Abstract
A glycerophospholipid (1-O-tuberculostearoyl-2-O-palmitoyl-sn-glycero-3-phosphoethanolamine) from Mycobacterium tuberculosis was isolated from the reference strain H37Rv. The molecular structure of this tuberculostearoyl [(R)-10-methyloctadecyl] and palmitoyl containing phosphatidylethanolamine (PE) has been resolved. The substitution pattern on the glycerol backbone could be determined by comparison of the isolate to the two synthetically prepared regioisomers. MS/MS analysis was used to determine its molecular structure. Production of this synthetic version of mycobacterial PE in high yield, with a stereochemically correct and pathogen-specific fatty acyl group, can be used as a standard in LC-MS based lipidomic analyses to detect trace amounts of mycobacterial PE in human blood, sputum, or tissues as a marker of infection by mycobacteria.
Keywords: tuberculostearic acid; MS/MS analysis; asymmetric 1,4-addition
Glycerophospholipids are found in all biological membranes and play an essential role in the structure of cellular membranes (1). The general structure of glycerophospholipids consists of a glycerol core with a phosphate group localized at the sn-3 position. The two remaining hydroxyl functionalities are substituted with at least one O-acyl, O-alkyl, or O-alk-1’-enyl residue. The two acyl-substituents can be either identical or different and affect membrane packing in ways that influence bacterial survival and drug transport. Some bacteria, such as Mycobacterium tuberculosis, have very specific glycerophospholipids, which can in principle be used to detect and identify a specific species. For physiological studies and potential early detection of tuberculosis, identification of pure cell wall and cell membrane components is of paramount importance. However, isolated compounds are often not pure and for this reason errors in the structural assignment of natural compounds have been made in the past (2). The characterization of isolated natural compounds by comparison with the corresponding synthetic material is therefore highly desirable.
The partial structural assignment of glycerophospholipids with two different acyl residues had already been studied by Smith et al. (3) in 1995 and later by Cabrera et al. (4) in 2000. Negative ion electrospray ionization tandem mass spectrometry was used to compare the fragmentation pattern of two commercially available regioisomers, l-O-palmitoyl-2-O-oleoyl-sn-glycero-3-phosphoethanolamine and l-O-oleoyl-2-O-palmitoyl-sn-glycero-3-phosphoethanolamine (3). As suggested by Jensen et al. (5) in 1987, cleavage of the acyl residues occurred preferentially at the sn-2 position of the glycerol backbone, resulting in a higher abundance of the sn-2 positioned carboxylate such that it was observed approximately in a 2:1 ratio. An extensive MS/MS analysis study by Hsu and Turk (6–11) also supports the conclusion that there is a preferred cleavage at the sn-2 position in phosphatidyl compounds and gives detailed mechanisms, involving participation of the phosphate head group, thereby explaining this result.
Gilleron et al. (12) used the same principle in 2001 to determine the fatty acid distribution of mycobacterial phosphatidyl-myo-inositol mannosides. They observed the preferred sn-2-glycerol cleavage in mass analysis and assigned a tuberculostearic acid (TBSA) residue to the sn-2-position of the glycerol backbone. Later, the structure was reassigned, placing the TBSA residue at the sn-1 position (13, 14). Dyer et al. (15) subsequently published an elegant synthesis of phosphatidylinositol dimannoside in 2007. This sn-1 palmitoyl, sn-2 tuberculostearoyl containing phosphatidylinositol dimannoside synthesis was based on the initial structural assignment (2001) and therefore turned out to provide the unnatural regioisomer.
Here, we describe the structure elucidation of 1-O-tuberculostearoyl-2-O-palmitoyl-sn-glycero-3-phosphoethanolamine, a genus specific form of phosphatidylethanolamine (PE) from M. tuberculosis, with regards to acyl substitutions on the glycerol backbone. Using tandem mass spectrometry and novel synthetic methods, we unambiguously determine the position of the two acyl residues and demonstrate that this specific glycerophospholipid is distinct from common mammalian forms of PE such that the synthetic molecule can be used as a standard to investigate candidate natural biomarkers of infection by mycobacteria.
EXPERIMENTAL PROCEDURES
Isolation of PE from M. tuberculosis
Total lipid extracts of M. tuberculosis strain H37Rv were prepared as previously described (16). One gram of lipid, extracted into 2:1 (v/v) of chloroform: methanol, was separated over a 75 ml open silica column (Alltech, Deerfield, IL) by sequential elution with five column volumes of chloroform, acetone, and methanol, respectively. The methanol eluting lipids were recovered, and 15 mg was separated by one-dimensional thin layer chromatography on 200 μm silica plates resolved with chloroform, methanol, and water (v/v/v 60:30:6). Bands were visualized by spraying with water prior to scraping. Lipids were serially extracted from scraped bands with chloroform and methanol (v/v 2:1, 1:2) and pure methanol. Lipid content was analyzed by positive-mode electrospray ionization mass spectrometry (ThermoFinnigan LCQ Advantage). PE molecular species were detected in a band with Rf of 0.47. Further purification of molecular species of PE was achieved by high performance liquid chromatography/mass spectrometry. Approximately 50 μg of PE containing palmitic and tuberculostearic acids was obtained with >95% purity as assessed by MS and 1H-NMR.
Synthesis of TBSA
To unambiguously establish the structure of the isolated mycobacterial glycerophospholipid, we decided to synthesize the two possible regioisomers 1 and 2 and use them as authentic standards for comparison to natural molecules in MS/MS analysis.
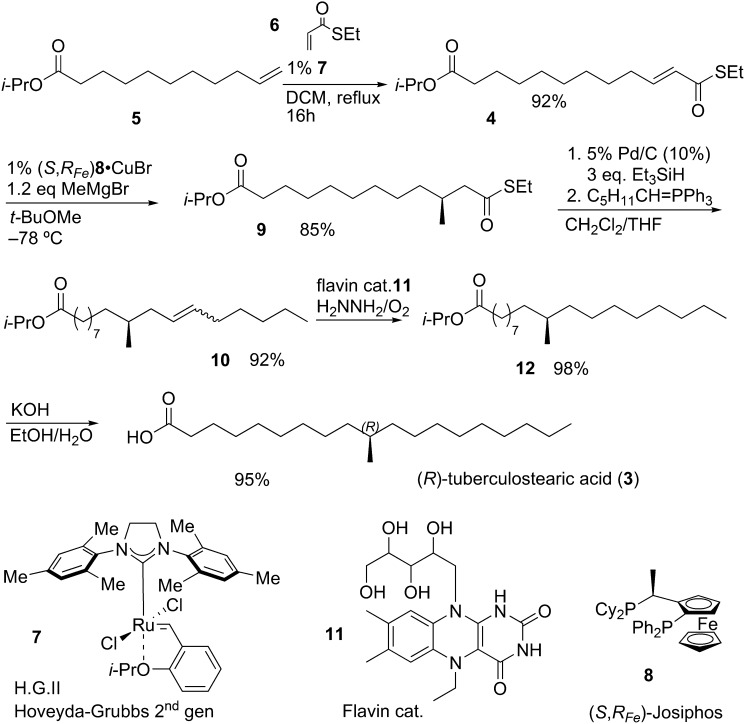
Several synthetic routes have been described for (R)-TBSA, but all routes provide the racemate (17–24) or are based on chiral pool strategies (15, 25, 26), so we designed a new catalytic, enantioselective route. Unsaturated thioester 4 (Fig. 1) was obtained in 92% yield by a ruthenium-catalyzed olefin metathesis of olefin 5 and S-ethyl thioacrylate 6 using Hoveyda-Grubbs second generation catalyst 7. This strategy for the construction of unsaturated thioesters from terminal olefins was recently reported by our group (27). Thioester 4 was subjected to a copper catalyzed asymmetric conjugate addition with methylmagnesium bromide and (S,RFe)-Josiphos 8 as the ligand (28). The use of a copper catalyzed asymmetric conjugate addition of Grignard reagents on unsaturated thioesters has recently been applied in a number of natural product syntheses (28–33). Methyl-branched thioester 9 was isolated in 85% yield with an enantiomeric excess of 90% (see supplemental data). The spectroscopic data of the Mosher ester derivative of compound 9, which was used for the ee determination, also confirmed the absolute configuration of the introduced methyl substituent (see supplemental data). The thioester functionality was selectively reduced, in the presence of the propyl oxo-ester, to the corresponding aldehyde under Fukuyama conditions (34). Wittig reagent C5H11CH = PPh3 was added to the reaction and a mixture of E- and Z-isomers of 10 was obtained in 92% yield from 9. Olefin 10 was reduced under transition metal-free conditions to avoid any double bond isomerization and thereby potentially racemization of the methyl-branched stereocenter. Flavin catalyst 11 in combination with hydrazine produces diimide in situ, which reduced olefin 10 in an excellent yield based on a recently published method (35). Saturated propylester 12 was hydrolyzed with KOH, leading to the desired natural occurring R-enantiomer of TBSA in 5 steps with an overall yield of 67% (92% on average per step).
Fig. 1.
Total synthesis of (R)-TBSA (3).
Synthesis of 1-O-tuberculostearoyl-2-O-palmitoyl- sn-glycero-3-phosphoethanolamine
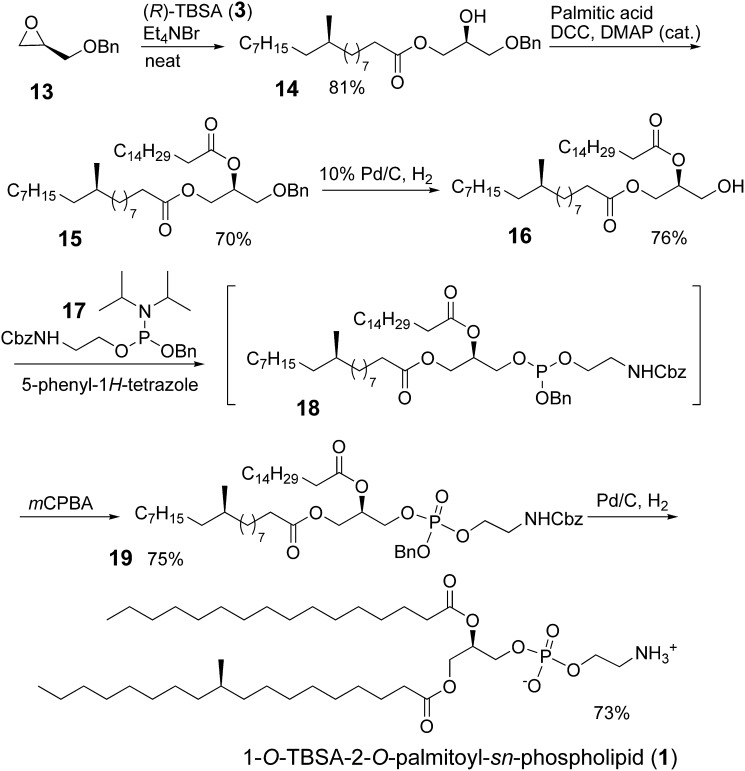
Commercially available (R)-benzyl glycidyl ether 13 was heated to 100°C together with one equivalent of (R)-TBSA, as a melt with catalytic amounts of Bu4NBr (Fig. 2) (15). Ester 14 was obtained in 81% yield together with a small amount of the other regioisomer, which was removed by column chromatography. The 2-position was acylated with palmitic acid (C15H31COOH) and N,N’-dicyclohexylcarbodiimide (DCC) as the coupling agent in 70% yield. Palladium-catalyzed hydrogenolysis of the benzyl ether in a 1:1 mixture of ethanol and acetic acid resulted in alcohol 16 in 76% yield. Basic conditions were avoided as this could easily lead to undesired acyl migration. We observed only traces of the 1,3-sn-diacyl glycerol product, which could be removed by flash chromatography. Initially, we tried methods (36, 37) for the introduction of the PE group starting from the phosphoryl chloride, which invariably gave unsatisfactory results. Finally, the PE group was introduced in a two-step, one-pot procedure. Reagent 17 (38) was added to 16 together with 5-phenyl-1H-tetrazole (39). The reaction was followed by TLC and after full conversion of the starting material to 18, m-chloroperbenzoic acid (mCPBA) was added as the oxidant. Using this procedure, benzyl and carboxybenzyl (Cbz) protected 19 was obtained in 75% yield. Finally, phosphate ester 19 was treated under hydrogenolysis conditions (39) to remove the benzyl and Cbz protecting groups in one step to yield the desired product 1, 1-O-TBSA-2-O-palmitoyl-sn-phospholipid in 73% yield.
Fig. 2.
Synthesis of 1-O-TBSA-2-O-palmitoyl-sn-phospholipid (1).
The regioisomer of 1-O-TBSA-2-O-palmitoyl-sn-phospholipid, i.e. 1-O-palmitoyl-2-O-TBSA-sn-phospholipid, 2, was synthesized in the same way, the only difference being the order of the introduction of the acyl residues. Palmitic acid was first introduced followed by (R)-TBSA.
HPLC/MS, HPLC/MS/MS, and 1H-NMR analysis of synthetic compounds and natural isolate
Natural and synthetic compounds were analyzed on a 1200 series HPLC with a quadrupole accurate mass time of flight (Q-TOF) 6520 mass spectrometer (Agilent Technologies, Santa Clara, CA). Voltage 3.5 kV, source temperature 325°C, drying gas 5L/min, nebulizer pressure 30 psi, and ions were detected in the negative mode. Approximately 2 μg of each of the synthetic compounds and 1 μg of purified natural phospholipid were injected into a 250 mm × 4.6 mm C18 bonded silica column (Vydac, Deerfield, IL) with a gradient elution program in which Solvent A, consisting of 50% methanol, 30% acetonitrile, 20% water, and Solvent B, consisting of 90% isopropanol and 10% hexanes (additives were 0.1% formic acid, 0.01% ammonium acetate, 0.02% trifluoroacetic acid, 0.02% hexafluoroisopropylalcohol). Solvent B was run at 60% from 0 to 4 min and then increased to 100% from 16 min to 28 min. To compare M. tuberculosis and mammalian phospholipids, approximately 20 μg of acetone precipitable lipids from mouse lung homogenate were injected onto a 150 mm × 2.0 mm mono chrom 3 diol column (Varian), and eluted with a gradient program in which solvent A consisted of methanol (additives were 0.1% formic acid and 0.05% ammonium hydroxide) and solvent B consisted of 60% hexanes and 40% isopropanol (additives were 0.1% formic acid and 0.05% ammonium hydroxide). The gradient was run from 95% to 85% solvent B from 0 to 6.6 min, to 0% solvent B until 16.2 min, then increased back to 95% solvent B from 22.8 to 26 min.
HPLC purification of the mycobacterial phospholipid
HPLC/MS (Thermo Scientific LXQ, Waltham, MA) using a 4.6 mm × 250 mm C18 bonded silica column (Vydac, Deerfield, IL) as the stationary phase and isocratic elution with a mobile phase consisting of 63% isopropanol, 15% methanol, 9% acetonitrile, 7% hexanes, and 6% water (additives were 0.1% formic acid, 0.01% ammonium acetate, 0.02% trifluoroacetic acid, 0.02% hexafluoroisopropylalcohol) was used to separate crude lipids by manual injection and fraction collection.
The two synthetic isomers 1 and 2 were compared with the natural product by MS/MS analysis in order to establish the correct structure of the natural isolate. The mass of the natural isolate (m/z 732.550) and the synthetic compounds 1-O-TBSA-2- O-palmitoyl-sn-phospholipid (m/z 732.551) and 1-O-palmitoyl-2-O- TBSA-sn-phospholipid (m/z 732.552) agreed well and were within 4 ppm of the calculated value for C40H79NO8P− (m/z 732.555). Additional information confirming the presence of the TBSA acyl residue was found by comparing the 1H-NMR spectrum of the natural product with that of the synthetic compounds. The CH3 branch has a distinct chemical shift and coupling constant in 1H-NMR. For both the natural isolate and the synthetic compounds a doublet at 0.83 ppm was observed with J = 6.4 Hz.
Tandem mass spectrometry
MS/MS was performed on LCQ Advantage (Thermo Finnigan, Waltham, MA). Compounds were analyzed by nanospray ESI-MS using borosilicate glass pipettes pulled to a 2 μm orifice (internal electrode 1.2 kV). Ions were detected in the negative mode and collided at 20% energy (see supplementary data).
RESULTS
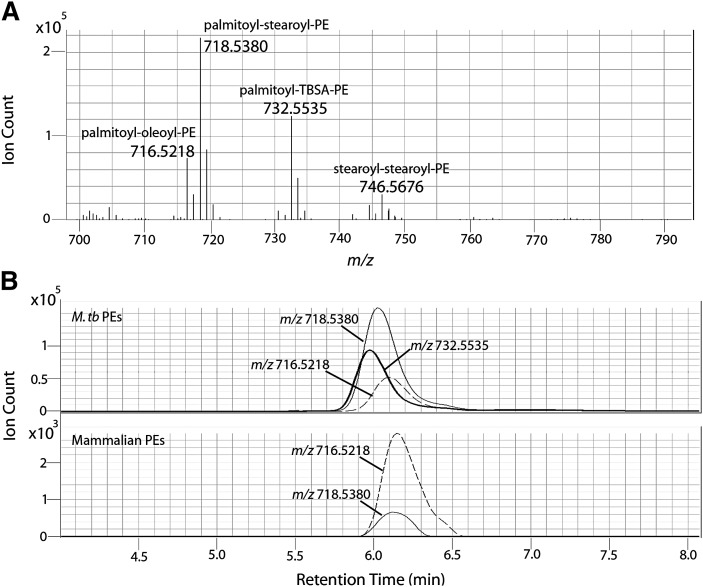
As part of ongoing research on immunologically active compounds from M. tuberculosis, we isolated an abundant glycerophospholipid from a virulent reference strain of M. tuberculosis known as H37Rv. The isolate was consistent with PE glycerol having two acyl residues, tuberculostearoyl [(R)-10-methyloctadecyl] and palmitoyl (Fig. 3). The molecular structure of TBSA is well documented and the position of the C10-methyl branch was already elucidated by Spielman (18) in 1934. This glycerophospholipid could be distinguished from mammalian PEs isolated from mouse lung based on ions that contain a C19 tuberculostearoyl unit. The presence of tuberculostearoyl residues in PEs from M. tuberculosis was reported in 1965 (40). Lipidomic surveys of mycobacterial lipids for candidate biomarkers are normally focused on ions that are abundant, universally present in pathogenic mycobacterial species, absent in mammalian lipids, and absent in nonmycobacterial pathogens and commensal organisms. Mycobacterial PEs fit all four criteria because a large subset of the PE molecular species carries a C19 TBSA, which is not made by mammalian cells and is exclusively found in mycobacteria. Negative mode mass spectrometry of the bacterial extracts from M. tuberculosis detected several ions corresponding to the expected mass of TBSA containing PE, with the strongest being m/z 732.5535 (Fig. 4A). Because mammalian cells rarely produce fatty acids with an odd number of methylene units, it was expected that mammalian PE would not contain a compound with the same mass. PE species not containing tuberculostearic acid were detected in both tuberculosis and mouse lipid preparations. However, 732.5535 was detected only in M. tuberculosis lipid preps (Fig. 4B). The resolution of our instrument suggests that if 732.5535 is present in mammalian lipid preps, its abundance is at least 1000-fold less (data not shown). The specific presence of a TBSA residue in PEs from M. tuberculosis indicates that this molecule is a candidate biomarker of infection by mycobacteria.
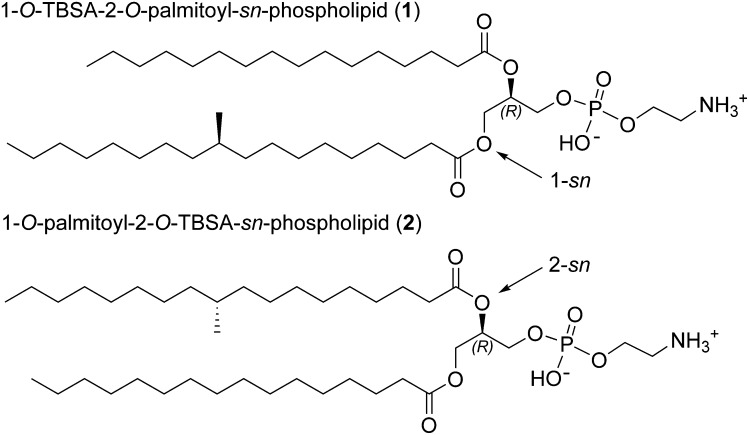
Fig. 3.
The two possible structures of the natural phospholipid; 1-O-TBSA-2-O-palmitoyl-sn-phospholipid (1) and 1-O-palmitoyl-2-O-TBSA-sn-phospholipid (2).
Fig. 4.
A: Negative mode mass analysis of detected M. tuberculosis phosphatidylethanolamines (PE). B: Extracted ion chromatograms of PE's detected in M. tuberculosis compared to mammalian PE's from non-infected mouse lung homogenate, no ion at m/z 732.55 was detected in this region of the chromatogram for the mammalian PEs.
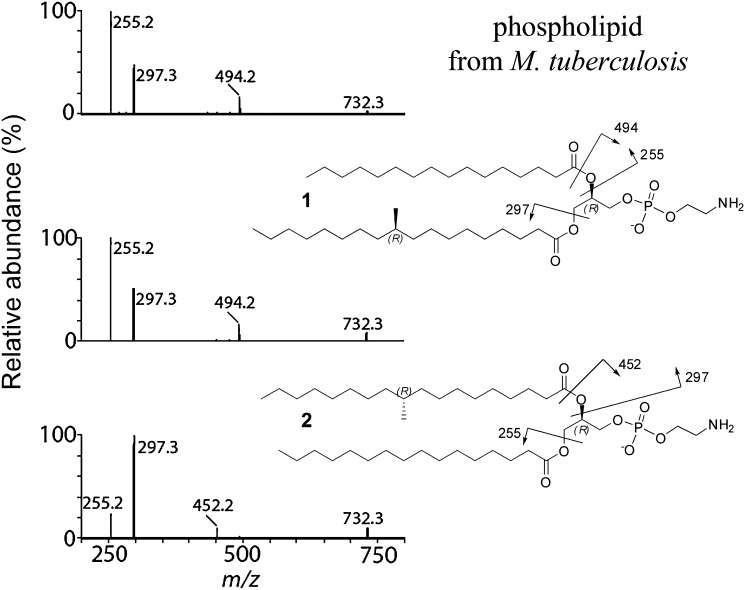
To determine the position of the acyl residues in the natural product, we compared the fragmentation pattern of the natural compound to each of the two synthetic regioisomers 1 and 2 (Fig. 5). MS/MS (ESI) in the negative mode shows a clear difference in the fragmentation pattern. The acyl groups at the sn-2-position of the glycerol backbone are preferentially cleaved as predicted. Cleavage can occur between the carbonyl carbon atom and the oxygen atom with transfer of hydrogen to leave a hydroxy group on the resulting fragment ion (phosphatidyl fragment), m/z 494 and m/z 452 for regioisomer 1 and 2, respectively. Alternatively, cleavage occurs between the oxygen and glycerol backbone, resulting in a negatively charged carboxylate fragment (m/z 255 and m/z 297 for palmitic acid and TBSA, respectively). These types of fragmentation occur preferentially at the sn-2-position of the glycerol backbone compared with the sn-1-position. The fragmentation pattern of the natural product matches with synthetic 1, whereas that of isomer 2 does not match with the natural product.
Fig. 5.
MS/MS (ESI) in negative mode.
With both synthetic 1-O-TBSA-2-O-palmitoyl-sn-phospholipid and 1-O-palmitoyl-2-O-TBSA-sn-phospholipid in hand, we conclude that it is straightforward to determine the position of two acyl side chains in diacylglycerol-phosphatidylethanolamine lipids by MS/MS analysis. The acyl residue at the sn-2-position will give a higher abundance in the ESI mass spectrum compared with the acyl residue at the sn-1-position. This is consistent with the reports of Hsu and Turk (6–11).
Finally, we compared HPLC retention times of the two synthetic compounds and the natural one (see supplemental data) to further study their chemical characteristics. Although retention times are similar, a difference was observed. The natural product and synthetic 1 elute at 6.1 min and regioisomer 2 elutes at 6.3 min.
CONCLUSIONS
A newly isolated glycerophospholipid from M. tuberculosis was characterized and analyzed by MS/MS analysis. Two regioisomers of the phospholipid were successfully synthesized and compared with the natural compound. MS/MS analysis was used to determine the position of the acyl residues. The natural product was identified as 1-O-TBSA-2-O-palmitoyl-sn-phosphotidylethanolamine. Additional evidence was obtained by 1H-NMR. Different di-acyl PEs can quickly be analyzed and identified by mass analysis and the relative position of the acyl residues can be determined by comparison of the mass fragmentation pattern. Production of this synthetic version of mycobacterial PE, with a stereochemically correct and pathogen-specific fatty acyl group in high yield, can be used as a standard in LC-MS based lipidomic analyses to detect trace amounts of mycobacterial PE in human blood, sputum, or tissues as a marker of infection by mycobacteria.
Acknowledgments
The authors thank A. Kiewiet (Stratingh Institute for Chemistry, University of Groningen) for technical support.
Footnotes
This work was supported by The Netherlands Organization for Scientific Research (NWO/CW) as well as the NIAID R01 049313, the Broad Institute Spark program, and the Burroughs Wellcome Program for Translational Research.
The online version of this article (available at http://www.jlr.org) contains supplementary data.
REFERENCES
- 1.Vance J. E. 2008. Phosphatidylserine and phosphatidylethanolamine in mammalian cells: two metabolically related aminophospholipids. J. Lipid Res. 49: 1377–1387. [DOI] [PubMed] [Google Scholar]
- 2.Nicolaou K. C., Snyder S. A. 2005. Chasing molecules that were never there: misassigned natural products and the role of chemical synthesis in modern structure elucidation. Agnew. Chem. Int. Ed. 44: 1012–1044. [DOI] [PubMed] [Google Scholar]
- 3.Smith P. B., Snyder A. P., Harden C. S. 1995. Characterization of bacterial phospholipids by electrospray ionization tandem mass spectrometry. Anal. Chem. 67: 1824–1830. [DOI] [PubMed] [Google Scholar]
- 4.Cabrera G. M., Fernández Murga M. L., Font de Valdez G., Seldes A. M. 2000. Direct analysis by electrospray ionisation tandem mass spectrometry of mixtures of phosphatidyldiacylglycerols from Lactobacillus. J. Am. Soc. Mass Spectrom. 35: 1452–1459. [DOI] [PubMed] [Google Scholar]
- 5.Jensen N. J., Tomer K. B., Gross M. L. 1987. FAB MS/MS for phosphatidylinostitol,-glycerol,-ethanolamine and other complex phospholipids. Lipids. 22: 480–489. [DOI] [PubMed] [Google Scholar]
- 6.Hsu F-F., Turk J. 2000. Characterization of phosphatidylinositol, phosphatidylinositol-4-phosphate, and phosphatidylinositol-4,5-bisphosphate by electrospray ionization tandem mass spectrometry: a mechanistic study. J. Am. Soc. Mass Spectrom. 11: 986–999. [DOI] [PubMed] [Google Scholar]
- 7.Hsu F-F., Turk J. 2000. Charge-remote and charge-driven fragmentation processes in diacyl glycerophosphoethanolamine upon low-energy collisional activation: a mechanistic proposal. J. Am. Soc. Mass Spectrom. 11: 892–899. [DOI] [PubMed] [Google Scholar]
- 8.Hsu F-F., Turk J. 2000. Charge-driven fragmentation processes in diacyl glycerophosphatidic acids upon low-energy collisional activation. A mechanistic proposal. J. Am. Soc. Mass Spectrom. 11: 797–803. [DOI] [PubMed] [Google Scholar]
- 9.Hsu F-F., Turk J. 2004. Studies on sulfatides by quadrupole ion-trap mass spectrometry with electrospray ionization: structural characterization and the fragmentation processes that include an unusual internal galactose residue loss and the classical charge-remote fragmentation. J. Am. Soc. Mass Spectrom. 15: 536–546. [DOI] [PubMed] [Google Scholar]
- 10.Hsu F-F., Turk J., Owens R. M., Rhoades E. R., Russel D. G. 2007. Structural characterization of phosphatidyl-myo-inositol mannosides from Mycobacterium bovis bacillus Calmette Guérin by multiple-stage quadrupole ion-trap mass spectrometry with electrospray ionization. I. PIMs and lyso-PIMs. J. Am. Soc. Mass Spectrom. 18: 466–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsu F-F., Turk J., Owens R. M., Rhoades E. R., Russel D. G. 2007. Structural characterization of phosphatidyl-myo-inositol mannosides from Mycobacterium bovis bacillus Calmette Gúerin by multiple-stage quadrupole ion-trap mass spectrometry with electrospray ionization. II. Monoacyl- and diacyl-PIMs. J. Am. Soc. Mass Spectrom. 18: 479–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilleron M., Ronet C., Mempel M., Monsarrat B., Gachelin G., Puzo G. 2001. Acylation state of the phosphatidylinositol mannosides from Mycobacterium bovis bacillus Calmette Guérin and ability to induce granuloma and recruit natural killer T cells. J. Biol. Chem. 276: 34896–34904. [DOI] [PubMed] [Google Scholar]
- 13.Gilleron M., Quesniaux V. F. J., Puzo G. 2003. Acylation state of the phosphatidylinositol hexamannosides from Mycobacterium bovis bacillus Calmette Guérin and Mycobacterium tuberculosis H37Rv and its implication in Toll-like receptor response. J. Biol. Chem. 278: 29880–29889. [DOI] [PubMed] [Google Scholar]
- 14.Gilleron M., Lindner B., Puzo G. 2006. MS/MS approach for characterization of the fatty acid distribution on mycobacterial phosphatidyl-myo-inositol mannosides. Anal. Chem. 78: 8543–8548. [DOI] [PubMed] [Google Scholar]
- 15.Dyer B. S., Jones J. D., Ainge G. D., Denis M., Larsen D. S., Painter G. F. 2007. Synthesis and structure of phosphatidylinositol dimannoside. J. Org. Chem. 72: 3282–3288. [DOI] [PubMed] [Google Scholar]
- 16.Matsunaga I., Bhatt A., Young D. C., Cheng T-Y., Eyles S. J., Besra G. S., Briken V., Porcelli S. A., Costello C. E., Jacobs W. R., Jr, et al. 2004. Mycobacterium tuberculosis pks12 produces a novel polyketide presented by CD1c to T cells. J. Exp. Med. 200: 1559–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderson R. J. 1927. A study of the phosphatide fraction of tubercle bacilli. J. Biol. Chem. 74: 537–551. [Google Scholar]
- 18.Spielman M. A. 1934. The chemistry of the lipids of tubercle bacilli. XXXIX. The constitution of tuberculostearic acid. J. Biol. Chem. 106: 87–96. [Google Scholar]
- 19.Prout F. S., Cason J., Ingersoll A. W. 1947. The synthesis of tuberculostearic acid. J. Am. Chem. Soc. 69: 1233. [DOI] [PubMed] [Google Scholar]
- 20.Prout F. S., Cason J., Ingersoll A. W. 1948. Branched-chain fatty acids. V. The synthesis of optically active 10-methyloctadecanoic acids. J. Am. Chem. Soc. 70: 298–305. [Google Scholar]
- 21.Linstead R. P., Lunt J. C., Weedon B. C. L. 1950. Anodic syntheses. Part II. Synthesis of (±)-tuberculostearic acid. J. Chem. Soc. 3331–3333. [Google Scholar]
- 22.Linstead R. P., Lunt J. C., Weedon B. C. L. 1951. Anodic syntheses. Part IV. Synthesis of (+)- and (–)-tuberculostearic acids. J. Chem. Soc. 1130–1132. [Google Scholar]
- 23.Hünig S., Salzwedel M. 1966. Synthesen mit Enaminen, X. Systematische Synthese methylverzweigter Carbonsäuren. Chem. Ber. 99: 823–842. [Google Scholar]
- 24.Wallace P. A., Minnikin D. E., McCrudden K., Pizzarello A. 1994. Synthesis of (R,S)-10-methyloctadecanoic acid (tuberculostearic acid) and key chiral 2-methyl branched intermediates. Chem. Phys. Lipids. 71: 145–162. [DOI] [PubMed] [Google Scholar]
- 25.Liu X., Stocker B. L., Seeberger P. H. 2006. Total synthesis of phosphatidylinositol mannosides of Mycobacterium tuberculosis. J. Am. Chem. Soc. 128: 3638–3648. [DOI] [PubMed] [Google Scholar]
- 26.Roberts I. O., Baird M. S. 2006. A new short synthesis of 10R-tuberculostearic acid and its enantiomer. Chem. Phys. Lipids. 142: 111–117. [DOI] [PubMed] [Google Scholar]
- 27.Van Zijl A. W., Minnaard A. J., Feringa B. L. 2008. Straightforward synthesis of alpha,beta-unsaturated thioesters via ruthenium-catalyzed olefin cross-metathesis with thioacrylate. J. Org. Chem. 73: 5651–5653. [DOI] [PubMed] [Google Scholar]
- 28.Mazery R., Pullez M., López F., Harutyunyan S. R., Minnaard A. J., Feringa B. L. 2005. An iterative catalytic route to enantiopure deoxypropionate subunits: asymmetric conjugate addition of Grignard reagents to α,β-unsaturated thioesters. J. Am. Chem. Soc. 127: 9966–9967. [DOI] [PubMed] [Google Scholar]
- 29.Ter Horst B., Feringa B. L., Minnaard A. J. 2007. Catalytic asymmetric synthesis of mycocerosic acid. Chem. Commun. (Camb.). 5: 489–491. [DOI] [PubMed] [Google Scholar]
- 30.Ter Horst B., Feringa B. L., Minnaard A. J. 2007. Catalytic asymmetric synthesis of phthioceranic acid, a heptamethyl-branched acid from Mycobacterium tuberculosis. Org. Lett. 9: 3013–3015. [DOI] [PubMed] [Google Scholar]
- 31.Howell G. P., Fletcher S. P., Geurts K., ter Horst B., Feringa B. L. 2006. Catalytic asymmetric synthesis of acyclic arrays by tandem 1,4-addition-aldol reactions. J. Am. Chem. Soc. 128: 14977–14985. [DOI] [PubMed] [Google Scholar]
- 32.Harutyunyan S. R., Zhao Z., den Hartog T., Bouwmeester K., Minnaard A. J., Feringa B. L., Govers F. 2008. Biologically active Phytophthora mating hormone prepared by catalytic asymmetric total synthesis. Proc. Natl. Acad. Sci. USA. 105: 8507–8512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Zijl A. W., Szymanski W., López F., Minnaard A. J., Feringa B. L. 2008. Catalytic enantioselective synthesis of vicinal dialkyl arrays. J. Org. Chem. 73: 6994–7002. [DOI] [PubMed] [Google Scholar]
- 34.Fukuyama T., Tokuyama H. 2004. Palladium-mediated synthesis of aldehydes and ketones from thiol esters. Aldrichim Acta. 37: 87–96. [Google Scholar]
- 35.Smit C., Fraaije M. W., Minnaard A. J. 2008. Reduction of carbon-carbon double bonds using organocatalytically generated diimide. J. Org. Chem. 73: 9482–9485. [DOI] [PubMed] [Google Scholar]
- 36.Eibl H. 1978. Phospholipid synthesis: oxazaphospholanes and dioxaphospholanes as intermediates. Proc. Natl. Acad. Sci. USA. 75: 4074–4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baer E., Buchnea D. 1958. Synthesis of unsaturated alpha-phosphatidic acids and alpha-bisphosphatidic acids; cardiolipin substitutes. IV. Arch. Biochem. Biophys. 78: 294–305. [DOI] [PubMed] [Google Scholar]
- 38.Rzepecki P. W., Prestwich G. D. 2002. Synthesis of hybrid lipid probes: derivatives of phosphatidylethanolamine-extended phosphatidylinositol 4,5-bisphosphate (Pea-PIP2). J. Org. Chem. 67: 5454–5460. [DOI] [PubMed] [Google Scholar]
- 39.Campbell A. S., Fraser-Reid B. 1994. Support studies for installing the phosphodiester residues of the Thy-1 glycoprotein membrane anchor. Bioorg. Med. Chem. 2: 1209–1219. [DOI] [PubMed] [Google Scholar]
- 40.Subrahmanyam D. 1965. Fatty acid composition of phosphatidyl ethanolamine of Mycobacterium tuberculosis. Indian J. Biochem. 2: 274–275. [PubMed] [Google Scholar]