte contractions stimulated by Iso. These findings significantly extend our previous investigation using an adenoviral transfer of Pak1 cDNA. In those studies (22), we reported evidence that the presence of active Pak1 is able to depress myofilament phosphorylation most likely through the activation of phosphatase PP2A (22). Our studies on Pak1 activation provide new evidence on mechanisms by which BK is cardioprotective. Previous studies in our laboratory (1) as well as others (46) have linked increases in myofilament Ca2+ sensitivity (a result of cTnI dephosphorylation) with resistance to cardiac stressors. The dephosphorylation of PLB would be expected to hinder the maladaptive SR Ca2+ load, a factor in ischemia-reperfusion injury (38). The results presented in Fig. 6 indicate that BK treatment induces a resistance to β-adrenergic stimulation in the cellular Ca2+ flux response, potentially by a slowed reuptake of Ca2+ to the SR or an extrusion from the cell or increased by an intracellular Ca2+ buffering. Along these lines, we recently reported that the activation of Pak1 by the drug FTY720 and sphingosine-1-phosphate are able to prevent ischemia-reperfusion-induced arrhythmias (12).
There is evidence from studies in noncardiac cells that support our findings. Studies in fibroblasts first suggested an activation of Pak1 by BK. Treatment of the fibroblasts with BK produced the same morphological change as the expression of constitutively active cdc42Hs, which is an upstream controller of Pak activity (25). Here we provide direct evidence that BK increased the phosphorylation of Thr423 of Pak1 in cardiac myocytes, and this increased autophosphorylation on Pak1 is correlated with the reduced phosphorylation of myofilament proteins and PLB. A connection between Pak1 and PP2A activity was first described in studies in neuronal tissue reported by Westphal et al. (47), who demonstrated that Pak1 and PP2A formed a signaling module in neuronal cells. This is also the case in cardiac myocytes (22). Other downstream kinase effectors in the heart for BK, especially protein kinase C (PKC), have been suggested but were poorly defined. Preconditioning following the treatment of hearts with BK was proposed to be related to PKC activation (10). Phospholipases C and D in rat ventricular myocytes were activated by BK, endothelin-1, and phenylephrine (6, 7). In a model of remote preconditioning, PKC inhibitors abolished the preconditioning effect (48). PKC was also suggested to be downstream of the adenosine and Gi/Go signaling pathways (26). Although there is evidence indicating that the signaling through PKC may be activated by BK, our data support the idea that the net effect of BK may reflect a balance between the activation of the activity of kinases and phosphatases.
Our finding of a BK-related activation of PP2A has important implications with regard to earlier studies in cardiac myocytes in which it was demonstrated that adenosine and acetylcholine signaling through Gi/Go may regulate the activity of PP2A without a significant effect on cAMP accumulation (17, 18, 37). These studies suggested an uncharacterized mechanism that regulates the activity of PP2A in response to adenosine and cholinergic stimulation. Our data indicate that the activation of Pak1 could be that mechanism (22). The physical association of Pak1 with PP2A in the heart suggested that Pak1 may execute its function through PP2A. The activity of PP2A is regulated by tyrosine phosphorylation (5), methylation (45), a large family of regulatory B subunits (34), and an autophosphorylation-regulated protein kinase (16). Recent studies of ours and others have also indicated that Pak1 activity has antiandrenergic effects (21) in the heart and may regulate some cardioprotective factors. Rac1/Pak1 activity is linked to the generation of nitric oxide (39) and cGMP (15), both of which have cardioprotective effects. Pak1 is also involved in the generation of reactive oxygen species, which is currently believed to have an important role in both the pre- and postconditioning effect of the heart during ischemia.
We think that the signaling mechanism in the transduction between BK receptor binding in cardiac myocytes and Pak1 activation is through Gi. In the heart, signaling through Gi is associated with the dephosphorylation of cTnI and PLB (30). Acetylcholine and adenosine, which signal through Gi, have also been demonstrated to induce an effect similar to BK on protein dephosphorylation in cardiac myocytes (17, 18, 30). Gi has also been demonstrated to couple to BK receptors (27, 28), and the inhibition of Gi protein by low-density lipoprotein attenuates the BK-stimulated release of endothelial-derived nitric oxide (27). In leukocytes, the activation of Pak1 is regulated by pertussis toxin treatment (23). Moreover, in fibroblasts, Cdc42 may be a component in the BK-initiated signaling cascade (25). Further support for the existence of this signaling cascade comes from the observation that the expression of constitutively active Cdc42 in fibroblasts can override the inhibitory effect of pertussis toxin on lysophosphatidic acid-induced cell morphological changes (44). Our recent studies also indicated that the activation of sphingosine-1 phosphate receptors in the heart may deliver signals to Pak1 via the inhibitory G proteins (12).
Yet, understanding the coordination of the molecular events in response to BK and other agonist stimulation in cardiac myocytes remains a significant challenge. When compared with PKA and PKC, Pak1 is a novel Ser/Thr protein kinase. BK and Pak1 may represent two interrelated coordinators that regulate both myofilament activities and cellular Ca2+ fluxes. Although there is strong evidence indicating that Pak1 is activated by BK, other signaling factors may also be partially responsible for the BK effect in cardiomyocytes. For example, BK had a broader effect in the reduction of the phosphorylation of myofilament proteins in adult rat ventricular myocytes than Pak1. The expression of the constitutively active Pak1 in the myocytes had no effect on the phosphorylation of cardiac troponin T and myosin regulatory light chain (MLC2v) (22), whereas in the present experiments, BK induced the dephosphorylation of cardiac troponin T and MLC2 (data not shown), as well as cTnI and myosin binding protein C. A further complication is that the Pak1 family consists of six members, and their functional role in cardiac muscle cells is just beginning to be understood. A further investigation of the regulation of Pak1 activity in cardiac muscle is likely to provide important data regarding both the short-term and long-term control of cardiac excitation and contraction.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants RO1-HL-64035 (to R. J. Solaro); P01-HL-62426, Project 1 (to R. J. Solaro); T32-HL-07692 (to Y. Ke and K. A. Sheehan); and F32-HL-076984 (to K. A. Sheehan) and by grants from The Wellcome Trust and the British Heart Foundation (to M. Lei).
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
We thank Dr. J. Chernoff for providing the antibody for Pak1 phosphorylated at Thr423.
REFERENCES
- 1.Arteaga GM, Warren CM, Milutinovic S, Martin AF, Solaro RJ. Specific enhancement of sarcomeric response to Ca2+ protects murine myocardium against ischemia-reperfusion dysfunction. Am J Physiol Heart Circ Physiol 289: H2183–H2192, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Barr E, Carroll J, Kalynych AM, Tripathy SK, Kozarsky K, Wilson JM, Leiden JM. Efficient catheter-mediated gene transfer into the heart using replication-defective adenovirus. Gene Ther 1: 51–58, 1994 [PubMed] [Google Scholar]
- 3.Baxter GF, Ebrahim Z. Role of bradykinin in preconditioning and protection of the ischaemic myocardium. Br J Pharmacol 135: 843–854, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cannell MB, Cheng H, Lederer WJ. Spatial non-uniformities in [Ca2+]i during excitation-contraction coupling in cardiac myocytes. Biophys J 67: 1942–1956, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen J, Parsons S, Brautigan DL. Tyrosine phosphorylation of protein phosphatase 2A in response to growth stimulation and v-src transformation of fibroblasts. J Biol Chem 269: 7957–7962, 1994 [PubMed] [Google Scholar]
- 6.Clerk A, Gillespie-Brown J, Fuller SJ, Sugden PH. Stimulation of phosphatidylinositol hydrolysis, protein kinase C translocation, and mitogen-activated protein kinase activity by bradykinin in rat ventricular myocytes: dissociation from the hypertrophic response. Biochem J 317: 109–118, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clerk A, Sugden PH. Regulation of phospholipases C and D in rat ventricular myocytes: stimulation by endothelin-1, bradykinin and phenylephrine. J Mol Cell Cardiol 29: 1593–1604, 1997 [DOI] [PubMed] [Google Scholar]
- 8.Cohen MV, Baines CP, Downey JM. Ischemic preconditioning: from adenosine receptor to KATP channel. Annu Rev Physiol 62: 79–109, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Critz SD, Cohen MV, Downey JM. Mechanisms of acetylcholine- and bradykinin-induced preconditioning. Vascul Pharmacol 42: 201–209, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Downey JM, Davis AM, Cohen MV. Signaling pathways in ischemic preconditioning. Heart Fail Rev 12: 181–188, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Ebrahim Z, Yellon DM, Baxter GF. Bradykinin elicits “second window” myocardial protection in rat heart through an NO-dependent mechanism. Am J Physiol Heart Circ Physiol 281: H1458–H1464, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Egom EE, Ke Y, Musa H, Mohamed TM, Wang T, Cartwright E, Solaro RJ, Lei M. FTY720 prevents ischemia/reperfusion injury-associated arrhythmias in an ex vivo rat heart model via activation of Pak1/Akt signaling. J Mol Cell Cardiol 48: 406–414, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fentzke RC, Buck SH, Patel JR, Lin H, Wolska BM, Stojanovic MO, Martin AF, Solaro RJ, Moss RL, Leiden JM. Impaired cardiomyocyte relaxation and diastolic function in transgenic mice expressing slow skeletal troponin I in the heart. J Physiol 517: 143–157, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gohlke P, Tschope C, Unger T. Bradykinin and cardiac protection. Adv Exp Med Biol 432: 159–172, 1997 [DOI] [PubMed] [Google Scholar]
- 15.Guo D, Tan YC, Wang D, Madhusoodanan KS, Zheng Y, Maack T, Zhang JJ, Huang XY. A Rac-cGMP signaling pathway. Cell 128: 341–355, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo H, Damuni Z. Autophosphorylation-activated protein kinase phosphorylates and inactivates protein phosphatase 2A. Proc Natl Acad Sci USA 90: 2500–2504, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta RC, Neumann J, Boknik P, Watanabe AM. M2-specific muscarinic cholinergic receptor-mediated inhibition of cardiac regulatory protein phosphorylation. Am J Physiol Heart Circ Physiol 266: H1138–H1144, 1994 [DOI] [PubMed] [Google Scholar]
- 18.Gupta RC, Neumann J, Durant P, Watanabe AM. A1-adenosine receptor-mediated inhibition of isoproterenol-stimulated protein phosphorylation in ventricular myocytes. Evidence against a cAMP-dependent effect. Circ Res 72: 65–74, 1993 [DOI] [PubMed] [Google Scholar]
- 19.Huser J, Lipsius SL, Blatter LA. Calcium gradients during excitation-contraction coupling in cat atrial myocytes. J Physiol 494: 641–651, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ke Y, Lei M, Collins TP, Rakovic S, Mattick PA, Yamasaki M, Brodie MS, Terrar DA, Solaro RJ. Regulation of L-type calcium channel and delayed rectifier potassium channel activity by p21-activated kinase-1 in guinea pig sinoatrial node pacemaker cells. Circ Res 100: 1317–1327, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Ke Y, Lei M, Solaro RJ. Regulation of cardiac excitation and contraction by p21 activated kinase-1. Prog Biophys Mol Biol 98: 238–250, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ke Y, Wang L, Pyle WG, de Tombe PP, Solaro RJ. Intracellular localization and functional effects of P21-activated kinase-1 (Pak1) in cardiac myocytes. Circ Res 94: 194–200, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Knaus UG, Morris S, Dong HJ, Chernoff J, Bokoch GM. Regulation of human leukocyte p21-activated kinases through G protein-coupled receptors. Science 269: 221–223, 1995 [DOI] [PubMed] [Google Scholar]
- 24.Kohno M, Yokokawa K, Minami M, Yasunari K, Maeda K, Kano H, Hanehira T, Yoshikawa J. Plasma levels of nitric oxide and related vasoactive factors following long-term treatment with angiotensin-converting enzyme inhibitor in patients with essential hypertension. Metabolism 48: 1256–1259, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Kozma R, Ahmed S, Best A, Lim L. The Ras-related protein Cdc42Hs and bradykinin promote formation of peripheral actin microspikes and filopodia in Swiss 3T3 fibroblasts. Mol Cell Biol 15: 1942–1952, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee HT, Emala CW. Protein kinase C and G(i/o) proteins are involved in adenosine- and ischemic preconditioning-mediated renal protection. J Am Soc Nephrol 12: 233–240, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Liao JK. Inhibition of Gi proteins by low density lipoprotein attenuates bradykinin-stimulated release of endothelial-derived nitric oxide. J Biol Chem 269: 12987–12992, 1994 [PubMed] [Google Scholar]
- 28.Liebmann C, Offermanns S, Spicher K, Hinsch KD, Schnittler M, Morgat JL, Reissmann S, Schultz G, Rosenthal W. A high-affinity bradykinin receptor in membranes from rat myometrium is coupled to pertussis toxin-sensitive G-proteins of the Gi family. Biochem Biophys Res Commun 167: 910–917, 1990 [DOI] [PubMed] [Google Scholar]
- 29.Lipp P, Pott L, Callewaert G, Carmeliet E. Simultaneous recording of Indo-1 fluorescence and Na+/Ca2+ exchange current reveals two components of Ca2+-release from sarcoplasmic reticulum of cardiac atrial myocytes. FEBS Lett 275: 181–184, 1990 [DOI] [PubMed] [Google Scholar]
- 30.Liu Q, Hofmann PA. Modulation of protein phosphatase 2a by adenosine A1 receptors in cardiomyocytes: role for p38 MAPK. Am J Physiol Heart Circ Physiol 285: H97–H103, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Manser E, Huang HY, Loo TH, Chen XQ, Dong JM, Leung T, Lim L. Expression of constitutively active alpha-PAK reveals effects of the kinase on actin and focal complexes. Mol Cell Biol 17: 1129–1143, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manser E, Leung T, Salihuddin H, Zhao ZS, Lim L. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature 367: 40–46, 1994 [DOI] [PubMed] [Google Scholar]
- 33.Margolius HS. Theodore Cooper Memorial Lecture. Kallikreins and kinins. Some unanswered questions about system characteristics and roles in human disease. Hypertension 26: 221–229, 1995 [DOI] [PubMed] [Google Scholar]
- 34.McCright B, Rivers AM, Audlin S, Virshup DM. The B56 family of protein phosphatase 2A (PP2A) regulatory subunits encodes differentiation-induced phosphoproteins that target PP2A to both nucleus and cytoplasm. J Biol Chem 271: 22081–22089, 1996 [DOI] [PubMed] [Google Scholar]
- 35.McEachern AE, Shelton ER, Bhakta S, Obernolte R, Bach C, Zuppan P, Fujisaki J, Aldrich RW, Jarnagin K. Expression cloning of a rat B2 bradykinin receptor. Proc Natl Acad Sci USA 88: 7724–7728, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mombouli JV, Vanhoutte PM. Kinins and endothelial control of vascular smooth muscle. Annu Rev Pharmacol Toxicol 35: 679–705, 1995 [DOI] [PubMed] [Google Scholar]
- 37.Neumann J, Gupta RC, Jones LR, Bodor GS, Bartel S, Krause EG, Pask HT, Schmitz W, Scholz H, Watanabe AM. Interaction of beta-adrenoceptor and adenosine receptor agonists on phosphorylation. Identification of target proteins in mammalian ventricles. J Mol Cell Cardiol 27: 1655–1667, 1995 [DOI] [PubMed] [Google Scholar]
- 38.Said M, Becerra R, Palomeque J, Rinaldi G, Kaetzel MA, Diaz-Sylvester PL, Copello JA, Dedman JR, Mundina-Weilenmann C, Vittone L, Mattiazzi A. Increased intracellular Ca2+ and SR Ca2+ load contribute to arrhythmias after acidosis in rat heart. Role of Ca2+/calmodulin-dependent protein kinase II. Am J Physiol Heart Circ Physiol 295: H1669–H1683, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sawada N, Salomone S, Kim HH, Kwiatkowski DJ, Liao JK. Regulation of endothelial nitric oxide synthase and postnatal angiogenesis by Rac1. Circ Res 103: 360–368, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schanstra JP, Alric C, Marin-Castano ME, Girolami JP, Bascands JL. Renal bradykinin receptors: localisation, transduction pathways and molecular basis for a possible pathological role (review). Int J Mol Med 3: 185–191, 1999 [DOI] [PubMed] [Google Scholar]
- 41.Sells MA, Pfaff A, Chernoff J. Temporal and spatial distribution of activated Pak1 in fibroblasts. J Cell Biol 151: 1449–1458, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sheehan KA, Ke Y, Wolska BM, Solaro RJ. Expression of active p21-activated kinase-1 induces Ca2+ flux modification with altered regulatory protein phosphorylation in cardiac myocytes. Am J Physiol Cell Physiol 296: C47–C58, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thomas D, Tovey SC, Collins TJ, Bootman MD, Berridge MJ, Lipp P. A comparison of fluorescent Ca2+ indicator properties and their use in measuring elementary and global Ca2+ signals. Cell Calcium 28: 213–223, 2000 [DOI] [PubMed] [Google Scholar]
- 44.Ueda H, Morishita R, Yamauchi J, Itoh H, Kato K, Asano T. Regulation of Rac and Cdc42 pathways by G(i) during lysophosphatidic acid-induced cell spreading. J Biol Chem 276: 6846–6852, 2001 [DOI] [PubMed] [Google Scholar]
- 45.Wei H, Ashby DG, Moreno CS, Ogris E, Yeong FM, Corbett AH, Pallas DC. Carboxymethylation of the PP2A catalytic subunit in Saccharomyces cerevisiae is required for efficient interaction with the B-type subunits Cdc55p and Rts1p. J Biol Chem 276: 1570–1577, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Westfall MV, Rust EM, Metzger JM. Slow skeletal troponin I gene transfer, expression, and myofilament incorporation enhances adult cardiac myocyte contractile function. Proc Natl Acad Sci USA 94: 5444–5449, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Westphal RS, Coffee RL, Jr, Marotta A, Pelech SL, Wadzinski BE. Identification of kinase-phosphatase signaling modules composed of p70 S6 kinase-protein phosphatase 2A (PP2A) and p21-activated kinase-PP2A. J Biol Chem 274: 687–692, 1999 [DOI] [PubMed] [Google Scholar]
- 48.Wolfrum S, Schneider K, Heidbreder M, Nienstedt J, Dominiak P, Dendorfer A. Remote preconditioning protects the heart by activating myocardial PKCepsilon-isoform. Cardiovasc Res 55: 583–589, 2002 [DOI] [PubMed] [Google Scholar]
- 49.Yin H, Chao J, Bader M, Chao L. Differential role of kinin B1 and B2 receptors in ischemia-induced apoptosis and ventricular remodeling. Peptides 28: 1383–1389, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]